Abstract
IFN-α prevents Ag-induced arthritis (AIA), and in this study we investigated the role of IDO1 and TGF-β signaling for this anti-inflammatory property of IFN-α. Arthritis was induced by methylated BSA (mBSA) in mBSA-sensitized wild-type (WT), Ido1−/−, or Ifnar−/− mice, treated or not with IFN-α or the IDO1 product kynurenine (Kyn). Enzymatic IDO1 activity, TGF-β, and plasmacytoid dendritic cells (pDC) were neutralized by 1-methyltryptophan and Abs against TGF-β and pDC, respectively. IDO1 expression was determined by RT-PCR, Western blot, and FACS, and enzymatic activity by HPLC. Proliferation was measured by 3H-thymidine incorporation and TGF-β by RT-PCR and ELISA. WT but not Ido1−/− mice were protected from AIA by IFN-α, and Kyn, the main IDO1 product, also prevented AIA, both in WT and Ifnar−/− mice. Protective treatment with IFN-α increased the expression of IDO1 in pDC during AIA, and Ab-mediated depletion of pDC, either during mBSA sensitization or after triggering of arthritis, completely abrogated the protective effect of IFN-α. IFN-α treatment also increased the enzymatic IDO1 activity (Kyn/tryptophan ratio), which in turn activated production of TGF-β. Neutralization of enzymatic IDO1 activity or TGF-β signaling blocked the protective effect of IFN-α against AIA, but only during sensitization and not after triggering of arthritis. Likewise, inhibition of the IDO1 enzymatic activity in the sensitization phase, but not after triggering of arthritis, subdued the IFN-α–induced inhibition of mBSA-induced proliferation. In conclusion, presence of IFN-α at Ag sensitization activates an IDO1/TGF-β–dependent anti-inflammatory program that upon antigenic rechallenge prevents inflammation via pDC.
Introduction
Antigen-specific tolerance could be a way of controlling autoimmune diseases and allergy without broad immune suppression (1). IFN-α can inhibit Ag-specific proliferation and prevent inflammation (2, 3), but its direct usage is hampered by proinflammatory properties, for example, as those observed in systemic lupus erythematosus and myositis (4). Although type I IFNs (IFN-α and IFN-β) also show clear anti-inflammatory properties (5, 6), for example, in multiple sclerosis (7), the way by which type I IFN prevents inflammation remains largely obscure.
By administering IFN-α or dsRNA, a type I IFN inducer, at the time of Ag sensitization, type I IFN signaling protects against Ag-induced arthritis (AIA) (2). This 28-d arthritis model consists of a sensitization phase (days 1–20), in which Ag-specific immunity develops, and an effector phase (days 21–28) triggered by intra-articular Ag rechallenge. To prevent development of arthritis, type I IFN signaling must be activated during sensitization, because the same regimen of IFN-α treatment given at the onset of the effector phase has no protective effect. The protection by IFN-α is accompanied by long-lasting inhibition of Ag-specific proliferation of splenocytes (2) and inhibition of Ag-specific production of proinflammatory cytokines (IL-1β, IL-12, IL-17, IFN-γ, and TNF) both ex vivo and in vitro (3). Presence of IFN-α at the time of Ag sensitization also enhances production of the immunoregulatory cytokine TGF-β, including prevention of Ag-induced suppression of TGF-β in the effector phase of AIA (3).
One tolerogenic component activated by TGF-β is expression of IDO1 (8, 9), which can also be induced by both type I and type II IFNs during inflammation (10, 11). IDO1 exerts immunoregulatory effects through enzymatic conversion of tryptophan (Trp) to kynurenine (Kyn) (12) that results in multiple effects on T lymphocytes, including inhibition of proliferation and differentiation toward a regulatory phenotype (13, 14). Nonenzymatic IDO1 signaling is activated by TGF-β and relies on phosphorylation of ITIMs within IDO1 and consequent activation of the noncanonical NF-κB pathway, which establishes stable IDO1 expression in plasmacytoid dendritic cells (pDC), resulting in long-term immune tolerance (9).
In this article, we showed that the protection exerted by IFN-α in AIA requires pDC and is dependent on both TGF-β and IDO1, and that the protective effect of IFN-α could be replaced by the IDO1 metabolite Kyn.
Materials and Methods
Mice
SV129EV and IFNARKO mice were from B&K. Ido1−/−, LysM Cre+, and Tgfb1fl/fl mice were from Jackson ImmunoResearch Laboratories. All mice were further bred at the Animal Facility of Linköping University. Ido1−/− mice were bred heterozygously, resulting in both Ido1−/− and wild-type (WT) offsprings. LysM Cre+ and Tgfb1fl/fl mice were crossbred to produce LysM Cre+/−Tgfbr2fl/fl. Offspring were genotyped using DNA from ear tissues, according to protocols by Jackson ImmunoResearch Laboratories. WT littermates and LysM Cre−Tgfbr2fl/fl were used as controls for Ido1−/− and LysM Cre+Tgfbr2fl/fl mice, respectively. Experiments were performed following the regulation of the Swedish Animal Welfare Act and approved by the Ethical Committee Board, Linköping University (Dnr 1-12), and South Stockholm regional court (N-271/14).
Ag-induced arthritis
Arthritis was triggered, as described previously (15), by intra-articular injection of 30 μg methylated BSA (mBSA; 20 μl) in the left knee joint (with PBS as control in the right knee) in female mice (8–13 wk age) presensitized 2 and 3 wk earlier with 100 and 200 μg mBSA, respectively, with or without 1000 U IFN-α (IFN-αA; PBL Assay Science, Piscataway, NJ), emulsified in IFA (Sigma-Aldrich, St. Louis, MO). In some experiments, 15 mg/kg Kyn (Sigma-Aldrich) was included in the emulsion at each immunization. At day 28, mice were sacrificed, and arthritis severity in the knee joints was analyzed by means of a score 0–3, as earlier described (2).
In vivo treatment with anti–TGF-β Abs, anti-pDC Abs, and 1-methyltryptophan
For administration in drinking water, dl 1-methyltryptophan (1-MT; Sigma-Aldrich) was initially dissolved in 0.05 M NaOH, adjusted to 5 mg/ml with MilliQ water and to pH 9 with HCl or NaOH. Freshly prepared 1-MT was provided every 3 d during treatment. Control mice received drinking water adjusted to pH 9. On average, each mouse drank 2–2.5 ml water per day, which corresponds to 10–12.5 mg 1-MT per day. Alternatively, a pellet continuously releasing 1-MT (5 mg/day for 28 d; Innovative Research of America, Sarasota, FL) was surgically inserted s.c. in the back of the mouse. IDO1 inhibition was confirmed by HPLC determination of Kyn levels (see below and Supplemental Fig. 1A). TGF-β was neutralized by daily i.p. injection for the indicated time of 150 μg anti–TGF-β Ab (clone 1D11, reactive to mouse TGF-β1/β3; EPIRUS Biopharmaceuticals Netherlands BV). As control, 200 μl PBS was injected i.p. pDC were depleted by repeated i.p. injection of 150 μg 120G8 clone (Dendritics, Lyon, France), according to the manufacturer’s instructions, at the indicated time points. A total of 91.6% of spleen pDC was depleted 3 d after the first injection (Supplemental Fig. 1E). As control, 200 μl PBS was injected i.p.
Ag-specific proliferation
At day 28 of AIA, spleen and lymph node (axillary, inguinal, popliteal, and caudal) cells were left untreated or restimulated for 72 h in vitro with 50 μg/ml mBSA, as earlier described (2). Radioactive thymidine (0.5 μCi/well) was added 20 h ahead of the final culture, and counts were determined in a beta counter to assess proliferation.
Evaluation of IDO1 expression and signaling
Spleens and lymph nodes were collected at indicated time points and stored in RNAlater, tissue storage reagent (Ambion, Foster City, CA), until analysis. In some instances, pDC were isolated from spleens before analysis by the mouse Plasmacytoid Dendritic Cell Isolation Kit (Miltenyi Biotec), according to the manufacturer’s instructions. Total mRNA was extracted using TRIzol reagent (Sigma-Aldrich), converted to cDNA by reverse transcriptase according to the manufacturer’s instructions (Life Technologies, Carlsbad, CA), and used as template in real-time PCR using universal PCR mastermix and specific TaqMan primer probes obtained from Life Technologies (IDO1, Mm 00492586_m1; Actinß, Mm 00607939_s1) on a ABI Prism 7500 fast PCR machine (Applied Biosystems). β-actin (Actb) was used as endogenous reference gene. All samples along with the naive control and no-template control were run in duplets. The Δ cycle threshold values were determined as the difference of mean cycle threshold values of Ido1 and Actb gene expression. Ido1, Tgfb1, Ifna11, and Ifnb1 expression was calculated using the 2−ΔΔCt method by normalizing with ΔCt values of spleen or lymph nodes from naive mice (16). Western blot and densitometry analysis was performed on lysates, as described previously (17), with a rabbit anti-mouse IDO mAb (cv152) (18), and the control Ab anti–β-tubulin (Sigma-Aldrich) was used as a loading control.
Trp and Kyn measurements
Samples were analyzed using a Waters 2695 separation module equipped with a Waters 2475 multiwavelength fluorescence detector, controlled by Empower software. Isocratic separation on a XBridge C18 3.5-μm 3.0 × 150-mm column with a Gemini C18 4*2.0 security guard cartridge, hold at 30°C, was used to separate Trp, Kyn, and the internal standard methylguanosine, with a mobile phase consisting of 4:96 acetonitrile:10 mM acetic acid at a flow rate of 0.5 ml/min. The substances were monitored by programmed wavelength detection setting, that is, Kyn excitation at 365 nm and emission 480 nm, Trp excitation at 300 nm and emission 350 nm, and internal standard excitation at 310 nm and emission 390 nm. Standards and controls were prepared in an albumin solution. Samples were prepared by adding 100 μl methanol solution of the internal standard to 50 μl standards, controls, or samples. Thereafter, samples were vortexed and centrifuged at 10,000 rpm for 10 min. Before injection, samples were diluted five times in 10 mM acetic acid, and, finally, 10 μl was injected onto the column.
TGF-β ELISA
The levels of TGF-β in serum (diluted 1:100) or culture supernatants (diluted 1:4) were determined by ELISA, according to the manufacturer’s instructions (human/mouse TGF-β1 ELISA Ready-SET-Go Kit; eBioscience, San Diego, CA).
In vitro cultures and FACS analysis
Spleen cells, either naive or isolated at day 10 of AIA, were seeded onto 96-well plates at 1 × 106 cell/ml in a total volume of 200 μl and stimulated with 50 μg/ml mBSA plus 0, 100, or 250 U/ml IFN-αA, with or without anti–TGF-β (40 μg/ml) or with or without 5 μM 1-MT. At 0, 6, 24, and 72 h, cells were harvested and subjected to RT-PCR to measure Ido1 and Tgfb1 gene expression, as described above. For FACS analysis of IDO1 protein expression, cells were stimulated with 0, 100, or 250 U/ml IFN-αA, with or without anti–TGF-β (40 μg/ml) and harvested after 72-h culture. Cells were washed and stained with anti-mPDCA1 (anti-mPDCA-1-allophycocyanin, clone JF05-1C2.4.1; Miltenyi Biotec), according to the manufacturer’s instructions, followed by fixation in 4% paraformaldehyde, permeabilization in intracellular staining buffer set (eBioscience), and subsequently stained with anti-IDO1 (anti-mouse IDO PerCP-eFluor 710; eBioscience, clone: mIDO-48), according to the manufacturer’s instructions. Between each step, a 3× wash in PBS/0.5% FBS was included. Stained cells, unstained cells, and fluoroscence minus one controls were analyzed on a Gallios instrument (Beckman Coulter, Brea, CA), and data were analyzed with Kaluza Flow Analysis Software (Beckman Coulter; version 1.2). Gates were set using fluoroscence minus one controls, as earlier described (19).
Statistical analysis
Differences in arthritis severity and differences in Kyn/Trp levels between two groups with different treatments were evaluated by Mann–Whitney U test. Levels of gene expression (RT-PCR) and number of IDO1-expressing pDC (FACS) were evaluated by Student t test. A p value <0.05 was considered significant.
Results
The protective effect of IFN-α in AIA is mediated by TGF-β
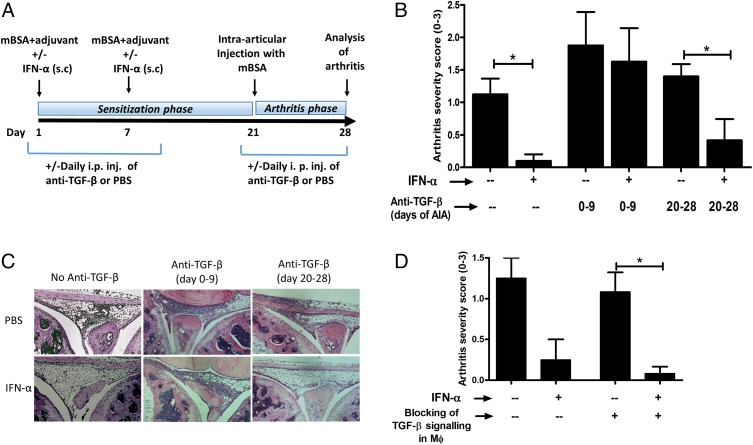
To determine whether protection by IFN-α in AIA could be mediated by TGF-β, anti–TGF-β Abs were administered daily i.p. during the sensitization phase (day 1–9) or the arthritis phase (day 20–28) of AIA (Fig. 1A). In line with our previous studies (2, 3), administration of IFN-α at the time of Ag sensitization protected mice from arthritis (Fig. 1B). The protection was clearly abrogated by neutralizing TGF-β Abs on day 0–9 in the sensitization phase (Fig. 1B, 1C) but IFN-α was still able to protect from arthritis when TGF-β was blocked on day 20–28 (Fig. 1B, 1C). Therefore, TGF-β appeared to be indispensable for the IFN-α–protective effect in the sensitization phase, but not in the arthritis (i.e., effector) phase of AIA.
FIGURE 1.
The protective effect of IFN-α against AIA is mediated by TGF-β signaling in the sensitization phase. (A) Schematic representation of the AIA model, as described in Materials and Methods. Briefly, AIA was induced in WT Sv129 mice and LysM Cre+/−Tgfbr2fl/fl with or without IFN-α treatment. TGF-β signaling was blocked in WT mice by daily i.p. injection of 150 μg anti–TGF-β Ab for the first 0–8 or 20–28 d of AIA. (B) The level of arthritis is expressed as severity score (mean ± SEM, n ≥ 4) from WT mice with or without IFN-α and anti–TGF-β treatment. (C) Representative histochemical slides showing inflammation of the knee joint from each group. (D) Level of arthritis expressed as severity score (mean ± SEM, n ≥ 6) from LysM Cre+/−Tgfbr2fl/fl mice with or without IFN-α treatment. Comparison of arthritis severity score between different treatment groups was done by Mann–Whitney U test (*p < 0.05).
Previously, we observed an increased expression of TGF-β in macrophages (Mφ) in ex vivo restimulated leukocytes from IFN-α–treated mice during AIA (3). To determine whether Mφ-producing TGF-β could be important for IFN-α–driven protection of AIA, we evaluated the protective effect of IFN-α in LysM Cre+Tgfbfl/fl mice, lacking the TGF-β encoding gene only in Mφ. In these mice, IFN-α protected against AIA to the same extent as observed in mice with intact TGF-β activity in Mφ (Fig. 1D).
The protective effect of IFN-α in AIA is accompanied by induction of IDO1 expression and activity
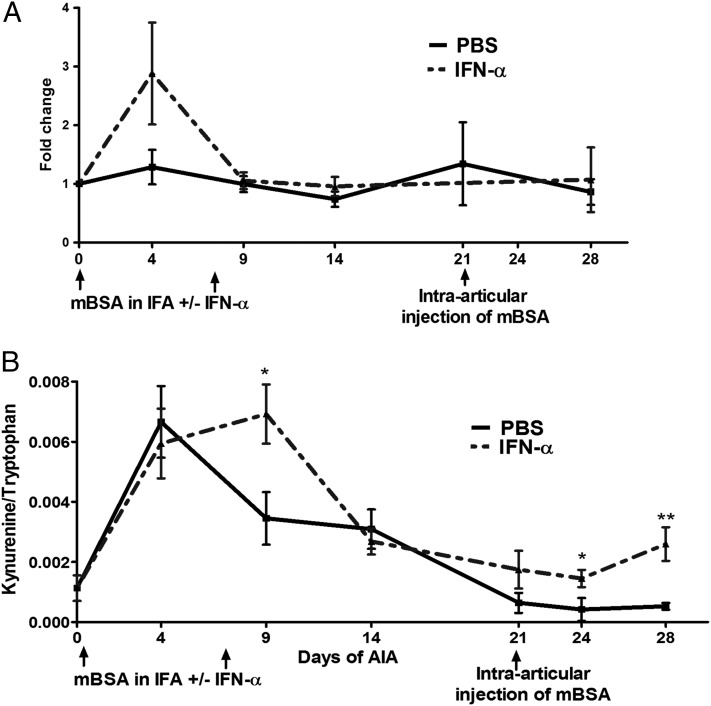
IDO1 is a TGF-β– and IFN-α–induced protein that contributes to immune suppression and tolerance (20). We therefore investigated IDO1 induction in mice treated with IFN-α during AIA. To this end, we monitored Ido1 expression over time (days 0, 4, 9, 14, 21, and 28 of AIA) by real-time PCR in splenocytes and lymph nodes purified from mice untreated or treated with IFN-α. We found an increase, although not significant (p = 0.13), in Ido1 mRNA at day 4 in spleen cells from IFN-α–treated mice as compared with controls, with no differences at all other analyzed time points (Fig. 2A). The same pattern, that is, an early increase in Ido1 mRNA, was observed in lymph node cells isolated from IFN-α–treated mice (data not shown).
FIGURE 2.
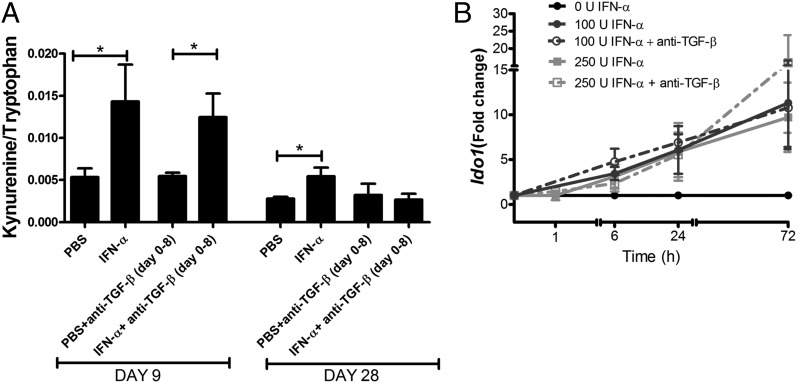
The protective effect of IFN-α in AIA is accompanied by induction of IDO1 expression and activity. (A) During AIA activated with or without IFN-α treatment, spleens were collected at days 0, 4, 10, 14, 21, and 28 and analyzed for relative Ido1 mRNA by real-time PCR. Data are expressed as fold change normalized to the reference gene Actb and Ido1 gene expression in spleens from naive mice, n ≥ 4. (B) Sera were collected at days 0, 4, 10, 14, 21, 24, and 28 of AIA and analyzed for Trp and Kyn concentration by HPLC. Data are expressed as the ratio of serum levels of Kyn to Trp, n ≥ 7. Comparisons of Kyn/Trp and Ido1 mRNA levels were done by Mann–Whitney U test. *p < 0.05, **p < 0.01.
Because Ido1 mRNA expression may not necessarily reflect the presence of the active IDO1 protein, we investigated the effect of IFN-α on IDO1 enzymatic activity during AIA. To this purpose, in the same mice and at the same time points as above, we determined the Kyn to Trp ratio (the main IDO1 product and substrate, respectively) in sera. The Kyn/Trp ratio increased at day 4 postsensitization in sera from both controls and IFN-α–treated animals (Fig. 2B). However, systemic IDO1 activity rapidly decreased thereafter in the control group and maintained at basal levels in the effector phase of arthritis. In contrast, in the IFN-α–treated group, the Kyn/Trp ratio further increased at day 9 (p < 0.03) as compared with controls before decreasing at days 14 and 21, after which it raised a little again (day 24, p < 0.03 and day 28, p < 0.006, as compared with the control group) (Fig. 2B).
IDO1 expression is required for the protective effect of IFN-α
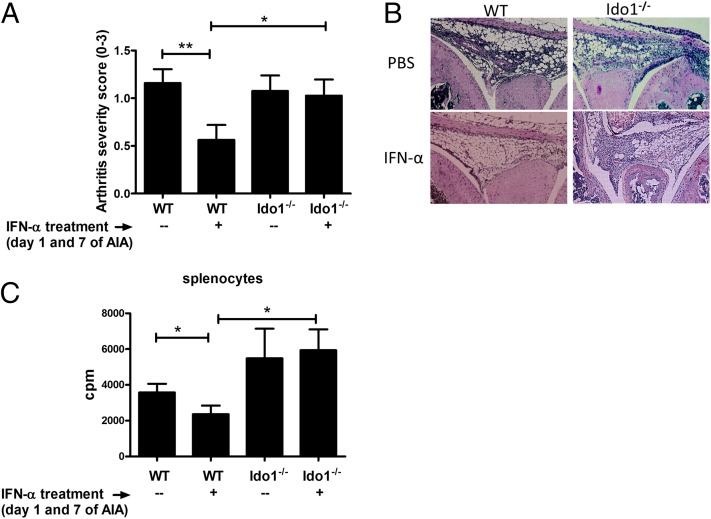
To determine whether IDO1 could mediate AIA protection conferred by IFN-α, we investigated the anti-inflammatory effects of IFN-α administration in mice lacking IDO1 expression. Similarly to experiments reported in Fig. 1, we administered IFN-α into WT and Ido1−/− C57BL/6 mice at the time of mBSA sensitization, and arthritis was triggered by intra-articular injection of mBSA at day 21. As depicted in Fig. 3A, both WT and Ido1−/− mice developed arthritis with a similar degree of severity. However, the protective effect of IFN-α, evident in WT animals, was lost in mice deficient for IDO1 expression. In fact, the disease severity in Ido1−/− mice treated with IFN-α did not differ from Ido1−/− mice not treated with IFN-α and, importantly, was significantly higher than that of IFN-α–treated WT mice (Fig. 3A, 3B). Also, the antiproliferative effect of IFN-α was abolished in Ido1−/− mice (Fig. 3C). Thus, our data suggested that IDO1 is required for the anti-inflammatory effect of IFN-α in AIA.
FIGURE 3.
IDO1 expression is required for the protective effect of IFN-α. AIA was induced in female Ido1−/− mice and their wild type littermates with or without 1000 U IFN-α as described in Materials and Methods. (A) The level of arthritis evaluated at day 28 of AIA is expressed as severity score (mean ± SEM, n ≥ 16) from Ido1−/− mice and their WT littermates. (B) Representative histochemical slide of the knee joints from each group is presented. (C) Splenocytes from day 28 of AIA were restimulated ex vivo with 50 μg/ml of mBSA for 72 h and pulsed with radioactive thymidine for the last 20 h. The incorporated radioactivity (cpm value) was determined by a beta counter. From the expressed values (mean cpm ± SEM, n ≥ 6), the radioactivity (cpm) of the corresponding mock (medium) stimulation was subtracted. Comparison of arthritis severity score and cpm values for proliferation between different treatment groups was done by the Mann–Whitney U test (*p < 0.05, **p < 0.01).
The enzymatic activity of IDO1 is required for the protective effect of IFN-α in the sensitization but not effector phase of AIA
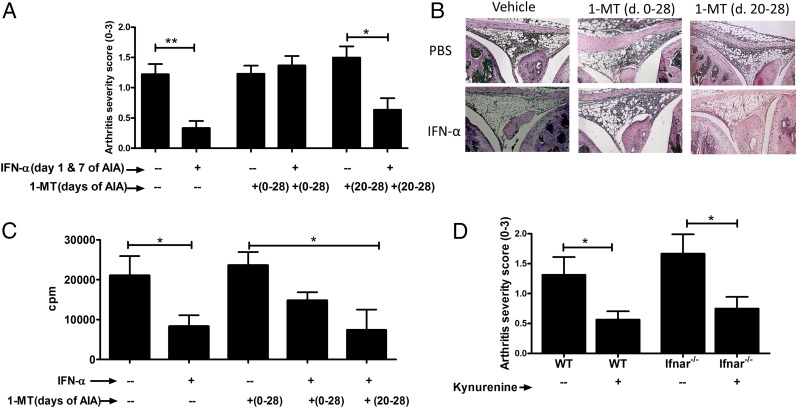
IDO1 exerts regulation via its enzymatic as well as signaling activity (21). To determine whether IDO1 enzymatic activity was required for the protective effect of IFN-α in AIA, we used 1-MT, a gold standard compound that inhibits the IDO1-mediated conversion of Trp into Kyn. In the AIA model, we administered 1-MT continuously starting from the day before the first mBSA immunization until day 28, or only in the effector phase (day 20–28), either in drinking water or as slow-releasing pellets surgically implanted s.c. into WT mice. Irrespectively of the route of administration, control mice receiving 1-MT, either on days 0–28 or days 20–28, developed arthritis with a similar degree of severity as the control group receiving vehicle alone (Fig. 4A, Supplemental Fig. 1B). The protective effect of IFN-α was abolished in mice in which the enzymatic activity of IDO1 was inhibited from day 0 to 28, but not from day 20 to 28, by continuous delivery of 1-MT, either by an implanted pellet releasing 1-MT in vivo (Fig. 4A) or in drinking water (Supplemental Fig. 1B). Thus, these data indicated that IDO1 catalytic activity is required in the sensitization phase, but is apparently redundant in the effector phase of AIA, for IFN-α to fulfill its protective effects. Likewise, 1-MT, administered continuously from day −1 to day 28, but not on days 21–28, hampered the antiproliferative effect of IFN-α in mBSA-stimulated splenocytes and lymph node cells isolated at day 28 (Fig. 4C, Supplemental Fig. 1D). Thus, the dampening effect of IFN-α on Ag-specific proliferation in the arthritis phase follows that of the protective effect of IFN-α in AIA, that is, it is dependent on IDO1 catalytic activity during the sensitization but not arthritis phase.
FIGURE 4.
The enzymatic activity of IDO1 is required for the protective effect of IFN-α in the sensitization, but not in the disease onset phase. AIA was induced in female mice with or without IFN-α treatment, as described in Materials and Methods. To inhibit the enzymatic activity of IDO1, 1-MT was administered either through drinking water or by surgical insertion of a 1-MT pellet (Supplemental Fig. 1B) at day −1 to day 28 or day 20–28 of AIA. (A) The level of arthritis expressed as severity score (mean ± SEM, n ≥ 7) from WT mice that received 1-MT or vehicle and with or without IFN-α treatment. (B) Representative histochemical slides of the knee joints from each group. (C) Splenocytes from WT mice at day 28 of AIA were restimulated ex vivo with 50 μg/ml mBSA for 72 h and pulsed with radioactive thymidine for the last 20 h. The incorporated radioactivity (cpm value) was determined by a beta counter. From the expressed values (mean cpm ± SEM, n ≥ 7), the radioactivity (cpm) of the corresponding mock (medium) stimulation was subtracted. (D) The level of arthritis expressed as severity score (mean ± SEM, n ≥ 7) from WT or IFNΑRKO mice with or without Kyn treatment (15 mg/kg mouse weight) during immunization of AIA. Comparison of arthritis severity score and cpm values for proliferation between groups was done by the Mann–Whitney U test (*p < 0.05, **p < 0.01).
Kyn, the major IDO1 product, ameliorates arthritis
The increased conversion of Trp to Kyn induced by IFN-α (Fig. 2B) and the pivotal role of IDO1 enzymatic activity for the protective effects of IFN-α (Fig. 4A, 4B) prompted us to determine whether Kyn could prevent development of AIA in the absence of IFN-α treatment. To this end, mice were administered with 15 mg/kg body weight of Kyn at days 1 and 7 of AIA with mBSA. Mice receiving Kyn developed a significantly milder form of arthritis than control animals receiving vehicle alone (Fig. 4D).
Because Kyn can be generated downstream of IFN-α signaling (Fig. 2B) and, conversely, because IDO1 signaling can also induce pDC production of IFN-α, which can sustain IDO1 immunoregulatory effects over the long-term (9), we evaluated whether the anti-arthritic effect of Kyn was effective upstream or downstream of type I IFN signaling. To this purpose, Kyn was administered to Ifnar−/− mice, that is, lacking the receptor for type I IFNs, at the time of mBSA priming, and arthritis was induced by intra-articular injection of the same Ag at day 21. Ifnar−/− mice were protected by Kyn at a level comparable to that of WT mice (Fig. 4D), suggesting that the Kyn-induced protection of AIA is independent of endogenous type I IFN signaling.
The enzymatic activity of IDO1 acts upstream of TGF-β signaling in the sensitization phase
Because both TGF-β and the enzymatic activity of IDO1 were required in the sensitization phase for the protective effect of IFN-α, we further investigated whether the IFN-α–induced activation of IDO1 enzymatic activity (Fig. 2B) was dependent on TGF-β signaling, and vice versa, whether the IFN-α–induced expression of TGF-β (3) was dependent on the enzymatic activity of IDO1. Administration of anti–TGF-β Abs in the sensitization phase (day 0–8), which clearly prevented the protective effect of IFN-α (Fig. 1), did not affect the increased IDO1 activity day 9, which was comparable in mice receiving IFN-α and mice receiving IFN-α plus anti–TGF-β (Fig. 5A). Kinetic IDO1 mRNA studies of splenocytes from immunized mice restimulated ex vivo with IFN-α with or without anti–TGF-β also showed that IFN-α–induced IDO1 mRNA expression was unaffected by inhibition of TGF-β signaling (Fig. 5B). Thus, IFN-α activates the enzymatic activity and expression of IDO1 independently of TGF-β. However, the second phase of IDO1 enzymatic activity observed in IFN-α–treated mice in the arthritis phase (Fig. 2B) was somewhat subdued by inhibition of TGF-β signaling (Fig. 5A).
FIGURE 5.
IFN-α–induced activation of IDO1 in the sensitization phase of AIA and in vitro occurs independently of TGF-β. Female mice were immunized days 1 and 7 with mBSA with or without IFN-α treatment, with or without anti–TGF-β Abs administered i.p. days 1–8 as in Fig. 1 and as described for AIA in Materials and Methods. (A) Sera were collected at days 9 and 28 and analyzed for Trp and Kyn concentration by HPLC. Data are expressed as the ratio of serum levels of Kyn to Trp, n ≥ 7. Comparisons between groups were made by Mann–Whitney U test. (B) Splenocytes isolated day 10 from mBSA-sensitized mice were restimulated ex vivo with 50 μg/ml mBSA with or without 100 U/ml IFN-α, with or without 40 μg/ml anti–TGF-β. Data are expressed as fold change normalized to the reference gene Actb and Ido1 expression in mBSA-stimulated cultured cells from the same mice, n = 5. Paired Student t test was used to evaluate differences between treatments. *p < 0.05.
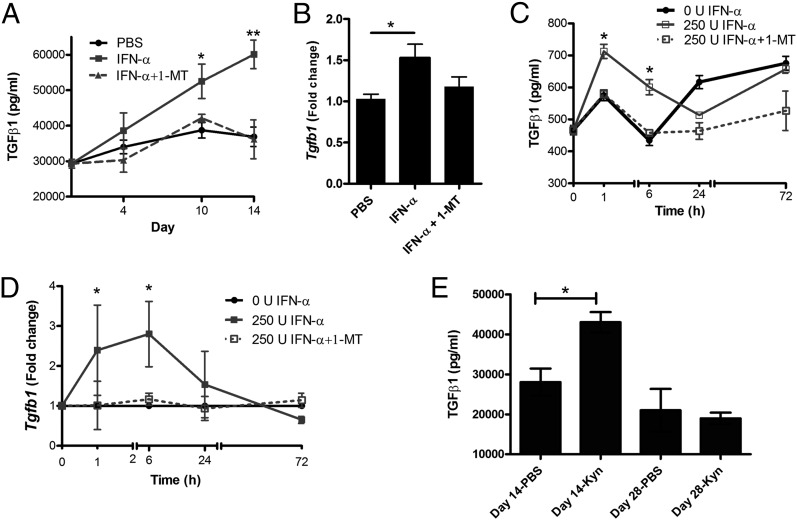
To determine whether the enzymatic activity of IDO1 affects TGF-β production induced by IFN-α, we measured TGF-β production in mice treated with the IDO1 inhibitor 1-MT. As depicted in Fig. 6A, mice immunized in the presence of IFN-α showed significantly higher serum levels of TGF-β production [in line with earlier (2, 3) observations], but in the presence of 1-MT, this increase was efficiently reduced (Fig. 6A) to the lower levels observed in control mice (which were not affected by 1-MT; data not shown). This pattern of inhibited TGF-β protein expression in the presence of 1-MT (Fig. 7A) was confirmed at the mRNA level day 10 of AIA (Fig. 6B), although only a trend of decreased TGF-β mRNA expression was observed in 1-MT–treated mice. To further study the kinetics of this IDO1-mediated regulation of TGF-β production, splenocytes from immunized mice were restimulated ex vivo with IFN-α with or without 1-MT. As depicted in Fig. 6C, already after 1 h of stimulation, the protein levels of TGF-β increased in IFN-α–stimulated cultures, but this increase was immediately hampered in the presence of 1-MT (Fig. 6C), which was also the case for the increased expression of TGF-β mRNA determined in the same cultures (Fig. 6D). Taken together, these inhibition studies showed that IFN-α activates the enzymatic activity of IDO1, which in turn mediates the IFN-α–induced activation of TGF-β production. In line with this, direct administration of Kyn, the metabolite of IDO1 enzymatic activity, clearly increased the serum levels of TGF-β in the sensitization phase of AIA (Fig. 6E).
FIGURE 6.
The enzymatic activity of IDO1 regulates IFN-α–induced TGF-β production in vivo and in vitro. (A and B) Female mice were immunized days 1 and 7 with mBSA with or without IFN-α treatment, with or without 1-MT administered in drinking water, as described for AIA in Materials and Methods. Levels of TGF-β in serum were determined by ELISA at the indicated time points after the first immunization (A), and TGF-β mRNA levels in spleens were determined by RT-PCR at day 10 (B). For in vitro studies, splenocytes isolated day 10 from mBSA-sensitized mice were restimulated ex vivo with 50 μg/ml mBSA with or without 250 U IFN-α, with or without 5 μM 1-MT. Depicted in (C) are the levels of TGF-β in culture supernatants determined by ELISA, and in (D) the TGF-β mRNA levels in cultures cells as determined by RT-PCR at the indicated time points. In (D), data are expressed as fold change normalized to reference gene Actb and Tgfβ expression in mBSA-stimulated cultured cells from the same mice. Comparison of TGF-β levels between groups in vivo (A, n = 8; B, n < 5) was done by the Mann–Whitney U test and in vitro (C and D, n = 5) by Student t test for paired observations (*p < 0.05, **p < 0.01). (E) Female mice were immunized days 1 and 7 with mBSA with or without Kyn treatment, and the levels of TGF-β in serum were determined by ELISA at day 14 (n = 4–6) and day 28 (n = 3) after the first immunization. Comparison between groups was made by Mann–Whitney U test.
FIGURE 7.
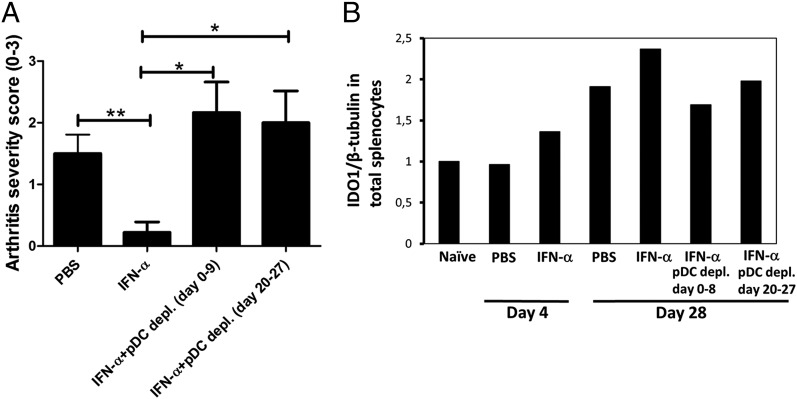
The protective effect of IFN-α in AIA requires the presence of pDC both in the sensitization and arthritis phase. AIA was induced in female mice with IFN-α treatment as described in Materials and Methods, with or without pDC-depletion by daily injections of the 120G8 Ab day 0–8 (sensitization phase) or day 20–27 (arthritis phase). (A) The level of arthritis is expressed as severity score (mean ± SEM, n ≥ 6). Comparison between groups was made by Mann–Whitney U test. *p < 0.05, **p < 0.01. (B) Quantitative densitometry analysis of western blots performed for IDO1 expression at days 4 and 28 in total splenocytes of mice subjected to AIA with IFN-α treatment with or without depletion of pDC in the sensitization or arthritis phase, as in (A). Data are presented as IDO1/β tubulin ratio and are representative of three experiments.
The protective effect of IFN-α in AIA requires the presence of pDC both in the sensitization and arthritis phase in AIA
Because pDC are a principal source of IDO1, we evaluated the importance of pDC for the protective effect of IFN-α. To this end, a pDC-depleting Ab (120 G8; Dendritics) was administered either during the time of Ag sensitization (day 0–8) or just before the time of arthritis triggering and onward (day 20–27). As depicted in Fig. 7A, depletion of pDC in the sensitization phase (day 0–8) clearly hampered the ability of IFN-α to protect against arthritis. Also, and in apparent contrast to the enzymatic activity of IDO1 and TGF-β signaling, which were required only in the sensitization phase, depletion of pDC in the arthritis phase (day 20–27) also abolished the protective effect of IFN-α (Fig. 7A). We next analyzed the effect of pDC depletion on the levels of IDO1 expression. As depicted in Fig. 7B, a decrease in IDO1 expression was apparent day 28, irrespectively, if depletion was carried out in the sensitization (day 0–8) or arthritis phase (day 20–27).
In vivo and ex vivo treatment with IFN-α induces IDO1 expression in pDC
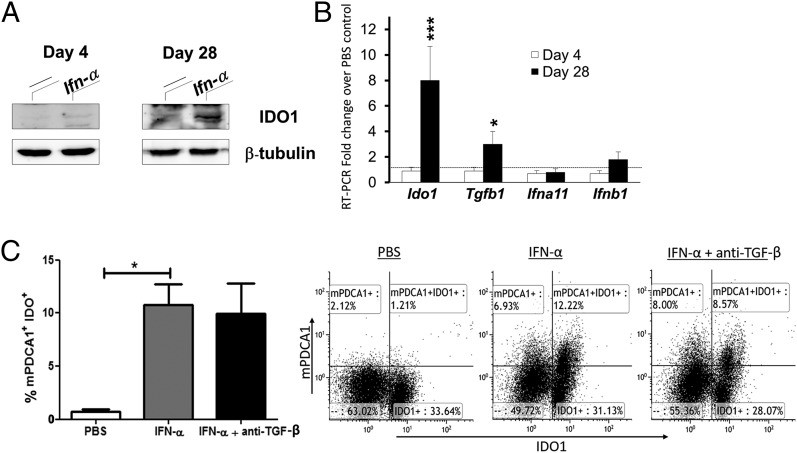
To further study the expression profile of IDO1 in pDC, pDC were isolated from control and IFN-α–treated mice in the sensitization and arthritis phase of AIA. As depicted in Fig. 8, presence of IFN-α at immunization activated IDO1 expression in pDC isolated from IFN-α–treated animals, to a minor extent initially, in which a weak signal was detected day 4 by Western blot (Fig. 8A), but not confirmed by mRNA levels in sorted pDC (Fig. 8B), and a very strong signal detected day 28 (Fig. 8A), which was confirmed by an 8-fold increase in Ido1 mRNA expression (Fig. 8B). The ability of IFN-α to induce expression of IDO1 in pDC, independently of TGF-β, was also confirmed in vitro by restimulation ex vivo of splenocytes from immunized mice (Fig. 8C). In line with the ability of IDO1 to enhance TGF-β production (Fig. 6), we also observed increased TGF-β mRNA levels in pDC day 28 (Fig. 8B), but no further modulation of type I IFN genes or noncanonical activation of NF-κB (data not shown), despite the known ability of TGF-β to activate this pathway in pDC (9).
FIGURE 8.
IFN-α induces IDO1 expression in splenic pDC in vitro and in vivo independently of TGF-β. AIA was induced in female mice with IFN-α treatment, as described in Materials and Methods. At days 4 and 28, mice were sacrificed and pDC were purified from their spleens. (A) Western blot analysis of IDO1 protein expression in pDC. (B) Real-time PCR analysis of Ido1, Tgfb1, Ifna11, and Ifnb1 transcripts in pDC isolated from spleen cells at days 4 and 28 of AIA from mice treated with IFN-α, normalized to the expression of Actb, and presented relative to results of pDC isolated from control mice not treated with IFN-α (dotted line, 1-fold). (C) Splenocytes were isolated at day 10 of AIA, stimulated with 50 μ/ml mBSA plus 250 U/ml IFN-α, with or without anti–TGF-β (40 μg/ml) for 72 h, and analyzed for IDO1-positive pDC by FACS (see Materials and Methods). The bar diagram (left) depicts the percentage of mPDCA+IDO1+ cells among the live lymphocyte population, and the FACS plots (right) show representative dot plots from one of five mice. Data in (A) are the representative of four experiments, and (B) and (C) (mean ± SD, n = 6) were analyzed by Student t test. *p < 0.05, ***p < 0.001.
Discussion
IFN-α has well-described immunostimulatory effects, but its immunosuppressive properties are only beginning to unfold (22). Our earlier work showed that administration of IFN-α at the time of Ag sensitization prevents arthritis induced by Ag rechallenge (2, 3). We showed in this work that this anti-inflammatory property of IFN-α is critically dependent on IDO1-mediated activation of TGF-β and pDC. The enzymatic IDO1 activity and TGF-β signaling are critical for the protective effect of IFN-α during Ag sensitization, and pDC, a source of IFN-α–induced IDO1 during AIA, are critically required both during sensitization and after Ag rechallenge for the anti-inflammatory effect of IFN-α in AIA.
An IDO1-dependent mechanism is crucial for this anti-inflammatory effect because Ido1−/− mice developed arthritis despite treatment with IFN-α at Ag sensitization (Fig. 3A, 3B). Likewise, protection against collagen-induced arthritis by oral collagen sensitization is also propagated via an IDO1-dependent mechanism (23). An important source for IDO1 is the pDC (24) (Fig. 8C). Already 4 d after the first immunization in AIA we observed a small increase in IDO1 expression in pDC isolated from IFN-α–treated animals by Western blot (Fig. 8A), although no increase in mRNA could be observed at this time point (Fig. 8B). In the arthritis phase, we observed an 8-fold increase in IDO1 mRNA and a strong IDO1 protein expression in pDC from mice treated with IFN-α. In line with this, in vivo depletion of pDC reduced the total levels of IDO1 protein in spleens (Fig. 7B). Thus, pDC are an important source of IDO1 expression activated by IFN-α, and this has also been observed in humans treated with IFN-α (25). An anti-inflammatory effect of IDO1-expressing pDC has earlier been anticipated in a number of experimental studies, including prevention of atherosclerosis (26), regulation of HIV responses (27, 28), and amelioration of experimental encephalitis (29), to name a few. In line with such observations, we showed in this work that pDC were critically involved in IFN-α–mediated protection against AIA (Fig. 7A). As indicated above, presence of pDC was required both in the sensitization and arthritis phase for IFN-α to prevent joint inflammation. Mechanistically, these two phases are clearly distinct in that the anti-inflammatory effect of IFN-α requires enzymatic IDO1 activity and TGF-β signaling in the sensitization phase, whereas these entities are redundant for the protective effect once inflammation is triggered by intra-articular injection of mBSA (Figs. 1, 4). Therefore, and as outlined below, IDO1-expressing pDC may have different roles in the sensitization versus arthritis phase to confer protection against AIA.
In the sensitization phase, the enzymatic activity of IDO1 is critical for the protective effect of IFN-α. First, 1-MT, the inhibitor of IDO1-mediated Trp to Kyn conversion, clearly abrogated the protective (Fig. 4A) and antiproliferative (Fig. 4C) effect of IFN-α in AIA if present in the sensitization phase, but not if administered in the arthritis phase. Second, direct administration of the IDO1 product Kyn in the sensitization phase also protected against AIA (Fig. 4D). A principal role of enzymatic IDO1 activity for protection in the sensitization phase is also supported by the much higher enzymatic activity in IFN-α–treated mice in this phase as compared with the arthritis phase (Fig. 2B). The described effect of enzymatic IDO1 activity induced by IFN-α in the sensitization phase of AIA is in line with numerous earlier observations of the antiproliferative (30, 31) and anti-inflammatory effect of the IDO1 (9, 32), and we show in this work that, once activated, this enzymatic activity induces an anti-inflammatory state that, weeks later, independently of continuous enzymatic activity, results in inhibition of Ag-induced inflammation (Fig. 4A) and proliferation (Fig. 4C).
Downstream of IFN-α–induced IDO1 enzymatic activity, we observed production of TGF-β (Fig. 6), and the activation of expression and enzymatic activity of IDO1 in the sensitization phase induced by IFN-α was furthermore clearly independent on signaling via TGF-β. This was evident in vivo in IFN-α–treated mice receiving Abs blocking TGF-β (Fig. 5A), and also confirmed in spleen cell cultures stimulated with IFN-α in vitro (Fig. 5B). This is in line with earlier findings that described IFN-γ–induced activation of enzymatic IDO1 activity independently of TGF-β. In these studies, a clear dichotomy was established in that TGF-β activated a nonenzymatic anti-inflammatory effect of IDO1 and IFN-γ activated the enzymatic IDO1 activity (9). In analogy with the effect of IFN-γ, we show in this study the same property of IFN-α, and we also describe a novel downstream effect in that this IFN-α–induced enzymatic IDO1 activity mediates the IFN-α–induced production of TGF-β. This is evident from the observation that activation of TGF-β production by IFN-α was clearly inhibited in the presence of 1-MT, both in vivo during AIA (Fig. 6A) and in vitro (Fig. 6C, 6D). This shows that the IFN-α–induced activation of IDO1 and TGF-β may be part of a common pathway in which IFN-α first activates the enzymatic activity of IDO1 and thereby the generation of Kyn (Fig. 2B), which in turn activates production of TGF-β, as shown in vivo in Fig. 6E.
In earlier studies, we observed increased TGF-β levels in IFN-α–treated mice, particularly in Mφ both in the sensitization phase and at the start of the arthritis phase (3). Although TGF-β production by Mφ was clearly dispensable for IFN-α–mediated protection against AIA (Fig. 1D), we found that TGF-β signaling in general was an integral part of the IFN-α–mediated protection against AIA (Fig. 1). This was shown using anti–TGF-β Abs in vivo, and, in analogy with the requirement of IDO1 enzymatic activity, TGF-β signaling was only required in the sensitization phase to confer protection (Fig. 1). Thus, both enzymatic IDO1 activity and downstream TGF-β signaling are required in the sensitization phase for the protective effect of IFN-α.
For protection induced by IFN-α, the sensitization phase also required presence of pDC (Fig. 7A), which expressed IDO1 in response to IFN-α, both in vivo (Fig. 8A, 8B) and in vitro (Fig. 8C). One way by which IDO1 expressing pDC may contribute to the observed antiproliferative (Figs. 3B, 4C) and anti-arthritic effect of IFN-α (Figs. 1, 3A, 4A, 7A) is by promotion of regulatory T cells (Tregs) with ability to restrain inflammation (33). pDC may employ type I IFN signaling to induce Tregs (34), and this ability has been shown to require the enzymatic activity of IDO1 (35). By generating Kyn from Trp, IDO1+ pDC can activate the aryl hydrocarbon receptor on naive T cells, which favors their development into Foxp3+ Tregs. Using pDC–T cell cocultures in the presence of IFN-α–inducing stimuli (CpG-DNA), Mezrich et al. (36) showed that T cells required functional aryl hydrocarbon receptor expression for optimal Treg development. The IDO1-induced TGF-β production (Fig. 6) may also contribute to the development of Tregs (37), and, of note, once IDO1 have induced Foxp3 expression in CD4+ cells via TGF-β, Foxp3 expression is sustained independently of TGF-β signaling (9). Likewise, once generated from Trp by IDO1, Kyn is able to activate a suppressive pathway, for example, inhibition of delayed-type hypersensitivity, also in cells not expressing IDO1 (38). Thus, if Foxp3+ Tregs are generated by IDO1+ pDC via Kyn and TGF-β in the sensitization phase of AIA, these observations (9, 38) may explain the requirement for IDO1 enzymatic activity and TGF-β signaling in the sensitization phase and their redundancy in the arthritis phase. Thus, once generated by Kyn and TGF-β, Tregs may inhibit inflammation without the need for further IDO1 enzymatic activity and TGF-β signaling. We currently evaluate the importance of enzymatic IDO1 activity and TGF-β for the generation of Tregs induced by IFN-α in AIA, and our unpublished observations point to a critical role of Tregs in the arthritis phase of AIA for the protective effect of IFN-α.
In the arthritis phase, the pDC are also critical for the protective effect of IFN-α (Fig. 7A), but the enzymatic activity of IDO1 is not because 1-MT administered in this phase did not abrogate the protective effect of IFN-α (Fig. 4A, 4B). In line with this, the IFN-α–induced IDO1 enzymatic activity clearly decreased after its peak in the sensitization phase, although a minor increase sustained by TGF-β signaling, as earlier reported (11), was yet detected day 28 (Fig. 2). One anti-inflammatory effect of IDO1-expressing pDC that is independent of the enzymatic activity of IDO1 is the nonenzymatic signaling function of IDO1. A prominent feature of this is phosphorylation of ITIMs within IDO1, which further results in noncanonical NF-κB activation and thereby sustained IDO1 production over a long time (9). We did not find evidence of phosphorylation of IDO1, or noncanonical activation of NF-κB in pDC isolated in the arthritis phase from mice protected against AIA by IFN-α (data not shown). We did, however, find increased IDO1 expression in the arthritis phase (8-fold activation compared with controls not receiving IFN-α; Fig. 8B), but the significance of this remains to be determined.
Anti-inflammatory effects of pDC independent of the IDO1 enzymatic activity include inhibition of IL-17 and IFN-γ production by CD4+ T cells, as observed by pDC depletion in the acute or relapse phase of experimental autoimmune encephalomyelitis using the IDO1 inhibitor 1-MT (29). Both IL-17 and IFN-γ are downregulated in CD4+ T cells by IFN-α during AIA, resulting in significantly lower IL-17 and IFN-γ serum levels in the arthritis phase in protected animals (3). Inhibition of IL-17 and IFN-γ production in CD4+ T cells may thus be one way by which pDC, independently of the IDO1 enzymatic activity, protects against AIA during the arthritis phase. Possibly, pDC achieves this protection downstream of Treg signaling. In such a setting, inhibitory CTLA-4 on activated Tregs would bind its receptors CD80/CD86 (B7) on the pDC, which impairs B7 interaction with the activating T cell molecule CD28 (39). This prevents activation of pathogenic T cells (39), which would prevent their production of IL-17 and IFN-γ. In this way, pDC may be responsible for the inhibition of IL-17 and IFN-γ production in CD4+ T cells, as observed in the arthritis phase in mice protected from AIA by IFN-α (3).
Another example of how Tregs regulate the function of dendritic cells (DC) is by regulating their Trp catabolism (40). Binding of CTLA-4 to CD80/86 (B7) on DC regulates IDO1-mediated Trp catabolism in DC and thereby their immunosuppressive capacity (28, 41). In fact, CTLA-4–mediated activation of IDO1 may be a natural way to prevent development of rheumatoid arthritis (RA) because point mutations in the CTLA-4 gene in RA patients result in lower IDO1 enzymatic activity, which may explain the decreased serum levels of Kyn in a cohort of RA patients (42).
Induction of Ag-specific tolerance can be a way to treat chronic inflammatory diseases, but the therapeutic use of type I IFNs is, however, impaired by proinflammatory (5) and toxic side effects. Thus, identification of (43) the pathways governing the anti-inflammatory properties of type I IFNs is warranted. In this study, we identified the enzyme IDO1 and its metabolite Kyn, a small compound with apparently no detectable adverse effects (18, 44), and TGF-β as key molecules in IFN-α–mediated protection against arthritis. We have thus delineated one pathway by which type I IFNs exert anti-inflammatory actions and pointed out new targets for anti-inflammatory therapies.
Supplementary Material
Acknowledgments
We thank Liv Gröntoft for technical assistance in preparing the joint specimen, and Hammoudi Alkaissi and Ratnesh Bhai Mehta for technical assistance in setting up RT-PCR experiments.
This work was supported by Swedish Research Council (Vetenskapsrådet), Reumatikerförbundet, Magnus Bergvall Foundation, and Linköping University.
The online version of this article contains supplemental material.
- AIA
- Ag-induced arthritis
- DC
- dendritic cell
- Kyn
- kynurenine
- Mφ
- macrophage
- mBSA
- methylated BSA
- 1-MT
- 1-methyltryptophan
- pDC
- plasmacytoid DC
- RA
- rheumatoid arthritis
- Treg
- regulatory T cell
- Trp
- tryptophan
- WT
- wild-type.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Garber K. 2014. Immunology: A tolerant approach. Nature 507: 418–420. [DOI] [PubMed] [Google Scholar]
- 2.Ying F., Chalise J. P., Narendra S. C., Magnusson M. 2011. Type I IFN protects against antigen-induced arthritis. Eur. J. Immunol. 41: 1687–1695. [DOI] [PubMed] [Google Scholar]
- 3.Chalise J., Narendra S., Paudyal B., Magnusson M. 2013. Interferon alpha inhibits antigen-specific production of proinflammatory cytokines and enhances antigen-specific transforming growth factor beta production in antigen-induced arthritis. Arthritis Res. Ther. 15: R143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banchereau J., Pascual V. 2006. Type I interferon in systemic lupus erythematosus and other autoimmune diseases. Immunity 25: 383–392. [DOI] [PubMed] [Google Scholar]
- 5.Trinchieri G. 2010. Type I interferon: friend or foe? J. Exp. Med. 207: 2053–2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.González-Navajas J. M., Lee J., David M., Raz E. 2012. Immunomodulatory functions of type I interferons. Nat. Rev. Immunol. 12: 125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stübgen J. P. 2009. Recombinant interferon-beta therapy and neuromuscular disorders. J. Neuroimmunol. 212: 132–141. [DOI] [PubMed] [Google Scholar]
- 8.Belladonna M. L., Orabona C., Grohmann U., Puccetti P. 2009. TGF-beta and kynurenines as the key to infectious tolerance. Trends Mol. Med. 15: 41–49. [DOI] [PubMed] [Google Scholar]
- 9.Pallotta M. T., Orabona C., Volpi C., Vacca C., Belladonna M. L., Bianchi R., Servillo G., Brunacci C., Calvitti M., Bicciato S., et al. 2011. Indoleamine 2,3-dioxygenase is a signaling protein in long-term tolerance by dendritic cells. Nat. Immunol. 12: 870–878. [DOI] [PubMed] [Google Scholar]
- 10.Belladonna M. L., Puccetti P., Orabona C., Fallarino F., Vacca C., Volpi C., Gizzi S., Pallotta M. T., Fioretti M. C., Grohmann U. 2007. Immunosuppression via tryptophan catabolism: the role of kynurenine pathway enzymes. Transplantation 84(1, Suppl)S17–S20. [DOI] [PubMed] [Google Scholar]
- 11.Belladonna M. L., Volpi C., Bianchi R., Vacca C., Orabona C., Pallotta M. T., Boon L., Gizzi S., Fioretti M. C., Grohmann U., Puccetti P. 2008. Cutting edge: Autocrine TGF-beta sustains default tolerogenesis by IDO-competent dendritic cells. J. Immunol. 181: 5194–5198. [DOI] [PubMed] [Google Scholar]
- 12.Brown R. R., Ozaki Y., Datta S. P., Borden E. C., Sondel P. M., Malone D. G. 1991. Implications of interferon-induced tryptophan catabolism in cancer, auto-immune diseases and AIDS. Adv. Exp. Med. Biol. 294: 425–435. [DOI] [PubMed] [Google Scholar]
- 13.Mellor A. L., Keskin D. B., Johnson T., Chandler P., Munn D. H. 2002. Cells expressing indoleamine 2,3-dioxygenase inhibit T cell responses. J. Immunol. 168: 3771–3776. [DOI] [PubMed] [Google Scholar]
- 14.Fallarino F., Grohmann U., You S., McGrath B. C., Cavener D. R., Vacca C., Orabona C., Bianchi R., Belladonna M. L., Volpi C., et al. 2006. The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell receptor zeta-chain and induce a regulatory phenotype in naive T cells. J. Immunol. 176: 6752–6761. [DOI] [PubMed] [Google Scholar]
- 15.van den Berg W. B., Joosten L. A., van Lent P. L. 2007. Murine antigen-induced arthritis. Methods Mol. Med. 136: 243–253. [DOI] [PubMed] [Google Scholar]
- 16.Livak K. J., Schmittgen T. D. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25: 402–408. [DOI] [PubMed] [Google Scholar]
- 17.Matino D., Gargaro M., Santagostino E., Di Minno M. N., Castaman G., Morfini M., Rocino A., Mancuso M. E., Di Minno G., Coppola A., et al. 2015. IDO1 suppresses inhibitor development in hemophilia A treated with factor VIII. J. Clin. Invest. 125: 3766–3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Romani L., Fallarino F., De Luca A., Montagnoli C., D’Angelo C., Zelante T., Vacca C., Bistoni F., Fioretti M. C., Grohmann U., et al. 2008. Defective tryptophan catabolism underlies inflammation in mouse chronic granulomatous disease. Nature 451: 211–215. [DOI] [PubMed] [Google Scholar]
- 19.Perfetto S. P., Chattopadhyay P. K., Roederer M. 2004. Seventeen-colour flow cytometry: unravelling the immune system. Nat. Rev. Immunol. 4: 648–655. [DOI] [PubMed] [Google Scholar]
- 20.Munn D. H., Mellor A. L. 2013. Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends Immunol. 34: 137–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fallarino F., Grohmann U., Puccetti P. 2012. Indoleamine 2,3-dioxygenase: from catalyst to signaling function. Eur. J. Immunol. 42: 1932–1937. [DOI] [PubMed] [Google Scholar]
- 22.Crouse J., Kalinke U., Oxenius A. 2015. Regulation of antiviral T cell responses by type I interferons. Nat. Rev. Immunol. 15: 231–242. [DOI] [PubMed] [Google Scholar]
- 23.Park M. J., Park K. S., Park H. S., Cho M. L., Hwang S. Y., Min S. Y., Park M. K., Park S. H., Kim H. Y. 2012. A distinct tolerogenic subset of splenic IDO(+)CD11b(+) dendritic cells from orally tolerized mice is responsible for induction of systemic immune tolerance and suppression of collagen-induced arthritis. Cell. Immunol. 278: 45–54. [DOI] [PubMed] [Google Scholar]
- 24.Fallarino F., Gizzi S., Mosci P., Grohmann U., Puccetti P. 2007. Tryptophan catabolism in IDO+ plasmacytoid dendritic cells. Curr. Drug Metab. 8: 209–216. [DOI] [PubMed] [Google Scholar]
- 25.Chevolet I., Schreuer M., Speeckaert R., Neyns B., Hoorens I., van Geel N., Krüse V., Hennart B., Allorge D., Van Gele M., Brochez L. 2015. Systemic immune changes associated with adjuvant interferon-α2b-therapy in stage III melanoma patients: failure at the effector phase? Melanoma Res. 25: 357–361. [DOI] [PubMed] [Google Scholar]
- 26.Yun T. J., Lee J. S., Machmach K., Shim D., Choi J., Wi Y. J., Jang H. S., Jung I. H., Kim K., Yoon W. K., et al. 2016. Indoleamine 2,3-dioxygenase-expressing aortic plasmacytoid dendritic cells protect against atherosclerosis by induction of regulatory T cells. Cell Metab. 23: 852–866. [DOI] [PubMed] [Google Scholar]
- 27.Manches O., Munn D., Fallahi A., Lifson J., Chaperot L., Plumas J., Bhardwaj N. 2008. HIV-activated human plasmacytoid DCs induce Tregs through an indoleamine 2,3-dioxygenase-dependent mechanism. J. Clin. Invest. 118: 3431–3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manches O., Fernandez M. V., Plumas J., Chaperot L., Bhardwaj N. 2012. Activation of the noncanonical NF-κB pathway by HIV controls a dendritic cell immunoregulatory phenotype. Proc. Natl. Acad. Sci. USA 109: 14122–14127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bailey-Bucktrout S. L., Caulkins S. C., Goings G., Fischer J. A., Dzionek A., Miller S. D. 2008. Cutting edge: central nervous system plasmacytoid dendritic cells regulate the severity of relapsing experimental autoimmune encephalomyelitis. J. Immunol. 180: 6457–6461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matysiak M., Stasiołek M., Orłowski W., Jurewicz A., Janczar S., Raine C. S., Selmaj K. 2008. Stem cells ameliorate EAE via an indoleamine 2,3-dioxygenase (IDO) mechanism. J. Neuroimmunol. 193: 12–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Terness P., Bauer T. M., Röse L., Dufter C., Watzlik A., Simon H., Opelz G. 2002. Inhibition of allogeneic T cell proliferation by indoleamine 2,3-dioxygenase-expressing dendritic cells: mediation of suppression by tryptophan metabolites. J. Exp. Med. 196: 447–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nguyen N. T., Nakahama T., Le D. H., Van Son L., Chu H. H., Kishimoto T. 2014. Aryl hydrocarbon receptor and kynurenine: recent advances in autoimmune disease research. Front. Immunol. 5: 551 doi:10.3389/fimmu.2014.00551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Notley C. A., Ehrenstein M. R. 2010. The yin and yang of regulatory T cells and inflammation in RA. Nat. Rev. Rheumatol. 6: 572–577. [DOI] [PubMed] [Google Scholar]
- 34.Pan, Z. J., C. G. Horton, C. Lawrence, and A. D. Farris. 2016. Plasmacytoid dendritic cells and type 1 IFN promote peripheral expansion of Foxp3 regulatory T cells specific for the ubiquitous RNA-binding nuclear antigen La/SS-B. Clin. Exp. Immunol. doi:10.1111/cei.12817. [DOI] [PMC free article] [PubMed]
- 35.Chen W., Liang X., Peterson A. J., Munn D. H., Blazar B. R. 2008. The indoleamine 2,3-dioxygenase pathway is essential for human plasmacytoid dendritic cell-induced adaptive T regulatory cell generation. J. Immunol. 181: 5396–5404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mezrich J. D., Fechner J. H., Zhang X., Johnson B. P., Burlingham W. J., Bradfield C. A. 2010. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J. Immunol. 185: 3190–3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hadaschik E. N., Enk A. H. 2015. TGF-β1-induced regulatory T cells. Hum. Immunol. 76: 561–564. [DOI] [PubMed] [Google Scholar]
- 38.Belladonna M. L., Grohmann U., Guidetti P., Volpi C., Bianchi R., Fioretti M. C., Schwarcz R., Fallarino F., Puccetti P. 2006. Kynurenine pathway enzymes in dendritic cells initiate tolerogenesis in the absence of functional IDO. J. Immunol. 177: 130–137. [DOI] [PubMed] [Google Scholar]
- 39.Onishi Y., Fehervari Z., Yamaguchi T., Sakaguchi S. 2008. Foxp3+ natural regulatory T cells preferentially form aggregates on dendritic cells in vitro and actively inhibit their maturation. Proc. Natl. Acad. Sci. USA 105: 10113–10118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fallarino F., Grohmann U., Hwang K. W., Orabona C., Vacca C., Bianchi R., Belladonna M. L., Fioretti M. C., Alegre M. L., Puccetti P. 2003. Modulation of tryptophan catabolism by regulatory T cells. Nat. Immunol. 4: 1206–1212. [DOI] [PubMed] [Google Scholar]
- 41.Grohmann U., Orabona C., Fallarino F., Vacca C., Calcinaro F., Falorni A., Candeloro P., Belladonna M. L., Bianchi R., Fioretti M. C., Puccetti P. 2002. CTLA-4-Ig regulates tryptophan catabolism in vivo. Nat. Immunol. 3: 1097–1101. [DOI] [PubMed] [Google Scholar]
- 42.Cribbs A. P., Kennedy A., Penn H., Read J. E., Amjadi P., Green P., Syed K., Manka S. W., Brennan F. M., Gregory B., Williams R. O. 2014. Treg cell function in rheumatoid arthritis is compromised by ctla-4 promoter methylation resulting in a failure to activate the indoleamine 2,3-dioxygenase pathway. Arthritis Rheumatol. 66: 2344–2354. [DOI] [PubMed] [Google Scholar]
- 43.Sleijfer S., Bannink M., Van Gool A. R., Kruit W. H., Stoter G. 2005. Side effects of interferon-alpha therapy. Pharm. World Sci. 27: 423–431. [DOI] [PubMed] [Google Scholar]
- 44.Bessede A., Gargaro M., Pallotta M. T., Matino D., Servillo G., Brunacci C., Bicciato S., Mazza E. M., Macchiarulo A., Vacca C., et al. 2014. Aryl hydrocarbon receptor control of a disease tolerance defence pathway. Nature 511: 184–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.