Structured Abstract
Objective
The objective of this study was to determine the relationships between echocardiography-derived measures of myocardial mechanics and cancer therapeutics-related cardiac dysfunction (CTRCD).
Background
Doxorubicin and trastuzumab are highly effective breast cancer therapies, but have a substantial risk of CTRCD. There is a critical need for the early detection of patients at increased risk of toxicity.
Methods
We performed a prospective, longitudinal cohort study of breast cancer participants undergoing doxorubicin and/or trastuzumab therapy. Echocardiography was performed prior to therapy initiation (baseline) and at standardized follow-up intervals during and after completion of therapy. Ejection fraction (EF), strain, strain rate, and ventricular-arterial coupling (Ea/Eessb) were quantitated. CTRCD was defined as a ≥10% reduction in EF from baseline to <50%. Multivariable logistic regression models were used to determine the associations between baseline levels and changes from baseline in echocardiographic measures and CTRCD. Receiver operating characteristic curves were used to evaluate the predictive ability of these measures.
Results
In total, 135 participants contributed 517 echocardiograms to the analysis. Over a median follow-up time of 1.9 years (Interquartile range 0.9, 2.4), 21 participants (15%) developed CTRCD. In adjusted models, baseline levels and changes in Ea/Eessb, circumferential strain, and circumferential strain rate were associated with 21 to 38% increased odds of CTRCD (p<0.001). Changes in longitudinal strain (p=0.037), radial strain (p=0.015), and radial strain rate (p=0.006) were also associated with CTRCD. Ea/Eessb [Area under the curve (AUC) 0.703 (95% confidence interval (CI) 0.583–0.807)] and circumferential strain [AUC 0.655 (95% CI 0.517–0.767)] demonstrated the greatest predictive utility. Sensitivity analyses using an alternative CTRCD definition did not impact our results.
Conclusions
Over an extended follow-up time, ventricular-arterial coupling and circumferential strain were strongly predictive of CTRCD. Our findings suggest a noninvasive strategy to identify high-risk patients prior to, during, and after cardiotoxic cancer therapy.
Keywords: Mechanics, Cardio-oncology, Echocardiography
Introduction
Doxorubicin and trastuzumab (Herceptin®) are highly effective cancer therapies used widely in the treatment of cancer that have led to important survival gains. However, these agents carry a substantial risk of cardiotoxicity when used in combination. Doxorubicin results in a dose-dependent risk of cardiomyopathy, which may occur in ~10% of patients at dosages of 250mg/m2.(1) Furthermore, doxorubicin-induced cardiomyopathy carries a poor prognosis, with a 3.5-fold increased risk of death or cardiac transplantation compared to idiopathic cardiomyopathy.(2) When used in combination, doxorubicin and trastuzumab may result in left ventricular (LV) dysfunction in up to 27% of individuals, and heart failure (HF) in up to 4%.(3) Despite the magnitude of this problem, a fundamental question remains: How can we identify the patient who is at high risk for cancer therapeutics-related cardiac dysfunction (CTRCD)?(4) Early detection of cardiac dysfunction could enable the implementation of cardioprotective strategies prior to late, potentially irreversible changes in cardiac function.
Currently, cardiac function prior to, during, and after cancer therapy is determined by assessment of ejection fraction (EF), typically by echocardiography or multi-gated acquisition scanning. Although a valid measure, EF lacks the sensitivity to detect early changes and can underestimate the degree of subclinical myocardial damage.(5) Recent studies in cardio-oncology have suggested that newer echocardiography-derived measures of myocardial mechanics, such as strain and strain rate, provide important insight into cardiac function.(6, 7) Strain and strain rate quantify the fractional change and rate of change in myocardial length during each cardiac cycle, and can be assessed in the longitudinal, circumferential, or radial dimensions. However, insight into the relevance of early changes in these measures and subsequent CTRCD, particularly after cancer therapy completion, remains limited.
Moreover, other measures of myocardial mechanics such as ventricular-arterial coupling (Ea/Eessb), have not previously been studied in cardio-oncology. Ea/Eessb is the ratio of effective arterial elastance (Ea), which integrates arterial load, and LV end-systolic elastance (Eessb), which quantifies chamber stiffness and contractility. As an index of the interaction between the ventricular and arterial system, it provides an assessment of cardiovascular performance and efficiency. Higher ratios of Ea/Eessb reflect compromised ventricular-vascular matching and are prognostic in HF.(8) As data suggest that arterial stiffening may be caused by doxorubicin and trastuzumab, we hypothesized that these agents might also result in ventricular-vascular uncoupling and that this ratio may help diagnose and predict CTRCD.(9, 10)
The overall objective of this study was to define the cross-sectional and longitudinal relationships between measures of myocardial mechanics and doxorubicin- and trastuzumab-induced CTRCD, to characterize their diagnostic and predictive utility. We comprehensively evaluated the associations of strain indices and ventricular-arterial coupling (Ea/Eessb) with CTRCD at the same visit and subsequent visit in a prospective longitudinal cohort of women with breast cancer.
Methods
Study Population
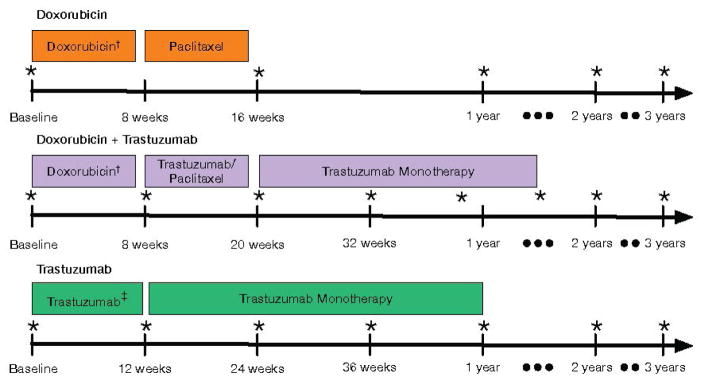
The Cardiotoxicity of Cancer Therapy (CCT) study is an ongoing, prospective longitudinal cohort study of women with breast cancer recruited from the Rena Rowan Breast Cancer Center of the Abramson Cancer Center at the University of Pennsylvania (Philadelphia, PA). The primary inclusion criteria were women at least 18 years of age diagnosed with breast cancer, prescribed doxorubicin and/or trastuzumab therapy. The only exclusion criterion was pregnancy. Treatment regimens were at the discretion of the oncology provider, and consisted of one of the following three main combinations: 1) doxorubicin (240 mg/m2) and cyclophosphamide, followed by paclitaxel; 2) doxorubicin (240 mg/m2) and cyclophosphamide followed by paclitaxel and trastuzumab; or 3) cyclophosphamide or carboplatin with docetaxel and trastuzumab (Figure 1). Trastuzumab dosing was prescribed as per standard guidelines.(11)
Figure 1. Echocardiography Protocol According to Treatment Regimen.
Echocardiograms were performed at baseline and standardized time intervals for each regimen. *Timing of echocardiograms across regimens;†includes cyclophosphamide;‡includes cyclophosphamide/docetaxel or carboplatin/docetaxel.
Prior to initiation of chemotherapy and at each follow-up visit, participants provided detailed clinical data via standardized questionnaires. Clinical data were verified via review of medical records. Transthoracic echocardiograms were performed at standardized intervals according to treatment regimen (Figure 1). Briefly, those patients treated with doxorubicin without trastuzumab underwent echocardiograms at baseline, at completion of chemotherapy, and annually. Those patients treated with doxorubicin and trastuzumab underwent an echocardiogram at baseline, after doxorubicin completion, every 3 months during trastuzumab therapy, and annually. Those patients treated with trastuzumab without doxorubicin underwent echocardiograms at baseline, every 3 months during trastuzumab therapy, and annually. The current analysis was limited to participants enrolled between August 2010 and March 2014 with at least two echocardiograms performed and quantitated.
This study was approved by the University of Pennsylvania Institutional Review Board and all participants provided written informed consent.
Echocardiography Acquisition
Transthoracic echocardiograms were acquired by a dedicated sonographer team at an Intersocietal Accreditation Commission laboratory according to a specific protocol at baseline and standardized time intervals. Two-dimensional images were acquired using Vivid 7 or E9 machines (GE Healthcare, Milwaukee, WI) in the parasternal short-axis view at the mid-papillary level and in the apical views at 60 to 80 frames/second, and digitally archived at the acquisition frame rate.
EF and Cancer Therapeutics-Related Cardiac Dysfunction
Echocardiograms were quantitated at the University of Pennsylvania Center for Quantitative Echocardiography (Philadelphia, PA). Quantitation of left ventricular volumes and strain measures was performed using 2D Cardiac Performance Analysis (TomTec Imaging Systems, Unterschleissheim, Germany).(12–14) Apical 4-chamber LV end-diastolic volume (EDV) and end-systolic volume (ESV) were calculated using the Simpson’s method of discs as recommended by the American Society of Echocardiography (ASE), with papillary muscles and trabecular structures defined as being intracavitary.(15, 16) These volumes were used to derive stroke volume (SV) and EF. The primary outcome measure of CTRCD was defined as a reduction in EF by ≥10% from baseline, prior to chemotherapy, to an absolute value of <50%, assessed at each visit. These criteria were chosen based upon previously published seminal breast cancer and cardio-oncology studies.(17, 18)
Myocardial Strain and Ventricular-Arterial Coupling
Longitudinal, circumferential, and radial peak systolic strain and strain rate measurements were performed on digitally archived images. The LV endocardial border was manually traced at the end-systolic frame of one cardiac cycle from the parasternal short-axis view at the mid-papillary level and apical 4-chamber views. In individual segments with poor tracking, the borders were manually readjusted. Peak strain and strain rate values were computed automatically and averaged across all segments.(16, 19)
Ea/Eessb, an estimate of ventricular-arterial coupling, was derived by the ratio of Ea (effective arterial elastance) to Eessb (end-systolic elastance). Ea was derived from the end-systolic pressure (ESP)/SV, where ESP was estimated as 0.90 x systolic pressure measured by manual blood pressure cuff measurement at the time of the echocardiogram.(20) The end-systolic elastance (Eessb) was determined using a modified single-beat algorithm described and validated by Chen, et al. (Supplementary Materials).(20)
Reproducibility Analyses
All LV volume and strain measurements were performed by a single, blinded observer with over 30 years of experience dedicated to echocardiography quantitation (T.P.). Reproducibility analyses (n=100) demonstrated that the intra-observer coefficients of variation (CV) were as follows: EF 4.9%; SV 7.7%; longitudinal strain 10.9%, circumferential strain 9.4%; and radial strain 26.2%. All Doppler analyses were performed by two highly experienced sonographers. The intra-observer CVs for Ea/Eessb were 6.4–7.3% and inter-observer CV was 9.5%. Overall, 5% of images were unanalyzable. Quantitation was performed blinded to subject characteristics and timing of echocardiograms.
Statistical Methods
Standard descriptive statistics were used to summarize participant characteristics at baseline. Measures of mechanics were graphically depicted over time, grouped by those participants who developed CTRCD at any time and those who did not. Linear regression models adjusting for treatment regimen and time since initiation of therapy were used to derive residuals between strain parameters and EF, and scatterplots of these residuals were used to explore whether the association between strain parameters and EF was consistent across treatment regimens after adjustment for time. We found consistency across treatment regimens, and therefore adjusted for, rather than stratified by, treatment regimen in subsequent analyses (Supplementary Figures 1A–C).
In a step-wise manner, we first identified the associations of measures of mechanics and CTRCD at the same visit (‘diagnostic’) and subsequently determined their ability to predict CTRCD at the subsequent visit (‘prognostic’). Logistic regression models were used to estimate the association of strain, strain rate, and ventricular-arterial coupling parameters with the odds of CTRCD. Variables that quantified the baseline levels and changes from baseline were included in all models. This cross-sectional analysis focused on the association of baseline levels and changes from baseline at a particular visit with the odds of CTRCD at the same visit, and was performed to provide insight into the diagnostic utility of these measures. For all models, generalized estimating equations with a robust variance estimator were used to account for the correlation arising from collecting repeated measures on participants over time.(21) Three sets of adjustment variables were considered. The first set included treatment regimen and time since therapy initiation. The second set additionally included: age, race, and heart rate. For the third set, we first evaluated a comprehensive list of potential confounders including breast cancer side, radiation therapy, diabetes, hypertension, hyperlipidemia, tobacco use, body mass index, and time-varying measures of systolic blood pressure, cardiac medication use (angiotensin converting enzyme inhibitor, angiotensin receptor blocker, beta blocker), and statin use for potential inclusion in the model. However, of these variables, only diabetes, hypertension, hyperlipidemia, and cardiac medication use improved model fit, and were therefore retained. A variable that improved model fit for any of the echocardiographic parameters was retained in all models, to maintain consistency and facilitate comparison across models. The fit of fully adjusted models was compared to that of minimally adjusted models. Throughout, model fit was assessed using QIC, an adaptation of the Akaike information criterion for repeated measures regression models.(22) Consistency of point estimates and confidence intervals across models was evaluated to assess model stability.
Receiver operating characteristic (ROC) curves were used to evaluate the ability of strain, strain rate, and ventricular-arterial coupling parameters to discriminate between those participants who experienced CTRCD and those who did not. This ‘prognostic’ analysis focused on baseline levels and changes from baseline at a particular visit and the occurrence of CTRCD at the subsequent visit. For each parameter, a logistic regression model was used to combine the baseline level and changes from baseline, adjusted for time since initiation of therapy; the linear predictor from this model was used in the ROC analysis.(23) Leave-one-out cross-validation was used so that the value of the linear predictor for each participant was calculated from a model fit to the data on all other participants. The leave-one-out approach ameliorates the potential for bias from developing and evaluating a model using the same data, and avoids arbitrarily splitting the data into derivation and evaluation cohorts. The area under the ROC curve (AUC) was used to measure prognostic accuracy. Confidence intervals (CI) for the AUC were based on 1000 cluster bootstrap samples, in which participants were selected with replacement.
All analyses were completed using R 3.1.2 (R Development Core Team, Vienna, Austria), including the geepack extension package.
Results
Study Population
Overall, 135 participants contributed 517 echocardiograms to the analysis, with a median of 3 (interquartile range [IQR] 2, 5) echocardiograms per participant. The median follow-up time (time to last echocardiogram) was 1.9 years (IQR 0.9, 2.4). Characteristics of the cohort are summarized in Table 1. At baseline, the median age was 48 years (IQR 41, 57). The population consisted of 60% Caucasian and 30% African American women. Cardiovascular risk factors were highly prevalent: 8% had diabetes, 27% had hypertension, 17% had hyperlipidemia, 43% had a history of tobacco use, and the majority were overweight, reflective of the population of patients seen in everyday clinical practice. Participants were most commonly treated with doxorubicin without trastuzumab (67%); the rest were treated with doxorubicin followed by trastuzumab (18%) or trastuzumab without doxorubicin (15%).
Table 1.
Characteristics of study participants at baseline
| All participants (n=135) | |
|---|---|
| Demographic characteristics | |
| Age, years | 48 (41, 57) |
| Race, n (%) | |
| Caucasian | 81 (60) |
| Black | 40 (30) |
| Other or unknown | 13 (10) |
| Cancer and related therapies | |
| Breast cancer side, n (%) | |
| Left | 65 (48) |
| Right | 60 (44) |
| Bilateral | 8 (6) |
| Breast Cancer Stage, n (%) | |
| Stage 1 | 25 (18) |
| Stage 2 | 71 (53) |
| Stage 3 | 35 (26) |
| Stage 4 | 4 (3) |
| Radiotherapy, n (%) | 90 (67) |
| Chemotherapy regimen, n (%) | |
| Doxorubicin | 91 (67) |
| Trastuzumab | 20 (15) |
| Doxorubicin + Trastuzumab | 24 (18) |
| Medical history and risk factors | |
| Diabetes, n (%) | 11 (8) |
| Hypertension, n (%) | 36 (27) |
| Hyperlipidemia, n (%) | 23 (17) |
| Tobacco use, n (%) | |
| Current | 8 (6) |
| Former | 50 (37) |
| Never | 77 (57) |
| Body mass index, kg/m2 | 26.6 (24.1, 31.0) |
| Systolic blood pressure, mmHg | 124 (116, 132) |
| Diastolic blood pressure, mmHg | 74 (68, 80) |
| Heart rate, beats/min | 77 (70, 89) |
| Cardiac medications | |
| ACE inhibitor or angiotensin receptor blocker, n (%) | 11 (8) |
| Beta blocker, n (%) | 10 (7) |
| HMG CoA reductase inhibitor, n (%) | 8 (6) |
| Echocardiogram measurements | |
| Longitudinal strain, % | −16.1 (−17.7, −14.0) |
| Circumferential strain, % | −26.5 (−29.2, −22.2) |
| Radial strain, % | 46.5 (34.1, 62.5) |
| Longitudinal strain rate, 1/s | −1.06 (−1.26, −0.86) |
| Circumferential strain rate, 1/s | −2.01 (−2.53, −1.55) |
| Radial strain rate, 1/s | 2.70 (2.05, 3.52) |
| Ventricular-arterial coupling (Ea/Eessb) | 1.02 (0.89, 1.22) |
| Left ventricular ejection fraction, % | 53.9 (50.9, 55.8) |
Baseline Echocardiographic Measures
At baseline, the median EF was 53.9%, mean EF 53.5%, and Ea/Eessb was 1.02 (Table 1). Median strain values were: −16.1% for longitudinal, −26.5% for circumferential, and 46.5% for radial. Median strain rate values were: −1.06 s−1 for longitudinal, −2.01 s−1 for circumferential, and 2.70 s−1 for radial. Compared to published literature from non-cancer cohorts largely derived from participants without cardiovascular risk factors, median values for longitudinal strain, EF, and Ea/Eessb were at the limits of normal, while circumferential and radial strain approximated published median values.(16, 24, 25)
Cancer Therapeutics-Related Cardiac Dysfunction (CTRCD) Outcomes
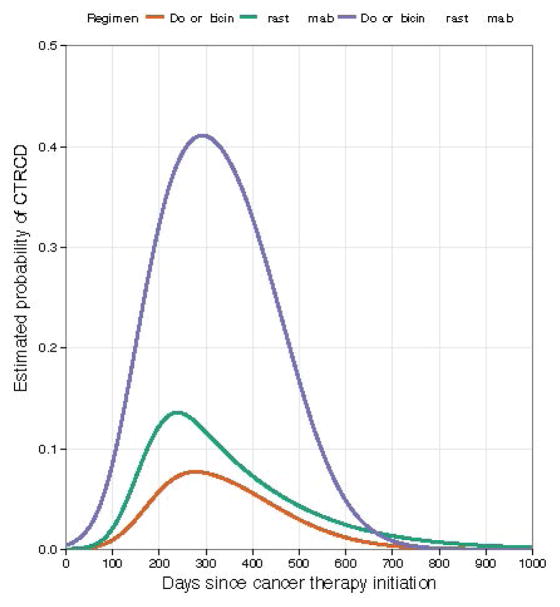
During the study period, 21 participants (15%) contributed 35 CTRCD events to the analyses, as defined by a reduction in EF by ≥10% from baseline to <50%. The predicted probabilities and timing of CTRCD according to treatment regimen are shown in Figure 2. Of these 21 participants, 11 participants had symptoms, dose adjustments, and/or were started on cardiac medications. Specifically, 8 experienced dose interruptions or early termination of therapy and 7 initiated cardiac medications. One participant refused to start cardiac medications. The remaining 10 were asymptomatic with no dose interruptions, 4 of whom developed cardiac dysfunction after completion of cancer therapy. Of the 21 participants who developed CTRCD, 9 had multiple events representing persistence or recurrence and 7 did not experience recovery by the time of the last assessment. No participants died of cardiovascular causes, though 14 participants died of breast cancer. For these participants, analyses were censored at the time of death.
Figure 2. Probability of CTRCD According to Treatment Regimen.
The predicted probabilities of CTRCD for doxorubicin (orange), doxorubicin followed by trastuzumab (purple), and trastuzumab (green).
The Association between Myocardial Mechanics Measures and CTRCD
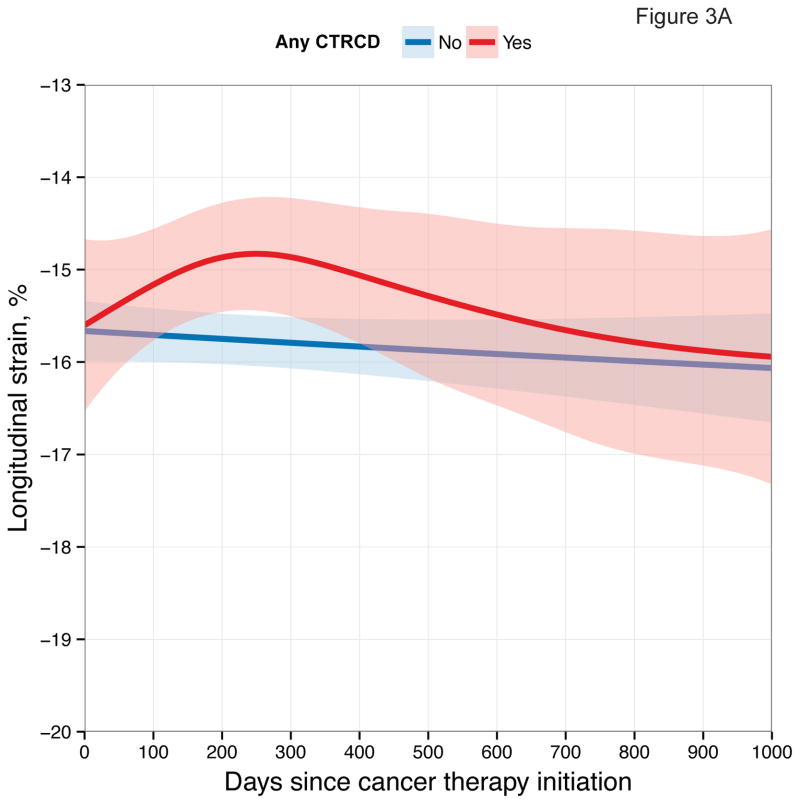
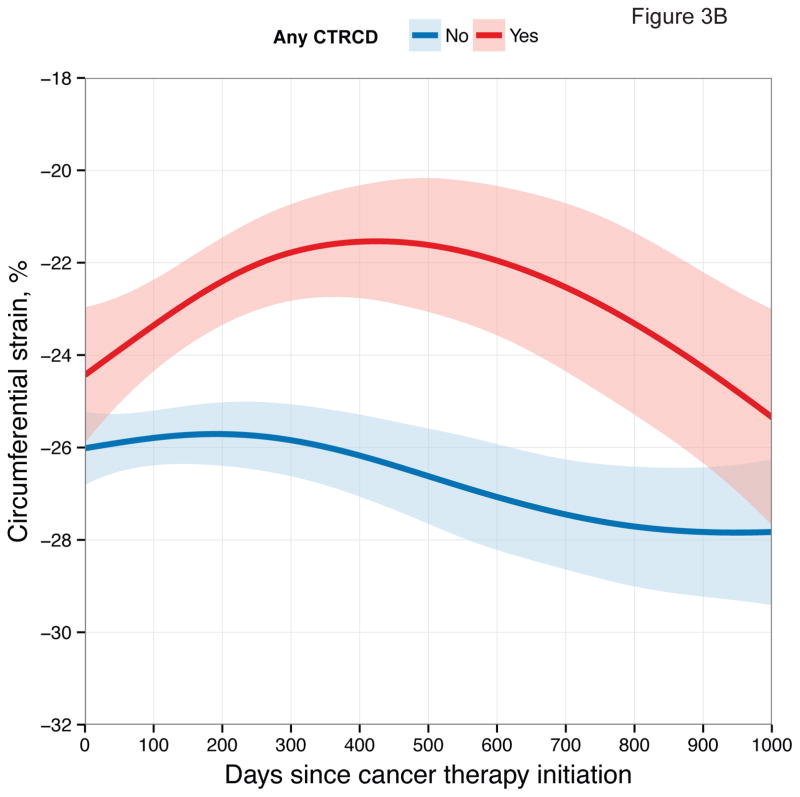
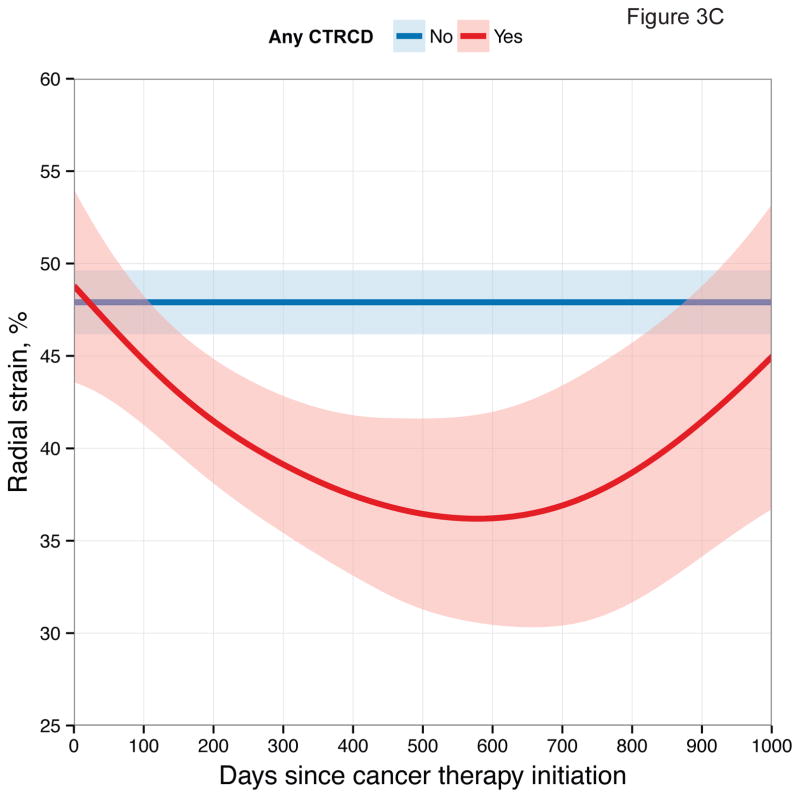
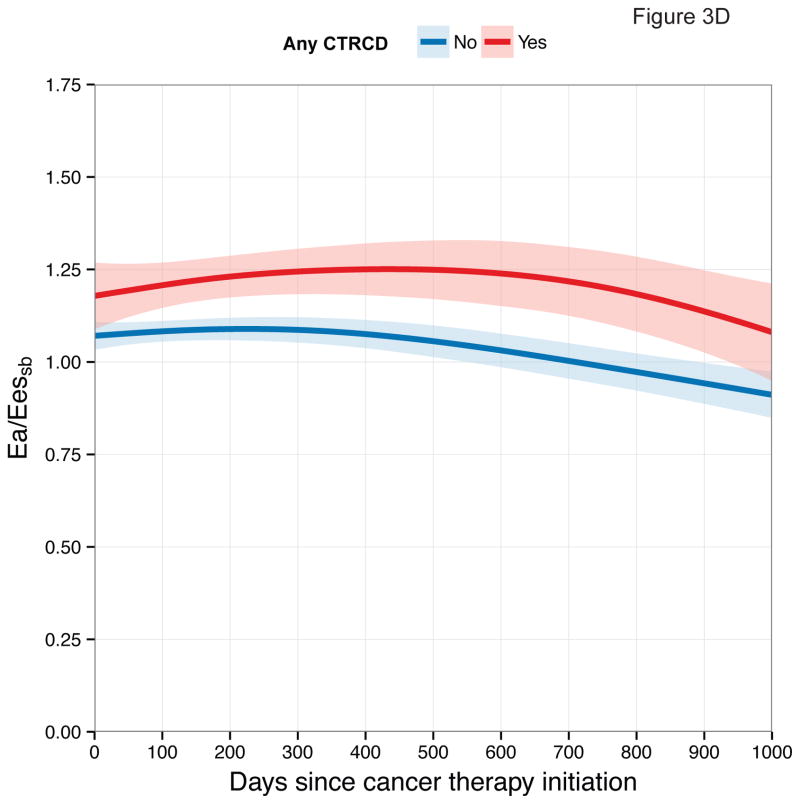
First, we examined longitudinal patterns in echocardiographic measures according to CTRCD. These graphical depictions demonstrate differences in each measure between participants who developed CTRCD at any time point and those who did not (Figure 3).
Figure 3. Longitudinal Patterns of Myocardial Mechanics According to CTRCD.
Smoothing splines with point-wise confidence bands for (A) longitudinal strain; (B) circumferential strain; (C) radial strain; (D) Ea/Eessb.
In our models, baseline measures of Ea/Eessb, circumferential strain, and circumferential strain rate were each strongly associated with CTRCD (Table 2). Here, a 0.1 unit difference in Ea/Eessb, a 1% difference in circumferential strain, and a 0.1 s−1 difference in circumferential strain rate were individually associated with a 21 to 38% increased odds of CTRCD. No other baseline mechanics measures were associated with CTRCD.
Table 2.
Associations of strain, strain rate, and ventricular-arterial coupling parameters with odds of Cancer Therapeutics-Related Cardiac Dysfunction (CTRCD)
| Model 1* | Model 2† | Model 3‡ | |||||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | ΔQIC§ | |
| Strain | |||||||
| Longitudinal strain, % | |||||||
| Baseline|| | 1.13 (0.88, 1.44) | 0.34 | 1.24 (0.89, 1.75) | 0.21 | 1.25 (0.89, 1.75) | 0.20 | −12.5 |
| Change¶ | 1.17 (1.00, 1.37) | 0.051 | 1.26 (1.04, 1.53) | 0.017 | 1.25 (1.01, 1.53) | 0.037 | |
| Circumferential strain, % | |||||||
| Baseline|| | 1.23 (1.10, 1.36) | <0.001 | 1.28 (1.14, 1.42) | <0.001 | 1.31 (1.16, 1.47) | <0.001 | −31.8 |
| Change¶ | 1.13 (1.05, 1.21) | 0.002 | 1.17 (1.06, 1.30) | 0.002 | 1.21 (1.10, 1.34) | <0.001 | |
| Radial strain, % | |||||||
| Baseline|| | 1.00 (0.97, 1.03) | 0.97 | 0.99 (0.95, 1.03) | 0.74 | 0.98 (0.95, 1.02) | 0.39 | −31.2 |
| Change¶ | 0.98 (0.96, 1.00) | 0.075 | 0.97 (0.94, 0.99) | 0.034 | 0.97 (0.94, 0.99) | 0.015 | |
| Strain rate | |||||||
| Longitudinal strain rate, 0.1/s | |||||||
| Baseline# | 0.99 (0.81, 1.22) | 0.93 | 0.99 (0.77, 1.29) | 0.95 | 0.97 (0.78, 1.22) | 0.81 | −12.6 |
| Change†† | 1.02 (0.86, 1.21) | 0.82 | 1.05 (0.86, 1.29) | 0.60 | 1.09 (0.91, 1.32) | 0.33 | |
| Circumferential strain rate, 0.1/s | |||||||
| Baseline# | 1.14 (1.04, 1.25) | 0.004 | 1.19 (1.08, 1.30) | <0.001 | 1.21 (1.09, 1.35) | <0.001 | −32.9 |
| Change†† | 1.12 (1.06, 1.18) | <0.001 | 1.15 (1.07, 1.23) | <0.001 | 1.17 (1.09, 1.26) | <0.001 | |
| Radial strain rate, 0.1/s | |||||||
| Baseline# | 1.00 (0.96, 1.05) | 0.99 | 0.99 (0.94, 1.04) | 0.65 | 0.98 (0.93, 1.04) | 0.56 | −32.0 |
| Change†† | 0.98 (0.95, 1.01) | 0.15 | 0.96 (0.93, 0.99) | 0.023 | 0.96 (0.93, 0.99) | 0.006 | |
| Ventricular-arterial coupling | |||||||
| Ea/Eessb | |||||||
| Baseline‡‡ | 1.40 (1.22, 1.60) | <0.001 | 1.39 (1.21, 1.59) | <0.001 | 1.38 (1.18, 1.60) | <0.001 | −15.0 |
| Change§§ | 1.26 (1.14, 1.40) | <0.001 | 1.26 (1.13, 1.40) | <0.001 | 1.23 (1.10, 1.37) | <0.001 | |
Adjusted for treatment regimen and time since initiation of therapy.
Adjusted for age, race, heart rate, treatment regimen, and time since initiation of therapy.
Adjusted for age, race, heart rate, history of diabetes, history of hypertension, history of hyperlipidemia, any cardiac medication use (angiotensin converting enzyme inhibitor, angiotensin receptor blocker, beta blocker), treatment regimen, and time since initiation of therapy.
QIC is the difference in QIC between Model 3 and Model 1 and compares the overall fit between models; negative numbers indicate that Model 3 provided a better fit.
Odds of CTRCD between two populations whose baseline value differs by 1%.
Odds of CTRCD between a population with a 1% increase from baseline and a population with no change.
Odds of CTRCD between two populations whose baseline value differs by 0.1/s.
Odds of CTRCD between a population with a 0.1/s increase from baseline and a population with no change.
Odds of CTRCD between two populations whose baseline value differs by 0.1.
Odds of CTRCD between a population with a 0.1 increase from baseline and a population with no change.
We also evaluated the relationships between changes in measures from baseline and CTRCD. Again, we found that changes in Ea/Eessb, circumferential strain, and circumferential strain rate were associated with CTRCD (Table 2). A 0.1 unit change in Ea/Eessb, a 1% change in circumferential strain, and a 0.1 s−1 change in circumferential strain rate were individually associated with a 17 to 23% increased odds of CTRCD. Furthermore, we determined that a worsening in longitudinal strain, radial strain, and radial strain rate were associated with CTRCD. A 1% change in longitudinal strain, a 1% change in radial strain, and a 0.1 s−1 change in radial strain rate were associated with a 3 to 25% increased odds of CTRCD. Overall, both baseline levels and changes in circumferential strain and Ea/Eessb were associated with CTRCD; only changes in longitudinal and radial strain were associated with CTRCD.
Post-hoc logistic regression models of the individual parameters Ea and Eessb were generated, suggesting that the relationship observed between ventricular-arterial coupling and CTRCD was primarily driven by Ea (Supplementary Materials).
Myocardial Mechanics Measures as Predictors of CTRCD
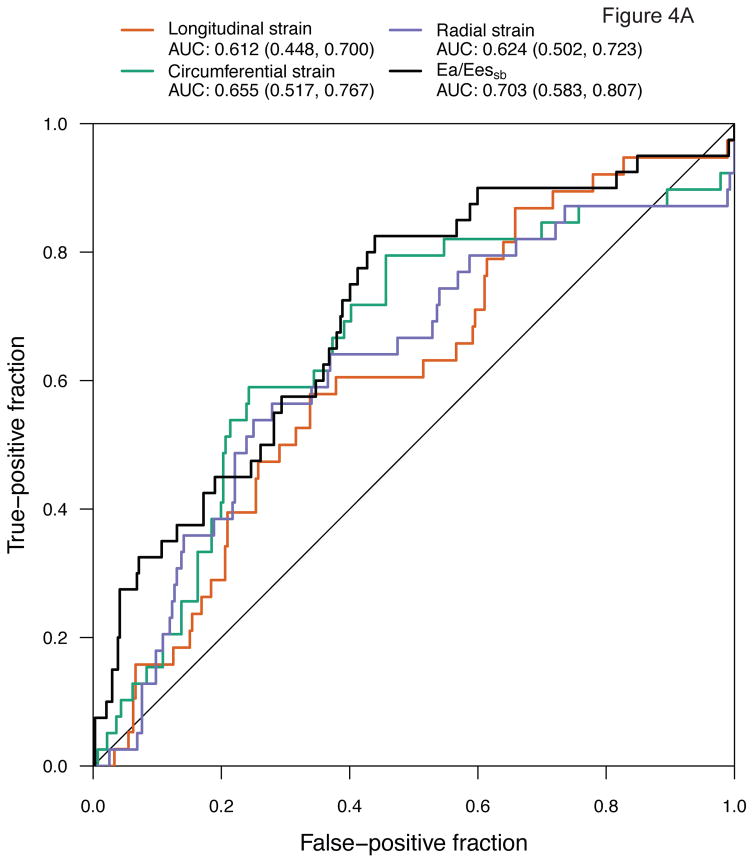
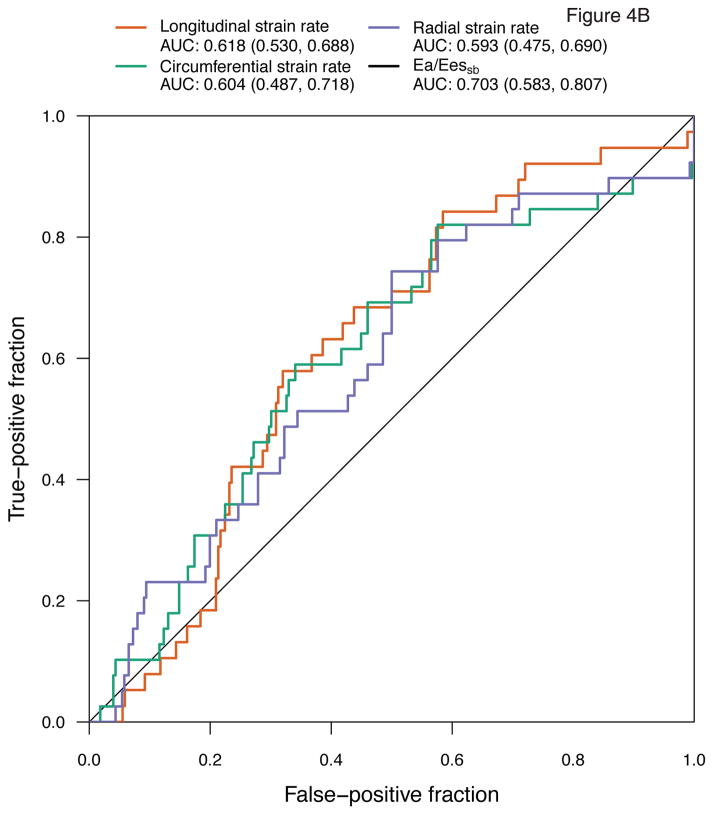
We then determined the role of mechanics measures as predictors of subsequent CTRCD using both baseline measures and changes from baseline, adjusted for treatment regimen and time. The median time to the subsequent visit was 166 days (IQR 89, 322), or 5.5 months. Ea/Eessb [AUC 0.703 (95% CI 0.583, 0.807)] and circumferential strain [AUC 0.655 (95% CI 0.517, 0.767)] had the strongest predictive ability (Figure 4). Radial strain [AUC 0.624 (95% CI 0.502, 0.723)] and longitudinal strain rate [AUC 0.618 (95% CI 0.530, 0.688)] also demonstrated modest predictive ability. However, longitudinal strain [AUC 0.612 (0.448, 0.700)], circumferential strain rate [AUC 0.604 (0.487, 0.718)], and radial strain rate [AUC 0.593 (95% CI 0.475, 0.690)] lacked statistical significance. These results largely paralleled the findings from our association models above, with Ea/Eessb and circumferential strain demonstrating the greatest relevance to CTRCD.
Figure 4. Receiver Operating Characteristic Curves of Myocardial Mechanics for Cancer CTRCD Prediction at the Subsequent Visit.
Analyses adjusted for treatment regimen and time demonstrate the area under the curve (AUC) for (A) strain and Ea/Eessb; (B) strain rate and Ea/Eessb.
Sensitivity Analyses
Approximately 20% of the images obtained from the apical 2-chamber view demonstrated some degree of foreshortening of the LV and/or obscured visualization of the apex. Thus, we restricted our primary analysis to longitudinal strain derived from the apical 4-chamber view alone. However, we performed additional analyses to ensure that quantitative assessment of longitudinal strain in the apical 4-chamber view alone did not impact our results. We observed a close correlation between apical 4-chamber and apical 2-chamber measurements of longitudinal strain and strain rate for those images that could be adequately analyzed (R~0.74, p<0.001). We also performed sensitivity analysis using the average of the 2- and 4-chamber longitudinal strain in those echocardiograms with adequate apical 2-chamber images. Using these averaged measures, our findings were similar with changes being associated, but not predictive of CTRCD (Supplementary Materials).
We also examined the impact of the alternative definition of CTRCD recently established by the ASE and European Association of Cardiovascular Imaging (EACVI), as an EF decline >10% to <53%.(26) Utilizing this definition of CTRCD, there were 5 additional events. Again, our findings were unchanged with Ea/Eessb and circumferential strain being most strongly predictive (Supplementary Materials).
Discussion
In this prospective, longitudinal cohort study of 135 women and analyses of 517 echocardiograms, we determined that Ea/Eessb and circumferential strain were mst strongly associated with and predictive of CTRCD. Overall, our data provide several new insights into echocardiography-derived measures of myocardial mechanics and CTRCD. First, these measures can be used to predict CTRCD across a broad range of time and treatment regimens. Second, ventricular-arterial coupling is a promising new measure to predict CTRCD. Third, circumferential strain may have more relevance to the cardio-oncology population than previously reported. Finally, noninvasive measures of myocardial mechanics may help to identify high-risk patients both before and during therapy.
Our results point towards the importance of ventricular-arterial coupling, a measure that has not been previously studied in cardio-oncology. These findings complement observations made in chronic HF, which have shown that noninvasive measures of ventricular-arterial coupling are predictive of outcomes.(8) Interestingly, our study demonstrates that increased arterial elastance and alterations in ventricular-vascular coupling occur with and in advance of EF deterioration. This association appears to be driven primarily by Ea. Further research in additional cohorts and clinical trials is needed to determine the relative importance of these measures in the prediction of CTRCD, and whether the coupling ratio provides incremental predictive utility in comparison to Ea alone, or in comparison to strain-based measures.
Our study is also the first to highlight the predictive value of circumferential strain in cardio-oncology. While deterioration of circumferential strain has been demonstrated in cancer patients treated with cardiotoxic regimens (7, 10), these prior studies have not identified predictive utility of this measure. Interestingly, studies in non-cancer populations have suggested that abnormalities in circumferential strain reflect an inherent vulnerability to cardiac dysfunction. In the Framingham Offspring Study, reduced circumferential strain was strongly associated with parental HF, suggesting that this measure at baseline may be an indicator of inherited HF susceptibility.(12) Additionally, our finding that circumferential strain is associated with and predictive of cardiotoxicity is consistent with findings in other HF populations that demonstrate significant associations between circumferential strain and clinical outcomes.(13, 27, 28)
Prior studies have emphasized the importance of changes in longitudinal strain in CTRCD.(6, 7) Although we similarly identified robust cross-sectional associations between changes in longitudinal strain and CTRCD supporting its relevance, longitudinal strain was not significantly predictive. In order to explore the possible reasons for these findings, we performed multiple sensitivity analyses. First, we determined the impact of evaluating longitudinal strain in the apical 4-chamber view alone. We found a strong correlation of 0.74 between longitudinal strain derived from the apical 4- and 2-chamber views, similar to the correlation reported in the literature of 0.92 between longitudinal strain derived from the 4-chamber view and averaged across all three apical views.(29) An additional sensitivity analysis within our own study evaluating longitudinal strain averaged across the apical 2- and 4-chamber views did not alter our findings. Second, we evaluated alternative definitions of CTRCD, such as a decrease in LVEF of ≤10% to <53%. This had no effect on our findings. Third, we explored the influence of our strain analysis platform on our results. We found a strong correlation between longitudinal strain measures derived from 2D Cardiac Performance Analysis and EchoPAC PC (GE Healthcare, Milwaukee, WI) in our laboratory (n=10, r=0.806, p=0.008). Of note, the ASE/EACVI does not endorse one analyses program over another (16, 19, 26) and our analysis program has been used in many prior studies.(12, 30, 31)
Overall, these analyses suggest that there was limited potential for bias introduced by analysis package and the use of the 4-chamber view alone, though this possibility cannot be entirely excluded. We postulate that the differences in our findings compared to others are primarily related to our participant characteristics with a high prevalence of cardiovascular risk factors; statistical approach of predicting CTRCD at the subsequent visit over a range of times rather than a fixed time interval; a longer duration of follow-up time; and the possible contribution of limited sample size. It is plausible that the predictive utility of longitudinal strain varies according to the time from cancer therapy exposure, and additional research is needed to determine the validity of this hypothesis.
We acknowledge the potential limitations of our study. First, it is possible that our sample size limited our ability to detect a significant association with one or more echocardiographic measures and cardiac dysfunction. Second, quantitation in our experienced core laboratory is highly reproducible; however, there is the potential for systematic differences between different core laboratories. Given the repeated measures and longitudinal assessment, these potential systematic differences would be unlikely to affect the significance of our analyses, but they could impact the interpretation of specific values and cutpoints. Similarly, these cutpoints could also differ according to strain software package. Third, further research with larger samples sizes and randomized clinical trials is of necessity in order to determine the external validity of our findings and to establish robust cutpoints that can be used in clinical practice.(32) However, our study does provide an important proof-of-concept that supports the relevance of baseline measures and changes in measures in predicting subsequent cardiac dysfunction over a broad range of follow-up time. Finally, the leave-one-out cross-validation ensured the internal validity of our prediction models and studying a non-clinical trial population with minimal exclusion criteria and high prevalence of cardiovascular risk factors enhanced external validity. However, studies in other populations should be performed to further enhance the generalizability of our results.
In conclusion, in this prospective longitudinal cohort study of women treated with cardiotoxic breast cancer therapy, we comprehensively defined noninvasive measures of mechanics. We determined that ventricular-arterial coupling and circumferential strain were most strongly predictive of CTRCD over an extensive follow-up time. Overall, our findings suggest a noninvasive strategy to identify high-risk patients prior to, during, and after cardiotoxic cancer therapy.
Supplementary Material
Scatterplots of ejection fraction residuals against strain residuals for each strain parameter. Residuals were obtained from linear regression models that adjusted for treatment, time since initiation of therapy, and their interaction. Lines depict smoothing splines stratified by treatment regimen: doxorubicin (orange); trastuzumab (green); and doxorubicin followed by trastuzumab (purple); and all participants across all treatment regimens (black). These plots are demonstrated for (A) longitudinal strain; (B) circumferential strain; and (C) radial strain.
CTRCD: Cancer Therapeutics-Related Cardiac Dysfunction
Supplementary Table 1: Associations of Ea and Eessb and Cancer Therapeutics-Related Cardiac Dysfunction (CTRCD)*
Supplementary Table 2A: Associations of Averaged Apical 2 Chamber and 4 Chamber Longitudinal Strain and Strain Rate with Cancer Therapeutics-Related Cardiac Dysfunction (CTRCD)*
Supplementary Table 2B: Area under the ROC Curve for Averaged Apical 2 Chamber and 4 Chamber Longitudinal Strain and Strain Rate with Cancer Therapeutics-Related Cardiac Dysfunction
Supplementary Table 3: Area under the ROC Curve for Prediction of Cancer Therapeutics-Related Cardiac Dysfunction Defined as EF Decline >10% to <53%
Perspectives.
Competency in medical knowledge
Echocardiographic measures of myocardial mechanics are associated with and predictive of cardiac dysfunction in women receiving breast cancer therapy.
Competency in patient care and procedural skills
Cardiac dysfunction is a relatively common side effect of chemotherapy. Echocardiographic measures of myocardial mechanics may play an important role in identifying those patients at highest risk.
Translational outlook
Future research should focus on understanding the mechanisms of anthracycline and trastuzumab-mediated cardiac dysfunction, arterial stiffening, and ventricular-vascular uncoupling in humans. Larger clinical studies will be required to determine the utility of myocardial mechanics for risk prediction in clinical practice.
Acknowledgments
Funding Sources: This work was supported by NHLBI R01-HL118018 (Ky), McCabe Fellow Award (Philadelphia, PA, Ky), American Cancer Society Institutional Research Grant -78-002-30 (Atlanta, Georgia, Ky), NHLBI K23-HL095661 (Ky). Dr. Khan was supported by NHLBI T32-HL007891 and Dr. Narayan by NICHD T32-HD060550.
Abbreviations
- LV
left ventricular
- CTRCD
cancer therapeutics-related cardiac dysfunction
- HF
heart failure EF, ejection fraction
- Ea/Eessb
ventricular arterial coupling
- Ea
effective arterial elastance
- Eessb
end-systolic elastance
- EDV
end-diastolic volume
- ESV
end-systolic volume
- SV
stroke volume
Footnotes
Disclosures: There are no relationships with industry to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Hari K. Narayan, Email: narayanh@email.chop.edu.
Benjamin French, Email: bcfrench@mail.med.upenn.edu.
Abigail M. Khan, Email: abbymaykhan@gmail.com.
Theodore Plappert, Email: theodore.plappert@uphs.upenn.edu.
David Hyman, Email: david.hyman@uphs.upenn.edu.
Akinyemi Bajulaiye, Email: akinyemi.bajulaiye@uphs.upenn.edu.
Susan Domchek, Email: susan.domchek@uphs.upenn.edu.
Angela DeMichele, Email: angela.demichele@uphs.upenn.edu.
Amy Clark, Email: amy.clark@uphs.upenn.edu.
Jennifer Matro, Email: Jennifer.matro@uphs.upenn.edu.
Angela Bradbury, Email: angela.bradbury@uphs.upenn.edu.
Kevin Fox, Email: kevin.fox@uphs.upenn.edu.
Joseph R. Carver, Email: jrc2@mail.med.upenn.edu.
References
- 1.Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: A retrospective analysis of three trials. Cancer. 2003;97:2869–79. doi: 10.1002/cncr.11407. [DOI] [PubMed] [Google Scholar]
- 2.Felker GM, Thompson RE, Hare JM, et al. Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. N Engl J Med. 2000;342:1077–84. doi: 10.1056/NEJM200004133421502. [DOI] [PubMed] [Google Scholar]
- 3.Seidman A, Hudis C, Pierri MK, et al. Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol. 2002;20:1215–21. doi: 10.1200/JCO.2002.20.5.1215. [DOI] [PubMed] [Google Scholar]
- 4.Shelburne N, Adhikari B, Brell J, et al. Cancer treatment-related cardiotoxicity: current state of knowledge and future research priorities. J Natl Cancer Inst. 2014;106 doi: 10.1093/jnci/dju232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Altena R, Perik PJ, Van Veldhuisen DJ, et al. Cardiovascular toxicity caused by cancer treatment: strategies for early detection. Lancet Oncol. 2009;10:391–9. doi: 10.1016/S1470-2045(09)70042-7. [DOI] [PubMed] [Google Scholar]
- 6.Negishi K, Negishi T, Hare JL, et al. Independent and Incremental Value of Deformation Indices for Prediction of Trastuzumab-Induced Cardiotoxicity. J Am Soc Echocardiogr. 2013;26:493–8. doi: 10.1016/j.echo.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 7.Sawaya H, Sebag IA, Plana JC, et al. Assessment of Echocardiography and Biomarkers for the Extended Prediction of Cardiotoxicity in Patients Treated With Anthracyclines, Taxanes, and Trastuzumab. Circ Cardiovasc Imaging. 2012;5:596–603. doi: 10.1161/CIRCIMAGING.112.973321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ky B, French B, May Khan A, et al. Ventricular-Arterial Coupling, Remodeling, and Prognosis in Chronic Heart Failure. J Am Coll Cardiol. 2013;62:1165–72. doi: 10.1016/j.jacc.2013.03.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hedhli N, Kalinowski A, Russell SK. Cardiovascular Effects of Neuregulin-1/ErbB Signaling: Role in Vascular Signaling and Angiogenesis. Curr Pharm Des. 2014;20:4899–905. doi: 10.2174/1381612819666131125151058. [DOI] [PubMed] [Google Scholar]
- 10.Drafts BC, Twomley KM, D’Agostino R, et al. Low to Moderate Dose Anthracycline-Based Chemotherapy Is Associated With Early Noninvasive Imaging Evidence of Subclinical Cardiovascular Disease. JACC Cardiovasc Imaging. 2013;6:877–85. doi: 10.1016/j.jcmg.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Theriault RL, Carlson RW, Allred C, et al. Breast cancer, version 3. 2013. J Natl Compr Canc Netw. 2013;11:753–61. doi: 10.6004/jnccn.2013.0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng S, McCabe EL, Larson MG, et al. Left ventricular mechanical function: clinical correlates, heritability, and association with parental heart failure: Heritability of left ventricular strain. Eur J Heart Fail. 2015;17:44–50. doi: 10.1002/ejhf.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang KW, French B, Khan AM, et al. Strain improves risk prediction beyond ejection fraction in chronic systolic heart failure. J Am Heart Assoc. 2014;3:e000550. doi: 10.1161/JAHA.113.000550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagata Y, Takeuchi M, Mizukoshi K, et al. Intervendor Variability of Two-Dimensional Strain Using Vendor-Specific and Vendor-Independent Software. J Am Soc Echocardiogr. 2015;28:630–41. doi: 10.1016/j.echo.2015.01.021. [DOI] [PubMed] [Google Scholar]
- 15.St John Sutton M, Plappert T. Core lab, no core lab or automated LVEF? Eur J Echocardiogr. 2007;8:239–40. doi: 10.1016/j.euje.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 16.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39. e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Rüscho J, Suto T, Greatorex V, Ward C, Straehle C, McFadden E, Dolci MS, Gelber RD, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–72. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 18.Cardinale D, Colombo A, Sandri MT, et al. Prevention of High-Dose Chemotherapy-Induced Cardiotoxicity in High-Risk Patients by Angiotensin-Converting Enzyme Inhibition. Circulation. 2006;114:2474–81. doi: 10.1161/CIRCULATIONAHA.106.635144. [DOI] [PubMed] [Google Scholar]
- 19.Voigt J-U, Pedrizzetti G, Lysyansky P, et al. Definitions for a Common Standard for 2D Speckle Tracking Echocardiography: Consensus Document of the EACVI/ASE/Industry Task Force to Standardize Deformation Imaging. J Am Soc Echocardiogr. 2015;28:183–93. doi: 10.1016/j.echo.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 20.Chen C-H, Fetics B, Nevo E, et al. Noninvasive single-beat determination of left ventricular end-systolic elastance in humans. J Am Coll Cardiol. 2001;38:2028–34. doi: 10.1016/s0735-1097(01)01651-5. [DOI] [PubMed] [Google Scholar]
- 21.Liang K-Y, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 22.Pan W. Akaike’s information criterion in generalized estimating equations. Biometrics. 2001;57:120–5. doi: 10.1111/j.0006-341x.2001.00120.x. [DOI] [PubMed] [Google Scholar]
- 23.French B, Saha-Chaudhuri P, Ky B, et al. Development and evaluation of multi-marker risk scores for clinical prognosis. Stat Methods Med Res. 2012 doi: 10.1177/0962280212451881. Internet. Available from: http://smm.sagepub.com/content/early/2012/07/04/0962280212451881.abstract. [DOI] [PMC free article] [PubMed]
- 24.Cheng S, Larson MG, McCabe EL, et al. Age-and sex-based reference limits and clinical correlates of myocardial strain and synchrony: the Framingham Heart Study. Circ Cardiovasc Imaging. 2013;6:692–9. doi: 10.1161/CIRCIMAGING.112.000627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Borlaug BA, Redfield MM, Melenovsky V, et al. Longitudinal Changes in Left Ventricular Stiffness: A Community-Based Study. Circ Heart Fail. 2013;6:944–52. doi: 10.1161/CIRCHEARTFAILURE.113.000383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Plana JC, Galderisi M, Barac A, et al. Expert Consensus for Multimodality Imaging Evaluation of Adult Patients during and after Cancer Therapy: A Report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2014;27:911–39. doi: 10.1016/j.echo.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 27.Hung C-L, Verma A, Uno H, et al. Longitudinal and Circumferential Strain Rate, Left Ventricular Remodeling, and Prognosis After Myocardial Infarction. J Am Coll Cardiol. 2010;56:1812–22. doi: 10.1016/j.jacc.2010.06.044. [DOI] [PubMed] [Google Scholar]
- 28.Cho G-Y, Marwick TH, Kim H-S, et al. Global 2-Dimensional Strain as a New Prognosticator in Patients With Heart Failure. J Am Coll Cardiol. 2009;54:618–24. doi: 10.1016/j.jacc.2009.04.061. [DOI] [PubMed] [Google Scholar]
- 29.Farsalinos KE, Daraban AM, Ünlü S, et al. Head-to-Head Comparison of Global Longitudinal Strain Measurements among Nine Different Vendors: The EACVI/ASE Inter-Vendor Comparison Study. J Am Soc Echocardiogr Off Publ Am Soc Echocardiogr. 2015;28:1171–81. e2. doi: 10.1016/j.echo.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 30.Kutyifa V, Kloppe A, Zareba W, et al. The Influence of Left Ventricular Ejection Fraction on the Effectiveness of Cardiac Resynchronization Therapy. J Am Coll Cardiol. 2013;61:936–44. doi: 10.1016/j.jacc.2012.11.051. [DOI] [PubMed] [Google Scholar]
- 31.Kraigher-Krainer E, Shah AM, Gupta DK, et al. Impaired Systolic Function by Strain Imaging in Heart Failure With Preserved Ejection Fraction. J Am Coll Cardiol. 2014;63:447–56. doi: 10.1016/j.jacc.2013.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Royston P, Altman DG, Sauerbrei W. Dichotomizing continuous predictors in multiple regression: a bad idea. Stat Med. 2006;25:127–41. doi: 10.1002/sim.2331. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Scatterplots of ejection fraction residuals against strain residuals for each strain parameter. Residuals were obtained from linear regression models that adjusted for treatment, time since initiation of therapy, and their interaction. Lines depict smoothing splines stratified by treatment regimen: doxorubicin (orange); trastuzumab (green); and doxorubicin followed by trastuzumab (purple); and all participants across all treatment regimens (black). These plots are demonstrated for (A) longitudinal strain; (B) circumferential strain; and (C) radial strain.
CTRCD: Cancer Therapeutics-Related Cardiac Dysfunction
Supplementary Table 1: Associations of Ea and Eessb and Cancer Therapeutics-Related Cardiac Dysfunction (CTRCD)*
Supplementary Table 2A: Associations of Averaged Apical 2 Chamber and 4 Chamber Longitudinal Strain and Strain Rate with Cancer Therapeutics-Related Cardiac Dysfunction (CTRCD)*
Supplementary Table 2B: Area under the ROC Curve for Averaged Apical 2 Chamber and 4 Chamber Longitudinal Strain and Strain Rate with Cancer Therapeutics-Related Cardiac Dysfunction
Supplementary Table 3: Area under the ROC Curve for Prediction of Cancer Therapeutics-Related Cardiac Dysfunction Defined as EF Decline >10% to <53%