Abstract
Mitochondria are highly abundant in and essential to the beat-to-beat contractile performance of hearts. However, relatively few cardiac diseases have been attributed to primary mitochondrial dysfunction. The paucity of evidence for “primary mitochondrial cardiac diseases” may be because such an entity doesn’t exist. Alternately, the consequences of mitochondrial dysfunction on hearts may be so severe that long-term viability is severely impaired and affected individuals are therefore not included in standard genetic screens of adult heart disease subjects. Here, I review accumulating experimental evidence that impairing mitochondrial fission or fusion causes cardiomyopathy in otherwise normal mice, and consider how these data could motivate screening of perinatal cardiomyopathy subjects for damaging mutations of mitochondrial fission and fusion factors.
Introduction
Mitochondria are frequently described as “highly dynamic”, meaning that their position within cells and their morphology (size, shape, and interconnectedness with other mitochondria) are variable. This characterization is accurate for many different types of cultured cells in which mitochondria have been studied using live-cell microscopy. Indeed, plasticity in location and structure of mitochondrial networks appears essential for normal cell mobility and replication. However, in vitro studies in cultured fibroblasts or similar cells do not reproduce the milieu of differentiated cells in vivo, and caution is warranted in extrapolating findings derived from tissue culture to normal or pathological conditions in developing or fully developed animals, or human subjects. Indeed, studying the most mitochondria-rich of all mammalian organs, the heart has uncovered unsuspected non-canonical functioning of mitochondrial dynamics proteins. Importantly, mitochondria of adult mammalian cardiomyocytes are seemingly static, only rarely exhibiting morphological evidence of fission or fusion; they also turn over very slowly [1–5]. Perhaps because normal cardiomyocyte mitochondrial dynamism is sluggish, genetic manipulation of mitochondrial dynamics factors in the heart has revealed important roles for mitochondrial fission and fusion as central regulators of mitochondrial quality control and metabolic transitioning. Evolving concepts derived from these experimental models have suggested novel mechanisms for cardiac disease, and seem likely to catalyze human genetic studies exploring causal links between damaging mutations of mitochondrial dynamism factors and clinical cardiomyopathy.
The dynamism of cardiac mitochondria dynamics proteins
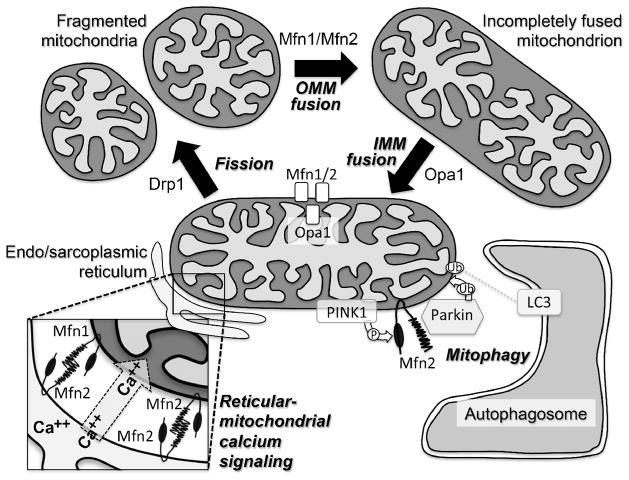
Mechanisms by which mitochondria undergo fusion and fission in hearts and elsewhere have been elaborated in detail and recently reviewed [6,7]. Briefly, mitochondrial fusion is a three-step process (Figure): 1. tethering of two mitochondria by reversible trans association of outer mitochondrial membrane mitofusin (Mfn) 1 or Mfn2; 2. GTPase-dependent fusion of outer mitochondrial membranes mediated by the same two Mfn proteins; and 3. GTPase-dependent fusion of inner mitochondrial membranes mediated by optic atrophy (Opa) 1. Although mitochondrial fusion is infrequent in normal adult mouse hearts, the mRNA transcripts and proteins of these fusion factors are abundant in mouse myocardium. Moreover, these fusion factors are regulated during cardiac stress or disease. Thus, Mfn1 levels reportedly are decreased in cardiac hypertrophy [8]. Mfn2 is reportedly increased after oxidant stress [9], but is decreased in diabetic hearts [10] and late after myocardial infarction [11]. And, Opa1 levels appeared to decrease in heart failure [12] and late after myocardial infarction [11], but were increased in hypertrophied hypertensive hearts [13] and in cardiomyocytes treated with insulin [14]. Mfn1, Mfn2, and Opa transcripts were all concordantly decreased in hearts having genetically-induced lipid overload [15].
Figure. Canonical (top) and non-canonical (bottom) functions of mitochondrial dynamics factors.
Schematic depiction of mitochondrial fission-fusion cycle, reticular-mitochondrial calcium signaling, and mitophagy. The processes mediated by Drp1, Mfn1 and 2, and Opa1 are indicated by arrows; ablation/inhibition of these factors results in accumulation of mitochondria like those before the arrow. Mfn1 and Mfn2 are both located at mitochondria and are largely redundant for OMM fusion (top), but only Mfn2 localizes to endo/sarcoplasmic reticulum (bottom left). Mfn2 also uniquely acts as a mitochondrial receptor for Parkin, facilitating its ubiquitination of OMM proteins and recruitment of autophagosomes (bottom right).
Mitochondrial fission is mechanistically more straightforward than fusion, and is primarily mediated by dynamin-related protein (Drp) 1 (Figure). This normally cytosolic protein is recruited to mitochondria where it decorates areas of mitochondria-endoplamsic reticulum contacts and (through nose-to-tail oligomerization and constriction) ligates and severs the parent mitochondrion into two daughter organelles [16]. Consistent with its constitutive expression and induced mitochondrial localization/activation, cardiac Drp1 activity can be regulated postranslationally by activating (S616, S622) or inhibitory (S637) phosphorylation[17,18], activating SIRT3-dependent deacetylation (K926/931) [19], and activating O-GlcNAcylation [20] (Drp1 regulation is reviewed in detail in [21]). Cardiac Drp1 levels are decreased in chronically hypertrophied, hypertensive rat hearts [13], but cardiomyocyte Drp1 levels are increased by the pro-hypertrophic neurohormone norepinephrine [22]. Regulated cardiac expression of Drp1, Mfn1, Mfn2, and Opa1 have all been linked to transcriptional regulatory activity of PGC-1 [23]. Altered expression or activation of mitochondrial dynamics factors in cardiac disease implied possible pathophysiological roles for mitochondrial fission and fusion in the heart, which established a conceptual foundation for hypothesis-testing in vitro and in vivo experimental manipulation.
Mitochondrial dynamism, cardiomyocyte differentiation, and cardiac development
As introduced above, mitochondria of adult cardiomyocytes appear as individually distinct, hypodynamic organelles. Yet, genetic deletion of mitochondrial fusion-promoting Mfn1 and Mfn2 (in combination) provokes mitochondrial fragmentation [3,24,25], whereas genetic deletion of mitochondrial fission-promoting Drp1 provokes mitochondrial enlargement [25,26], demonstrating that mitochondrial fission and fusion occur (albeit slowly) in adult hearts. Thus, when either fusion or fission are genetically abrogated in adult hearts, the resulting imbalance in mitochondrial dynamism reveals low basal activity of the opposing mitochondrial dynamics process.
By comparison with adult cardiomyocytes, mitochondria of cardiac stem cells and embryonic or developing cardiomyocytes are more dynamic and interconnected [27–29]. Indeed, mitochondrial remodeling has profound effects on myocyte differentiation that seemingly involve all three major categories of mitochondrial dynamics proteins: outer membrane mitofusins [28], inner membrane pro-fusion Opa1 [28,30], and fission-promoting Drp1 [31]. It is likely that perturbations of mitochondrial remodeling can alter cardiomyocyte and myogenic differentiation. One intriguing possibility is that pathologically fragmented mitochondria act as calcium sponges, disrupting calcineurin-NFAT signaling and downstream Notch1-regulated gene expression programs essential to cardiomyocyte differentiation and myocardial development [28]. Developmental abnormalities of human hearts have not yet been linked to disturbances in mitochondrial dynamism, but this is an understudied area that will require whole-genome or exome sequencing of large congenital heart disease patient cohorts to adequately examine the pathological consequences of rare, damaging mutations of mitochondrial fission or fusion factors on fetal, embryonic and perinatal hearts.
Mitochondrial fission and experimental heart disease
Pharmacological approaches to inhibit Drp1 were evaluated in experimental cardiac injury and suggested that Drp1-mediated mitochondrial fission can be an important mediator of cardiac dysfunction. Inhibition of Drp1 with Mdivi-1 [32–34] or P110 [35], or genetic inhibition of Drp1 with an adenovirally expressed dominant negative mutant (Drp1 K38A; [36]) or transgenically expressed Pim-1 [37], all were beneficial in models of cardiac ischemia.
Based on associations between mitochondrial fragmentation, cardiomyocyte apoptosis, and cardiac dysfunction in the studies described above, it was inferred that mitochondrial fission is inherently pathological. If so, interruption of fission by genetic Drp1 ablation should, like like Mdivi-1 and P110, be cardioprotective. However, germ-line Drp1 knockout mice died with neurological defects as E12.5 embryos [38], and Cre-mediated muscle-restricted Drp1 ablation induced lethal neonatal cardiomyopathy [39]. Even cardiomyocyte-specific Drp1 gene recombination after birth provoked cardiomyopathies and lethality in juvenile mice [25,26]. Drp1 gene recombination in adult hearts was equally deadly: conditional (tamoxifen-activated modified estrogen receptor [MER] conjugated Cre) cardiomyocyte-specific Drp1 deletion in adult mice induced fatal cardiomyopathies after ~6–13 weeks [25,40]. The predicted morphological consequence of suppressing mitochondrial fission (i.e. mitochondrial elongation) was observed in each of the cardiac Drp1 knockout models, but seemed unrelated to the dramatic heart failure phenotypes. Indeed, cardiomyocyte-specific ablation of fusion-promoting Mfn2 caused a similar degree of mitochondrial enlargement, with much less severe functional consequences in otherwise normal hearts [41,42].
The aggressive phenotypes evoked by cardiomyocyte Drp1 ablation have been linked by two groups to increased mitophagy signaling. Our laboratory described enhanced mitophagy signaling (mitochondrial-associated ubiquitin, p62 and LC3) in adult Drp1 knockout mice [25] and subsequently linked this to upregulation and activation of Parkin [43]. The Sesaki group likewise reported increased mitophagy signaling (mitochondrial protein ubiquitination and p62 accumulation) in a neonatal cardiac Drp1 knockout mouse model [26]. They further observed that combined absence of Parkin (germ line deletion) and Drp1 (cardiomyocyte-specific deletion) “exacerbated the integrity of mitochondria”, suggesting that Parkin and Drp1 were important for maintaining mitochondrial quality. Because mitophagy signaling persisted in the combined cardiomyocyte-specific Drp1 knockout mice and germ-line Parkin null mice however, the authors concluded that the mitophagy stimulated by Drp1 insufficiency was Parkin-independent. How can these and our data be reconciled? I would argue that the Sasaki data do not refute a role for Parkin in Drp1 deficiency, but simply reveal the presence of one or more Parkin-independent mechanisms of mitophagy. Indeed, by developing a cardiomyocyte-specific Parkin knockout mouse and exploring the consequences of genetic complentation between it and our adult cardiomyocyte-specific Drp1 knockout mouse, we subsequently provided seemingly incontrovertible evidence that Parkin is both induced by, and responsible for, mitochondrial loss and cardiac failure in cardiomyocyte Drp1 deficiency [43]. We further demonstrated how cardiac phenotypes in germ-line Parkin knockout mice can be confounded by compensatory induction of Parkin-independent mitophagy [51].
Sadoshima’s group published what at first glance seems to be an outlier study in which decreased autophagic flux was reported in adult cardiomyocyte-specific Drp1 knockout mice [40]. Importantly, these researchers observed mitochondrial engulfment by autophagosomes (i.e. “mitochondrial autophagy”) but did not actually measure targeted mitophagy (mitochondrial-associated uniquitin, p62, or LC3) as would be induced by Parkin or functionally overlapping pathways. Their experimental read-outs were all measures of macroautophagy, which of course can provoke non-specific mitochondrial engulfment by autophagosomes, and so there is no discrepancy. Taking all available data together we conclude that cardiac dysfunction provoked by a deficiency of Drp1 is at least in part a consequence of hyper-mitophagic mitochondrial loss [25,26,43].
It is noteworthy that pharmacological Drp1 inhibition is described in the literature as beneficial, whereas genetic Drp1 ablation is lethal. The exact reasons for apparent benefits of acute pharmacological Drp1 inhibition (vide supra), but catastrophic effects of even conditional cardiomyocyte-specific Drp1 inhibition are not currently known, but several possibilities deserve consideration. The first is the context of the intervention: Mdivi-1 and P110 were studied in the setting of an acute cardiac injury, and so their effects would likely impact reactive mitochondrial fission (as observed during apoptosis) to a greater extent than homeostatic mitochondrial fission. The reciprocal is true for cardiomyocyte-targeted Drp1 gene ablation, either at the time of birth or in adult mice; as there is no cardiac injury the effects of gene ablation would be on house keeping or normal homeostatic mitochondrial fission. Another possibility is that Mdivi-1 and P110 exert effects independent of or in addition to inhibiting Drp1. While some degree of drug non-specificity is an almost universal finding, the distinct mechanisms of actions of these two agents, but their similar effects on ischemic hearts, argues that their benefits derive from their shared suppression of Drp1-mediated mitochondrial fission.
The discovery of a Drp1 mutation (A395D) that impairs GTPase activity and Drp1 self-assembly in an infant who succumbed to severe metabolic defects [44,45] suggests that loss of function of Drp1 can contribute to human cardiac disease. Cardiomyopathy caused by a Drp1 mutation in the same domain, C452F in so-called Python mice [46,47], is likewise associated with GTPase abnormalities and defective Drp1 assembly and further supports deep sequencing efforts to uncover disease-related Drp1 mutants in otherwise unexplained clinical cardiomyopathy.
Mitochondrial fusion and experimental heart disease
As introduced earlier, the initial requisite step for mitochondrial fusion is reversible inter-mitochondrial tethering mediated by Mfn1 or Mfn2. The second step, irreversible outer membrane fusion, is also mediated by (either of the) mitofusins. Because Mfn1 and Mfn2 are largely redundant for both of these initial steps, and pharmacological inhibitors of mitofusins have not yet been reported, the classic experimental manipulation to prevent mitochondrial fusion has been genetic deletion of Mfn1 and Mfn2 in combination [48,49]. Conditional genetic deletion of Mfn1 and Mfn2 in murine cardiac myocytes during the early stages of embryonic heart development (using Nkx2.5-Cre) was uniformly lethal after ~E13 [3], provoking hypoplastic myocardium and perturbed calcium signaling that were mechanistically linked by interruption of the transcriptional program for myocardial development normally regulated by calcineurin and Notch1 [28]. Mitochondria of E12.5 Mfn1/Mfn2 double knockout mouse hearts exhibited marked heterogeneity in size of spherical-shaped organelles, with absence of the elongated mitochondrial morphology that typifies embryonic cardiomyocytes; many organelles also showed severe degeneration or loss of internal cristae [28]. The same concomitant genetic ablation of Mfn1 and Mfn2 in 8 week old adult mouse hearts (Myh6-driven MER-Cre-MER activated with tamoxifen) [3] similarly produced “fragmented” mitochondria with degenerated cristae, but the gross heart morphology provoked by interrupting mitochondrial fusion was eccentric remodeling and myocardial hypertrophy [25].
Fusion-mediated mitochondrial remodeling is central to transcriptional control of embryonic heart growth, whereas in adult hearts an intact mitochondrial fusion apparatus seems more important for mitochondrial quality control [25]. Accumulating data support the necessity for mitochondrial fusion in normal metabolic adaptation [24] and to prevent mitochondria-mediated cytotoxicity [25]. Promotion of normal perinatal myocardial metabolic transitioning by Mfn1 or Mfn2 [24] and failure of mitochondrial quality control mechanisms in Mfn1/Mfn2 deficient hearts [25] is explained by loss of Mfn2-specific niche functioning in the Parkin pathway of mitophagy, now recognized as important for both processes [50,51] (Figure). Thus, interpretation of combined genetic Mfn1 and Mfn2 ablation in hearts (and elsewhere) is confounded by impairment of Mfn2-regulated mitophagy and mitochondrial-reticular calcium cross-talk (reviewed in [52,53]) (Figure); phenotypes cannot therefore be confidently attributed only to interruption of mitochondrial fusion. Likewise, because the inner mitochondrial membrane fusion protein Opa1 is essential for maintaining normal cristae structure [54], cardiomyopathies provoked by myocyte-specific Opa1 gene deletion or altered processing may be caused by defective mitochondrial inner membrane architecture [55] or interruption of mitochondrial fusion [54,56,57]. Further complicating experimental interpretation, outer mitochondrial membrane fusion is not abrogated by Opa1 ablation, but is interrupted after outer membrane fusion [58]. Considerations of the complex functional profiles of mitochondrial fusion proteins have sometime been overlooked when assigning causality by process [59]. We suggest that unambiguous conclusions regarding the various roles of mitochondrial fusion factors in hearts will likely require development of subtle genetic or pharmacological “separation of function” tools that inhibit and promote mitochondrial fusion at its initial stages without primarily impacting mitochondrial calcium uptake, mitophagy or cristae organization.
Mitochondrial fission and fusion – a matter of balance?
Taken together, the above research indicates that interruption of either mitochondrial fission (Drp1 ablation) or fusion (Mfn1/Mfn2 ablation; Opa1 ablation) causes cardiomyopathies in mice, although the respective cardiomyopathies differed in their gross cardiac, cellular and molecular characteristics [25]. Incontrovertibly, these data demonstrate the necessity for both mitochondrial fission and fusion in normal hearts. It is reasonable to ask whether pathology is caused by absolute or relative defects in fission and fusion. In other words, is it the presence of a given dynamism defect that is maladaptive, or is it the resulting imbalance between mitochondrial fission and fusion. This is an important question because if it is the former, then the only possible means of correcting cardiomyopathies evoked by defective fission is to restore fission. Alternately, if balance is more important than absolute rate of fission or fusion, then benefits could be obtained by inducing a parallel reduction in fusion that re-establishes balanced dynamism at a lower rate. David Chan’s group recently examined this hypothesis in mice having defective mitochondrial fission caused by genetic deletion of a mitochondrial Drp1 receptor protein, mitochondrial fission factor (Mff) [60]. Although germ-line ablation of Mff had multi-systemic effects, the mice died at ~13 weeks of age most likely from dilated cardiomyopathy. Mff-deficient cardiac mitochondria exhibited morphological heterogeneity, were decreased in abundance, developed respiratory defects and showed evidence for increased mitophagy; these findings recapitulate the cardinal features of conditional cardiac Drp1 ablation [25,43]. Although generalized embryonic Mfn1 deficiency is lethal [61], crossing Mff knockout and Mfn1 knockout mice reciprocally rescued each [60]. A similar benefit was obtained by germ-line deletion of a single Mfn1 allele in the Mff knockout mouse background. In contrast, and likely because cardiac Mfn2 is central to mitophagy signaling [51,62] and reticular-mitochondrial calcium transport [42], combined deletion of Mfn2 with Mff did not evoke the same salubrious effects as deleting Mff and Mfn1 [60]. Because Mff deletion conferred only a partial defect in mitochondrial fission, it remains to be determined if concomitant suppression of mitochondrial fusion can rescue the cardiomyopathy provoked by directly ablating cardiac Drp1. Nevertheless, these fascinating results support potential efficacy of therapeutic measures restoring a balance between mitochondrial fission and fusion in conditions where one or the other process is dysregulated.
Acknowledgments
Supported by National Institutes of Health grants HL59888 and HL128071.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Huang X, Sun L, Ji S, Zhao T, Zhang W, Xu J, Zhang J, Wang Y, Wang X, Franzini-Armstrong C, et al. Kissing and nanotunneling mediate intermitochondrial communication in the heart. Proc Natl Acad Sci U S A. 2013;110:2846–2851. doi: 10.1073/pnas.1300741110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fujioka H, Tandler B, Hoppel CL. Mitochondrial division in rat cardiomyocytes: an electron microscope study. Anat Rec (Hoboken) 2012;295:1455–1461. doi: 10.1002/ar.22523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen Y, Liu Y, Dorn GW., 2nd Mitochondrial fusion is essential for organelle function and cardiac homeostasis. Circ Res. 2011;109:1327–1331. doi: 10.1161/CIRCRESAHA.111.258723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim TY, Wang D, Kim AK, Lau E, Lin AJ, Liem DA, Zhang J, Zong NC, Lam MP, Ping P. Metabolic labeling reveals proteome dynamics of mouse mitochondria. Mol Cell Proteomics. 2012;11:1586–1594. doi: 10.1074/mcp.M112.021162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kasumov T, Dabkowski ER, Shekar KC, Li L, Ribeiro RF, Jr, Walsh K, Previs SF, Sadygov RG, Willard B, Stanley WC. Assessment of cardiac proteome dynamics with heavy water: slower protein synthesis rates in interfibrillar than subsarcolemmal mitochondria. Am J Physiol Heart Circ Physiol. 2013;304:H1201–1214. doi: 10.1152/ajpheart.00933.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dorn GW., 2nd Mitochondrial dynamics in heart disease. Biochim Biophys Acta. 2013;1833:233–241. doi: 10.1016/j.bbamcr.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dorn GW., 2nd Mitochondrial dynamism and heart disease: changing shape and shaping change. EMBO Mol Med. 2015;7:865–877. doi: 10.15252/emmm.201404575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fang L, Moore XL, Gao XM, Dart AM, Lim YL, Du XJ. Down-regulation of mitofusin-2 expression in cardiac hypertrophy in vitro and in vivo. Life Sci. 2007;80:2154–2160. doi: 10.1016/j.lfs.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 9.Shen T, Zheng M, Cao C, Chen C, Tang J, Zhang W, Cheng H, Chen KH, Xiao RP. Mitofusin-2 is a major determinant of oxidative stress-mediated heart muscle cell apoptosis. J Biol Chem. 2007;282:23354–23361. doi: 10.1074/jbc.M702657200. [DOI] [PubMed] [Google Scholar]
- 10.Gao Q, Wang XM, Ye HW, Yu Y, Kang PF, Wang HJ, Guan SD, Li ZH. Changes in the expression of cardiac mitofusin-2 in different stages of diabetes in rats. Mol Med Rep. 2012;6:811–814. doi: 10.3892/mmr.2012.1002. [DOI] [PubMed] [Google Scholar]
- 11.Javadov S, Rajapurohitam V, Kilic A, Hunter JC, Zeidan A, Said Faruq N, Escobales N, Karmazyn M. Expression of mitochondrial fusion-fission proteins during post-infarction remodeling: the effect of NHE-1 inhibition. Basic Res Cardiol. 2011;106:99–109. doi: 10.1007/s00395-010-0122-3. [DOI] [PubMed] [Google Scholar]
- 12.Chen L, Gong Q, Stice JP, Knowlton AA. Mitochondrial OPA1, apoptosis, and heart failure. Cardiovasc Res. 2009;84:91–99. doi: 10.1093/cvr/cvp181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang Y, Mi C, Liu J, Gao F, Long J. Compromised mitochondrial remodeling in compensatory hypertrophied myocardium of spontaneously hypertensive rat. Cardiovasc Pathol. 2014;23:101–106. doi: 10.1016/j.carpath.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Parra V, Verdejo HE, Iglewski M, Del Campo A, Troncoso R, Jones D, Zhu Y, Kuzmicic J, Pennanen C, Lopez-Crisosto C, et al. Insulin stimulates mitochondrial fusion and function in cardiomyocytes via the Akt-mTOR-NFkappaB-Opa-1 signaling pathway. Diabetes. 2014;63:75–88. doi: 10.2337/db13-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elezaby A, Sverdlov AL, Tu VH, Soni K, Luptak I, Qin F, Liesa M, Shirihai OS, Rimer J, Schaffer JE, et al. Mitochondrial remodeling in mice with cardiomyocyte-specific lipid overload. J Mol Cell Cardiol. 2015;79:275–283. doi: 10.1016/j.yjmcc.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang CR, Blackstone C. Dynamic regulation of mitochondrial fission through modification of the dynamin-related protein Drp1. Ann N Y Acad Sci. 2010;1201:34–39. doi: 10.1111/j.1749-6632.2010.05629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang YW, Chang YT, Wang Q, Lin JJ, Chen YJ, Chen CC. Quantitative phosphoproteomic study of pressure-overloaded mouse heart reveals dynamin-related protein 1 as a modulator of cardiac hypertrophy. Mol Cell Proteomics. 2013;12:3094–3107. doi: 10.1074/mcp.M113.027649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zaja I, Bai X, Liu Y, Kikuchi C, Dosenovic S, Yan Y, Canfield SG, Bosnjak ZJ. Cdk1, PKCdelta and calcineurin-mediated Drp1 pathway contributes to mitochondrial fission-induced cardiomyocyte death. Biochem Biophys Res Commun. 2014;453:710–721. doi: 10.1016/j.bbrc.2014.09.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Samant SA, Zhang HJ, Hong Z, Pillai VB, Sundaresan NR, Wolfgeher D, Archer SL, Chan DC, Gupta MP. SIRT3 deacetylates and activates OPA1 to regulate mitochondrial dynamics during stress. Mol Cell Biol. 2014;34:807–819. doi: 10.1128/MCB.01483-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gawlowski T, Suarez J, Scott B, Torres-Gonzalez M, Wang H, Schwappacher R, Han X, Yates JR, 3rd, Hoshijima M, Dillmann W. Modulation of dynamin-related protein 1 (DRP1) function by increased O-linked-beta-N-acetylglucosamine modification (O-GlcNAc) in cardiac myocytes. J Biol Chem. 2012;287:30024–30034. doi: 10.1074/jbc.M112.390682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hall AR, Burke N, Dongworth RK, Hausenloy DJ. Mitochondrial fusion and fission proteins: novel therapeutic targets for combating cardiovascular disease. Br J Pharmacol. 2014;171:1890–1906. doi: 10.1111/bph.12516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pennanen C, Parra V, Lopez-Crisosto C, Morales PE, Del Campo A, Gutierrez T, Rivera-Mejias P, Kuzmicic J, Chiong M, Zorzano A, et al. Mitochondrial fission is required for cardiomyocyte hypertrophy mediated by a Ca2+-calcineurin signaling pathway. J Cell Sci. 2014;127:2659–2671. doi: 10.1242/jcs.139394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23•.Martin OJ, Lai L, Soundarapandian MM, Leone TC, Zorzano A, Keller MP, Attie AD, Muoio DM, Kelly DP. A role for peroxisome proliferator-activated receptor gamma coactivator-1 in the control of mitochondrial dynamics during postnatal cardiac growth. Circ Res. 2014;114:626–636. doi: 10.1161/CIRCRESAHA.114.302562. The authors used selective transcriptional profiling of PGC-1 deficient hearts to uncover a regulatory link between mitochondrial biogenesis and mitochondrial dynamism. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Papanicolaou KN, Kikuchi R, Ngoh GA, Coughlan KA, Dominguez I, Stanley WC, Walsh K. Mitofusins 1 and 2 are essential for postnatal metabolic remodeling in heart. Circ Res. 2012;111:1012–1026. doi: 10.1161/CIRCRESAHA.112.274142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25••.Song M, Mihara K, Chen Y, Scorrano L, Dorn GW., 2nd Mitochondrial fission and fusion factors reciprocally orchestrate mitophagic culling in mouse hearts and cultured fibroblasts. Cell Metab. 2015;21:273–285. doi: 10.1016/j.cmet.2014.12.011. This comprehensive comparison of fusion- and fission-defective mouse hearts uncovered reciprocal defects in mitophagy leading to different types of cardiomyopathy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kageyama Y, Hoshijima M, Seo K, Bedja D, Sysa-Shah P, Andrabi SA, Chen W, Hoke A, Dawson VL, Dawson TM, et al. Parkin-independent mitophagy requires Drp1 and maintains the integrity of mammalian heart and brain. EMBO J. 2014;33:2798–2813. doi: 10.15252/embj.201488658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rehman J. Empowering self-renewal and differentiation: the role of mitochondria in stem cells. J Mol Med (Berl) 2010;88:981–986. doi: 10.1007/s00109-010-0678-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kasahara A, Cipolat S, Chen Y, Dorn GW, 2nd, Scorrano L. Mitochondrial fusion directs cardiomyocyte differentiation via calcineurin and Notch signaling. Science. 2013;342:734–737. doi: 10.1126/science.1241359. [DOI] [PubMed] [Google Scholar]
- 29.Song M, Dorn GW., 2nd Mitoconfusion: noncanonical functioning of dynamism factors in static mitochondria of the heart. Cell Metab. 2015;21:195–205. doi: 10.1016/j.cmet.2014.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sin J, Andres AM, Taylor DJ, Weston T, Hiraumi Y, Stotland A, Kim BJ, Huang C, Doran KS, Gottlieb RA. Mitophagy is required for mitochondrial biogenesis and myogenic differentiation of C2C12 myoblasts. Autophagy. 2015 doi: 10.1080/15548627.2015.1115172. ePub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim B, Kim JS, Yoon Y, Santiago MC, Brown MD, Park JY. Inhibition of Drp1-dependent mitochondrial division impairs myogenic differentiation. Am J Physiol Regul Integr Comp Physiol. 2013;305:R927–938. doi: 10.1152/ajpregu.00502.2012. [DOI] [PubMed] [Google Scholar]
- 32.Ong SB, Subrayan S, Lim SY, Yellon DM, Davidson SM, Hausenloy DJ. Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation. 2010;121:2012–2022. doi: 10.1161/CIRCULATIONAHA.109.906610. [DOI] [PubMed] [Google Scholar]
- 33.Sharp WW, Fang YH, Han M, Zhang HJ, Hong Z, Banathy A, Morrow E, Ryan JJ, Archer SL. Dynamin-related protein 1 (Drp1)-mediated diastolic dysfunction in myocardial ischemia-reperfusion injury: therapeutic benefits of Drp1 inhibition to reduce mitochondrial fission. FASEB J. 2014;28:316–326. doi: 10.1096/fj.12-226225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharp WW, Beiser DG, Fang YH, Han M, Piao L, Varughese J, Archer SL. Inhibition of the mitochondrial fission protein dynamin-related protein 1 improves survival in a murine cardiac arrest model. Crit Care Med. 2015;43:e38–47. doi: 10.1097/CCM.0000000000000817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Disatnik MH, Ferreira JC, Campos JC, Gomes KS, Dourado PM, Qi X, Mochly-Rosen D. Acute inhibition of excessive mitochondrial fission after myocardial infarction prevents long-term cardiac dysfunction. J Am Heart Assoc. 2013;2:e000461. doi: 10.1161/JAHA.113.000461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zepeda R, Kuzmicic J, Parra V, Troncoso R, Pennanen C, Riquelme JA, Pedrozo Z, Chiong M, Sanchez G, Lavandero S. Drp1 loss-of-function reduces cardiomyocyte oxygen dependence protecting the heart from ischemia-reperfusion injury. J Cardiovasc Pharmacol. 2014;63:477–487. doi: 10.1097/FJC.0000000000000071. [DOI] [PubMed] [Google Scholar]
- 37.Din S, Mason M, Volkers M, Johnson B, Cottage CT, Wang Z, Joyo AY, Quijada P, Erhardt P, Magnuson NS, et al. Pim-1 preserves mitochondrial morphology by inhibiting dynamin-related protein 1 translocation. Proc Natl Acad Sci U S A. 2013;110:5969–5974. doi: 10.1073/pnas.1213294110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ishihara N, Nomura M, Jofuku A, Kato H, Suzuki SO, Masuda K, Otera H, Nakanishi Y, Nonaka I, Goto Y, et al. Mitochondrial fission factor Drp1 is essential for embryonic development and synapse formation in mice. Nat Cell Biol. 2009;11:958–966. doi: 10.1038/ncb1907. [DOI] [PubMed] [Google Scholar]
- 39.Ishihara T, Ban-Ishihara R, Maeda M, Matsunaga Y, Ichimura A, Kyogoku S, Aoki H, Katada S, Nakada K, Nomura M, et al. Dynamics of mitochondrial DNA nucleoids regulated by mitochondrial fission is essential for maintenance of homogeneously active mitochondria during neonatal heart development. Mol Cell Biol. 2015;35:211–223. doi: 10.1128/MCB.01054-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ikeda Y, Shirakabe A, Maejima Y, Zhai P, Sciarretta S, Toli J, Nomura M, Mihara K, Egashira K, Ohishi M, et al. Endogenous Drp1 mediates mitochondrial autophagy and protects the heart against energy stress. Circ Res. 2015;116:264–278. doi: 10.1161/CIRCRESAHA.116.303356. [DOI] [PubMed] [Google Scholar]
- 41.Papanicolaou KN, Khairallah RJ, Ngoh GA, Chikando A, Luptak I, O’Shea KM, Riley DD, Lugus JJ, Colucci WS, Lederer WJ, et al. Mitofusin-2 maintains mitochondrial structure and contributes to stress-induced permeability transition in cardiac myocytes. Mol Cell Biol. 2011;31:1309–1328. doi: 10.1128/MCB.00911-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen Y, Csordas G, Jowdy C, Schneider TG, Csordas N, Wang W, Liu Y, Kohlhaas M, Meiser M, Bergem S, et al. Mitofusin 2-containing mitochondrial-reticular microdomains direct rapid cardiomyocyte bioenergetic responses via interorganelle Ca(2+) crosstalk. Circ Res. 2012;111:863–875. doi: 10.1161/CIRCRESAHA.112.266585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43•.Song M, Gong G, Burelle Y, Gustafsson AB, Kitsis RN, Matkovich SJ, Dorn GW., 2nd Interdependence of Parkin-Mediated Mitophagy and Mitochondrial Fission in Adult Mouse Hearts. Circ Res. 2015;117:346–351. doi: 10.1161/CIRCRESAHA.117.306859. This follow-up study to reference 25 revealed that parkin-mediated mitophagy is an inducible response in hearts, and rescued the cardiomyopathy provoked by ablating Drp1 by also ablating Parkin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Waterham HR, Koster J, van Roermund CW, Mooyer PA, Wanders RJ, Leonard JV. A lethal defect of mitochondrial and peroxisomal fission. N Engl J Med. 2007;356:1736–1741. doi: 10.1056/NEJMoa064436. [DOI] [PubMed] [Google Scholar]
- 45.Chang CR, Manlandro CM, Arnoult D, Stadler J, Posey AE, Hill RB, Blackstone C. A lethal de novo mutation in the middle domain of the dynamin-related GTPase Drp1 impairs higher order assembly and mitochondrial division. J Biol Chem. 2010;285:32494–32503. doi: 10.1074/jbc.M110.142430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ashrafian H, Docherty L, Leo V, Towlson C, Neilan M, Steeples V, Lygate CA, Hough T, Townsend S, Williams D, et al. A mutation in the mitochondrial fission gene Dnm1l leads to cardiomyopathy. PLoS Genet. 2010;6:e1001000. doi: 10.1371/journal.pgen.1001000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cahill TJ, Leo V, Kelly M, Stockenhuber A, Kennedy NW, Bao L, Cereghetti G, Harper AR, Czibik G, Lao C, et al. Resistance of Dynamin-related Protein 1 Oligomers to Disassembly Impairs Mitophagy, Resulting in Myocardial Inflammation and Heart Failure. J Biol Chem. 2015;290:25907–25919. doi: 10.1074/jbc.M115.665695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen H, McCaffery JM, Chan DC. Mitochondrial fusion protects against neurodegeneration in the cerebellum. Cell. 2007;130:548–562. doi: 10.1016/j.cell.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 49.Chen H, Vermulst M, Wang YE, Chomyn A, Prolla TA, McCaffery JM, Chan DC. Mitochondrial fusion is required for mtDNA stability in skeletal muscle and tolerance of mtDNA mutations. Cell. 2010;141:280–289. doi: 10.1016/j.cell.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Song M, Chen Y, Gong G, Murphy E, Rabinovitch PS, Dorn GW., 2nd Super-suppression of mitochondrial reactive oxygen species signaling impairs compensatory autophagy in primary mitophagic cardiomyopathy. Circ Res. 2014;115:348–353. doi: 10.1161/CIRCRESAHA.115.304384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51••.Guohua Gong MS, Csordas Gyorgy, Kelly Daniel P, Matkovich Scot J, Dorn GW., 2nd Parkin-mediated mitophagy directs perinatal cardiac metabolic maturation in mice. Science. 2015;350:AAD2459. doi: 10.1126/science.aad2459. This study builds upon insights from reference 62 to discover a completely novel role for Parkin in hearts - promotion of mitochodrial turnover that enables normal developmental metabolic transitioning. The data further link mitochondrial dynamism, mitophagy, and mitochondrial biogenesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dorn GW, 2nd, Maack C. SR and mitochondria: calcium cross-talk between kissing cousins. J Mol Cell Cardiol. 2013;55:42–49. doi: 10.1016/j.yjmcc.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 53.Shirihai OS, Song M, Dorn GW., 2nd How mitochondrial dynamism orchestrates mitophagy. Circ Res. 2015;116:1835–1849. doi: 10.1161/CIRCRESAHA.116.306374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Frezza C, Cipolat S, Martins de Brito O, Micaroni M, Beznoussenko GV, Rudka T, Bartoli D, Polishuck RS, Danial NN, De Strooper B, et al. OPA1 controls apoptotic cristae remodeling independently from mitochondrial fusion. Cell. 2006;126:177–189. doi: 10.1016/j.cell.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 55•.Varanita T, Soriano ME, Romanello V, Zaglia T, Quintana-Cabrera R, Semenzato M, Menabo R, Costa V, Civiletto G, Pesce P, et al. The OPA1-dependent mitochondrial cristae remodeling pathway controls atrophic, apoptotic, and ischemic tissue damage. Cell Metab. 2015;21:834–844. doi: 10.1016/j.cmet.2015.05.007. This study of Opa1 deficiency links cardiomyopathy to altered cristae structure. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56•.Wai T, Garcia-Prieto J, Baker MJ, Merkwirth C, Benit P, Rustin P, Rupérez FJ, Barbas Coral, Ibañez B, Langer R. Imbalanced OPA1 processing and mitochondrial fragmentation cause heart failure in mice. Science. 2015;350:aad0116–0111. doi: 10.1126/science.aad0116. In contrast to reference 55; this study of altered Opa1 processing linked cardiomyopathy to altered mitochodnrial fusion. [DOI] [PubMed] [Google Scholar]
- 57.Cogliati S, Frezza C, Soriano ME, Varanita T, Quintana-Cabrera R, Corrado M, Cipolat S, Costa V, Casarin A, Gomes LC, et al. Mitochondrial cristae shape determines respiratory chain supercomplexes assembly and respiratory efficiency. Cell. 2013;155:160–171. doi: 10.1016/j.cell.2013.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Song Z, Ghochani M, McCaffery JM, Frey TG, Chan DC. Mitofusins and OPA1 mediate sequential steps in mitochondrial membrane fusion. Mol Biol Cell. 2009;20:3525–3532. doi: 10.1091/mbc.E09-03-0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Roberta A, Gottlieb DB. Mitochondria shape cardiac metabolism. Science. 2015;350:1162–1163. doi: 10.1126/science.aad8222. [DOI] [PubMed] [Google Scholar]
- 60••.Chen H, Ren S, Clish C, Jain M, Mootha V, McCaffery JM, Chan DC. Titration of mitochondrial fusion rescues Mff-deficient cardiomyopathy. J Cell Biol. 2015;211:795–805. doi: 10.1083/jcb.201507035. These authors show that cardiomyopathy provoked by interrupting Drp1-dependent mitochondrial fission (Mff knokcout) can be rescued by concomitantly suppressing mitochondrial fusion (Mfn1 knockout), revealing a central role for fission/fusion balance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen H, Detmer SA, Ewald AJ, Griffin EE, Fraser SE, Chan DC. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol. 2003;160:189–200. doi: 10.1083/jcb.200211046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen Y, Dorn GW., 2nd PINK1-phosphorylated mitofusin 2 is a Parkin receptor for culling damaged mitochondria. Science. 2013;340:471–475. doi: 10.1126/science.1231031. [DOI] [PMC free article] [PubMed] [Google Scholar]