Abstract
Tissue formation and cell differentiation depend on a properly assembled extracellular matrix (ECM). Fibronectin is a key constituent of the pericellular ECM, forming essential connections between cell surface integrin receptors and structural components of the ECM. Recent studies using vertebrate models, conditional gene knockouts, tissue explants, and cell culture systems have identified developmental processes that depend on fibronectin and its receptor α5β1 integrin. We describe requirements for fibronectin matrix in the cardiovascular system, somite and precartilage development, and epithelial-mesenchymal transition. Information about molecular mechanisms shows the importance of fibronectin and integrins during tissue morphogenesis and cell differentiation, as well as their cooperation with growth factors to mediate changes in cell behaviors.
Introduction
The extracellular matrix (ECM) is a critical regulator of cell behaviors [1,2]. Cell- ECM binding provides chemical signals, structural support, and local cues organized to promote integration of environmental information by intracellular pathways. Cell interactions with the ECM are dynamic, usually involving new ECM protein polymerization and matrix remodeling. Cell-mediated assembly of ECM proteins into multimeric structures directs cell and tissue organization and controls cell differentiation, making the assembly process a key player in tissue morphogenesis. Assembly of the ubiquitous ECM protein fibronectin (FN) into fibrils requires coordination of FN expression levels with integrin receptor activity and connections to cytoskeletal components [3,4]. FN fibrils then form the foundation upon which collagens and many other ECM proteins are deposited. This review summarizes recent data on the critical role of FN matrix during tissue development with emphasis on how the ECM cooperates with other molecules to promote morphogenetic processes.
Fibronectin matrix in cardiovascular development
FN dimers are assembled into fibrils primarily by the integrin receptor α5β1 and both ligand and receptor play central roles in cardiovascular development. FN-null mice die as embryos with defects in formation of the heart and early embryonic and extraembryonic blood vessels [5,6]. A closer examination of heart development in the absence of FN showed defects in the cardiac outflow tract and right ventricle that were linked to decreased proliferation of cardiac precursor cells [7]. Similar cardiac defects were observed in conditional mouse knockouts of FN or α5 targeted to the pharyngeal region [8] as well as in fibroblast growth factor 8 (Fgf8) hypomorphs [9]. Transcriptional targets of Fgf8 signaling were down-regulated in FN-null or α5-null embryos implicating FN matrix in the potentiation of Fgf8 signaling at least for this stage of heart development [7].
FN and α5 have also been linked to the migration of cardiac precursor cells. In zebrafish between 18 and 24 hpf, myocardial precursors migrate to the midline and then fuse to form a single heart tube [10]. Knockdown of the transcription factor Snail1b causes a reduction in α5 expression and discontinuous FN matrix in the region of cell migration [11]. Both FN matrix assembly and cardiac fusion were rescued by injection of α5 mRNA indicating that precursor cell migration depends on Snail1b-mediated regulation of α5 expression and subsequent FN matrix assembly. Perhaps Snail1b is acting downstream of sphingosine-1-phosphate (S1P). Mutation of the S1P transporter Spns2, which is required for S1P secretion, combined with knockdown of FN caused a severe, two-heart phenotype in zebrafish [12]. Cardiac progenitor cell differentiation appeared normal but cell movements needed for cardiac fusion did not occur.
Analysis of epicardial cell migration in vitro has uncovered a novel mechanism that is executed by two proteins, Bves and NDRG4, previously implicated in vesicular trafficking [13–15]. Knockdown of the Bves/NDRG4 complex increased FN accumulation in cytoplasmic vesicles and decreased deposition of vesicular FN onto the substrate [13]**. These changes in FN subcellular localization accompanied loss of directional persistence in cell migration suggesting an interesting model in which autocrine FN recycling supports de novo matrix assembly to direct epicardial cell migration.
Conditional knockout of α5 in the anterior mesoderm, the source of cardiac tissue and the anterior vasculature, identified a surprising connection between mesoderm and neural crest development. Differentiation of cardiac neural crest into vascular smooth muscle cells (VSMCs) was affected by the absence of this integrin in mesodermal cells which caused morphogenetic defects in aortic arch arteries [16]**. These results suggest a model in which the ECM assembled by α5β1 on mesodermal cells influences neural crest-derived cells to develop into VSMCs. FN-binding integrins are also needed in the neural-crest derived VSMCs. Double knockout of α5 and αv integrins in VSMCs, both of which bind to the RGD domain of FN, caused embryonic lethality with cardiovascular defects similar to the anterior mesoderm α5 knockout mice [17]; single integrin knockout showed no defects in cardiac development. Both VSMCs and the vessel wall ECM were abnormal in α5/αv-null mice, which corresponded with severe defects in FN matrix assembly in cultured double-null VSMCs [17]*. Matrix incorporation of a number of other ECM proteins was deficient. Of particular note is latent transforming growth factor β (TGFβ) binding protein (Ltbp1); its deficiency disrupted Smad signaling downstream of TGFβ, a likely contributor to the observed cardiovascular defects [17]. Together these analyses of cardiac neural crest differentiation demonstrate a central role for integrins and their FN matrix in controlling cell differentiation by providing pericellular signals from the ECM itself as well as by presenting exogenous factors like TGFβ. Other FN-dependent signals may participate in development of a VSMC-phenotype [18,19]. Interestingly, activation of Notch was limited to those neural crest cells that express FN and α5β1 and Notch activation was needed for VSMC differentiation by neural crest. These findings suggest the novel idea that FN induces an autocrine signaling response by cells [19]*. Future interpretations of FN’s effects should consider potentially distinct roles of paracrine and autocrine mechanisms of FN action.
Synergy between FN-integrin and homophilic cadherin interactions
Matrix assembly and integrin activity determine somite border formation and recent genetic studies in zebrafish identified Rap1 GTPase as an inside-out activator of α5 integrin in this process. Whereas loss of α5 has an effect on anterior somite formation with a modest reduction in FN matrix [20], knockdown of both Rap1 and α5 caused loss of all somite borders and complete disruption of FN matrix [21]. The regulation of integrin activation and FN matrix assembly during somitogenesis occurs by a unique mechanism. Homophilic cell-cell interactions between cadherin 2 molecules in the presomitic mesoderm stabilize the association of α5 integrins on adjacent cells to maintain them in an inactive state [22]**. The unusual integrin-integrin association was suggested when cells lacking α5 were introduced into a mutant host zebrafish that lacks proper segmental patterning and these cells rescued α5 activation and FN matrix formation. Cadherin 2-null zebrafish have ectopic FN matrix assembly and α5 clustering, supporting its role as an integrin regulator [22]. Eph/ephrin signaling can activate integrins at somite boundaries [23] suggesting that a pathway from ephrin through Rap1 activates α5 and promotes FN matrix assembly during morphogenesis of somite boundaries.
Cadherins have also been linked to FN matrix assembly in chondrocyte differentiation and salivary gland cleft formation. The early events in chondrogenic differentiation such as cell condensation and changes in gene expression are recapitulated in a micromass culture system using mesenchymal stem cells [24]. We showed that cell aggregation depends on FN matrix assembly [25]. As mesenchymal stem cells condense, FN and N-cadherin levels are up-regulated and matrix assembly increases. Once cells have condensed into an aggregate, N-cadherin is down-regulated but FN matrix assembly continues. During this period, cells proliferate and initiate the differentiation program, which requires induction of the Sox9 transcription factor, which is essential for cartilage-specific gene expression. Either the blockade of FN polymerization [25] or the knockdown of N-cadherin [26] in micromass cultures prevents cell condensation. Incomplete condensation prevents up-regulation of Sox9 expression and cell differentiation into chondrocytes. These data and the role of cadherins in somite morphogenesis suggest that cadherin interactions bring or keep cells juxtaposed during the initial stages of morphogenesis but then FN matrix assembly takes over to maintain cell connections while allowing cell rearrangements needed for subsequent steps in tissue development.
Differential regulation of cadherins and FN matrix assembly may be a common mechanism for cell aggregation as it has been connected to differences in cohesion of glioblastoma cells that vary in invasiveness [27]. Cell clustering, as shown on a collagen matrix substrate, depends not only on FN and α5β1 but also on development of a pro- contractile phenotype. LPA treatment stimulates contractility and clustering concomitant with FN matrix assembly [28]. In fact, the clustering appears to depend on a balance between pro-contractile signals that promote matrix formation and pro-migratory signals such as PDGF and the matrix metalloprotease MMP-2 that induce matrix turnover [29]. This balance may be a common mechanism for cell clustering in development and disease.
Regulated contractility is also proposed to explain cleft formation during branching morphogenesis in salivary gland explants. A localized increase in FN fibril formation decreased expression of E-cadherin allowing cleft formation sites to form on the surface of the explant [30]. Myosin phosphatase appears to be a central regulator of this process. By binding to either myosin light chain or a deacetylase (HDAC6), it controls the balance between cell contractility and microtubule acetylation [31]**. Binding to HDAC6 caused hyperacetylation of microtubules, which increased adhesion maturation and induced FN matrix formation over the entire surface of the explant resulting in decreased cleft formation. On the other hand, dephosphorylation of myosin light chain by the phosphatase reduced contractility sufficiently to allow localized changes that promote cleft formation [31].
Another type of FN-cell interaction involving exosomes has recently been implicated in intercellular communication. Exosomes are endosome-derived extracellular vesicles involved in intercellular signaling and delivery of cargo such as signaling proteins, lipids and miRNAs, to neighboring cells [32]. Tumor-derived exosomes can promote tumor progression and cell invasion [33]. FN has now been shown to play an important role as a mediator of myeloma-derived exosome targeting. Interestingly, FN binds to heparan sulfate on the surface of exosomes and acts as a bridge to heparan sulfate proteoglycans on the target cells [34]*. Blocking antibodies against FN prevented exosome delivery suggesting a potential new approach to modulate tumor progression and other functional effects downstream of exosome targeting.
Cooperation between FN matrix and growth factors
TGFβ has essential roles in both normal and pathogenic cell differentiation and tissue morphogenesis. It is a well-known inducer of expression of genes involved in cell adhesion and matrix assembly. In myofibroblast differentiation, for example, expression of ECM proteins such as FN and type I collagen and of cell contractility molecules (e.g., α-smooth muscle actin) are up-regulated [35,36] and FN matrix assembly is enhanced [37]. However, in some cells, co-regulation of ECM molecules by TGFβ can modulate the extent of FN assembly. Hyaluronan (HA) and FN were both increased in the matrix of TGFβ-induced human lung myofibroblasts. However, FN levels could be further enhanced by degradation of HA or inhibition of HA synthase (HAS) [38] suggesting that HA in the matrix has an inhibitory effect on FN assembly. Changes in ECM protein levels are not necessarily a direct effect of TGFβ stimulation. For example, the transcription factor SOX4 is induced by TGFβ and then up-regulates mesenchymal genes including FN [39]. Therefore, it is important to control the timing of experiments in order to distinguish signals directly downstream of TGFβ from those that are several steps removed from TGFβ action.
During development, FN matrix potentiates morphogenetic signals such as Fgf8 and S1P, as mentioned above. Epithelial-mesenchymal transition (EMT), another important morphogenetic process, is induced by TGFβ with dramatic changes in gene expression and cell phenotype [40]. FN matrix signaling has recently been shown to cooperate with TGFβ to stimulate EMT [41]**. While definitive changes in EMT can take many hours or days in some cases, significant up-regulation of key EMT proteins, including FN, Snail, and N-cadherin, was detected within 6 hrs of TGFβ treatment in mammary epithelial cells attached to FN but not in cells on laminin [41]. Smad2 phosphorylation was also enhanced by FN. Notably, interactions with FN allowed cells to respond to lower concentrations of TGFβ indicating that FN and TGFβ signals cooperate to help cells reach a threshold needed for induction of EMT. While these results implicate the canonical Smad pathway in the cooperative effects, a non- canonical pathway with PEAK1 as a node connecting TGFβ receptor signals with integrin signals through Src and ERK has also been reported [42]. Another EMT inducer, the matrix metalloprotease MMP-3, also relies on FN-cell interactions since EMT was activated by MMP-3 treatment of mammary epithelial cells only when the cells were growing on a FN substrate; cells on laminin did not undergo EMT [43]. These results with mammary epithelial cells and EMT show that cell-FN interactions cooperate with, and prolong the cellular responses to, growth factors and other extracellular inducers.
Concluding remarks
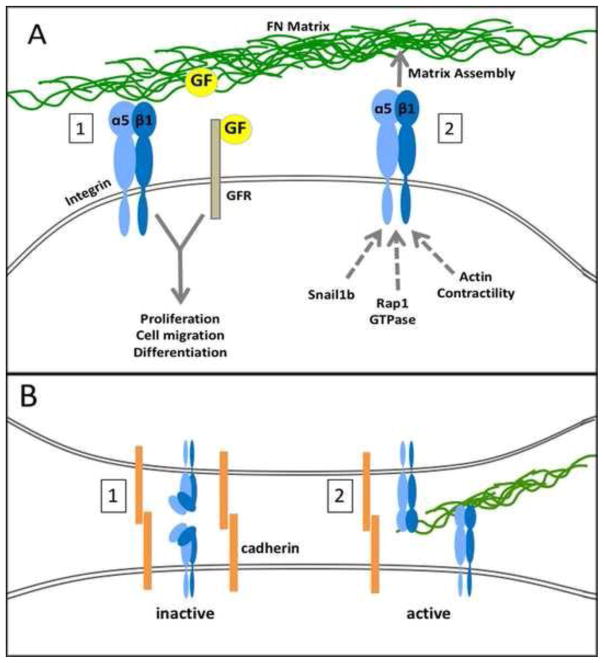
While FN matrix stimulates cells directly through α5β1 integrin to the actin cytoskeleton and intracellular pathways, it also collaborates with other extracellular signals to regulate morphogenesis and differentiation. The effects of soluble extracellular signaling factors are potentiated by outside-in signals involving FN matrix to promote cell growth, motility, and differentiation (Figure 1A-1). Intracellularly, α5β1 is affected by other pathways to induce assembly of a FN matrix. In vivo evidence shows that inside-out signaling can be downstream of transcriptional regulation, other transmembrane receptor signals, and cell contractility (Figure 1A-2). Integrin-cadherin collaboration appears to have a dual role in morphogenesis (Figure 1B), maintaining integrins in an inactive state during somitogenesis and bringing cells together to induce matrix assembly in precartilage condensation. Outside-in and inside-out signals have been described before but these latest results show mechanistic significance in discrete developmental events. Furthermore, not only does FN enhance the effects of other signals, it also appears to collaborate with itself to promote cell migration and differentiation through autocrine signaling.
Figure 1.
Figure 1A. Bidirectional signaling by a FN matrix.
(1) Outside-in signaling is initiated by α5β1 (blue) binding to FN matrix (green). FN matrix provides binding sites for soluble growth factors (GF) which facilitates GF binding and activation of its receptor (GFR). Intracellular integration of FN and GF signals stimulates specific cell responses. Pathways mentioned in the text that collaborate with FN matrix include TGFβ, Fgf8, S1P, and Notch.
(2) Inside-out signaling promotes FN matrix assembly by activating α5β1 integrins. Mechanisms include stimulation of integrin activity by cytoplasmic pathways that involve Rap1 GTPase or actin contractility and by α5 expression induced by Snail1b transcription factor.
Figure 1B. Collaboration between integrins and cadherins to regulate FN matrix assembly.
(1) Homophilic interactions between cadherins (orange) on neighboring presomitic mesodermal cells maintain integrins (blue) in an inactive folded conformation.
(2) Downregulation of cadherins allows integrin-mediated FN matrix assembly between neighboring cells, as observed in precartilage condensation.
Acknowledgments
The authors wish to acknowledge NIH for funding CA160611 and EB001046 (to J.E.S).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hynes RO, Yamada KM. In: Extracellular Matrix Biology. Schubach RSMSD, editor. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press; 2012. [Google Scholar]
- 2.Mecham RP. In: The Biology of Extracellular Matrix. Mecham RP, editor. Springer- Verlag; Berlin Heidelberg Springer: 2011. [Google Scholar]
- 3.Singh P, Carraher C, Schwarzbauer JE. Assembly of fibronectin extracellular matrix. Annu Rev Cell Dev Biol. 2010;26:397–419. doi: 10.1146/annurev-cellbio-100109-104020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwarzbauer JE, DeSimone DW. Fibronectins, their fibrillogenesis, and in vivo functions. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a005041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.George EL, Baldwin HS, Hynes RO. Fibronectins are essential for heart and blood vessel morphogenesis but are dispensable for initial specification of precursor cells. Blood. 1997;90:3073–3081. [PubMed] [Google Scholar]
- 6.George EL, Georges-Labouesse EN, Patel-King RS, Rayburn H, Hynes RO. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development. 1993;119:1079–1091. doi: 10.1242/dev.119.4.1079. [DOI] [PubMed] [Google Scholar]
- 7.Mittal A, Pulina M, Hou SY, Astrof S. Fibronectin and integrin alpha 5 play requisite roles in cardiac morphogenesis. Dev Biol. 2013;381:73–82. doi: 10.1016/j.ydbio.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen D, Wang X, Liang D, Gordon J, Mittal A, Manley N, Degenhardt K, Astrof S. Fibronectin signals through integrin alpha5beta1 to regulate cardiovascular development in a cell type-specific manner. Dev Biol. 2015;407:195–210. doi: 10.1016/j.ydbio.2015.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park EJ, Ogden LA, Talbot A, Evans S, Cai CL, Black BL, Frank DU, Moon AM. Required, tissue-specific roles for Fgf8 in outflow tract formation and remodeling. Development. 2006;133:2419–2433. doi: 10.1242/dev.02367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stainier DY. Zebrafish genetics and vertebrate heart formation. Nat Rev Genet. 2001;2:39–48. doi: 10.1038/35047564. [DOI] [PubMed] [Google Scholar]
- 11.Qiao L, Gao H, Zhang T, Jing L, Xiao C, Xiao Y, Luo N, Zhu H, Meng W, Xu H, et al. Snail modulates the assembly of fibronectin via alpha5 integrin for myocardial migration in zebrafish embryos. Sci Rep. 2014;4:4470. doi: 10.1038/srep04470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hisano Y, Ota S, Takada S, Kawahara A. Functional cooperation of spns2 and fibronectin in cardiac and lower jaw development. Biol Open. 2013;2:789–794. doi: 10.1242/bio.20134994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13**.Benesh EC, Miller PM, Pfaltzgraff ER, Grega-Larson NE, Hager HA, Sung BH, Qu X, Baldwin HS, Weaver AM, Bader DM. Bves and NDRG4 regulate directional epicardial cell migration through autocrine extracellular matrix deposition. Mol Biol Cell. 2013;24:3496–3510. doi: 10.1091/mbc.E12-07-0539. Benesh et al. The authors report that a complex of blood vessel epicardial substrate (Bves) and N-Myc downstream regulated gene 4 (NDRG4) regulates directional migration of epicardial cells through a novel mechanism that recycles vesicular FN to the basal cell surface for substrate deposition. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qu X, Jia H, Garrity DM, Tompkins K, Batts L, Appel B, Zhong TP, Baldwin HS. Ndrg4 is required for normal myocyte proliferation during early cardiac development in zebrafish. Dev Biol. 2008;317:486–496. doi: 10.1016/j.ydbio.2008.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kachhap SK, Faith D, Qian DZ, Shabbeer S, Galloway NL, Pili R, Denmeade SR, DeMarzo AM, Carducci MA. The N-Myc down regulated Gene1 (NDRG1) Is a Rab4a effector involved in vesicular recycling of E-cadherin. PLoS One. 2007;2:e844. doi: 10.1371/journal.pone.0000844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16**.Liang D, Wang X, Mittal A, Dhiman S, Hou SY, Degenhardt K, Astrof S. Mesodermal expression of integrin alpha5beta1 regulates neural crest development and cardiovascular morphogenesis. Dev Biol. 2014;395:232–244. doi: 10.1016/j.ydbio.2014.09.014. Liang et al. An anterior mesoderm-specific knockout of α5 integrin shows defects in neural crest differentiation suggesting a novel model in which mesodermal α5β1 and its FN matrix signal between the mesoderm and neural crest cells, thereby regulating neural crest development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17*.Turner CJ, Badu-Nkansah K, Crowley D, van der Flier A, Hynes RO. alpha5 and alphav integrins cooperate to regulate vascular smooth muscle and neural crest functions in vivo. Development. 2015;142:797–808. doi: 10.1242/dev.117572. Turner et al. Mice that are double null for α5/αv in VSMCs have defects in cardiovascular development and VSMC differentiation. Several ECM proteins including Ltbp1 are absent from the vessel wall ECM indicating that the defects result, at least in part, from reduced TGFβ signaling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi F, Long X, Hendershot A, Miano JM, Sottile J. Fibronectin matrix polymerization regulates smooth muscle cell phenotype through a Rac1 dependent mechanism. PLoS One. 2014;9:e94988. doi: 10.1371/journal.pone.0094988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19*.Wang X, Astrof S. Neural crest cell-autonomous roles of fibronectin in cardiovascular development. Development. 2016;143:88–100. doi: 10.1242/dev.125286. Wang and Astrof. Using a cardiac neural crest cell-specific knockout of FN and α5 integrin, the authors show the requirement for FN matrix during differentiation of to VSMCs, in part by mediating activation of Notch signaling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Julich D, Geisler R, Holley SA, Tubingen Screen C. Integrinalpha5 and delta/notch signaling have complementary spatiotemporal requirements during zebrafish somitogenesis. Dev Cell. 2005;8:575–586. doi: 10.1016/j.devcel.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 21.Lackner S, Schwendinger-Schreck J, Julich D, Holley SA. Segmental assembly of fibronectin matrix requires rap1b and integrin alpha5. Dev Dyn. 2013;242:122–131. doi: 10.1002/dvdy.23909. [DOI] [PubMed] [Google Scholar]
- 22**.Julich D, Cobb G, Melo AM, McMillen P, Lawton AK, Mochrie SG, Rhoades E, Holley SA. Cross-Scale Integrin Regulation Organizes ECM and Tissue Topology. Dev Cell. 2015;34:33–44. doi: 10.1016/j.devcel.2015.05.005. Julich et al. Using fluorescence cross-correlation spectroscopy of zebrafish paraxial mesoderm, the authors demonstrate interaction between α5 integrins on adjacent cells. These integrin interactions are stabilized by Cadherin 2 and maintain the integrins in an inactive state. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Julich D, Mould AP, Koper E, Holley SA. Control of extracellular matrix assembly along tissue boundaries via Integrin and Eph/Ephrin signaling. Development. 2009;136:2913–2921. doi: 10.1242/dev.038935. [DOI] [PubMed] [Google Scholar]
- 24.Singh P, Schwarzbauer JE. Fibronectin and stem cell differentiation - lessons from chondrogenesis. J Cell Sci. 2012;125:3703–3712. doi: 10.1242/jcs.095786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh P, Schwarzbauer JE. Fibronectin matrix assembly is essential for cell condensation during chondrogenesis. J Cell Sci. 2014;127:4420–4428. doi: 10.1242/jcs.150276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bobick BE, Chen FH, Le AM, Tuan RS. Regulation of the chondrogenic phenotype in culture. Birth Defects Res C Embryo Today. 2009;87:351–371. doi: 10.1002/bdrc.20167. [DOI] [PubMed] [Google Scholar]
- 27.Shannon S, Vaca C, Jia D, Entersz I, Schaer A, Carcione J, Weaver M, Avidar Y, Pettit R, Nair M, et al. Dexamethasone-Mediated Activation of Fibronectin Matrix Assembly Reduces Dispersal of Primary Human Glioblastoma Cells. PLoS One. 2015;10:e0135951. doi: 10.1371/journal.pone.0135951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.da Rocha-Azevedo B, Ho CH, Grinnell F. Fibroblast cluster formation on 3D collagen matrices requires cell contraction dependent fibronectin matrix organization. Exp Cell Res. 2013;319:546–555. doi: 10.1016/j.yexcr.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.da Rocha-Azevedo B, Ho CH, Grinnell F. PDGFstimulated dispersal of cell clusters and disruption of fibronectin matrix on three-dimensional collagen matrices requires matrix metalloproteinase-2. Mol Biol Cell. 2015;26:1098–1105. doi: 10.1091/mbc.E14-09-1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sakai T, Larsen M, Yamada KM. Fibronectin requirement in branching morphogenesis. Nature. 2003;423:876–881. doi: 10.1038/nature01712. [DOI] [PubMed] [Google Scholar]
- 31**.Joo EE, Yamada KM. MYPT1 regulates contractility and microtubule acetylation to modulate integrin adhesions and matrix assembly. Nat Commun. 2014;5:3510. doi: 10.1038/ncomms4510. Joo et al. A balance between cell contractility and microtubule acetylation modulates branching morphogenesis in salivary gland explants. This balance is reached through the action of myosin phosphatase on either myosin light chain or HDAC6 to decrease or increase contractility, respectively. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang HG, Grizzle WE. Exosomes: a novel pathway of local and distant intercellular communication that facilitates the growth and metastasis of neoplastic lesions. Am J Pathol. 2014;184:28–41. doi: 10.1016/j.ajpath.2013.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thompson CA, Purushothaman A, Ramani VC, Vlodavsky I, Sanderson RD. Heparanase regulates secretion, composition, and function of tumor cell- derived exosomes. J Biol Chem. 2013;288:10093–10099. doi: 10.1074/jbc.C112.444562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34*.Purushothaman A, Bandari SK, Liu J, Mobley JA, Brown EE, Sanderson RD. Fibronectin on the Surface of Myeloma Cell-derived Exosomes Mediates Exosome-Cell Interactions. J Biol Chem. 2016;291:1652–1663. doi: 10.1074/jbc.M115.686295. Purushothaman et al. Analysis of myeloma cell-derived exosomes shows that FN bound to heparan sulfate on the extracellular surface of exosomes mediates exosome interactions with target cells to stimulate endothelial cell invasion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Desai VD, Hsia HC, Schwarzbauer JE. Reversible modulation of myofibroblast differentiation in adipose-derived mesenchymal stem cells. PLoS One. 2014;9:e86865. doi: 10.1371/journal.pone.0086865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hinz B, Phan SH, Thannickal VJ, Prunotto M, Desmouliere A, Varga J, De Wever O, Mareel M, Gabbiani G. Recent developments in myofibroblast biology: paradigms for connective tissue remodeling. Am J Pathol. 2012;180:1340–1355. doi: 10.1016/j.ajpath.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Torr EE, Ngam CR, Bernau K, Tomasini-Johansson B, Acton B, Sandbo N. Myofibroblasts exhibit enhanced fibronectin assembly that is intrinsic to their contractile phenotype. J Biol Chem. 2015;290:6951–6961. doi: 10.1074/jbc.M114.606186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Evanko SP, Potter-Perigo S, Petty LJ, Workman GA, Wight TN. Hyaluronan Controls the Deposition of Fibronectin and Collagen and Modulates TGF- beta1 Induction of Lung Myofibroblasts. Matrix Biol. 2015;42:74–92. doi: 10.1016/j.matbio.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vervoort SJ, Lourenco AR, van Boxtel R, Coffer PJ. SOX4 mediates TGF-beta- induced expression of mesenchymal markers during mammary cell epithelial to mesenchymal transition. PLoS One. 2013;8:e53238. doi: 10.1371/journal.pone.0053238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41**.Park J, Schwarzbauer JE. Mammary epithelial cell interactions with fibronectin stimulate epithelial-mesenchymal transition. Oncogene. 2014;33:1649–1657. doi: 10.1038/onc.2013.118. Park and Schwarzbauer. Using mammary epithelial cells, the authors show that FN, but not laminin, induces EMT and that cell interactions with FN potentiate an EMT response such that sub-threshold levels of TGFβ are able to induce an EMT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Agajanian M, Campeau A, Hoover M, Hou A, Brambilla D, Kim SL, Klemke RL, Kelber JA. PEAK1 Acts as a Molecular Switch to Regulate Context- Dependent TGFbeta Responses in Breast Cancer. PLoS One. 2015;10:e0135748. doi: 10.1371/journal.pone.0135748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen QK, Lee K, Radisky DC, Nelson CM. Extracellular matrix proteins regulate epithelial-mesenchymal transition in mammary epithelial cells. Differentiation. 2013;86:126–132. doi: 10.1016/j.diff.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]