Abstract
AIMS--To understand the mechanism for regulation of intraocular pressure, human trabecular cells were examined to determine whether they could respond to the change in hydraulic pressure. METHODS--Human trabecular cells were cultured from trabeculum tissue fragments excised during trabeculectomy in four eyes of three patients with primary open angle glaucoma and exposed to the change of hydraulic pressure in a tissue culture flask connected to a glass syringe. The pressure was exerted by automatic infusion of the piston of the syringe and monitored by a pressure gauge. The intracellular calcium concentration was measured in real time with a calcium binding fluorescent dye, fluo-3. RESULTS--A small number (about 10%) of cells appearing morphologically to be trabecular cells showed transient elevations or oscillations of the intracellular calcium concentration in response to the elevation of hydraulic pressure to 20-30 mm Hg, indicating that a part of the human trabecular cells could sense the change in hydraulic pressure. CONCLUSION--Some cells in the human trabecular tissue seem to sense the change in intraocular pressure and might play a role in its regulation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
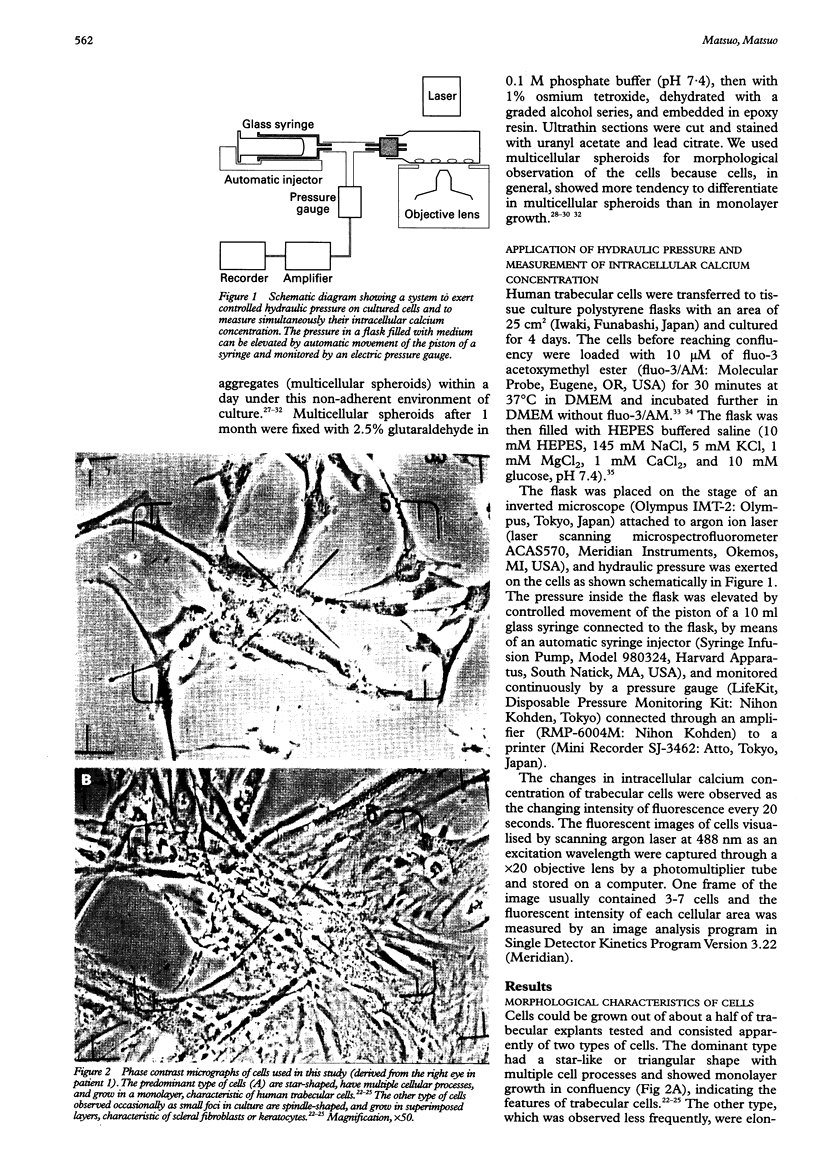
- Alvarado J. A., Wood I., Polansky J. R. Human trabecular cells. II. Growth pattern and ultrastructural characteristics. Invest Ophthalmol Vis Sci. 1982 Oct;23(4):464–478. [PubMed] [Google Scholar]
- Alvarado J., Murphy C., Juster R. Trabecular meshwork cellularity in primary open-angle glaucoma and nonglaucomatous normals. Ophthalmology. 1984 Jun;91(6):564–579. doi: 10.1016/s0161-6420(84)34248-8. [DOI] [PubMed] [Google Scholar]
- Ando J., Komatsuda T., Kamiya A. Cytoplasmic calcium response to fluid shear stress in cultured vascular endothelial cells. In Vitro Cell Dev Biol. 1988 Sep;24(9):871–877. doi: 10.1007/BF02623896. [DOI] [PubMed] [Google Scholar]
- Ando J., Ohtsuka A., Katayama Y., Korenaga R., Ishikawa C., Kamiya A. Intracellular calcium response to directly applied mechanical shearing force in cultured vascular endothelial cells. Biorheology. 1994 Jan-Feb;31(1):57–68. doi: 10.3233/bir-1994-31105. [DOI] [PubMed] [Google Scholar]
- Arora P. D., Bibby K. J., McCulloch C. A. Slow oscillations of free intracellular calcium ion concentration in human fibroblasts responding to mechanical stretch. J Cell Physiol. 1994 Nov;161(2):187–200. doi: 10.1002/jcp.1041610202. [DOI] [PubMed] [Google Scholar]
- Belmonte C., Simon J., Gallego A. Effects of intraocular pressure changes on the afferent activity of ciliary nerves. Exp Eye Res. 1971 Nov;12(3):342–355. doi: 10.1016/0014-4835(71)90159-x. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Calcium oscillations. J Biol Chem. 1990 Jun 15;265(17):9583–9586. [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and calcium signalling. Nature. 1993 Jan 28;361(6410):315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Bill A. Some aspects of aqueous humour drainage. Eye (Lond) 1993;7(Pt 1):14–19. doi: 10.1038/eye.1993.4. [DOI] [PubMed] [Google Scholar]
- Coroneo M. T., Korbmacher C., Flügel C., Stiemer B., Lütjen-Drecoll E., Wiederholt M. Electrical and morphological evidence for heterogeneous populations of cultured bovine trabecular meshwork cells. Exp Eye Res. 1991 Apr;52(4):375–388. doi: 10.1016/0014-4835(91)90032-a. [DOI] [PubMed] [Google Scholar]
- DeLong G. R. Histogenesis of fetal mouse isocortex and hippocampus in reaggregating cell cultures. Dev Biol. 1970 Aug;22(4):563–583. doi: 10.1016/0012-1606(70)90169-7. [DOI] [PubMed] [Google Scholar]
- GLOSTER J., GREAVES D. P. Effect of diencephalic stimulation upon intra-ocular pressure. Br J Ophthalmol. 1957 Sep;41(9):513–532. doi: 10.1136/bjo.41.9.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabelt B. T., Kaufman P. L. Prostaglandin F2 alpha increases uveoscleral outflow in the cynomolgus monkey. Exp Eye Res. 1989 Sep;49(3):389–402. doi: 10.1016/0014-4835(89)90049-3. [DOI] [PubMed] [Google Scholar]
- Goligorsky M. S. Mechanical stimulation induces Ca2+i transients and membrane depolarization in cultured endothelial cells. Effects on Ca2+i in co-perfused smooth muscle cells. FEBS Lett. 1988 Nov 21;240(1-2):59–64. doi: 10.1016/0014-5793(88)80340-5. [DOI] [PubMed] [Google Scholar]
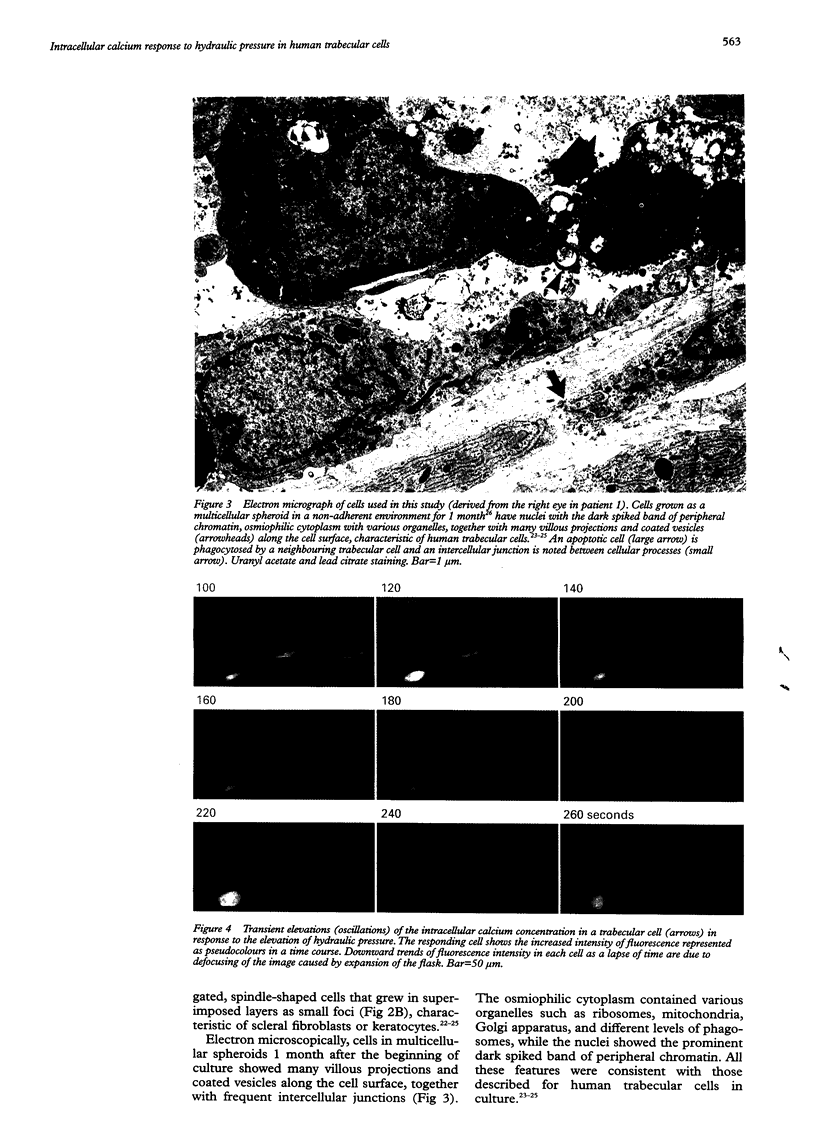
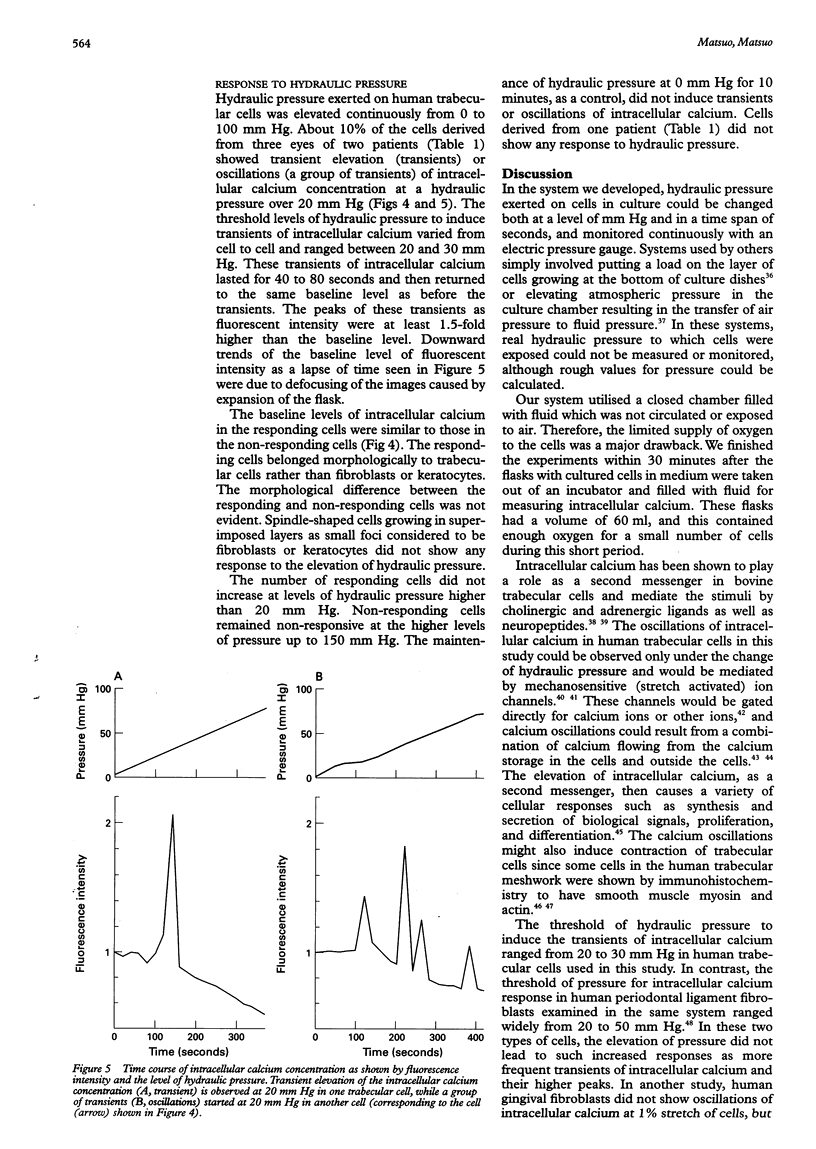
- Grierson I., Marshall J., Robins E. Human trabecular meshwork in primary culture: a morphological and autoradiographic study. Exp Eye Res. 1983 Oct;37(4):349–365. doi: 10.1016/0014-4835(83)90172-0. [DOI] [PubMed] [Google Scholar]
- Hernandez M. R., Weinstein B. I., Schwartz J., Ritch R., Gordon G. G., Southren A. L. Human trabecular meshwork cells in culture: morphology and extracellular matrix components. Invest Ophthalmol Vis Sci. 1987 Oct;28(10):1655–1660. [PubMed] [Google Scholar]
- Kao J. P., Harootunian A. T., Tsien R. Y. Photochemically generated cytosolic calcium pulses and their detection by fluo-3. J Biol Chem. 1989 May 15;264(14):8179–8184. [PubMed] [Google Scholar]
- Koide N., Sakaguchi K., Koide Y., Asano K., Kawaguchi M., Matsushima H., Takenami T., Shinji T., Mori M., Tsuji T. Formation of multicellular spheroids composed of adult rat hepatocytes in dishes with positively charged surfaces and under other nonadherent environments. Exp Cell Res. 1990 Feb;186(2):227–235. doi: 10.1016/0014-4827(90)90300-y. [DOI] [PubMed] [Google Scholar]
- LELE P. P., GRIMES P. The role of neural mechanisms in the regulation of intraocular pressure in the cat. Exp Neurol. 1960 Jun;2:199–220. doi: 10.1016/0014-4886(60)90009-1. [DOI] [PubMed] [Google Scholar]
- Lepple-Wienhues A., Rauch R., Clark A. F., Grássmann A., Berweck S., Wiederholt M. Electrophysiological properties of cultured human trabecular meshwork cells. Exp Eye Res. 1994 Sep;59(3):305–311. doi: 10.1006/exer.1994.1112. [DOI] [PubMed] [Google Scholar]
- MOSCONA A. Rotation-mediated histogenetic aggregation of dissociated cells. A quantifiable approach to cell interactions in vitro. Exp Cell Res. 1961 Jan;22:455–475. doi: 10.1016/0014-4827(61)90122-7. [DOI] [PubMed] [Google Scholar]
- Merritt J. E., McCarthy S. A., Davies M. P., Moores K. E. Use of fluo-3 to measure cytosolic Ca2+ in platelets and neutrophils. Loading cells with the dye, calibration of traces, measurements in the presence of plasma, and buffering of cytosolic Ca2+. Biochem J. 1990 Jul 15;269(2):513–519. doi: 10.1042/bj2690513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minta A., Kao J. P., Tsien R. Y. Fluorescent indicators for cytosolic calcium based on rhodamine and fluorescein chromophores. J Biol Chem. 1989 May 15;264(14):8171–8178. [PubMed] [Google Scholar]
- Morris C. E. Mechanosensitive ion channels. J Membr Biol. 1990 Feb;113(2):93–107. doi: 10.1007/BF01872883. [DOI] [PubMed] [Google Scholar]
- Mueller-Klieser W. Multicellular spheroids. A review on cellular aggregates in cancer research. J Cancer Res Clin Oncol. 1987;113(2):101–122. doi: 10.1007/BF00391431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakago-Matsuo C., Matsuo T., Nakago T. Intracellular calcium response to hydraulic pressure in human periodontal ligament fibroblasts. Am J Orthod Dentofacial Orthop. 1996 Mar;109(3):244–248. doi: 10.1016/s0889-5406(96)70147-6. [DOI] [PubMed] [Google Scholar]
- Ohuchi T., Tanihara H., Yoshimura N., Kuriyama S., Ito S., Honda Y. Neuropeptide-induced [Ca2+]i transients in cultured bovine trabecular cells. Invest Ophthalmol Vis Sci. 1992 Apr;33(5):1676–1684. [PubMed] [Google Scholar]
- PERKINS E. S. Sensory mechanisms and intraocular pressure. Exp Eye Res. 1961 Dec;1:160–167. doi: 10.1016/s0014-4835(61)80022-5. [DOI] [PubMed] [Google Scholar]
- Polansky J. R., Weinreb R. N., Baxter J. D., Alvarado J. Human trabecular cells. I. Establishment in tissue culture and growth characteristics. Invest Ophthalmol Vis Sci. 1979 Oct;18(10):1043–1049. [PubMed] [Google Scholar]
- Polansky J. R., Wood I. S., Maglio M. T., Alvarado J. A. Trabecular meshwork cell culture in glaucoma research: evaluation of biological activity and structural properties of human trabecular cells in vitro. Ophthalmology. 1984 Jun;91(6):580–595. doi: 10.1016/s0161-6420(84)34241-5. [DOI] [PubMed] [Google Scholar]
- Rohen J. W. Why is intraocular pressure elevated in chronic simple glaucoma? Anatomical considerations. Ophthalmology. 1983 Jul;90(7):758–765. doi: 10.1016/s0161-6420(83)34492-4. [DOI] [PubMed] [Google Scholar]
- STEINBERG M. S. Reconstruction of tissues by dissociated cells. Some morphogenetic tissue movements and the sorting out of embryonic cells may have a common explanation. Science. 1963 Aug 2;141(3579):401–408. doi: 10.1126/science.141.3579.401. [DOI] [PubMed] [Google Scholar]
- Sackin H. Mechanosensitive channels. Annu Rev Physiol. 1995;57:333–353. doi: 10.1146/annurev.ph.57.030195.002001. [DOI] [PubMed] [Google Scholar]
- Schachtshabel D. O., Bigalke B., Rohen J. W. Production of glycosaminoglycans by cell cultures of the trabecular meshwork of the primate eye. Exp Eye Res. 1977 Jan;24(1):71–80. doi: 10.1016/0014-4835(77)90286-x. [DOI] [PubMed] [Google Scholar]
- Schwarz G., Callewaert G., Droogmans G., Nilius B. Shear stress-induced calcium transients in endothelial cells from human umbilical cord veins. J Physiol. 1992 Dec;458:527–538. doi: 10.1113/jphysiol.1992.sp019432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamer W. D., Seftor R. E., Williams S. K., Samaha H. A., Snyder R. W. Isolation and culture of human trabecular meshwork cells by extracellular matrix digestion. Curr Eye Res. 1995 Jul;14(7):611–617. doi: 10.3109/02713689508998409. [DOI] [PubMed] [Google Scholar]
- Tamm E. R., Flügel C., Stefani F. H., Lütjen-Drecoll E. Nerve endings with structural characteristics of mechanoreceptors in the human scleral spur. Invest Ophthalmol Vis Sci. 1994 Mar;35(3):1157–1166. [PubMed] [Google Scholar]
- Tanihara H., Ohuchi T., Yoshimura N., Negishi M., Ito S. Heterogeneous response in calcium signaling by adrenergic and cholinergic stimulation in cultured bovine trabecular cells. Exp Eye Res. 1991 Apr;52(4):393–396. doi: 10.1016/0014-4835(91)90034-c. [DOI] [PubMed] [Google Scholar]
- Trapp B. D., Honegger P., Richelson E., Webster H. D. Morphological differentiation of mechanically dissociated fetal rat brain in aggregating cell cultures. Brain Res. 1979 Jan 5;160(1):117–130. doi: 10.1016/0006-8993(79)90605-x. [DOI] [PubMed] [Google Scholar]
- Tripathi R. C., Tripathi B. J. Human trabecular endothelium, corneal endothelium, keratocytes, and scleral fibroblasts in primary cell culture. A comparative study of growth characteristics, morphology, and phagocytic activity by light and scanning electron microscopy. Exp Eye Res. 1982 Dec;35(6):611–624. doi: 10.1016/s0014-4835(82)80074-2. [DOI] [PubMed] [Google Scholar]
- Tsien R. W., Tsien R. Y. Calcium channels, stores, and oscillations. Annu Rev Cell Biol. 1990;6:715–760. doi: 10.1146/annurev.cb.06.110190.003435. [DOI] [PubMed] [Google Scholar]
- VON SALLMANN L., FUORTES M. G., MACRI F. J., GRIMES P. Study of afferent electric impulses induced by intraocular pressure changes. Am J Ophthalmol. 1958 Apr;45(4 Pt 2):211–220. doi: 10.1016/0002-9394(58)90245-9. [DOI] [PubMed] [Google Scholar]
- VON SALLMANN L., LOWENSTEIN O. Responses of intraocular pressure, blood pressure, and cutaneous vessels to electric stimulation in the diencephalon: the ninth Francis I. Proctor Lecture. Am J Ophthalmol. 1955 Apr;39(4 Pt 2):11–29. doi: 10.1016/0002-9394(55)90148-3. [DOI] [PubMed] [Google Scholar]
- Weinreb R. N., Mitchell M. D., Polansky J. R. Prostaglandin production by human trabecular cells: in vitro inhibition by dexamethasone. Invest Ophthalmol Vis Sci. 1983 Dec;24(12):1541–1545. [PubMed] [Google Scholar]
- Weinreb R. N., Polansky J. R., Alvarado J. A., Mitchell M. D. Arachidonic acid metabolism in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 1988 Nov;29(11):1708–1712. [PubMed] [Google Scholar]
- Wiederholt M., Bielka S., Schweig F., Lütjen-Drecoll E., Lepple-Wienhues A. Regulation of outflow rate and resistance in the perfused anterior segment of the bovine eye. Exp Eye Res. 1995 Aug;61(2):223–234. doi: 10.1016/s0014-4835(05)80042-9. [DOI] [PubMed] [Google Scholar]
- Yousefian J., Firouzian F., Shanfeld J., Ngan P., Lanese R., Davidovitch Z. A new experimental model for studying the response of periodontal ligament cells to hydrostatic pressure. Am J Orthod Dentofacial Orthop. 1995 Oct;108(4):402–409. doi: 10.1016/s0889-5406(95)70038-2. [DOI] [PubMed] [Google Scholar]
- Zuazo A., Ibañez J., Belmonte C. Sensory nerve responses elicited by experimental ocular hypertension. Exp Eye Res. 1986 Nov;43(5):759–769. doi: 10.1016/s0014-4835(86)80007-0. [DOI] [PubMed] [Google Scholar]
- de Kater A. W., Shahsafaei A., Epstein D. L. Localization of smooth muscle and nonmuscle actin isoforms in the human aqueous outflow pathway. Invest Ophthalmol Vis Sci. 1992 Feb;33(2):424–429. [PubMed] [Google Scholar]
- de Kater A. W., Spurr-Michaud S. J., Gipson I. K. Localization of smooth muscle myosin-containing cells in the aqueous outflow pathway. Invest Ophthalmol Vis Sci. 1990 Feb;31(2):347–353. [PubMed] [Google Scholar]