Abstract
Kayexalate is an ion exchange resin that is commonly used to acutely treat patients with hyperkalemia. Bowel ulceration and necrosis is a rare and uncommonly recognized complication of kayexalate administration. More often, concomitant administration with sorbitol is reported to damage the bowel; however, there are reports of kayexalate administration causing bowel necrosis without sorbitol. We present a case of a critically ill patient who underwent total colectomy for colonic necrosis secondary to oral kayexalate administration that was not recognized until late in the pathologic process. We also review the literature to further investigate this progression.
INTRODUCTION
There is a known relationship between kayexalate administration and colon necrosis, ulceration and perforation [1]. This relationship, although rare, is important to consider in the management of patients with abdominal pain following kayexalate administration. We present a critically ill patient requiring a total abdominal colectomy following kayexalate administration whose disease process was not revealed until late in the course due to her critical illness.
CASE PRESENTATION
A 55-year-old Caucasian female former smoker with a past medical history of hypertension and chronic obstructive pulmonary disease (COPD) presented to an outside hospital with altered mental status and acute renal failure 3 days after an uncomplicated right total hip arthroplasty performed for avascular necrosis of the femoral head secondary to chronic steroid use. Initial workup included computed tomography (CT) imaging of the chest, abdomen and pelvis and was significant only for aspirated material in the trachea. She was urgently transferred to our tertiary referral center for further workup and management. On admission, she was obtunded, hypotensive, found to have a well-healing right hip wound, and her abdomen was noted to be soft, non-distended and non-tender. She was intubated for hypercarbic respiratory failure, started on broad-spectrum antibiotics, and given stress dose steroids for hypotension.
She underwent an extensive workup for a source of infection including blood cultures, urinalysis, lumbar puncture, viral assays and repeat CT imaging of the chest abdomen and pelvis on hospital day (HD) 2 that was significant only for mildly dilated loops of small bowel consistent with an ileus. Blood cultures returned positive on HD2 for Pseudomonas aeruginosa and Klebsiella oxytoca. She had a mild leukocytosis and normal lactate. During the following week, she had continued need for critical care although she was weaned off of vasopressors and was tolerating enteral nutrition. Within that period, she was also started on a heparin drip for a questionable deep vein thrombosis. On HD11 her kidney injury acutely worsened leading to hyperkalemia for which she received a single 30 g dose of oral kayexalate without sorbitol. Later that day, she was placed on continuous renal replacement therapy (CRRT) for oliguric renal failure. On HD13, she was noted to have bloody diarrhea with increased abdominal distention on exam. The leukocytosis was found elevated to 24 000/mm3 with a normal lactate. A repeat abdominal/pelvic CT scan at that time demonstrated improvement in bowel distention and absence of abscess, pneumatosis, pneumoperitoneum or other abdominal pathology. The heparin drip and enteral feedings were stopped and gastroenterology consulted for an anticipated flexible sigmoidoscopy. The bloody diarrhea resolved within 24 h of stopping the heparin drip; thus the endoscopy was deferred. On HD19, bloody stools returned prompting a flexible sigmoidoscopy, which identified several ulcerations that were biopsied, later revealing ischemic necrosis of the bowel. Clostridum difficile toxin assays as well as other colonic bacterial and viral pathogens were repeatedly negative throughout the course. During this time, she continued on broad-spectrum antibiotics.
On HD20, she was noted to have a more distended abdomen and peritonitis. She underwent an urgent exploratory laparotomy and was discovered to have frank stool contamination of the peritoneal cavity, necrosis of the colon extending from the sigmoid to the cecum, including 40 cm of the terminal ileum, as well as a large cecal perforation. She underwent a total abdominal colectomy followed by multiple washouts and eventual end ileostomy. After a prolonged hospital course she was discharged to a long-term rehab center.
At pathological examination, the mucosa was diffusely hemorrhagic with extensive multifocal ulcerations. Crystalloid particles consistent with Kayexalate were identified throughout the bowel wall (Figs 1 and 2). There was no evidence of neoplasia or viral inclusions and cytomegalovirus immunostains were negative. Thus, a diagnosis of kayexalate-induced colon ischemia and necrosis was made.
Figure 1:
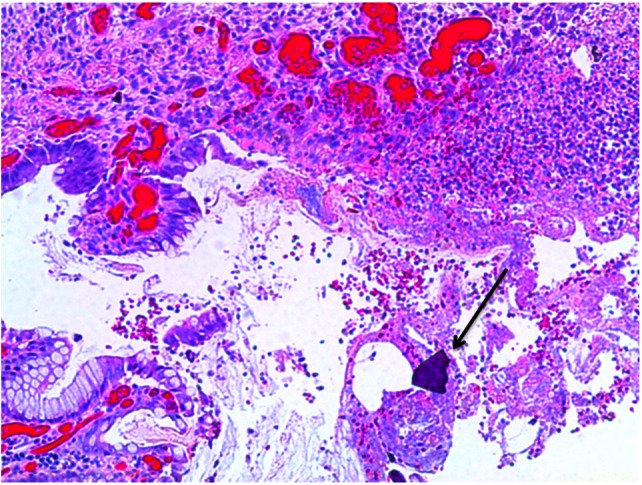
Colonic ulceration with necrosis and kayexalate crystal identified.
Figure 2:
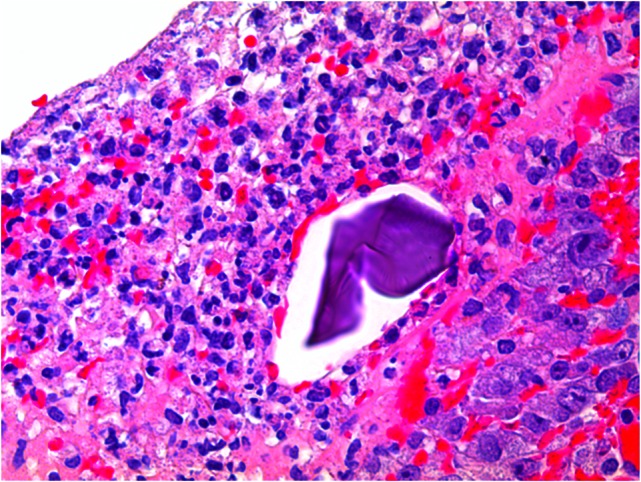
Kayexalate crystal within colonic mucosa and surrounding necrosis.
DISCUSSION
Kayexalate was first approved by the FDA in 1958 and has been commonly used to treat hyperkalemia from various causes since that time [1]. It is a cation-exchange resin that can be administered orally or rectally. Its mechanism is to exchange its bound sodium with potassium in the colon promoting overall excretion of potassium. It is not an ideal agent for acute reduction of hyperkalemia as its mechanism occurs over hours to days [2]. Its administration with sorbitol has a well-documented association with bowel necrosis and ulceration, prompting a black box warning on rectally administered Kayexalate as well as the removal of 70% sorbitol containing kayexalate from the market by the FDA in 2006 [3, 4]. Since that time, case reports continue to be published throughout the literature linking kayexalate administration without sorbitol to colon ulceration, necrosis and ischemia. The incidence of colon necrosis following kayexalate administration is reported to be 0.14–1.8% [1, 3, 5]. These cases, although rare, are often detected early in the pathological process due to the patient's complaints of increased abdominal pain and distention. There are also occasional reports of free air on imaging studies leading to laparotomy [6].
The first cases of kayexalate associated colon ulceration and necrosis were reported by Lillemoe et al. [7]. They described five cases of colon perforation associated with kayexalate in sorbitol administration, four of which died from their illness. They corroborated their findings using animal models to further prove the theory that kayexalate was the culprit of the colon necrosis. Many early cases of colon necrosis described the use of kayexalate enema preparations only; now there is an established relationship by oral or enema route [8]. There has also been found to be upper gastrointestinal ulceration secondary to kayexalate administration, however, none of these cases have required surgical intervention [2]. A reported 91% of patients with colon perforation following kayexalate administration had a history of acute kidney injury, chronic kidney disease or end stage renal disease [1]. One proposed mechanism of injury is the elevated renin seen in patients with renal failure. Renin's activation of angiotensin II and subsequent splanchnic vasoconstriction can lead to non-occlusive mesenteric ischemia which predisposes the colonic mucosa to injury following dramatic electrolyte and in turn fluid shifts [9].
The presence of kayexalate crystals on pathology specimens distinguishes kayexalate-induced necrosis from ischemic necrosis. Otherwise, these entities have a similar presentation. Identification of rhomboid or triangular, basophilic crystals with a mosaic pattern on hematoxylin and eosin (H & E) stain is pathognomonic for the presence of kayexalate (Figs 1 and 2). These crystals are identified adherent to the mucosa or imbedded within inflammatory milieu and ulceration [10].
We present this case as a grave reminder to any provider that utilizes kayexalate of the rare yet devastating complications of kayexalate administration, specifically colon ulceration and necrosis. Ischemic colitis has a virtually identical presentation to kayexalate-induced colon injury; the treatments are similar as well, though we propose that cessation of kayexalate administration is prudent in such circumstances. An increased clinical suspicion should be raised in the patient that has abdominal pain, bloody stools, or peritonitis after the administration of kayexalate in hopes of recognizing this uncommon complication.
REFERENCES
- 1.Harel Z, Harel S, Shah PS, Wald R, Perl J, Bell CM. Gastrointestinal adverse events with sodium polystyrene sulfonate (kayexalate) use: a systematic review. Am J Med 2013;126:264. e9–24. [DOI] [PubMed] [Google Scholar]
- 2.Abraham SC, Bhagavan BS, Lee LA, Rashid A, Wu TT. Upper gastrointestinal tract injury in patients receiving kayexalate (sodium polystyrene sulfonate) in sorbitol: clinical, endoscopic, and histopathologic findings. Am J Surg Pathol 2001;25:637–44. [DOI] [PubMed] [Google Scholar]
- 3.Watson M, Baker TP, Nguyen A, Sebastianelli ME, Stewart HL, Oliver DK, et al. Association of prescription of oral sodium polystyrene sulfonate with sorbitol in an inpatient setting with colonic necrosis: a retrospective cohort study. Am J Kidney Dis 2012;60:409–16. [DOI] [PubMed] [Google Scholar]
- 4.Sterns RH, Rojas M, Bernstein P, Chennupati S. Ion-exchange resins for the treatment of hyperkalemia: are they safe and effective. J Am Soc Nephrol 2010;21:733–5. [DOI] [PubMed] [Google Scholar]
- 5.Chou YH, Wang HY, Hsieh MS. Colonic necrosis in a young patient receiving oral kayexalate in sorbitol: case report and literature review. Kaohsiung J Med Sci 2011;27:155–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goutrobe P, Montcriol A, Lacroix G, Bordes J, Meaudre E, Souraud JB. Intestinal necrosis associated with orally administered calcium polystyrene sulfonate without sorbitol. Ann Pharmacother 2011;45:e13. [DOI] [PubMed] [Google Scholar]
- 7.Lillemoe KD, Romolo JL, Hamilton SR, Pennington LR, Burdick JF, Williams GM. Intestinal necrosis due to sodium polystyrene (kayexalate) in sorbitol enemas: clinical and experimental support for the hypothesis. Surgery 1987;101:267–72. [PubMed] [Google Scholar]
- 8.Dardik, Moesinger RC, Efron G, Barbul A, Harrison MG. Acute abdomen with colonic necrosis induced by kayexalae-sorbitol. South Med J 2000;93:511–14. [PubMed] [Google Scholar]
- 9.Rogers FB, Li SC. Acute colonic necrosis associated with sodium polystyrene sulfonate (kayexalate) enemas in a critically ill patient: a case report and review of the literature. J Trauma 2001;51:395–7. [DOI] [PubMed] [Google Scholar]
- 10.Parfitt J, Driman D. Pathological effects of drugs on the gastrointestinal tract: a review. Hum Pathol 2007;38:527–36. [DOI] [PubMed] [Google Scholar]


