Abstract
Background
The technologies currently available to detect the presence of foetal genetic abnormalities are complex, and undergoing prenatal diagnostic testing can have wide‐ranging repercussions. Before individuals can decide with certainty whether or not to take these tests, they first need to grasp the many psychosocial and clinical dimensions of prenatal genetic testing.
Objective
To test a model integrating key psychosocial and clinical factors as predictors of decisional conflict in decisions about whether or not to undergo prenatal genetic testing.
Method
Adults (n = 457) read one of four hypothetical scenarios asking them to imagine expecting a child and considering the option of a prenatal test able to detect a genetic condition; age of condition onset (birth vs. adulthood) and its curability (no cure vs. curable) were manipulated. Participants completed measures of decisional conflict, perceived benefits from normal results, test response efficacy, condition coherence, child‐related worry, perceived disagreement with the other parent's preference, motivation to comply with doctors' perceived preferences, and parity.
Results
Prenatal testing decisional conflict was positively predicted by perceiving normal results as beneficial, doubting the test's reliability, lacking understanding of the genetic condition, worrying about the health of the foetus, perceiving differences of opinion from partner/spouse, wanting to follow doctors' preferences, and being childless.
Discussion
These results, of growing relevance given the increasing availability of new technologies in pregnancy care, can inform communication strategies that facilitate coupless' decision making.
Conclusion
This study provides insights into factors that might complicate prenatal testing decision making.
Keywords: Decisional Conflict, Genetic Testing, Prenatal Testing, Decision‐making
Introduction
With the rapid development of prenatal tests for genetic conditions,1, 2 expectant parents are increasingly facing decisions as to whether or not to undergo testing to determine whether their unborn children have anomalies linked with specific health conditions. And as the numbers of Western women becoming pregnant later in life increase,3, 4 so do their risks of carrying a foetus with Down syndrome5 and other chromosomal abnormalities.6 Diagnostic tests (e.g., amniocentesis) can detect foetal chromosomal anomalies but are associated with risk miscarriage risks (e.g., approximately 1 in 300 to 500 for amniocentesis).7 Given these risks, individuals deciding whether to undergo prenatal diagnostic testing are likely to experience decisional conflict.8 This state of psychological distress is central to the decision‐making theory,9 which posits that decisions are difficult to make if they are time constrained and involve options that are risky, irreversible, and emotionally laden. Uncertainty and subsequent distress‐associated prenatal diagnostic testing could be minimized by health professionals if concerns are identified and addressed through patient education and counselling. Yet, to date, theory and research on predictors of decisional conflict over prenatal diagnostic testing are lacking. Previous studies on decisional conflict in the context of prenatal testing have shown that informed decision making is associated with lower decisional conflict,10, 11 and that decision aids (i.e., educational materials) and genetic counselling can reduce feelings of conflict related to making these choices.12, 13 Yet to our knowledge, no studies have examined which values, beliefs, social influences, personal experiences, or condition characteristics predict decisional conflict over prenatal diagnostic testing. This study was conducted to address this research gap.
Psychosocial and clinical predictors of decisional conflict with prenatal diagnostic testing
Drawing on theories of health decision making and behaviour and guided by a review of empirical evidence, we developed a model integrating psychosocial and clinical factors likely to influence decisional conflict with prenatal diagnostic testing experienced by would‐be parents, irrespective of their age. This proposed model (Fig. 1) integrates factors identified by utility theories,14, 15 Common‐Sense Model of Health Behavior,16 Protection Motivation Theory14 and Theory of Planned Behavior17 as motivating health‐related behaviours. We developed a new model because none of these existing models account for decisional conflict; instead, they explain intentions and behaviour. Integrated models can advance theory and research by combining factors found to be potent predictors and mechanisms within related behavioural domains to enhance explanatory power in predicting behaviour in a new domain.18, 19 We integrated those factors that are likely to influence prenatal testing decision making; to date, their contributions to decisional conflict have not yet been empirically evaluated. These factors include perceived benefits from normal results (Utility Theories14, 15, 20), test response efficacy (Protection Motivation Theory14, 21, 22), condition coherence (Common‐Sense Model of Health Behavior16, 23), child‐related worry (Common‐Sense Model of Health Behavior7, 16, 24, 25, 26), and motivation to comply with doctors' perceived preferences (Theory of Planned Behavior6, 27). In addition, factors suggested by empirical research but which have not been clearly integrated into theory include perceived disagreement with other parent's preference,28 parity,20, 29, 30 and the characteristics of the condition tested for.29, 31, 32, 33, 34
Figure 1.
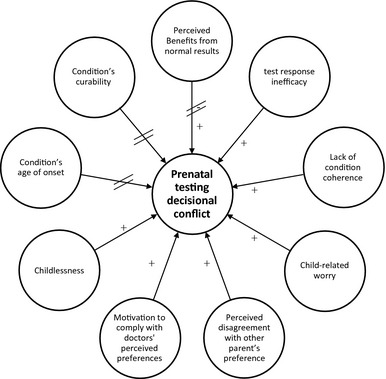
Proposed integrated model of psychosocial factors influencing prenatal diagnostic testing decisional conflict. Lines with crossbars indicate relationships not supported by the study findings.
Prospective parents engaged in deliberative decision making will consider the pros and cons of receiving test results.15 The benefits from normal test results include reassurance about the health of the foetus.20 Not perceiving benefits from normal test results should promote decisional conflict.
Test response efficacy, defined as perceptions of the test's ability to accurately detect abnormalities, can also influence decisions to undergo testing.21, 22 If couples believe prenatal diagnostic testing can provide reliable information, then they are likely to value the test and feel more confident about their decision.16 Lower test response efficacy, however, should lead to greater decisional conflict.
In reaching a decision about prenatal diagnostic testing, couples typically attempt to gain a better understanding of the genetic condition (e.g., possible phenotypes, clinical outcomes etc.) to form a coherent account of the condition. This condition coherence (i.e., the extent to which a health threat ‘makes sense’) is a key determinant of protective behaviours such as genetic screening.23 When presented with a health threat, individuals combine several cognitive attributes (e.g., the cause of the threat, potential treatment) to make sense of the threat and to create their own ‘condition coherence’, or understanding of the condition. As such, individuals who appreciate the genetic aetiology of the condition should feel relatively confident that genetic diagnostic testing is the appropriate tool to detect that condition, whereas those with low condition coherence should experience greater decisional conflict.
Child‐related worry has also been shown to predict prenatal testing intentions.7, 24, 25 Theory and research demonstrate that worry significantly influences health decisions and does so independently of risk‐related cognitions.26 Decisional conflict should be greater for prospective parents worried about the health of the foetus and thus likely to deliberate the pros and cons of testing.
Interpersonal dynamics within the wider social environment in which prenatal testing decisions take place can also influence the decision‐making process.17, 35 Motivations to comply with the perceived preferences of others can critically influence decisional conflict, particularly given the extent to which being accountable to others and maintaining harmonious relationships with significant others are important determinants of satisfactory reproductive decision making.36 Perceiving differences of opinion from that of the other parent may complicate prenatal testing decision making and arouse decisional conflict.28 Therefore, we expect that perceived disagreement will lead to greater decisional conflict.
Health professionals are also influential in prenatal diagnostic testing decision making.27 Perceptions of their views on prenatal testing can contribute to decisional conflict. They are ethically bound to operate in a non‐directive manner, but the simple fact that they offer diagnostic testing to prospective parents can be viewed as a sign of endorsement.37 Couples who feel compelled to comply with their doctors' perceived preferences may feel less certain that their decision reflects their personal preferences. Hence, greater decisional conflict may arise for couples high in motivation to comply with doctors' perceived preferences.
Evidence indicates that uncertainty surrounding reproductive decisions is also likely to be influenced by first‐hand experiences with pregnancies,20, 38, 39 and the age of onset and curability of the condition.29 Previous experience with pregnancies can provide experiential knowledge of the issues associated with prenatal testing.30, 37 Compared with parents, childless couples are less likely to have already faced issues about prenatal testing and could therefore experience greater decisional conflict. With regard to the role of conditions’ age of onset, Canadian and American surveys showed that genetic testing is more acceptable when used to diagnose early‐onset illnesses than adult‐onset diseases.31, 32 Consequently, decisional conflict should be greater when the conditions tested for are late‐onset. Finally, the severity of a condition can also influence decisions around prenatal testing.29 One study revealed that participants were more accepting of reproductive technologies when used to test for conditions perceived to reduce lifespan and quality of life.33 Managing certain diseases (e.g. sickle cell disease, an incurable birth onset condition) may require lifelong medical care and surveillance, as well as reliance on experts and specialized medical centres. The clinical severity of the condition, as well as the psychological and economic impacts of the disease, is likely to trigger internal ethical debates that call upon personal judgements about the quality of life the individual would have if born with the condition.40, 41 Such considerations, weighed against the benefits of being born at all, would need time to be carefully deliberated. However, decisions about prenatal testing are time pressured as they need to be made within a given time frame to allow termination of pregnancy if the parents decided to do so. According to decision‐making theory,9 decisions made under time pressure lead to decisional conflict. Hence, parents caught between their parental duties and the perceived quality of life of their future children are likely to experience great uncertainty about prenatal testing. Decisional conflict should be greater for curable conditions, than for non‐curable, fatal conditions.
Objective and hypotheses
We conducted an online study to test the proposed model of psychosocial (perceived benefits from normal results; test response efficacy; condition coherence; child‐related worry; perceived disagreement with other parent's preference; motivation to comply with doctors' perceived preferences) and clinical characteristics (condition's age of onset and curability) promoting decisions conflict about prenatal diagnostic testing. Aware that presenting information about foetal abnormalities and birth defects could trigger stress amongst expectant parents, we recruited adults from the general population to respond to hypothetical scenarios describing prenatal diagnostic testing for genetic conditions. We predicted that lower perceived benefits from normal results, lower test response efficacy, lower condition coherence, greater child‐related worry, perceived disagreement with the other parent, and greater motivation to comply with doctors' perceived preferences would independently predict greater decisional conflict. Furthermore, we hypothesized that being childless and considering testing for a curable or an adult‐onset condition would each predict greater decisional conflict.
Method
Recruitment and participants
The university's ethics committee approved this study. Participants were recruited through announcements to community and web‐based organizations throughout New Zealand. Eligibility criteria included fluency in English, age of 18 years or over, and current involvement in a romantic relationship. Altogether, 345 women and 112 men (M age = 32.68 years; SD = 8.61 years) completed the study. Approximately two‐thirds (n = 291) had one child or more. The majority either had no religious affiliation (Agnostic: n = 22; Atheist: n = 33; no religion: n = 176) or affiliated with Christianity (n = 188). Participants who self‐identified with more than one ethnicity were categorized using a standard procedure for prioritizing ethnicity.42 Most participants were New Zealand European (n = 328), other European (n = 54), or Māori (n = 25); 10.4% identified with other ethnicities.
Design and procedures
The study utilized a 2 (Onset: Birth vs. Adulthood) × 2(Curability: No Cure vs. Available Cure) between‐subjects design. After entering the study website and providing consent, participants were randomly assigned to read one of four hypothetical scenarios, which varied only in terms of the age of onset (‘symptoms appear slowly between the ages of 30 and 50 years’ vs ‘symptoms are present from birth’) and curability of the condition (‘no cure’ vs ‘a cure’) (Appendix A). The adulthood ages of onset reflected those of existing diseases, such as early‐onset Alzheimer's disease.43, 44, 45 Participants then completed measures of decisional conflict, perceived benefits from receiving normal results, test response efficacy, condition coherence, child‐related worry, perceived disagreement with other parent's preference motivation to comply with doctors' perceived preferences, and demographic information (including parenthood status). The questionnaire ended with debriefing information about prenatal testing and sources to consult for those wanting more information about genetic conditions.
Measures
Unless otherwise stated, all items were rated from −3 (strongly disagree) to +3 (strongly agree). Items were averaged to generate scores after reverse‐scoring negatively worded items (rev).
Decisional conflict
We adapted the most conceptually relevant subscale of the Decisional Conflict scale,46 that is, the decisional uncertainty subscale. The six items, scored from 0 (strongly disagree) to 6 (strongly agree), were as follows: ‘The decision to undergo or not prenatal testing would be hard for me to make’; ‘I feel I know the risks and benefits of the procedure involved (rev)’; ‘I am unsure about what I would do in this situation’; ‘I would need more advice and information about my options before making a decision about prenatal testing’; ‘It is clear to me what choice would be the best for me (rev)’; and ‘It would be hard to decide which are the most important to me: the risks or the benefits associated with the test’. Internal consistency was high; Cronbach's α = 0.86.
Perceived benefits from receiving normal results
Four items was used to assess anticipated benefits from receiving normal test results: ‘Knowing that my unborn child did not have the genetic mutation… 1)… would help me feel less anxious about the pregnancy, 2)… would make me feel reassured about the health of my unborn child, 3)… would increase my confidence regarding the progress/outcomes of the pregnancy’, and 4) ‘The test results would resolve the uncertainty about whether or not my unborn child has this condition’; α = 0.85.
Test response efficacy
A three‐item measure was used to assess beliefs that prenatal genetic testing can reliably detect foetal genetic abnormalities: ‘Undergoing this prenatal test would clearly indicate the presence of this condition’; ‘I do not feel confident that this prenatal test would give accurate information about whether or not my unborn child would have the condition (rev)’; and ‘This prenatal test could indicate whether or not something is wrong’; α = 0.71.
Condition coherence
An adapted subscale from the Illness Perceptions Questionnaire‐Revised47 included the items: ‘The symptoms of this condition are puzzling to me (rev)’; ‘I have a clear picture/understanding of this condition’; ‘This condition is a mystery to me (rev)’; ‘I don't fully understand this condition (rev)’; and ‘This condition makes sense to me’; α = 0.89.
Child‐related worry
The child‐related worry measure48 included three items: ‘If I/we were expecting a child, I would worry about it being affected with this genetic condition’; ‘I am concerned my child may be born with this genetic condition’; and ‘The thought of giving birth to a child with his genetic condition bothers me’; α = 0.87.
Perceived disagreement
Anticipated differences of opinions from partners were assessed using scores of testing interest and normative beliefs–partner. Testing interest (i.e., interest in undergoing prenatal testing) was assessed by a six‐item measure: ‘Undergoing this prenatal test would be too distressing for me (rev)’; ‘For me, even a slight increase in the chance of miscarriage would be unacceptable (rev)’; ‘This prenatal test would expose my unborn child to unnecessary risk (rev)’; ‘Prenatal testing would be of no benefit to myself or my family (rev)’; ‘It would be important to get the test’; and ‘I would request the test’; α = 0.91. Normative beliefs–partner was assessed with ‘How much would your partner/spouse want you to undergo prenatal testing?’ (0: not at all; 6: very much). First, the z scores for both scales were computed. Testing interest z scores ranged from −1.88 to 1.99, and normative beliefs – partner z scores ranged from −1.47 to 1.26. Next, a constant of 2 was added to both z scores so that all values would be positive. Z scores now ranged from 0.12 to 3.99 for testing interest and from 0.53 to 3.26 for normative beliefs – partner. Scores were plotted (Fig. 2), and participants were categorized into four groups based on where their scores fell within these four quadrants; a method frequently used by health researchers.49, 50, 51 The first quadrant ‘self against/other for testing’ comprised participants who were against prenatal testing but who perceived their partner/spouse would be in favour (n = 100, 21.9%). The second quadrant ‘perceived agreement for testing’ comprised 185 individuals (40.5%). The third quadrant ‘perceived agreement against testing’ included 151 individuals (33%). The final quadrant ‘self for/other against testing’ included participants who were in favour of prenatal testing but who perceived their partner/spouse would be against it (n = 21, 4.6%).
Figure 2.
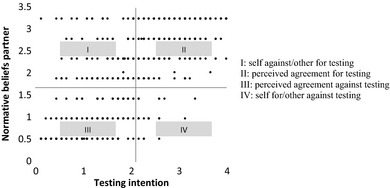
Own testing interest versus partners'/spouses' perceived preferences.
Motivation to comply with doctors' perceived preferences
Willingness to follow doctors' perceived preferences for prenatal testing was measured with the item: ‘I would undergo prenatal testing if it was important to my doctor/obstetrician/midwife’.
Demographic information
Participants reported their gender, age, relationship status, number of children, religious affiliation, and ethnicity.
Analytical strategy
Data were analysed using SPSS v20. Differences in socio‐demographic characteristics between the four Onset and Curability conditions and the four Differences of Opinions groups were assessed with Pearson χ2 (for categorical variables) and anova (for continuous variables). Correlation coefficients were computed to assess the bivariate relationships between decisional conflict and the other variables. The main and interaction effects of curability, age of onset, and number of children (none vs. at least one) on decisional conflict were tested using regression analyses. A set of three dummy variables were created to compare the four Differences of Opinion groups, with each dummy variable comparing ‘the self against/other for testing’ group with one of the other three groups. The relationships between decisional conflict and its hypothesized predictors were tested through regression analyses. Significance level was set at P < 0.05.
Results
Demographic differences for the four condition groups and the four differences of opinions groups
The four Onset and Curability conditions were equivalent in terms of gender, age, number of children, religious affiliation, and ethnicity (all P's > 0.05). Similarly, the four Differences of Opinions groups did not differ in terms of assignment to Onset conditions, assignment to Curability conditions, gender, age, number of children, religious affiliation, and ethnicity (all P's > 0.05).
Descriptive statistics and correlational relationships for decisional conflict and the demographic, clinical, and psychosocial variables
Overall, participants reported moderate decisional conflict and varied in their interest in testing (Table 1). They slightly agreed that receiving normal test results was beneficial and moderately agreed that prenatal testing could reliably detect genetic conditions. Participants varied in condition coherence. They generally had a slight tendency to worry about the health of the unborn child and would be somewhat motivated to undergo prenatal testing if this was important to their doctors.
Table 1.
Range of mean scores, means (M), standard deviations (SD), and correlation coefficients between decisional conflict and the study variables
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Range of mean scores | 0 to 6 | – | – | – | −3 to +3 | −3 to +3 | −3 to +3 | −3 to +3 | −3 to +3 | −3 to +3 |
| M | 2.77 | – | – | – | 0.78 | 1.40 | 0.33 | 0.06 | 0.63 | −0.08 |
| SD | 1.50 | – | – | – | 0.88 | 1.20 | 0.95 | 1.38 | 2.00 | 1.55 |
| 1. Decisional conflict | – | |||||||||
| 2. Curability (not fatal vs. fatal) | −0.02 | – | ||||||||
| 3. Onset age (birth vs. adult) | −0.07 | −0.04 | – | |||||||
| 4. Number of children (0 vs. 1+) | −0.17** | −0.02 | −0.01 | – | ||||||
| 5. Benefits from normal results | 0.17** | 0.08 | −0.02 | 0.03 | – | |||||
| 6. Test response efficacy | −0.12* | 0.01 | 0.04 | 0.05 | 0.39** | – | ||||
| 7. Condition coherence | −0.22** | 0.04 | 0.09* | 0.03 | 0.12* | 0.27** | – | |||
| 8. Child‐related worry | 0.24** | 0.12* | −0.07 | −0.01 | 0.41** | 0.13** | −0.07 | – | ||
| 9. Motivation to comply–doctors | 0.29** | 0.00 | 0.03 | −0.12* | 0.39** | 0.22** | 0.01 | 0.31** | – | |
| 10. Testing interest | 0.10* | 0.01 | −0.06 | −0.09* | 0.48** | 0.28** | 0.12* | 0.47** | 0.49** | – |
**P < 0.01 level; *P < 0.05 level.
Decisional conflict did not correlate with curability or onset. As predicted, decisional conflict significantly correlated with all of the psychosocial variables. Greater decisional conflict was associated with perceiving normal results as beneficial, doubting the test's reliability, lacking a coherent understanding of the condition, worrying about the health of the unborn child, being motivated to comply with doctors' perceived preference regarding testing, being interested in undergoing prenatal testing, and being childless (Table 1). In addition, decisional conflict did not correlate with most demographic variables, including gender.
Psychosocial and clinical variables as independent predictors of decisional conflict
Regression analyses were conducted to test the main and interaction effects of curability (fatal vs. not fatal) and age of onset (early vs. late) on decisional conflict. Contrary to predictions, curability and age of onset did not influence decisional conflict (P's > 0.05). Hence, they were excluded from the final analyses.
Contrary to hypotheses, greater (not lower) perceived benefits from negative results predicted greater decisional conflict (Table 2). As hypothesized, lower test response efficacy, lower condition coherence, greater child‐related worry, perceived differences in opinions between parents, greater motivation to comply with doctors' perceived preferences, and being childless independently predicted greater decisional conflict.
Table 2.
Regression analyses on decisional conflict in prenatal testing
| B | SE B | β | R 2 | F | |
|---|---|---|---|---|---|
| Self against, other for vs. perceived agreement for | −0.48 | 0.12 | −0.24*** | 0.25 | 16.24*** |
| Motivation to comply with doctors' perceived preference | 0.17 | 0.04 | 0.22*** | ||
| Self against, other for vs. perceived agreement against | −0.37 | 0.13 | −0.18** | ||
| Self against, other for vs. self for, other against | 0.55 | 0.22 | 0.18** | ||
| Test response efficacy | −0.21 | 0.06 | −0.17*** | ||
| Child‐related worry | 0.18 | 0.05 | 0.17*** | ||
| Number of children (0 vs. 1+) | −0.38 | 0.13 | −0.12** | ||
| Condition coherence | −0.21 | 0.07 | −0.13** | ||
| Benefits from normal results | 0.18 | 0.09 | 0.11* |
***P < 0.001; **P < 0.01; *P < 0.05.
We conducted exploratory analyses (at P < 0.01) to test whether Differences of Opinions interacted with the study variables in predicting decisional conflict, No clear patterns of trends emerged, suggesting that the observed patterns of relationships of other factors with decisional conflict are unaffected by perceived differences of opinions in the couple.
Finally, analyses conducted on the parents yielded the same patterns of associations between the predictor variables and decisional conflict.
Discussion and conclusions
For couples, having to choose between accepting and declining prenatal diagnostic testing is likely to trigger decisional conflict because each option is emotionally charged and involves weighing risks against benefits.52 Health professionals can best help prospective parents make this decision if they understand what factors may create decisional conflict. Given the paucity of theory and research on predictors of decisional conflict in the context of prenatal testing, we proposed and tested a model of psychosocial and clinical factors contributing to prenatal diagnostic testing decisional conflict (Fig. 1). The findings largely supported this model.
Motivation to comply with doctors' perceived preferences and perceived disagreement with partner emerged as the strongest predictors of decisional conflict. Participants who were motivated to follow doctors' preferences and/or who perceived disagreement with their partner/spouse were the most conflicted about testing. These findings extend previous evidence on the roles of social influences in reproductive choices53 by demonstrating the strong links of these two social influence factors on decisional conflict. During pregnancy, women tend to seek different providers for different types of support.54, 55 For informational support (e.g., provision of facts), expectant parents usually consult medical professionals.55 Our findings suggest that motivations to comply with perceived recommendations of health professionals are associated with greater uncertainty about prenatal testing. It is also possible that the causal relationship is reversed, and that greater uncertainty about prenatal testing enhances motivations to follow perceived health professionals' preferences. Either way, our findings suggest that parents who express preferences to act in accordance with medical experts may feel particularly confused by the complex issues surrounding prenatal testing and may benefit from genetic counselling. This is of noteworthy importance as non‐directiveness is central to genetic counselling, which means that professionals cannot deliberately withhold information or influence patients' decisions.56, 57, 58
For emotional support (e.g., empathetic listening and reassurance), expectant parents typically turn to their partners59 with the expectations that, together, they will deliberate the issues at stake, reach an agreement, and provide each other with reassurance over their choice.20 Family planning decisions reflect people's values as independent individuals, but also as united couples.60 The need for partnership between men and women in these moments has been widely recognized.61, 62, 63 These findings provide evidence that perceived incongruence of opinions can aggravate decisional conflict. This perceived lack of unity may complicate the deliberative process, potentially inducing emotional distress and leading them to hesitate about prenatal testing. From a counselling perspective, probes into the individual preferences of both prospective parents could reveal discrepancies of opinions that can be targeted and potentially resolved through guided discussions.
These findings on the roles of expectations of partners and providers in generating decisional conflict are particularly important in the light of sociological and anthropological research suggesting that social expectations about prenatal testing are growing stronger. With the wide availability of prenatal testing in Western countries, it is likely to be perceived as self‐evident.64, 65 Increasingly, individuals are likely to feel social pressures to be a ‘good parent’ and to not only take the test in the ‘best interests’ of the unborn child,66 but also to accept that child unconditionally, except in the case of a severe disability resulting in a life of suffering where termination would arguably be less morally objectionable.67 For some parents, however, the concepts of ‘best interests’ and ‘unconditional acceptance’ may not always be compatible. On the one hand, these parents may view prenatal testing as being in the interest of the child and therefore lean towards taking the test. Simultaneously, they may hesitate to take the test by fear of receiving abnormal results, a situation that would make them consider termination because of their doubts towards their own abilities to raise a child special needs.25, 68 These individuals, caught in a perceived ‘reproductive accountability’,70 may fear being misunderstood by significant others, such as the other parent of their unborn child or their health provider. Hence, parents‐to‐be could feel torn between their desire to fulfil their parental duties and the need to resolve the moral and psychological implications related to abnormal test results; these anxieties are likely to be greater for first‐time parents who lack experiential knowledge.70
Other factors independently predicting decisional conflict included response efficacy and condition coherence. Response efficacy has been demonstrated to influence decisions about protective behaviours, and these findings show that its role extends to the domains of decisional conflict and prenatal testing. If prenatal tests are perceived to be unreliable, then people are likely to experience doubts that exacerbate decisional conflict. Related to response efficacy is condition coherence (i.e. or understanding the link between the genetic origin of a health threat and the genetic mutation being tested for). Condition coherence can increase motivations to obtain the genetic test.71, 72 Our findings suggest the importance of educating expectant couples about the genetic conditions tested for and the tests' detection rates, so as to increase informed decisions and decrease uncertainty.
Perceiving normal results as beneficial and worrying about the health of the foetus predicted higher decisional conflict. Feelings of worry are often key motivators of screening and protection behaviours.48, 73 This relationship was reflected in our study by the positive correlations linking worry about the health of the unborn child, interest in undergoing prenatal testing, and perceived benefits from receiving normal results. Parents‐to‐be may be divided between their desires to be provided with (anxiety‐reducing) normal results and their fears to receive (anxiety‐provoking) abnormal test results. It may be that understanding the value of normal results and therefore perceiving the possible presence of foetal abnormalities enhances the salience of risks about these abnormalities which, in turn, increases decisional conflict. Hence, parents concerned about the health of the foetus may report interest but also hesitation in prenatal testing if they fear that test result could bring about an undesired outcome. Further studies could assess the respective roles of perceived benefits from normal results and anticipated harms from abnormal results in decisional conflict.
Decisional conflict did not vary by the age of onset or curability of the genetic condition, suggesting that prenatal testing decisions may be equally difficult regardless of these condition characteristics. Decisional conflict did not vary by the age of onset or curability of the genetic condition, suggesting that decisions about prenatal testing for this type of neurological condition may be equally difficult regardless of these condition characteristics. The neurological condition described could be sufficiently threatening even when its onset is in adulthood and its potential severity is low (i.e., it is described as curable) that the range in perceived severity of the four conditions was too minimal to lead to differences in the extent to which it influences decisional conflict. Nevertheless, age of onset and curability could influence decisional conflict for tests of other conditions, particularly those for which potential parents have considerable familiarity and which involve familiar treatments (e.g., blindness, hereditary cancer, or cystic fibrosis).
Whilst decisional conflict did not vary by gender, it did so as a function of parity: Childless participants reported greater hesitation relative to participants with children. Differences in experiential knowledge (i.e. everyday experiences) may account for these findings. Abel and Browner74 differentiated empathic knowledge (acquired through interactions with others) from embodied knowledge (derived from personal physical experience as such pregnancy). Both types of subjective knowledge can shape health behaviours. Indeed, individuals can draw from these past experiences and make relatively informed health‐related decisions in the future.75, 76 Contrary to childless participants, parents can draw from both types of experiential knowledge: women from having ‘embodied’ pregnancy, and partners from having been closely associated to it. Such familiarity with pregnancy and related issues would facilitate future reproductive choices. It might also reduce the ‘burden of anticipation’ (Wexler, 1979, cited in Ref. 77, p. 63) experienced by individuals facing a new situation, such as childless individuals facing decisions about prenatal testing. In the study, although some childless couples may have experienced non‐viable pregnancies, the majority are unlikely to have had first‐hand experience with prenatal care or to have previously engaged in debates about reproductive issues, and they may be more conflicted about making a decision about prenatal testing as a consequence.60
Several study limitations warrant comment. First, we used hypothetical scenarios to elicit views on prenatal testing in the general population, for conditions described but not named; an approach that may be criticized for potentially producing findings not easily generalizable to ‘real life’ situations. However, this well‐accepted means of investigation23, 77, 78, 79, 80 allowed us to systematically manipulate information hypothesized to influence decision conflict (i.e., age of onset or curability) and to test our model with a sample who is likely to face these types of prenatal testing decisions in the future under conditions safe from creating significant distress. Nonetheless, caution is warranted when extending the present findings to actual would‐be parents and a critical next step in this research area is to conduct a study testing the model with expecting parents who are facing prenatal testing decisions. Second, our choice of hypothetical scenarios prevented us from conducting follow‐up analyses on decisional satisfaction and regret. A critical step for future research would be to assess decisional satisfaction and regret in individuals experiencing decisional conflict during pregnancy. These studies would yield valuable information on how to maximize decision satisfaction in individuals initially unsure about their choice. Finally, the correlational nature of the findings precludes inferences of causality. The integrative model can be used to guide experimental studies in which these factors are manipulated (e.g., through health communications) to determine their influence on decisional conflict.
The present findings have potential clinical and educational implications. For some individuals, choosing between accepting and declining prenatal testing could create conflict. Health professionals providing pregnancy care can facilitate decision making and reduce distress by addressing the key psychosocial aspects of prenatal testing identified by the integrated model. Clinicians may be able to reassure hesitant parents‐to‐be by ‘normalizing’ their state of anxiety as feeling conflicted about prenatal testing is common amongst expectant parents, especially amongst first‐time parents. In keeping with the patient‐centred approach, professionals could reassure their patients and emphasize that many other couples in a similar situation also feel anxious. This emphasis might help patients fell less isolated and more inclined to discuss concerns they might have otherwise kept to themselves by fear of being judged for having unique unreasonable fears.81, 82 Anxious patients might also benefit from tasks aimed at reducing uncertainty, such as values clarification exercises present in some decision aids.83, 84 Health professionals may be able to minimize couples' conflict by clarifying issues important to them, such as the prospects of receiving test results, the reliability of the test, and worries about the health of the unborn child. More time may be needed to explore these issues with new parents than with couples who already have children. The deliberative process about whether or not to take the test is likely to continue at home,20 especially if future parents disagree. These conflicting couples might find decision aids (i.e. informational resources, supposed to be an adjunct to counselling) particularly helpful in reaching a common decision.13, 85, 86
Conclusion
By drawing on several decision‐making theories to identify factors with established associations with health behaviour decisions,16, 17, 21, 22, 46, 80, 87 we developed an integrated model delineating their independent roles in exacerbating or minimizing conflict over behavioural decisions in the context of prenatal genetic testing. This multitheory approach18 provided a rich understanding of the issues at stake in prenatal testing decision making. Our findings suggest that couples faced with prenatal testing may experience decisional conflict, but that psychological distress could be reduced if the key psychosocial concerns are addressed.
Sources of funding
None received.
Conflict of interest
None declared.
Appendix A. Hypothetical scenarios read by participants
The manipulated information is italicised below for clarity purposes, but not in the texts read by participants.
Vividly imagine you are expecting a baby and are at the beginning (under 12 weeks) of the pregnancy. You hear about a pre‐birth test that can detect with more accuracy than an ultrasound can whether the fetus is affected with a condition.
- Now imagine that this condition is a disease caused by a genetic mutation. The condition involves a progressive deterioration in:
- Knowledge and understanding (cognitive deterioration)
- Movements, with occurrence of involuntary movements (neurological deterioration), and
- Personality (deterioration of emotional systems)
The condition is a progressive ‘adult‐onset’ condition: symptoms appear slowly between the ages of 30 and 50 years. [‘birth‐onset’ condition: symptoms are present from birth].On average, in the entire population, parents have 1 chance in 200 (0.5%) of having a child with this condition. No cure is currently available. Individuals with this condition will live approximately 10 to 15 years after the onset of illness [A cure is currently available. Individuals with this condition who receive the treatment will live after the onset of illness and with minimal effects of the condition.]
The test consists of obtaining a small sample of placenta or amniotic fluid (these surround the fetus). Sometimes under local anaesthetic and with ultrasound guidance, a syringe is used to collect small samples of the required tissues.
This procedure may be mildly uncomfortable for the mother, as some angling to get good views of the baby may be required. There may also be discomfort due to bruising and some cramps may be experienced. However, these usually resolve within 24 h.
The risk of procedure‐related miscarriage is between 0.5 and 1%. This means that between 1 in 200 and 2 in 200 women will miscarry following the procedure. There is also a risk of natural miscarriage of 2% that is present in all pregnancies at 10 weeks gestation, whether or not the test is performed.
This procedure is done as an outpatient procedure and partners (or a support person/Whānau/family support) can attend. The test is performed between 10 and 15 weeks of pregnancy (depending of the sample required). Results are available 10–14 days following the procedure. In New Zealand and Australia, the test is usually free of charge.
References
- 1. Amor DJ. Future of whole genome sequencing. Journal of Paediatrics and Child Health, 2014; 51: 251–254. [DOI] [PubMed] [Google Scholar]
- 2. Chiu RWK. Noninvasive prenatal testing by maternal plasma DNA analysis: current practice and future applications. Scandinavian Journal of Clinical & Laboratory Investigation, 2014; 74: 48–53. [DOI] [PubMed] [Google Scholar]
- 3. Statistics New Zealand . Births and deaths, 2010. Available at: http://www.stats.govt.nz/browse_for_stats/population/births/BirthsAndDeaths_HOTPYeDec10.aspx. Accessed 10.02.2012.
- 4. Martin JA, Hamilton BE, Ventura SJ, Osterman MJK, Wilson EC, Mathews TJ. Births: Final Data for 2010. Centre for Disease Control and Prevention, 2012. Document available at: http://www.cdc.gov/nchs/data/nvsr/nvsr61/nvsr61_01.pdf. Accessed 10.02.2012. [PubMed] [Google Scholar]
- 5. Morris JK, Wald NJ, Mutton DE, Alberman E. Comparison of models of maternal age‐specific risk for Down syndrome live births. Prenatal Diagnosis, 2003; 23: 252–258. [DOI] [PubMed] [Google Scholar]
- 6. Hook EB. Rates of chromosome abnormalities at different maternal ages. Obstetrics & Gynecology, 1981; 58: 282–285. [PubMed] [Google Scholar]
- 7. The American College of Obstetricians and Gynecologists . ACOG Practice Bulletin No. 88: invasive prenatal testing for aneuploidy. Obstetrics & Gynecology, 2007; 110: 1459–1467. [DOI] [PubMed] [Google Scholar]
- 8. Bell M, Stoneman Z. Reactions to prenatal testing: reflection of religiosity and attitudes toward abortion and people with disabilities. American Journal of Mental Retardation, 2000; 105: 1–13. [DOI] [PubMed] [Google Scholar]
- 9. Janis IL, Mann L. Decision Making. New York: The Free Press, 1977. [Google Scholar]
- 10. van den Berg M, Timmermans DRM, Kate LP, van Vugt JMG, van der Wal G. Are pregnant women making informed choices about prenatal screening? Genetics in Medicine, 2005; 7: 332–338. [DOI] [PubMed] [Google Scholar]
- 11. Kaiser AS, Ferris LE, Pastuszakm AL et al The effects of prenatal group genetic counselling on knowledge, anxiety and decisional conflict: issues for nuchal translucency screening. Journal of Obstetrics & Gynaecology, 2002; 22: 246–255. [DOI] [PubMed] [Google Scholar]
- 12. Bekker HL, Hewison J, Thornton JG. Applying decision analysis to facilitate informed decision making about prenatal diagnosis for Down syndrome: a randomised controlled trial. Prenatal Diagnosis, 2004; 24: 265–275. [DOI] [PubMed] [Google Scholar]
- 13. Nagle C, Gunn J, Bell R et al Use of a decision aid for prenatal testing of fetal abnormalities to improve women's informed decision making: a cluster randomised controlled trial. British Journal of Obstetrics and Gynaecology, 2008; 115: 339–347. [DOI] [PubMed] [Google Scholar]
- 14. Boer R, Seydel ER. Protection motivation theory In: Conner M, Norman P. (eds) Predicting Health Behaviour: Research and Practice With Social Cognition Models. Buckingham: Open University Press, 1995: 95–120. [Google Scholar]
- 15. vonNeumann J , Morgenstern O. Theory of Games and Economic Behavior (2d rev. ed.), xviii. Princeton, NJ: Princeton University Press, 1947: 641 p. [Google Scholar]
- 16. Leventhal H, Brissette I, Leventhal EA. The common‐sense model of self‐regulation of health and illness In: Cameron LD, Leventhal H. (eds) The Self‐Regulation of Health and Illness Behaviour. London: Routledge, 2003: 42–65. [Google Scholar]
- 17. Ajzen I. The Theory of Planned Behavior. Organizational Behavior and Human Decision Processes, 1991; 50: 179–211. [Google Scholar]
- 18. Broadstock M, Michie S. Processes of patient decision making: theoretical and methodological issues. Psychology and Health, 2000; 15: 191–204. [Google Scholar]
- 19. Michie S, Webb TL, Sniehotta FF. The importance of making explicit links between theoretical constructs and behaviour change techniques. Addiction,2010; 105: 1897–1898. [DOI] [PubMed] [Google Scholar]
- 20. Humphreys L, Cappelli M, Hunter A, Allanson J, Zimak A. What is the significance of attendance by the partner at genetic counselling for advanced maternal age? Psychology, Health & Medicine, 2003; 8: 266–278. [Google Scholar]
- 21. Rogers RW. A protection motivation theory of fear appeals and attitude change. The Journal of Psychology, 1975; 91: 93–114. [DOI] [PubMed] [Google Scholar]
- 22. Rogers RW. Cognitive and physiological processes in fear appeals and attitude change: a revised theory of protection motivation In: Cacioppo JT, Petty RE. (eds) Social Psychophysiology: A Sourcebook. New York: The Guildford Press, 1983: 153–176. [Google Scholar]
- 23. Cameron LD, Marteau TM, Brown PM, Klein W, Sherman K. Communication strategies for enhancing understanding of the behavioral implications of genetic and biomarker tests for disease risk: the role of coherence. Journal of Behavioral Medicine, 2012; 5: 286–298. [DOI] [PubMed] [Google Scholar]
- 24. van den Berg M, Timmermans DRM, Knol DL et al Understanding pregnant women's decision making concerning prenatal screening. Health Psychology, 2008; 27: 430–437. [DOI] [PubMed] [Google Scholar]
- 25. Muller C, Cameron LD. Trait anxiety, information modality, and responses to communications about prenatal genetic testing. Journal of Behavioral Medicine, 2014; 37: 988–999. [DOI] [PubMed] [Google Scholar]
- 26. Cameron LD, Chan CKY. Designing health communications: harnessing the power of affect, imagery, and self‐regulation. Social and Personality Psychology Compass, 2008; 2: 262–282. [Google Scholar]
- 27. Ahmed S, Bryant LD, Tizro Z, Shickle D. Is advice incompatible with autonomous informed choice? Women's perceptions of advice in the context of antenatal screening: a qualitative study. Health Expectations, 2014; 17: 555–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wätterbjörk I, Blomberg K, Nilsson K, Sahlberg‐Blom E. Decision‐making process of prenatal screening described by pregnant women and their partners. Health Expectations, 2013. DOI: 10.1111/hex.12147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Asplin N, Wessel H, Marions L, Öhman SG. Pregnant women's perspectives on decision‐making when a fetal malformation is detected by ultrasound examination. Sexual & Reproductive Healthcare, 2013; 4: 79–84. [DOI] [PubMed] [Google Scholar]
- 30. Anderson G. Patient decision‐making for clinical genetics. Nursing Inquiry, 2007; 14: 13–22. [DOI] [PubMed] [Google Scholar]
- 31. Genetics and Public Policy Center . Reproductive genetic testing: What America thinks, 2004. Available at: http://www.dnapolicy.org/images/reportpdfs/ReproGenTestAmericaThinks.pdf. Accessed 10.02.2012.
- 32. Martin S. Most Canadians welcome genetic testing. Canadian Medical Association Journal, 2000; 163: 200. [PMC free article] [PubMed] [Google Scholar]
- 33. Muller C, Shepherd D. Attitudes towards reproductive technologies for humans. New Zealand Journal of Social Sciences Online, 2009; 4: 225–238. [Google Scholar]
- 34. Boardman FK. The expressivist objection to prenatal testing: the experiences of families living with genetic disease. Social Science and Medicine, 2014; 107: 18–25. [DOI] [PubMed] [Google Scholar]
- 35. Vennum A, Fincham FD. Assessing decision making in young adult romantic relationships. Psychological Assessment, 2011; 23: 739–751. [DOI] [PubMed] [Google Scholar]
- 36. Browner CH, Preloran HM, Cox SJ. Ethnicity, bioethics, and prenatal diagnosis: the amniocentesis decisions of Mexican‐origin women and their partners. American Journal of Public Health, 1999; 89: 1658–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Potter BK, O'Reilly N, Etchegary H et al Exploring informed choice in the context of prenatal testing: findings from a qualitative study. Health Expectations, 2008; 11: 355–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bryant LD, Hewison J, Green J. Attitudes towards prenatal diagnosis and termination in women who have a sibling with Down's syndrome. Journal of Reproductive and Infant Psychology, 2005; 23: 181–198. [Google Scholar]
- 39. France EF, Locock L, Hunt K, Ziebland S, Field K, Wyke S. Imagined futures: how experiential knowledge of disability affects parents' decision making about fetal abnormality. Health Expectations, 2012; 15: 139–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wonkam A, de Vries J, Royal CD, Ramesar R, Angwafo FF 3rd. Would you terminate a pregnancy affected by sickle cell disease? Analysis of views of patients in Cameroon. Journal of Medical Ethics, 2014; 40: 615–620. [DOI] [PubMed] [Google Scholar]
- 41. García E, Timmermans DR, van Leeuwen E. Parental duties and prenatal screening: does an offer of prenatal screening lead women to believe that they are morally compelled to test? Midwifery, 2012; 28: e837–e843. [DOI] [PubMed] [Google Scholar]
- 42. Frazer . Selected health professional workforce New Zealand 2002. In: Service NZHI (ed.). 2003. Available at: https://www.health.govt.nz/system/files/documents/publications/healthprofs02.pdf. Accessed 10.02.2012.
- 43. Josefson D. Doctors successfully screen embryos for gene mutation linked to early onset Alzheimer's. British Medical Jornal, 2002; 324: 564. [Google Scholar]
- 44. Larner AJ, Doran M. Reply to Dr Raux et al.: molecular diagnosis of autosomal dominant early onset Alzheimer's disease: an update. Journal of Medical Genetics, 2005; 42: 793–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Spriggs M. Genetically selected baby free of inherited predisposition to early‐onset Alzheimer's disease. Journal of Medical Ethics, 2002; 28: 290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. O'Connor AM. Validation of a decisional conflict scale. Medical Decision Making, 1995; 15: 25–30. [DOI] [PubMed] [Google Scholar]
- 47. Moss‐Morris R, Weinman J, Petrie KJ, Horne R, Cameron LD, Buick D. The revised illness perception questionnaire (IPQ‐R). Psychology & Health, 2002; 17: 1–16. [Google Scholar]
- 48. Cameron LD, Reeve J. Risk perceptions, worry, and attitudes about genetic testing for breast cancer susceptibility. Psychology & Health, 2006; 21: 211–230. [DOI] [PubMed] [Google Scholar]
- 49. Morrison MA, Morrison T. The Psychology of Modern Prejudice. Hauppauge, NY: Nova Science Publishers, 2008; 332‐viii. [Google Scholar]
- 50. McDonell MG, Kerbrat AH, Comtois KA, Russo J, Lowe JM, Ries RK. Validation of the co‐occurring disorder quadrant model. Journal of Psychoactive Drugs, 2012; 44: 266–273. [DOI] [PubMed] [Google Scholar]
- 51. Shedler J, Mayman M, Manis M. The illusion of mental health. American Psychologist, 1993; 48: 1117–1131. [DOI] [PubMed] [Google Scholar]
- 52. van den Berg M, Timmermans DR, ten Kate LP, van Vugt JM, van der Wal G. Informed decision making in the context of prenatal screening. Patient Education and Counseling, 2006; 63: 110–117. [DOI] [PubMed] [Google Scholar]
- 53. Rowe H, Fisher J, Quinlivan J. Women who are well informed about prenatal genetic screening delay emotional attachment to their fetus. Journal of Psychosomatic Obstetrics & Gynecology, 2009; 30: 34–41. [DOI] [PubMed] [Google Scholar]
- 54. Kukulu K, Buldukoglu K, Keser I et al Psychological effects of amniocentesis on women and their spouses: importance of the testing period and genetic counseling. Journal of Psychosomatic Obstetrics & Gynecology, 2006; 27: 9–15. [DOI] [PubMed] [Google Scholar]
- 55. Rini C, Dunkel Schetter C, Hobel CJ, Glynn LM, Sandman CA. Effective social support: Antecedents and consequences of partner support during pregnancy. Personal Relationships, 2006; 13: 207–29. [Google Scholar]
- 56. McGillivray G, Rosenfeld JA, McKinlay Gardner RJ, Gillam LH. Genetic counselling and ethical issues with chromosome microarray analysis in prenatal testing. Prenatal Diagnosis, 2012; 32: 389–395. [DOI] [PubMed] [Google Scholar]
- 57. Pergament E, Pergament D. Reproductive decisions after fetal genetic counselling. Best Practice & Research. Clinical Obstetrics & Gynaecology, 2012; 26: 517–529. [DOI] [PubMed] [Google Scholar]
- 58. Wessels T, Koole T, Penn C. ‘And then you can decide’ – antenatal foetal diagnosis decision making in South Africa. Health Expectations, 2014. DOI: 10.1111/hex.12322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Rini C, Dunkel Schetter C. The effectiveness of social support transactions in intimate relationships In: Davila J, Sullivan K. (eds) Support Processes in Intimate Relationships. New York, NY: Oxford, 2010: 26–67. [Google Scholar]
- 60. Kuppermann M, Norton ME, Gates E et al Computerized prenatal genetic testing decision‐assisting tool: a randomized controlled trial. Obstetrics and Gynecology, 2009; 113: 53–63. [DOI] [PubMed] [Google Scholar]
- 61. Caleshu C, Shiloh S, Price C, Sapp J, Biesecker B. Invasive prenatal testing decisions in pregnancy after infertility. Prenatal Diagnosis, 2010; 30: 575–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Durand M‐A, Stiel M, Boivin J, Elwyn G. Information and decision support needs of parents considering amniocentesis: interviews with pregnant women and health professionals. Health Expectations, 2010; 13: 125–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. García E, Timmermans DRM, van Leeuwen E. Rethinking autonomy in the context of prenatal screening decision‐making. Prenatal Diagnosis, 2008; 28: 115–120. [DOI] [PubMed] [Google Scholar]
- 64. De Jong A, Dondorp WJ, de Die‐Smulders CEM, Frints SGM, de Wert GMWR. Non‐invasive prenatal testing: ethical issues explored. European Journal of Human Genetics, 2010; 18: 272–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Suter SM. The Routinization of prenatal testing. American Journal of Law and Medicine, 2002; 8: 233–270. [PubMed] [Google Scholar]
- 66. Green J, Hewison J, Bekker H, Bryant L, Cuckle H. Psychosocial aspects of genetic screening of pregnant women and newborns: a systematic review. Health Technology Assessment, 2004; 8: 1–109. [DOI] [PubMed] [Google Scholar]
- 67. Garcίa E, Timmermans DRM, van Leeuwen E. Reconsidering prenatal screening: an empirical – ethical approach to understand moral dilemmas as a question of personal preferences. Journal of Medical Ethics, 2009; 35: 410–414. [DOI] [PubMed] [Google Scholar]
- 68. Knoppers BM, Bordet S, Isasi RM. Preimplantation genetic diagnosis: an overview of socio‐ethical and legal considerations. Annual Review of Genomics and Human Genetics, 2006; 7: 201–221. [DOI] [PubMed] [Google Scholar]
- 69. Burgess MM, D'Agincourt‐Canning L. Genetic testing for hereditary disease: attending to relational responsibility. Journal of Clinical Ethics, 2001; 12: 361–372. [PubMed] [Google Scholar]
- 70. Etchegary H, Potter B, Howley H et al The influence of experiential knowledge on prenatal screening and testing decisions. Genetic Testing, 2008; 12: 115–124. [DOI] [PubMed] [Google Scholar]
- 71. Henneman L, Timmermans DRM, van der Wal G. Public experiences, knowledge and expectations about medical genetics and the use of genetic information. Community Genetics, 2004; 7: 33–43. [DOI] [PubMed] [Google Scholar]
- 72. Human Genetics Commission . Public Attitudes to Human Genetic Information. People's Panel Quantitative Study Conducted for the Human Genetics Commission. London: Crown, 2001. [Google Scholar]
- 73. Weinstein ND. Effects of personal experience on self‐protective behavior. Psychological Bullertin, 1989; 105: 31–50. [DOI] [PubMed] [Google Scholar]
- 74. Abel EK, Browner CH. Selective compliance with biomedical authority and the uses of experiential knowledge In Lock M, Kaufert PA. (eds) Pragmatic Women and Body Politics. Cambridge: Cambridge University Press, 1998: 310–326. [Google Scholar]
- 75. Rees G, Fry A, Cull A. A family history of breast cancer: women's experiences from a theoretical perspective. Social Science & Medicine, 2001; 52: 1433–1440. [DOI] [PubMed] [Google Scholar]
- 76. d'Agincourt‐Canning L. The effect of experiential knowledge on construction of risk perception in hereditary breast/ovarian cancer. Journal of Genetic Counseling, 2005; 14: 55–69. [DOI] [PubMed] [Google Scholar]
- 77. Kruglanski AW, Webster DM, Klem A. Motivated resistance and openness to persuasion in the presence or absence of prior information. Journal of Personality & Social Psychology, 1993; 65: 861–876. [DOI] [PubMed] [Google Scholar]
- 78. Sanderson SC, Michie S. Genetic testing for heart disease susceptibility: potential impact on motivation to quit smoking. Clinical Genetics, 2007; 71: 501–510. [DOI] [PubMed] [Google Scholar]
- 79. Wright AJ, Sutton SR, Hankins M, Whitwell SCL, Macfarlane A, Marteau TM. Why does genetic causal information alter perceived treatment effectiveness? An analogue study. British Journal of Health Psychology, 2012; 17: 294–313. [DOI] [PubMed] [Google Scholar]
- 80. Claassen L, Henneman L, De Vet R, Knol D, Marteau T, Timmermans D. Fatalistic responses to different types of genetic risk information: exploring the role of Self‐Malleability. Psychology & Health, 2009; 25: 183–196. [DOI] [PubMed] [Google Scholar]
- 81. Hickerton CL, Aitken M, Hodgson J, Delatycki MB. “Did you find that out in time?”: new life trajectories of parents who choose to continue a pregnancy where a genetic disorder is diagnosed or likely. American Journal of Medical Genetics Part A, 2012; 158A: 373–383. [DOI] [PubMed] [Google Scholar]
- 82. Scully JL, Porz R, Rehmann‐Sutter C. ‘You don't make genetic test decisions from one day to the next’–using time to preserve moral space. Bioethics, 2007; 21 : 208–217. [DOI] [PubMed] [Google Scholar]
- 83. O'Connor AM, Bennett CL, Stacey D et al Decision aids for people facing health treatment or screening decisions. Cochrane Database Systematic Review, 2003; 2: CD001431. [DOI] [PubMed] [Google Scholar]
- 84. Sapp JC, Hull SC, Duffer S et al Ambivalence toward undergoing invasive prenatal testing: an exploration of its origins. Prenatal Diagnosis, 2010; 30: 77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Arimori N. Randomized controlled trial of decision aids for women considering prenatal testing: the effect of the Ottawa Personal Decision Guide on decisional conflict. Japan Journal of Nursing Science, 2006; 3: 119–130. [Google Scholar]
- 86. Jackson C, Cheater FM, Reid I. A systematic review of decision support needs of parents making child health decisions. Health Expectations, 2008; 11: 232–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Marteau TM, Weinman J. Self‐regulation and the behavioural response to DNA risk information: a theoretical analysis and framework for future research. Social Science & Medicine, 2006; 62: 1360–1368. [DOI] [PubMed] [Google Scholar]


