Abstract
Objective
To develop and validate new regret scales and examine whether a decision aid affects different aspects of regret in the treatment choice for prostate cancer.
Methods
This was a multicentre trial (three sites) with imbalanced randomization (1 : 2). From 2008 to 2011, patients with localized prostate cancer were randomized 1 : 2 to usual care (N = 77) or usual care plus a decision aid presenting risks and benefits of different treatments (N = 163). The treatments were surgery and (external or interstitial) radiotherapy. Regret was assessed before, and 6 and 12 months after treatment, using the Decisional regret scale by Brehaut et al. (Medical Decision Making, 23, 2003, 281), and three new scales focusing on process, option and outcome regret. The relation between decision aid and regret was analysed by anova.
Results
The concurrent validity of the new regret scales was confirmed by correlations between regret and anxiety, depression, decision evaluation scales and health‐related quality of life. With a decision aid, patient participation was increased (P = 0.002), but regret was not. If anything, in patients with serious morbidity the decision aid resulted in a trend to less option regret and less Brehaut regret (P = 0.075 and P = 0.061, with effect sizes of 0.35 and 0.38, respectively). Exploratory analyses suggest that high‐risk patients benefitted most from the decision aid.
Conclusion
The new regret scales may be of value in distinguishing separate aspects of regret. In general, regret was not affected by the decision aid. In patients with serious morbidity, a trend to lower option regret with a decision aid was observed.
Keywords: decision aid, patient participation, prostate cancer, regret
Introduction
For patients facing a choice between different treatment options, decision aids have been developed. However, these tools are still not widely implemented in daily oncology practice, possibly for fear of increasing anxiety1 or uncertainty.2 The fear of negative effects, for example reducing hope or increasing regret, makes some physicians hesitant to share all outcome information,3 despite the fact that studies did not find negative effects. When patients with cancer were informed about a poor prognosis, hope was maintained.4 And when they were involved in the treatment choice, regret was not increased.5, 6, 7
This study focuses in more detail on different aspects of regret. To date, regret has been mainly studied using the Brehaut regret scale8 (a questionnaire on decisional regret), or by separate questions such as ‘Would you choose the same treatment again?’.9 Such measures mainly focus on which treatment option was chosen. With respect to decision making, however, three types of regret have been distinguished:10 (i) process regret, referring to the process leading up to the choice; (ii) option regret, referring to the treatment chosen; and (iii) outcome regret, referring to the results of the treatment. This study is the first to develop and validate separate regret subscales in order to measure different types of regret in the context of an actual treatment decision for cancer, that is prostate cancer.
Decision support and regret
Two opposing hypotheses can be formulated with regard to the relation between decision support and regret. The Decision Justification Theory10, 11 posits that people tend to ask themselves whether a choice was justified. A ‘careful and thorough (i.e. justified)’ decision is expected to reduce feelings of regret.10 This would suggest that a decision aid would decrease regret.
The medical psychological model, on the other hand, emphasizes the vulnerability of patients. Patients may prefer to avoid threatening or complicated information,12, 13, 14 and they may prefer to avoid responsibility for the possible negative consequences of the choice.15, 16 This would suggest that a decision aid would increase regret, particularly in patients experiencing a bad treatment outcome. In line with this reasoning, we examined patients with bad outcome as a separate group.
The study focused on two questions: (i) whether a decision aid affects regret, and (ii) what the effect is in patients with poor outcome in terms of serious side‐effects.
Methods
Regret scales
To measure different aspects of regret, 18 regret statements were developed (Table 1), in part derived from previous studies.8, 9, 11, 17, 18, 19 Patients indicated to what extent they agreed with the statements, on a scale from 1 (completely disagree) to 5 (completely agree). Three aspects of regret10 were distinguished: (i) process regret, about the process leading up to the choice, with items such as ‘I made a well‐informed choice’, and (ii) option regret, about the treatment chosen, with items such as ‘I would choose the same treatment again’ and (iii) outcome regret, about the treatment results, with items such as ‘I regret the way the treatment turned out for me’. Within each domain, items were averaged, recoding negatively worded items, to arrive at a regret score of 1 (no regret) to 5 (strong regret).
Table 1.
Items included in the regret subscales and their mean scores on a 5‐point scale1
| Items | Mean (SD) | ||
|---|---|---|---|
| Process regret | At t2 | ||
| I made a well‐informed choice | 4.3 (0.8) | ||
| I want a clearer advice | 2.2 (1.5) | ||
| I know the pros and cons of the treatment | 4.2 (0.8) | ||
| I want more information about this decision | 2.4 (1.4) | ||
| I am satisfied with the information I received | 4.2 (0.9) | ||
| I regret the way the decision was reached | 1.6 (1.2) | ||
| I weighed the pros and cons of the treatment against each other | 4.4 (0.8) | ||
| Option regret | At t3 | At t4 | |
| It was the right decision | 4.5 (0.7) | 4.4 (0.7) | |
| I regret the choice that was made | 1.5 (0.8) | 1.5 (0.7) | |
| I would go for the same choice if I had to do it over again | 4.5 (0.7) | 4.4 (0.8) | |
| The treatment was the wrong one for me | 1.4 (0.6) | 1.5 (0.8) | |
| Looking back, another treatment would've been a better choice | 1.5 (0.8) | 1.6 (0.8) | |
| I'm satisfied with the treatment | 4.4 (0.7) | 4.3 (0.8) | |
| The decision was a wise one | 4.4 (0.7) | 4.3 (0.8) | |
| Outcome regret | |||
| I regret the way the treatment turned out for me | 1.8 (1.0) | 1.8 (1.0) | |
| The choice did me a lot of harm | 1.9 (1.1) | 2.0 (1.1) | |
| I'm satisfied with the outcome of the treatment | 4.2 (0.9) | 4.0 (1.0) | |
| I regret the side effects I experienced | 3.0 (1.3) | 2.9 (1.3) | |
t2 = 2 weeks after the decision was made, before treatment started.
t3 = 6 months after treatment.
t4 = 12 months after treatment.
1Item scores run from 1 (completely disagree) to 5 (completely agree).
Trial design
The methods used in this trial have previously been described elsewhere.20, 21 This was a prospective, parallel‐group, multicentre randomized controlled trial between usual care and usual care plus decision aid (Fig. 1). Patients and caregivers could not be blinded to the intervention.
Figure 1.
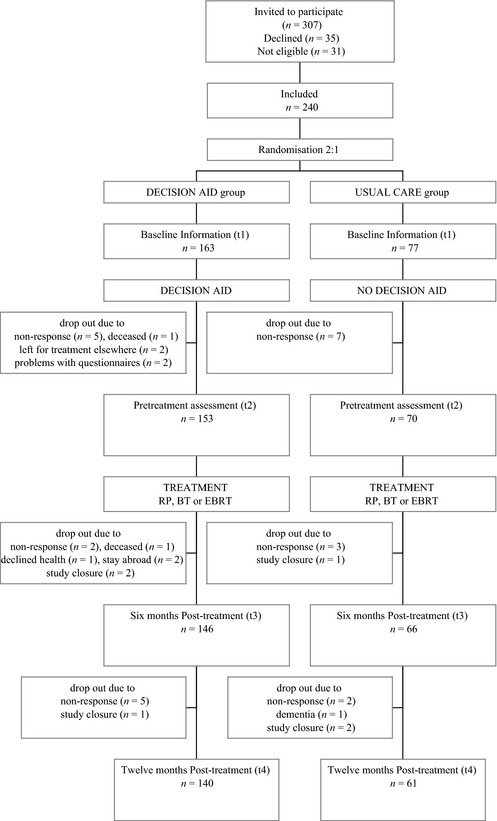
Patient flow and study design.
Setting and patients
From 2008 to 2011, patients with primary localized prostate cancer (T1‐3a), eligible for both radical prostatectomy and radiotherapy, were enrolled in three hospitals in the Netherlands, that is Radboud UMC Nijmegen, Canisius Wilhelmina Hospital in Nijmegen and Rijnstate Hospital in Arnhem. The latter are two large non‐academic centres. Given the focus on the effect of serious side‐effects, patients wanting active surveillance were not included in the study. Other exclusion criteria were mental/cognitive problems, and inadequate knowledge of the Dutch language, as assessed by the physician. The study was approved by the ethics committees of the participating hospitals.
Randomization
Enrolled patients were individually randomized to (i) the usual care group, which discussed the treatment choice with their specialist, or (ii) the decision aid group, which, in addition, had the decision aid presented by the researcher (JvTG). Randomization was imbalanced (1 : 2) to have a large enough decision aid group to answer separate research questions, reported elsewhere.20 Randomization was centralized to avoid allocation bias and was blocked in groups of 3 per hospital, thus stratifying for hospital site.
Procedure
During the first consultation, the urologist mentioned that different treatment options were available. Patients who wanted active surveillance were not eligible for the study. The urologists mentioned the study to all remaining eligible patients. For these patients, urologists were instructed to describe the treatment options briefly and not to decide on a treatment within the first consultation. Written informed consent was obtained after the patients received additional information about the study from the researcher. Subsequently, patients were randomized to the usual care group or the decision aid group, as described above. The decision aid was presented to the decision aid group only, about a week after the consultation with the urologist, in a separate consultation with the researcher. A single researcher was used to obtain a standardized presentation of the decision aid, thus minimizing the effect of variation between caregivers. Finally, all patients had a second consultation with the urologist to discuss and decide on the treatment choice.
Decision aid
The decision aid was developed according to the IPDAS criteria.22 It explained that there are different treatment options with different pros and cons. Radical prostatectomy (by open, laparoscopic or robot‐assisted procedure) and external beam radiotherapy were presented to all patients. A third option, brachytherapy, was presented only to eligible patients.
During the decision aid consultation with the researcher, first the main features of each treatment were described in terms of procedures involved. Risk information regarding the outcome (bNED and survival) and side‐effects (erectile, urinary and bowel) was based on a literature search20 and was provided in frequencies and visual aid formats. Figure 2 shows the information presented to most patients, that is low‐/intermediate‐risk patients (PSA ≤ 20 and Gleason ≤ 7 and T1T2). For patients at higher risk (PSA > 20, Gleason > 7 and/or T3a), the 10‐year risk of prostate cancer specific mortality was adapted to 10 and 18% after radical prostatectomy and external beam radiotherapy, respectively. The information was also given to the patients to take home.
Figure 2.
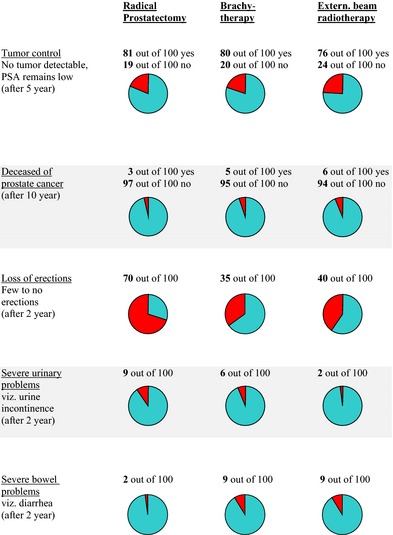
Decision aid for patients with PSA ≤ 20, Gleason ≤ 7, T1T2 and eligible for brachytherapy.
To clarify values, patients were encouraged to consider and discuss which pros and cons were most important to them, both during the decision aid consultation and at home using the written take‐home information. The decision aid has been described in more detail elsewhere.20
Outcome measures and follow‐up
Questionnaires were sent at baseline (t1), that is before the treatment choice was made; about 2 weeks later at pre‐treatment (t2), that is after the treatment was chosen, but before it was carried out; and at 6 months (t3) and 12 months (t4) after the surgery or the last radiotherapy session. The first questionnaire (t1) collected baseline demographic and medical patient characteristics. Decision‐related outcome measures (including regret) were assessed at t2 and/or later (see below).
Patients’ characteristics
Tumour characteristics were extracted from the patients’ medical records. Demographic variables were collected by questionnaire at t1.
Regret
Process regret was assessed at t2, right after the decision‐making process and before treatment. Option regret and outcome regret were assessed 6 and 12 months after treatment, at t3 and t4. These new regret scales range from 1 (no regret) to 5 (strong regret). In addition, the Brehaut regret scale8 was assessed at t3 and t4, ranging from 0 to 100, with items partly similar to our option regret scale.
Patient participation
At t2, perceived participation was assessed by asking ‘Who decided on the treatment choice?’,23 with answers ranging from ‘only the physician’ to ‘only me’. To be able to correct for possible baseline differences, baseline participation preference was measured at t1: ‘Who should, in your opinion, decide on the treatment choice?’
Measures for validation
For the purpose of validating the regret scales, anxiety and depression from the HADS24 and Satisfaction–Uncertainty and Decision Control from the Decisional Evaluation Scales18 were assessed at t2, t3 and t4. The prostate‐specific HR‐QOL was assessed at t1, t3 and t4 by means of the EPIC scale.25 Scores ranged from 0 to 100, with higher scores reflecting better functioning.
Statistical analysis
The validity of the newly developed regret scales was examined by factor analysis on the items (oblique rotation) and by analysing the correlations with anxiety/depression, the Decision Evaluation Scales and health‐related quality of life. In addition, Crohnbach's alphas were calculated. Furthermore, the regret scores at 6 and 12 months after treatment were compared to examine the reproducibility of the scales.
To examine differences between the decision aid group and the usual care group, t‐tests were used for continuous variables and chi‐square tests for ordinal or dichotomous variables. When data were missing, scale values were calculated only if at least half of the items were filled out, using the mean of the scored items. For analysis, the participation level was dichotomized as patient involved (‘together with physician’, ‘mainly me’ or ‘only me’) vs. physician decided (‘mainly physician’ or ‘only physician’). The effect of the decision aid on regret was analysed by anova.
Our primary research question for the intervention was whether a decision aid had an impact on regret. The effect of the decision aid on Brehaut regret, process regret, option regret and outcome regret was analysed. Variables were considered as possible confounding factors if they were related to the regret score and differed at baseline (P < 0.15) between the decision aid group and the usual care group. None of the demographic, medical or decision‐related variables, however, were significantly related to any of the regret scales. Therefore, subsequent analyses (anova) were not corrected for confounders, and unadjusted means are presented.
Our second a priori research question was whether the effect was different in patients with a bad treatment outcome. Therefore, the above analyses were repeated on those patients that had serious morbidity at t4. Serious morbidity, that is poor functional outcome, was defined as a decrease of 15 points or more on the 100‐point urinary, bowel and/or sexual summary EPIC score25 compared to the patient's baseline score. This criterion represents a minimal important difference (MID) of half a standard deviation.26 As standard deviations for the scales ranged from 8 to 30, the upper limit of 30 was chosen, resulting in a MID of 15.
Results
Patients
The patients in this study were described in more detail elsewhere.20, 21 In total, 307 patients were approached for the study, of whom 36 declined (12%), representing an informed consent rate of 88%.21 The patients who declined had a similar mean age as those who gave informed consent (65 vs. 64 years). Of the remaining 271 patients, 31 were not eligible; 14 were excluded because of other health problems, including cardiac problems and other tumours; and 17 because they chose active surveillance after all. Thus, 240 patients were included. Patient characteristics in the decision aid group and the usual care group were comparable for education, age, baseline physical functioning and tumour characteristics.20 Within the decision aid group, 91 patients (56%) were eligible for brachytherapy and 16 patients (10%) were high‐risk patients. Overall, 169 (71%) were treated by RP, 28 (12%) by BT and 42 (18%) by EBRT.20
The new regret scales
Tables 2 and 3 show results on the newly developed regret scales. Process regret was assessed by seven items. We categorized the other eleven items a priori into two subscales, option regret (7 items) and outcome regret (4 items); the loadings of the items in a factor analysis confirmed this categorization both at 6 and 12 months after treatment (Table 2). Cronbach's alphas for the scales on process regret and outcome regret were 0.95 and 0.79, respectively. For the option regret scale, Cronbach's alpha was 0.94, compared to 0.83 for the validated Brehaut scale.
Table 2.
Factor loadings (Pattern Matrix) of 11 items in two subscales
| Option regret | Outcome regret | |
|---|---|---|
| At 6 months after treatment | ||
| It was the right decision | 0.78 | |
| I regret the choice that was made | −0.62 | |
| I would go for the same choice if I had to do it over again | 0.86 | |
| The treatment was the wrong one for me | −0.90 | |
| Looking back, another treatment would've been a better choice | −0.71 | |
| I'm satisfied with the treatment | 0.63 | −0.30 |
| The decision was a wise one | 0.74 | |
| I regret the way the treatment turned out for me | 0.67 | |
| The choice did me a lot of harm | 0.70 | |
| I'm satisfied with the outcome of the treatment | 0.42 | −0.37 |
| I regret the side effects I experienced | 0.88 | |
| At 12 months after treatment | ||
| It was the right decision | −0.82 | |
| I regret the choice that was made | 0.85 | |
| I would go for the same choice if I had to do it over again | −0.91 | |
| The treatment was the wrong one for me | 0.91 | |
| Looking back, another treatment would've been a better choice | 0.82 | |
| I'm satisfied with the treatment | −0.70 | |
| The decision was a wise one | −0.90 | |
| I regret the way the treatment turned out for me | 0.66 | |
| The choice did me a lot of harm | 0.72 | |
| I'm satisfied with the outcome of the treatment | 0.32 | −0.57 |
| I regret the side effects I experienced | 0.91 | |
Extraction Method: Principal Component Analysis. Rotation Method: Oblimin with Kaiser Normalization.
Correlations smaller than 0.30 were suppressed.
Table 3.
Correlations of process regret (at t2) and option and outcome regret (at t4) with Decision Evaluation Scales (Satisfaction/Uncertainty, Decision control, Informed choice), anxiety and depression. Perceived responsibility and quality of life (QOL) scores at the corresponding point in time (t2 or t4)
| Process regret (t2) | Option regret (t4) | Outcome regret (t4) | |
|---|---|---|---|
| Satisfaction/Uncertainty | −0.641 | −0.561 | −0.431 |
| Decision control | −0.571 | −0.611 | −0.351 |
| Informed choice | −0.971 | NA | NA |
| Anxiety | 0.202 | 0.241 | 0.261 |
| Depression | 0.192 | 0.251 | 0.361 |
| Feeling responsible for decision | −0.382 | −0.401 | −0.202 |
| Feeling responsible for outcome | −0.093 | −0.202 | −0.251 |
| QOL scores (EPIC)3 | |||
| Urinary score | NA | −0.182 | −0.461 |
| Bowel score | NA | −0.182 | −0.251 |
| Sexual score | NA | −0.103 | −0.271 |
NA, not assessed at given time.
1 P < 0.001, 2 P < 0.02, 3Not Significant. 4Higher EPIC scores reflect better quality of life.
To quantify the reproducibility of the scales, the scores at 6 months and 12 months after treatment were compared. Correlations between the scores at 6 and 12 months were 0.65 (P < 0.001) for outcome regret and 0.66 (P < 0.001) for option regret, compared to 0.56 (P < 0.001) for the Brehaut scale. This time difference is quite large, resulting in an underestimate of the reproducibility.
For the purpose of validating the regret scales, correlations were examined of process regret (t2) and option and outcome regret (t4) with other measures at the corresponding point in time (t2 or t4) (Table 3). Process regret, option regret and outcome regret correlated significantly with the Decision Evaluation Scales (Satisfaction/Uncertainty, Informed choice, Decision control), anxiety and depression (Table 3). Outcome regret also correlated well with health‐related quality of life (urinary, bowel and sexual scores). All correlations were in the expected direction; that is, more regret was associated with more anxiety/depression and with less decision satisfaction, less informed choice, less decision control and lower scores on health‐related QOL.
Effect of the decision aid
At baseline (t1), the preferred participation level was high; 86 and 88% of the patients in the decision aid group and the usual care group, respectively, indicated at baseline that the patient should be involved in the treatment decision (‘together with the physician’, or ‘mainly me’ or ‘only me’). At t2, 95% of the patients in the decision aid group indicated that they actually had been involved in the decision, compared to 83% in the usual care group (P = 0.002). As such, the decision aid had the expected effect on patient participation.
At the same time, the decision aid did not affect regret scores at t4, when analysed in all patients (Table 4). In Table 5, regret was examined separately in patients with or without toxicity. Regret was not affected in patients without serious side‐effects. However, in patients with serious side‐effects, if anything, a trend to less option regret and less Brehaut regret was found when a decision aid had been used (P = 0.075 and P = 0.061, respectively, and effect sizes of 0.35 and 0.38, respectively).
Table 4.
Regret scores in the decision aid group and the usual care group (scores and analyses unadjusted)
| Regret scales | Decision aid Mean (SD) | Usual care Mean (SD) | P |
|---|---|---|---|
| Process regret1 | |||
| t2 (N = 219) | 1.85 (0.50) | 1.83 (0.54) | 0.78 |
| Option regret1 | |||
| t3 (N = 210) | 1.49 (0.55) | 1.53 (0.54) | 0.59 |
| t4 (N = 201) | 1.58 (0.65) | 1.68 (0.62) | 0.30 |
| Outcome regret1 | |||
| t3 (N = 209) | 2.06 (0.82) | 2.22 (0.86) | 0.19 |
| t4 (N = 201) | 2.16 (0.86) | 2.29 (0.91) | 0.32 |
| Brehaut regret2 | |||
| t3 (N = 212) | 14.2 (14.9) | 15.7 (15.3) | 0.52 |
| t4 (N = 201) | 16.1 (16.2) | 19.4 (16.6) | 0.19 |
t2 = 2 weeks after the decision was made, before treatment started.
t3 = 6 months after treatment.
t4 = 12 months after treatment.
1Regret scales run from 1 (=no regret) to 5 (=strong regret).
2Brehaut regret scale runs from 0 to 100 with higher scores reflecting more regret.
Table 5.
Regret scores for patients with and without serious side‐effects at t4, 12 months after treatment, in the decision aid group and the usual care group (scores and analyses unadjusted)
| Decision aid Mean (SD) | Usual care Mean (SD) | P | Effect size | |
|---|---|---|---|---|
| Without serious side‐effects1 | ||||
| Option regret3 (N = 61) | 1.42 (0.71) | 1.40 (0.42) | 0.91 | 0.03 |
| Outcome regret3 (N = 61) | 1.76 (0.75) | 1.81 (0.49) | 0.82 | −0.07 |
| Brehaut regret2 (N = 61) | 11.4 (17.8) | 12.8 (11.0) | 0.76 | −0.09 |
| With serious side‐effects1 | ||||
| Option regret3 (N = 120) | 1.61 (0.57) | 1.83 (0.70) | 0.075 | −0.354 |
| Outcome regret3 (N = 120) | 2.35 (0.88) | 2.58 (1.04) | 0.223 | −0.254 |
| Brehaut regret2 (N = 121) | 17.8 (14.7) | 23.9 (18.9) | 0.061 | −0.384 |
1Having serious side‐effects was defined as a decrease of at least 15 points from baseline in EPIC score for either urinary, bowel and/or sexual functioning.
2Brehaut regret scale runs from 0 to 100 with higher scores reflecting more regret.
3Option and outcome regret scales run from 1 (=no regret) to 5 (=strong regret).
4These effect sizes are considered small (0.2) to medium (0.5) effects.
Discussion
The decision aid did not affect regret in general. However, in patients experiencing side‐effects the use of the decision aid tended to lower regret compared to usual care.
Development of the regret scales
As part of this study, we developed regret subscales. The Crohnbach's alphas and the reproducibility of the regret scores were good. For the purpose of validating these scales, their correlations with other measures were examined. All correlations were in the expected direction; that is, more regret was associated with more anxiety/depression and with less decisional conflict and lower QOL. These findings confirm the concurrent validity of our regret scales.
Previous research showed a shift in how patients evaluated the decision‐making process, depending on the treatment outcome: the better the outcome of the treatment, the easier they perceived the decision‐making process in retrospect.27 Others suggested that patients experiencing problems after treatment may shift more responsibility about the treatment decision to others and may believe to have had little choice.28 To avoid such bias by treatment experience, process regret was only measured right after the decision‐making process (t2).
Relation between decision support and regret
Previous studies examining the effect of decision support reported less regret5, 29, 30 or no effect,6, 7 which is in line with our results. In our study, the lack of an effect on regret in general could not be attributed to the decision aid being ineffective, because several effects of the decision aid were found on the level of participation and on treatment choice, as reported elsewhere.20 Thus, with regard to our first research question, we can conclude that the decision aid did not induce regret in general.
However, a different result was found for an important patient group, that is those with serious side‐effects. For these patients, a trend to less regret was found when a decision aid had been used compared to usual care. This effect was not caused by a difference in outcome or treatment choice between the decision aid group and the usual care group. In itself, poor outcome (in terms of functional or biochemical outcome) can lead to more regret, as is illustrated in Table 5 and as has been shown by previous research.31 The decision aid, however, did not influence functional outcome, as a similar proportion of patients were faced with serious side‐effects in the decision aid group and the control group, that is 66.7 and 66.0%, respectively. Secondly, to control for the effect of the decision aid on treatment choice, we separately analysed the data of patients who had all received the same treatment (i.e. radical prostatectomy). Again, we found no effect of the decision aid in patients with good functional outcome, and again, the decision aid was associated with a trend to less option and Brehaut regret in patients with poor functional outcome, with identical effect sizes of 0.35 and 0.38, respectively. These effects support the Decision Justification Theory,10, 11 in that decision support may help patients to reach a careful decision, thus reducing feelings of regret later on. One could hypothesize that regret is also influenced by risk classification, with higher risk patients possibly experiencing less regret when faced with side‐effects. Analyses showed that in patients with side‐effects, regret did not differ between risk groups. Option regret, for example, is 1.71, 1.66 and 1.58 for low, intermediate and high risk, respectively (P = 0.75). However, explorative analyses with risk group included in the model for option regret, showed a main effect of decision aid (P = 0.047) and an interaction between risk group and decision aid (P = 0.013). In the setting of side‐effects, the decision aid had more of a lowering effect on regret in intermediate‐ or high‐risk patients than in low‐risk patients. Option regret with or without decision aid was 1.77 vs. 1.53, respectively, in low‐risk patients, 1.49 vs. 2.00 in intermediate‐risk patients and 1.39 vs. 1.92 in high‐risk patients.
Limitations and strengths
The study was carried out in the context of prostate cancer, limiting generalization to other cancer types. Another limitation is the follow‐up duration of 1 year post‐treatment, which may be too short to fully capture the development of regret. In addition, the effect of poor outcome could only be analysed in terms of functional outcome, that is side‐effects. Poor biochemical outcome such as PSA relapse was too scarce within the first year to be analysed separately. Furthermore, there is debate about the presentation of probabilities. The pie chart format in itself has been reported to lead to more errors and longer reaction times compared to other formats.32 However, adding numbers to the pie charts, as in our risk presentation, eliminated this negative effect.33 As numbers were provided next to the pie charts, our format appears to be suitable.
Another limitation is the exclusion of patients preferring active surveillance. The second research question in this study focused on the effect of serious side‐effects. The treatment option of active surveillance does not lead to side‐effects. Therefore, patients on active surveillance were not suited for the second research question and were not included in the study. Moreover, at the time of the development of the decision aid, outcome data were not available for active surveillance in the same detail as for other treatments, and therefore, this option was not included in the overview (Fig. 2). Still, exclusion of patients on active surveillance is a limitation, as they constitute a relevant patient group which may also be at risk for regret.
The values clarification in decision aids can be implicit (e.g. stimulating the patient to think about which treatment aspects are most important to him) or explicit (rating or ranking different treatment aspects). Our approach involves an implicit values clarification rather than an explicit one. In recent years, there is debate on whether explicit values clarification exercises actually improve the quality of decision making.34, 35
A strength of this study is that patient participation and other effects of the decision aid were assessed before the treatment was executed, avoiding bias by treatment experiences. In addition, this is a prospective study, eliminating recall bias. This study is the first to measure different types of regret in the context of an actual treatment decision. Most studies to date used the Brehaut scale, focusing on option regret. Our study provided new insights for two additional aspects of regret, namely process regret and outcome regret.
Conclusion
Within the context of treatment choice for prostate cancer, regret was not increased by a decision aid in this study, nor in previous studies.5, 6, 7, 29 If anything, our data and reports by others5, 29, 30 suggest that decision support may tend to lower regret, particularly for an important patient group, that is patients with serious morbidity. Exploratory analyses suggest that intermediate‐ and high‐risk patients benefitted most from the decision aid. Although more research is needed, the newly developed regret scales may be of value in distinguishing between different aspects of regret, that is process, option and outcome regret.
Conflict of interests
There are no conflict of interests.
Source of funding
Financial support for this study was provided by a grant (2007‐3809) from the Dutch Cancer Society, Amsterdam, the Netherlands.
Trial registration: NTR1334.
Presented in part at the 35th SMDM meeting and the 14th SMDM European meeting.
References
- 1. Shepherd HL, Butow PN, Tattersall MH. Factors which motivate cancer doctors to involve their patients in reaching treatment decisions. Patient Education and Counseling, 2011; 84: 229–235. [DOI] [PubMed] [Google Scholar]
- 2. Legare F, Ratte S, Gravel K, Graham ID. Barriers and facilitators to implementing shared decision‐making in clinical practice: update of a systematic review of health professionals' perceptions. Patient Education and Counseling, 2008; 73: 526–535. [DOI] [PubMed] [Google Scholar]
- 3. Mack JW, Smith TJ. Reasons why physicians do not have discussions about poor prognosis, why it matters, and what can be improved. Journal of Clinical Oncology, 2012; 30: 2715–2717. [DOI] [PubMed] [Google Scholar]
- 4. Smith TJ, Dow LA, Virago E, Khatcheressian J, Lyckholm LJ, Matsuyama R. Giving honest information to patients with advanced cancer maintains hope. Oncology (Williston Park), 2010; 24: 521–525. [PubMed] [Google Scholar]
- 5. Peate M, Meiser B, Cheah BC et al Making hard choices easier: a prospective, multicentre study to assess the efficacy of a fertility‐related decision aid in young women with early‐stage breast cancer. British Journal of Cancer, 2012; 106: 1053–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wakefield CE, Meiser B, Homewood J, Ward R, O'Donnell S, Kirk J. Randomized trial of a decision aid for individuals considering genetic testing for hereditary nonpolyposis colorectal cancer risk. Cancer, 2008; 113: 956–965. [DOI] [PubMed] [Google Scholar]
- 7. Berry DL, Wang Q, Halpenny B, Hong F. Decision preparation, satisfaction and regret in a multi‐center sample of men with newly diagnosed localized prostate cancer. Patient Education and Counseling, 2012; 88: 262–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brehaut JC, O'Connor AM, Wood TJ et al Validation of a decision regret scale. Medical Decision Making, 2003; 23: 281–292. [DOI] [PubMed] [Google Scholar]
- 9. Clark JA, Bokhour BG, Inui TS, Silliman RA, Talcott JA. Measuring patients' perceptions of the outcomes of treatment for early prostate cancer. Medical Care, 2003; 41: 923–936. [DOI] [PubMed] [Google Scholar]
- 10. Connolly T, Reb J. Regret in cancer‐related decisions. Health Psychology, 2005; 24: S29–S34. [DOI] [PubMed] [Google Scholar]
- 11. Connolly T, Zeelenberg M. Regret in decision making. Current Directions in Psychological Science, 2002; 11: 212–216. [Google Scholar]
- 12. Lamont EB, Christakis NA. Prognostic disclosure to patients with cancer near the end of life. Annals of Internal Medicine, 2001; 134: 1096–1105. [DOI] [PubMed] [Google Scholar]
- 13. Kaplowitz SA, Campo S, Chiu WT. Cancer patients' desires for communication of prognosis information. Health Communication, 2002; 14: 221–241. [DOI] [PubMed] [Google Scholar]
- 14. Leydon GM, Boulton M, Moynihan C et al Cancer patients' information needs and information seeking behaviour: in depth interview study. BMJ, 2000; 320: 909–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. de Haes H. Dilemmas in patient centeredness and shared decision making: a case for vulnerability. Patient Education and Counseling, 2006; 62: 291–298. [DOI] [PubMed] [Google Scholar]
- 16. Livaudais JC, Franco R, Fei K, Bickell NA. Breast cancer treatment decision‐making: are we asking too much of patients? Journal of General Internal Medicine, 2013; 28: 630–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McCammon KA, Kolm P, Main B, Schellhammer PF. Comparative quality‐of‐life analysis after radical prostatectomy or external beam radiation for localized prostate cancer. Urology, 1999; 54: 509–516. [DOI] [PubMed] [Google Scholar]
- 18. Stalmeier PF, Roosmalen MS, Verhoef LC et al The decision evaluation scales. Patient Education and Counseling, 2005; 57: 286–293. [DOI] [PubMed] [Google Scholar]
- 19. O'Connor AM. Validation of a decisional conflict scale. Medical Decision Making, 1995; 15: 25–30. [DOI] [PubMed] [Google Scholar]
- 20. van Tol‐Geerdink JJ, Willem Leer J, Weijerman PC et al Choice between prostatectomy and radiotherapy when men are eligible for both: a randomized controlled trial of usual care vs decision aid. BJU International, 2013; 111: 564–573. [DOI] [PubMed] [Google Scholar]
- 21. van Tol‐Geerdink JJ, Leer JW, van Oort IM et al Quality of life after prostate cancer treatments in patients comparable at baseline. British Journal of Cancer, 2013; 108: 1784–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Elwyn G, O'Connor A, Stacey D et al Developing a quality criteria framework for patient decision aids: online international Delphi consensus process. BMJ, 2006; 333: 417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Deber RB, Kraetschmer N, Irvine J. What role do patients wish to play in treatment decision making? Archives of Internal Medicine, 1996; 156: 1414–1420. [PubMed] [Google Scholar]
- 24. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatrica Scand, 1983; 67: 361–370. [DOI] [PubMed] [Google Scholar]
- 25. Wei JT, Dunn RL, Litwin MS, Sandler HM, Sanda MG. Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health‐related quality of life in men with prostate cancer. Urology, 2000; 56: 899–905. [DOI] [PubMed] [Google Scholar]
- 26. Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health‐related quality of life: the remarkable universality of half a standard deviation. Medical Care, 2003; 41: 582–592. [DOI] [PubMed] [Google Scholar]
- 27. Larsson US, Svardsudd K, Wedel H, Saljo R. Patient involvement in decision‐making in surgical and orthopaedic practice. Effects of outcome of operation and care process on patients' perception of their involvement in the decision‐making process. Scandinavian Journal of Caring Sciences, 1992; 6: 87–96. [DOI] [PubMed] [Google Scholar]
- 28. Street RL Jr, Voigt B. Patient participation in deciding breast cancer treatment and subsequent quality of life. Medical Decision Making, 1997; 17: 298–306. [DOI] [PubMed] [Google Scholar]
- 29. Krones T, Keller H, Sonnichsen A et al Absolute cardiovascular disease risk and shared decision making in primary care: a randomized controlled trial. Annals of Family Medicine, 2008; 6: 218–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Feldman‐Stewart D, Tong C, Siemens R et al The impact of explicit values clarification exercises in a patient decision aid emerges after the decision is actually made: evidence from a randomized controlled trial. Medical Decision Making, 2012; 32: 616–626. [DOI] [PubMed] [Google Scholar]
- 31. Davis JW, Kuban DA, Lynch DF, Schellhammer PF. Quality of life after treatment for localized prostate cancer: differences based on treatment modality. Journal of Urology, 2001; 166: 947–952. [PubMed] [Google Scholar]
- 32. Feldman‐Stewart D, Kocovski N, McConnell BA, Brundage MD, Mackillop WJ. Perception of quantitative information for treatment decisions. Medical Decision Making, 2000; 20: 228–238. [DOI] [PubMed] [Google Scholar]
- 33. Feldman‐Stewart D, Brundage MD, Zotov V. Further insight into the perception of quantitative information: judgments of gist in treatment decisions. Medical Decision Making, 2007; 27: 34–43. [DOI] [PubMed] [Google Scholar]
- 34. Fagerlin A, Pignone M, Abhyankar P et al Clarifying values: an updated review. BMC Medical Informatics and Decision Making, 2013; 13(Suppl 2): S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pieterse AH, de Vries M. On the suitability of fast and frugal heuristics for designing values clarification methods in patient decision aids: a critical analysis. Health Expectations, 2013; 16: e73–e79. [DOI] [PMC free article] [PubMed] [Google Scholar]


