Abstract
Maltreated youths in foster care often experience negative developmental and psychological outcomes, which have been linked with poor response inhibition. Recent evidence suggests that childhood maltreatment is also associated with alterations in the neural circuitry underlying response inhibition. However, a burgeoning line of research has begun to explore the mitigating effects of preventive interventions on neural functioning. The current study used event-related functional magnetic resonance imaging to explore the impact of early childhood maltreatment and a preventive intervention on response inhibition in early adolescence. Thirty-six demographically similar adolescents (ages 9–14 years) completed a Go/NoGo task. The sample included nonmaltreated adolescents (n = 14) and maltreated adolescents who were in foster care as preschoolers and randomly assigned to receive services as usual (n = 11) or a preventive intervention, Multidimensional Treatment Foster Care for Preschoolers (n = 11). The groups demonstrated similar behavioral performance but significantly different neural patterns. The maltreated adolescents who received services as usual demonstrated subcortical hypoactivity during successful response inhibition and subcortical hyperactivity during unsuccessful response inhibition. In contrast, the nonmaltreated adolescents and maltreated adolescents who received the intervention exhibited strikingly similar neural patterns during successful response inhibition, but the maltreated adolescents who received the intervention demonstrated prefrontal hypoactivity during unsuccessful response inhibition. These findings offer preliminary evidence that early childhood maltreatment alters the neural patterns underlying response inhibition in early adolescence and that participating in a preventive intervention could mitigate maltreatment-related effects on these neural systems.
Childhood maltreatment describes caregiver behaviors that result in harm, threat to harm, or potential to harm a child, including neglect and physical, emotional, and sexual abuse (Leeb, Paulozzi, Melanson, Simon, & Arias, 2008). In the United States, approximately 400,000 children reside in foster care due to maltreatment, and 250,000 children enter foster care annually (U.S. Department of Health and Human Services, 2011). Childhood maltreatment is associated with severe, long-term consequences on developmental outcomes such as academic achievement (Hart & Rubia, 2012) and is a chief antecedent for major mental health problems (Green et al., 2010). A valuable step in understanding the effects of childhood maltreatment on such outcomes is to examine its impact on the neural bases of related cognitive abilities. We investigated the neural correlates of response inhibition in adolescents who experienced early childhood maltreatment compared to demographically similar, nonmaltreated adolescents and examined the effects of a preventive intervention for maltreated children in foster care on these neural patterns.
A common consequence of childhood maltreatment is increased attention and externalizing problems. In one study, nearly 40% of the maltreated youths met diagnostic criteria for attention deficit hyperactivity disorder (ADHD), oppositional defiant disorder, and/or conduct disorder (Garland et al., 2001). Recent developmental traumatology theories posit that childhood maltreatment disrupts structural and functional neural development, consequently elevating the risk for cognitive impairments (Hart & Rubia, 2012) and psychopathology (De Bellis, 2002). Brain regions characterized by high glucocorticoid-receptor densities and protracted neural development (e.g., prefrontal cortex) are considered to be at the greatest risk (Pechtel & Pizzagalli, 2011). Consistent with these theories, childhood maltreatment is associated with alterations within lateral and ventromedial fronto-cortical, fronto-striatal, and fronto-limbic networks (Hart & Rubia, 2012), which have been linked to attention and externalizing problems (Norman et al., 2011; Woltering, Granic, Lamm, & Lewis, 2011). There is also evidence that childhood maltreatment impairs cognitive abilities associated with prefrontal functioning (e.g., sustained attention and response inhibition; Beers & De Bellis, 2002) and alters electrophysiological activity believed to reflect attention and error-monitoring abilities (Bruce, McDermott, Fisher, & Fox, 2009; McDermott, Westerlung, Zeanah, Nelson, & Fox, 2012).
One of the most commonly used paradigms for assessing response inhibition is the Go/NoGo (GNG) task, which requires participants to respond to frequent Go stimuli and inhibit prepotent responses to infrequent NoGo stimuli. Adults recruit a right-lateralized prefrontal, striatal, and parietal network, including the inferior frontal gyrus, medial frontal gyrus/anterior cingulate cortex, middle/superior frontal gyri, insula, caudate, putamen, thalamus, inferior parietal lobule, posterior cingulate cortex/precuneus, and occipital regions (Swick, Ashley, & Turken, 2011; Wager et al., 2005), while youths recruit a right-lateralized prefrontal, parietal, and temporal network, including the inferior frontal gyrus, medial frontal gyrus/anterior cingulate cortex, middle/superior frontal gyri, precentral gyrus, primary motor cortex, posterior cingulate cortex, and middle/superior temporal gyri (Bunge, Dudukovic, Thomason, Vaidya, & Gabrielli, 2002; Durston et al., 2002; Durston, Mulder, Casey, Ziermans, & van Engeland, 2006; Tamm, Menon, & Reiss, 2002). Developmental research findings suggest that response inhibition and its underlying neural circuitry mature throughout childhood, with children demonstrating adult-like behavioral performance by approximately age 12 years (Levin et al., 1991). Furthermore, there is a developmental shift in neural recruitment, with increased recruitment of task-specific regions (e.g., inferior frontal gyrus, anterior cingulate cortex, insula, and striatum) and reduced recruitment of task-general regions (e.g., dorsolateral prefrontal cortex and posterior parietal cortex) from childhood to adulthood (Bunge et al., 2002; Durston et al., 2002, 2006; Rubia, Smith, Taylor, & Brammer, 2007; Rubia et al., 2006; Tamm et al., 2002).
To date, only a few studies have investigated response inhibition in maltreated youths. Adolescents with histories of interpersonal trauma and posttraumatic stress symptoms (ages 10–16 years, Mage = 13.7 years) demonstrated similar behavioral performance but distinct neural patterns during a GNG task compared to nonmaltreated adolescents (Carrion, Garrett, Menon, Weems, & Reiss, 2008). Specifically, maltreated adolescents displayed middle frontal hypoactivation and medial frontal, inferior temporal, and occipital hyperactivation. Similarly, Bruce et al. (2013) found no group differences in task accuracy but significant group differences in neural patterns during a GNG task. Maltreated children (ages 9–12 years, Mage = 10.9 years) demonstrated medial frontal, middle frontal, and occipital hypoactivation during successful response inhibition and inferior parietal, precuneus, and occipital hyperactivation during unsuccessful response inhibition. In a related study, Mueller et al. (2010) used a Change task to study response inhibition and switching abilities in adolescents who experienced adverse care (Mage = 13.2 years). During successful switching, maltreated adolescents demonstrated comparable accuracy but hyperactivation of a left-lateralized frontal and striatal network, including the inferior frontal gyrus, anterior cingulate cortex, precentral and postcentral gyri, insula, caudate, and putamen. During unsuccessful switching, maltreated adolescents displayed hypoactivation in the inferior frontal gyrus, precentral gyrus, and insula. Generalizations across these studies should be made with caution given their somewhat inconsistent results, but these findings offer preliminary evidence for atypical recruitment of lateral and medial prefrontal, subcortical, and posterior (i.e., inferior temporal, inferior and medial parietal, and occipital) regions by maltreated youths during response inhibition.
Few studies have investigated the impact of preventive interventions on neural functioning in maltreated youths; however, results from electrophysiological studies are promising. For example, McDermott, Westerlund, Zeanah, Nelson, and Fox (2012) examined the impact of severe deprivation on electrophysiological activity during a GNG task. During NoGo trials, postinstitutionalized children who received a foster care intervention demonstrated similar frontal recruitment and error-related negativity (ERN) amplitudes to children who had never been institutionalized, whereas postinstitutionalized children who did not receive the intervention displayed frontal hypoactivation and diminished P300 and ERN amplitudes. Furthermore, a preventive intervention designed for maltreated children in foster care, Multidimensional Treatment Foster Care for Preschoolers (MTFC-P), has been associated with positive outcomes, including increased secure attachment-related behaviors, more normative stress response system functioning, and (most germane to the current study) more typical error monitoring, as indexed by a more pronounced feedback-related negativity (Bruce et al., 2009; Fisher & Kim, 2007; Fisher, Stoolmiller, Gunnar, & Burraston, 2007). Taken together, these findings suggest that preventive interventions could ameliorate the negative impact of childhood maltreatment on response inhibition abilities on the neural level. Notably, no previous study has used functional magnetic resonance imaging (fMRI) to investigate the long-term impact of an early preventive intervention for maltreated youths, making the current study highly novel.
The current study used event-related fMRI during a GNG task to examine the impact of early childhood maltreatment and a preventive intervention on behavioral and neural patterns of response inhibition in early adolescence. Participants represented three groups: nonmaltreated adolescents who were raised by their biological parents and were demographically similar to the maltreated adolescents (community comparison; CC), maltreated adolescents who were in foster care as preschoolers and were randomly assigned to receive services as usual (regular foster care; RFC), and maltreated adolescents who were in foster care as preschoolers and were randomly assigned to receive the intervention (MTFC-P).
On the behavioral level, we did not predict group differences in Go trial accuracy or mean reaction time but did predict group differences in NoGo trial accuracy: CC adolescents would demonstrate the greatest accuracy, MTFC-P adolescents would demonstrate intermediate accuracy, and RFC adolescents would demonstrate the worst accuracy. On the neural level, we predicted that maltreated (RFC and MTFC-P) adolescents would exhibit atypical recruitment of prefrontal, subcortical, and posterior regions during successful and unsuccessful response inhibition compared to CC adolescents but that MTFC-P adolescents, relative to RFC adolescents, would recruit neural patterns that more closely resembled those of CC adolescents. For example, during successful response inhibition, we predicted that RFC and MTFC-P adolescents would demonstrate hypoactivation of task-specific regions (ventral prefrontal cortex [e.g., anterior cingulate cortex and inferior frontal gyrus] and striatal regions [e.g., caudate and putamen]) and hyperactivation of task-general regions (dorsolateral prefrontal cortex [e.g., middle/superior frontal gyri] and posterior regions [e.g., temporal, parietal, and occipital cortices]) but that RFC adolescents would demonstrate more atypical neural patterns than MTFC-P adolescents.
Method
Participants
Thirty-nine adolescents (ages 9–14 years) and their parents were recruited from a larger, longitudinal randomized controlled trial of MTFC-P. Three adolescents were excluded from the current analyses due to major visually detected artefacts in the imaging data on both runs (i.e., 10 or more volumes; n = 1) or poor task performance on both runs (i.e., failed to respond to at least 30% of Go trials; n = 2). Thus, 36 adolescents were included in the final analyses: 14 CC adolescents, 11 RFC adolescents, and 11 MTFC-P adolescents. The RFC and MTFC-P adolescents were originally referred by the local child welfare system office at ages 3–6 years and were entering new nonrelative foster care placements (i.e., entering foster care for the first time, reentering foster care following failed reunification attempts, or transitioning to new foster care placements). The CC adolescents were recruited via flyers and newspaper advertisements at ages 3–6 years, had lived consistently with at least one biological parent, and had no verified involvement with child welfare services. To ensure that group differences were not attributable to socioeconomic status, the CC families were required to have an annual household income of less than $30,000 and a parental education of less than a 4-year college degree. Additional exclusionary criteria for the current study included MRI contraindications, history of head injury or epilepsy, and current psychotropic medication usage (stimulants were withheld 24 hours prior to scanning).
The adolescents were primarily Caucasian (CC = 86%, RFC = 91%, MTFC-P = 91%). As is shown in Table 1, the groups did not differ on age at assessment, F(2, 33) = 0.29, ns, sex, χ2(2, n = 36) = 0.47, ns, race, χ2(2, n = 36) = 0.24, ns, or pubertal status, F(2, 33) = 0.08, ns (Petersen, Crocket, Richards, & Boxer, 1988). Furthermore, the groups did not differ on general cognitive ability, as measured by the Block Design subscale, F(2, 33) = 0.17, ns, and Vocabulary subscale, F(2, 33) = 0.31, ns, of the Wechsler Intelligence Scale for Children, 4th edition (WISC-IV; Weschler, 2003).
Table 1.
Demographic Information and Behavioral Performance
| CC | RFC | MTFC-P | |
|---|---|---|---|
| Sex (male:female) | 7:7 | 5:6 | 4:8 |
| Age | 11.81 (1.33) | 12.02 (1.21) | 12.22 (1.44) |
| Pubertal status | 2.19 (0.56) | 2.09 (0.70) | 2.20 (0.83) |
| Block Design | 10.21 (1.42) | 10.00 (2.68) | 10.55 (2.51) |
| Vocabulary | 10.07 (2.34) | 10.45 (1.92) | 10.82 (2.75) |
| Go trial accuracy | 95.24 (43.26) | 93.18 (7.07) | 90.91 (6.77) |
| NoGo trial accuracy | 54.76 (29.08) | 56.82 (22.68) | 48.99 (12.44) |
| Reaction time | 527.74 (95.15) | 523.81 (64.55) | 522.48 (71.33) |
Note. Means and standard deviations are reported unless otherwise noted. CC = community comparison; MTFC-P = Multidimensional Treatment Foster Care for Preschoolers; RFC = regular foster care.
The maltreated adolescents had encountered a range of adverse experiences prior to participating in the randomized controlled trial of MTFC-P. According to their child welfare services records, 95% of these adolescents experienced multiple types of maltreatment, including physical or supervisory neglect (RFC = 100%, MTFC-P = 82%), physical abuse (RFC = 27%, MTFC-P = 27%), emotional abuse (RFC = 100%, MTFC-P = 100%), and sexual abuse (RFC = 18%, MTFC-P = 36%). The average age at first placement into foster care was 3.21 years (SD = 1.28). The average number of foster care transitions was 3.50 (SD = 2.41) at the time of the randomized controlled trial and 5.27 (SD = 2.81) at the time of the current study. However, at time of the current study, the maltreated adolescents had been living with a biological parent (n = 8) or adoptive parent (n = 14) for an average of 5.45 years (SD = 1.94).
Intervention
MTFC-P is a family-based preventive intervention of enhanced foster care designed to reduce child behavioral problems and enhance child regulatory abilities through positive reinforcement and consistent, nonpunitive limit-setting (Fisher & Chamberlain, 2000; Fisher, Ellis, & Chamberlain, 1999). MTFC-P is delivered via a multidisciplinary team (i.e., foster parent consultants, behavioral specialists, and family therapists) to preschoolers (ages 3–6 years) in foster care, their foster parents, and their biological or adoptive parents. Prior to placement, foster parents are trained to provide high rates of reinforcement for positive behaviors and effective consequences for negative behaviors. After placement, foster parents are given extensive support through 24-hr crisis intervention (as needed), daily telephone contact, and weekly support groups. Children receive services in their homes and preschools from behavioral specialists and attend weekly therapeutic playgroup sessions designed to foster self-regulatory and socioemotional skills. Family therapists provide training and services to the biological or adoptive parents to facilitate continuity in parenting and successful transitions. Services are typically provided for 6–9 months.
The RFC adolescents received services as usual through the child welfare system as preschoolers. These services often include individual child psychotherapy, early childhood education programs, and services such as speech therapy. No attempt was made to influence the type or amount of services given to these adolescents or their parents.
Procedure
The adolescents completed two assessments at the Lewis Center for Neuroimaging at the University of Oregon. During the first assessment, they received instructions on imaging procedures, participated in an MRI simulation scan, practiced the GNG task, and completed the WISC-IV. The adolescents and their parents also completed a packet of questionnaires. During the second assessment, the adolescents completed an MRI scan. Informed assent and consent was obtained from all adolescents and parents, respectively.
Measures
Child Behavior Checklist
To investigate emotional and behavioral problems, the parents completed the Child Behavior Checklist (CBCL; Achenbach, 1991). Given the increased prevalence rates of attention and externalizing problems among maltreated youths, we examined T-scores from the Attention Problems scale and Externalizing scale, which comprise the Delinquent Behavior and Aggressive Behavior scales. There were no group differences on these scales, F(2, 33) = 0.68, ns, and F(2, 33) = 0.35, ns, respectively.
GNG task
To investigate the neural correlates of response inhibition, the adolescents completed an MRI-compatible, event-related GNG task (Durston et al., 2002). The task included two runs, counterbalanced across adolescents, with 72 trials per run (75% Go trials and 25% NoGo trials). Stimuli consisted of white, single-digit numbers presented on a black background. The adolescents were instructed to respond as quickly as possible to every number (Go stimuli) except a specific nontarget number (NoGo stimulus), which varied across adolescents. Stimuli were presented for 500ms, and an interstimulus interval of 2500ms, 5000ms, or 7500ms was presented between trials. Each run was preceded by a 3000ms fixation cross, which served as a resting baseline.
Stimuli were projected onto a computer screen at the back of a scanner bore via a digital projector and reverse-screen display system. The adolescents viewed the stimuli via a mirror attached to a birdcage head coil and responded using a fiber-optic response box. The response hand was counterbalanced across adolescents. We used Presentation (Neurobehavioral Systems, Inc.) to present the stimuli and record responses and reaction times. We provided foam padding to prevent head movement and earplugs and headphones to protect hearing.
Imaging data acquisition and preprocessing
Imaging data were acquired using a Siemens Allegra 3-Tesla head-only MRI scanner (Siemens Medical Solutions USA, Inc.). Blood oxygen-level dependent, echo-planar images were acquired across the whole brain with a T2*-weighted gradient echo sequence (TR/TE = 2000/30ms, flip angle = 80°, matrix size = 64 × 64, 32 interleaved slices). A high-resolution T2-weighted structural scan was acquired coplanar to the functional sequence (TR/TE = 2500/4.38ms, flip angle = 8°, matrix size = 256 × 192), which included a Prospective Acquisition CorrEction sequence (PACE; Thesen, Heid, Mueller, & Schad, 2000). PACE prospectively minimizes the effect of head motion by performing real-time adjustments of slice alignment and orientation of each volume prior to acquiring subsequent volumes.
BOLD-EPI data were preprocessed and analyzed in MATLAB using NeuroElf (http://neuroelf.net) and Statistical Parametric Mapping 8.0 (SPM; Wellcome Department of Imaging Neuroscience, London, UK) software. Images were converted into Neuroimaging Informatics Technology Initiative format using MRIConvert (http://lcni.uoregon.edu/~jolinda/MRIConvert), robustly skull-stripped using the Brain Extraction Tool implemented in FMRIB Software Library, and manually reoriented to the AC-PC line by two researchers to ensure quality control. The high-resolution structural image was normalized to SPM’s canonical T1-structural template. Functional images were slice-time corrected, realigned to the mean functional image, coregistered to the structural image, segmented, normalized, and smoothed using a 6-mm full-width, half-maximum isotropic Gaussian kernel.
Data Analysis
Behavioral data
Behavioral data were analyzed using SPSS 21.0. To investigate group differences in behavioral performance, we conducted a repeated measures ANOVA for accuracy (Go trial accuracy [number of correct Go trials divided by the total number of Go trials] and NoGo trial accuracy [number of correct NoGo trials divided by the total number of NoGo trials]), with group as the between-subjects factor and condition as the within-subjects factor, and a one-way ANOVA for mean reaction time (mean time to respond to correct Go trials), with group as the between-subjects factor.
Imaging data
To investigate the impact of early childhood maltreatment and participation in MTFC-P on the neural correlates of response inhibition, we compared the neural patterns underlying successful and unsuccessful response inhibition across groups. For each participant, condition effects were estimated according to the general linear model using a canonical hemodynamic response function. A 128s high-pass filter removed low-frequency noise, and an autoregressive model, AR(1), estimated temporal autocorrelation. Individual runs were excluded from the analyses due to poor task performance (i.e., failed to respond to at least 30% of Go trials; n = 3) or slice prescription error (n = 1). Single subject-level models included three regressors representing correct Go, correct NoGo, and incorrect NoGo trials. The average number of correct Go, correct NoGo, and incorrect NoGo trials were as follows: CC M (SD) = 102.86 (4.67), 19.71 (10.47), and 16.29 (10.47); RFC M (SD) = 87.64 (26.15), 16.73 (7.09), and 14.36 (9.37); MTFC-P M (SD) = 94.64 (19.57), 16.91 (5.34), and 17.45 (5.09), respectively. There was no significant main effect of group, F(2,33) = 2.26, ns, or group × condition interaction, F(2,33) = 2.08, ns, for the number of trials. Nuisance regressors included incorrect Go trials (due to low frequency), six rigid-body motion parameters representing translations and rotations during motion correction, and volumes that included major visually detected artefacts. (Separate regressors were modeled for each nonconsecutive trial with major artefacts.) There were no group differences in the number of artefact regressors: CC M (SD) = 1.57 (2.03), RFC M (SD) = 2.36 (3.07), MTFC-P M (SD) = 1.91 (3.81), F(2, 33) = 0.22, ns.
Planned contrasts were created to identify regions where activity was greater for correct NoGo relative to correct Go trials (Correct NoGo > Correct Go), representing successful response inhibition; for incorrect NoGo relative to correct NoGo trials (Incorrect NoGo > Correct NoGo), representing unsuccessful response inhibition; and for each condition relative to the resting baseline (Correct Go > Rest, Correct NoGo > Rest, and Incorrect NoGo > Rest). To estimate population effects, the Correct NoGo > Correct Go and Incorrect NoGo > Correct NoGo contrasts were entered at the group level. No explicit masks were used.
First, we examined the neural patterns underlying successful and unsuccessful response inhibition across all adolescents. We conducted whole-brain ANOVAs for the Correct NoGo > Correct Go and Incorrect NoGo > Correct NoGo contrasts, collapsed across groups. Whole-brain magnitude- and cluster-extent thresholds were calculated using Monte Carlo simulations with AlphaSim, resulting in p < 0.005 and k = 57 and k = 62 (corresponding to p < 0.05 family-wise error correction) for successful and unsuccessful response inhibition, respectively. Resulting clusters served as group-independent, task-related regions of interest (ROIs). To examine group-specific neural patterns underlying successful and unsuccessful response inhibition, we conducted whole-brain ANOVAs for the Correct NoGo > Correct Go and Incorrect NoGo > Correct NoGo contrasts, separated by group. Activation maps were thresholded at p < 0.005 and k = 20, as recommended by Lieberman and Cunningham (2009). Due to the relatively small sample sizes within each group, we also examined activity at a more liberal threshold of p < 0.01 and k = 20. To investigate associations between the neural patterns underlying successful and unsuccessful response inhibition and response inhibition abilities and clinical symptomatology, we extracted parameter estimates from each group-independent, task-related ROI using the MarsBaR toolbox for SPM and conducted correlational analyses between these parameter estimates and task performance (Go trial accuracy and NoGo trial accuracy) and CBCL variables (Attention Problems and Externalizing T-scores), collapsed across groups.
Second, we examined group differences in the neural patterns underlying successful and unsuccessful response inhibition. We first compared recruitment of the group-independent, task-related ROIs by conducting an ANOVA for each ROI, with group as the between-subjects factor. Next, we conducted our primary analyses and examined group differences in the neural patterns underlying successful and unsuccessful response inhibition across the whole brain. We conducted whole-brain ANOVAs, with group as the between-subjects factor, for the Correct NoGo > Correct Go and Incorrect NoGo > Correct NoGo contrasts. Activation maps were thresholded at p < 0.005 and k = 20. To determine which conditions drove group differences, we extracted parameter estimates from clusters representing each condition within a given contrast relative to the resting baseline and entered them into post-hoc, independent-samples t-tests, thresholded at p < 0.05. To explore the associations between group differences in the neural patterns underlying successful and unsuccessful response inhibition and response inhibition abilities and clinical symptomatology, we conducted correlational analyses between these parameter estimates and task performance and CBCL variables, collapsed across groups. Anatomical labels were determined by visual inspection and automated Talairach conversion tools. Coordinates are presented in MNI space.
Results
Behavioral Data
Accuracy was compared across groups and conditions. The main effect of condition was significant, F(1, 33) = 95.23, p < 0.001. The adolescents demonstrated greater accuracy on Go trials, M (SD) = 93.10% (1.10%), than on NoGo trials, M (SD) = 53.50% (3.90%). Accuracy was comparable across groups; thus, the main effect of group and the group × condition interaction were nonsignificant, F(2, 33) = 0.52, ns, and F(2, 33) = 0.16, ns, respectively. Similarly, the main effect of group for mean reaction time was nonsignificant, F(2, 33) = 0.02, ns. (See Table 1.)
Imaging Data
Activity underlying successful response inhibition
To investigate the neural patterns underlying successful response inhibition, we compared activity during correct NoGo relative to correct Go trials, collapsed across groups. Successful response inhibition was associated with a primarily right-lateralized lateral frontal and temporal network, including the right inferior frontal gyrus (extending into the insula and precentral gyrus), left inferior frontal gyrus (extending into the insula), right superior frontal gyrus, right medial frontal gyrus/dorsal anterior cingulate cortex (extending into the left hemisphere), right inferior parietal lobule/superior temporal gyrus (extending into the supramarginal gyrus), and left middle temporal gyrus. (See Table 2.) These regions served as group-independent, task-related ROIs for successful response inhibition.
Table 2.
Group-Independent Activity Underlying Successful Response Inhibition
| Region | x | y | z | t | k |
|---|---|---|---|---|---|
| Inferior frontal gyrus | 30 | 18 | −18 | 8.14 | 407 |
| Inferior frontal gyrus | −27 | 18 | −18 | 6.07 | 304 |
| Superior frontal gyrus | 12 | 24 | 60 | 5.01 | 124 |
| Medial frontal gyrus/dorsal anterior cingulate cortex | 6 | 27 | 36 | 4.82 | 583 |
| Inferior parietal lobule/superior temporal gyrus | 51 | −45 | 39 | 4.23 | 68 |
| Middle temporal gyrus | −60 | −27 | −12 | 4.00 | 57 |
Note. Activation maps thresholded at p < 0.005 and k = 57.
We next investigated group-specific neural patterns underlying successful response inhibition. The CC group recruited a bilateral lateral frontal and temporal network, including the bilateral inferior frontal gyri/insula, right medial/superior frontal gyri, and bilateral middle temporal gyrus; at a more liberal threshold, they also recruited the left thalamus. The RFC group recruited activity limited to the right precuneus; at a more liberal threshold, they also recruited bilateral inferior frontal gyri and right insula. The MTFC-P group, similar to the CC group, recruited a bilateral lateral frontal and temporal network, including the right inferior frontal gyrus (extending into the insula and precentral gyrus), left insula (extending into the inferior frontal gyrus), left medial frontal gyrus/dorsal anterior cingulate cortex, right middle frontal gyrus, and left middle temporal gyrus. (See Table 3.)
Table 3.
Group-Specific Activity Underlying Successful Response Inhibition
| Region | x | y | z | t | k |
|---|---|---|---|---|---|
| CC | |||||
| Inferior frontal gyrus | 42 | 24 | −3 | 4.98 | 169 |
| Inferior frontal gyrus | −27 | 18 | −18 | 4.42 | 117 |
| Middle temporal gyrus | 51 | −30 | −6 | 4.38 | 31 |
| Middle temporal gyrus | −54 | −33 | −6 | 3.61 | 42 |
| Medial frontal gyrus/superior frontal gyrus | 3 | 27 | 51 | 3.71 | 43 |
| RFC | |||||
| Precuneus | 9 | −66 | 42 | 3.11 | 23 |
| MTFC-P | |||||
| Inferior frontal gyrus | 30 | −18 | −15 | 7.72 | 375 |
| Dorsal anterior cingulate cortex | −9 | 33 | 15 | 5.54 | 736 |
| Insula | −36 | 15 | −6 | 5.17 | 222 |
| Middle temporal gyrus | −60 | −24 | −12 | 4.39 | 34 |
| Middle frontal gyrus | 24 | 51 | 15 | 3.94 | 35 |
| Middle frontal gyrus | 33 | 30 | 24 | 3.68 | 55 |
Note. Activation maps thresholded at p < 0.005 and k = 20. CC = community comparison; MTFC-P = Multidimensional Treatment Foster Care for Preschoolers; RFC = regular foster care.
We then conducted correlational analyses between the parameter estimates extracted from the group-independent, task-related ROIs and task performance and CBCL variables, collapsed across groups. Activity within the right inferior frontal gyrus correlated negatively with Go trial accuracy, r(36) = −0.36, p = 0.032. Activity within the right superior frontal gyrus correlated negatively with Externalizing T-scores, r(34) = −0.41, p = 0.013. Activity within the left middle temporal gyrus correlated negatively with Attention Problems T-scores, r(34) = −0.33, p = 0.050.
Activity underlying unsuccessful response inhibition
To investigate the neural patterns underlying unsuccessful response inhibition, we compared activity during incorrect NoGo relative to correct NoGo trials, collapsed across groups. Unsuccessful response inhibition was associated with a primarily left-lateralized subcortical, parietal, and cerebellar/occipital network, including the left cerebellum (extending into the lingual gyrus, red nucleus, and bilateral thalamus), left insula (extending into the precentral gyrus), left inferior parietal lobule (supramarginal gyrus), left precuneus, and right parahippocampus. (See Table 4.) These regions served as group-independent, task-related ROIs for unsuccessful response inhibition.
Table 4.
Group-Independent Activity Underlying Unsuccessful Response Inhibition
| Region | x | y | z | t | k |
|---|---|---|---|---|---|
| Cerebellum | −6 | −39 | −6 | 5.72 | 1789 |
| Insula | −39 | 9 | 0 | 3.90 | 82 |
| Inferior parietal lobule | −51 | −42 | 45 | 3.88 | 74 |
| Precuneus | −3 | −51 | 51 | 3.29 | 102 |
| Parahippocampus | 27 | 3 | −21 | 3.28 | 90 |
Note. Activation maps thresholded at p < 0.005 and k = 62.
We next investigated group-specific neural patterns underlying unsuccessful response inhibition. The CC group recruited activity limited to the cerebellum (extending into the lingual gyrus); at a more liberal threshold, they also recruited a wider bilateral lateral frontal and subcortical network, including the bilateral middle frontal/precentral gyri, right medial frontal gyrus, middle cingulate cortex, left posterior cingulate cortex, right striatum (caudate and thalamus), and left cerebellum. The RFC group recruited a primarily left-lateralized lateral frontal, subcortical, parietal, and cerebellar network, including the left middle frontal gyrus, right insula, bilateral thalamus, left inferior parietal lobule, left precuneus, and left cerebellum (extending into the lingual gyrus); at a more liberal threshold, they also recruited the right inferior frontal gyrus, bilateral precentral gyrus, middle cingulate cortex, and right putamen, and activity within the left thalamus extended into the insula, cerebellum, red nucleus, and lingual gyrus. The MTFC-P group recruited a primarily left-lateralized subcortical and cerebellar network, including the left thalamus, red nucleus, and left cerebellum; at a more liberal threshold, they also recruited the right precentral gyrus, left inferior parietal lobule, left precuneus, right parahippocampus (extending into the claustrum), and right thalamus. (See Table 5.)
Table 5.
Group-Specific Activity Underlying Unsuccessful Response Inhibition
| Region | x | y | z | t | k |
|---|---|---|---|---|---|
| CC | |||||
| Cerebellum | 0 | −48 | 0 | 4.53 | 204 |
| RFC | |||||
| Cerebellum | −3 | −57 | −6 | 3.09 | 21 |
| Thalamus | −18 | −27 | 0 | 4.20 | 173 |
| Thalamus | 18 | −24 | −6 | 3.80 | 97 |
| Insula | 36 | −15 | 3 | 3.79 | 26 |
| Middle frontal gyrus | −27 | 9 | 42 | 3.68 | 31 |
| Inferior parietal lobule | −57 | −39 | 33 | 3.55 | 25 |
| Precuneus | −9 | −51 | 51 | 3.29 | 30 |
| MTFC-P | |||||
| Cerebellum | −27 | −51 | −18 | 3.87 | 28 |
| Cerebellum | −3 | −57 | −6 | 3.48 | 35 |
| Thalamus | −12 | −18 | 6 | 3.67 | 36 |
| Midbrain | 0 | −24 | −15 | 3.41 | 34 |
Note. Activation maps thresholded at p < 0.005 and k = 20. CC = community comparison; MTFC-P = Multidimensional Treatment Foster Care for Preschoolers; RFC = regular foster care.
We then conducted correlational analyses between the parameter estimates extracted from the group-independent, task-related ROIs and task performance and CBCL variables, collapsed across groups. Activity within the left cerebellum, left insula, and right parahippocampus correlated negatively with Externalizing T-scores, r(33) = −0.43, p = 0.009, r(33) = −0.42, p = 0.011, and r(33) = −0.45, p = 0.007, respectively. Activity within the left precuneus and right hippocampus correlated negatively with Go trial accuracy, r(35) = −0.35, p = 0.037, and r(35) = −0.34, p = 0.046, respectively.
Group differences in activity underlying successful response inhibition
To investigate group differences in the neural patterns underlying successful response inhibition, we first compared activity within the group-independent, task-related ROIs. Group differences were nonsignificant within the right inferior frontal gyrus, F(2, 33) = 0.44, ns, left inferior frontal gyrus, F(2, 33) = 0.59, ns, right superior frontal gyrus, F(2, 33) = 0.13, ns, right medial frontal gyrus/dorsal anterior cingulate cortex, F(2, 33) = 0.94, ns, right supramarginal gyrus, F(2, 33) = 0.09, ns, and left middle temporal gyrus, F(2, 33) = 1.69, ns.
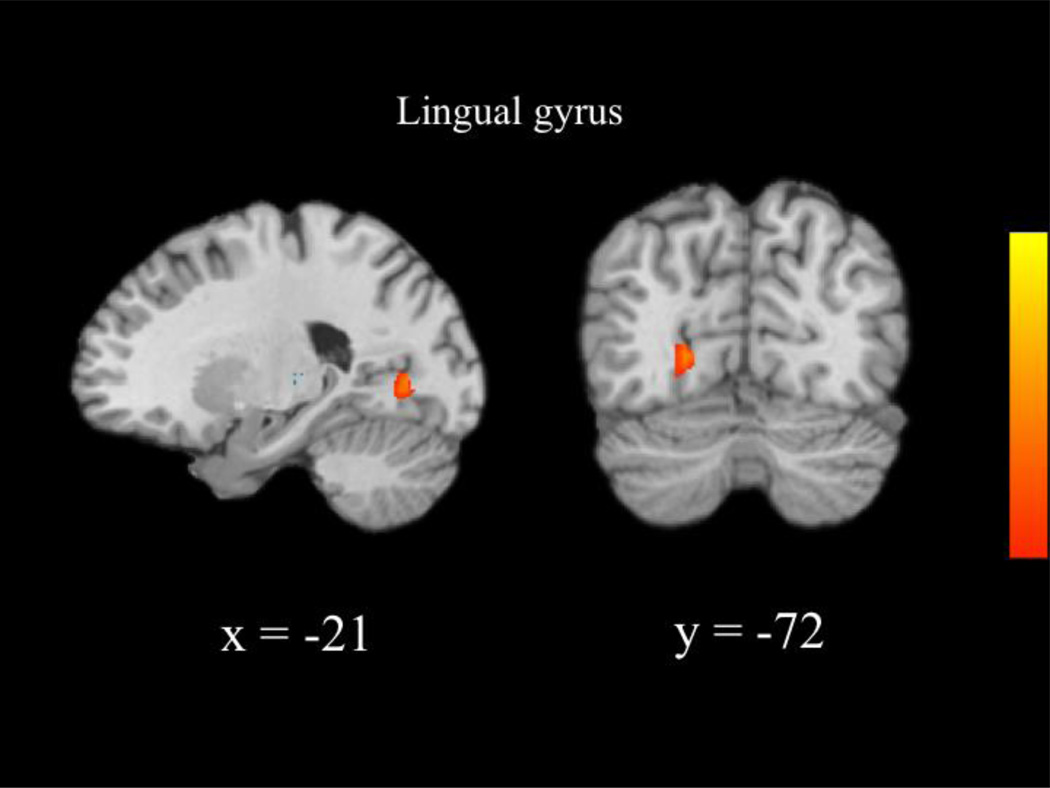
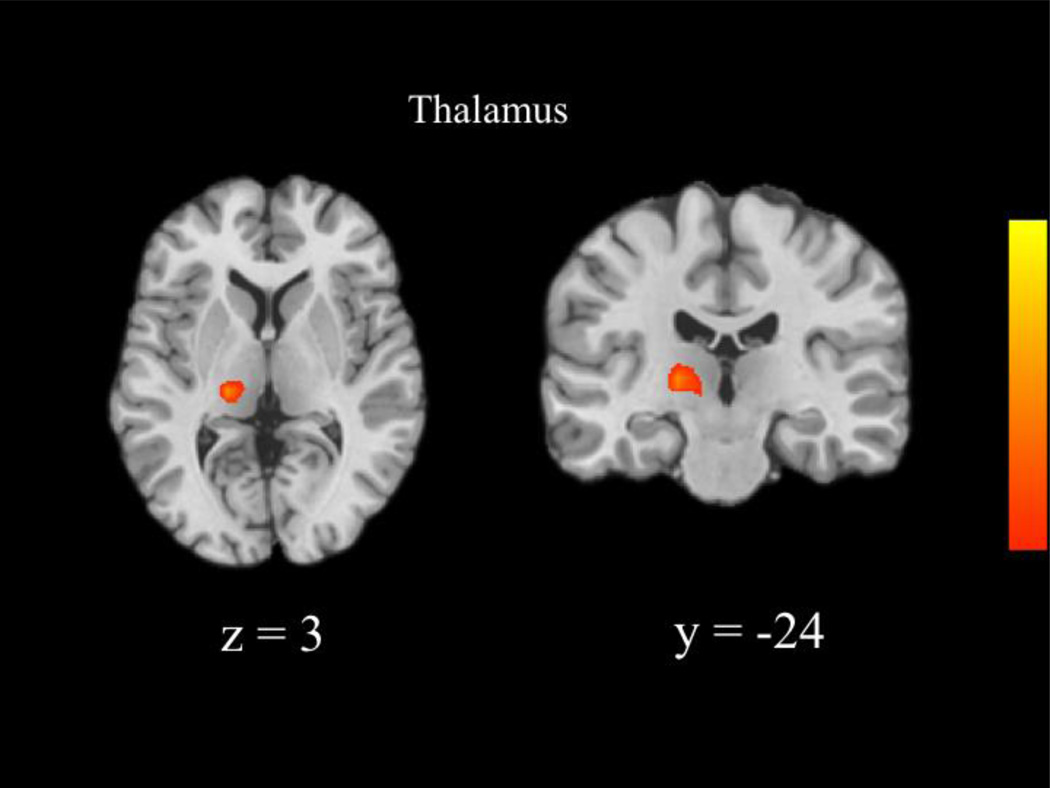
We next compared the neural patterns recruited by each group during correct NoGo relative to correct Go trials across the whole brain. The MTFC-P group recruited greater activity than the RFC group within the left lingual gyrus (see Table 6 and Figure 1). Post-hoc t-tests revealed nonsignificant group differences between the RFC and MTFC-P groups during correct NoGo trials, t(23) = −1.71, ns, and correct Go trials, t(23)= −1.00, ns. Furthermore, the CC group recruited greater activity than the RFC group within the left thalamus (see Table 6 and Figure 2). Post-hoc t-tests revealed that the RFC group recruited greater activity than the CC group during correct Go trials, t(23) = −3.24, p = 0.004. There were no significant differences between the CC and MTFC-P groups. Correlational analyses revealed that activity within the right lingual gyrus and right thalamus was not significantly related to any variables of interest.
Table 6.
Group Differences in Activity Underlying Successful Response Inhibition
| Region | x | y | z | t | k |
|---|---|---|---|---|---|
| MTFC-P > RFC | |||||
| Lingual gyrus | −21 | −72 | 0 | 4.15 | 22 |
| CC > RFC | |||||
| Thalamus | −18 | −24 | 3 | 4.27 | 35 |
Note. Activation maps thresholded at p < 0.005 and k = 20. CC = community comparison; MTFC-P = Multidimensional Treatment Foster Care for Preschoolers; RFC = regular foster care.
Figure 1.
Group differences between the MTFC-P and RFC groups in activity underlying successful response inhibition
Figure 2.
Group differences between the CC and RFC groups in activity underlying successful response inhibition
Group differences in activity underlying unsuccessful response inhibition
To investigate group differences in the neural patterns underlying unsuccessful response inhibition, we first compared activity within the group-independent, task-related ROIs. Group differences were nonsignificant within the left cerebellum, F(2, 32) = 1.39, ns, left insula, F(2, 32) = 0.93, ns, left inferior parietal lobule, F(2, 32) = 1.42, ns, left precuneus, F(2, 32) = 2.72, ns, and right parahippocampus, F(2, 32) = 0.57, ns.
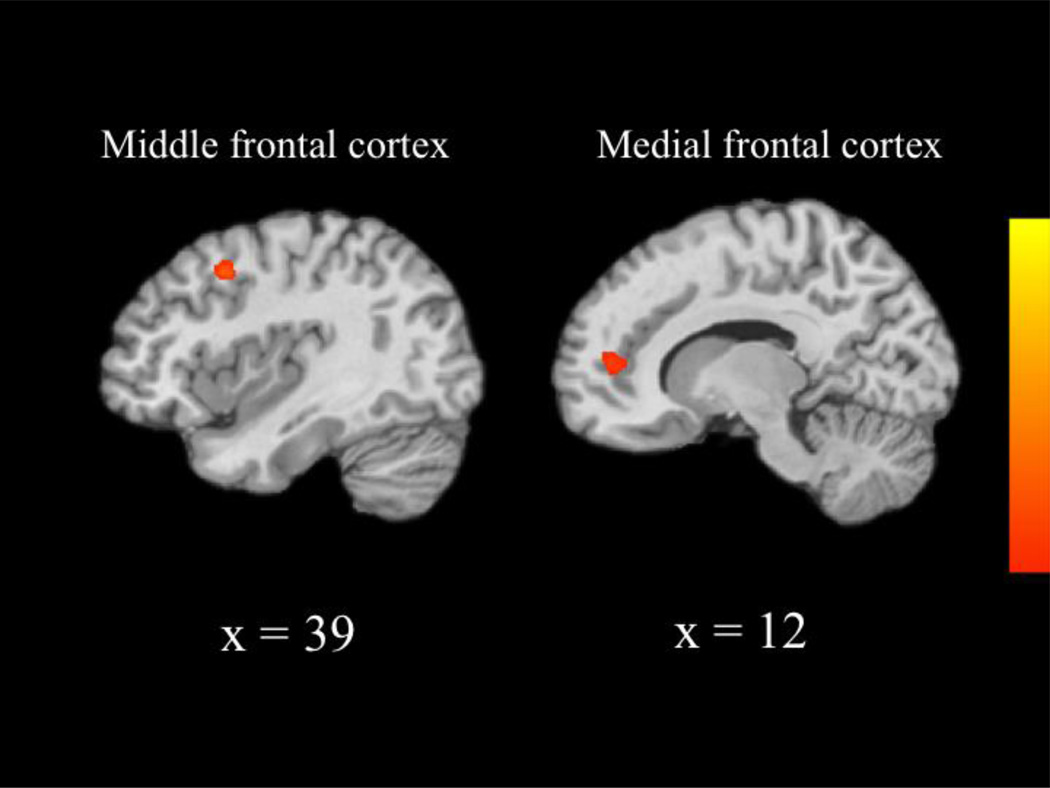
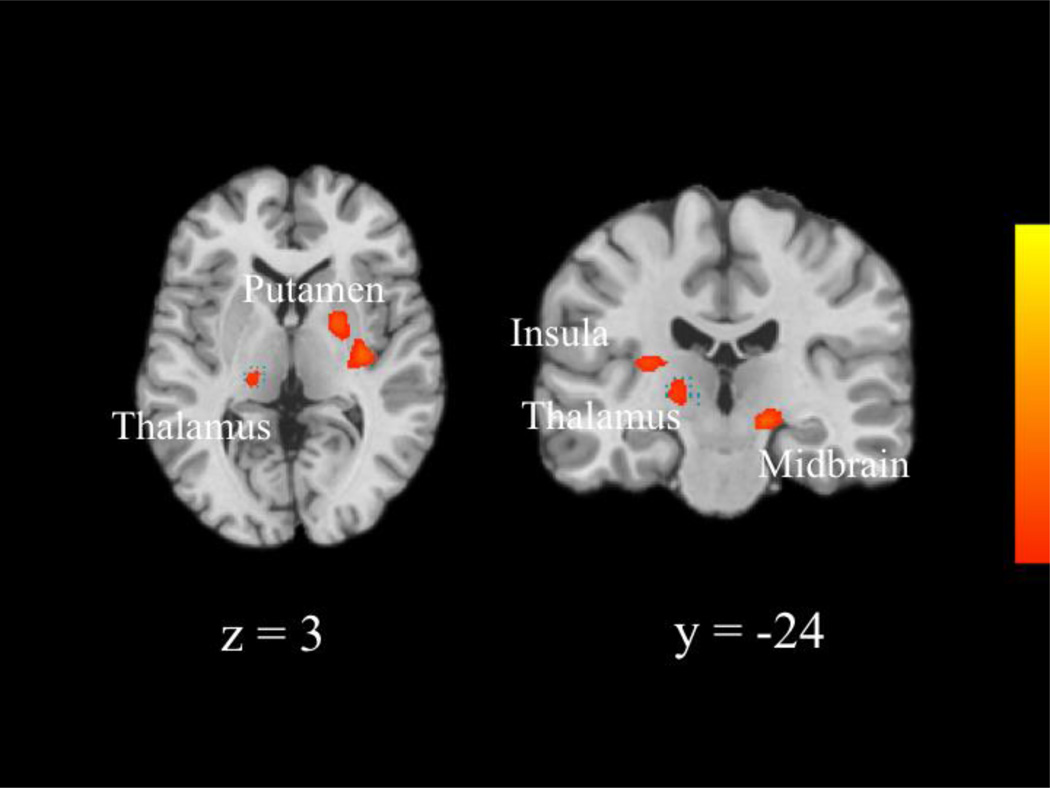
We next compared the neural patterns recruited by each group during incorrect NoGo relative to correct NoGo trials across the whole brain. The CC group recruited greater activity than the MTFC-P group within the right middle frontal gyrus (extending into the precentral gyrus and superior frontal gyrus; see Table 7 and Figure 3). Post-hoc t-tests revealed that the CC group recruited greater activity during incorrect NoGo trials, t(22) = 2.17, p = 0.041, and the MTFC-P group recruited marginally greater activity during correct NoGo trials, t(18.05) = −2.07, p = 0.053. (Results for correct NoGo trials were adjusted due to unequal variances, Levene’s Test, F[1, 23] = 5.02, p = 0.035.) Additionally, the CC group recruited greater activity than the MTFC-P group within the right medial frontal gyrus (extending into the anterior cingulate cortex). Post-hoc t-tests revealed that the CC group recruited marginally greater activity during incorrect NoGo trials, t(22) = 1.97, p = 0.061, and the MTFC-P group recruited greater activity during correct NoGo trials, t(23) = −2.19, p = 0.039. Furthermore, the RFC group recruited greater activity than the CC group within the right putamen (extending into the claustrum), right midbrain (extending into the thalamus and substantia nigra), left thalamus (extending into the parahippocampus), and left insula (extending into the claustrum). (See Table 7 and Figure 4.) Post-hoc t-tests revealed that the RFC group recruited greater activity than the CC group within all four regions during incorrect NoGo trials, t(12.59) = −3.06, p = 0.009, t(14.90) = −2.66, p = 0.018, t(22) = −2.88, p = 0.009, and t(12.08) = −2.95, p = 0.012, respectively. (Results for the putamen, thalamus, and insula were adjusted due to unequal variances, Levene’s Test, F[1, 22] = 8.86, p = 0.007, F[1, 22] = 5.40, p = 0.030, and F[1, 22] = 7.43, p = 0.012, respectively.) There were no significant differences between the MTFC-P and RFC groups. Correlational analyses revealed that activity within the right middle frontal gyrus, which differed between the CC and MTFC-P groups, correlated negatively with Externalizing T-scores, r(33) = −0.34, p = 0.047. Activity within the right putamen and left insula, which differed between the CC and RFC groups, correlated negatively with Externalizing T-scores, r(33) = −0.36, p = 0.034, and r(33) = −0.37, p = 0.031, respectively. No other correlational analyses reached significance.
Table 7.
Group Differences in Activity Underlying Unsuccessful Response Inhibition
| Region | x | y | z | t | k |
|---|---|---|---|---|---|
| CC > MTFC-P | |||||
| Middle frontal gyrus | 39 | 9 | 42 | 3.77 | 43 |
| Medial frontal gyrus | 12 | 48 | 9 | 3.19 | 27 |
| RFC > CC | |||||
| Thalamus | −18 | −27 | 0 | 3.53 | 26 |
| Insula | −27 | −24 | 12 | 3.43 | 31 |
| Putamen | 21 | 0 | 6 | 4.16 | 87 |
| Midbrain | 18 | −21 | −9 | 3.87 | 36 |
Note. Activation maps thresholded at p < 0.005 and k = 20. CC = community comparison; MTFC-P = Multidimensional Treatment Foster Care for Preschoolers; RFC = regular foster care.
Figure 3.
Group differences between the CC and MTFC-P groups in activity underlying unsuccessful response inhibition
Figure 4.
Group differences between the RFC and CC groups in activity underlying unsuccessful response inhibition
Discussion
The current study investigated the impact of early childhood maltreatment and a preventive intervention on the neural patterns underlying response inhibition in early adolescence. Overall, the observed patterns of behavioral performance and neural recruitment were consistent with prior research using the GNG task (Bunge et al., 2002; Durston et al., 2002, 2006; Tamm et al., 2002). However, while there were no group differences in behavioral performance, significant group differences in neural recruitment were observed, particularly within dorsolateral and medial prefrontal and subcortical regions during unsuccessful response inhibition. While the current study is highly novel, these findings should be interpreted as preliminary evidence to guide future studies.
Behavioral Performance
Consistent with previous research (Bruce et al., 2013; Tamm et al., 2002), the adolescents were more accurate in responding to Go trials than inhibiting responses to NoGo trials. However, group differences in behavioral performance were nonsignificant. Although we predicted group differences in behavioral performance due to past findings of impaired response inhibition in maltreated youths (De Bellis, Hooper, Spratt, & Woolley, 2009; Hart & Rubia, 2012), previous studies investigating maltreated children and children with ADHD have similarly reported significant group differences using neural measures despite a lack of group differences using behavioral measures (Bruce et al., 2009, 2013; Carrion et al., 2008; Durston, et al., 2006; Mueller et al., 2010). This pattern might represent subtle differences in cognitive processing that do not result in differences in behavioral performance (e.g., accuracy or reaction time) but impact day-to-day functioning in more complex settings.
Activity Underlying Successful Response Inhibition
During successful response inhibition, the adolescents as a whole recruited a primarily right-lateralized lateral and medial prefrontal, parietal, and temporal network, including the inferior frontal gyrus, superior frontal gyrus, and medial frontal gyrus/anterior cingulate cortex. These results converge with findings from previous research investigating response inhibition in typically developing youths (Bunge et al., 2002; Durston et al., 2002, 2006; Tamm et al., 2002) and maltreated children (Bruce et al., 2013). The consistency of these findings across studies and populations highlights the importance of this neural network in successful response inhibition.
Our examination of group-specific neural patterns revealed that all three groups recruited the inferior frontal gyrus, which is considered a key region in successful response inhibition (Durston et al., 2006; Rubia, Smith, Brammer, & Taylor, 2003; Rubia et al., 2006). However, while the nonmaltreated adolescents and maltreated adolescents who received MTFC-P recruited this region robustly, the maltreated adolescents who received services as usual recruited qualitatively weaker activity that only reached significance at a reduced threshold. Furthermore, the nonmaltreated adolescents and maltreated adolescents who received MTFC-P recruited strikingly similar neural patterns of robust prefrontal and temporal activity, including the inferior frontal gyrus, middle/superior frontal gyri, medial frontal gyrus/anterior cingulate cortex, and middle temporal gyrus. Prior findings have highlighted these regions as the prototypical network underlying successful response inhibition (Rubia et al., 2007). Intriguingly, results from the current study parallel recent findings that nonmaltreated children recruit greater middle and medial frontal activity during successful response inhibition than maltreated children (Bruce et al., 2013), suggesting that more robust middle and medial frontal recruitment might be more normative. Moreover, in the current study, greater lateral frontal and temporal recruitment during successful response inhibition was associated with reduced attention and externalizing problems. Overall, these findings suggest that participating in an early preventive intervention, such as MTFC-P, might mitigate the effects of early childhood maltreatment on the neural circuitry underlying successful response inhibition.
Although our quantitative comparisons revealed no significant group differences within the group-independent, task-related ROIs, the nonmaltreated adolescents recruited significantly greater activity within the left thalamus compared to the maltreated adolescents who received services as usual. In particular, these maltreated adolescents recruited greater activity during correct Go trials. The thalamus supports selective attention, response selection, and motor responding and plays a crucial role in the fronto-striato-pallidal pathway that supports correctly responding to Go trials (Aron & Poldrack, 2006; Band & van Boxtel, 1999; Booth et al., 2003). Of particular relevance, recent findings suggest that postnatal stress significantly impairs fronto-subcortical networks, particularly connectivity between the frontal lobes and the thalamus (Pollak et al, 2010). These findings suggest that early childhood maltreatment alters thalamic development, resulting in attenuated recruitment during successful response inhibition.
In addition, the maltreated adolescents who received MTFC-P recruited greater lingual gyral activity than the maltreated adolescents who received services as usual. The lingual gyrus has been linked to response execution, response inhibition, and selective and sustained attention (Booth et al., 2003; Menon, Adleman, White, Glover, & Reiss, 2001; Velanova, Wheeler, & Luna, 2009). In a previous study, nonmaltreated children recruited greater lingual gyral activity during successful response inhibition than maltreated children (Bruce et al., 2013). Taken together, these findings suggest that youths who experience early childhood maltreatment recruit reduced occipital activity during successful response inhibition but that participating in an early preventive intervention, such as MTFC-P, could mitigate these maltreatment-related effects.
Activity Underlying Unsuccessful Response Inhibition
During unsuccessful response inhibition, the adolescents as a whole recruited a primarily left-lateralized lateral frontal, subcortical, parietal, temporal, cerebellar, and occipital network. In previous research, typically developing youths and adults recruited a similar, widely distributed network of regions (Chevrier, Noseworthy, & Schachar, 2007; Garavan, Ross, Murphy, Roche, & Stein, 2002; Menon et al., 2001; Rubia et al., 2003, 2007). Further highlighting the importance of these regions, greater recruitment of the insula, parahippocampus, and cerebellum during unsuccessful response inhibition was associated with fewer externalizing problems in the current study. Similarly, in a recent study with adults with ADHD, greater insular activity during unsuccessful response inhibition was associated with less severe symptomatology (Cubillo et al., 2010).
Our examination of group-specific neural patterns revealed that all three groups recruited the cerebellum, which plays an important role in response execution (Steele et al., 2013). However, our results also revealed interesting qualitative differences. Compared to the nonmaltreated adolescents, the maltreated adolescents recruited qualitatively more robust and diffuse subcortical activity during unsuccessful response inhibition. Furthermore, the maltreated adolescents who received services as usual recruited qualitatively more widespread activity within middle frontal, insular, and parietal regions than the maltreated adolescents who received MTFC-P. These results suggest that early childhood maltreatment might be associated with more diffuse neural recruitment, particularly within subcortical regions, during unsuccessful response inhibition.
Although quantitative comparisons revealed no significant group differences within the group-independent, task-related ROIs, the maltreated adolescents who received services as usual recruited significantly greater activity within the left thalamus during incorrect NoGo trials than the nonmaltreated adolescents. Interestingly, these maltreated adolescents also recruited this region of the thalamus to a greater extent during correct Go trials. Taken together, these results suggest that early childhood maltreatment is broadly associated with thalamic hyperactivation during motor execution. In addition, relative to the nonmaltreated adolescents, the maltreated adolescents who received services as usual recruited significantly greater activity within multiple subcortical and adjacent cortical areas, such as the right midbrain, right thalamus, right putamen, and left insula, during incorrect NoGo trials. Prior findings suggest that a similar basal ganglia-thalamo-cortical circuit supports motor planning and might be key in selecting motor actions in the context of multiple alternatives (Boecker, Jankowski, Ditter, & Scheef, 2008; Jankowski, Scheef, Huppe, & Boecker 2009; Sommer, 2003). Specifically, the thalamus is associated with motor response selection (Aron & Poldrak, 2006), the midbrain prevents the execution of motor demands (Band & van Boxtel, 1999), the insula supports error processing following unsuccessful response inhibition (Menon et al., 2001), and the putamen supports behavioral adjustment following errors (Garavan et al., 2002). These findings suggest that early childhood maltreatment is associated with hyperactivation within a network of subcortical and adjacent cortical regions, which might represent alterations in multiple aspects of response inhibition (e.g., motor planning, motor response selection, motor response withdrawal, error processing, and behavioral adjustment following errors).
There were also significant group differences between the nonmaltreated adolescents and maltreated adolescents who received MTFC-P. During unsuccessful response inhibition, the nonmaltreated adolescents recruited significantly greater activity within the right middle frontal gyrus and right medial frontal gyrus (extending into the anterior cingulate cortex). While the nonmaltreated adolescents recruited greater middle and medial frontal activity during incorrect NoGo trials, the maltreated adolescents who received MTFC-P recruited greater activity in these regions during correct NoGo trials. A recent review highlighted the role of these medial frontal regions in unsuccessful response inhibition, specifically in conflict detection, error processing, and behavioral adjustment following errors (Verbruggen & Logan, 2008). Consistent with this theory, adults recruit the anterior cingulate cortex and presupplementary motor area following incorrect responses and recruit greater activity during behavioral adjustments following errors (Garavan et al., 2002). Thus, our findings suggest that, relative to the nonmaltreated adolescents, the maltreated adolescents who received MTFC-P recruited reduced activity during unsuccessful response inhibition and greater activity during successful response inhibition in regions involved in error monitoring and behavioral adjustment.
It is noteworthy that the two maltreated groups displayed distinct neural patterns during unsuccessful response inhibition relative to the nonmaltreated adolescents, suggesting that different cognitive processes might drive unsuccessful response inhibition within maltreated groups. While the maltreated adolescents who received services as usual demonstrated hyperactivation within subcortical and adjacent cortical regions relative to the nonmaltreated adolescents, the maltreated adolescents who received MTFC-P demonstrated middle and medial frontal hypoactivation relative to the nonmaltreated adolescents. Similarly, Chevrier et al. (2007) posited that unsuccessful response inhibition is supported by dissociable neural correlates; the inferior frontal gyrus and subcortical regions support motor response withdrawal, while the anterior cingulate cortex and middle frontal gyrus support error-processing and error-monitoring abilities. Overall, these findings suggest that the maltreated adolescents who received services as usual experience multiple impairments in response inhibition but that the maltreated adolescents who received MTFC-P experience specific impairments in error monitoring.
Strengths, Limitations, and Avenues for Future Research
Our study exhibits several strengths and provides multiple avenues for future research. First, in contrast to prior studies that used a block design or did not examine correct and incorrect NoGo trials separately, we used an event-related design, which permitted us to compare activity during successful and unsuccessful response inhibition. Second, the groups were closely matched on key factors, including adolescent age, pubertal status, and general cognitive abilities. In addition, the maltreated groups were randomly assigned to receive MTFC-P or services as usual.
A limitation of the current study is its small sample size. It is challenging to collect neuroimaging data from maltreated youths; however, we included comparable sample sizes to previous neuroimaging studies that investigated maltreated populations (Bruce et al., 2013; Carrion et al., 2008; Mueller et al., 2010). Nevertheless, the results of this study should be interpreted with caution and viewed as preliminary evidence for future research with larger samples. A larger sample would offer greater power for detecting less robust group differences and conducting group-specific correlational analyses. It would also permit researchers to compare the neural patterns associated with distinct types of maltreatment (e.g., neglect and physical, emotional, and sexual abuse) and examine the effects of the onset, chronicity, severity, and recency of maltreatment. Relatedly, specific characteristics of the preventive intervention, such as timing of the intervention, should be considered in future research examining intervention effects on the neural correlates of response inhibition. Future research should also investigate group differences in functional connectivity, particularly within networks that have been shown to differ structurally and functionally in maltreated populations (e.g., the fronto-striatal network).
Conclusion
Our findings provide evidence that early childhood maltreatment alters the neural patterns underlying successful and unsuccessful response inhibition in early adolescence. Our results also converge with previous findings suggesting that maltreated youths recruit atypical activity within prefrontal, subcortical, and posterior regions. In particular, maltreated adolescents demonstrated hyperactivation within subcortical regions during unsuccessful response inhibition, suggesting that maltreated youths might process multiple aspects of response inhibition atypically. However, our results also suggest that neural consequences of early childhood maltreatment is responsive to intervention. Compared to the maltreated adolescents who received services as usual, the nonmaltreated adolescents and maltreated adolescents who received MTFC-P recruited strikingly similar neural patterns of robust and diffuse activity within the prototypical response inhibition network during successful response inhibition. These results suggest that participating in an early preventive intervention, such as MTFC-P, could ameliorate the effects of early childhood maltreatment on the neural circuitry underlying successful response inhibition in early adolescence.
Research Highlights.
The current study examined the impact of early childhood maltreatment and a preventive intervention on the neural correlates of response inhibition in early adolescence.
Compared to the nonmaltreated adolescents, the maltreated adolescents who were in foster care as preschoolers and randomly assigned to receive services as usual demonstrated subcortical hypoactivation during successful response inhibition and subcortical hyperactivation during unsuccessful response inhibition.
The maltreated adolescents who were in foster care as preschoolers and randomly assigned to receive a preventive intervention exhibited strikingly similar neural patterns to the nonmaltreated adolescents during successful response inhibition but exhibited prefrontal hypoactivation during unsuccessful response inhibition.
These findings suggest that early childhood maltreatment alters the neural patterns underlying response inhibition in early adolescence and that participating in a preventive intervention could mitigate maltreatment-related effects on these neural systems.
Acknowledgments
Support for this research was provided by the following grants: MH078105, NIMH, U.S. PHS; and DA026501, NIDA, U.S. PHS. Kathryn Jankowski was supported by National Science Foundation Graduate Research Fellowship 2011122786. The authors thank the adolescents and parents who participated in this study, the staff at the Robert and Beverly Lewis Center for Neuroimaging at the University of Oregon for technical assistance, Drs. Jennifer Pfeifer and Elliot Berkman for feedback on data analysis, and Matthew Rabel for editorial assistance.
References
- Achenbach TM. Manual for the Child Behavior Checklist/4–18 and 1991 profile. Burlington, VT: University of Vermont, Department of Psychiatry; 1991. [Google Scholar]
- Aron AR, Poldrack RA. Cortical and subcortical contributions to stop signal response inhibition: Role of the subthalamic nucleus. Journal of Neuroscience. 2006;26:2424–2433. doi: 10.1523/JNEUROSCI.4682-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Band GP, van Boxtel GJ. Inhibitory motor control in stop paradigms: Review and reinterpretation of neural mechanisms. Acta Psychologica. 1999;101:179–211. doi: 10.1016/s0001-6918(99)00005-0. [DOI] [PubMed] [Google Scholar]
- Beers SR, De Bellis MD. Neuropsychological function in children with maltreatment-related posttraumatic stress disorder. American Journal of Psychiatry. 2002;159:483–486. doi: 10.1176/appi.ajp.159.3.483. [DOI] [PubMed] [Google Scholar]
- Boecker H, Jankowski J, Ditter P, Scheef L. A role of the basal ganglia and midbrain nuclei for initiation of motor sequences. Neuroimage. 2008;39:1356–1369. doi: 10.1016/j.neuroimage.2007.09.069. [DOI] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Lei Z, Trommer BL, Davenport ND, Mesulam MM. Neural development of selective attention and response inhibition. Neuroimage. 2003;20:737–751. doi: 10.1016/S1053-8119(03)00404-X. [DOI] [PubMed] [Google Scholar]
- Bruce J, Fisher PA, Graham AM, Moore WE, Peake SJ, Mannering AM. Patterns of brain activation in foster children and nonmaltreated children during an inhibitory control task. Developmental Psychopathology. 2013;25:931–941. doi: 10.1017/S095457941300028X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce J, McDermott JM, Fisher PA, Fox NA. Using behavioral and electrophysiological measures to assess the effects of a preventive intervention: A preliminary study with preschool-aged foster children. Prevention Science. 2009;10:129–140. doi: 10.1007/s11121-008-0115-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge SA, Dudukovic NM, Thomason ME, Vaidya CJ, Gabrieli JDE. Immature frontal lobe contributions to cognitive control in children: Evidence from fMRI. Neuron. 2002;33:301–311. doi: 10.1016/s0896-6273(01)00583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrion VG, Garrett A, Menon V, Weems CF, Reiss AL. Posttraumatic stress symptoms and brain function during a response-inhibition task: An fMRI study in youth. Depression and Anxiety. 2008;25:514–526. doi: 10.1002/da.20346. [DOI] [PubMed] [Google Scholar]
- Chevrier AD, Noseworthy MD, Schachar R. Dissociation of response inhibition and performance monitoring in the stop signal task using event-related fMRI. Human Brain Mapping. 2007;28:1347–1358. doi: 10.1002/hbm.20355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubillo A, Halari R, Ecker C, Giampietro V, Taylor E, Rubia K. Reduced activation and inter-regional functional connectivity of fronto-striatal networks in adults with childhood Attention-Deficit Hyperactivity Disorder (ADHD) and persisting symptoms during tasks of motor inhibition and cognitive switching. Journal of Psychiatric Research. 2010;44:629–639. doi: 10.1016/j.jpsychires.2009.11.016. [DOI] [PubMed] [Google Scholar]
- De Bellis MD. Developmental traumatology: A contributory mechanism for alcohol and substance use disorders. Psychoneuroendocrinology. 2002;27:155–170. doi: 10.1016/s0306-4530(01)00042-7. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Hooper SR, Spratt EG, Woolley DP. Neuropsychological findings in childhood neglect and their relationships to pediatric PTSD. Journal of the International Neuropsychological Society. 2009;15:868–878. doi: 10.1017/S1355617709990464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durston S, Thomas KM, Yang Y, Ulug AM, Zimmerman RD, Casey BJ. A neural basis for the development of inhibitory control. Developmental Science. 2002;5:F9–F16. [Google Scholar]
- Durston S, Mulder M, Casey BJ, Ziermans T, van Engeland H. Activation in ventral prefrontal cortex is sensitive to genetic vulnerability for attention-deficit hyperactivity disorder. Biological Psychiatry. 2006;60:1062–1070. doi: 10.1016/j.biopsych.2005.12.020. [DOI] [PubMed] [Google Scholar]
- Fisher PA, Chamberlain P. Multidimensional treatment foster care a program for intensive parenting, family support, and skill building. Journal of Emotional and Behavioral Disorders. 2000;8:155–164. [Google Scholar]
- Fisher PA, Ellis BH, Chamberlain P. Early intervention foster care: A model for preventing risk in young children who have been maltreated. Children's Services: Social Policy, Research, and Practice. 1999;2:159–182. [Google Scholar]
- Fisher PA, Kim HK. Intervention effects on foster preschoolers' attachment-related behaviors from a randomized trial. Prevention Science. 2007;8:161–170. doi: 10.1007/s11121-007-0066-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher PA, Stoolmiller M, Gunnar MR, Burraston BO. Effects of a therapeutic intervention for foster preschoolers on diurnal cortisol activity. Psychoneuroendocrinology. 2007;32:892–905. doi: 10.1016/j.psyneuen.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Murphy K, Roche RA, Stein EA. Dissociable executive functions in the dynamic control of behavior: Inhibition, error detection, and correction. Neuroimage. 2002;17:1820–1829. doi: 10.1006/nimg.2002.1326. [DOI] [PubMed] [Google Scholar]
- Garland AF, Hough RL, McCabe KM, Yeh M, Wood PA, Aarons GA. Prevalence of psychiatric disorders in youths across five sectors of care. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40:409–418. doi: 10.1097/00004583-200104000-00009. [DOI] [PubMed] [Google Scholar]
- Green JG, McLaughlin KA, Berglund PA, Gruber MJ, Sampson NA, Zaslavsky AM, Kessler RC. Childhood adversities and adult psychiatric disorders in the national comorbidity survey replication I: Associations with first onset of DSM-IV disorders. Archives of General Psychiatry. 2010;67:113–123. doi: 10.1001/archgenpsychiatry.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart H, Rubia K. Neuroimaging of child abuse: A critical review. Frontiers in Human Neuroscience. 2012;6(52):1–24. doi: 10.3389/fnhum.2012.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski J, Scheef L, Huppe C, Boecker H. Distinct striatal regions for planning and executing novel and automated movement sequences. Neuroimage. 2009;44:1369–1379. doi: 10.1016/j.neuroimage.2008.10.059. [DOI] [PubMed] [Google Scholar]
- Leeb RT, Paulozzi L, Melanson C, Simon T, Arias I. Child maltreatment surveillance: Uniform definitions for public health and recommended data elements. Atlanta, GA: Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; 2008. [Google Scholar]
- Levin HS, Culhane KA, Hartmann J, Evankovich K, Mattson AJ, Harward H, Fletcher JM. Developmental changes in performance on tests of purported frontal lobe functioning. Developmental Neuropsychology. 1991;7:377–395. [Google Scholar]
- Lieberman MD, Cunningham WA. Type I and Type II error concerns in fMRI research: Re-balancing the scale. Social Cognitive and Affective Neuroscience. 2009;4:423–428. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott JM, Westerlund A, Zeanah CH, Nelson CA, Fox NA. Early adversity and neural correlates of executive function: Implications for academic adjustment. Developmental Cognitive Neuroscience. 2012;2:S59–S66. doi: 10.1016/j.dcn.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, Adleman NE, White CD, Glover GH, Reiss AL. Error-related brain activation during a Go/NoGo response inhibition task. Human Brain Mapping. 2001;12:131–143. doi: 10.1002/1097-0193(200103)12:3<131::AID-HBM1010>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SC, Maheu FS, Dozier M, Peloso E, Mandell D, Leibenluft E, Ernst M. Early-life stress is associated with impairment in cognitive control in adolescence: An fMRI study. Neuropsychologia. 2010;48:3037–3044. doi: 10.1016/j.neuropsychologia.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman AL, Pulido C, Squeglia LM, Spadoni AD, Paulus MP, Tapert SF. Neural activation during inhibition predicts initiation of substance use in adolescence. Drug and Alcohol Dependence. 2011;119:216–223. doi: 10.1016/j.drugalcdep.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechtel P, Pizzagalli DA. Effects of early life stress on cognitive and affective function: An integrated review of human literature. Psychopharmacology. 2011;214:55–70. doi: 10.1007/s00213-010-2009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen A, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Pollak SD, Nelson CA, Schlaak MF, Roeber BJ, Wewerka SS, Wiik KL, Gunnar MR. Neurodevelopmental effects of early deprivation in postinstitutionalized children. Child Development. 2010;81:224–236. doi: 10.1111/j.1467-8624.2009.01391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Brammer MJ, Taylor E. Right inferior prefrontal cortex mediates response inhibition while mesial prefrontal cortex is responsible for error detection. Neuroimage. 2003;20:351–358. doi: 10.1016/s1053-8119(03)00275-1. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Taylor E, Brammer M. Linear age-correlated functional development of right inferior fronto-striato-cerebellar networks during response inhibition and anterior cingulate during error-related processes. Human Brain Mapping. 2007;28:1163–1177. doi: 10.1002/hbm.20347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Woolley J, Nosarti C, Heyman I, Taylor E, Brammer M. Progressive increase of frontostriatal brain activation from childhood to adulthood during event-related tasks of cognitive control. Human Brain Mapping. 2006;27:973–993. doi: 10.1002/hbm.20237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer MA. The role of the thalamus in motor control. Current Opinion in Neurobiology. 2003;13:663–670. doi: 10.1016/j.conb.2003.10.014. [DOI] [PubMed] [Google Scholar]
- Steele VR, Aharoni E, Munro GE, Calhoun VD, Nyalakanti P, Stevens MC, Kiehl KA. A large scale (N = 102) functional neuroimaging study of response inhibition in a Go/NoGo task. Behavioural Brain Research. 2013;256:529–536. doi: 10.1016/j.bbr.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swick D, Ashley V, Turken U. Are the neural correlates of stopping and not going identical? Quantitative meta-analysis of two response inhibition tasks. Neuroimage. 2011;56:1655–1665. doi: 10.1016/j.neuroimage.2011.02.070. [DOI] [PubMed] [Google Scholar]
- Tamm L, Menon V, Reiss AL. Maturation of brain function associated with response inhibition. Journal of the American Academy of Child & Adolescent Psychiatry. 2002;41:1231–1238. doi: 10.1097/00004583-200210000-00013. [DOI] [PubMed] [Google Scholar]
- Thesen S, Heid O, Muller E, Schad LR. Prospective acquisition correction for head motion with image-based tracking for real-time fMRI. Magnetic Resonance in Medicine. 2000;44:457–465. doi: 10.1002/1522-2594(200009)44:3<457::aid-mrm17>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Velanova K, Wheeler ME, Luna B. The maturation of task set-related activation supports late developmental improvements in inhibitory control. Journal of Neuroscience. 2009;29:12558–12567. doi: 10.1523/JNEUROSCI.1579-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen F, Logan GD. Response inhibition in the stop-signal paradigm. Trends in Cognitive Science. 2008;12:418–424. doi: 10.1016/j.tics.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Sylvester CY, Lacey SC, Nee DE, Franklin M, Jonides J. Common and unique components of response inhibition revealed by fMRI. Neuroimage. 2005;27:323–340. doi: 10.1016/j.neuroimage.2005.01.054. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children. 4th. San Antonio, TX: The Psychological Corporation; 2003. [Google Scholar]
- Woltering S, Granic I, Lamm C, Lewis MD. Neural changes associated with treatment outcome in children with externalizing problems. Biological Psychiatry. 2011;70:873–879. doi: 10.1016/j.biopsych.2011.05.029. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. The AFCARS report: Preliminary FY 2010 estimates as of July 2011. Washington, DC: Author; 2011. [Google Scholar]