Abstract
Background
Subjective survival probabilities (SSPs) are considered relevant in relation to lifestyle as lifestyle improvements may improve health and lower mortality risk.
Objective
To study individuals' SSP in a population of elderly (i.e. 60 years and older) from 15 European countries.
Methods
Data from the second wave of the Survey of Health, Ageing and Retirement in Europe (SHARE) were used. Individuals were asked about their chances to live up to age [T] or more. These SSPs were related to general characteristics, health and lifestyle. In addition, cross‐country comparisons were made. The validity of the probabilistic elicitation format used for collecting SSPs was also addressed.
Results
The average subjective probability of surviving the next 9–15 years was around 57%. Mean SSPs varied significantly across age, with lower means at higher ages. Cross‐country comparisons showed lowest mean in the Czech Republic (42%) and the highest in Denmark (64%). SSPs correlated with socio‐demographic, socio‐economic and also strongly with (objective) health characteristics except for obesity. Smokers reported significantly lower SSPs compared to non‐smokers, but no difference was found between non‐smokers and quitters. Excessive alcohol consumers reported significantly higher SSPs than moderate consumers and abstainers, but this only held for female excessive drinkers. Physical inactivity was negatively associated with SSPs, but this relation was attenuated at higher ages. In this context, important cross‐country differences were found.
Conclusions
Subjective survival probabilities are informative and relevant in relation to lifestyle decisions and can be validly obtained in elder people. The results from this study provide interesting implications for health policy, health communication strategies and future research.
Keywords: elderly, Europe, health, lifestyle, subjective survival probabilities
Introduction
Individuals' own perception of remaining lifetime is increasingly considered relevant in relation to lifestyle behaviours such as smoking. Individuals generally face uncertainty regarding their own mortality risk or may have inaccurate expectations regarding longevity and the impact of health behaviours thereon. If people underestimate or even ignore the health consequences (i.e. longevity reductions) of unhealthy behaviour, they may more easily adopt and maintain an unhealthy lifestyle. Therefore, more insight into how individuals assess their own mortality risks in relation to unhealthy behaviours may help understand health‐behavioural decision‐making. This is even more important as modifiable unhealthy behaviours are an increasing threat to global mortality and morbidity and even small lifestyle improvements may importantly improve health and lower mortality risk.1 In this paper, we therefore investigate the relations between individuals' subjective survival probabilities (SSPs) and socio‐demographic characteristics, health and especially health behaviour. The paper adds to the literature by investigating these issues in a population of elderly (i.e. 60 years and older) from 15 European countries, using data from the Survey of Health, Ageing and Retirement in Europe (SHARE).
The importance of lifespan uncertainty in the decision‐making process of individuals was already emphasized by Yaari.2 When maximizing lifetime utility, people trade‐off subjectively expected gains and costs. To predict and explain individual behaviour, economists traditionally used assumptions about individual subjective expectations rather than actual data.3 More recently, data on subjective expectations and probabilities of survival have been collected in large household surveys.
Several studies have investigated the congruity between SSPs and actuarial survival probabilities to assess whether individuals' beliefs about their remaining lifetime are accurate.4, 5, 6, 7, 8, 9, 10, 11 Less congruity may imply inaccurate subjective expectations, but may also signal individuals' private information beyond what is accounted for in life tables.9 Such information may be used by individuals when making economic decisions.12, 13 Studies have also highlighted the relation between SSP and economic decisions regarding retirement, social security claiming,8, 14, 15 saving, consumption and bequests.12, 13, 16, 17 These studies suggest that SSPs are indeed important in economic decision‐making processes of individuals. Another stream of research has focused on whether SSPs predict individuals' actual mortality7, 18, 19, 20, 21 and the relation between SSPs and socio‐demographic characteristics and socio‐economic status,5, 22, 23 but also, for example, parental longevity.19 Note that research on subjective life expectancy has been conducted using point estimates or verbal answers rather than probabilities. Our focus here is on studies that used SSPs as elicitation method.
In line with these research applications in the field of economic decision‐making, SSPs have been found relevant for lifestyle decisions as well. Regarding tobacco use, there is large consensus that smoking decreases longevity, possibly up to 10 years.24 Hurd and McGarry5 found that SSPs indeed vary systematically with smoking. Lower SSPs for smokers compared to non‐smokers are reported, although among smokers little variation in SSPs was found according to intensity of smoking.25 Schoenbaum26 found that heavy smokers (≥25 cigarettes per day) fail to adjust their survival expectations downwards in line with life tables, while expectations of never, former and light smokers (<25 cigarettes per day) resembled actuarial predictions. Khwaja et al.27 also concluded that smokers expect to live longer than objective longevity figures predict. Balia28 used the first wave of the SHARE data to study the formation of SSPs in relation to smoking and individual perception of health risks, with a particular focus on the short‐ and long‐term effects of smoking and the reversibility of these effects.
Besides smoking, obesity is an important public health issue. While the consequences of obesity on morbidity are commonly acknowledged, the relation between obesity and life expectancy is less straightforward. It seems that obesity is more harmful in terms of reduced longevity among younger adults than among older people.29, 30 Walter et al.31 did not find evidence that increased body weight decreases life expectancy among older people. In terms of impact of obesity on SSPs, Falba and Busch32 reported lower SSPs among respondents who were overweight or obese compared to normal weight respondents. These authors concluded, however, that obese individuals do not fully update (i.e. lower) their subjective survival chances in line with the excess mortality risk associated with obesity as estimated in life tables used in their study. Hurd and McGarry5 even found no association between SSPs and (over)weight.
Other lifestyle‐related risk factors in part related to obesity, such as alcohol consumption and physical activity, also seem to be systematically related to SSPs. In line with epidemiological data, moderate alcohol consumers report higher SSPs than heavy drinkers (five or more glasses per day) and people who abstain from drinking.5, 10 In addition, people who are physically active report, on average, higher longevity expectations.5
In general, previous research findings regarding the association between SSPs and lifestyle‐related health risk factors suggest that SSPs in general vary with risk factors in a fairly systematic way, commonly in expected directions. The relation between obesity and SSPs is more diverse. In this study, we add to this empirical and theoretical literature in a number of ways. First, we provide descriptive statistics of SSPs using cross‐national European data and perform a country comparison among thirteen countries. Second, we investigate whether the SSPs vary with socio‐demographic characteristics and socio‐economic status, objective health status and, in particular, lifestyle, which is the main objective of our study. Finally, we address the validity of the probabilistic elicitation format for collecting data on individuals’ longevity perception, as it is unclear whether respondents are capable of expressing their survival expectations using probabilities. The remainder of this paper is structured as follows: first, in the next section, we describe our data, measures and analyses. After that, we present our results. We end the paper with a discussion of our main findings.
Data and methods
Data source and description
For our study, we used data from the second wave (2006/2007) of the Survey of Health, Aging, and Retirement in Europe (SHARE). SHARE is a cross‐national and multidisciplinary panel database with microlevel information on health, socio‐economic status, and social and family networks. Its format is analogous to the US Health Retirement Study (HRS) and the English Longitudinal Study of Ageing (ELSA). The SHARE database contains data from more than 22 000 households in 15 countries across Europe. Based on probability samples and using a computer‐assisted personal interviewing technique (for details, see Börsch‐Supan et al.33, Börsch‐Supan and Jürges34), information is collected of non‐institutionalized individuals aged 50 and older and their spouses (who may also be younger than 50 years). More documentation and information on SHARE can be found at http://www.share-project.org. We excluded respondents aged under 60 and over 90 years, as explained in the next section, and respondents from Ireland or Israel because complete data were not available at the time of our study. We also left out respondents that had item non‐response on any covariate under study, except for household income. We used logistic regression to test whether responding to the survival probability question was attributable to particular characteristics.
Measurement
Exploratory variable
SHARE provides an indicator of individuals’ SSP. In the ‘Expectations’ module of the SHARE questionnaire, after a warm‐up question and several other questions about expectations, respondents were asked to state their SSP on a scale from 0 to 100 as follows:
What are the chances that you will live to be age [T] or more?
The target age T (75, 80, 85, etc.) presented to the respondent depends on the age of the respondent. Respondents aged between 50 and 65 at the time of the interview were presented a target age of 75 years implying time horizons, that is the target age minus current age, ranging from 9 to 25 years. Respondents aged 66 through 90 years were presented with target ages using time horizons varying systematically between 9 and 15 years. For example, respondents aged 65 through 70 received a target age of 80, while those aged 80 through 85 received a target age of 95. Respondents older than 90 years got increasingly shorter time horizons with a minimum of six years. For congruity reasons, we decided to limit the variety of time horizons and therefore to retain only those respondents aged between 60 and 90 years old, who were all presented with a target age T which was in the range of 9–15 years from their current age. As time horizons differ between respondents, conditioning of the distribution of SSPs on age and target age is necessary.
Covariates
The covariates used in our analyses (and their reference categories) are displayed in Appendix A. Below we highlight some variables that need further explanation.
Education was operationalized using a recategorization into four levels of the 1997 International Standard Classification of Education (ISCED‐97). Respondents who indicated that they were still in school or have had an ‘other type of education’ were assigned to one of the four levels according to the number of years of education. Household income concerned the overall income received in Euros, net of tax, by all household members together in an average month in the last year. Missing values for income were imputed based on age, gender, country, household size, years of education and work status. Income value was adjusted (i) for household size, by dividing household income by the square root of the number of persons in the household, and (ii) for the purchasing power of different currencies using the PPP exchange rate of the year in which the interview was administered (i.e. 2006 or 2007). Because the distribution of income was skewed, income was dichotomized using the overall sample median (net) average income per month (€ 991).
We used the following health behaviour variables: smoking, alcohol consumption and physical (in)activity. We differentiated between non‐smokers, past smokers (i.e. smoked at least for a year in the past) and current smokers. Data on alcohol consumption were used to construct a binary variable identifying respondents that consumed more alcohol than the recommended levels in the Netherlands (two glasses per day for men, one glass per day for women) in the last three months prior to the interview.35 Respondents were considered to be physically inactive when they hardly ever or never engaged in moderate (e.g. gardening, walking) or vigorous physical activity (e.g. sports).
Analyses
We provide descriptive statistics of the SSP variable with particular attention to the variation in SSP according to age and country. Intuitively, one may expect that the age of a respondent will have a considerable influence on his SSP to some future age. We used analysis of variance to this end. Furthermore, as we used data from 13 countries across Europe, we look for any particular response patterns of SSP across countries.
Multivariate analysis
We used multivariate ordinary least squares regression to examine the association between SSP and the covariates. We defined four models, each consecutive model nested in the previous one. The first model investigated the association of SSP with socio‐demographic characteristics and socio‐economic status, including a squared term of age to adjust for a nonlinear effect. In the second model, we added health indicators, and in the third model, we added lifestyle factors. Finally, in the fourth model, we tested for several interactions between socio‐demographic variables and lifestyle variables and subsequently added two statistically significant interaction terms: excessive alcohol consumption × gender, and physical inactivity × age. In addition, to explore possible country‐specific associations, we estimated the fourth model for each country separately.
Reliability
It is important to understand whether respondents are willing and/or able to answer probabilistic questions.3 We investigated the reliability of SSP responses using two criteria from the SHARE database. First, we took the sum of two related questions about the chance that the standard of living will be better or worse 5 years from now. To be internally consistent, answers to these questions should not add up to more than 100%. Considering some margin of error, a tolerance level of 10% was applied.28 Second, we used a numeracy test. Respondents were asked: ‘If the chance of getting a disease is 10 per cent, how many people out of the 1000 would be expected to get the disease?’ The possible answers were categorized as follows: 100, 10, 90, 900 and ‘other’. To check the sensitivity of our findings to the reliability of SSP responses, we repeated our multivariate regression analysis using a subsample consisting of the respondents that provided valid answers to both criteria.
Analyses were conducted using stata 11 IC (StataCorp, College Station, TX, USA).
Results
Sample characteristics
From the SHARE Wave 2 database (n = 33 281), 20 421 respondents were selected based on their age and country of residence. Furthermore, 345 respondents (1.7%) with target ages outside the range of 9–15 were excluded. SSP response rate in this subsample was about 89%; hence, 2225 more respondents were dropped. Logistic regression analysis showed that, besides significant country differences, a higher age, a lower educational level and being physically inactive decreased SSP response rate significantly, χ2(36) = 808.44, P < 0.001. Finally, from the 17 851 respondents left, we excluded observations with item non‐response on any of the included covariates except household income (n = 1556), leaving 16 295 (81% of the relevant sample) respondents as our final sample for further analyses.
Overall subsample sizes varied by country, from approximately 1600 in Italy and Belgium to around 800 in Austria and Switzerland. The overall composition of the sample by country was as follows: Austria 5.1%, Belgium 9.8%, Czech Republic 7.2%, Denmark 7.9%, France 7.4%, Germany 8.6%, Greece 8.8%, Italy 10.5%, the Netherlands 8.0%, Spain 5.7%, Sweden 9.0% and Switzerland 4.9%. Males were slightly underrepresented (47%). Table 1 provides the characteristics of our final sample.
Table 1.
Sample characteristics (n = 16 295)
| Variable | Category | % |
|---|---|---|
| Age (mean [SD]) | 70.3 (7.2) | |
| Male (%) | 47.2 | |
| Living alone (%) | 27.6 | |
| Parent(s) alive (%) | 9.6 | |
| Child(ren) (%) | 90.8 | |
| Educational level (%) | ISCED 0 or 1 | 37.0 |
| ISCED 2 | 17.6 | |
| ISCED 3 or 4 | 29.6 | |
| ISCED 5 or 6 | 15.8 | |
| Working (%) | 8.2 | |
| Income high (%) | 50.0 | |
| Living in rural area or small town (%) | 51.8 | |
| Chronic disease (%) | 81.3 | |
| Depressed (%) | 24.3 | |
| Obese (%) | 19.1 | |
| Doctor visits high (%) | 45.7 | |
| Drug use (%) | 78.9 | |
| Hospital stay overnight (%) | 16.4 | |
| ADL limitations (%) | 11.4 | |
| iADL limitations (%) | 18.8 | |
| Smoking status (%) | Never | 55.0 |
| No, stopped | 30.2 | |
| Yes | 14.8 | |
| Alcohol consumption – excessive (%) | 34.1 | |
| Physically inactive (%) | 12.4 |
Subjective survival probabilities
The mean time horizon in eliciting SSP was 12.4 years (SD 1.5), and the mean SSP was 56.5% (SD 30.4). Figure 1 presents the distribution of SSPs, which took 58 different values in the range of 0 to 100. Seven per cent of the respondents thought that they had no chance of surviving until their target age, and 12.7% thought this chance was 100%. Almost one quarter of the sample stated a SSP of 50%. A large majority of the respondents rounded their SSP: 93.6% of the answers were rounded to tens (50, 60, 70, etc.) and 98.7% to fives or tens (50, 55, 60, etc.).
Figure 1.
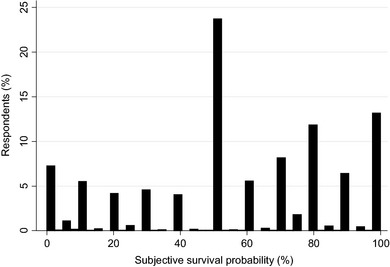
Distribution of subjective survival probabilities (n = 16 295).
Age groups
We constructed six age categories with 5‐year age bands. Table 2 presents these categories, the number of respondents, the time horizon and the mean SSP for each age group.
Table 2.
Time horizon and subjective survival probabilities by age group (n = 16 295)
| Age categories | n | Time horizon M (SD) | Survival probability M (SD) |
|---|---|---|---|
| Age 60–65 years | 4807 | 12.6 (1.5) | 68.7 (25.2) |
| Age 65–70 years | 3939 | 11.6 (1.5) | 62.9 (27.6) |
| Age 70–75 years | 3183 | 12.6 (1.4) | 54.0 (29.1) |
| Age 75–80 years | 2403 | 12.7 (1.5) | 44.1 (30.6) |
| Age 80–85 years | 1427 | 12.8 (1.4) | 35.4 (29.5) |
| Age 85–90 years | 536 | 13.0 (1.3) | 28.3 (30.4) |
| Total | 16 295 | 12.4 (1.5) | 56.5 (30.4) |
Mean SSP varied significantly across all age groups, F(5, 16294) = 595.68, P < 0.001. As expected, the youngest age group reported the highest SSP (68.7%) and the oldest group reported the lowest (28.3%). Time horizons differed across age groups, for example the mean time horizon of the age group 65–70 years was significantly lower compared to all other age groups.
Mean SSP varied significantly across countries, F(12, 16 282)= 61.72, P < 0.001. The means in the Czech Republic and Poland were clearly the lowest, that is 42.1% and 44.3%, respectively. The average SSP in Belgium (lowest), Germany, Austria, France, Sweden, Spain and Greece (highest) ranged from 53.6% to 59.5%. In four countries (Netherlands, Italy, Denmark and Switzerland), mean survival probabilities were higher than 60%, with highest mean SSP reported in Denmark (64.1%). Mean time horizons were all within the range of 12.25 years (Germany) to 12.47 years (Greece), while mean ages across countries varied from 69.6 years to 71.9 years for the Netherlands and Spain, respectively.
Figure 2 presents the mean SSP by age group and country. All countries showed a similar pattern to that in Table 2, with some deviation in the patterns of Poland and the Czech Republic. Note that the mean SSP in these two countries were significantly lower than in all other countries. In Sweden, the range of mean survival probabilities, from the lowest age group (i.e. 60–65 years) to the highest age group (i.e. 85–90 years), was greatest, while this range was smallest in Poland.
Figure 2.
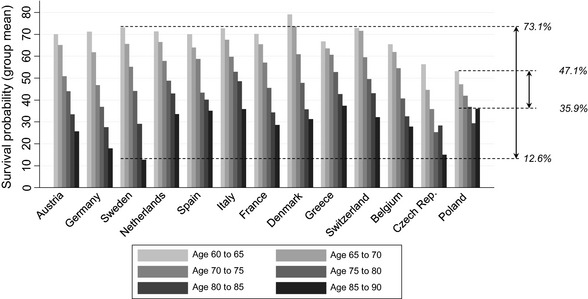
Subjective survival probability by age category and country.
Multivariate analysis
Table 3 presents the results of the multivariate analysis of SSP including all the covariates shown in Table 1. Results indicated that the final model explains 26% of the variance in SSP. The R 2 significantly increased with each consecutive model, although the actual increments in R 2 were relatively small. In general, the (signs of the) coefficients of the covariates were fairly stable across the four models, except for the first, most restrictive model.
Table 3.
| Variables3 | Model 1 | Model 2 | Model 3 | Model 4 |
|---|---|---|---|---|
| Time horizon | −1.62*** (0.144) | −1.65*** (0.140) | −1.66*** (0.140) | −1.65*** (0.140) |
| Male | −0.51 (0.416) | −2.31*** (0.410) | −2.65*** (0.451) | −1.55*** (0.552) |
| Age | −1.24*** (0.075) | −1.14*** (0.073) | −1.14*** (0.073) | −1.15*** (0.073) |
| (Age)2 | −0.02*** (0.004) | −0.01** (0.004) | −0.01* (0.004) | −0.01*** (0.004) |
| Germany | −4.55*** (1.315) | −4.72*** (1.253) | −4.92*** (1.252) | −4.87*** (1.252) |
| Sweden | 0.46 (1.374) | −1.51 (1.330) | −2.00 (1.334) | −2.05 (1.332) |
| Netherlands | 2.93** (1.290) | 1.33 (1.235) | 1.00 (1.242) | 1.05 (1.241) |
| Spain | 2.63* (1.530) | 2.84** (1.448) | 2.90** (1.449) | 2.97** (1.447) |
| Italy | 6.66*** (1.301) | 7.59*** (1.239) | 7.77*** (1.240) | 7.90*** (1.239) |
| France | 1.67 (1.320) | 2.01 (1.262) | 1.64 (1.263) | 1.68 (1.264) |
| Denmark | 6.77*** (1.366) | 5.11*** (1.309) | 4.70*** (1.317) | 4.63*** (1.317) |
| Greece | 3.41*** (1.257) | 2.48** (1.207) | 2.33* (1.207) | 2.39** (1.207) |
| Switzerland | 5.13*** (1.441) | 2.91** (1.394) | 2.54* (1.397) | 2.65* (1.396) |
| Belgium | −2.20* (1.237) | −1.79 (1.183) | −2.19* (1.184) | −2.18* (1.184) |
| Czech Republic | −14.33*** (1.397) | −14.41*** (1.330) | −14.32*** (1.328) | −14.32*** (1.328) |
| Poland | −10.81*** (1.434) | −6.96*** (1.380) | −6.64*** (1.382) | −6.51*** (1.382) |
| Living alone | −2.82*** (0.559) | −1.98*** (0.541) | −1.78*** (0.541) | −1.75*** (0.541) |
| Parent(s) alive | 3.13*** (0.680) | 3.04*** (0.662) | 2.99*** (0.660) | 2.93*** (0.659) |
| Child(ren) | 1.50* (0.799) | 1.75** (0.775) | 1.59** (0.775) | 1.56** (0.774) |
| ISCED 2 | 0.82 (0.677) | −0.30 (0.657) | −0.35 (0.656) | −0.32 (0.656) |
| ISCED 3 or 4 | 2.69*** (0.623) | 1.00 (0.606) | 0.80 (0.607) | 0.78 (0.607) |
| ISCED 5 or 6 | 4.01*** (0.722) | 1.92*** (0.706) | 1.61** (0.704) | 1.56** (0.704) |
| Working | 4.44*** (0.764) | 3.09*** (0.758) | 3.12*** (0.757) | 2.97*** (0.755) |
| Income high | 1.18* (0.606) | 0.75 (0.589) | 0.63 (0.588) | 0.54 (0.588) |
| Rural area | −1.15** (0.482) | −1.24*** (0.467) | −1.31*** (0.467) | −1.34*** (0.467) |
| Chronic disease | −2.18*** (0.623) | −2.20*** (0.623) | −2.19*** (0.623) | |
| Depressed | −9.66*** (0.567) | −9.32*** (0.569) | −9.31*** (0.569) | |
| Obese | −0.66 (0.552) | −0.74 (0.552) | −0.66 (0.552) | |
| Doctor visits high | −3.63*** (0.473) | −3.64*** (0.472) | −3.62*** (0.471) | |
| Drug use | −2.89*** (0.622) | −2.90*** (0.621) | −2.83*** (0.621) | |
| Hospital stay (night) | −1.06* (0.618) | −0.84 (0.619) | −0.84 (0.618) | |
| ADL limitations | −2.64*** (0.808) | −1.92** (0.821) | −2.10** (0.822) | |
| iADL limitations | −5.73*** (0.676) | −5.09*** (0.686) | −5.16*** (0.684) | |
| Stopped smoking | 0.43 (0.513) | 0.36 (0.513) | ||
| Currently smoking | −2.85*** (0.639) | −2.87*** (0.639) | ||
| Alcohol cons. (exc.) | 1.82*** (0.472) | 0.46 (0.611) | ||
| Physically inactive | −3.62*** (0.816) | −6.54*** (1.185) | ||
| Exces. alc × female | 3.07*** (0.867) | |||
| Phys. inact × age | 0.33*** (0.096) | |||
| Constant | 82.24*** (2.309) | 93.27*** (2.279) | 93.69*** (2.284) | 93.53*** (2.285) |
| F stat | 164.67 | 167.14 | 151.75 | 145.48 |
| R 2 | 0.21 | 0.26 | 0.26 | 0.26 |
| Adj. R 2 | 0.21 | 0.25 | 0.26 | 0.26 |
1 n = 16 295.
2Robust standard errors in parentheses.
3Austria is country reference group. For education, ISCED 0 or 1 is reference group. For smoking status, non‐smokers are reference group.
***P < 0.01, **P < 0.05, *P < 0.10.
A longer time horizon was negatively associated with respondents’ SSP. Men had lower SSPs than women (not confirmed in Model 1), while age showed an accelerating declining effect on SSP (given the significant quadratic term). Country coefficients varied to a great extent in line with the results from Fig. 2. Other important predictors of SSPs are mental health and the variables related to the social environment of the respondent. In particular, individuals who indicated that one or both parents were still alive reported significantly higher probabilities (Model 4: coefficient 2.95), having children had a similar, albeit smaller effect. The effect of living alone was also similar, but negative. Living in a rural area or small town was associated with lower SSPs.
In Model 4, all health indicators, except for obesity and hospital stay overnight, were negatively associated with SSP. Regarding the lifestyle covariates, respondents who smoked or were physically inactive reported lower SSPs, while having quit smoking was not related to SSP. Interestingly, excessive alcohol consumption was associated with higher SSP in the third model, but when adding the interaction term with gender, the main effect was no longer significant. The interaction term, however, was significant indicating that the ‘positive’ effect from the third model related only to women. In other words, only women who drink excessively (i.e. more than one glass per day) had significantly higher SSP. Age significantly attenuated the negative effect of being physically inactive on SSP.
The estimation of the fourth regression model for each country separately revealed a similar pattern for many variables in terms of sign and magnitude of the coefficient, for example, for ‘time horizon’, ‘age’, ‘depression’ and ‘doctor visits’. Table 4 shows estimation results for six countries (the other countries are shown in Appendix B). Coefficients of some other variables, however, differed substantially. Among others, the effects of gender and having children from the previous analysis were not present in any of the six countries, while smoking only had a significant negative association with SSP in the Netherlands. Former smokers from Spain and the Czech Republic reported significantly higher SSP than non‐smokers, while quitters from the Netherlands reported lower probabilities. The results for alcohol consumption also differed from the original analysis, while the outcomes for the variable ‘physically inactive’ were similar to those in Table 3. Note that the standard errors are much larger due to smaller sample sizes.
Table 4.
Country differences subjective survival probability: regression analysis (Model 4) by country 1
| Variables | Sweden | Netherlands | Spain | Italy | Greece | Czech Rep |
|---|---|---|---|---|---|---|
| Time horizon | −1.35*** (0.509) | −1.86*** (0.442) | −1.98*** (0.601) | −1.29*** (0.451) | −0.65 (0.411) | −1.86*** (0.512) |
| Male | 1.05 (1.839) | 1.31 (1.824) | −2.73 (2.095) | −1.77 (1.649) | 2.49 (1.588) | 1.18 (2.074) |
| Age | −1.02*** (0.285) | −1.44*** (0.236) | −1.16*** (0.345) | −0.84*** (0.228) | −0.27 (0.209) | −2.15*** (0.251) |
| (Age)2 | −0.06*** (0.015) | 0.01 (0.014) | 0.00 (0.019) | −0.01 (0.015) | −0.04*** (0.013) | 0.06*** (0.016) |
| Living alone | −2.07 (1.927) | 1.06 (1.860) | −2.45 (2.934) | −3.22* (1.927) | −4.13*** (1.409) | 2.71 (1.746) |
| Parent(s) alive | 1.35 (2.030) | −1.10 (2.210) | 8.32*** (3.045) | 4.55** (2.188) | 1.77 (1.736) | 1.16 (2.707) |
| Child(ren) | 4.65 (2.942) | 2.68 (2.775) | 4.06 (4.097) | −2.27 (2.456) | −0.82 (1.821) | 4.60 (2.970) |
| ISCED 2 | 3.70* (2.088) | −2.22 (2.083) | −2.68 (2.576) | −1.31 (1.916) | −1.25 (1.920) | −1.04 (2.144) |
| ISCED 3 or 4 | 6.71*** (1.988) | 0.73 (2.393) | 2.51 (3.405) | −3.88* (2.132) | 2.83* (1.713) | −2.78 (2.257) |
| ISCED 5 or 6 | 0.20 (2.059) | −1.83 (2.484) | 0.34 (3.549) | −6.31** (2.678) | 1.68 (2.170) | −1.77 (3.077) |
| Working | 5.02** (2.126) | 1.83 (2.527) | 4.88 (3.711) | 2.56 (3.272) | −1.06 (1.968) | 1.06 (3.319) |
| Income high | −2.18 (3.955) | 2.63 (2.138) | −6.36*** (2.183) | 2.30 (1.593) | 1.58 (1.340) | −25.67*** (3.592) |
| Rural area | −2.87* (1.577) | 0.83 (1.501) | −1.14 (2.082) | −2.14 (1.648) | 1.80 (1.506) | −3.53** (1.631) |
| Chronic disease | −1.54 (1.963) | −3.37* (1.900) | −3.68 (2.769) | −2.40 (2.334) | −1.12 (1.857) | −4.86* (2.553) |
| Depressed | −3.75 (2.306) | −7.35*** (2.042) | −12.86*** (2.337) | −13.23*** (1.666) | −7.88*** (1.749) | −9.24*** (1.910) |
| Obese | 1.23 (2.068) | 2.95 (1.968) | −5.21** (2.151) | −0.44 (1.748) | 0.21 (1.427) | −1.29 (1.714) |
| Doctor visits high | −1.50 (1.607) | −5.42*** (1.644) | −7.44*** (2.045) | −0.10 (1.456) | −3.96*** (1.248) | −6.43*** (1.777) |
| Drug use | −5.53*** (1.979) | −3.79** (1.747) | 2.52 (2.907) | −1.34 (2.239) | −1.20 (1.861) | −2.82 (2.782) |
| Hosp. stay (night) | 3.48 (2.383) | −1.62 (2.319) | −6.11** (2.676) | 1.88 (2.010) | −4.13* (2.275) | −0.28 (2.061) |
| ADL limitations | −0.23 (3.069) | −0.50 (3.315) | 2.71 (3.656) | −7.19*** (2.722) | −8.33*** (3.033) | −2.31 (2.637) |
| iADL limitations | −2.35 (2.768) | −6.34*** (2.232) | −12.51*** (3.072) | −5.08** (2.109) | 2.71 (1.667) | −4.00* (2.311) |
| Stopped smoking | −1.23 (1.598) | −3.04* (1.571) | 4.05* (2.436) | 0.96 (1.684) | 2.77 (1.706) | 3.44* (2.011) |
| Currently smok. | −3.71 (2.380) | −7.66*** (2.232) | −1.84 (3.186) | 0.24 (2.105) | −1.41 (1.557) | −0.96 (2.439) |
| Alc. cons. (exc.) | −2.61 (2.100) | −3.75* (2.026) | −1.43 (2.817) | 0.33 (1.929) | 3.91** (1.659) | −4.70** (2.299) |
| Phys. Inactive | −18.83*** (6.009) | −3.33 (4.569) | −8.85* (4.680) | −6.89** (2.786) | −14.79*** (4.727) | −9.46*** (3.013) |
| Exc. alc. × female | 6.48** (2.992) | 6.94** (2.776) | 10.11* (5.654) | 3.46 (3.484) | 2.43 (2.553) | 4.33 (3.373) |
| Phys. inact × age | 1.33*** (0.469) | 0.35 (0.408) | 0.41 (0.395) | 0.48* (0.260) | 1.07*** (0.361) | 0.16 (0.274) |
| Constant | 85.76*** (7.326) | 98.03*** (6.674) | 102.38*** (8.754) | 97.65*** (6.717) | 75.21*** (5.861) | 83.63*** (7.414) |
| Observations | 1471 | 1302 | 923 | 1715 | 1428 | 1179 |
| R 2 | 0.32 | 0.23 | 0.28 | 0.18 | 0.21 | 0.27 |
| Adj. R 2 | 0.30 | 0.22 | 0.26 | 0.17 | 0.20 | 0.25 |
1Robust standard errors in parentheses.
***P < 0.01, **P < 0.05, *P < 0.10.
Reliability
The final analyses concerned the reliability of the answers to the SSP question. 435 respondents (2.7%) did not answer either one or both of the two questions about the standard of living in five years. Of the remaining 15 860 respondents, 96.9% provided a reliable answer. In addition, 77% (n = 12 550) of the respondents answered the numeracy question correctly. In total, a subsample of 11 918 respondents (75.2%) answered both questions validly.
Re‐estimation of the fourth regression model (from Table 3) with this subsample showed that this model performed similarly to the initial model (using our original sample) in terms of adjusted R 2 (0.24 vs. 0.26) (results not shown here). Although the coefficients varied somewhat in size between both analyses, almost all variables retained their sign and statistical significance.
Discussion
In this paper, we have presented the SSP from respondents aged 60 through 90 years from several European countries, using data from the second wave of SHARE and related them to general characteristics, (mental) health and, our main focus, lifestyle factors. The average SSP of surviving the next 9–15 years was around 57%. In general, the findings from this study show associations between SSP and socio‐demographic, socio‐economic, social context and (objective) health indicators in line with previous research.
Regarding socio‐economic status, one would expect a positive significant relation between measures like education and income and SSP, considering the fact that richer and higher educated people may have better living conditions as well as better access to (better) health‐care services. Indeed, our results suggest that higher educated respondents report higher SSP, but this effect diminishes once we introduce health and lifestyle variables into our model. The same pattern holds for income, where we only find a significant association between a higher income and SSP in our first model, although the sign remains consistent in all models.
Obesity is not significantly associated with SSP. Although several previous studies did report such an association, it is coherent with the recent literature that indicates that being obese at older ages does not necessarily shorten remaining life expectancy but instead increases morbidity and disability. It would therefore be interesting to investigate whether obese individuals adjust their expectations regarding future quality of life accordingly.
As expected, smokers reported significantly lower survival chances compared to non‐smokers. Interestingly, however, no SSP difference was found between non‐smokers and past smokers. This is interesting, since risk of disease and early death from most smoking‐related causes only declines to the level of never‐smokers after many years. A number of possible explanations for this somewhat surprising finding may be given. First, quitters may have already stopped smoking for many years and therefore take all the benefits of quitting into consideration. Alternatively, more recent quitters may be overoptimistic regarding the benefits of stopping and incorporate this into their survival chances. Finally, our findings may also indicate that non‐smokers underestimate their SSPs.
The association between SSP and alcohol consumption is rather striking. Our third regression model showed that individuals who drink excessively report significantly higher SSP than moderate drinkers and abstainers. However, after introducing the interaction term excessive alcohol consumption × gender, this result only held for female excessive drinkers. A possible explanation for this has to do with the (believed) protective effect of (light–)moderate alcohol consumption. Female excessive drinkers might not consider themselves to be heavy drinkers and therefore belief to have benefits from their alcohol use. This relates to the fact that excessive drinking among women, according to the Dutch alcohol norm, starts from one glass of alcohol per day. Another possible explanation comes from the increasing risk of harmful health effects as the amount of drinking increases. From our data, it turned out that men, next to being more likely than women to drink excessively, consume more alcohol also excessively.
Finally, physical inactivity was negatively associated with SSP. It is important to emphasize that such an association need not signal causality. Indeed, being physically inactive may result from poor health rather than being a lifestyle decision. The fourth regression model indicated that a higher age attenuates the negative relation between physical inactivity and SSP. This may be partly explained by the acceptance of less mobility or a poorer health state at more advanced stages of life. If a declining physical functionality is considered a normal part of ageing, then its relation with SSP may become weaker. Instead, physical inactivity at younger ages can be an outcome of a serious health issue that individuals may believe to influence their longevity.
Cross‐country differences
Our results showed important cross‐country differences in terms of average SSP, the range between the lowest and highest age group, and some associations between SSP and the covariates, such as alcohol consumption and having stopped smoking. Regarding the latter, current smokers in the Netherlands reported by far the lowest SSP relatively to non‐smokers and quitters. This may signal that Dutch smokers are informed about and aware of the negative effects of smoking on life expectancy. Therefore, despite the success of tobacco control policies in declining smoking rates and raising awareness about the harmful effects of smoking, the Dutch government may reconsider its prioritization in tobacco control, aiming, for example, at higher prices of cigarettes and better treatment (coverage) to help smokers stop, instead of increasing consumer information. These results show the value of examining the differences in the public uptake of preventive strategies between countries.
Furthermore, a striking finding in this study was the fact that, overall, respondents living in a rural area or small town reported significantly lower expected survival chances than respondents living in an urban environment. In our country analyses, we found this negative significant relationship only for Sweden and the Czech Republic, although for most countries the signs were consistent with our main model. This clear impact of living in a rural area deserves more attention in future studies. While it may reflect differences in lifestyles, access to health‐care facilities or working conditions, the current study cannot answer these questions.
Overall, results from Poland and the Czech Republic seemed to be somewhat deviant compared to other countries. A possible explanation is that both Eastern European countries were not included in the first wave of SHARE. This means that both the Czech and Polish respondents were probably unfamiliar with the probabilistic format as well as with subjective longevity questions, which may have had some influence on their responses. Alternatively, respondents from other countries were observed for the second time and may have gained knowledge about their longevity expectations prior to the second wave (so‐called learning effect). It would therefore be interesting to see whether the result patterns between Eastern and Western European samples are more similar in the fourth wave of SHARE. These considerations also raise the question whether it is more informative to analyse all country samples separately instead of aggregating these subsamples to one sample. In that context, a recent cross‐sectional study, Péntek et al.36 found that subjective life expectancy patterns (using a point estimate) in Hungary and the Netherlands were similar, despite differences in actuarial life expectancy and cultural diversity. This supports the idea that results across European samples may be comparable.
Probabilistic format
The use of the probabilistic format when investigating subjective longevity expectations is increasingly embraced in the literature for reasons of interpretation, interpersonal comparability and comparisons with known event frequencies, and the possibility to investigate the consistency of responses.37 It is still important, however, to understand the willingness and capability of individuals to answer probabilistic questions. A promising result from our study that supports the use of SSPs is the relatively high SSP response rate (89%), especially considering the average age of our sample (around 70 years). It is unclear how much of the non‐response can be ascribed to misunderstanding, cognition or observation error. Our analysis showed that, among other things, non‐response was higher in certain countries and among older and lower educated people. This indicates that the use of probabilities as an elicitation method may be less valid under some circumstances. Recently, initiatives using visual aids have been employed that could be useful in respondents who are less capable or willing to answer probability questions verbally. See Delavande et al., 38 for instance, for a review on methods for eliciting SSPs in developing countries.
We have addressed this issue also by identifying ‘reliable’ respondents according to two validity criteria. Outcomes using only reliable responses (around 75% of our sample) were very similar to our original analysis. This is indicative of good validity of the SSP question, but still it is worth‐investigating further whether respondents should be systematically excluded based on such criteria. In a similar context, however, Hurd and McGarry5 argue that even lower quality responses contain information that is worthwhile. Furthermore, they argue that a certain amount of inconsistency (i.e. as found in our study) is acceptable and most likely similar to inconsistencies and errors found in many of the predictor variables.
Related issues are that of rounding numerical responses and ‘focal‐point responses’, which are common in SSP data.8, 12 From the distribution shown in Fig. 1, it becomes evident that this is also the case in our study. For example, the (often highest) spike at 50% is problematic in terms of interpretation. Bruine de Bruin et al.39 suggest that this response may reflect more fundamental uncertainty (similar to ‘don't know’) or the cognitive inability to answer probabilistic questions rather than real probabilistic thinking. However, while focal values probably represent measurement error, it is still believed that focal‐point responses do contain valuable information. Therefore, the general practice is to take numerical answers at face value instead of correcting for biases, including those at 0%, 50% and 100%.8, 40 This line was also taken here.
Limitations
Several issues deserve attention when interpreting the results from our study. First, a drawback of our study is a consequence of the fact that SHARE only includes one SSP question with one individual target age instead of a sequence of questions using different target ages. Therefore, SHARE does not provide the opportunity to estimate a whole distribution of probabilities of the expected ‘time of death’.
Another limitation concerns the measures used for assessing objective health. The input for our health indicators, like the amount of doctor visits, were given by the respondents and not objectively measured. This arguably introduces measurement errors. More objective health measures, such as the measurement of how fast a respondent can expel air from his/her longs and walking speed, were present in the dataset of SHARE but available only for certain age groups. Moreover, response rates were relatively low, possibly because respondents did not feel safe performing the tests. We deliberately opted for the most objective measure of health status available instead of a subjective measure, such as self‐assessed health. Previous research has shown that self‐assessed health is indeed a good predictor of SSP and predicts mortality rather well,28 but more objective health indicators reduce problems of endogeneity.
Third, as SSP was measured on a continuous scale that took on a value within a defined range from 0 to 100, ordinary least squares regression may prove inadequate as it does not necessarily constrain inference about the outcome values to the predefined range.41 To test the influence on our results, we also performed a generalized ordered logit model for ordinal dependent variables. SSP was restructured into three ordinal categories: 0–33% (n = 3860), 34–66% (n = 5530) and 67–100% (n = 6905). The results (not shown here) showed largely the same outcomes as the ordinary least squares regression (in terms of both signs of the coefficients and significance levels). The most salient results were the lack of significance for the variables ‘age squared’ and ‘having children’. Other differences from the original analysis were merely related to the degree of significance. Some covariates, like ‘age’, ‘depression’, ‘physical inactivity’ and two country dummies, violated the parallel lines assumption, which means that their effect is not the same across the three SSP categories (but these differences are related more to the strength of the observed relationships than more fundamental differences). Overall, this lends support to our choice for interpreting the independent variable as continuous and for presenting the results from the ordinary least squares regression.
A final, important issue is related to the use of cross‐sectional data. It is clear that using panel data allows us to better understand the formation of SSPs. The use of panel data would provide the opportunity to see whether people update their SSP according to new information and events (e.g. quit smoking). However, the objective in our study was mainly of descriptive nature and, moreover, at the time of this study, only two ‘prospective’ waves were available. Only few people that would fit our criteria of sample inclusion and that participated in the first wave had experienced relevant new events or passed away before the start of the second wave. Moreover, it needs to be stressed that even when using panel data, statements about causality regarding the relation between SSP and behaviour remain tentative and contentious since this relation is to a large extent circular. In other words, it is unclear whether SSPs are affected by lifestyle decisions and/or vice versa. Other, advanced econometric methods may be used to tackle this issue of endogeneity.
Conclusions
The findings from our study suggest that SSPs are useful, informative and important in relation to lifestyle decisions and can be validly obtained in elder people. Although negative health effects of certain lifestyle decisions are widely publicized, individuals may not adequately personalize the possible consequences of such decisions. Unawareness or underestimation of health risks related to unhealthy behaviour (e.g. excessive alcohol consumption) or the possible benefits from lifestyle improvements impede the effectiveness of health policy aimed at improving lifestyle and with that reducing avoidable premature mortality. Our results show that the relation between SSP and lifestyle differs among subgroups. This was most markedly for excessive alcohol consumption among men and women and, to a lesser extent, for physical inactivity at higher ages. Obese respondents did not report lower SSP than respondents with normal weight, despite the fact that research shows that obese adults recognize the adverse health effects of obesity.42 Moreover, significant cross‐country differences regarding SSP and its relation to lifestyle were observed. These findings provide interesting implications for health policy and, for instance, targeting health communication strategies. Moreover, they warrant the further exploration of not only SSPs, but also of expectations of future quality of life in relation to lifestyle decisions.
Acknowledgements
This paper uses data from SHARE wave 1 and 2 release 2.5.0, as of 24 May 2011. The SHARE data collection has been primarily funded by the European Commission through the 5th Framework Programme (project QLK6‐CT‐2001‐00360 in the thematic programme Quality of Life), through the 6th Framework Programme (projects SHARE‐I3, RII‐CT‐2006‐062193, COMPARE, CIT5‐CT‐2005‐028857, and SHARELIFE, CIT4‐CT‐2006‐028812) and through the 7th Framework Programme (SHARE‐PREP, No. 211909, SHARE‐LEAP, No. 227822 and SHARE M4, No. 261982). Additional funding from the U.S. National Institute on Aging (U01 AG09740‐13S2, P01 AG005842, P01 AG08291, P30 AG12815, R21 AG025169, Y1‐AG‐4553‐01, IAG BSR06‐11 and OGHA 04‐064) and the German Ministry of Education and Research as well as from various national sources is gratefully acknowledged (see www.share-project.org for a full list of funding institutions). This study was part of the project ‘Living longer in good health’, which was financially supported by Netspar. The opinions expressed in the paper are those of the authors. The authors have no conflict of interests that are directly relevant to the content of this article.
Appendix A.
Glossary of variables
| Variable | Variable definition/categories |
|---|---|
| SSP | Subjective survival probability (range 0–100) |
| Time horizon | Target age – current age (range 9–15 years) |
| Male | 1 if male, 0 if female |
| Age | Age in years |
| (Age)2 | Age in years squared |
| Country (dummy variables)1 | Austria (reference group), Germany, Sweden, Netherlands, Spain, Italy, France, Denmark, Greece, Switzerland, Belgium, Czech Republic, Poland |
| Living alone | 1 if living alone, 0 if living with spouse or partner |
| Parent(s) alive | 1 if one or both parents alive, 0 if no parents alive |
| Child(ren) | 1 if respondent has child(ren), 0 if respondent has no child(ren) |
| ISCED 22 | 1 if lower secondary education or second stage of basic education, 0 otherwise |
| ISCED 3 or 42 | 1 if (upper) secondary education or post‐secondary non‐tertiary education, 0 otherwise |
| ISCED 5 or 62 | 1 if first or second stage of tertiary education, 0 otherwise |
| Working | 1 if worker (both employed and self‐employed), 0 otherwise |
| Income high3 | 1 if above median income, 0 if below median income |
| Rural area | 1 if living in a small town, rural area or village, 0 if living in a city, suburb or large town. |
| Chronic disease4 | 1 if chronically ill, 0 if not chronically ill |
| Depressed5 | 1 if depressed, 0 if not depressed |
| Obese | 1 if obese (BMI ≥ 30 kg/m2), 0 otherwise |
| Doctor visits high6 | 1 if above country median number of contacts with medical doctor in the last 12 months, 0 otherwise |
| Drug user | 1 if used physician prescribed drugs in the last week, 0 otherwise |
| Hospital stay (night) | 1 if a respondent stayed in a hospital overnight during the last 12 months, 0 otherwise |
| ADL limitation | 1 if at least one limitation with daily activities of daily living (e.g. dressing), 0 otherwise |
| iADL limitation | 1 if at least one limitation with instrumental activities of daily living (e.g. preparing hot meal), 0 otherwise |
| Stopped smoking7 | 1 if quit smoking, 0 otherwise |
| Currently smoking7 | 1 if currently smoking, 0 otherwise |
| Alcohol consump. (excessive) | 1 if drinking more than recommended levels or excessively, 0 otherwise |
| Physically inactive | 1 if physically inactive, 0 otherwise |
| Alcohol consump. × female | 1 if female and drinking more than recommended levels or excessively, 0 otherwise |
| Physically inactive × age | Physically inactive multiplied by age |
1Reference category (indicated by 0) is Austria, because the mean SSP was closest to the overall sample mean SSP.
2Reference group is ISCED 0 or 1, that is no education, pre‐primary education, primary education and first stage of basic education.
3Overall sample median (net) average income per month is € 991, adjusted for household size and PPP.
4A respondent was considered to be chronically ill in case he/she reported to be diagnosed with a chronic disease by a doctor.
5Depression was assessed using the 12‐item EURO‐D scale with scores above three indicating a clinically significant level of depression.
6Dentist visits were disregarded.
7Reference group is non‐smokers.
Appendix B1.
| Variables | Austria | Germany | France | Denmark | Switzerland | Belgium | Poland |
|---|---|---|---|---|---|---|---|
| Time horizon | −2.86*** (0.600) | −2.94*** (0.473) | −1.20** (0.531) | −1.27** (0.497) | −1.88*** (0.649) | −1.79*** (0.437) | −1.22** (0.532) |
| Male | −2.56 (2.230) | −3.40* (1.972) | −5.98** (2.319) | −3.60 (2.481) | −3.33 (2.568) | −1.32 (1.887) | −3.14 (2.193) |
| Age | −1.50*** (0.325) | −1.94*** (0.295) | −1.20*** (0.268) | −1.53*** (0.254) | −0.94*** (0.338) | −1.13*** (0.212) | −0.98*** (0.283) |
| (Age)2 | 0.00 (0.019) | 0.02 (0.017) | −0.00 (0.018) | −0.00 (0.015) | −0.03 (0.020) | −0.02 (0.014) | 0.03 (0.019) |
| Living alone | 3.32 (2.243) | −0.49 (2.140) | −2.62 (1.925) | −4.87** (1.942) | −0.12 (2.373) | 0.23 (1.659) | −3.53* (2.092) |
| Parent(s) alive | 1.35 (3.246) | 8.31*** (2.213) | 2.49 (2.203) | 0.88 (2.333) | 3.48 (2.828) | 3.41* (2.027) | 1.98 (3.250) |
| Child(ren) | 6.33** (2.809) | −0.19 (3.025) | −1.42 (2.648) | −0.42 (3.177) | −1.04 (2.888) | 0.20 (2.234) | 11.33*** (3.652) |
| ISCED 2 | 5.66* (3.166) | −2.14 (10.088) | 4.77* (2.770) | 0.23 (3.917) | −5.53 (3.435) | 0.89 (1.764) | 13.59** (6.669) |
| ISCED 3 or 4 | 4.99** (2.494) | −0.21 (9.939) | 1.43 (1.943) | 3.26 (2.221) | −1.62 (3.021) | −1.24 (1.861) | 2.16 (2.105) |
| ISCED 5 or 6 | 8.30** (3.285) | 3.11 (10.091) | 3.48 (2.284) | 2.54 (2.269) | 0.77 (3.944) | 2.56 (1.935) | 2.07 (3.694) |
| Working | 6.22 (3.905) | 3.22 (2.672) | 4.99 (3.158) | 1.99 (2.188) | −0.45 (2.722) | −2.24 (3.031) | 1.11 (6.311) |
| Income high | 3.48 (2.305) | 4.42* (2.281) | 0.85 (2.067) | −3.91 (2.984) | 0.32 (2.227) | 2.18 (1.605) | −1.91 (2.916) |
| Rural area | −0.40 (2.122) | −2.55 (1.566) | 0.27 (1.695) | −2.09 (1.533) | −0.85 (2.183) | −1.59 (1.395) | −2.31 (1.997) |
| Chronic disease | −4.79* (2.477) | 1.09 (2.054) | −4.16* (2.213) | −2.71 (1.935) | −2.33 (2.412) | 1.24 (1.982) | −6.55** (3.333) |
| Depressed | −11.40*** (2.692) | −10.76*** (2.195) | −11.15*** (1.735) | −7.77*** (2.559) | −3.98 (3.244) | −7.90*** (1.660) | −9.59*** (1.879) |
| Obese | −3.03 (2.201) | −2.41 (2.161) | 2.87 (2.146) | −1.40 (2.178) | −2.54 (3.165) | −0.54 (1.675) | 1.34 (2.051) |
| Doctor visits high | −1.55 (1.981) | −3.90** (1.635) | −4.58*** (1.766) | −4.99*** (1.681) | −5.34** (2.182) | −3.96*** (1.514) | −2.53 (1.896) |
| Drug use | −1.32 (2.500) | −4.82** (2.021) | −0.72 (2.379) | −3.52* (1.887) | 1.01 (2.568) | −3.00 (1.993) | −1.15 (2.956) |
| Hospital stay overnight | −1.05 (2.223) | 0.46 (1.978) | −2.12 (2.287) | 0.81 (2.224) | 1.42 (2.909) | −0.78 (1.845) | −5.51** (2.150) |
| ADL limitations | −7.36** (3.245) | −1.12 (2.836) | −0.32 (2.832) | 2.05 (3.757) | −2.26 (5.408) | −3.24 (2.282) | −0.69 (2.205) |
| iADL limitations | −9.21*** (2.917) | −7.61*** (2.852) | −1.51 (2.418) | −8.51*** (3.066) | −9.01*** (4.348) | −3.49 (2.171) | −8.38*** (2.288) |
| Stopped smoking | −0.44 (2.355) | 1.10 (1.801) | 2.87 (1.968) | 0.96 (1.651) | −2.06 (2.354) | −2.89* (1.547) | −0.97 (2.219) |
| Currently smoking | −3.81 (2.814) | −3.46 (2.269) | 0.34 (2.771) | −2.26 (2.029) | −1.37 (2.843) | −7.03*** (2.251) | −4.65* (2.548) |
| Alcohol cons. (exc.) | 1.45 (2.766) | −0.14 (2.077) | 3.16 (2.456) | −0.68 (2.288) | −1.04 (2.796) | 3.57* (1.909) | 4.80* (2.518) |
| Physically inactive | −4.28 (4.255) | −4.42 (5.810) | −2.27 (4.135) | −8.12 (6.566) | −7.51 (10.093) | −3.65 (3.862) | 4.75 (3.064) |
| Exces. alc. × female | −1.37 (3.702) | 3.84 (2.988) | −4.42 (3.187) | 3.93 (3.047) | −0.33 (3.717) | 1.53 (2.647) | −1.21 (3.867) |
| Phys. inact × age | 0.45 (0.327) | −0.12 (0.420) | −0.40 (0.323) | −0.24 (0.423) | 0.09 (0.703) | 0.11 (0.296) | −0.15 (0.296) |
| Constant | 99.09*** (9.191) | 106.05*** (11.928) | 90.73*** (7.886) | 100.14*** (7.312) | 104.31*** (9.575) | 90.40*** (6.450) | 71.15*** (8.155) |
| Observations | 829 | 1403 | 1206 | 1280 | 794 | 1597 | 1168 |
| R 2 | 0.33 | 0.31 | 0.27 | 0.32 | 0.22 | 0.24 | 0.17 |
| Adj. R 2 | 0.31 | 0.30 | 0.25 | 0.31 | 0.20 | 0.23 | 0.15 |
1Robust standard errors in parentheses.
***P < 0.01, **P < 0.05, *P < 0.10.
References
- 1. Khaw K‐T, Wareham N, Bingham S et al Combined impact of health behaviours and mortality in men and women: the EPIC‐Norfolk Prospective Population Study. PLoS Medicine, 2008; 5: e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yaari ME. Uncertain lifetime, life insurance, and the theory of the consumer. The Review of Economic Studies, 1965; 32: 137–150. [Google Scholar]
- 3. Manski CF. Measuring expectations. Econometrica, 2004; 72: 1329–1376. [Google Scholar]
- 4. Hamermesh DS. Expectations, life expectancy, and economic behaviour. The Quarterly Journal of Economics, 1985; 100: 389–408. [Google Scholar]
- 5. Hurd MD, McGarry K. Evaluation of the subjective probabilities of survival in the Health and Retirement Study. Journal of Human Resources, 1995; 30: S268–S292. [Google Scholar]
- 6. Mirowsky J. Subjective life expectancy in the US: correspondence to actuarial estimates by age, sex and race. Social Science & Medicine, 1999; 49: 967–979. [DOI] [PubMed] [Google Scholar]
- 7. Elder TE. Subjective survival probabilities in the Health and Retirement Study: Systematic bias and predictive validity. MRRC Working Paper 2007. Available at: http://www.mrrc.isr.umich.edu/publications/papers/pdf/wp159.pdf, accessed 16 February 2012.
- 8. O'Donnell O, Teppa F, van Doorslaer E. Can Subjective Survival Expectations Predict Retirement Behaviour? Amsterdam: DNB, 2008. [Google Scholar]
- 9. Perozek M. Subjective expectations to forecast longevity: do survey respondents know something we don't know? Demography, 2008; 45: 95–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hurd MD. Subjective probabilities in household surveys. Annual Review of Economics, 2009; 1: 543–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Peracchi F, Perotti V. Subjective survival probabilities and life tables: Evidence from Europe. EIEF Working Paper Series 2011. Available at: http://www.eief.it/files/2012/09/wp-16-subjective-survival-probabilities-and-life-tables_evidence-from-europe.pdf, accessed 23 May 2012.
- 12. Gan L, Gong G, Hurd MD, McFadden DL. Subjective mortality risk and bequest. NBER Working Paper Series 2004. Available at: http://www.nber.org/papers/w10789.pdf?new_window=1, accessed 14 February 2012.
- 13. Salm M. Subjective mortality expectations and consumption and savings behaviours among the elderly. Canadian Journal of Economics, 2010; 43: 1040–1057. [Google Scholar]
- 14. Hurd MD, Smith JP, Zissimopoulos JM. The effects of subjective survival on retirement and social security claiming. Journal of Applied Econometrics, 2004; 19: 761–775. [Google Scholar]
- 15. Benitez‐Silva H, Dwyer DS. The rationality of retirement expectations and the role of new information. Review of Economics and Statistics, 2005; 87: 587–592. [Google Scholar]
- 16. de Nardi M, French E, Jones JB. Life expectancy and old age savings. American Economic Review, 2009; 99: 110–115. [Google Scholar]
- 17. Post T, Hanewald K. Longevity risk, subjective survival expectations, and individual saving behavior. Netspar Discussion Papers 2011. Available at: http://arno.uvt.nl/show.cgi?fid=114096, accessed 23 May 2012.
- 18. Smith VK, Taylor DH Jr, Sloan FA. Longevity expectations and death: can people predict their own demise? American Economic Review, 2001; 91: 1126–1134. [Google Scholar]
- 19. Hurd MD, McGarry K. The predictive validity of subjective probabilities of survival. Economic Journal, 2002; 112: 966–998. [Google Scholar]
- 20. Siegel M, Bradley EH, Kasl SV. Self‐rated life expectancy as a predictor of mortality: evidence from the HRS and AHEAD Survey. Gerontology, 2003; 49: 265–271. [DOI] [PubMed] [Google Scholar]
- 21. Winter J. Expectations and attitudes In: Börsch‐Supan A, Brugiavini A, Jürges H. et al (eds) First Results From the Survey of Health, Ageing and Retirement in Europe (2004‐2007) – Starting the Longitudinal Dimension. Mannheim: Mannheim Research Institute for the Economics of Aging (MEA), 2008: 306–311. [Google Scholar]
- 22. Mirowsky J, Ross CE. Socioeconomic status and subjective life expectancy. Social Psychology Quarterly, 2000; 63: 133–151. [Google Scholar]
- 23. Delavande A, Rohwedder S. Differential survival in Europe and the United States: estimates based on subjective probabilities of survival. Demography, 2011; 48: 1377–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking: 50 years’ observations on male British doctors. British Medical Journal, 2004; 328: 1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Smith VK, Taylor DH Jr, Sloan FA, Johnson FR, Desvousges WH. Do smokers respond to health shocks? The Review of Economics and Statistics, 2011; 83: 675–687. [Google Scholar]
- 26. Schoenbaum M. Do smokers understand the mortality effects of smoking? Evidence from the Health and Retirements Survey. American Journal of Public Health, 1997; 87: 755–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Khwaja A, Sloan F, Chung S. The relationship between individual expectations and behaviors: mortality expectations and smoking decisions. Journal of Risk Uncertainty, 2007; 35: 179–201. [Google Scholar]
- 28. Balia S. Survival expectations, subjective health and smoking: Evidence from European countries. HEDG Working Paper 2011. Available at: http://www.york.ac.uk/media/economics/documents/herc/wp/11_30.pdf, accessed 14 February 2012.
- 29. Fontaine KR, Redden DT, Wang C, Westfall AO, Allisson DB. Years of life lost due to obesity. The Journal of the American Medical Association, 2003; 289: 187–193. [DOI] [PubMed] [Google Scholar]
- 30. Peeters A, Barendregt JJ, Willekens F, Mackenbach JP, Mamun AA, Bonneux L. Obesity in adulthood and its consequences for life expectancy: a life‐table analysis. Annals of Internal Medicine, 2003; 138: 24–32. [DOI] [PubMed] [Google Scholar]
- 31. Walter S, Kunst AE, Mackenbach JP, Hofman A, Tiemeier H. Mortality and disability: the effect of overweight and obesity. International Journal of Obesity, 2009; 33: 1410–1418. [DOI] [PubMed] [Google Scholar]
- 32. Falba TA, Busch SH. Survival expectations of the Obese: is excess mortality reflected in perceptions? Obesity Research, 2005; 13: 754–761. [DOI] [PubMed] [Google Scholar]
- 33. Börsch‐Supan A, Brugiavini A, Jürges H et al First Results From the Survey of Health, Ageing and Retirement in Europe (2004‐2007). Starting the Longitudinal Dimension. Mannheim: Mannheim Research Institute for the Economics of Aging (MEA), 2008. [Google Scholar]
- 34. Börsch‐Supan A, Jürges H. The Survey of Health, Ageing and Retirement in Europe – Methodology. Mannheim: Mannheim Research Institute for the Economics of Aging (MEA), 2005. [Google Scholar]
- 35. Health Council of the Netherlands . Guidelines for a Healthy Diet 2006. The Hague: Health Council of the Netherlands, 2006. [Google Scholar]
- 36. Péntek M, Brodszky V, Gulácsi ÁL et al Subjective expectations regarding length and health‐related quality of life in Hungary: results from an empirical investigation. Health Expectations, 2014; 17: 696–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dominitz J, Manski CF. Using expectations data to study subjective income expectations. Journal of the American Statistical Association, 1997; 92: 855–867. [Google Scholar]
- 38. Delavande A, Giné X, McKenzie D. Measuring subjective expectations in developing countries: a critical review and new evidence. Journal of Developmental Economics, 2011; 94: 151–163. [Google Scholar]
- 39. Bruine dBW, Fischbeck PS, Stiber NA, Fischhoff B. What number is fifty‐fifty? Redistributing excessive 50% responses in elicited probabilities Risk Analysis, 2002; 22: 713–723. [DOI] [PubMed] [Google Scholar]
- 40. Manski CF, Molinari F. Rounding probabilistic expectations in surveys. Journal of Business & Economic Statistics, 2010; 28: 219–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bottai M, Cai B, McKeown RE. Logistic quantile regression for bounded outcomes. Statistics in Medicine, 2010; 29: 309–317. [DOI] [PubMed] [Google Scholar]
- 42. Finkelstein EA, Brown DS, Douglas EW. Do obese persons comprehend their personal health risks? American Journal of Health Behaviour, 2008; 32: 508–516. [DOI] [PubMed] [Google Scholar]


