Abstract
Corydalis yanhusuo, a well-known herbaceous plant, is commonly used in the treatment of inflammation, injury and pain. One natural agent isolated from Corydalis yanhusuo, 13-methyl-palmatrubine, was found to have a cytotoxic effect on cancer cells as reported in published studies. In the present study, we synthesized a potential anti-lung tumor agent, 13-methyl-palmatrubine and analyzed its activity. 13-Methyl-palmatrubine exhibited a cytotoxic effect on a panel of cancer cell lines in a time- and concentration-dependent manner. Among all the tested cancer cell lines, lung cancer A549 cells were most sensitive to 13-methyl-palmatrubine treatment. Meanwhile 13-methyl-palmatrubine showed less cytotoxicity in human normal cells. Our investigation revealed that 13-methyl-palmatrubine induced apoptosis and cell cycle arrest in A549 cells in a dose-dependent manner. Furthermore, 13-methyl-palmatrubine treatment caused activation of P38 and JNK pathways and blocked the EGFR pathway. In conclusion, our findings demonstrated that 13-methyl-palmatrubine inhibited the growth of A549 cells mediated by blocking of the EGFR signaling pathway and activation of the MAPK signaling pathway and provides a better understanding of the molecular mechanisms of 13-methyl-palmatrubine.
Keywords: A549 cells, apoptosis, cell cycle arrest, 13-methyl-palmatrubine, EGFR-MAPK
Introduction
Cancer is still a serious clinical issue with significant social and economic impact on the human health care system (1). Despite modern advancements in diagnosis, prevention and therapy, cancer still affects millions of patients worldwide, reduces their quality of life and is one of the leading causes of mortality worldwide (1,2). Natural products including plants, microorganisms and marines provide rich resources for anticancer drug discovery (3,4). Based on ancient and modern Chinese herbal medicine books and Pharmacopoeia of China, there are many natural anticancer plants or herbal formulations which provide a guide, along with clinical evidence, for the identification of new anticancer agents or a natural source of alternative cancer therapy, and these have recently received increasing scientific attention (5,6).
Corydalis yanhusuo, also named Rhizoma Corydalis, is a well-known herbaceous plant commonly used in the treatment of pain, injuries and coronary diseases in Traditional Chinese Medicine (7,8). Alkaloids are important biological active constituents of Corydalis yanhusuo (9). 13-Methyl-palmatrubine was isolated as a natural protoberberine alkaloid from the methanol extract of the tubes of Corydalis yanhusuo (10). The chemical structure of 13-methyl-palmatrubine is shown in Fig. 1A. One study found that 13-methyl-palmatrubine exhibited antitumor activity in 3 types of human cancer cell lines (11). In our large scale screening for suitable anticancer agents from herbal plants, 13-methyl-palmatrubine exhibited an antiproliferative effect on various cell lines. Further experiments indicated that 13-methyl-palmatrubine induced apoptosis and arrested the cell cycle in lung cancer A549 cells, suggesting that 13-methyl-palmatrubine may be an antitumor compound for lung cancer treatment.
Figure 1.
Growth inhibition effect of 13-methyl-palmatrubine on several cell lines. (A) The chemical structure of 13-methyl-palmatrubine. (B) The inhibition effect of 13-methyl-palmatrubine on 5 human cancer cell lines at 48 h. (C) Cell viability of A549 cells following treatment with increasing concentrations of 13-methyl-palmatrubine for 48 h. (D) Effect of increasing concentrations of 13-methyl-palmatrubine on HEK293 and L02 cells for 48 h. (E) The body weights of nude A549 mouse models in vivo. (F) 13-Methyl-palmatrubine suppressed tumor growth in A549 nude mouse models in vivo. (G) MMP collapse in A549 cells at 24 h following 13-methyl-palmatrubine exposure. (H) 13-methyl-palmatrubine exposure induced caspase-9 activation in the A549 cells following a 48-h treatment. (I) 13-Methyl-palmatrubine exposure induced caspase-3 activation in the A549 cells following a 48-h treatment. Statistical differences were considered significant at the levels of *P<0.05, **P<0.01 or ***P<0.001.
Materials and methods
Materials
RPMI-1640 medium, fetal bovine serum (FBS), pancreatin, penicillin and streptomycin were obtained from Gibco (Carlsbad, CA, USA). Terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end-labeling (TUNEL) Apo-Green Detection kit was supplied by Roche (Roche, Basel, Switzerland). The Annexin V and PI kit was purchased from Biotool (Selleck Chemicals, Houston, TX, USA). Protein and RNA extraction kits, BCA protein assay kit, propidium iodide (PI), caspase-3 and -9 activity kit, dimethyl sulfoxide (DMSO), 4′,6-diamidino-2-phenylindole (DAPI) and Hoechst 33243 were purchased from Beyotime Institute of Biotechnology (Beyotime, Haimeng, China). 13-Methyl-palmatrubine standard preparation (purity >98%) was purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). The JC-10 Mitochondrion Membrane Potential Assay Kit was obtained from AAT Bioquest (Sunnyvale, CA, USA). ECL Advanced Detection kit was provided by Thermo Fisher (Waltham, USA). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl-2H tetrazolium bromide (MTT) and bovine serum albumin (BSA) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Primary antibodies and HRP-labeled secondary anti-mouse/anti-rabbit antibodies were provided by Cell Signaling Technology (CST; Beverly, MA, USA). All other chemicals needed were of analytic grade.
Cell lines and cell culture
The human cell lines used in the present study included A549, a human lung cancer cell line; HCT116, a human colon carcinoma cell line; MCF-7, a human breast cancer cell line; MKN-45, a human cancer cell line; HepG2, a human hepatocellular carcinoma cell line, L02, a human normal liver cell line; and HEK293 a human kidney normal cell line obtained from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). Cells were grown in a humidified atmosphere of 5% CO2 at 37°C with RPMI-1640 medium. Cells were supplemented with 10% fetal calf serum containing antibiotics (100 IU/ml penicillin and 100 IU/ml streptomycin).
Cytotoxicity assay in vitro
Cells were seeded and treated with 13-methyl-palmatrubine at increasing concentrations. The 13-methyl-palmatrubine standard was dissolved in DMSO at a concentration of 300 µg/ml as the initial dose. Then, the stock solution was maintained at −80°C. The solutions were diluted to the desired concentrations when used. 13-Methyl-palmatrubine was dissolved in DMSO with a final DMSO concentration <0.1%. Cytotoxicity of the control and treated cells was measured using the MTT assay. Cells (5×103) were cultured in 96-well plates treated with the indicated concentratins for the indicated times. The absorbance of the treated and control cells at 570 nm was assessed using a microplate reader (PerkinElmer, Inc., Waltham, MA, USA).
Cell cycle distribution assay by flow cytometry
Cell cycle analysis was determined by PI staining. Briefly, A549 cells were treated with 13-methyl-palmatrubine. Treated and control cells were harvested, washed twice with phosphate-buffered saline (PBS) and fixed with pre-cooled 70% ethanol for 4 h at 4°C. Fixed cells were washed, pelleted, re-suspended in 500 µl PBS containing 50 µg RNase A at 37°C and then stained with 5 µg PI in the dark at room temperature for 30 min. Finally, cell cycle distributions were immediately assessed using a FACSCalibur cytometer (BD Biosciences, San Jose, CA, USA).
Cell morphological assay
Hoechst 33342 staining
The non-small cell lung cancer (NSCLC) A549 cells were cultured in 6-well plates and treated with increasing doses of 13-methyl-palmatrubine. The cells were fixed with 4% paraformaldehyde and incubated with Hoechst 33342 (5 µg/ml) for 10 min. After washing with cold PBS, the A549 cells after treatment and the control cells were observed by inverted fluorescence microscopy (D5100; Nikon, Tokyo, Japan).
TUNEL staining
The NCSLC A549 cells were cultured and treated on a specific glass cover in 6-well plates and treated as mentioned above. TUNEL kit was used to determine DNA fragmentation in apoptotic cells according to the manufacturer's instructions. The cells were stained by TUNEL and DAPI for image analysis. The samples after treatment and the control cells were viewed by Apo-Green fluorescence at 520 nm and blue DAPI at 460 nm using a fluorescence microscope (D5100).
Apoptosis analysis with Annexin V/PI
The A549 cells were cultured and treated as mentioned above. A FITC-Annexin V/PI cell apoptosis assay was conducted to evaluate the apoptosis ratio in the cells according to the kit manufacturer's instructions. In brief, the untreated and treated cells were washed and harvested in 100 µl 1X binding buffer, and 5 µl Annexin V-FITC solution and 5 µl PI staining solution were added to each 100 µl of cell suspension. Then, the cells were incubated for 15 min. Binding buffer (400 µl) was added to the cell suspensions before determination by FACSCalibur flow cytometry (BD Biosciences).
Mitochondrial membrane potential (MMP) assay
The AAT JC-10 assay kit was used to evaluate the changes in MMP. The kit was used according to the manufacturer's instructions. Briefly, A549 cells were seeded in a 96-well plate, and 50 µl/well JC-10 solution was added into the cell suspensions. Cell suspensions were incubate in a 37°C incubator with 5% CO2 for 30 min. The cells were washed twice with pre-cold PBS, re-suspended in assay buffer B and immediately examined on a microplate reader under excitation filter of 490 nm while emission filter of 525 and 590 nm, separately (PerkinElmer, Inc.).
Animals
The present study was strictly conducted according to the Declaration of Helsinki and the Guide for the Care and the Use of Laboratory Animals as adopted and promulgated by the United States National Institutes of Health. All animal experiments were approved by the Institutional Animal Care and Use Committee of FuDan University. Nude mice (6 weeks), half males and half females, were provided by Shanghai SLAC Laboratory Animal Center. Healthy A549 cells were harvested, washed 3 times, and suspended in cold PBS. Each mouse was injected intraperitoneally with 1×106 A549 cells. The nude mice were then divided into four groups: control, low dose, medium dose and high dose groups, with 6 mice in each group. When the volume of the tumor reached 100 mm3, 13-methyl-palmatrubine was injected into the nude mice, while saline was injected in the control group. All groups were administered the injection every 3 days for a total of 7 treatments. The nude mice were sacrificed 24 h after the last treatment, and the tumor xenografts were removed and measured. Tumor sizes were measured every 3 days using micrometer calipers, and tumor weights were calculated at the conclusion of the experiments.
Immunohistochemistry
The paraffin-embedded implanted tumor samples were stained using cleaved-caspase-3 and Ki67 antibodies separately for immunohistochemistry according to the manufacture's instructions. Images were captured by a fluorescence microscope (D5100).
Western blotting
A549 cells were planted and treated with the designated concentrations of 13-methyl-palmatrubine for the desired times. The cells were harvested, washed and lysed in RIPA lysis buffer. Then, the lysate was centrifuged at 12,000 rpm for 10 min at 4°C. The BCA kit was used to determine the protein level of the supernatant. Proteins were separated with 8–15% SDS-PAGE, transferred to polyvinylidene fluoride (PVDF) membranes, and incubated with the respective primary antibody at 4°C overnight. Then, the proteins were subsequently incubated with the secondary antibody. The signals were determined by system (BD Biosciences). The β-actin antibody was chosen as the control.
Analysis of data
Triplicate experiments were performed with independent samples. The results are expressed as means ± standard deviation (SD). The results were analyzed using ANOVA t-test to assess statistical significance. A P-value at <0.05 was considered to indicate a statistically significant result. All statistical analyses were performed using commercially available statistical software (SPSS 19.0; SPSS, Inc., Chicago, IL, USA). Statistical differences were considered at the P<0.05, P<0.01 or P<0.001 level vs. the control group as indicated in the figures and legends.
Results
13-Methyl-palmatrubine inhibits proliferation and induces cell cycle arrest and apoptosis in the A549 cells in vitro
To verify the effect of 13-methyl-palmatrubine on cancer cell growth, cells were treated with various concentrations of 13-methyl-palmatrubine for 48 h. MTT assay was used to assess the cell viability after treatment. As shown in Fig. 1B, the viability of five cancer cell lines was decreased in a concentration-dependent manner. Compared with the A549 cells, the other human cancer cell lines demonstrated more resistance to the 13-methyl-palmatrubine treatment, indicating that A549 human lung cancer cells are more susceptible to 13-methyl-palmatrubine. Furthermore, cell-induced apoptosis by 13-methyl-palmatrubine in A549 cells was further confirmed in a time- and dose-dependent manner, as shown in Fig. 1C. The most efficacious 13-methyl-palmatrubine treatment time was 48 h. The IC50 value of 13-methyl-palmatrubine in the A549 cells was 58.57±3.58 µg/ml at 48 h. Significantly higher IC50 values for 13-methyl-palmatrubine in two normal human cell lines (L02 and HEK293) are shown in Fig. 1D, suggesting the relative safety of 13-methyl-palmatrubine.
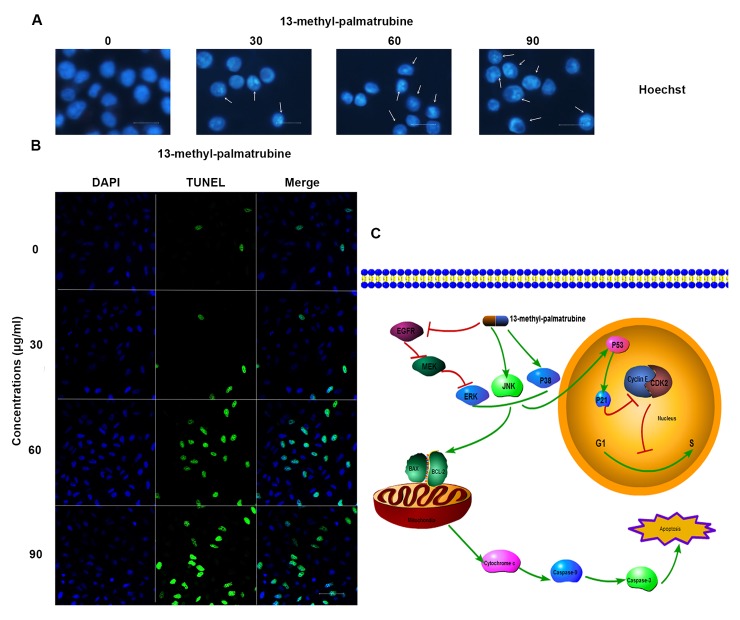
In order to investigate the inhibitory effect on proliferation induced by 13-methyl-palmatrubine treatment, multiple assays were conducted to analyze whether 13-methyl-palmatrubine induces apoptosis in A549 cells. Based on the positive preliminary MTT results, we conducted the Annexin V/PI assay to determine the apoptotic population in the 13-methyl-palmatrubine-treated and control cells. 13-Methyl-palmatrubine treatment significantly increased the percentage of apoptotic cells when compared with the control, as shown in Fig. 2. Hoechst staining exhibited differences in cell morphology displying cell shrinkage, nuclear fragmentation and chromatin compaction (Fig. 4A), which commonly represents typical apoptosis in most apoptotic cases. Consistently, TUNEL staining demonstrated an increased ratio of TUNEL (green) in the 13-methyl-palmatrubine-treated cells (Fig. 4B).
Figure 2.
13-Methyl-palmatrubine induces apoptosis in the A549 cell line. A549 cells were treated with increasing concentrations of 13-methyl-palmatrubine or combined with EGF for 48 h, and then the cells were assessed using Annexin V/PI flow cytometric assay. Statistical differences were considered significant at the levels of *P<0.05, **P<0.01 or ***P<0.001.
Figure 4.
Effect of 13-methyl-palmatrubine on apoptosis and cell cycle arrest in the A549 cells. Cells were exposed to increasing concentrations of 13-methyl-palmatrubine for 48 h. (A) Immunofluorescence microscopy images of Hoechst staining of the 13-methyl-palmatrubine-treated A549 cells in vitro; scale bar, 10 µm. (B) Immunofluorescence microscopy image of TUNEL staining of the 13-methyl-palmatrubine-treated A549 cells for DAPI (blue), TUNEL FITC (green) and their merge in vitro; scale bar, 50 µm. (C) Illustration of the mechanism involved in the effect of 13-methyl-palmatrubine on A549 cells.
Subsequently, analysis of cell cycle distribution by flow cytometry was performed. As shown in Fig. 3, 13-methyl-palmatrubine treatment at increasing concentrations in the A549 cells led to a G1 phase accumulation of cells. These results showed that 13-methyl-palmatrubine treatment induced an accumulation of A549 cells in the G1/S phase in a dose-dependent manner. Collectively, these results showed that 13-methyl-palmatrubine inhibited A549 cell proliferation via G1/S cell cycle phase arrest and apoptosis.
Figure 3.
13-Methyl-palmatrubine induces cell cycle arrest in the A549 cell line. A549 cells were treated with increasing concentrations of 13-methyl-palmatrubine or combined with EGF for 48 h, then the cells were assessed using cell cycle flow cytometric assay. Statistical differences were considered significant at the levels of *P<0.05, **P<0.01 or ***P<0.001.
13-Methyl-palmatrubine inhibits proliferation and induces cell cycle arrest and apoptosis in A549 cells in vivo
An in vivo study was conducted to evaluate the antiproliferative effect of 13-methyl-palmatrubine. During the study, no marked change in mouse body weight was noted (Table I and Fig. 1E). This implied that injection of 13-methyl-palmatrubine was not significantly toxic to the nude mice. After treatment for 21 days, the tumors treated with 13-methyl-palmatrubine were smaller than that noted in the control group (Table I and Fig. 1F). Therefore, we suggested that 13-methyl-palmatrubine may be a promising approach toward antitumor treatment. The results were consistent with the in vitro study.
Table I.
Inhibitory effect of 13-methyl-palmatrubine on A549 implantation tumor growth in BALB/c-nu mice.
| Groups | Surviving animals (n) | Body weight (g)
|
Tumor weight (g) | Inhibition rate (%) | |
|---|---|---|---|---|---|
| Start | End | ||||
| Control | 6 | 20.2±0.30 | 24.98±1.17 | 1.40±0.18 | |
| Treatment | |||||
| 3 mg/kg | 6 | 20.88±0.36 | 24.58±1.22 | 0.91±0.23a | 34.59 |
| 6 mg/kg | 6 | 20.77±0.47 | 24.61±1.08 | 0.62±0.23c | 55.78 |
| 9 mg/kg | 6 | 20.58±0.58 | 23.90±1.40 | 0.38±0.16c | 73.18 |
P<0.05 vs. the vehicle control;
P<0.01 vs. the vehicle control;
P<0.001 vs. the vehicle control. Treatment groups were administered 13-methyl-palmatrubine at different doses.
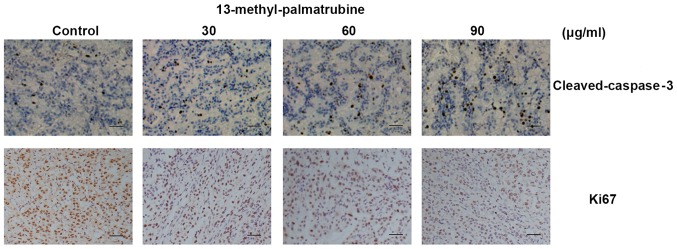
We found a significant decrease in the Ki67-positive level in cells in the treatment group as compared with that noted in the control group. Meanwhile, cleaved-caspase-3-positive cells were increased in a concentration-dependent manner (Fig. 5), which indicated the apoptotic and antiproliferative effects of 13-methyl-palmatrubine in vivo.
Figure 5.
Antitumor effect of 13-methyl-palmatrubine on A549 cells in vivo after 21 days of administration. Effect of 13-methyl-palmatrubine on cleaved-caspase-3 and Ki67 levels in the A549 nude model; scale bar, 100 µm.
The mechanism of 13-methyl-palmatrubine in apoptosis
The JC-10 assay indicated the loss of ΔΨm after 13-methyl-palmatrubine treatment. Loss of ΔΨm is a typical event in the early phase of the mitochondrial apoptotic pathway. Thus, we choose 24 h as the experiment time in the JC-10 assay to investigate the loss of MMP. As shown in Fig. 1G, our findings indicated that the red fluorescence (normal cells) was decreased while the green fluorescence (apoptotic cells) increased in the 13-methyl-palmatrubine treated cells. The decrease in the ratio of red/green fluorescence indicated that 13-methyl-palmatrubine induced the collapse of MMP (ΔΨm) in the A549 cells. Caspase-9 and caspase-3 activity assay demonstrated activation of caspase-9 and caspase-3. As shown in Fig. 1H and I, 13-methyl-palmatrubine induced a significant activation of caspase-3 and -9 in a dose-dependent manner, and there was a significant difference between the control and 13-methyl-palmatrubine-treated cells.
Western blotting of the 13-methyl-palmatrubine-treated A549 cells vs. the control cells indicated the related apoptotic mechanism (Fig. 6). The epidermal growth factor receptor (EGFR) pathway leads to numerous effects such as anti-apoptosis, cell cycle arrest. MAPK is one of its multiple downstream pathways. In the present study, 13-methyl-palmatrubine treatment caused downregulation of EGFR, RAS and ERK. Notably, there was marked upregulation in the MAPK expression level. Phosphorylated (p)-P38 and p-JNK were increased in a concentration-dependent manner, while there were no changes in the total P38 and total JNK levels. The Bax/Bcl-2 ratio was increased. The induction of apoptotic released c-Myc, and subsequent activation of caspase-9 and -3 and cleaved PARP.
Figure 6.
Effect of 13-methyl-palmatrubine on EGFR-MAPK-related protein levels by western blot assay. A549 cells were exposed to increasing doses of 13-methyl-palmatrubine for 48 h.
Mechanism of 13-methyl-palmatrubine in cell cycle arrest
As above mentioned, there was an accumulation of cells undergoing cell cycle progression in the G1/S phase in a dose-dependent manner following 13-methyl-palmatrubine treatment in the A549 cells. Then, we investigated whether 13-methyl-palmatrubine arrested the cell cycle through expression of cyclin E and CDK2. Western blotting showed an appreciable downregulation of cyclin E and upregulation of CDK2 with increasing concentrations of 13-methyl-palmatrubine. Thus, we examined the expression level of P53 and P21, which are two important proteins that lie upstream of cyclin E/CDK2 and commonly result in cell cycle arrest and apoptosis. Our results indicated that treatment with 13-methyl-palmatrubine arrested the cell cycle in the G1/S phase in a dose-dependent manner in the A549 cells through upregulation of P53, P21 and inhibition of CDK2 and cyclin E complex (Fig. 6).
Discussion
The antitumor effect of 13-methyl-palmatrubine was reported 10 years ago. 13-Methyl-palmatrubine was found to exhibit a moderate cytotoxic effect in several carcinoma cell lines (11). The present study discovered the antiproliferative effect of 13-methyl-palmatrubine in a panel of cancer cell lines. Meanwhile, the IC50 value of 13-methyl-palmatrubine in normal cells was significantly higher than this value noted in the cancer cell lines. These results suggested that 13-methyl-palmatrubine may be a promising anticancer agent. Thus, exploration of the mechanism of 13-methyl-palmatrubine treatment was required.
The epidermal growth factor receptor (EFGR) protein is a 170-kDa glycoprotein which consists of an extracellular ligand-binding domain, a transmembrane domain containing a single hydrophobic anchor sequence and an intracellular domain with tyrosine. Phosphorylated EGFR initiates the activation of downstream pathways, including Janus kinase (JAK) signal transducer and activator of transcription (STAT), phosphatidylinositol 3-kinase (PI3K)/AKT and mitogen-activated protein kinase (MAPK) cascades (12,13). Activation of the EGFR downstream pathway leads to cell proliferation, migration, adhesion, anti-apoptosis, angiogenesis and metastasis (13,14). High expression of EGFR is closely associated with multiple epithelial-derived tumors, for example, breast (15), colon (16), ovarian (17), gastric (18) and lung cancer (19).
At present, novel therapeutic approaches, particularly antitumor agents, targeting the EGFR signaling pathway family and their downstream pathways have been developed, such as gefitinib (20) and afatinib (21). In the present study, we evaluated the impact of 13-methyl-palmatrubine treatment on the EGFR pathway. 13-Methyl-palmatrubine at a moderate concentration inhibited activation of the EGFR pathway with a downstream effect on Ras/Raf/MEK/ERK. This effect was closely associated with cell apoptosis and cell proliferation. The MAPK signaling pathway lies downstream of EGFR and accepts the signal transmission of EGFR (22). Numerous antitumor agents focus on MAPK as a molecular target (23,24). The ERK of MAPK is inhibited through mediation of the EGFR pathway (17). Notably, 13-methyl-palmatrubine treatment induced phosphorylation of JNK and P38 pathways which are other important signaling pathways in MAPK. The combination effect of EGFR and MAPK led to a subsequent cascade apoptotic reaction in 13-methyl-palmatrubine-induced cells.
MMPs play a crucial role in the apoptotic cascade pathways (25). MMP collapse allows release of cytochrome c from the space between the outer and inner mitochondrial membranes into the cytosol, and therefore subsequently triggers caspase activation and other apoptotic processes (26,27). In the present study, 13-methyl-palmatrubine treatment elicited MMP collapse, and induced the release of cytochrome c which is associated with the activation of caspase-3 and -9, and cleavage of PARP. Thereby, 13-methyl-palmatrubine treatment triggers A549 cell death. The present study suggested that 13-methyl-palmatrubine induced cells to undergo apoptosis by initiating the intrinsic mitochondrial-mediated pathway.
Serial study
In addition, we conducted a serial study to confirm the EGFR-MAPK signaling pathway activity in 13-methyl-palmatrubine-treated A549 cells. As known, EGF stimulates activation of the EGFR signaling pathway (28). At first, the apoptosis and cell cycle in the A549 cells treated with 13-methyl-palmatrubine at medium concentrations followed by the addition of EGF to 100 ng/ml were evaluated. The apoptosis in the 13-methyl-palmatrubine combined with EGF group was decreased compared with the 13-methyl-palmatrubine only treated group, while the cell cycle was also arrested (Figs. 2 and 3). The EGFR protein and downstream ERK protein levels were upregulated in the combination group (Fig. 7). These results demonstrated that the EGFR signaling pathway plays an important role in the activity of 13-methyl-palmatrubine in the A549 cells.
Figure 7.
Effect of 13-methyl-palmatrubine on EGFR-MAPK-related protein levels by western blot assay. A549 cells were exposed to 13-methyl-palmatrubine (0 and 60 µg/ml) or 13-methyl-palmatrubine (60 µg/ml) combined with EGF (100 ng/ml) for 48 h, SP600125 (JNK inhibitor, 5 µM) for 9 h and SB203580 (P38 inhibitor, 5 µM) for 9 h.
Secondly, SP600125 and SB203580 are commonly used to abolish JNK and P38 signaling pathway phosphorylation, separately. Thus, they were employed to further investigation the role of the MAPK signaling pathway in the 13-methyl-palmatrubine-treated A549 cells. As shown in Fig. 7, SP600125 suppressed JNK phosphorylation while it exerted no impact on other signaling pathways. SB203580 inhibited P38 phosphorylation while it elicited no impact on other signaling pathways. In conclusion, EGFR inhibition, JNK activation and P38 activation may run separately and contribute combination apoptotic effects.
P53 is a critical protein which causes a cellular response to cell DNA damage in the apoptotic pathway (29). Meanwhile, P53 also plays a crucial role in stimulating the transcription that arrests the cell cycle (30). The regulation of the cell cycle is also an important target of cancer therapy (31). Anticancer drugs usually arrest the cell cycle at the G1/S or G2/M phase (32,33). In the present study, 13-methyl-palmatrubine induced a significant increase in G1/S arrest at increasing concentrations. P53 and its downstream pathway genes, such as P21, are tightly linked to cell proliferation, apoptosis and differentiation (34,35). As mentioned above, western blot analysis demonstrated a significant increase in P53 and P21 expression. The G1 phase to S phase cell progression is activated by phosphorylated Rb which is affected by CDK2/cyclin E complexes. Our western blot results showed that 13-methyl-palmatrubine inhibited the expression of CDK2 and cyclin E.
In conclusion, in the present study, 13-methyl-palmatrubine was found to exert an antitumor effect via induced apoptosis and cell cycle arrest. The EGFR signaling pathway and downstream MAPK signaling pathway played important roles in the 13-methyl-palmatrubine-induced antitumor effect on the A549 cells (Fig. 4C). In conclusion, the results suggest that 13-methyl-palmatrubine may serve as a potential therapeutic anticancer compound against human lung tumors.
Acknowledgments
The State Administration of Traditional Chinese Medicine 'Twelfth Five Year Plan' Key Specialty (Chinese Medicine Geriatrics) and Shanghai 'XinLin New Star Plan' (ZY3-RCPY-2-2031) provided financial support for the present study. The technological support was provided by the Shanghai Key Laboratory of Clinical Geriatric Medicine.
References
- 1.Saika K, Sobue T. Cancer statistics in the world. Gan To Kagaku Ryoho. 2013;40:2475–2480. In Japanese. [PubMed] [Google Scholar]
- 2.DeSantis C, Naishadham D, Jemal A. Cancer statistics for African Americans, 2013. CA Cancer J Clin. 2013;63:151–166. doi: 10.3322/caac.21173. [DOI] [PubMed] [Google Scholar]
- 3.Safarzadeh E, Sandoghchian Shotorbani S, Baradaran B. Herbal medicine as inducers of apoptosis in cancer treatment. Adv Pharm Bull. 2014;4(Suppl 1):S421–S427. doi: 10.5681/apb.2014.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hiruma W, Suruga K, Kadokura K, Tomita T, Sekino Y, Komatsu Y, Kimura M, Ono N. Antitumor effects of a plant extract mixture. Yakugaku Zasshi. 2013;133:487–491. doi: 10.1248/yakushi.12-00278-1. In Japanese. [DOI] [PubMed] [Google Scholar]
- 5.Lu Y, Li CS, Dong Q. Chinese herb related molecules of cancer-cell-apoptosis: A minireview of progress between Kanglaite injection and related genes. J Exp Clin Cancer Res. 2008;27:31. doi: 10.1186/1756-9966-27-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng S, Yang H, Zhang S, Wang X, Yu L, Lu J, Li J. Initial study on naturally occurring products from traditional Chinese herbs and vegetables for chemoprevention. J Cell Biochem Suppl. 1997;27:106–112. doi: 10.1002/(SICI)1097-4644(1997)27+<106::AID-JCB17>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 7.Leung WC, Zheng H, Huen M, Law SL, Xue H. Anxiolytic-like action of orally administered dl-tetrahydropalmatine in elevated plus-maze. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:775–779. doi: 10.1016/S0278-5846(03)00108-8. [DOI] [PubMed] [Google Scholar]
- 8.Sagare AP, Lee YL, Lin TC, Chen CC, Tsay HS. Cytokinin-induced somatic embryogenesis and plant regeneration in Corydalis yanhusuo (Fumariaceae) - a medicinal plant. Plant Sci. 2000;160:139–147. doi: 10.1016/S0168-9452(00)00377-0. [DOI] [PubMed] [Google Scholar]
- 9.Wu G, Qian Z, Guo J, Hu D, Bao J, Xie J, Xu W, Lu J, Chen X, Wang Y. Ganoderma lucidum extract induces G1 cell cycle arrest, and apoptosis in human breast cancer cells. Am J Chin Med. 2012;40:631–642. doi: 10.1142/S0192415X12500474. [DOI] [PubMed] [Google Scholar]
- 10.Cheng XY, Shi Y, Zheng SL, Jin W, Sun H. Two new protoberberine quaternary alkaloids from Corydalis yanhusuo. J Asian Nat Prod Res. 2008;10:1117–1121. doi: 10.1080/10286020802410615. [DOI] [PubMed] [Google Scholar]
- 11.Ikekawa T, Ikeda Y. Antitumor activity of 13-methyl-berberrubine derivatives. J Pharmacobiodyn. 1982;5:469–474. doi: 10.1248/bpb1978.5.469. [DOI] [PubMed] [Google Scholar]
- 12.Lurje G, Lenz HJ. EGFR signaling and drug discovery. Oncology. 2009;77:400–410. doi: 10.1159/000279388. [DOI] [PubMed] [Google Scholar]
- 13.Uribe P, Gonzalez S. Epidermal growth factor receptor (EGFR) and squamous cell carcinoma of the skin: Molecular bases for EGFR-targeted therapy. Pathol Res Pract. 2011;207:337–342. doi: 10.1016/j.prp.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 14.Hynes NE, Lane HA. ERBB receptors and cancer: The complexity of targeted inhibitors. Nat Rev Cancer. 2005;5:341–354. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- 15.Normanno N, Campiglio M, Maiello MR, De Luca A, Mancino M, Gallo M, D'Alessio A, Menard S. Breast cancer cells with acquired resistance to the EGFR tyrosine kinase inhibitor gefitinib show persistent activation of MAPK signaling. Breast Cancer Res Treat. 2008;112:25–33. doi: 10.1007/s10549-007-9830-2. [DOI] [PubMed] [Google Scholar]
- 16.LaBonte MJ, Wilson PM, Fazzone W, Russell J, Louie SG, El-Khoueiry A, Lenz HJ, Ladner RD. The dual EGFR/HER2 inhibitor lapatinib synergistically enhances the antitumor activity of the histone deacetylase inhibitor panobinostat in colorectal cancer models. Cancer Res. 2011;71:3635–3648. doi: 10.1158/0008-5472.CAN-10-2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kandala PK, Wright SE, Srivastava SK. Blocking epidermal growth factor receptor activation by 3,3′-diindolylmethane suppresses ovarian tumor growth in vitro and in vivo. J Pharmacol Exp Ther. 2012;341:24–32. doi: 10.1124/jpet.111.188706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhen Y, Guanghui L, Xiefu Z. Knockdown of EGFR inhibits growth and invasion of gastric cancer cells. Cancer Gene Ther. 2014;21:491–497. doi: 10.1038/cgt.2014.55. [DOI] [PubMed] [Google Scholar]
- 19.Gadgeel SM, Ali S, Philip PA, Ahmed F, Wozniak A, Sarkar FH. Response to dual blockade of epidermal growth factor receptor (EGFR) and cycloxygenase-2 in nonsmall cell lung cancer may be dependent on the EGFR mutational status of the tumor. Cancer. 2007;110:2775–2784. doi: 10.1002/cncr.23100. [DOI] [PubMed] [Google Scholar]
- 20.Kobayashi S, Boggon TJ, Dayaram T, Jänne PA, Kocher O, Meyerson M, Johnson BE, Eck MJ, Tenen DG, Halmos B. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med. 2005;352:786–792. doi: 10.1056/NEJMoa044238. [DOI] [PubMed] [Google Scholar]
- 21.Huguet F, Fernet M, Giocanti N, Favaudon V, Larsen AK. Afatinib, an irreversible EGFR family inhibitor, shows activity toward pancreatic cancer cells, alone and in combination with radiotherapy, independent of KRAS status. Target Oncol. 2016;11:371–381. doi: 10.1007/s11523-015-0403-8. [DOI] [PubMed] [Google Scholar]
- 22.Dhillon AS, Hagan S, Rath O, Kolch W. MAP kinase signalling pathways in cancer. Oncogene. 2007;26:3279–3290. doi: 10.1038/sj.onc.1210421. [DOI] [PubMed] [Google Scholar]
- 23.Tang Y, Yan G, Song X, Wu K, Li Z, Yang C, Deng T, Sun Y, Hu X, Yang C, et al. STIP overexpression confers oncogenic potential to human non-small cell lung cancer cells by regulating cell cycle and apoptosis. J Cell Mol Med. 2015;19:2806–2817. doi: 10.1111/jcmm.12670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang G, Tang B, Tang K, Dong X, Deng J, Liao L, Liao Z, Yang H, He S. Isoquercitrin inhibits the progression of liver cancer in vivo and in vitro via the MAPK signalling pathway. Oncol Rep. 2014;31:2377–2384. doi: 10.3892/or.2014.3099. [DOI] [PubMed] [Google Scholar]
- 25.Kasahara A, Scorrano L. Mitochondria: From cell death executioners to regulators of cell differentiation. Trends Cell Biol. 2014;24:761–770. doi: 10.1016/j.tcb.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 26.Ly JD, Grubb DR, Lawen A. The mitochondrial membrane potential (deltapsi(m)) in apoptosis; An update. Apoptosis. 2003;8:115–128. doi: 10.1023/A:1022945107762. [DOI] [PubMed] [Google Scholar]
- 27.Estaquier J, Vallette F, Vayssiere JL, Mignotte B. The mitochondrial pathways of apoptosis. Adv Exp Med Biol. 2012;942:157–183. doi: 10.1007/978-94-007-2869-1_7. [DOI] [PubMed] [Google Scholar]
- 28.Li L, Gao Y, Zhang L, Zeng J, He D, Sun Y. Silibinin inhibits cell growth and induces apoptosis by caspase activation, downregulating survivin and blocking EGFR-ERK activation in renal cell carcinoma. doi: 10.1016/j.canlet.2008.06.033. [DOI] [PubMed] [Google Scholar]
- 29.Danial NN, Korsmeyer SJ. Cell death: Critical control points. Cell. 2004;116:205–219. doi: 10.1016/S0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 30.Seifrtová M, Cochlarová T, Havelek R, Řezáčová M. Benfluron induces cell cycle arrest, apoptosis and activation of p53 pathway in MOLT-4 leukemic cells. Folia Biol. 2015;61:147–155. doi: 10.14712/fb2015061040147. [DOI] [PubMed] [Google Scholar]
- 31.Malumbres M. Cyclin-dependent kinases. Genome Biol. 2014;15:122. doi: 10.1186/gb4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhuo Z, Hu J, Yang X, Chen M, Lei X, Deng L, Yao N, Peng Q, Chen Z, Ye W, et al. Ailanthone inhibits Huh7 cancer cell growth via cell cycle arrest and apoptosis in vitro and in vivo. Sci Rep. 2015;5:16185. doi: 10.1038/srep16185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chien CC, Wu MS, Shen SC, Ko CH, Chen CH, Yang LL, Chen YC. Activation of JNK contributes to evodiamine-induced apoptosis and G2/M arrest in human colorectal carcinoma cells: A structure-activity study of evodiamine. PLoS One. 2014;9:e99729. doi: 10.1371/journal.pone.0099729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ondrouskova E, Vojtesek B. Programmed cell death in cancer cells. Klin Onkol. 2014;27(Suppl 1):S7–S14. doi: 10.14735/amko20141S7. In Czech. [DOI] [PubMed] [Google Scholar]
- 35.Lai CY, Tsai AC, Chen MC, Chang LH, Sun HL, Chang YL, Chen CC, Teng CM, Pan SL. Aciculatin induces p53-dependent apoptosis via MDM2 depletion in human cancer cells in vitro and in vivo. PLoS One. 2012;7:e42192. doi: 10.1371/journal.pone.0042192. [DOI] [PMC free article] [PubMed] [Google Scholar]