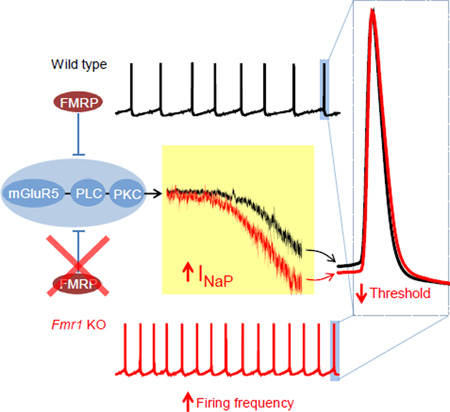
SUMMARY
Altered neuronal excitability is one of the hallmarks of fragile X syndrome (FXS), but the mechanisms underlying this critical neuronal dysfunction are poorly understood. Here we find that pyramidal cells in the entorhinal cortex of Fmr1 KO mice, an established FXS mouse model, display a decreased AP threshold and increased neuronal excitability. The AP threshold changes in Fmr1 KO mice are caused by increased persistent sodium current (INaP). Our results indicate that this abnormal INaP in Fmr1 KO animals is mediated by increased mGluR5-PLC-PKC signaling. These findings identify Na+ channel dysregulation as a major cause of neuronal hyperexcitability in cortical FXS neurons and uncover a mechanism by which abnormal mGluR5 signaling causes neuronal hyperexcitability in a FXS mouse model.
Graphical abstract
INTRODUCTION
Fragile X syndrome (FXS), the most common cause of inherited intellectual disability, is typically associated with cognitive, behavioral and social impairments, as well as a wide range of neurological abnormalities (Santoro et al., 2012). A prominent characteristic of neurological defects in FXS is neuronal hyperexcitability, which is believed to cause a variety of symptoms, including hyperactivity, increased sensitivity to sensory stimuli, and a high incidence of seizures (Contractor et al., 2015). These phenotypes account for a considerable part of the disease pathophysiology. However, the underlying mechanisms responsible for increased neuronal excitability in FXS remain poorly understood.
Altered neuronal excitability has been reported in various brain regions of FXS models (Deng et al., 2013; Gibson et al., 2008; Kalmbach et al., 2015; Myrick et al., 2015; Tang and Alger, 2015; Zhang et al., 2014). Among these regions, the parahippocampal cortices, and particularly the entorhinal cortex (EC), play an essential role in generation and maintenance of a wide range of seizure syndromes (Chatzikonstantinou, 2014). Anatomically, EC mediates the majority of connections between various brain regions and the hippocampus, which is one of the most heavily investigated brain areas implicated in the pathology of FXS (Santoro et al., 2012). The EC is thus regarded as the gateway to the hippocampus and has been implicated as one of the key epileptogenic brain areas (Chatzikonstantinou, 2014). Despite its critical role in cortico-hippocampal network excitability, little is known about pathophysiological changes in the EC that occur in FXS models. EC thus represents a highly relevant model system to investigate excitability defects in FXS.
Action potential (AP) threshold is one of the key determinants of neuronal excitability (Bean, 2007). The threshold determines when an AP is initiated, sets the neuron’s firing rate and shape neuronal computations, including coincidence detection, temporal coding and feature selectivity (Bean, 2007). AP threshold is governed predominately by Na+ channel availability and activation propensity near the threshold, whereas K+ channels and other conductances can dynamically modulate AP threshold in an adaptive way (Carter and Bean, 2009; Hu et al., 2009; Platkiewicz and Brette, 2010). In addition to the fast transient Na+ current (INaT) underlying AP rising phase, the Na+ channels can give rise to a noninactivating persistent Na+ current (INaP) that activates at subthreshold voltages (Crill, 1996). Although the amplitude of INaP is generally small relative to the INaT, it is highly functionally significant and may strongly influence transduction of synaptic inputs into AP generation (Crill, 1996; Hu et al., 2009). Here we demonstrate that INaP is abnormally increased in the EC layer III excitatory pyramidal neurons of Fmr1 KO mice, leading to decreased AP threshold and increased neuronal excitability. Our results suggest that this enhanced INaP is caused by exaggerated mGluR5 signaling acting via phospholipase C (PLC) and protein kinase C (PKC), a signaling mechanism distinct from the well-established mGluR5 signaling cascade affecting local translation in Fmr1 KO animals. These findings identify Na+ channel dysregulation as a major cause of neuronal hyperexcitability in cortical FXS neurons and uncover a previously unrecognized mechanism by which abnormal mGluR5 causes neuronal hyperexcitability in an FXS mouse model. Our findings may thus provide a therapeutic strategy to ameliorate neuronal excitability defects in FXS.
RESULTS
Increased Pyramidal Cell Excitability in the Entorhinal Cortex of Fmr1 KO Mice
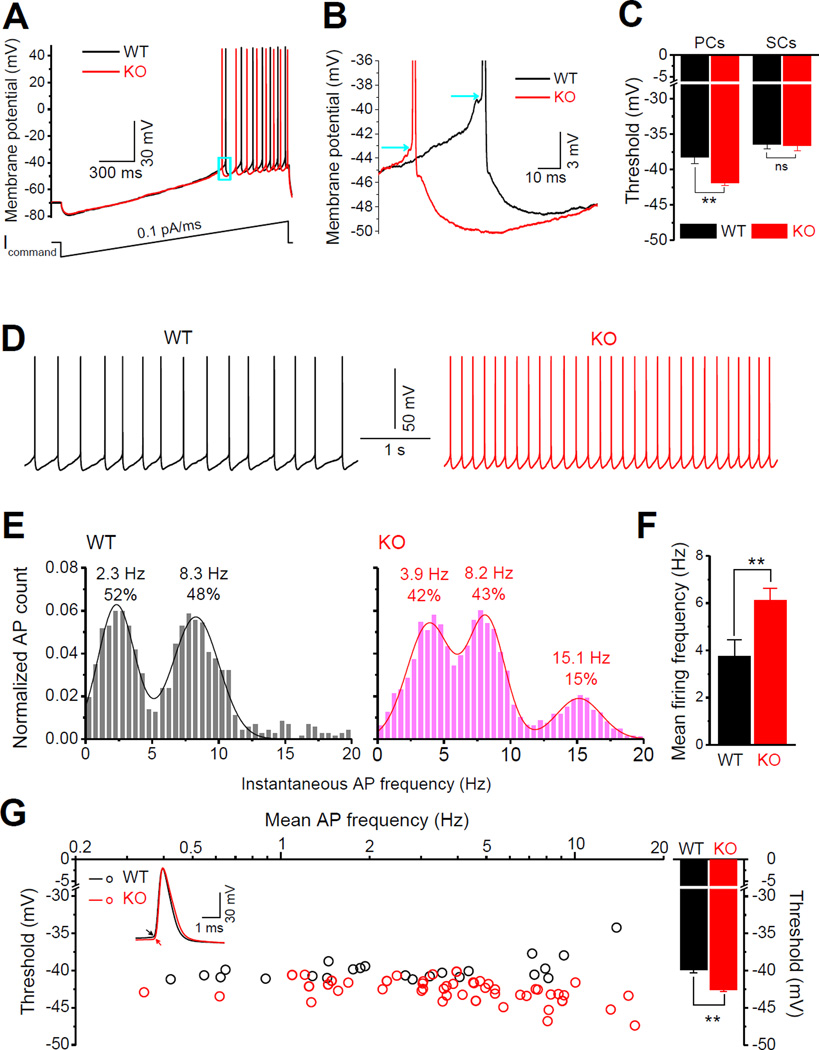
Superficial layers (layers II–III) of the EC serve as an information gateway to the hippocampus. Accordingly, we first asked whether the excitability of the principal neurons in EC superficial layers (i.e., layer II stellate cells and layer III pyramidal cells (PCs)) is altered in Fmr1 KO mice. APs were evoked by a ramp current injection (Yamada-Hanff and Bean, 2013) (Figure 1A). Only the first APs were used to determine AP threshold to avoid the effect of ramp current cumulatively inactivating Na+ channels on the threshold of following APs (Figure 1A,B). No changes in AP threshold was observed in the stellate cells of Fmr1 KO mice (WT −36.50 ± 0.56 mV, n = 7; KO −36.65 ± 0.69 mV, n = 11, p = 0.517, Figure 1C). In contrast, we found that the threshold potential of layer III PCs had a significant hyperpolarizing shift in Fmr1 KO mice (WT −38.31 ± 0.82 mV, n = 6; KO −41.88 ± 0.33 mV, n = 6, p = 0.0096; Figure 1C).
Figure 1. Increased Neuronal Excitability in the EC Layer III PCs of Fmr1 KO Mice.
(A) Determination of AP threshold. APs were evoked by a ramp current injection (~0.1pA/ms, lower trace). Representative AP traces from EC layer III PCs are shown (upper traces). Only the 1st AP was used to estimate AP threshold (box area).
(B) Enlargement of boxed area in (A) showing a hyperpolarizing shift of threshold voltage in Fmr1 KO neuron. Arrows denote the thresholds in WT and Fmr1 KO neurons.
(C) Decreased threshold voltage was found in the PCs, but not stellate cells (SCs), in the EC superficial layers.
(D) Sample traces of spontaneous APs recorded at −51 mV in WT and Fmr1 KO neurons.
(E) AP frequency distribution. A bin size of 0.5 Hz was used to calculate AP firing frequency distribution from a 20-s long trace per cell. The number of APs within a bin was divided by the total number of APs for pooling the data from all tested cells. Insert numbers denote the peak frequency and percentile estimated from Gaussian fits.
(F) Mean AP firing frequency in Fmr1 KO and WT EC layer III PCs, data averaged from (E).
(G) Threshold estimates for spontaneous APs. Each point represents an averaged AP threshold in one cell (from all APs in a 20s-long trace in each cell). Insert AP traces show a hyperpolarizing shift of threshold in Fmr1 KO neurons (arrows). Bar graph shows mean thresholds for all cells.
** p < 0.01; ns, not significant. All data are mean ± SEM.
We further examined the excitability of EC layer III PCs by setting the resting membrane potential at −51 mV through automatic current injection to induce AP firing spontaneously. At this membrane potential, about 70% of WT neurons and all tested Fmr1 KO neurons fired spontaneously (data not shown). Our results confirmed the increased excitability of these neurons in Fmr1 KO mice, as evident by the increased mean firing frequency (WT 3.75 ± 0.70 Hz, n = 23; KO 6.12 ± 0.52 Hz, n = 48, p=0.0082; Figure 1D,E,F) and decreased AP threshold (WT −39.93 ± 0.33 mV; KO −42.61 ± 0.21 mV, p<0.00001; Figure 1G). We further verified the increased excitability of Fmr1 KO neurons by examining the distribution of instantaneous AP firing frequency in the tested neurons (Figure 1E). Specifically, we noted that in Fmr1 KO neurons the first peak of firing frequency shifted from 2.3 to 3.9 Hz, and an additional 3rd peak appeared at ~15 Hz (Figure 1E). We therefore used the layer III PCs as a model to elucidate the underlying mechanisms of this defect.
Abnormal Persistent Na+ Current Causes Increased Excitability in EC Layer III Pyramidal Cells of Fmr1 KO Mice
Because the intrinsic membrane properties play a major role in setting neuronal excitability, we compared the resting membrane potential (RMP), input resistance and membrane capacitance in EC layer III PCs of Fmr1 KO and WT animals, but found no significant differences in any of these parameters between genotypes (Figure S1A-C). We further found that changes in tonic excitatory/inhibitory inputs do not account for the increased excitability of EC Layer III PCs in Fmr1 KO mice (Figure S2). In fact, we noted that sIPSC frequency was increased in Fmr1 KO neurons (Figure S2B) suggesting a compensatory action of tonic inhibition. However, this effect of tonic inhibition was insufficient to counter-balance the increased excitability of Layer III PCs in Fmr1 KO mice since our observations of decreased AP threshold and increased firing in these neurons were made in the intact EC circuits (Figure 1D-G), in the presence of this compensatory effect of tonic inhibition.
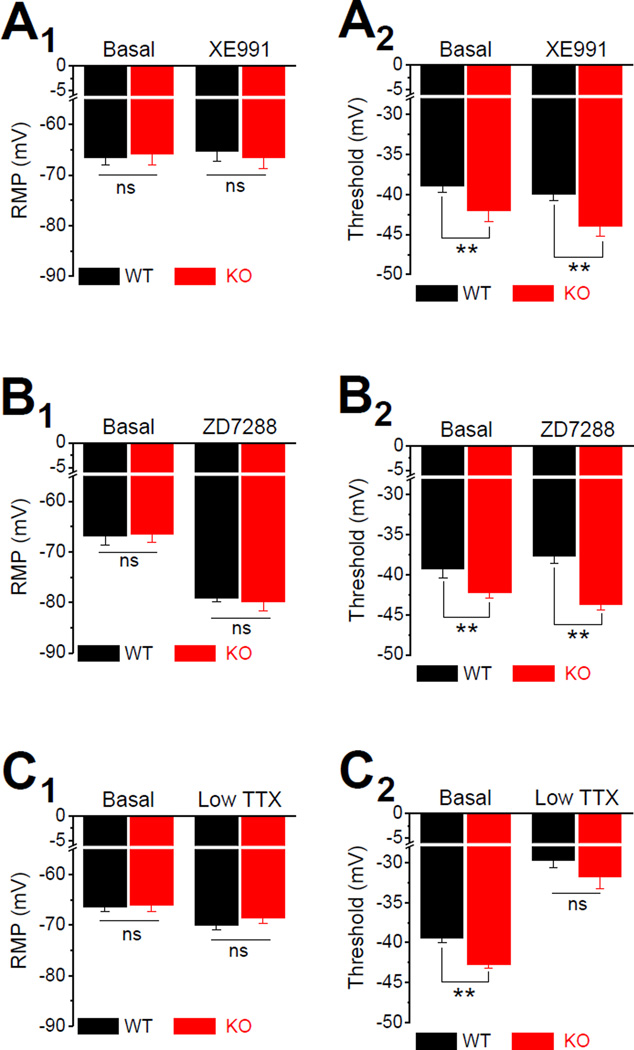
Since sub-threshold currents are believed to have critical role in controlling RMP and regulating AP initiation and thus setting neuronal excitability, we then probed whether changes in sub-threshold currents play a role in the increased excitability in Fmr1 KO neurons. There are 3 major types of sub-threshold currents in central neurons: M current (IM, carried by Kv7 channels), H current (Ih, carried by HCN channels) and INaP (Honigsperger et al., 2015; Stafstrom, 2007; Yamada-Hanff and Bean, 2013, 2015). We thus used specific inhibitors of IM, Ih or INaP to probe their role in regulating neuronal excitability in EC Layer III PCs. The Kv7 channel inhibitor XE991 (10 µM) had no detectable effect on RMP (Figure 2A1), suggesting a low activity of these channels at the potentials around RMP in EC Layer III PCs. XE991 slightly decreased the threshold potential in both WT and Fmr1 KO neurons, but it failed to affect the differences in threshold between genotypes (XE991: WT −40.02 ± 0.71 mV, n=7; KO −44.01 ± 1.25 mV, n=6, p=0.0051; Figure 2A2). Unlike XE991, the HCN channel blocker ZD7288 (10 µM) markedly hyperpolarized the RMP both in WT and Fmr1 KO neurons to the same extend (ZD7288: WT −79.20 ± 0.73, n=6; KO −79.83 ± 1.80 mV, n=6; p=0.769; Figure 2B1), suggesting a high activity of HCN channels at the potentials around RMP in both genotypes. However, ZD7288 failed to abolish the difference in AP threshold between genotypes (ZD7288: WT −37.63 ± 0.97 mV; KO −43.69 ± 0.66 mV; p=0.00047; Figure 2B2). Finally, low concentration of TTX (20 nM) to block INaP (Hammarstrom and Gage, 1998) had a small, but significant hyperpolarizing effect on RMP in both genotypes (Basal: WT −66.45 ± 0.78 mV, n=11; KO −66.12 ± 1.11 mV, n=7, p=0.816; TTX: WT −70.0 ± 0.89 mV; KO −68.57± 1.13 mV, p=0.336; Basal vs. TTX within genotype: WT p=0.0014; KO p=0.0023; Figure 2C1). Most importantly, TTX (20 nM) abolished the difference in AP threshold between genotypes (WT −29.75 ± 0.87 mV; KO −31.75 ± 1.48 mV; p=0.239; Figure 2C2). These results point to abnormal INaP, but not IM or Ih, as a potential cause of the decreased AP threshold in Fmr1 KO neurons.
Figure 2. Abnormal Persistent Na+ current in EC Layer III PCs of Fmr1 KO Mice.
(A) Effect of Kv7 channel inhibitor XE991 on RMP (A1) and AP threshold (A2).
(B) Same as (A) for HCN channel blocker, ZD7288.
(C) Same as (A) for INaP blocker, 20 nM TTX.
* p < 0.05, ** p < 0.01. All data are mean ± SEM.
Increased Persistent Sodium Current Underlies AP Threshold Changes in Fmr1 KO Mice
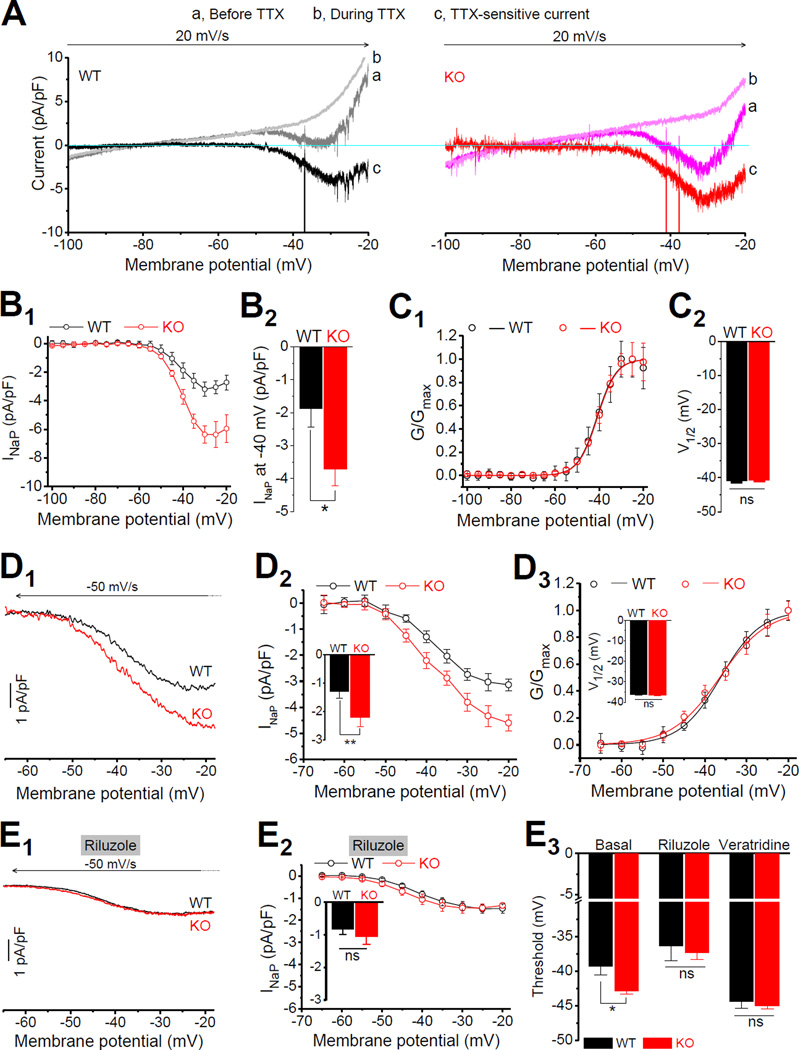
To verify the above observations and examine changes in INaP in Fmr1 KO neurons, we recorded INaP evoked by a slow depolarization ramp (20 mV/s) to measure its quasi-steady-state voltage dependence (Yamada-Hanff and Bean, 2015), using the same internal and external solutions as those used above in AP recordings. The TTX-sensitive current was first evident at ~ −65 mV (Figure 3A). AP currents that escaped voltage clamp control were present in most neurons. As expected, INaP was significantly increased in Fmr1 KO neurons (INaP at −40 mV: WT −1.88 ± 0.55 pA/pF, n=5; KO −3.71 ± 0.52 pA/pF, n=5; p=0.0297; Figure 3B), while the voltage-dependent activation of INaP was not altered in Fmr1 KO neurons (INaP half activation voltage V1/2: WT −41.1 ± 0.33 mV, n=5; KO −40.7 ± 0.19 mV, n=5; p=0.5638; Figure 3C).
Figure 3. Enhanced Persistent Na+ Current Underlies AP Threshold Changes in Fmr1 KO neurons.
(A) INaP evoked by a depolarizing voltage ramp (−100 to −20 mV, 20 mV/s) before (traces a) and during (traces b) application of TTX. TTX-sensitive current (traces c) was obtained by subtraction. Note that the large escaped AP currents were truncated to emphasize the INaP.
(B) I-V curves (B1) were constructed from the ramp evoked INaP (mean current value over 0.01 mV intervals from averages of 4–5 trials for each cell to approximating quasi-steady state current). Currents were normalized to corresponding cell capacitance for better comparison. (B2) INaP at −40 mV was significantly large in Fmr1 KO neurons.
(C) Voltage-dependent activation curve of INaP (C1). Data fitted by Boltzmann function. Summarized data of half activation voltage V1/2 (C2).
(D) INaP evoked by a repolarizing ramp (D1, +30 to −65 mV, −50 mV/s). For better comparison with (A), the traces are presented in the same direction as in (A) in a −65 to −20 mV range. Arrow indicates the time direction. (D2) The difference of I-V curves between WT and Fmr1 KO neurons. Insert: INaP at −40 mV. (D3) The voltage-dependent activation of INaP in Fmr1 KO and WT neurons. Insert: V1/2 of INaP activation.
(E) Effects of INaP inhibitor riluzole on the INaP and AP threshold in Fmr1 KO and WT neurons. (E1) Sample INaP traces. (E2) I-V curves. Insert, INaP at −40 mV. (E3) Effects of INaP inhibitor riluzole and INaP opener veratridine on AP threshold.
* p < 0.05; ** p < 0.01; ns, not significant. All data are mean ± SEM.
To further confirm these observations, we modified our protocol to avoid generation of escaped AP currents, and achieve more reliable INaP recordings. We also modified our recording solutions to include blockers of K+ and Ca2+ channels to minimize contamination of INaP measurements from Na+-activated K+ currents and other K+ and Ca2+ conductances. Briefly, we used a Cs+-based internal solution supplemented with 4-AP (2 mM) and TEA (10 mM), and also included TEA (20 mM, to replace equimolar NaCl) and CdCl2 (100 µM) in external solution. Under these conditions and using a repolarizing ramp voltage (+30 to −65 mV, −50mV/s) to evoked INaP, we could record INaP without any AP currents out of voltage clamp control. In these measurements we focused on the voltage in the range from −65 to −20 mV for better comparison with the above results. It is noteworthy that, within a certain voltage range, activation of INaP has been shown to be independent of polarity of the voltage ramp (Astman et al., 2006). Indeed, consistent with the findings above, we found that INaP was significantly larger in Fmr1 KO than WT neurons (INaP at −40mV: WT −1.29 ± 0.23 pA/pF, n=7; KO −2.21 ± 0.32 pA/pF, n=6; p=0.0066 Figure 3D1,2). As in experiments above, the voltage-dependent activation of INaP was not different between genotypes (V1/2: WT −36.27 ± 0.28 mV; KO −36.49 ± 0.41 mV, p=0.9738; Figure 3D3). We note that the half activation voltage V1/2 was shifted to a more depolarizing voltage in both genotypes compared to the above measurements (Figure 3C), presumably due to the changes in internal/external solutions (Na+ and K+ gradients, K+ and Ca2+ channel blockers). Finally, using a potent INaP inhibitor riluzole (10 µM) (Spadoni et al., 2002; Urbani and Belluzzi, 2000), we further verified that the observed differences between Fmr1 KO and WT are due to altered INaP. Indeed, riluzole (10 µM) eliminated differences in ramp-evoked current between genotypes (in riluzole at −40mV: WT −0.83 ± 0.16 pA/pF, n=6; KO −1.06 ± 0.24 pA/pF, n=6; p=0.195; Figure 3E1,2). Together, these results suggest that INaP is abnormally increased in Fmr1 KO neurons. Our measurements further indicate that within the voltage range used in our recordings depolarizing and repolarizing ramp measurements of INaP are largely equivalent. Therefore, to avoid contamination from escaped AP currents, in the following experiments we will use the repolarizing ramps to record INaP.
If reduced AP threshold in Fmr1 KO neurons results from increased INaP, then inhibition of INaP should abolish the differences in threshold between genotypes. Indeed, we found that riluzole (10 µM) abolished the difference in AP threshold between genotypes (WT −36.41 ± 2.15 mV, n=6; KO −37.38 ± 0.96, n=6; p=0.710; Figure 3E3). Moreover, we also found that the INaP opener veratridine (1 µM) shifted the threshold to a more hyperpolarizing voltages in both genotypes, and most importantly it also abolished the difference in AP threshold between genotypes (WT −44.36 ± 0.98 mV, n=6; KO −44.98 ± 0.45, n=6; p=0.613; Figure 3E3). The evidence that both INaP inhibitor riluzole and INaP opener veratridine abolished the difference in threshold between genotypes suggests that the abnormal INaP is unlikely caused by altered expression of Na+ channels in Fmr1 KO neurons. Taken together, these results suggest that an abnormally enhanced INaP causes the increased excitability of EC layer III PCs in Fmr1 KO mice.
Hyperexcitability of EC Layer III PCs in Fmr1 KO Mice Is Mediated by Exaggerated mGluR5 Signaling
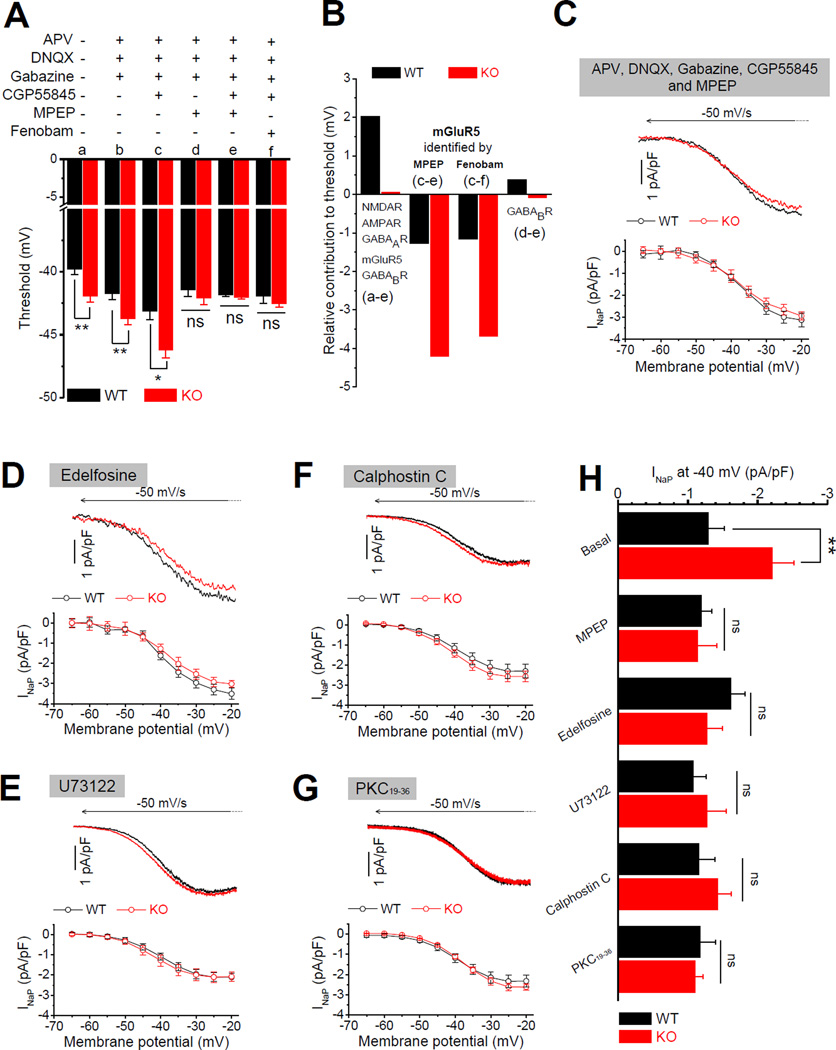
Na+ channel activity is continuously and extensively modulated by a variety of signaling pathways, including metabotropic neurotransmitter receptors. Given a number of studies implicating dysfunction of mGluR5 or GABABR signaling pathways in FXS models (Bear et al., 2004; Pacey et al., 2009; Wahlstrom-Helgren and Klyachko, 2015), both of which are known to modulate Na+ channel properties (Crill, 1996), we examined whether these signaling pathways play a role in the changes of AP threshold in Fmr1 KO neurons. We first pharmacologically isolated the cells from glutamatergic and GABAergic transmission networks by using a cocktail containing both fast and slow synaptic transmission blockers (in µM: 50 APV, 10 DNQX, 5 gabazine, 2 CGP55845 and 10 MPEP to block NMDA, AMPA, GABAA, GABAB and mGluR5 receptors, respectively). Surprisingly, the cocktail of these 5 blockers completely abolished the difference in AP threshold between genotypes (WT −41.82 ± 0.15 mV, n = 6; KO −42.01 ± 0.17 mV, n = 7, p = 0.462; Figure 4A), indicating that changes in AP threshold in Fmr1 KO neurons are mediated by activation of one or several signaling pathways coupled to these receptors. In line with our findings above that increased excitability could not be attributed to network changes in fast synaptic transmission, we found that inhibition of fast synaptic transmission alone (with APV, DNQX, and gabazine) failed to abolish the difference in threshold between genotypes (WT −41.75 ± 0.48 mV, n = 12; KO −43.71 ± 0.49 mV, n = 13, p = 0.0079; Figure 4A), pointing to abnormal metabotropic signaling pathways as mediators of AP threshold changes in Fmr1 KO neurons. We further found that inhibition of GABAB receptors with CGP55845 in combination with fast transmission blockers, shifted the threshold in a hyperpolarizing direction for both genotypes, but more importantly, it failed to reduce differences in threshold between genotypes (WT −43.10 ± 0.69 mV, n = 6; KO −46.21 ± 0.61 mV, n = 6, p = 0.032; Figure 4A). In contrast, the mGluR5 blocker MPEP in combination with the fast transmission blockers abolished differences in AP threshold between genotypes (WT −41.43 ± 0.53 mV, n = 7; KO −42.11 ± 0.50 mV, n = 6, p = 0.914; Figure 4A). These results single out the dysfunction of mGluR5 signaling as a mediator of AP threshold defect in EC Layer III PCs in Fmr1 KO mice. To verify this finding, we used another specific mGluR5 antagonist fenobam. As expected, fenobam (10 µM) (in combination with fast transmission blockers and CGP55845) abolished the difference in threshold between genotypes (WT −41.94 ± 0.58 mV, n = 7; KO – 42.53 ± 0.26 mV, n = 8, p = 0.786; Figure 4A).
Figure 4. Exaggerated mGluR5-PKC-PLC Signaling Causes Enhanced Persistent Na+ Current in Fmr1 KO Neurons.
(A) Effects of various combinations of blockers on AP threshold.
(B) Estimates for receptor contributions to cell excitability from (A).
(C) Sample INaP traces (upper panel) and summarized data (lower panel) for INaP measured in presence of mGluR5 inhibitor MPEP in combination with 4 other blockers (against NMDA, AMPA, GABAA and GABAB receptors).
(D) Same as (C) for INaP measured with intracellular application of selective PLC inhibitor edelfosine.
(E) Same as (C) for INaP measured with intracellular application of PLC inhibitor U73122.
(F) Same as (C) for INaP measured with intracellular application of PKC inhibitor calphostin C.
(G) Same as (C) for INaP measured with intracellular application of PKC inhibitor PKC19–36.
(H) INaP values at −40 mV summarized from (C-G).
* p < 0.05; ** p < 0.01; ns, not significant. All data are mean ± SEM.
To further clarify this issue, we estimated the relative contributions of the above-mentioned receptors to neuronal excitability through comparison of the changes in AP threshold by their specific blockers. In the WT neurons, the relative contribution of combining activation of these 5 receptors to AP threshold is about +2 mV as evident by subtracting threshold values obtained with and without all 5 blockers (Figure 4B). This can be interpreted to indicate that the network activity normally maintains the E/I balance via these 5 receptors with slight inhibitory dominance. In contrast, in Fmr1 KO neurons the net effect of the same 5 receptors is only about +0.06 mV, suggesting that the E/I balance in Fmr1 KO network shifts towards increased excitability. We note that addition of any blocker alters not only the individual cells we recorded from, but the entire network excitability; therefore the contribution of a specific receptor to cell excitability can only be determined while all other receptors are blocked. Thus by subtracting threshold values obtained with and without MPEP (or fenobam) in the presence of 4 other blockers, this analysis demonstrates the markedly increased contribution of mGluR5 signaling to AP threshold in Fmr1 KO compared with WT neurons (MPEP: WT −1.28 mV; KO −4.20 mV; fenobam: WT −1.16 mV; KO −3.69 mV, Figure 4B). In contrast, the contribution of GABABRs to AP threshold was very modest in both genotypes (Figure 4B). Taken together, these results indicate that the decreased AP threshold in Fmr1 KO neurons is caused by the abnormally elevated mGluR5 signaling.
Exaggerated mGluR5 signaling acting via PLC-PKC Pathway Causes Increased Persistent Na+ Current in Fmr1 KO neurons
Because our results demonstrate that enhanced INaP in Fmr1 KO neurons decreases AP threshold, and that the abnormal AP threshold is also attributed to the elevated mGluR5 activity, we then asked whether the elevated mGluR5 signaling causes enhanced INaP in Fmr1 KO neurons. To minimize the network influences from other glutamatergic or GABAergic receptors on INaP, we measured INaP in the presence of 4 blockers of NMDA, AMPA, GABAA and GABAB receptors. First, we found that INaP was still significantly larger in the Fmr1 KO than WT neurons in the presence of these 4 blockers (INaP at −40 mV: WT −1.40 ± 0.23 pA/pF, n = 7; KO −2.33 ± 0.18 pA/pF, n = 7, p = 0.0092; Figure S3A,B) and was nearly the same as INaP measured without the blockers (Figure 3D), while the voltage-dependent activation of INaP was unaffected in both genotypes (Figure S3C). These results support the notion that these four receptors modulate AP threshold through mechanisms other than INaP. In contrast, when mGluR5 blocker MPEP (10 µM) was combined with the other 4 blockers, it decreased the INaP in Fmr1 KO neurons (Figure 4C,H) and, most importantly, MPEP abolished the difference in INaP between genotypes (with MPEP, at −40 mV: WT −1.20 ± 0.15 pA/pF, n = 6; KO −1.14 ± 0.28 pA/pF, n = 6, p = 0.871; Figure 4C,H). We note that MPEP decreased INaP predominately in Fmr1 KO neurons, with negligible effect in WT neurons. These results indicate that elevated mGluR5 signaling leads to enhanced INaP in Fmr1 KO neurons.
Rapid normalization of INaP in Fmr1 KO neurons by acute inhibition of mGluR5 suggests that the effects of elevated mGluR5 signaling on INaP are mediated, at least in part, by modulation of Na+ channel activity. Indeed, pharmacological activation of Group I mGluRs has been shown to modulate activity of Na+ channels and specifically INaP (Carlier et al., 2006; D'Ascenzo et al., 2009; Dong and Ennis, 2013) in a PLC-dependent manner (D'Ascenzo et al., 2009). To probe the signaling pathway mediating mGluR5 actions on INaP in Fmr1 KO neurons, we examined the effects of disrupting the mGluR5-PLC signaling pathway using a selective PLC inhibitor, edelfosine. Application of edelfosine (1 µM) via the recording pipette (to avoid network effects) completely abolished the differences in INaP between genotypes (at −40 mV: WT −1.62 ± 0.20 pA/pF, n = 6, KO −1.28 ± 0.21 pA/pF, n = 6, p = 0.3089, Figure 4D,H). Intracellular application of another PLC inhibitor U73122 (1 µM) similarly abolished the difference in INaP between genotypes (at −40 mV: WT −1.10 ± 0.18 pA/pF, n = 6, KO −1.27 ± 0.28 pA/pF, n = 6, p =0.574, Figure 4E,H). PKC is the major downstream effector of PLC activation (Mochly-Rosen et al., 2012) and is a well known modulator of Na+ channel activity (Li et al., 1993). We therefore further tested if PKC activation downstream of PLC mediates the observed changes in INaP using two potent and selective PKC inhibitors calphostin C and PKC19–36 (a pseudosubstrate peptide inhibitor of PKC). Intracellular application of either calphostin C (10 µM) or PKC19–36 (2 µM) via the recording pipette completely abolished the differences in INaP between genotypes (Figure 4F, G, H) (calphostin C at −40 mV: WT −1.16 ± 0.23 pA/pF, n =6, KO −1.42 ± 0.20 pA/pF, n = 6, p = 0.50199; PKC19–36: WT −1.18 ± 0.21 pA/pF, n = 7, KO −1.11 ± 0.11 pA/pF, n = 6, p = 0.8308).
Taken together, our results suggest that an exaggerated activity of mGluR5 acting via PLCPKC signaling pathway enhances INaP in Fmr1 KO neurons, which in turn leads to decreased AP threshold and increased neuronal excitability in the EC layer III PCs of Fmr1 KO mice.
DISCUSSION
Here we demonstrate that PCs in the EC layer III of Fmr1 KO mice have a decreased AP threshold and increased excitability caused by dysregulation of Na+ channels. Our results indicate that this Na+ channel dysregulation is mediated by exaggerated mGluR5-PLC-PKC signaling that markedly increases persistent Na+ current in Fmr1 KO neurons. These findings suggest that Na+ channel dysregulation plays a major role in neuronal hyperexcitability in an FXS mouse model. Our results also reveal an important mechanism by which abnormal mGluR5 signaling causes neuronal hyperexcitability in the absence of FMRP. The finding that inhibition of INaP eliminates differences in AP threshold between Fmr1 KO and WT neurons suggests an avenue for development of therapeutic strategies to normalize neuronal hyperexcitability in FXS.
Emerging evidence links hyperexcitability in FXS with dysfunction in a number of ion channels, predominately K+ channels (Brown et al., 2010; Deng et al., 2013; Gross et al., 2011; Kalmbach et al., 2015; Lee and Jan, 2012; Myrick et al., 2015; Routh et al., 2013; Zhang et al., 2014) and HCN channels (Brager et al., 2012; Zhang et al., 2014). Among the conductances that modulate neuronal excitability, the sub-threshold voltage-dependent conductances are critical to both the membrane excitability state and synaptic integration via regulation of both RMP and AP threshold. IM, Ih and INaP are the three major sub-threshold conductances in central neurons that are active and do not inactivate at sub-threshold voltages, and thus play a critical role in setting neuronal excitability (Honigsperger et al., 2015; Yamada-Hanff and Bean, 2013, 2015). However, these three conductances have distinct pattern of voltage dependence: subthreshold depolarization enhances IM and INaP, but dampens Ih; in contrast, sub-threshold hyperpolarization dampens IM and INaP, but enhances Ih. As a result, these three conductances differentially contribute to regulation of RMP and AP threshold. In agreement with this notion, we found that reduced AP threshold of EC layer III PCs in Fmr1 KO mice is caused by enhanced 10 INaP, but not IM or Ih. We note that our results do not exclude a possibility that in addition to INaP, INaT is also altered in Fmr1 KO neurons. Indeed, the maximal rise speed of AP, a Na+ channel-dependent parameter, is increased in Fmr1 KO neurons (p = 0.00368, data not shown), which might indicate a change in INaT.
Our data further indicate that changes in both INaP and the AP threshold are mediated by exaggerated mGluR5 signaling in the absence of FMRP. It is well accepted that loss of FMRP causes abnormally elevated mGluR5 activity (Santoro et al., 2012). PLC is a well-defined effector of mGluR5 signaling (Kettunen et al., 2002) and is well known to modulate activity of voltage-gated ion channels and specifically INaP (D'Ascenzo et al., 2009). The receptor-dependent activation of PLC results in generation of diacylglycerol and IP3. IP3, in turn, triggers the release of calcium from the ER, causing a rise in cytosolic calcium concentration, which then activates PKC (Kettunen et al., 2002). Activation of PKC has been shown to increase neuronal excitability by enhancing INaP at subthreshold voltages in neocortical neurons (Astman et al., 2006). In addition, activation of mGluR5 by DHPG in WT mice has been shown to upregulate INaP (Carlier et al., 2006; D'Ascenzo et al., 2009). Our results suggest that the increased INaP in Fmr1 KO neurons is caused by exaggerated activity of mGluR5 acting via PLC-PKC pathway. This is a distinct signaling mechanism than the well-established signaling cascade by which abnormal mGluR5 activation impacts local translation in Fmr1 KO animals (Santoro et al., 2012). While we cannot rule out the possibility that changes in INaP are mediated in part by altered Na+ channel expression, our results indicate that the functional regulation of INaP by mGluR5 is sufficient to account for the changes in AP threshold in Fmr1 KO neurons. This notion is supported by the findings that either INaP inhibitors or INaP opener are sufficient to abolish the differences in AP threshold between genotypes. This is further supported by the observation that in PCs isolated from circuit activity (via blockade of both ionic and metabotropic transmission receptors), the maximal rise speed of AP is no longer different between genotypes (p = 0.758, data not shown)—suggesting that in the absence of circuit activity, Na+ channel properties are similar in Fmr1 KO and WT animals. Taken together, these results uncover a distinct mechanism by which abnormal mGluR5 signaling causes hyperexcitability in cortical neurons of Fmr1 KO mice.
Effects of excessive mGluR5 signaling on circuit excitability are reflected in prolongation of cortical UP states in Fmr1 KO mice (Hays et al., 2011) and our findings point to INaP as a potential target to alleviate these hyperexcitability defects. Indeed, although the amplitude of INaP is small relative to the transient Na+ current, this persistent component is highly functionally significant because it is activated ~10 mV negative to the threshold potential and is characterized by steep voltage dependence at subthreshold potentials, thus providing strongly positive feedback for further depolarization (Bean, 2007; Crill, 1996). These features make neurons with increased INaP particularly susceptible to hyperexcitability defects leading to seizures (Stafstrom, 2007), a common dysfunction in FXS. Indeed, pro-epileptic conditions have been associated with elevated INaP (Azouz et al., 1996; Somjen and Muller, 2000), and mutations in Na+ channels that cause increases in INaP have been found in patients with epilepsy (Meisler and Kearney, 2005; Vreugdenhil et al., 2004; Rhodes et al., 2005). Moreover, INaP inhibitors have been effective in treating both partial and generalized tonic-clonic seizures in humans (Stafstrom, 2007). A number of anti-epileptic drugs are INaP inhibitors and have no effects on transient Na+ current at therapeutic concentrations (Segal and Douglas, 1997; Spadoni et al., 2002; Taverna et al., 1998). Given that hyperexcitability-associated phenotypes are common in FXS, our finding that enhanced INaP has a profound effect on neuronal excitability in EC layer III PCs of Fmr1 KO mice suggests that it may play an important role in the pathophysiology of FXS. INaP may thus represent a therapeutic target for treating hyperexcitability defects in FXS.
EXPERIMENTAL PROCEDURES
Animals and slice preparation
Fmr1 KO and WT control mice on FVB background were obtained from The Jackson Laboratory. Slices were prepared as previously described (Deng et al., 2013). All animal procedures were in compliance with the NIH Guide for the Care and Use of Laboratory Animals, and conformed to Washington University Animal Studies Committee guidelines.
Electrophysiology
Whole-cell recordings using a Multiclamp 700B amplifier (Molecular Devices) were made from pyramidal or stellate cells of EC superficial layers. For INaP recordings, cell capacitance was compensated. Series resistance compensation was enabled with 80–90% correction and 16 µs lag. INaP was isolated by subtracting current in 1 µM TTX from that before TTX. All recordings were conducted at near-physiological temperature (33–34°C).
Statistical analysis
Data are presented as mean ± SEM. Student’s paired or unpaired t test was used for statistical analysis as appropriate; significance was set as p < 0.05. The n was number of cells tested.
Supplementary Material
Acknowledgments
This work was supported in part by a grant R01 NS081972 to VAK from the NINDS. We thank Mrs. Owyoung for her constructive comments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Additional details are available in Supplemental Experimental Procedures.
AUTHOR CONTRIBUTIONS
P.Y.D. and V.A.K. conceived and designed the study. P.Y.D. conducted the experiments, and analyzed the data. P.Y.D. and V.A.K. wrote the manuscript.
The authors declare no competing financial interests.
REFERENCES
- Astman N, Gutnick MJ, Fleidervish IA. Persistent sodium current in layer 5 neocortical neurons is primarily generated in the proximal axon. J Neurosci. 2006;26:3465–3473. doi: 10.1523/JNEUROSCI.4907-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azouz R, Jensen MS, Yaari Y. Ionic basis of spike after-depolarization and burst generation in adult rat hippocampal CA1 pyramidal cells. J Physiol. 1996;492(Pt 1):211–223. doi: 10.1113/jphysiol.1996.sp021302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean BP. The action potential in mammalian central neurons. Nat Rev Neurosci. 2007;8:451–465. doi: 10.1038/nrn2148. [DOI] [PubMed] [Google Scholar]
- Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004;27:370–377. doi: 10.1016/j.tins.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Brager DH, Akhavan AR, Johnston D. Impaired dendritic expression and plasticity of h-channels in the fmr1(-/y) mouse model of fragile X syndrome. Cell Rep. 2012;1:225–233. doi: 10.1016/j.celrep.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MR, Kronengold J, Gazula VR, Chen Y, Strumbos JG, Sigworth FJ, Navaratnam D, Kaczmarek LK. Fragile X mental retardation protein controls gating of the sodium-activated potassium channel Slack. Nat Neurosci. 2010;13:819–821. doi: 10.1038/nn.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlier E, Sourdet V, Boudkkazi S, Deglise P, Ankri N, Fronzaroli-Molinieres L, Debanne D. Metabotropic glutamate receptor subtype 1 regulates sodium currents in rat neocortical pyramidal neurons. J Physiol. 2006;577:141–154. doi: 10.1113/jphysiol.2006.118026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter BC, Bean BP. Sodium entry during action potentials of mammalian neurons: incomplete inactivation and reduced metabolic efficiency in fast-spiking neurons. Neuron. 2009;64:898–909. doi: 10.1016/j.neuron.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatzikonstantinou A. Epilepsy and the hippocampus. Front Neurol Neurosci. 2014;34:121–142. doi: 10.1159/000356435. [DOI] [PubMed] [Google Scholar]
- Contractor A, Klyachko VA, Portera-Cailliau C. Altered Neuronal and Circuit Excitability in Fragile X Syndrome. Neuron. 2015;87:699–715. doi: 10.1016/j.neuron.2015.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crill WE. Persistent sodium current in mammalian central neurons. Annu Rev Physiol. 1996;58:349–362. doi: 10.1146/annurev.ph.58.030196.002025. [DOI] [PubMed] [Google Scholar]
- D'Ascenzo M, Podda MV, Fellin T, Azzena GB, Haydon P, Grassi C. Activation of mGluR5 induces spike afterdepolarization and enhanced excitability in medium spiny neurons of the nucleus accumbens by modulating persistent Na+ currents. J Physiol. 2009;587:3233–3250. doi: 10.1113/jphysiol.2009.172593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng PY, Rotman Z, Blundon JA, Cho Y, Cui J, Cavalli V, Zakharenko SS, Klyachko VA. FMRP regulates neurotransmitter release and synaptic information transmission by modulating action potential duration via BK channels. Neuron. 2013;77:696–711. doi: 10.1016/j.neuron.2012.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson JR, Bartley AF, Hays SA, Huber KM. Imbalance of neocortical excitation and inhibition and altered UP states reflect network hyperexcitability in the mouse model of fragile X syndrome. J Neurophysiol. 2008;100:2615–2626. doi: 10.1152/jn.90752.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross C, Yao X, Pong DL, Jeromin A, Bassell GJ. Fragile X mental retardation protein regulates protein expression and mRNA translation of the potassium channel Kv4.2. J Neurosci. 2011;31:5693–5698. doi: 10.1523/JNEUROSCI.6661-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarstrom AK, Gage PW. Inhibition of oxidative metabolism increases persistent sodium current in rat CA1 hippocampal neurons. J Physiol. 1998;510:735–741. doi: 10.1111/j.1469-7793.1998.735bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays SA, Huber KM, Gibson JR. Altered neocortical rhythmic activity states in Fmr1 KO mice are due to enhanced mGluR5 signaling and involve changes in excitatory circuitry. J Neurosci. 2011;31:14223–14234. doi: 10.1523/JNEUROSCI.3157-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honigsperger C, Marosi M, Murphy R, Storm JF. Dorsoventral differences in Kv7/M-current and its impact on resonance, temporal summation and excitability in rat hippocampal pyramidal cells. J Physiol. 2015;593:1551–1580. doi: 10.1113/jphysiol.2014.280826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalmbach BE, Johnston D, Brager DH. Cell-Type Specific Channelopathies in the Prefrontal Cortex of the fmr1-/y Mouse Model of Fragile X Syndrome. eNeuro 2. 2015 doi: 10.1523/ENEURO.0114-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettunen P, Krieger P, Hess D, El Manira A. Signaling mechanisms of metabotropic glutamate receptor 5 subtype and its endogenous role in a locomotor network. J Neurosci. 2002;22:1868–1873. doi: 10.1523/JNEUROSCI.22-05-01868.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HY, Jan LY. Fragile X syndrome: mechanistic insights and therapeutic avenues regarding the role of potassium channels. Curr Opin Neurobiol. 2012;22:887–894. doi: 10.1016/j.conb.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, West JW, Numann R, Murphy BJ, Scheuer T, Catterall WA. Convergent regulation of sodium channels by protein kinase C and cAMP-dependent protein kinase. Science. 1993;261:1439–1442. doi: 10.1126/science.8396273. [DOI] [PubMed] [Google Scholar]
- Meisler MH, Kearney JA. Sodium channel mutations in epilepsy and other neurological disorders. J Clin Invest. 2005;115:2010–2017. doi: 10.1172/JCI25466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myrick LK, Deng PY, Hashimoto H, Oh YM, Cho Y, Poidevin MJ, Suhl JA, Visootsak J, Cavalli V, Jin P, et al. Independent role for presynaptic FMRP revealed by an FMR1 missense mutation associated with intellectual disability and seizures. Proc Natl Acad Sci U S A. 2015;112:949–956. doi: 10.1073/pnas.1423094112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacey LK, Heximer SP, Hampson DR. Increased GABA(B) receptor-mediated signaling reduces the susceptibility of fragile X knockout mice to audiogenic seizures. Mol Pharmacol. 2009;76:18–24. doi: 10.1124/mol.109.056127. [DOI] [PubMed] [Google Scholar]
- Platkiewicz J, Brette R. A threshold equation for action potential initiation. PLoS Comput Biol. 2010;6:e1000850. doi: 10.1371/journal.pcbi.1000850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes TH, Vanoye CG, Ohmori I, Ogiwara I, Yamakawa K, George AL., Jr Sodium channel dysfunction in intractable childhood epilepsy with generalized tonic-clonic seizures. J Physiol. 2005;569:433–445. doi: 10.1113/jphysiol.2005.094326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routh BN, Johnston D, Brager DH. Loss of functional A-type potassium channels in the dendrites of CA1 pyramidal neurons from a mouse model of fragile X syndrome. J Neurosci. 2013;33:19442–19450. doi: 10.1523/JNEUROSCI.3256-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro MR, Bray SM, Warren ST. Molecular mechanisms of fragile X syndrome: a twenty-year perspective. Annu Rev Pathol. 2012;7:219–245. doi: 10.1146/annurev-pathol-011811-132457. [DOI] [PubMed] [Google Scholar]
- Segal MM, Douglas AF. Late sodium channel openings underlying epileptiform activity are preferentially diminished by the anticonvulsant phenytoin. J Neurophysiol. 1997;77:3021–3034. doi: 10.1152/jn.1997.77.6.3021. [DOI] [PubMed] [Google Scholar]
- Somjen GG, Muller M. Potassium-induced enhancement of persistent inward current in hippocampal neurons in isolation and in tissue slices. Brain Res. 2000;885:102–110. doi: 10.1016/s0006-8993(00)02948-6. [DOI] [PubMed] [Google Scholar]
- Spadoni F, Hainsworth AH, Mercuri NB, Caputi L, Martella G, Lavaroni F, Bernardi G, Stefani A. Lamotrigine derivatives and riluzole inhibit INa,P in cortical neurons. Neuroreport. 2002;13:1167–1170. doi: 10.1097/00001756-200207020-00019. [DOI] [PubMed] [Google Scholar]
- Stafstrom CE. Persistent sodium current and its role in epilepsy. Epilepsy Curr. 2007;7:15–22. doi: 10.1111/j.1535-7511.2007.00156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang AH, Alger BE. Homer protein-metabotropic glutamate receptor binding regulates endocannabinoid signaling and affects hyperexcitability in a mouse model of fragile X syndrome. J Neurosci. 2015;35:3938–3945. doi: 10.1523/JNEUROSCI.4499-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taverna S, Mantegazza M, Franceschetti S, Avanzini G. Valproate selectively reduces the persistent fraction of Na+ current in neocortical neurons. Epilepsy Res. 1998;32:304–308. doi: 10.1016/s0920-1211(98)00060-6. [DOI] [PubMed] [Google Scholar]
- Urbani A, Belluzzi O. Riluzole inhibits the persistent sodium current in mammalian CNS neurons. Eur J Neurosci. 2000;12:3567–3574. doi: 10.1046/j.1460-9568.2000.00242.x. [DOI] [PubMed] [Google Scholar]
- Vreugdenhil M, Hoogland G, van Veelen CW, Wadman WJ. Persistent sodium current in subicular neurons isolated from patients with temporal lobe epilepsy. Eur J Neurosci. 2004;19:2769–2778. doi: 10.1111/j.1460-9568.2004.03400.x. [DOI] [PubMed] [Google Scholar]
- Wahlstrom-Helgren S, Klyachko VA. GABAB Receptor-Mediated Feed-Forward Circuit Dysfunction in the Mouse Model of Fragile X Syndrome. J Physiol. 2015;593:5009–5024. doi: 10.1113/JP271190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada-Hanff J, Bean BP. Persistent sodium current drives conditional pacemaking in CA1 pyramidal neurons under muscarinic stimulation. J Neurosci. 2013;33:15011–15021. doi: 10.1523/JNEUROSCI.0577-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada-Hanff J, Bean BP. Activation of Ih and TTX-sensitive sodium current at subthreshold voltages during CA1 pyramidal neuron firing. J Neurophysiol. 2015;114:2376–2389. doi: 10.1152/jn.00489.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Bonnan A, et al. Dendritic channelopathies contribute to neocortical and sensory hyperexcitability in Fmr1(-/y) mice. Nat Neurosci. 2014;17:1701–1709. doi: 10.1038/nn.3864. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.