Abstract
Methamphetamine (Meth) use is common among HIV-infected persons. It remains unclear whether Meth dependence is associated with long-lasting degenerative changes in the brain parenchyma and microvasculature of HIV-infected individuals. We examined the postmortem brains of 78 HIV-infected adults, twenty of whom were diagnosed with lifetime Meth dependence (18 past and two current at the final follow-up visit). Using logistic regression models, we analyzed associations of Meth with cerebral gliosis (immunohistochemistry for ionized calcium-binding adapter molecule-1 [Iba1] and glial fibrillary acidic protein [GFAP] in frontal, temporo-parietal, and putamen-internal capsule regions), synaptodendritic loss (confocal microscopy for synaptophysin [SYP] and microtubule-associated protein-2 [MAP2] in frontal cortex), β-amyloid plaque deposition (immunohistochemistry in frontal and temporo-parietal cortex and putamen), and arteriolosclerosis (histopathology in forebrain white matter). We found that Meth was associated with marked Iba1 gliosis in the temporo-parietal region (odds ratio 4.42 [95% confidence interval 1.36, 14.39], p=0.014, n=62), which remained statistically significant after adjusting for HIV encephalitis, white matter lesions, and opportunistic diseases (n=61); hepatitis C virus seropositivity (n=54); and lifetime dependence on alcohol, opiates, and cannabis (n=62). There was no significant association of Meth with GFAP gliosis, SYP or MAP2 loss, β-amyloid plaque deposition, or arteriolosclerosis. In conclusion, we found lifetime Meth dependence to be associated with focal cerebral microgliosis among HIV-infected adults, but not with other brain degenerative changes examined. Some of the changes in select brain regions might be reversible following extended Meth abstinence or, alternatively, might have not been induced by Meth initially.
Keywords: Arteriolosclerosis, β-Amyloid, Astrogliosis, Methamphetamine, Microgliosis, Small vessel disease
Introduction
Methamphetamine (Meth) use is a common comorbidity in HIV-infected persons (Cadet and Krasnova 2007). Meth dependence was found to increase the risk of cognitive impairment in HIV-infected individuals (Rippeth et al. 2004) particularly those who had advanced immune suppression (Carey et al. 2006). Additive effects of chronic Meth use and HIV infection were documented on cerebral metabolite abnormalities denoting neuronal injury and glial activation, measured with proton magnetic resonance (MR) spectroscopy (Chang et al. 2005). In another similar study (Taylor et al. 2007), although the effects of Meth on cerebral metabolite changes were not evident, Meth appeared to modify the effects of HIV in individuals with high plasma viral loads.
The pharmacokinetics of Meth in the human brain includes fast uptake, widespread distribution, and slow clearance, as shown in positron emission tomography (PET) studies with [11C]-d-Meth (Fowler et al. 2008; Volkow et al. 2010), suggesting extended brain exposure to the sympathomimetic and toxic effects of Meth. The widespread distribution of Meth was also observed in the postmortem brains of chronic Meth users with the presence of Meth in blood (Kalasinsky et al. 2001). Accordingly, chronic Meth use may induce long-lasting injury involving broad brain regions and not limited to structures containing presynaptic monoaminergic nerve terminals (Cadet and Krasnova 2007; Kuhn et al. 2011). In support of this notion, a PET study with [11C](R)-PK11195 (a radiotracer targeting the 18-kDa translocator protein enriched in activated microglia) showed higher activated microglial density in multiple brain regions of abstinent Meth users when compared to age-matched control participants (Sekine et al. 2008). Also, evidence of increased white matter hyperintensities on MR imaging, suggestive of ischemic lesions caused by cerebral small vessel disease (Ovbiagele and Saver 2006), was reported in men with lifetime Meth dependence (Bae et al. 2006).
Relatively few studies have addressed the effects of chronic Meth use on immunohistologic and gene expression changes in postmortem HIV-infected brains (Chana et al. 2006; Everall et al. 2005; Langford et al. 2003). For instance, Langford et al. (2003) reported that among HIV encephalitis brains, the brains of chronic Meth users had higher degrees of CD45-immunoreactive microgliosis and synaptophysin immunoreactivity loss in the frontal cortex. Nonetheless, it remains unclear whether Meth dependence is associated with long-lasting degenerative changes in the brain parenchyma and microvasculature of HIV-infected individuals with consideration of a variety of biological-relevant comorbid factors.
In the present study, we investigated associations of lifetime Meth dependence with cerebral microgliosis, astrogliosis, synaptodendritic loss, β-amyloid plaque deposition, and arteriolosclerosis in the postmortem brains of HIV-infected adults.
Methods
Autopsy cohort
We gathered 78 autopsy HIV-infected cases that were evaluated during life for Meth dependence [using either Psychiatric Research Interview for Substance and Mental Disorders (Hasin et al. 1996) or Composite International Diagnostic Interview (Robins et al. 1988)] and had archival brain tissue blocks available in the California NeuroAIDS Tissue Network (CNTN, #R458). The University of California San Diego Human Research Protections Program approved the project. All study participants provided written informed consent. The written consent to autopsy was obtained. There were 63 men (80.8%). The study participants died between 1999 and 2010 and ranged in age at death from 26 to 70 years (median 46 years, interquartile range [IQR] 39–54 years); 30 (38.5%) cases aged ≥50 years (older age). Forty-six cases (59.0%) were white, 14 (17.9%) Hispanic, 13 (16.7%) black, and five (6.4%) Asian or other race/ethnicity. The apolipoprotein-E (APOE) ε4 status [having one or two ε4 alleles, based on the allelic discrimination assay; Taqman SNP Genotyping Assays, Applied Biosystems, Carlsbad, CA, USA (Soontornniyomkij et al. 2012a)] was present in 21 (31.3%) of 67 cases examined. Hepatitis C virus (HCV) seropositivity was detected in 29 (41.4%) of 70 cases that underwent serological testing. Of all 78 cases, seven (9%) had diabetes mellitus, 24 (30.8%) had systemic arterial hypertension, and 14 (17.9%) had dyslipidemia. Lifetime cigarette smoking was found in 25 (32.5%) of 77 cases with available data. Of all 78 cases, lifetime dependence on alcohol was present in 35 (44.9%), opiates in ten (12.8%), and cannabis in eight (10.3%).
In the present study, highly active antiretroviral therapy (HAART) was defined as regimens containing three or more antiretroviral medications from at least two different drug classes (Soontornniyomkij et al. 2014). The antiretroviral regimens used at the last follow-up visit closest to death (median 14.6 weeks, IQR 3.5–31.3 weeks prior to death) were categorized into protease inhibitor-based HAART, non-protease inhibitor-based HAART, and no HAART (i.e., receiving non-HAART regimens, discontinuation of antiretroviral treatment, or never receiving antiretroviral treatment). The estimate of central nervous system penetration effectiveness (CPE) of individual antiretroviral drugs was ranked from 1 (poorest) to 4 (best), in accordance with the 2010 CPE ranking system (Letendre et al. 2010). The CPE score of each antiretroviral regimen was derived from adding all of the ranks. In the present study, the CPE scores ranged from 2 to 14 (median 7, IQR 6–8) in patients who were receiving antiretroviral treatment at the last follow-up visit closest to death (n=48).
The median HIV infection duration (i.e., the estimated duration of HIV infection from the patient’s first awareness to death) was 14.0 years (IQR 8.5–19.3 years, n=70). In the total follow-up period, the median on-antiretroviral duration (i.e., the estimated total duration of exposure to any antiretroviral regimens) was 2.7 years (IQR 0.7–6.6 years, n=71). The median off-antiretroviral duration (i.e., the HIV infection duration subtracted by the on-antiretroviral duration) was 9.7 years (IQR 4.5–14.3 years, n=65).
The median postmortem interval was 13 hours (IQR 12–28 hours, n=77). Of all 78 cases, 77 (98.7%) had AIDS-defining conditions. In addition, the systemic autopsy findings commonly included hepatic cirrhosis, atherosclerotic cardiovascular disease, myocardial infarction, malignant neoplasms (primarily lymphomas, lung carcinomas, and hepatocellular carcinoma), and pneumonia.
Brain histopathology
The neuropathologist (V.S.), who was blinded to clinical and autopsy information, reviewed hematoxylin and eosin-stained paraffin-embedded formalin-fixed brain tissue slides, as previously described (Soontornniyomkij et al. 2014). HIV encephalitis was histologically defined as multiple foci of microgliosis, multinucleated giant cells, and astrogliosis (Budka et al. 1991). White matter (WM) lesions were defined as foci of WM rarefaction with macrophage infiltration or astrogliosis in the forebrain in the absence of HIV encephalitis and progressive multifocal leukoencephalopathy. Arteriolosclerosis was defined as concentric intramural hyalinization of small arteries or arterioles in the forebrain WM, excluding those vessels in the ependymal vicinity, and was graded as absent (normal), mild (partial-thickness involvement), or severe (full-thickness involvement with or without perivascular space dilatation); in each case a grade of arteriolosclerosis was assigned based on the severest lesion (Soontornniyomkij et al. 2014) (Electronic Supplementary Table 1). Opportunistic infections (i.e., cytomegalovirus disease, cryptococcosis, progressive multifocal leukoencephalopathy, and toxoplasmosis) were found in 12 (15.8%), primary non-Hodgkin lymphomas in three (3.9%), and metastatic carcinomas in two (2.6%) of 76 cases examined.
Immunohistochemistry for Iba1, GFAP, and β-amyloid
Immunohistochemical staining was performed on five-µm-thick sections prepared from three paraffin-embedded formalin-fixed brain tissue blocks: frontal, temporal or parietal, and putamen-internal capsule. We used primary antibodies raised against ionized calcium-binding adapter molecule-1 (Iba1, microglia marker; rabbit polyclonal, #019-19741, Wako, Richmond, VA, USA; 1:1,000 dilution), glial fibrillary acidic protein (GFAP, astroglia marker; rabbit polyclonal, #Z0334, Dako, Carpinteria, CA, USA; 1:1,000), and β-amyloid (mouse monoclonal, clone 4G8, #SIG-39220, Covance, Princeton, NJ, USA; 1:20,000). Antigen retrieval was performed in 10-mM Tris/1-mM EDTA-2Na/0.05% Tween-20 buffer (pH 9) in 121°C autoclave (20 min) for Iba1 and GFAP, or 90% formic acid (5 min) for β-amyloid. Following 24-hour incubation at 4°C with the primary antibody, immunohistochemical signals were developed using species-appropriate ImmPRESS anti-IgG (peroxidase) polymer detection kits (Vector Laboratories, Burlingame, CA, USA; 40 min) and diaminobenzidine (ImmPACT DAB Peroxidase Substrate; Vector Laboratories; 5 min), as previously described (Soontornniyomkij et al. 2012b). The β-amyloid immunostained tissue sections were counterstained with Mayer hematoxylin. Isocortical tissue sections from an Alzheimer’s disease brain were used as the positive tissue control for β-amyloid immunohistochemical staining (Soontornniyomkij et al. 2012a). For the negative reagent control, the primary antibody was omitted.
For quantification of Iba1 and GFAP density, the DAB-immunostained tissue slides were scanned using a microscope slide scanner (20x objective lens, Aperio ScanScope GL, Leica Biosystems, Buffalo Grove, IL, USA). Using the Aperio ImageScope software, a square of 3,000 × 3,000 µm2 was extracted from the cortical gray matter (GM) and subcortical WM (of the frontal and temporo-parietal regions), putamen, and internal capsule separately. Using the Image-Pro Analyzer software (Version 6.3, Media Cybernetics, Bethesda, MD, USA), the DAB intensity was quantified within each area of interest, i.e., the cortical GM layers II–VI, subcortical WM, putamen, and internal capsule, as previously described (Soontornniyomkij et al. 2012b). The DAB intensity per unit area (i.e., DAB density) was calculated, as previously described (Soontornniyomkij et al. 2010). To adjust for the between-batch variation, one brain section from the same positive tissue control block was included in each immunostaining batch. The control DAB density (in a specified area) was used to normalize all the DAB density values of studied cases in the same batch, giving rise to the immunoreactivity density values.
In each case, β-amyloid plaque deposition was designated as present when β-amyloid plaques were found in any of the frontal and temporo-parietal cortical GM and putamen (Electronic Supplementary Table 1).
Immunofluorescence confocal microscopy for SYP and MAP2
Fresh frontal cortical GM tissue blocks were fixed in 4% paraformaldehyde overnight, serially cut at 40 µm thick with the vibratome, and stored at −30°C in cryoprotective medium (30% ethylene glycol, 30% glycerin, and 40% phosphate-buffered saline). Free-floating tissue sections were incubated with mouse monoclonal primary antibodies against synaptophysin (SYP, presynaptic terminal marker; clone SY38, #MAB5258, EMD Millipore, Billerica, MA, USA; 1:10 dilution) or microtubule-associated protein-2 (MAP2, dendritic marker; clone 5F9, #05-346, EMD Millipore; 1:20). Following overnight incubation, the sections were washed and then incubated for one hour with FITC-conjugated horse anti-mouse IgG secondary antibody (Vector Laboratories; 1:75), as described previously (Langford et al. 2003). The sections were washed and then transferred to microscope slides and mounted under glass coverslips with anti-fading medium.
For quantification of SYP and MAP2 density, fluorescence-immunostained tissue slides were imaged with the laser scanning confocal microscope (63x objective lens, MRC-1024, Bio-Rad, Hercules, CA, USA) and the LaserSharp software (Version 3.2, Bio-Rad). For each case, three microscopic fields were imaged and the fluorescence density was measured using the ImageJ software (National Institutes of Health, Bethesda, MD, USA) (Langford et al. 2003). The immunoreactivity density value (Electronic Supplementary Table 1) was derived from the average of three fluorescence density values.
Categorical transformation of Iba1, GFAP, SYP, and MAP2 density
In each of three brain regions (frontal, temporo-parietal, and putamen-internal capsule), the Iba1 immunoreactivity density in the GM correlated directly with that in the WM (n=64, rs=0.74; n=62, rs=0.78; and n=64, rs=0.67, respectively; p<0.0001 each region; Spearman’s rank correlation coefficient). In each of the frontal and putamen-internal capsule regions, the GFAP immunoreactivity density in the GM correlated directly with that in the WM (n=64, rs=0.48, p<0.0001; and n=64, rs=0.26, p=0.037, respectively). In the temporo-parietal region, the similar correlation did not reach statistical significance (n=62, rs=0.22, p=0.08).
In each of the three brain regions, we averaged the (Iba1 or GFAP) immunoreactivity density values in the GM and WM to generate the regional density value (Electronic Supplementary Table 1). Then, we transformed the regional density values (continuous variable) in the present study (78 cases) into three severity grades (categorical variable), based on their tertile ranks within our CNTN database (144 cases, of which the present cohort of 78 cases was a subset) of the distributions of regional density values specific for each marker in each brain region. Each of the regional Iba1 and GFAP density values was graded as mild (≤ the lower tertile), moderate (> the lower tertile and < the upper tertile), or marked (≥ the upper tertile) gliosis (Electronic Supplementary Table 1). Based on the same CNTN database, each of the frontal SYP and MAP2 density values was graded as mild (≥ the upper tertile), moderate (< the upper tertile and > the lower tertile), or marked (≤ the lower tertile) loss (Electronic Supplementary Table 1).
Statistical analysis
We performed logistic regression analyses to test associations between the categorical outcomes (severity grades of cerebral parenchymal changes and arteriolosclerosis) and predictor (lifetime Meth dependence). Biological-relevant factors, as well as factors that were distributed differently between non-Meth and Meth groups, were included as covariates in multivariable models. Since the outcomes were grading of severity, ordinal models that did not assume proportional odds were chosen for all pathologic changes examined. The odds ratio (OR) with its 95% confidence interval (CI) reflected the effect size. Mann-Whitney U test and Spearman’s rank correlation coefficient (rs) were employed to analyze continuous variables. Fisher’s exact test was used for comparing frequencies of categorical variables. Two-tailed p values were considered significant at p<0.05. The statistical analyses were carried out using R (Version 3.0.1, 2013, http://www.r-project.org).
Results
Cohort characteristics
Twenty of 78 cases (25.6%) were diagnosed with lifetime Meth dependence [i.e., either past (n=18) or current (n=2)] at the last follow-up visit closest to death (median 14.6 weeks, IQR 3.5–31.3 weeks prior to death) (Electronic Supplementary Table 1). We evaluated the distributions of demographic and biological-relevant factors (Table 1). Between non-Meth and Meth groups, none of these factors showed significant differences, except for the HCV seropositivity, HAART status, alcohol, and cannabis. The frequency of HCV seropositivity was higher in the Meth group (65% vs. 32%). The Meth group had lower frequency in using protease inhibitor-based HAART (20% vs. 53.4%) and higher frequency of the no-HAART status (70% vs. 36.2%). The frequencies of alcohol and cannabis were higher in the Meth group (65% vs. 37.9 % and 25% vs. 5.2%, respectively). Accordingly, in addition to the occurrence of HIV encephalitis or WM lesions and brain opportunistic diseases, the HCV seropositivity and lifetime dependence on substances other than Meth were included as covariates in multivariable models of the cerebral parenchymal changes and arteriolosclerosis (Beitner-Johnson et al. 1993; Letendre et al. 2007; Persidsky et al. 2011). The HAART status (Soontornniyomkij et al. 2014), in addition to diabetes (Pantoni 2010), was included as a covariate in multivariable models of arteriolosclerosis.
Table 1.
Distribution of demographic and biological-relevant factors in relation to lifetime methamphetamine (Meth) dependence
| Non-Meth | Meth | Statistical testa | |
|---|---|---|---|
| Age at death: median [IQR] years, n | 46 [39–55], 58 | 46 [38.8–53.8], 20 | p=0.80 |
| Older age (≥50 years) at death: n (%) | 22 (37.9) | 8 (40.0) | p=1.00 |
| Men: n (%) | 46 (79.3) | 17 (85.0) | p=0.75 |
| Race/ethnicity: n (%) | p=0.41 | ||
| White | 32 (55.2) | 14 (70.0) | |
| Hispanic | 12 (20.7) | 2 (10.0) | |
| Black | 9 (15.5) | 4 (20.0) | |
| Asian or other | 5 (8.6) | 0 (0.0) | |
| Apolipoprotein-E ε4 status: n (%) | 16 (32.7) | 5 (27.8) | p=0.77 |
| Hepatitis C virus seropositivity: n (%) | 16 (32.0) | 13 (65.0) | p=0.016* |
| Diabetes mellitus: n (%) | 3 (5.2) | 4 (20.0) | p=0.07 |
| Hypertension: n (%) | 17 (29.3) | 7 (35.0) | p=0.78 |
| Dyslipidemia: n (%) | 9 (15.5) | 5 (25.0) | p=0.33 |
| Cigarette smoking, lifetime: n (%) | 16 (28.1) | 9 (45.0) | p=0.18 |
| Alcohol dependence, lifetime: n (%) | 22 (37.9) | 13 (65.0) | p=0.042* |
| Opiate dependence, lifetime: n (%) | 7 (12.1) | 3 (15.0) | p=0.71 |
| Cannabis dependence, lifetime: n (%) | 3 (5.2) | 5 (25.0) | p=0.023* |
| HAART status: n (%) | p=0.02* | ||
| Protease inhibitor-based HAART | 31 (53.4) | 4 (20.0) | |
| Non-protease inhibitor-based HAART | 6 (10.3) | 2 (10.0) | |
| No HAARTb | 21 (36.2) | 14 (70.0) | |
| CPE score of antiretroviral regimenc: median [IQR], n | 7 [6–8], 41 | 7 [6.5–9], 7 | p=0.86 |
| HIV infection duration: median [IQR] years, n | 14.5 [8.3–18.2], 52 | 11.7 [9.9–20.4], 18 | p=0.39 |
| On-antiretroviral duration: median [IQR] years, n | 2.8 [0.7–6.6], 53 | 2.5 [0.7–7.8], 18 | p=0.93 |
| Off-antiretroviral duration: median [IQR] years, n | 8.7 [3.7–14.3], 49 | 10.1 [7.6–15.2], 16 | p=0.44 |
| Postmortem interval: median [IQR] hours, n | 13 [12–24], 57 | 15 [10–44], 20 | p=0.67 |
| HIV encephalitis or white matter lesions: n (%) | 13 (22.8) | 5 (26.3) | p=0.76 |
| Opportunistic diseases: n (%) | 8 (14.0) | 6 (31.6) | p=0.10 |
IQR interquartile range, HAART highly active antiretroviral therapy, CPE central nervous system penetration effectiveness
Mann-Whitney U test is used to compare continuous variables (median [IQR]) and Fisher’s exact test for categorical variables [n (%)]
Included in the No HAART category were patients who received non-HAART regimens or no longer received antiretroviral treatment at the last follow-up visit closest to death, and those who had never received antiretroviral treatment
Patients who were receiving antiretroviral treatment at the last follow-up visit closest to death
Statistically significant at p<0.05
Iba1 gliosis
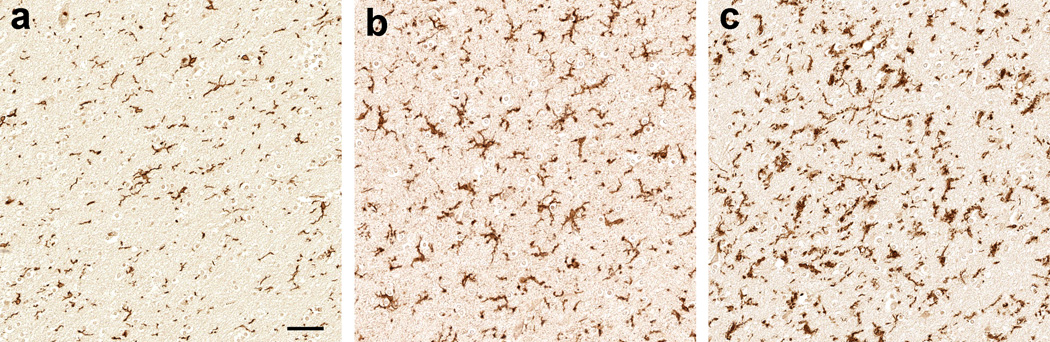
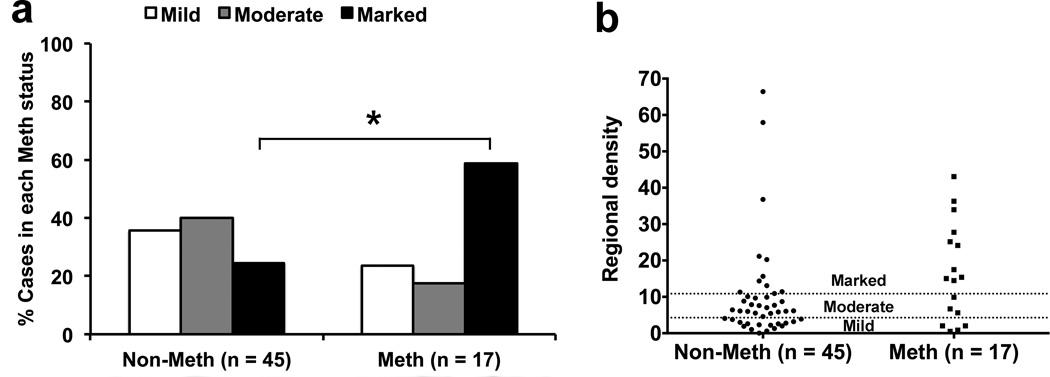
DAB immunohistochemistry for Iba1 showed parenchymal microglia and perivascular macrophages in both GM and WM (Fig. 1a–c). In the univariable logistic regression analysis (Table 2), lifetime Meth dependence was associated with marked Iba1 gliosis in the temporo-parietal region (n=62, OR 4.42 [95% CI 1.36, 14.39], p=0.014, Fig. 2a). This association remained significant after adjusting for HIV encephalitis, WM lesions, and opportunistic diseases (n=61, adjusted OR 5.76 [95% CI 1.67, 19.82], p=0.006); HCV seropositivity (n=54, adjusted OR 3.80 [95% CI 1.04, 13.84], p=0.043); and lifetime dependence on alcohol (n=62, adjusted OR 4.58 [95% CI 1.34, 15.65], p=0.015), opiates (n=62, adjusted OR 4.84 [95% CI 1.45, 16.15], p=0.010), and cannabis (n=62, adjusted OR 4.18 [95% CI 1.22, 14.32], p=0.023). Shown in Fig. 2b is the distribution of the temporo-parietal regional density of Iba1 immunoreactivity in non-Meth and Meth groups.
Fig. 1.
Immunoreactivity pattern for ionized calcium-binding adapter molecule-1 (Iba1) in the cerebral subcortical white matter of HIV-infected adults. Depicted are mild (a), moderate (b), and marked (c) Iba1-immunoreactive microgliosis; scale bar: 50 µm
Table 2.
Cerebral parenchymal changes and arteriolosclerosis in relation to lifetime methamphetamine (Meth) dependence: logistic regression analysis
| Pathologic change | ||||
|---|---|---|---|---|
| Brain region | Non-Meth: n (%) | Meth: n (%) | Odds ratio (OR) [95% CI], p valuea | |
| Iba1 gliosis: mild, moderate, marked | Marked/moderate vs. mild | Marked vs. moderate/mild | ||
| Frontal GM/WM | 15 (32.6), 13 (28.3), 18 (39.1) | 3 (16.7), 12 (66.7), 3 (16.7) | 2.42 [0.61, 9.66], p=0.21 | 0.31 [0.08, 1.23], p=0.10 |
| Temporo-parietal GM/WM | 16 (35.6), 18 (40.0), 11 (24.4) | 4 (23.5), 3 (17.6), 10 (58.8) | 1.79 [0.50, 6.42], p=0.37 | 4.42 [1.36, 14.39], p=0.014*b |
| Putamen/Internal capsule | 18 (37.5), 18 (37.5), 12 (25.0) | 4 (25.5), 4 (25.5), 8 (50.0) | 1.80 [0.50, 6.43], p=0.37 | 3.00 [0.92, 9.74], p=0.07 |
| GFAP gliosis: mild, moderate, marked | Marked/moderate vs. mild | Marked vs. moderate/mild | ||
| Frontal GM/WM | 19 (41.3), 10 (21.7), 17 (37.0) | 5 (27.8), 10 (55.6), 3 (16.7) | 1.83 [0.56, 5.99], p=0.32 | 0.34 [0.09, 1.35], p=0.13 |
| Temporo-parietal GM/WM | 16 (35.6), 14 (31.1), 15 (33.3) | 4 (23.5), 8 (47.1), 5 (29.4) | 1.79 [0.50, 6.42], p=0.37 | 0.83 [0.25, 2.80], p=0.77 |
| Putamen/Internal capsule | 14 (29.2), 14 (29.2), 20 (41.7) | 3 (18.8), 6 (37.5), 7 (43.8) | 1.78 [0.44, 7.24], p=0.42 | 1.09 [0.35, 3.41], p=0.88 |
| SYP loss: mild, moderate, marked | Marked/moderate vs. mild | Marked vs. moderate/mild | ||
| Frontal GMc | 16 (44.4), 14 (38.9), 6 (16.7) | 6 (37.5), 5 (31.3), 5 (31.3) | 1.33 [0.40, 4.46], p=0.64 | 2.27 [0.58, 8.97], p=0.24 |
| MAP2 loss: mild, moderate, marked | Marked/moderate vs. mild | Marked vs. moderate/mild | ||
| Frontal GM | 16 (44.4), 11 (30.6), 9 (25.0) | 6 (37.5), 5 (31.3), 5 (31.3) | 1.33 [0.40, 4.46], p=0.64 | 1.36 [0.37, 5.00], p=0.64 |
| β-Amyloid plaque deposition: absent, present | Present vs. absent | |||
| Frontal GM, temporo parietal GM, and putamend | 34 (69.4), 15 (30.6) | 14 (73.7), 5 (26.3) | 1.10 [0.36, 3.31], p=0.87 | |
| Arteriolosclerosis: absent, mild, severe | Severe/mild vs. absent | Severe vs. mild/absent | ||
| Forebrain WM | 10 (17.9), 18 (32.1), 28 (50.0) | 6 (33.3), 6 (33.3), 6 (33.3) | 0.43 [0.13, 1.44], p=0.17 | 0.50 [0.16, 1.52], p=0.22 |
CI confidence interval, Iba1 ionized calcium-binding adapter molecule-1, GFAP glial fibrillary acidic protein, SYP synaptophysin, MAP2 microtubule-associated protein-2, GM gray matter, WM white matter
Univariable logistic regression with ordinal models being used for all the outcomes (all pathologic changes examined)
In multivariable logistic regression models, lifetime Meth dependence is associated with marked Iba1 gliosis after adjusting for HIV encephalitis, WM lesions, and opportunistic diseases (n=61, adjusted OR 5.76 [95% CI 1.67, 19.82], p=0.006); hepatitis C virus seropositivity (n=54, adjusted OR 3.80 [95% CI 1.04, 13.84], p=0.043); and lifetime dependence on other substances (n=62; alcohol: adjusted OR 4.58 [95% CI 1.34, 15.65], p=0.015; opiates: adjusted OR 4.84 [95% CI 1.45, 16.15], p=0.010; and cannabis: adjusted OR 4.18 [95% CI 1.22, 14.32], p=0.023)
The presence of HIV encephalitis or WM lesions is associated with SYP loss after adjusting for lifetime Meth dependence and opportunistic diseases (n=51; adjusted OR [marked/moderate vs. mild] 11.77 [95% CI 1.35, 102.46], p=0.025; adjusted OR [marked vs. moderate/mild] 6.42 [95% CI 1.44, 28.65], p=0.015) in multivariable analysis
The apolipoprotein-E ε4 status is associated with β-amyloid plaque deposition after adjusting for lifetime Meth dependence and older age (≥50 years) (n=62, adjusted OR 3.23 [95% CI 1.04, 10.03], p=0.043) in multivariable analysis
Statistically significant at p<0.05
Fig. 2.
Lifetime methamphetamine (Meth) dependence categorized frequency distribution of microgliosis grades [derived from the regional density of ionized calcium-binding adapter molecule-1 (Iba1) immunoreactivity] in the temporo-parietal region. Lifetime Meth dependence is associated with marked Iba1 gliosis (relative to moderate/mild, odds ratio 4.42 [95% confidence interval 1.36, 14.39], p=0.014 [*], n=62) in the ordinal univariable logistic regression analysis (a). The scatter plot of the regional density of Iba1 immunoreactivity (arbitrary unit) is shown (b)
In either the frontal or putamen-internal capsule region, no significant association was found between Meth and Iba1 gliosis in either univariable analysis (n=64, Table 2) or multivariable analysis after adjusting for HIV encephalitis, WM lesions, and opportunistic diseases (n=63, p>0.09 and p>0.22, respectively), HCV seropositivity (n=57, p>0.10; and n=56, p>0.07, respectively), and lifetime dependence on alcohol (n=64, p>0.13 and p>0.21, respectively), opiates (n=64, p>0.07 and p>0.08, respectively), and cannabis (n=64, p>0.08 and p>0.12, respectively).
GFAP gliosis
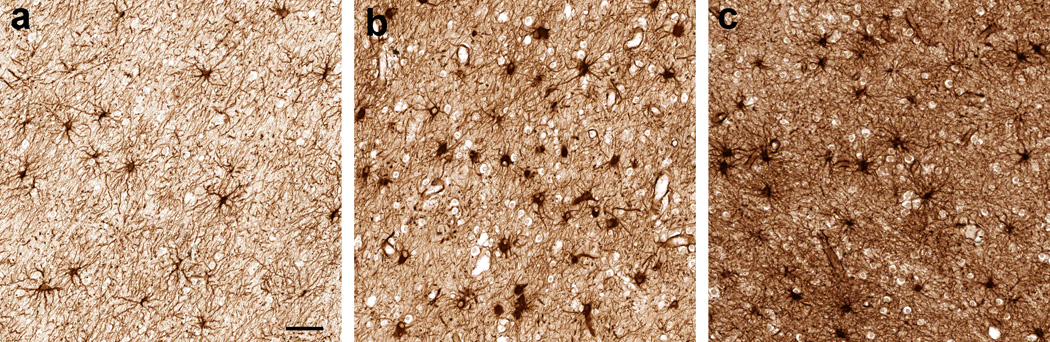
DAB immunohistochemistry for GFAP showed astroglia distributed singly in both GM and WM (Fig. 3a–c). In the logistic regression analysis, lifetime Meth dependence was not significantly associated with GFAP gliosis in any of the frontal, temporo-parietal, and putamen-internal capsule regions in either univariable analysis (n=64, n=62, and n=64, respectively; Table 2) or multivariable analysis after adjusting for HIV encephalitis, WM lesions, and opportunistic diseases (n=63, p>0.07; n=61, p>0.24; and n=63, p>0.41, respectively), HCV seropositivity (n=57, p>0.06; n=54, p>0.38; and n=56, p>0.66, respectively), and lifetime dependence on alcohol (n=64, p≥0.09; n=62, p>0.49; and n=64, p>0.32, respectively), opiates (n=64, p>0.07; n=62, p≥0.36; and n=64, p>0.24, respectively), and cannabis (n=64, p≥0.11; n=62, p≥0.26; and n=64, p>0.41, respectively).
Fig. 3.
Immunoreactivity pattern for glial fibrillary acidic protein (GFAP) in the cerebral subcortical white matter of HIV-infected adults. Depicted are mild (a), moderate (b), and marked (c) GFAP-immunoreactive astrogliosis; scale bar: 50 µm
SYP and MAP2 loss
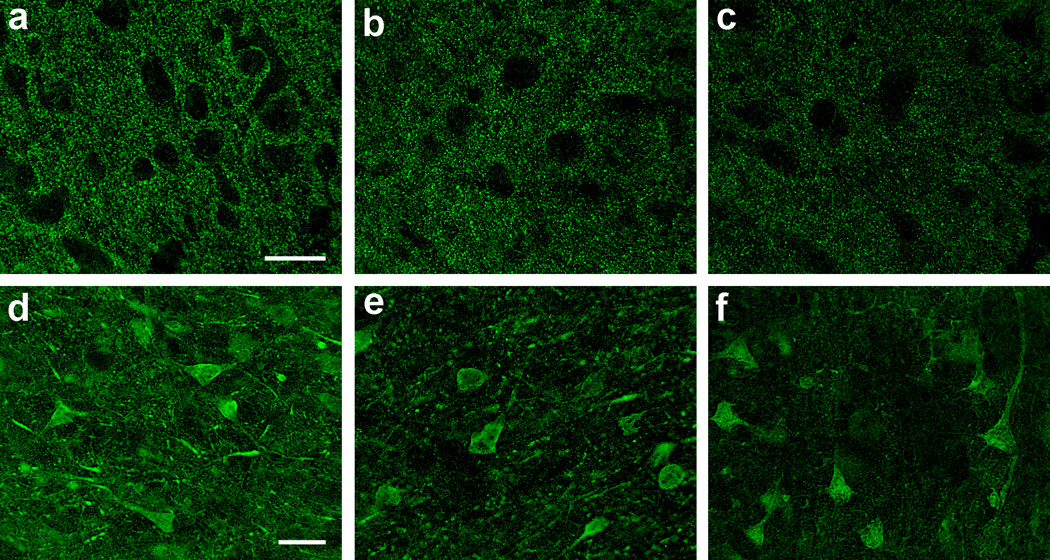
In the frontal cortical GM, immunofluorescence confocal microscopy for SYP showed punctate neuropil immunoreactivity between neuronal outlines (Fig. 4a–c), and that for MAP2 revealed immunoreactive neuronal soma and dendrites (Fig. 4d–f) (Bryant et al. 2015). The SYP density correlated directly with the MAP2 density (n=52, rs=0.78, p<0.0001, Spearman’s rank correlation coefficient).
Fig. 4.
Immunofluorescence confocal microscopy for synaptophysin (SYP, a–c) and microtubule-associated protein-2 (MAP2, d–f) in the frontal cortical gray matter of HIV-infected adults. Shown are mild (a), moderate (b), and marked (c) loss of SYP immunoreactivity in neuropil between neuronal outlines, as are mild (d), moderate (e), and marked (f) loss of MAP2 immunoreactivity in neuronal soma and dendrites; scale bars: 20 µm (for SYP and MAP2)
In the logistic regression analysis, there was no significant association between lifetime Meth dependence and SYP or MAP2 loss in the frontal cortical GM in either univariable analysis (n=52; Table 2) or multivariable analysis after adjusting for HIV encephalitis, WM lesions, and opportunistic diseases (n=51, p>0.43 and p>0.83, respectively), HCV seropositivity (n=48, p>0.53 and p>0.83, respectively), and lifetime dependence on alcohol (n=52, p>0.20 and p>0.36, respectively), opiates (n=52, p>0.17 and p≥0.37, respectively), and cannabis (n=52, p>0.23 and p>0.30, respectively). In the first multivariable model, the presence of HIV encephalitis or WM lesions was associated with SYP loss after adjusting for Meth and opportunistic diseases (n=51; adjusted OR [marked/moderate vs. mild] 11.77 [95% CI 1.35, 102.46], p=0.025; adjusted OR [marked vs. moderate/mild] 6.42 [95% CI 1.44, 28.65], p=0.015). For MAP2 loss, the similar association did not reach statistical significance (n=51; adjusted OR [marked/moderate vs. mild] 4.72 [95% CI 0.88, 25.32], p=0.07; adjusted OR [marked vs. moderate/mild] 1.34 [95% CI 0.32, 5.61], p=0.69).
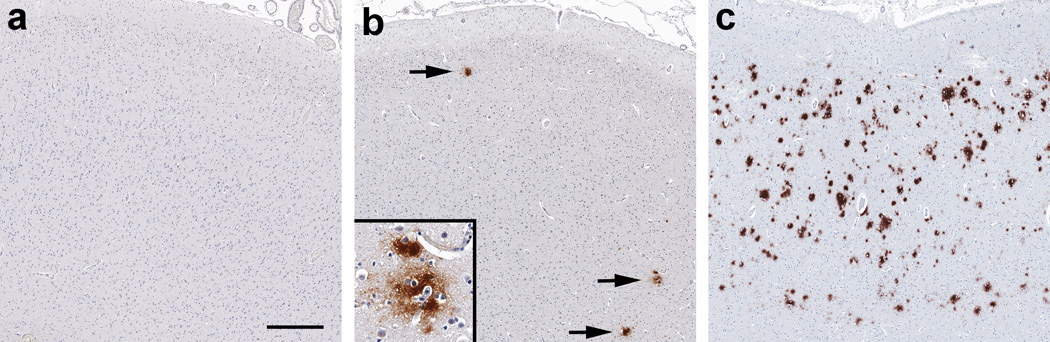
β-Amyloid plaque deposition
In a subset of cerebral tissue sections, DAB immunohistochemistry for β-amyloid revealed focal or widespread distribution of (extracellular) diffuse plaques in the GM neuropil, including perineuronal and perivascular localizations (Fig. 5a–c) (Soontornniyomkij et al. 2012a). In the logistic regression analysis, there was no significant association between lifetime Meth dependence and β-amyloid plaque deposition in either univariable analysis (n=68; Table 2) or multivariable analysis after adjusting for the APOE ε4 status and older age (n=62, p>0.99), HCV seropositivity (n=60, p>0.99), and lifetime dependence on alcohol (n=68, p=0.80), opiates (n=68, p=0.73), and cannabis (n=68, p=0.97). In the first multivariable model, the APOE ε4 status was associated with β-amyloid plaque deposition after adjusting for Meth and older age (n=62, adjusted OR 3.23 [95% CI 1.04, 10.03], p= 0.043).
Fig. 5.
Immunoreactivity pattern for β-amyloid in the cerebral cortical gray matter of HIV-infected adults. The absence (a), focal presence (b, arrows), and widespread presence (c) of diffuse β-amyloid plaques are depicted, scale bar: 500 µm. High magnification of (extracellular) diffuse β-amyloid plaques is shown (b, inset)
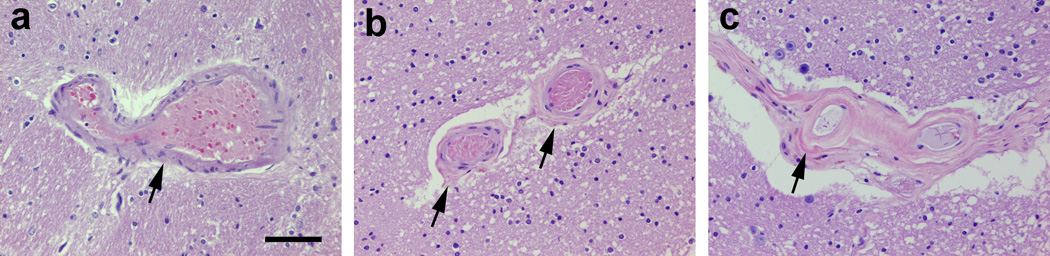
Arteriolosclerosis
Representative histopathologic features of arteriolosclerosis in the forebrain WM are shown in Fig. 6a–c. In the logistic regression analysis, lifetime Meth dependence was not significantly associated with arteriolosclerosis in the forebrain WM in either univariable analysis (n=74; Table 2) or multivariable analysis after adjusting for the HAART status (relative to no HAART) and diabetes (n=74, p>0.15), HCV seropositivity (n=66, p>0.19), and lifetime dependence on alcohol (n=74, p>0.06), opiates (n=74, p>0.15), and cannabis (n=74, p>0.27).
Fig. 6.
Histopathologic features of arteriolosclerosis in the forebrain white matter of HIV-infected adults. On hematoxylin and eosin staining, arteriolosclerosis is defined as concentric intramural hyalinization of small arteries or arterioles and graded as absent (normal, with intactness of vascular smooth muscle cells) (a, arrow), mild (partial thickness involvement) (b, arrows), and severe (full-thickness involvement) (c, arrow); scale bar: 100 µm
Discussion
The present clinico-pathological study of HIV-infected adults showed that lifetime Meth dependence was associated with marked Iba1 microgliosis in the temporo-parietal region even after statistically adjusting for biological-relevant covariates including HIV encephalitis, WM lesions, opportunistic diseases, HCV seropositivity, and lifetime dependence on alcohol, opiates, and cannabis. Nonetheless, there was no significant association between Meth and Iba1 microgliosis in the other two cerebral regions examined. In addition, we found no significant association of Meth with other brain pathologic changes including GFAP astrogliosis, SYP/MAP2 synaptodendritic loss, β-amyloid plaque deposition, and arteriolosclerosis.
Of note, only focal cerebral Iba1 microgliosis, but not other brain pathologic changes examined, was found to be associated with lifetime Meth dependence in HIV-infected adults in our present study. Of the 20 cases with lifetime Meth dependence, only two were diagnosed with current Meth dependence at the last follow-up visit closest to death. In both of these cases, Iba1 microgliosis was of moderate or marked severity in each of three brain regions examined (Electronic Supplementary Table 1). Therefore, it is likely that some of the changes in select brain regions might be reversible and have gradually diminished following extended abstinence from Meth. This notion is supported by several in vivo human functional neuroimaging studies. For example, a PET study with [11C](R)-PK11195 showed that brain microgliosis was increased in early Meth abstinence and diminished proportionately with the duration of continuous drug abstinence (Sekine et al. 2008). Volkow et al. (2001) reported PET evidence of dopamine transporter normalization in the striatum of abstinent Meth-dependent individuals followed longitudinally. In another PET study, the recovery of decreased glucose metabolism was observed after protracted Meth abstinence in the thalamus but not in the caudate or nucleus accumbens (Wang et al. 2004). Similarly, studies using photon MR spectroscopy revealed normalization of cerebral metabolite levels in the anterior cingulate cortex following long-term abstinence from Meth (Nordahl et al. 2005; Salo et al. 2011). Further, in a non-human primate model of long-term Meth self-administration, there were increases in myo-inositol in the anterior cingulate cortex and decreases in glutamate and glutamine in the caudate-putamen at one week following Meth withdrawal, both followed by a linear pattern of normalization by one year of abstinence (Yang et al. 2015).
It is also possible that some of brain pathologic changes examined in the present study might have not been induced by Meth at the beginning. In a postmortem study of Meth-intoxicated individuals, Kitamura et al. (2010) reported increased microgliosis, but not astrogliosis, in the striatum by immunohistochemistry. Still, Tong et al. (2014) found no increases in protein levels of microglia and astroglia markers in the striatum and frontal cortex tissue homogenates from chronic Meth users (about half of them died of drug intoxication). The differential susceptibility to Meth neurotoxicity might vary from one brain region to another (Kuhn et al. 2011; Wang et al. 2004). In the present study, we could not exclude a possibility of failure to detect immunohistologic alterations that might exist in some brain regions not examined.
A study by Langford et al. (2003) showed higher degrees of frontal microgliosis and SYP immunoreactivity loss in HIV encephalitis brains of chronic Meth users when compared to those brains of non-Meth subjects. Although we found lifetime Meth dependence to be associated with focal cerebral microgliosis even after statistically adjusting for multiple biological-relevant covariates including HIV encephalitis, we did not find any significant association of Meth with frontal SYP loss in either univariable or multivariable logistic regression models. This disagreement over SYP loss may be related to the fact that in our present study only a minority of cases had HIV encephalitis (Table 1). And we found that the presence of HIV encephalitis or WM lesions was associated with SYP loss after statistically adjusting for Meth and opportunistic diseases.
The presence of cerebral microgliosis in association with lifetime Meth dependence in HIV-infected individuals suggests that Meth alone or interactions between Meth and HIV (such as HIV-1 Tat and gp120 proteins) induce long-lasting microglial activation (Kuhn et al. 2011; Mediouni et al. 2015). It is also possible that microglial activation occurs in response to chronic Meth-induced neuronal injury (Bowyer et al. 2008). Microglia upon activation may have the capacity to become polarized into pro-inflammatory (M1-like, cytotoxic) and anti-inflammatory (M2-like, reparative) phenotypes, as well as various overlapping phenotypes. Still, analysis of gene and protein expression of microglia polarization may not suffice to determine the functional state of microglia (Prinz and Priller 2014). The complex interaction and contribution (either neurotoxic or neuroprotective) of microglial activation following Meth exposure remain to be elucidated in cell systems and animal models (Frank et al. 2016; Kuhn et al. 2011; Loftis and Janowsky 2014; McConnell et al. 2015).
The present study was limited by the absence of data on the route of drug consumption, lifetime quantity and duration of Meth use, and duration of continuous drug abstinence, which might affect the severity of Meth-induced pathologic changes observed in postmortem brains.
In conclusion, we found lifetime Meth dependence to be associated with focal cerebral microgliosis among HIV-infected adults, but not with other brain degenerative changes examined. Some of the changes in select brain regions might be reversible and have gradually diminished following extended abstinence from Meth. Alternatively, some of the changes might have not been induced by Meth in the first place. The hypothesis that some of the Meth-associated pathologic alterations in the brain can recover (at least partially) as a function of extended drug abstinence can be tested in prospective cohorts of HIV-infected individuals using PET or photon MR spectroscopy, as well as in animal models of Meth-HIV comorbidities (Bortell et al. 2015; Hoefer et al. 2015; Huitron-Resendiz et al. 2010).
Supplementary Material
Acknowledgments
This work was supported by the United States National Institutes of Health (NIH) grants P50 DA026306 (C. L. Achim, A. Umlauf, V. Soontornniyomkij), U24 MH100928 (C. L. Achim, D. J. Moore, E. Masliah, A. Umlauf, V. Soontornniyomkij), P30 MH062512 (C. L. Achim), R01 MH096648 (D. J. Moore, C. L. Achim, V. Soontornniyomkij), R01 AG043384 (E. Masliah, C. L. Achim, V. Soontornniyomkij), and R01 MH094159 (C. L. Achim, B. Soontornniyomkij); and TMARC-1 Pilot Grant 4 (PSTTMP4: V. Soontornniyomkij).
This publication was made possible from NIH funding through the NIMH and NINDS Institutes by the following grants: Manhattan HIV Brain Bank (MHBB): U24 MH100931, Texas NeuroAIDS Research Center (TNRC): U24 MH100930, National Neurological AIDS Bank (NNAB): U24 MH100929, California NeuroAIDS Tissue Network (CNTN): U24 MH100928, Data Coordinating Center (DCC): U24 MH100925. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the National NeuroAIDS Tissue Consortium (NNTC) or NIH.
The Translational Methamphetamine AIDS Research Center (TMARC) is supported by Center award P50 DA026306 from the National Institute on Drug Abuse (NIDA) and is affiliated with the University of California, San Diego (UCSD), the Sanford-Burnham Medical Research Institute (SBMRI), and the University of California, Irvine (UCI). The TMARC is composed of: Director – Igor Grant, M.D.; Co-Directors – Ronald J. Ellis, M.D., Ph.D., Scott L. Letendre, M.D., and Cristian L. Achim, M.D., Ph.D.; Center Manager – Mariana Cherner, Ph.D.; Associate Center Manager – Erin E. Morgan, Ph.D.; Assistant Center Manager – Aaron M. Carr, B.A.; Behavioral Assessment and Medical (BAM) Core–Neuromedical and Laboratory Unit (NLU): Scott L. Letendre, M.D. (Core Co-Director/NLU Chief), Ronald J. Ellis, M.D., Ph.D.; BAM Core–Neuropsychiatric Unit (NPU): Robert K. Heaton, Ph.D. (Core Co-Director/NPU Chief), J. Hampton Atkinson, M.D., Thomas D. Marcotte, Ph.D., Erin E. Morgan, Ph.D., Matthew Dawson (NPU Manager); Neuroimaging (NI) Core: Gregory G. Brown, Ph.D. (Core Director), Thomas T. Liu, Ph.D., Miriam Scadeng, Ph.D., Christine Fennema-Notestine, Ph.D., Sarah L. Archibald, M.A., John R. Hesselink, M.D., Mary Jane Meloy, Ph.D., Craig E.L. Stark, Ph.D.; Neuroscience and Animal Models (NAM) Core: Cristian L. Achim, M.D., Ph.D. (Core Director), Marcus Kaul, Ph.D., Virawudh Soontornniyomkij, M.D.; Pilot and Developmental (PAD) Core: Mariana Cherner, Ph.D. (Core Director), Stuart A. Lipton, M.D., Ph.D.; Pilot Study Leaders: Jennifer E. Iudicello, Ph.D., Assawin Gongvatana, Ph.D., Rachel D. Schrier, Ph.D., Virawudh Soontornniyomkij, M.D., Marta Massanella, Ph.D.; Administrative Coordinating Core (ACC)–Data Management and Information Systems (DMIS) Unit: Anthony C. Gamst, Ph.D. (Unit Chief), Clint Cushman, B.A. (Unit Manager); ACC–Statistics Unit: Florin Vaida, Ph.D. (Unit Chief), Ian S. Abramson, Ph.D., Reena Deutsch, Ph.D., Anya Umlauf, M.S.; ACC–Participant Unit: J. Hampton Atkinson, M.D. (Unit Chief), Jennifer Marquie-Beck, M.P.H. (Unit Manager); Project 1: Arpi Minassian, Ph.D. (Project Director), William Perry, Ph.D., Mark A. Geyer, Ph.D., Jared W. Young, Ph.D.; Project 2: Amanda B. Grethe, Ph.D. (Project Director), Susan F. Tapert, Ph.D., Assawin Gongvatana, Ph.D.; Project 3: Erin E. Morgan, Ph.D. (Project Director), Igor Grant, M.D.; Project 4: Svetlana Semenova, Ph.D. (Project Director), Athina Markou, Ph.D., James Kesby, Ph.D.; Project 5: Marcus Kaul, Ph.D. (Project Director). The views expressed in this article are those of the authors and do not reflect the official policy or position of the United States Government.
Footnotes
Part of this work was previously presented at the 13th International Symposium on NeuroVirology meeting, June 2–6, 2015, San Diego, California, USA.
Disclosure
The authors declare that they have no conflict of interest.
References
- Bae SC, Lyoo IK, Sung YH, Yoo J, Chung A, Yoon SJ, Kim DJ, Hwang J, Kim SJ, Renshaw PF. Increased white matter hyperintensities in male methamphetamine abusers. Drug Alcohol Depend. 2006;81:83–88. doi: 10.1016/j.drugalcdep.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Beitner-Johnson D, Guitart X, Nestler EJ. Glial fibrillary acidic protein and the mesolimbic dopamine system: regulation by chronic morphine and Lewis-Fischer strain differences in the rat ventral tegmental area. J Neurochem. 1993;61:1766–1773. doi: 10.1111/j.1471-4159.1993.tb09814.x. [DOI] [PubMed] [Google Scholar]
- Bortell N, Morsey B, Basova L, Fox HS, Marcondes MC. Phenotypic changes in the brain of SIV-infected macaques exposed to methamphetamine parallel macrophage activation patterns induced by the common gamma-chain cytokine system. Front Microbiol. 2015;6:900. doi: 10.3389/fmicb.2015.00900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowyer JF, Robinson B, Ali S, Schmued LC. Neurotoxic-related changes in tyrosine hydroxylase, microglia, myelin, and the blood-brain barrier in the caudate-putamen from acute methamphetamine exposure. Synapse. 2008;62:193–204. doi: 10.1002/syn.20478. [DOI] [PubMed] [Google Scholar]
- Bryant AK, Ellis RJ, Umlauf A, Gouaux B, Soontornniyomkij V, Letendre SL, Achim CL, Masliah E, Grant I, Moore DJ. Antiretroviral therapy reduces neurodegeneration in HIV infection. AIDS. 2015;29:323–330. doi: 10.1097/QAD.0000000000000553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budka H, Wiley CA, Kleihues P, Artigas J, Asbury AK, Cho ES, Cornblath DR, Dal Canto MC, DeGirolami U, Dickson D. HIV-associated disease of the nervous system: review of nomenclature and proposal for neuropathology-based terminology. Brain Pathol. 1991;1:143–152. doi: 10.1111/j.1750-3639.1991.tb00653.x. [DOI] [PubMed] [Google Scholar]
- Cadet JL, Krasnova IN. Interactions of HIV and methamphetamine: cellular and molecular mechanisms of toxicity potentiation. Neurotox Res. 2007;12:181–204. doi: 10.1007/BF03033915. [DOI] [PubMed] [Google Scholar]
- Carey CL, Woods SP, Rippeth JD, Gonzalez R, Heaton RK, Grant I. Additive deleterious effects of methamphetamine dependence and immunosuppression on neuropsychological functioning in HIV infection. AIDS Behav. 2006;10:185–190. doi: 10.1007/s10461-005-9056-4. [DOI] [PubMed] [Google Scholar]
- Chana G, Everall IP, Crews L, Langford D, Adame A, Grant I, Cherner M, Lazzaretto D, Heaton R, Ellis R, Masliah E. Cognitive deficits and degeneration of interneurons in HIV+ methamphetamine users. Neurology. 2006;67:1486–1489. doi: 10.1212/01.wnl.0000240066.02404.e6. [DOI] [PubMed] [Google Scholar]
- Chang L, Ernst T, Speck O, Grob CS. Additive effects of HIV and chronic methamphetamine use on brain metabolite abnormalities. Am J Psychiatry. 2005;162:361–369. doi: 10.1176/appi.ajp.162.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everall I, Salaria S, Roberts E, Corbeil J, Sasik R, Fox H, Grant I, Masliah E. Methamphetamine stimulates interferon inducible genes in HIV infected brain. J Neuroimmunol. 2005;170:158–171. doi: 10.1016/j.jneuroim.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Fowler JS, Volkow ND, Logan J, Alexoff D, Telang F, Wang GJ, Wong C, Ma Y, Kriplani A, Pradhan K, Schlyer D, Jayne M, Hubbard B, Carter P, Warner D, King P, Shea C, Xu Y, Muench L, Apelskog K. Fast uptake and long-lasting binding of methamphetamine in the human brain: comparison with cocaine. Neuroimage. 2008;43:756–763. doi: 10.1016/j.neuroimage.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Adhikary S, Sobesky JL, Weber MD, Watkins LR, Maier SF. The danger-associated molecular pattern HMGB1 mediates the neuroinflammatory effects of methamphetamine. Brain Behav Immun. 2016;51:99–108. doi: 10.1016/j.bbi.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, Trautman KD, Miele GM, Samet S, Smith M, Endicott J. Psychiatric Research Interview for Substance and Mental Disorders (PRISM): reliability for substance abusers. Am J Psychiatry. 1996;153:1195–1201. doi: 10.1176/ajp.153.9.1195. [DOI] [PubMed] [Google Scholar]
- Hoefer MM, Sanchez AB, Maung R, de Rozieres CM, Catalan IC, Dowling CC, Thaney VE, Piña-Crespo J, Zhang D, Roberts AJ, Kaul M. Combination of methamphetamine and HIV-1 gp120 causes distinct long-term alterations of behavior, gene expression, and injury in the central nervous system. Exp Neurol. 2015;263:221–234. doi: 10.1016/j.expneurol.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huitron-Resendiz S, Henriksen SJ, Barr MC, Testa MP, Crawford E, Parsons LH, Sanchez-Alavez M, Phillips TR. Methamphetamine and lentivirus interactions: reciprocal enhancement of central nervous system disease. J Neurovirol. 2010;16:268–278. doi: 10.3109/13550284.2010.497807. [DOI] [PubMed] [Google Scholar]
- Kalasinsky KS, Bosy TZ, Schmunk GA, Reiber G, Anthony RM, Furukawa Y, Guttman M, Kish SJ. Regional distribution of methamphetamine in autopsied brain of chronic human methamphetamine users. Forensic Sci Int. 2001;116:163–169. doi: 10.1016/s0379-0738(00)00368-6. [DOI] [PubMed] [Google Scholar]
- Kitamura O, Takeichi T, Wang EL, Tokunaga I, Ishigami A, Kubo S. Microglial and astrocytic changes in the striatum of methamphetamine abusers. Leg Med (Tokyo) 2010;12:57–62. doi: 10.1016/j.legalmed.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Kuhn DM, Angoa-Pérez M, Thomas DM. Nucleus accumbens invulnerability to methamphetamine neurotoxicity. ILAR J. 2011;52:352–365. doi: 10.1093/ilar.52.3.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford D, Adame A, Grigorian A, Grant I, McCutchan JA, Ellis RJ, Marcotte TD, Masliah E. Patterns of selective neuronal damage in methamphetamine-user AIDS patients. J Acquir Immune Defic Syndr. 2003;34:467–474. doi: 10.1097/00126334-200312150-00004. [DOI] [PubMed] [Google Scholar]
- Letendre S, Paulino AD, Rockenstein E, Adame A, Crews L, Cherner M, Heaton R, Ellis R, Everall IP, Grant I, Masliah E. Pathogenesis of hepatitis C virus coinfection in the brains of patients infected with HIV. J Infect Dis. 2007;196:361–370. doi: 10.1086/519285. [DOI] [PubMed] [Google Scholar]
- Letendre SL, Ellis RJ, Ances BM, McCutchan JA. Neurologic complications of HIV disease and their treatment. Top HIV Med. 2010;18:45–55. [PMC free article] [PubMed] [Google Scholar]
- Loftis JM, Janowsky A. Neuroimmune basis of methamphetamine toxicity. Int Rev Neurobiol. 2014;118:165–197. doi: 10.1016/B978-0-12-801284-0.00007-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell SE, O’Banion MK, Cory-Slechta DA, Olschowka JA, Opanashuk LA. Characterization of binge-dosed methamphetamine-induced neurotoxicity and neuroinflammation. Neurotoxicology. 2015;50:131–141. doi: 10.1016/j.neuro.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mediouni S, Garibaldi Marcondes MC, Miller C, McLaughlin JP, Valente ST. The cross-talk of HIV-1 Tat and methamphetamine in HIV-associated neurocognitive disorders. Front Microbiol. 2015;6:1164. doi: 10.3389/fmicb.2015.01164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordahl TE, Salo R, Natsuaki Y, Galloway GP, Waters C, Moore CD, Kile S, Buonocore MH. Methamphetamine users in sustained abstinence: a proton magnetic resonance spectroscopy study. Arch Gen Psychiatry. 2005;62:444–452. doi: 10.1001/archpsyc.62.4.444. [DOI] [PubMed] [Google Scholar]
- Ovbiagele B, Saver JL. Cerebral white matter hyperintensities on MRI: current concepts and therapeutic implications. Cerebrovasc Dis. 2006;22:83–90. doi: 10.1159/000093235. [DOI] [PubMed] [Google Scholar]
- Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9:689–701. doi: 10.1016/S1474-4422(10)70104-6. [DOI] [PubMed] [Google Scholar]
- Persidsky Y, Ho W, Ramirez SH, Potula R, Abood ME, Unterwald E, Tuma R. HIV-1 infection and alcohol abuse: neurocognitive impairment, mechanisms of neurodegeneration and therapeutic interventions. Brain Behav Immun. 2011;25(Suppl 1):S61–S70. doi: 10.1016/j.bbi.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz M, Priller J. Microglia and brain macrophages in the molecular age: from origin to neuropsychiatric disease. Nat Rev Neurosci. 2014;15:300–312. doi: 10.1038/nrn3722. [DOI] [PubMed] [Google Scholar]
- Rippeth JD, Heaton RK, Carey CL, Marcotte TD, Moore DJ, Gonzalez R, Wolfson T, Grant I. Methamphetamine dependence increases risk of neuropsychological impairment in HIV infected persons. J Int Neuropsychol Soc. 2004;10:1–14. doi: 10.1017/S1355617704101021. [DOI] [PubMed] [Google Scholar]
- Robins LN, Wing J, Wittchen HU, Helzer JE, Babor TF, Burke J, Farmer A, Jablenski A, Pickens R, Regier DA. The Composite International Diagnostic Interview. An epidemiologic instrument suitable for use in conjunction with different diagnostic systems and in different cultures. Arch Gen Psychiatry. 1988;45:1069–1077. doi: 10.1001/archpsyc.1988.01800360017003. [DOI] [PubMed] [Google Scholar]
- Salo R, Buonocore MH, Leamon M, Natsuaki Y, Waters C, Moore CD, Galloway GP, Nordahl TE. Extended findings of brain metabolite normalization in MA-dependent subjects across sustained abstinence: a proton MRS study. Drug Alcohol Depend. 2011;113:133–138. doi: 10.1016/j.drugalcdep.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekine Y, Ouchi Y, Sugihara G, Takei N, Yoshikawa E, Nakamura K, Iwata Y, Tsuchiya KJ, Suda S, Suzuki K, Kawai M, Takebayashi K, Yamamoto S, Matsuzaki H, Ueki T, Mori N, Gold MS, Cadet JL. Methamphetamine causes microglial activation in the brains of human abusers. J Neurosci. 2008;28:5756–5761. doi: 10.1523/JNEUROSCI.1179-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soontornniyomkij V, Moore DJ, Gouaux B, Soontornniyomkij B, Tatro ET, Umlauf A, Masliah E, Levine AJ, Singer EJ, Vinters HV, Gelman BB, Morgello S, Cherner M, Grant I, Achim CL. Cerebral β-amyloid deposition predicts HIV-associated neurocognitive disorders in APOE ε4 carriers. AIDS. 2012a;26:2327–2335. doi: 10.1097/QAD.0b013e32835a117c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soontornniyomkij V, Risbrough VB, Young JW, Wallace CK, Soontornniyomkij B, Jeste DV, Achim CL. Short-term recognition memory impairment is associated with decreased expression of FK506 binding protein 51 in the aged mouse brain. Age (Dordr) 2010;32:309–322. doi: 10.1007/s11357-010-9145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soontornniyomkij V, Soontornniyomkij B, Moore DJ, Gouaux B, Masliah E, Tung S, Vinters HV, Grant I, Achim CL. Antioxidant sestrin-2 redistribution to neuronal soma in human immunodeficiency virus-associated neurocognitive disorders. J Neuroimmune Pharmacol. 2012b;7:579–590. doi: 10.1007/s11481-012-9357-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soontornniyomkij V, Umlauf A, Chung SA, Cochran ML, Soontornniyomkij B, Gouaux B, Toperoff W, Moore DJ, Masliah E, Ellis RJ, Grant I, Achim CL. HIV protease inhibitor exposure predicts cerebral small vessel disease. AIDS. 2014;28:1297–1306. doi: 10.1097/QAD.0000000000000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor MJ, Schweinsburg BC, Alhassoon OM, Gongvatana A, Brown GG, Young-Casey C, Letendre SL, Grant I. Effects of human immunodeficiency virus and methamphetamine on cerebral metabolites measured with magnetic resonance spectroscopy. J Neurovirol. 2007;13:150–159. doi: 10.1080/13550280701194230. [DOI] [PubMed] [Google Scholar]
- Tong J, Fitzmaurice P, Furukawa Y, Schmunk GA, Wickham DJ, Ang LC, Sherwin A, McCluskey T, Boileau I, Kish SJ. Is brain gliosis a characteristic of chronic methamphetamine use in the human? Neurobiol Dis. 2014;67:107–118. doi: 10.1016/j.nbd.2014.03.015. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Franceschi D, Sedler M, Gatley SJ, Miller E, Hitzemann R, Ding YS, Logan J. Loss of dopamine transporters in methamphetamine abusers recovers with protracted abstinence. J Neurosci. 2001;21:9414–9418. doi: 10.1523/JNEUROSCI.21-23-09414.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Shumay E, Telang F, Thanos PK, Alexoff D. Distribution and pharmacokinetics of methamphetamine in the human body: clinical implications. PLoS One. 2010;5:e15269. doi: 10.1371/journal.pone.0015269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Chang L, Miller E, Sedler M, Hitzemann R, Zhu W, Logan J, Ma Y, Fowler JS. Partial recovery of brain metabolism in methamphetamine abusers after protracted abstinence. Am J Psychiatry. 2004;161:242–248. doi: 10.1176/appi.ajp.161.2.242. [DOI] [PubMed] [Google Scholar]
- Yang S, Belcher AM, Chefer S, Vaupel DB, Schindler CW, Stein EA, Yang Y. Withdrawal from long-term methamphetamine self-administration ‘normalizes’ neurometabolites in rhesus monkeys: a (1) H MR spectroscopy study. Addict Biol. 2015;20:69–79. doi: 10.1111/adb.12078. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.