Abstract
Many individuals with autism spectrum disorder (ASD) exhibit motor difficulties, but it is unknown whether manual motor skills improve, plateau, or decline in ASD in the transition from childhood into adulthood. Atypical development of manual motor skills could impact the ability to learn and perform daily activities across the life span. This study examined longitudinal grip strength and finger tapping development in individuals with ASD (n=90) compared to individuals with typical development (n=56), ages five to 40 years old. We further examined manual motor performance as a possible correlate of current and future daily living skills. The group with ASD demonstrated atypical motor development, characterized by similar performance during childhood but increasingly poorer performance from adolescence into adulthood. Grip strength was correlated with current adaptive daily living skills, and Time 1 grip strength predicted daily living skills eight years into the future. These results suggest that individuals with ASD may experience increasingly more pronounced motor difficulties from adolescence into adulthood and that manual motor performance in ASD is related to adaptive daily living skills.
Keywords: grip strength, finger tapping, motor speed, upper extremity, childhood, adolescence, adulthood
To track development in infancy, parents and clinicians emphasize a child’s ability to roll, crawl, and walk. However, with increasing age, the developmental focus shifts from motor milestones to cognitive markers (i.e., language, reading, or spatial reasoning). For many, motor skills from adolescence to middle adulthood are virtually ignored outside of the context of athletics or rehabilitation. However, in older adulthood, the emphasis on motor skills re-emerges, focusing on those who may be at risk for falls or who may have difficulty with everyday tasks due to motor declines. In other words, motor skills in typically developing individuals appear to be emphasized at points in the life span when motor ability impacts daily living skills and independence. However, for many children with developmental disorders, motor difficulties may impact functional limitations across the life span. Specifically, in children with cerebral palsy, gross and fine motor movements were found to predict adaptive daily living skills (Tseng, Chen, Shieh, Lu, & Huang, 2011). This finding is perhaps unsurprising given that motor difficulties are profound enough to be considered a primary diagnostic indicator of cerebral palsy. However, what is not known is whether individuals with persistent but mild motor difficulties have corresponding difficulties with adaptive daily living skills, even at ages when motor skills are not a developmental focus.
In individuals with Autism Spectrum Disorder (ASD), motor difficulties are not a core symptom, suggesting that motor impairments in this population may be more subtle than in disorders like cerebral palsy. However, a variety of gross and fine motor impairments in ASD have been commonly reported (for a meta-analyses see Fournier, Hass, Naik, Lodha, & Cauraugh, 2010). These motor difficulties include dyspraxia (Dewey, 1991; Dewey, Cantell, & Crawford, 2007; Dowell, Mahone, & Mostofsky, 2009; Dziuk et al., 2007; Minshew, Goldstein, & Siegel, 1997; Mostofsky et al., 2006; Rogers, Bennetto, McEvoy, & Pennington, 1996), challenges with postural stability (Fournier, Kimberg, et al., 2010; Kohen-Raz, Volkman, & Cohen, 1992; Molloy, Dietrich, & Bhattacharya, 2003; Papadopoulos et al., 2011; Travers, Powell, Klinger, & Klinger, 2013; Weimer, Schatz, Lincoln, Ballantyne, & Trauner, 2001), impaired motor anticipation (Rinehart et al., 2006; Rinehart, Bradshaw, Brereton, & Tonge, 2001), and weak grip strength (Kern et al., 2013; Travers et al., 2015). These motor difficulties have been reported across wide age ranges, but data are limited regarding the longitudinal development of motor ability in this population. Understanding at what ages individuals with ASD struggle most with motor difficulties may provide insight into the nature and real-world impact of motor difficulties in ASD. Given that service receipt for people with ASD decreases after high school (Shattuck, Wagner, Narendorf, Sterzing, & Hensley, 2011), understanding the developmental trajectory of motor skills could have important implications for interventions that might allow individuals with ASD to function better in adulthood. More broadly, understanding whether subtle motor difficulties do or do not impact adaptive daily living skills from childhood through middle adulthood, regardless of diagnosis, would have implications for how we conceptualize and monitor motor skills during these developmental periods. Therefore, the purpose of this study is to examine the developmental trajectory of motor ability in ASD from childhood into adulthood and to further explore the potential impact of manual motor difficulties on adaptive daily living skills.
Few studies have examined age-related changes in motor ability in individuals with ASD. Of these studies, one suggested improvements in motor skills with age, while the other studies suggested declines relative to typically developing peers. Specifically, a higher incidence of hypotonia and ataxia was reported in two-to-six-year olds with ASD compared to seven-to-18-year olds with ASD (Ming, Brimacombe, & Wagner, 2007), suggesting that motor difficulties in ASD may diminish from early childhood into adolescence. In contrast, multiple studies have demonstrated increasingly poorer motor skills in children with ASD one to four years of age. Specifically, a repeated measures analysis indicated atypical fine and gross motor ability at 12 to 24 months of age that became increasingly more impaired compared to typically developing age norms between 31 to 36 months of age (Lloyd, MacDonald, & Lord, 2013). Similarly, a longitudinal randomized controlled trial demonstrated increasingly poorer parent-reported motor skills in ASD from two to four years of age in the control group (Dawson et al., 2010), even though the active treatment group actually demonstrated increasingly better parent-reported motor skills. In toddlers at-risk for ASD, approximately 25% of the sample demonstrated declines in motor skills (Ben-Sasson & Gill, 2014). However, these motor declines occurred in conjunction with improvements in language skills, demonstrating the discontinuity in development across domains that can occur in children of this age. Collectively, these results suggest that motor skills in children with ASD and at-risk for ASD may appear increasingly less typical as they transition from infancy to toddlerhood, possibly varying according to type of early intervention received. In older individuals (5–52 years of age), cross-sectional analyses of postural adjustments suggested delayed postural development in ASD, with adults with ASD not reaching adult-level postural stability (Minshew, Sung, Jones, & Furman, 2004). Therefore, postural stability impairments in childhood may persist into adulthood. However, the differing samples and methodologies of these studies impede our understanding of how motor skills develop and change with age in ASD. Further, there is a strong need for a longitudinal examination of motor ability in ASD beyond toddlerhood.
Of the variety of motor skills that have been reported to be impaired in ASD, being able to grip objects and dexterously move one’s fingers is essential for completion of a variety of important tasks across the life span. Poor manual motor skills could build on one another, leading to increased disability over time. Thus, fundamental, manual motor assesments like grip strength and finger tapping speed may be particularly apt for a longitudinal examination, as these skills are diagnostic correlates for success during more complex hand movements. In infancy, effectively gripping objects and moving one’s fingers allow for children to explore the world around them, reaching for and manipulating objects. In childhood, grip strength and finger dexterity facilitate key academic tasks, such as handwriting. Indeed, grip strength was found to be correlated with pencil control and handwriting legibility in children with ASD (Alaniz, Galit, Necesito, & Rosario, 2015). Poor handwriting may have detrimental effects on tasks like taking notes and completing math problems, creating a lasting impact on academic performance. In adolescence and adulthood, tasks such as food preparation, dressing, caring for others, and driving involve gripping objects and skillfully moving one’s fingers. Furthermore, hand strength may provide insights into age-related changes from mid to later adulthood. Specifically, midlife grip strength has been shown to predict functional limitations (Rantanen et al., 1999) and mortality 25 years later (Al Snih, Markides, Ray, Ostir, & Goodwin, 2002; Fujita et al., 1995; Gale, Martyn, Cooper, & Sayer, 2007; Katzmarzyk & Craig, 2002; Metter, Talbot, Schrager, & Conwit, 2002; Rantanen et al., 2012; Sasaki, Kasagi, Yamada, & Fujita, 2007). Manual motor difficulties during development may also predict difficulties with daily living skills and later outcomes. In preschoolers with ASD, motor difficulties (along with sensory difficulties) correlated with adaptive daily living skills (Jasmin et al., 2009). Whether this relationship persists past preschool into later childhood and adulthood in ASD has not yet been tested. One possibility is that even subtle motor difficulties may be associated with concurrent daily living skills and predictive of future adaptive daily living skills, such that the cumulative effect of the manual motor difficulties during development could hinder the achievement of independent living skills once the child reaches adulthood. Indeed, adaptive daily living skills in ASD have been shown to have an atypical plateau during early adolescence (Smith, Maenner, & Seltzer, 2012), and we do not know whether motor difficulties may contribute to this plateau.
The aims of the present study were to 1) examine age-related changes in longitudinal measures of grip strength and finger tapping in ASD compared to typical development from early childhood into mid-adulthood, and 2) to examine manual motor performance as a predictor of concurrent and future adaptive daily living skills. Based on the previous literature identifying multiple motor impairments in individuals with ASD, we hypothesized that individuals with ASD would demonstrate atypical manual motor development across this age span. Furthermore, because manual motor ability underlies a number of adaptive daily living skills (i.e., dressing, hand writing, preparing food, etc.), we hypothesized that grip strength and finger tapping would be correlated with concurrent adaptive daily living skills and predictive of future adaptive daily livings skills.
Methods
Design
The present study is an analysis of data from a broader, 12-year longitudinal study that focused on age-related brain and behavior changes in males with ASD compared to typically developing controls. In the broader study, four waves of data collection (both neuroimaging and behavioral measures) occurred. There was approximately 2.5 years between each time point of data collection. Added measures of grip strength and finger tapping speed and measures of adaptive functioning were collected one to three times over an eight-year period (primarily at Times 1 and 3). In the group with ASD, 60% had two or more motor assessments over the course of the study, and in the group with typical development 50% had two or more motor assessments. Adaptive functioning was measured at Times 1, 3, and 4. Both the broader longitudinal study and the current study employed an accelerated longitudinal design (Harezlak, Ryan, Giedd, & Lange, 2005; Nesselroade & Baltes, 1979), which measured individual longitudinal changes in motor measurements across multiple age cohorts. In other words, participants of multiple age groups participated at Time 1 and then were followed at Times 2–4. Participants were included in the analyses even if they had measurements at only one time point. In these models, it is statistically acceptable to include all available data, balanced or not, as an empirical basis for estimating a growth curve model (even if the participant has just one observation (Lange & Laird, 1989). The rationale behind this is that the inclusion of all available data provides a more precise estimate of the longitudinal and cross-sectional mean functions as well as additional information on baselines, peaks, valleys, inflections, even though these latter three require estimation of higher order derivatives..
Participants
Participants’ consent was obtained according to the Declaration of Helsinki, and this research was approved by the University of Utah and the University of Wisconsin-Madison Institutional Review Boards. Ninety males with ASD and 56 age- and sex-matched typically developing controls (TDC) between the ages of five and 39 years (M = 18.0 years) participated in this study. These participants were selected from participants in the broader longitudinal neuroimaging study (110 ASD and 78 TDC), excluding participants with an atypical presentation of ASD or TDC, or participants who did not have an age match in the other group. Time 1 motor data from these participants have been reported previously (Duffield et al., 2013; Travers et al., 2015). The participants with ASD were diagnosed based on Autism Diagnostic Interview-Revised (Lord, Rutter, & Le Couteur, 1994), Autism Diagnostic Observation Scale (ADOS) (Lord et al., 2000), Diagnostic Statistical Manual-IV-TR (American Psychiatric Association, 2000), and ICD-10 criteria. Participants with ASD were included in the present study if they met criteria for a lifetime diagnosis of autistic disorder, Asperger’s syndrome, or pervasive developmental disorder not otherwise specified (89% met full criteria for autistic disorder). Exclusion criteria included medical causes of ASD (determined by patient history, physical exam, fragile-X testing, and karyotype), history of severe head injury, hypoxia-ischemia, seizure disorder, and other neurological disorders. Typically developing control participants were confirmed as having typical development through history, the ADOS, IQ testing, and neuropsychological and standardized psychiatric assessment. All participants had English as their first language and were verbal at the time of testing. On average, the group with ASD had average IQ (M = 101.07, SD = 16.59), whereas the group with typical development had above-average IQ (M = 117.54, SD = 14.7). See Table 1 for more detailed participant information.
Table 1.
Demographic characteristics of the longitudinal study sample and p-values for group comparisons
| ASD | Typical Development | p-value | |
|---|---|---|---|
| Number of Participants | 90 | 56 | -- |
| Number of Motor Assessments | 144 | 83 | -- |
| Motor Assessments per Participant, mean(SD) | 1.61(0.51) | 1.54(0.57) | .42 |
| Motor Assessments, range | 1–3 | 1–3 | -- |
| Right Hand Preferred | 88.2% | 96.4% | -- |
| Body Mass Index (BMI), mean(SD) | 23.41(6.86) | 22.63(5.06) | .41 |
| BMI, range | 14.47–46.33 | 13.74–39.33 | -- |
| Age in years, mean(SD) | 17.51(7.61) | 18.81(7.88) | .33 |
| Age in years, range | 6.38–37.78 | 6.90–39.78 | -- |
| FSIQ, mean(SD) | 101.07(16.59) | 117.54(14.7) | <.001 |
| FSIQ, range | 72–137 | 78–152 | -- |
| SRS Total Raw Score, mean(SD) | 96.52(27.87) | 17.03(13.28) | <.001 |
| SRS Total Raw Score, range | 21–157 | 0–60 | -- |
| Vineland Daily Living Standard Score, mean(SD) | 79.46(13.22) | 102.15(9.59) | <.001 |
| Vineland Daily Living Standard Score, range | 50–113 | 81–121 | -- |
| Vineland Adaptive Behavior Composite Standard Score, mean(SD) | 75.87(10.13) | 101.27(9.39) | <.001 |
| Vineland Adaptive Behavior Composite Standard Score, range | 56–109 | 78–121 | -- |
ASD, autism spectrum disorder; FSIQ, full-scale IQ; SRS, social responsiveness scale
Assessments
Grip strength
Grip strength was measured with a hand dynamometer according to the Halstead-Reitan Battery guidelines (Reitan Laboratories: www.reitanlabs.com; Heaton, Grant, & Matthews, 1991). Grip strength from the dynamometer was recorded and reported in kilograms (as is the industry standard). Participants were instructed to hold the upper part of the dynamometer in the palm of the hand and squeeze the stirrup with the fingers as tightly as possible. Participants completed two trials. However, if the strength in kilograms of these two trials were not within a five-point range, a third trial was completed. This procedure was carried out with both the preferred and nonpreferred hands. A third trial was administered as often in the typically developing group (64% of the preferred-hand assessments, 63% of the nonpreferred-hand assessments) as in the ASD group (69% of the preferred-hand assessments, 68% of the nonpreferred-hand assessments), preferred: χ2 = 0.37, p = .54, nonpreferred: χ2 = 0.13, p = .72. There was a very high correlation between the grip strength measurements in the preferred and non-preferred hands, r = +.96, p < .001. To increase comparability of our grip strength measures to previous studies (Dodds et al., 2014), a maximum grip strength score was calculated for each time point, taking the strongest grip strength among all trials, regardless of hand preference. Follow-up analyses examined preferred and nonpreferred hand grip strength separately, by averaging the trials for each hand.
Finger tapping
Finger tapping was measured by the number of taps performed by the pointer finger of each hand over a period of 10 seconds according to the Halstead-Reitan Battery (Reitan Laboratories: www.reitanlabs.com; Heaton et al., 1991). Participants were instructed to tap the pointer finger as many times as possible until the experimenter with the stopwatch said stop. The majority of participants completed the task on the manual finger tapping board, but the younger participants completed the task using the electronic finger tapping version. For each hand, participants completed five trials. However, if the scores from the five trials were not within five finger taps, additional trials for each hand were administered until this administration rule was met or until 10 total trials for each hand were administered. For each time point and each hand, the trials were averaged.
Adaptive functioning
The Vineland Adaptive Behavior Scales (VABS) parent rating forms (Sparrow & Cicchetti, 1989) were used to examine adaptive functioning in the participants at Times 1, 3, and 4. The VABS gathers information about the ability of the child to take care of him or herself and the ability to get along with others in light of the child’s age. Age-normed scores were derived for the adaptive behavior composite in addition to age-normed scores for the social skills, daily living skills, and communication subscales. For Time 1, 59 of the 90 individuals with ASD, and 30 of the 56 individuals with typical development had completed VABS measures. For Time 3, 22 of the 90 individuals with ASD and only 3 of the 56 individuals with typical development had completed VABS measures. For Time 4, 61 of the 90 individuals with ASD and 34 of the 56 individuals with typical development had completed VABS measures.
IQ
IQ was assessed in participants at each time point. Standard scores full scale IQ [FSIQ] are reported. At Time 1, participants completed the Differential Abilities Scales (Elliott, 1990), Wechsler Intelligence Scale for Children (3rd edition) (Wechsler, 1991), or Wechsler Adult Intelligence Scale (3rd edition) (Wechsler, 1997) (depending on age and verbal ability). At Time 3, participants completed the two-subtest version of the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999).
Data Analysis
Because age-related changes in motor skills may not be fully captured by parametric methods, we took a semi-parametric approach that used penalized smoothing splines (Wood, 2004) to examine age-related changes in manual motor performance. The general additive model smoothing splines do not restrict the fit to polynomials. The semiparametric smoothing splines bend to data and are penalized for each bend to minimize model complexity. In this way, the smoothing spline models provide the fewest bends that account for the most variance. These smoothing splines are more likely to capture nonlinear growth spurts or declines, which may improve our understanding of potentially divergent developmental trajectories in ASD compared to typical development. Further, these models may prevent fitted curve bias at the extremes of the data range, a curse of polynomial fits. As elsewhere, the mixed-effects aspect of these smoothing splines consider both between and repeated measurements from participants and are designed for our accelerated longitudinal design (Alexander-Bloch et al., 2014; Harezlak et al., 2005). All statistical analyses were performed in R version 3.1.1 (R. Core Team 2014). Specifically, we used the gamm4 package to perform generalized additive mixed models (Hastie, 1999; Wood, 2004, 2006; Wood & Scheipl, 2014). To ensure that our typically developing group was estimating typical motor performance curves, we compared the grip strength spline curves with the normative data derived from Dodds et al. (2014).
The penalized spline curves examined the effects of age and diagnosis on grip strength and on finger tapping speed in both the preferred and nonpreferred hands. Specifically, age and the interaction term between group and age were defined as smooth terms, whereas group was a fixed variable, and the subject was set as the random effect. The analyses were performed both with and without controlling for full scale IQ. Follow-up independent-samples t-tests were conducted to confirm the pattern of the spline results.
To examine whether manual motor performance measures could predict concurrent daily living skills and also living skills 5–9 years into the future, the ppcor package in R (Kim, 2012) was used to correlate Time 1 manual motor performance with Time 1 VABS daily living standard scores and then with Time 4 VABS daily living standard scores (controlling for age, as age was found to significantly relate to both grip strength and VABS daily living standard scores). To make sure that our results were not driven by IQ differences, additional partial correlations were performed controlling for both age and full scale IQ. Further, to examine if these relationships remained in an adult-only sample, we also looked at these partial correlations in those who were 18 years or older at Time 1. While Time 3 VABS results were available, these were not used in the analyses due to a smaller sample size and uncertainty about whether the data were missing at random. We employed p-values of standardized regression coefficients and Cohen’s d to assess statistical significance.
Results
Manual motor performance and developmental trajectory models
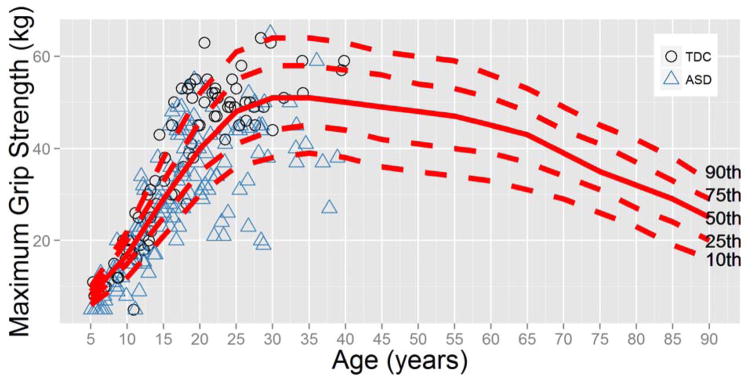
Prior to our case-control comparisons, we confirmed that our typically developing motor performance data was consistent with previously established norms. Specifically, we examined whether our measurements of maximum grip strength in the typically developing group fit with the age-related trajectories of Dodds et al. (2014), who developed norms for maximum grip strength based on approximately 50,000 participants. In Figure 1, the individual data points from our study may be seen in context of the Dodds et al. (2014) definition of 10th, 25th, 50th, 75th and 90th percentiles. The typically developing growth curves of grip strength in the present study are quite similar to that of the normative sample. However, further analysis at the individual level indicated that 37 of the 144 grip strength observations in the ASD group (25.7%) were below the 10th percentile of the normative data, compared to one of 83 observations (1.2%) in the typically developing group. Of the observations that fell below the 10th percentile, 32 participants with ASD (35.6%) had at least one measurement of grip strength below the 10th percentile (compared to 1.82% of participants in the typically developing sample). Therefore, while the normative data coincided with the typically developing data in the present study, more than one third of the participants with ASD had grip strengths below the 10th percentile. Intriguingly, 12 participants with ASD (13.3%) had at least one measurement of grip strength above the 90th percentile. An independent-samples t-test indicated that those within the ASD group who had grip strength above the 90th percentile did not differ from those within the ASD group who had grip strength below the 10th percentile in terms of age, t(42) = −0.45, p = .65, BMI, t(33) = 1.37, p = .18, or full scale IQ, t(42) = −1.00, p = .32.
Figure 1.
Maximum grip strength (in kilograms) measurements from the present study plotted in references to the age-specific maximum grip strength norms of Dodds et al. (2014). Approximately 25% of the grip strength measurements of the group with ASD were below the 10th percentile.
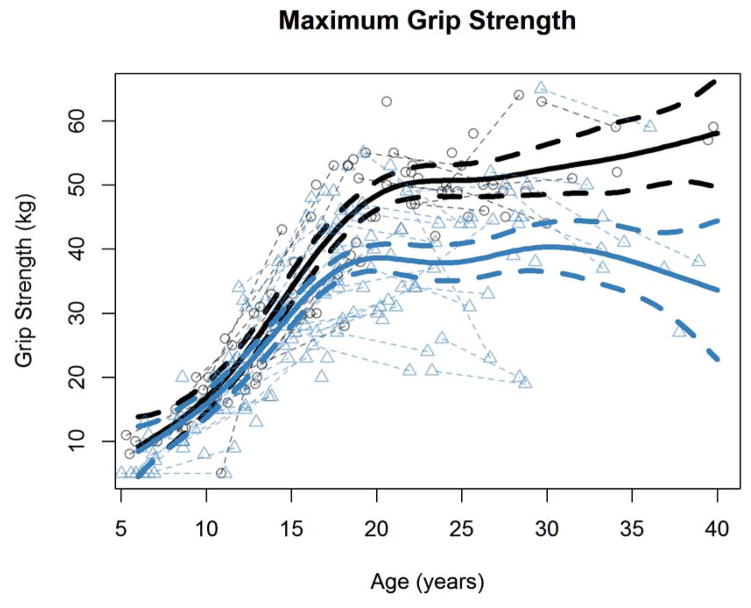
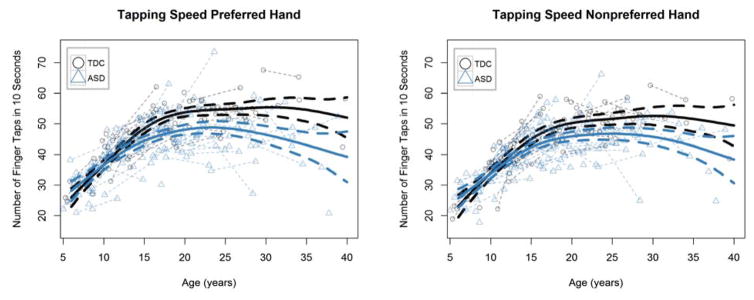
In order to examine if manual motor performance develops differently in ASD compared to typical development, we tested for case-control differences in both grip strength and finger tapping speed (preferred and nonpreferred hands separately) and their longitudinal trajectories. Maximum grip strength measurements from both groups are shown in Figure 2, and the finger tapping speed measurements from both groups are shown in Figure 3. Our generalized additive mixed effects model analysis found significant age-spline-by-group interactions for grip strength and finger tapping speed of both hands (all p values < = .001; Table 2), suggesting distinct developmental trajectories for motor performance in the group with ASD compared to the group with typical development. Even when running these models controlling for full scale IQ, the interaction effects remained significant (again all p values < = .001).
Figure 2.
Maximum grip strength (in kilograms) as a function of age in the ASD and typically developing groups.
Figure 3.
Tapping speed (both preferred and nonpreferred hands) as a function of age in the autism spectrum disorder (ASD) and typically developing control (TDC) groups.
Table 2.
Generalized additive mixed-effects models examining group and age-related changes in grip strength and finger tapping
| Group | Age spline | Age x group spline | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate | p-value | EDF | F | p-value | EDF | F | p-value | ||
| Grip strength | Maximum | ||||||||
| Both Groups | 3.65 | 0.20 | 6.24 | 67.03 | <.001 | 1.00 | 18.26 | <.001 | |
| ASD | -- | -- | 5.58 | 47.17 | <.001 | -- | -- | -- | |
| TDC | -- | -- | 5.29 | 94.24 | <.001 | -- | -- | -- | |
| Preferred Hand | |||||||||
| Both Groups | 2.64 | 0.34 | 6.23 | 61.47 | <.001 | 1 | 17.00 | <.001 | |
| ASD | -- | -- | 5.71 | 43.56 | <.001 | -- | -- | -- | |
| TDC | -- | -- | 5.21 | 81.31 | <.001 | -- | -- | -- | |
| Nonpreferred Hand | |||||||||
| Both Groups | 1.71 | 0.543 | 6.00 | 52.60 | <.001 | 1 | 10.76 | 0.001 | |
| ASD | -- | -- | 5.32 | 41.14 | <.001 | -- | -- | -- | |
| TDC | -- | -- | 5.63 | 59.12 | <.001 | -- | -- | -- | |
| Finger Tapping | Preferred Hand | ||||||||
| Both Groups | 2.89 | 0.22 | 4.62 | 46.82 | <.001 | 1 | 11.46 | <.001 | |
| ASD | -- | -- | 3.98 | 28.55 | <.001 | -- | -- | -- | |
| TDC | -- | -- | 4.94 | 60.89 | <.001 | -- | -- | -- | |
| Nonpreferred Hand | |||||||||
| Both Groups | 3.27 | 0.12 | 4.78 | 54.05 | <.001 | 1 | 11.36 | <.001 | |
| ASD | -- | -- | 4.09 | 38.02 | <.001 | -- | -- | -- | |
| TDC | -- | -- | 4.30 | 51.58 | <.001 | -- | -- | -- | |
ASD, autism spectrum disorder; EDF, estimated degrees of freedom for spline; F, f-statistic; TDC, typically developing control.
An examination of the spline plots (Figures 2 and 3) suggested that motor performance may begin to further deviate between the groups around the age of 15 years. To confirm this conjecture, we divided the sample by average age over the course of the longitudinal study (those on average older than 15 versus those on average younger than 15). In the ASD group, 38 participants (42%) were under the age of 15 years, whereas in the typically developing group, 19 participants (34%) were under the age of 15 years. In those younger than 15 years of age, the group with ASD demonstrated weaker grip strength (average across preferred and nonpreferred hands) (M = 15.71, SD = 8.90) than the group with typical development (M = 17.69, SD = 6.12), but this difference was not statistically significant and had a small-to-medium effect size, t(55) = 0.98, p = .33, d = 0.28. In contrast, in those older than 15 years of age, the group with ASD demonstrated significantly weaker grip strength (M = 33.13, SD = 8.95) than the group with typical development (M = 43.27, SD = 8.65), and this was a large effect size, t(87) = 5.38, p < .001, d = 1.16.
The finger tapping pattern of results was nearly identical. In those younger than 15 years, the group with ASD demonstrated fewer finger taps in a 10-second interval (average across preferred and nonpreferred hands) (M = 44.97, SD = 6.06) than the group with typical development (M = 37.99, SD = 7.03, but this difference was not statistically significant, t(55) = 1.01, p = .32, d = 0.28. In contrast, in those older than 15 years of age, the group with ASD demonstrated significantly slower finger tapping (M = 44.97, SD = 6.06) than the group with typical development (M = 50.46, SD = 5.48), and this was a large effect size, t(87) = 4.46, p < .001, d = 0.96.
Manual motor performance correlates with concurrent adaptive daily living skills
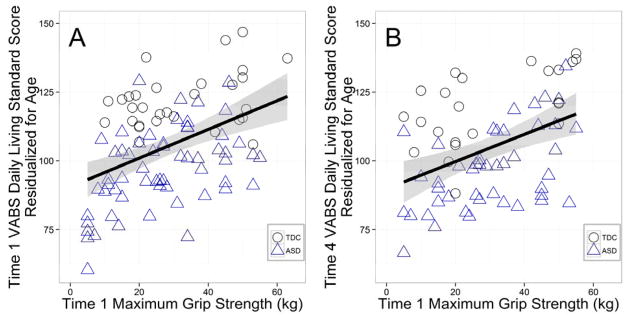
Partial correlations examined whether grip strength and finger tapping were associated with concurrent adaptive daily living scores. After accounting for age, Time 1 VABS daily living standard scores were correlated with both Time 1 maximum grip strength (Figure 4a), r= +.27, p = .009, n =89, and Time 1 finger tapping, r= +.23, p =.03, n =89. After accounting for both age and FSIQ, maximum grip strength still significantly correlated with daily living skills, r= +.24, p =.03, n =89, but finger tapping no longer significantly correlated with daily living skills, r= +.18, p =.08, n =89. These correlations correspond to small-to-medium effect sizes.
Figure 4.
Time 1 maximum grip strength correlations with concurrent (Time 1) and future (Time 4) Vineland Adaptive Behaviors Scale (VABS) Daily Living Scale Standard Scores. Time 4 measures were an average of eight years after Time 1 measures.
Manual motor performance predicts future adaptive daily living skills
We next examined whether Time 1 manual motor performance measures correlated with future (Time 4) adaptive daily living skills. We found that the average time between Time 1 and Time 4 sessions was 7.78 years (range: 5.71–8.63 years). After accounting for age, Time 4 VABS daily living standard scores were correlated with Time 1 grip strength, r= +.28, p = .02, n =68 (Figure 4b), but not by Time 1 finger tapping speed, r= +.20, p =.09, n =68. This pattern of results remained after controlling for both age and FSIQ (grip: r = +.24, p = .04; finger tapping: r = +.19, p = .11). When looking only at adult participants (18 years or older at Time 1), the relationship between Time 4 VABS daily living standard scores and Time 1 manual motor performance scores did not appear to meet the assumption of linearity. Consequently, a spearman’s rank partial correlation was employed. Nonparametric Spearman correlations demonstrated that Time 4 VABS daily living standard scores were correlated with both Time 1 grip strength, rs = +.41, p < .05, n =23, and Time 1 finger tapping speed, rs = +.56, p = .003, n=23 (accounting for both age and IQ). Again, these correlations correspond to small-to-medium effect sizes.
Discussion
The present study investigated performance and age-related changes in longitudinal measures of grip strength and finger tapping speed in ASD compared to typical development from early childhood into mid-adulthood. Our measures focused on fundamental aspects of developmental manual motor performance that likely serve as a foundation for more complicated hand movements. Our repeated measurements occurred at times in the life span when motor ability is not typically a developmental focus. Further, we examined manual motor performance as a correlate of concurrent adaptive daily living skills and future adaptive daily living skills. The results yielded three important findings: (1) atypical development of grip strength and finger tapping speed in ASD marked by progressively more robust group differences from adolescence into adulthood, (2) a large proportion of the ASD sample with maximum grip strength below the 10th percentile for age norms, and (3) manual motor performance skills (primarily maximum grip strength) being associated with concurrent and future daily living skills. Each of these results is discussed in more detail.
Atypical development of manual motor skills in autism
The group with ASD demonstrated atypical manual motor development. This development was characterized by similar performance on grip strength and finger tapping from five to 14 years of age but increasingly larger group differences from 15 to 39 years of age. Across both groups, adolescence was a time of increasing grip strength and finger tapping speed. However, the group with ASD exhibited an early plateau in these skills, suggesting that individuals with ASD may experience increasingly more pronounced manual motor performance difficulties (compared to their typically developing peers) in the transition from adolescence into adulthood. These longitudinal results help clarify some of the inconsistencies in the literature. For example, the present results extend upon the findings of atypical motor maturation in toddlers with and at-risk for ASD (Ben-Sasson & Gill, 2014; Lloyd et al., 2013). Our results are similar to those of Lloyd et al. (2013) who found that toddlers with ASD demonstrated increasingly more impaired motor skills compared to typically developing age norms as they got older. While there was no age overlap between the present study and the study by Lloyd and colleagues (2013), both studies suggest that motor performance in ASD may lag behind age-related motor improvements seen in typically developing individuals. Further, individuals with ASD demonstrating age-related increases in grip strength and finger tapping speed from childhood to adulthood is consistent with the results of Ming et al. (2007) that suggested that motor difficulties in ASD may diminish with age. However, our findings extend to show that the age-related improvements in motor skill in individuals with ASD may lag behind and never fully reach the age-related improvements observed in individuals with typical development. This lag was observed in both the grip strength and finger tapping data, and this finding is consistent with the postural control literature in ASD, which suggests that individuals with ASD may not reach the typical adult-levels of postural control (Minshew et al., 2004). Therefore, it is possible that fundamental manual motor skills and postural control may follow a similar developmental trajectory in ASD, a key area for future research.
There are a number of potential reasons for why developmental manual motor performance may become more impaired in ASD (in relation to same-aged typically developing peers) from adolescence into adulthood. One possibility is that these changes in manual motor performance in ASD may be due to biological factors, such as potential age-related brain changes. Indeed, a recent longitudinal brain imaging study with this sample suggested that whole brain volume goes from being higher to lower in the ASD group compared to the typically developing group during early adolescence (Lange et al., 2015), and Time 1 data indicated a correlation between white matter microstructure and manual motor performance in ASD (Travers et al., 2015). Future analyses will investigate whether longitudinal changes in brain volume or white matter may correspond to changes in manual motor performance. Additionally, models of atypical synaptic pruning in autism suggest that sensory and motor symptoms may be some of the earliest indicators of ASD (Thomas, Davis, Karmiloff-Smith, Knowland, & Charman, 2015). While these computational models do not address how these sensory and motor symptoms may progress into adolescence and adulthood, it is possible that over pruning or decreased brain connectivity could set off a developmental cascade that over time could contribute to the manual motor difficulties observed in the present study.
Another possible cause for the increased gap in motor performance between ASD and typically developing adolescents may be due to differences in physical activity over time. School-aged children with ASD have been found to be less physically active and less physically fit compared to same-aged children with typical development (Tyler, MacDonald, & Menear, 2014), and older children with ASD have been found to have even lower physical activity levels (Macdonald, Esposito, & Ulrich, 2011). Decreased physical activity levels during childhood could create a negative cascade that could culminate in adolescence as poorer motor performance. However, future research is needed to directly examine this possibility.
Given the atypical developmental trajectory of manual motor performance in the group with ASD from five to 40 years of age, another key question is what does motor performance look like in those with ASD older than 40 years. In typically developing individuals, motor performance is known to decline in later adulthood. With the early plateau in ASD, it is possible that older adults with ASD may experience differentially more difficulty with motor tasks in later years. Indeed, a recent study in a small sample suggested that a high proportion of adults with ASD over the age of 39 years exhibited Parkinson like symptoms, including tremors and postural instability (Starkstein, Gellar, Parlier, Payne, & Piven, 2015). Given the high prevalence of children with ASD who will eventually become older adults with ASD, the causes and consequences of atypical motor development across the life span should be investigated, especially since poorer manual motor skills could have negative impacts on quality of life and independence in this aging population.
Finally, the present developmental trajectories beg the question of whether a targeted motor intervention before early adolescence could potentially prevent the manual motor performance decline observed in ASD. A small study found that 12 weeks of hippotherapy in five-to-12 year olds with ASD decreased postural sway and increased adaptive functioning (Ajzenman, Standeven, & Shurtleff, 2013), suggesting that gains in motor performance and adaptive functioning may be made in a relatively short time in ASD. Whether or not manual motor performance is similarly affected is a key avenue for future research.
Grip strength in ASD in relation to age norms
We used grip strength age norms (Dodds et al., 2014) to confirm the validity of our typically developing data, and in doing so, we found that a substantial portion of grip strength observations in the group with ASD were far below age norms. Specifically, we found that over one third of the participants with ASD had at least one measurement of maximum grip strength below the 10th percentile (compared to only one participant in the typically developing sample). However, there was substantial within-group variability in grip strength, such that 13% of individuals with ASD actually had grip strength above the 90th percentile. Those with grip strength below the 10th percentile did not differ from those with grip strength above the 90th percentile in terms of IQ, BMI, or age. Therefore, it is unlikely that the grip strength variability within the ASD group was due to differences in cognitive level, weight, or chronological age.
The high proportion of individuals with low grip strength suggest that a number of the individuals with ASD had substantially weaker grip strength compared to the age norms, and these observations of very weak grip strength occurred throughout the age span of the study (5–40 years). These results are consistent with previous findings of group differences in grip strength between individuals with ASD and individuals with typical development (Kern et al., 2013). In high-ASD-risk infants (i.e., infant siblings of children with ASD), manual motor skills correlated with later expressive language (LeBarton & Iverson, 2013), and studies in later childhood and adulthood suggest that a variety of motor difficulties may be related to core ASD symptom severity (Bhat, Galloway, & Landa, 2012; Dowell et al., 2009; Dziuk et al., 2007; Gernsbacher, Sauer, Geye, Schweigert, & Hill Goldsmith, 2008; Hilton, Graver, & LaVesser, 2007; Linkenauger, Lerner, Ramenzoni, & Proffitt, 2012; MacDonald, Lord, & Ulrich, 2013; Papadopoulos et al., 2011; Radonovich, Fournier, & Hass, 2013; Travers et al., 2013, 2015). Therefore, poorer motor performance may not be just a peripheral aspect of autism but potentially a contributing factor to the core symptom severity of ASD.
It is also interesting to note that difficulties with manual motor control have been reported in ASD-related disorders, such as schizophrenia (Teremetz et al., 2014) and schizotypy (Lenzenweger & Maher, 2002). Autism and schizophrenia have been reported to share a number of common genetic contributions (Rapoport, Chavez, Greenstein, Addington, & Gogtay, 2009), and while it is unclear exactly how these genetic variants may impact motor development, this may be an important avenue for future research. It is a distinct possibility that manual motor performance difficulties are not specific to ASD but may reflect difficulties across disorders that are biologically similar to ASD.
Grip strength is a measure of muscle strength, not muscle tone. However, it is unclear whether the weaker grip strength in those with ASD may be related to the high incidence of low muscle tone often observed in this population. For example, hypotonia was identified in 51% of children with ASD (Ming et al., 2007), and the most common minor neurological dysfunctions identified in children with ASD were atypical muscle tone, poorer postural control, and poorer fine motor skills (De Jong, Punt, De Groot, Minderaa, & Hadders-Algra, 2011). Future research will be needed to differentiate the effects of muscle tone on grip strength in ASD, as they may have different underlying causes and consequences in ASD. Nevertheless, the present results suggest not only that there is a different developmental trajectory for grip strength in ASD but also that a number of individuals with ASD may have particularly low grip strength compared to age-related, typically developing norms.
Motor correlations with concurrent and future adaptive daily living skills
Manual motor skills (particularly maximum grip strength) were found to have a small-to-medium relation with both current and future daily living skills (both across our entire sample and just in the adults), which suggests that manual motor performance may be related to a portion of independent living skills in adulthood. While relationships between developmental motor skills and adaptive daily living skills have been reported in individuals with more profound motor difficulties, the present results suggest that even subtle motor difficulties impact adaptive daily living skills from childhood through middle adulthood. The results in our wide age span extend upon the results of Jasmin et al. (2009), who found that motor and sensory difficulties correlated with daily living skills in preschoolers with ASD. Even though our measurements of maximum grip strength occurred before the age of 40 years, our results are also consistent with studies of typical development, in finding that midlife maximum grip strength has been found to be related to later functional limitations (Rantanen et al., 1999). Other studies of midlife grip strength in typical development have also found that it predicts mortality 25 years later (Al Snih et al., 2002; Fujita et al., 1995; Gale et al., 2007; Katzmarzyk & Craig, 2002; Metter et al., 2002; Rantanen et al., 2012; Sasaki et al., 2007). Therapeutic techniques that target manual motor function and help people find strategies to compensate for motor difficulties may be beneficial for individuals who present with these subtle motor difficulties, not only in old age but perhaps across the life span.
The relation between manual motor performance and adaptive daily living skills may in part contribute to the developmental trajectory of adaptive daily living skills in ASD. Adaptive daily living skills in ASD have been shown to plateau during early adolescence (Smith et al., 2012), and the current results suggest that manual motor difficulties may contribute to this plateau. In older adults with typical development, grip strength has been found to relate to a number of different skills that are associated with independent living, such as opening jars or bottles, carrying grocery bags, holding books, wringing out towels, pouring liquids from containers, and stirring mixtures (Simard et al., 2012). It is possible that these tasks may be more difficult for those with lower grip strength in our sample, partially driving the relationship between grip strength and adaptive daily living skills.
Furthermore, the relationship between manual motor performance and future adaptive daily living skills remained even when we only examined the adults (18 years and older) in the study. Context variables such as schoolwork, independent vocation, and high quality relationships with family have been shown to lead to improvements in core autism features into adulthood (Taylor & Seltzer, 2010; Taylor, Smith, & Mailick, 2014; Woodman, Smith, Greenberg, & Mailick, 2015). However, little research has examined the role of motor ability in predicting adult outcomes in ASD, and our results suggest that manual motor performance might relate to daily living skills during adulthood in this population. Future research should examine if grip strength may be predictive of more general outcomes in adults with ASD, like employment, quality of life, or community engagement.
Limitations
The results of the present study have limited generalizability due to our sample size (especially for the adult-only analysis) and small number of repeated measures per participant. Developmental trajectories of motor skill will be further understood when based on more participants and more repeated measurements per participant (three or more data points). Further, the external validity of the present study is limited, in that our measurements of grip strength and finger tapping were not performed with a concrete goal of function in mind. In addition, we did not measure other manual motor skills that are likely related to adaptive daily living skills (i.e., reaching, grasping, object manipulation). Therefore, while the present study focused on fundamental manual motor skills that are likely diagnostic correlates for more complicated motor skills, future studies should include testing a variety of fine motor skills (including perceptual-motor abilities) and adaptive abilities during real-life, functional tasks.
Conclusions and Implications
Across the life span, use of our hands allows us to complete a number of meaningful tasks, such as preparing meals, dressing ourselves, writing, typing, holding hands, driving, and eating. Our results demonstrate that a substantial proportion of participants with ASD exhibit very weak grip strength, suggesting that grip strength may be a particular challenge for a good proportion of this population. Challenges with grip strength may lead to difficulties with effectively performing functional tasks such as handwriting or dressing. For example, a child’s difficulty with the foundational motor skill of gripping objects may lead to weak grip exerted on a writing utensil, which may lead to poor legibility and decreased motivation to practice handwriting. Further, our results suggest that individuals with ASD may experience increasingly more pronounced manual motor performance difficulties as they transition from adolescence into adulthood. Manual motor skills (particularly grip strength) were found to relate to both current and future daily living skills (both across our entire sample and just in the adults), which suggests that manual motor performance in ASD could be a limiting factor for developing independent living skills in adulthood.
Supplementary Material
Figure 5.
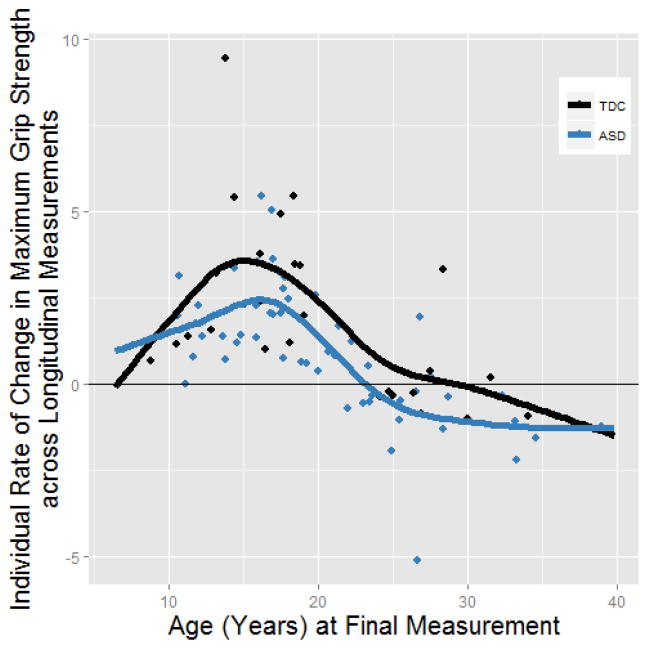
Change in grip strength across longitudinal measurement as a function of age. Because participants had more than two time points, we calculated the linear slope for grip strength as a function of age for each participant (rather than just subtracting the first measurement from the final measurement). These data suggest that the typically developing participants (TDC) were more likely to have positive increases in grip strength compared to participants with autism spectrum disorder (ASD). These data support the main findings of the study. However, Fig 2 is a more comprehensive picture of these same data, in that you see the change in grip strength over time in relation to the starting grip strength.
Figure 6.
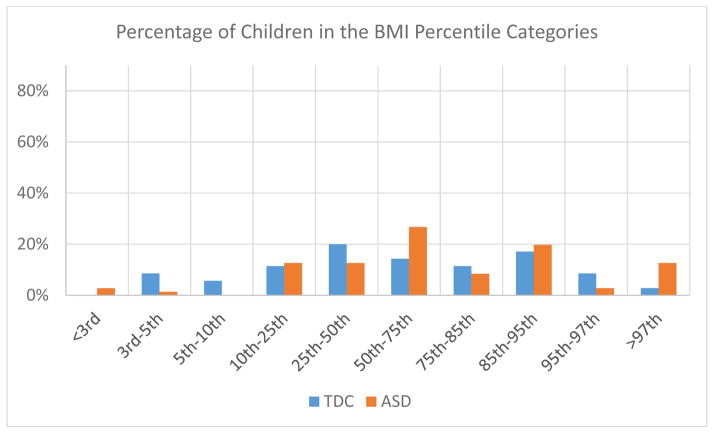
Body mass index (BMI) reported in reference to Center for Disease Control (CDC) BMI percentiles for children two to 20 years of age in the autism spectrum disorder (ASD) and typically developing control (TDC) groups. The two groups had similar distributions in terms of percentile BMI, although there appeared to be more extreme BMI’s (both on the low and high end) in the individuals with ASD. The groupings had n’s that were too small to perform Chi-Square analyses. Note that in Table 1, there are no significant group differences in raw BMI scores.
Research Highlights.
Severe manual motor difficulties can negatively impact independent living skills. However, it is unclear if persistent but less severe manual motor difficulties, as commonly reported in Autism Spectrum Disorder (ASD), also disrupt the development of daily living skills across the life span.
The group with ASD demonstrated atypical development of grip strength and finger tapping, marked by an early plateau and increasingly larger group differences from adolescence into adulthood.
Compared to age norms, over a third of the ASD sample had at least one maximum grip strength measurement below the 10th percentile.
Manual motor performance (primarily grip strength) was associated with concurrent adaptive daily living skills and adaptive daily living skills 8 years into the future, even after controlling for age and IQ.
Acknowledgments
This work was supported by the National Institute of Mental Health [RO1 MH080826 to JEL, ALA, NL, EDB; RO1 MH084795 to JEL, NL], the Eunice Kennedy Shriver National Institute of Child Health and Human Development [P30 HD003352 to the Waisman Center], the Hartwell Foundation [to BGT], the Brain and Behavior Research Foundation [to BGT], and the Poelman Foundation [to EDB]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health, the National Institute of Child Health & Development, or the National Institutes of Health. We thank Annahir Cariello and Celeste Knoles for their contributions to this project. We thank Karl S. Rosengren for helpful comments and questions during the revision of this manuscript. We sincerely thank all the individuals and families who participated in this study.
References
- Ajzenman HF, Standeven JW, Shurtleff TL. Effect of hippotherapy on motor control, adaptive behaviors, and participation in children with autism spectrum disorder: a pilot study. The American Journal of Occupational Therapy: Official Publication of the American Occupational Therapy Association. 2013;67(6):653–663. doi: 10.5014/ajot.2013.008383. http://doi.org/10.5014/ajot.2013.008383. [DOI] [PubMed] [Google Scholar]
- Alaniz ML, Galit E, Necesito CI, Rosario ER. Hand Strength, Handwriting, and Functional Skills in Children With Autism. The American Journal of Occupational Therapy: Official Publication of the American Occupational Therapy Association. 2015;69(4) doi: 10.5014/ajot.2015.016022. 6904220030p1–9. http://doi.org/10.5014/ajot.2015.016022. [DOI] [PubMed] [Google Scholar]
- Alexander-Bloch AF, Reiss PT, Rapoport J, McAdams H, Giedd JN, Bullmore ET, Gogtay N. Abnormal cortical growth in schizophrenia targets normative modules of synchronized development. Biological Psychiatry. 2014;76(6):438–446. doi: 10.1016/j.biopsych.2014.02.010. http://doi.org/10.1016/j.biopsych.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Snih S, Markides KS, Ray L, Ostir GV, Goodwin JS. Handgrip strength and mortality in older Mexican Americans. Journal of the American Geriatrics Society. 2002;50(7):1250–1256. doi: 10.1046/j.1532-5415.2002.50312.x. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic criteria from DSM-IV-TR. Arlington, VA: American Psychiatric Association; 2000. [Google Scholar]
- Ben-Sasson A, Gill SV. Motor and language abilities from early to late toddlerhood: using formalized assessments to capture continuity and discontinuity in development. Research in Developmental Disabilities. 2014;35(7):1425–1432. doi: 10.1016/j.ridd.2014.03.036. http://doi.org/10.1016/j.ridd.2014.03.036. [DOI] [PubMed] [Google Scholar]
- Bhat AN, Galloway JC, Landa RJ. Relation between early motor delay and later communication delay in infants at risk for autism. Infant Behavior & Development. 2012;35(4):838–846. doi: 10.1016/j.infbeh.2012.07.019. http://doi.org/10.1016/j.infbeh.2012.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Rogers S, Munson J, Smith M, Winter J, Greenson J, … Varley J. Randomized, controlled trial of an intervention for toddlers with autism: the Early Start Denver Model. Pediatrics. 2010;125(1):e17–23. doi: 10.1542/peds.2009-0958. http://doi.org/10.1542/peds.2009-0958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jong M, Punt M, De Groot E, Minderaa RB, Hadders-Algra M. Minor neurological dysfunction in children with autism spectrum disorder. Developmental Medicine and Child Neurology. 2011;53(7):641–646. doi: 10.1111/j.1469-8749.2011.03971.x. http://doi.org/10.1111/j.1469-8749.2011.03971.x. [DOI] [PubMed] [Google Scholar]
- Dewey D. Praxis and sequencing skills in children with sensorimotor dysfunction. Developmental Neuropsychology. 1991;7(2):197–206. http://doi.org/10.1080/87565649109540487. [Google Scholar]
- Dewey D, Cantell M, Crawford SG. Motor and gestural performance in children with autism spectrum disorders, developmental coordination disorder, and/or attention deficit hyperactivity disorder. Journal of the International Neuropsychological Society. 2007;13(02) doi: 10.1017/S1355617707070270. http://doi.org/10.1017/S1355617707070270. [DOI] [PubMed] [Google Scholar]
- Dodds RM, Syddall HE, Cooper R, Benzeval M, Deary IJ, Dennison EM, … Sayer AA. Grip strength across the life course: normative data from twelve British studies. PloS One. 2014;9(12):e113637. doi: 10.1371/journal.pone.0113637. http://doi.org/10.1371/journal.pone.0113637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowell LR, Mahone EM, Mostofsky SH. Associations of postural knowledge and basic motor skill with dyspraxia in autism: implication for abnormalities in distributed connectivity and motor learning. Neuropsychology. 2009;23(5):563–570. doi: 10.1037/a0015640. http://doi.org/10.1037/a0015640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffield TC, Trontel HG, Bigler ED, Froehlich A, Prigge MB, Travers B, … Lainhart J. Neuropsychological investigation of motor impairments in autism. Journal of Clinical and Experimental Neuropsychology. 2013;35(8):867–881. doi: 10.1080/13803395.2013.827156. http://doi.org/10.1080/13803395.2013.827156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziuk MA, Gidley Larson JC, Apostu A, Mahone EM, Denckla MB, Mostofsky SH. Dyspraxia in autism: association with motor, social, and communicative deficits. Developmental Medicine and Child Neurology. 2007;49(10):734–739. doi: 10.1111/j.1469-8749.2007.00734.x. http://doi.org/10.1111/j.1469-8749.2007.00734.x. [DOI] [PubMed] [Google Scholar]
- Elliott C. The Nature and Structure of Children’s Abilities: Evidence from the Differential Ability Scales. Journal of Psychoeducational Assessment. 1990;8:376–390. [Google Scholar]
- Fournier KA, Hass CJ, Naik SK, Lodha N, Cauraugh JH. Motor coordination in autism spectrum disorders: a synthesis and meta-analysis. Journal of Autism and Developmental Disorders. 2010;40(10):1227–1240. doi: 10.1007/s10803-010-0981-3. http://doi.org/10.1007/s10803-010-0981-3. [DOI] [PubMed] [Google Scholar]
- Fournier KA, Kimberg CI, Radonovich KJ, Tillman MD, Chow JW, Lewis MH, … Hass CJ. Decreased static and dynamic postural control in children with autism spectrum disorders. Gait & Posture. 2010;32(1):6–9. doi: 10.1016/j.gaitpost.2010.02.007. http://doi.org/10.1016/j.gaitpost.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita Y, Nakamura Y, Hiraoka J, Kobayashi K, Sakata K, Nagai M, Yanagawa H. Physical-strength tests and mortality among visitors to health-promotion centers in Japan. Journal of Clinical Epidemiology. 1995;48(11):1349–1359. doi: 10.1016/0895-4356(95)00533-1. [DOI] [PubMed] [Google Scholar]
- Gale CR, Martyn CN, Cooper C, Sayer AA. Grip strength, body composition, and mortality. International Journal of Epidemiology. 2007;36(1):228–235. doi: 10.1093/ije/dyl224. http://doi.org/10.1093/ije/dyl224. [DOI] [PubMed] [Google Scholar]
- Gernsbacher MA, Sauer EA, Geye HM, Schweigert EK, Hill Goldsmith H. Infant and toddler oral- and manual-motor skills predict later speech fluency in autism. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2008;49(1):43–50. doi: 10.1111/j.1469-7610.2007.01820.x. http://doi.org/10.1111/j.1469-7610.2007.01820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harezlak J, Ryan LM, Giedd JN, Lange N. Individual and population penalized regression splines for accelerated longitudinal designs. Biometrics. 2005;61:1037–48. doi: 10.1111/j.1541-0420.2005.00376.x. [DOI] [PubMed] [Google Scholar]
- Hastie T. Generalized additive models. Boca Raton, Fla: Chapman & Hall/CRC; 1999. [Google Scholar]
- Heaton R, Grant I, Matthews C. HRB Comprehensive Norms Computer Program. Psychological Assessment Resources, Inc; Odessa, Florida: 1991. [Google Scholar]
- Hilton C, Graver K, LaVesser P. Relationship between social competence and sensory processing in children with high functioning autism spectrum disorders. Research in Autism Spectrum Disorders. 2007;1(2):164–173. [Google Scholar]
- Jasmin E, Couture M, McKinley P, Reid G, Fombonne E, Gisel E. Sensori-motor and daily living skills of preschool children with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2009;39(2):231–241. doi: 10.1007/s10803-008-0617-z. http://doi.org/10.1007/s10803-008-0617-z. [DOI] [PubMed] [Google Scholar]
- Katzmarzyk PT, Craig CL. Musculoskeletal fitness and risk of mortality. Medicine and Science in Sports and Exercise. 2002;34(5):740–744. doi: 10.1097/00005768-200205000-00002. [DOI] [PubMed] [Google Scholar]
- Kern JK, Geier DA, Adams JB, Troutman MR, Davis GA, King PG, Geier MR. Handgrip strength in autism spectrum disorder compared with controls. Journal of Strength and Conditioning Research / National Strength & Conditioning Association. 2013;27(8):2277–2281. doi: 10.1519/JSC.0b013e31827de068. http://doi.org/10.1519/JSC.0b013e31827de068. [DOI] [PubMed] [Google Scholar]
- Kim S. ppcor: Partial and Semi-partial (Part) correlation. 2012 Retrieved from http://CRAN.R-project.org/package=ppcor.
- Kohen-Raz R, Volkman FR, Cohen DJ. Postural control in children with autism. Journal of Autism and Developmental Disorders. 1992;22(3):419–432. doi: 10.1007/BF01048244. http://doi.org/10.1007/BF01048244. [DOI] [PubMed] [Google Scholar]
- Lange N, Laird NM. The effect of covariance structure on variance estimation in balanced growth curve models with random parameters. Journal of the American Statistical Association. 1989;1989(84):241–247. [Google Scholar]
- Lange N, Travers BG, Bigler ED, Prigge MBD, Froehlich AL, Nielsen JA, … Lainhart JE. Longitudinal volumetric brain changes in autism spectrum disorder ages 6–35 years. Autism Research: Official Journal of the International Society for Autism Research. 2015;8(1):82–93. doi: 10.1002/aur.1427. http://doi.org/10.1002/aur.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBarton ES, Iverson JM. Fine motor skill predicts expressive language in infant siblings of children with autism. Developmental Science. 2013;16(6):815–827. doi: 10.1111/desc.12069. http://doi.org/10.1111/desc.12069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenzenweger MF, Maher BA. Psychometric schizotypy and motor performance. Journal of Abnormal Psychology. 2002;111(4):546–555. doi: 10.1037//0021-843x.111.4.546. [DOI] [PubMed] [Google Scholar]
- Linkenauger SA, Lerner MD, Ramenzoni VC, Proffitt DR. A perceptual-motor deficit predicts social and communicative impairments in individuals with autism spectrum disorders. Autism Research: Official Journal of the International Society for Autism Research. 2012;5(5):352–362. doi: 10.1002/aur.1248. http://doi.org/10.1002/aur.1248. [DOI] [PubMed] [Google Scholar]
- Lloyd M, MacDonald M, Lord C. Motor skills of toddlers with autism spectrum disorders. Autism: The International Journal of Research and Practice. 2013;17(2):133–146. doi: 10.1177/1362361311402230. http://doi.org/10.1177/1362361311402230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore PC, … Rutter M. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30(3):205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Macdonald M, Esposito P, Ulrich D. The physical activity patterns of children with autism. BMC Research Notes. 2011;4:422. doi: 10.1186/1756-0500-4-422. http://doi.org/10.1186/1756-0500-4-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald M, Lord C, Ulrich DA. The relationship of motor skills and social communicative skills in school-aged children with autism spectrum disorder. Adapted Physical Activity Quarterly: APAQ. 2013;30(3):271–282. doi: 10.1123/apaq.30.3.271. [DOI] [PubMed] [Google Scholar]
- Metter EJ, Talbot LA, Schrager M, Conwit R. Skeletal muscle strength as a predictor of all-cause mortality in healthy men. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 2002;57(10):B359–365. doi: 10.1093/gerona/57.10.b359. [DOI] [PubMed] [Google Scholar]
- Ming X, Brimacombe M, Wagner GC. Prevalence of motor impairment in autism spectrum disorders. Brain & Development. 2007;29(9):565–570. doi: 10.1016/j.braindev.2007.03.002. http://doi.org/10.1016/j.braindev.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Minshew NJ, Goldstein G, Siegel DJ. Neuropsychologic functioning in autism: profile of a complex information processing disorder. Journal of the International Neuropsychological Society: JINS. 1997;3(4):303–316. [PubMed] [Google Scholar]
- Minshew NJ, Sung K, Jones BL, Furman JM. Underdevelopment of the postural control system in autism. Neurology. 2004;63(11):2056–2061. doi: 10.1212/01.wnl.0000145771.98657.62. http://doi.org/10.1212/01.WNL.0000145771.98657.62. [DOI] [PubMed] [Google Scholar]
- Molloy CA, Dietrich KN, Bhattacharya A. Postural Stability in Children with Autism Spectrum Disorder. Journal of Autism and Developmental Disorders. 2003;33(6):643–652. doi: 10.1023/b:jadd.0000006001.00667.4c. http://doi.org/10.1023/B:JADD.0000006001.00667.4c. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Dubey P, Jerath VK, Jansiewicz EM, Goldberg MC, Denckla MB. Developmental dyspraxia is not limited to imitation in children with autism spectrum disorders. Journal of the International Neuropsychological Society. 2006;12(03) doi: 10.1017/s1355617706060437. http://doi.org/10.1017/S1355617706060437. [DOI] [PubMed] [Google Scholar]
- Nesselroade JR, Baltes PB. Longitudinal research in the study of behavior and development. Academic Press; 1979. Retrieved from http://books.google.com/books?id=5ZhpAAAAMAAJ. [Google Scholar]
- Papadopoulos N, McGinley J, Tonge B, Bradshaw J, Saunders K, Murphy A, Rinehart N. Motor Proficiency and Emotional/Behavioural Disturbance in Autism and Asperger’s Disorder: Another Piece of the Neurological Puzzle? Autism: The International Journal of Research and Practice; 2011. http://doi.org/10.1177/1362361311418692. [DOI] [PubMed] [Google Scholar]
- Radonovich KJ, Fournier KA, Hass CJ. Relationship between postural control and restricted, repetitive behaviors in autism spectrum disorders. Frontiers in Integrative Neuroscience. 2013;7:28. doi: 10.3389/fnint.2013.00028. http://doi.org/10.3389/fnint.2013.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rantanen T, Guralnik JM, Foley D, Masaki K, Leveille S, Curb JD, White L. Midlife hand grip strength as a predictor of old age disability. JAMA: The Journal of the American Medical Association. 1999;281(6):558–560. doi: 10.1001/jama.281.6.558. [DOI] [PubMed] [Google Scholar]
- Rantanen T, Masaki K, He Q, Ross GW, Willcox BJ, White L. Midlife muscle strength and human longevity up to age 100 years: a 44-year prospective study among a decedent cohort. Age (Dordrecht, Netherlands) 2012;34(3):563–570. doi: 10.1007/s11357-011-9256-y. http://doi.org/10.1007/s11357-011-9256-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport J, Chavez A, Greenstein D, Addington A, Gogtay N. Autism spectrum disorders and childhood-onset schizophrenia: clinical and biological contributions to a relation revisited. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48(1):10–18. doi: 10.1097/CHI.0b013e31818b1c63. http://doi.org/10.1097/CHI.0b013e31818b1c63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R. Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: 2013. Retrieved from http://www.R-project.org. [Google Scholar]
- Rinehart NJ, Bellgrove MA, Tonge BJ, Brereton AV, Howells-Rankin D, Bradshaw JL. An examination of movement kinematics in young people with high-functioning autism and Asperger’s disorder: further evidence for a motor planning deficit. Journal of Autism and Developmental Disorders. 2006;36(6):757–767. doi: 10.1007/s10803-006-0118-x. http://doi.org/10.1007/s10803-006-0118-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinehart NJ, Bradshaw JL, Brereton AV, Tonge BJ. Movement preparation in high-functioning autism and Asperger disorder: a serial choice reaction time task involving motor reprogramming. Journal of Autism and Developmental Disorders. 2001;31(1):79–88. doi: 10.1023/a:1005617831035. [DOI] [PubMed] [Google Scholar]
- Rogers SJ, Bennetto L, McEvoy R, Pennington BF. Imitation and pantomime in high-functioning adolescents with autism spectrum disorders. Child Development. 1996;67(5):2060–2073. [PubMed] [Google Scholar]
- Sasaki H, Kasagi F, Yamada M, Fujita S. Grip strength predicts cause-specific mortality in middle-aged and elderly persons. The American Journal of Medicine. 2007;120(4):337–342. doi: 10.1016/j.amjmed.2006.04.018. http://doi.org/10.1016/j.amjmed.2006.04.018. [DOI] [PubMed] [Google Scholar]
- Shattuck PT, Wagner M, Narendorf S, Sterzing P, Hensley M. Post-high school service use among young adults with an autism spectrum disorder. Archives of Pediatrics & Adolescent Medicine. 2011;165(2):141–146. doi: 10.1001/archpediatrics.2010.279. http://doi.org/10.1001/archpediatrics.2010.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard J, Chalifoux M, Fortin V, Archambault MJ, St-Cerny-Gosselin A, Desrosiers J. Could questions on activities of daily living estimate grip strength of older adults living independently in the community? Journal of Aging Research. 2012;2012:427109. doi: 10.1155/2012/427109. http://doi.org/10.1155/2012/427109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LE, Maenner MJ, Seltzer MM. Developmental trajectories in adolescents and adults with autism: the case of daily living skills. Journal of the American Academy of Child and Adolescent Psychiatry. 2012;51(6):622–631. doi: 10.1016/j.jaac.2012.03.001. http://doi.org/10.1016/j.jaac.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow SS, Cicchetti DV. Vineland Adaptive Behavior Scales. In: Newmark CS, editor. Major Psychological Assessment Instruments. Vol. 2. Boston, MA: Major Psychological Assessment Instruments; 1989. pp. 199–231. [Google Scholar]
- Starkstein S, Gellar S, Parlier M, Payne L, Piven J. High rates of parkinsonism in adults with autism. Journal of Neurodevelopmental Disorders. 2015;7(1):29. doi: 10.1186/s11689-015-9125-6. http://doi.org/10.1186/s11689-015-9125-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JL, Seltzer MM. Changes in the autism behavioral phenotype during the transition to adulthood. Journal of Autism and Developmental Disorders. 2010;40(12):1431–1446. doi: 10.1007/s10803-010-1005-z. http://doi.org/10.1007/s10803-010-1005-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JL, Smith LE, Mailick MR. Engagement in vocational activities promotes behavioral development for adults with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2014;44(6):1447–1460. doi: 10.1007/s10803-013-2010-9. http://doi.org/10.1007/s10803-013-2010-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teremetz M, Amado I, Bendjemaa N, Krebs MO, Lindberg PG, Maier MA. Deficient grip force control in schizophrenia: behavioral and modeling evidence for altered motor inhibition and motor noise. PloS One. 2014;9(11):e111853. doi: 10.1371/journal.pone.0111853. http://doi.org/10.1371/journal.pone.0111853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MSC, Davis R, Karmiloff-Smith A, Knowland VCP, Charman T. The over-pruning hypothesis of autism. Developmental Science. 2015 doi: 10.1111/desc.12303. http://doi.org/10.1111/desc.12303. [DOI] [PubMed]
- Travers BG, Bigler ED, Tromp DPM, Adluru N, Destiche D, Samsin D, … Lainhart JE. Brainstem white matter predicts individual differences in manual motor difficulties and symptom severity in Autism. Journal of Autism and Developmental Disorders. 2015 doi: 10.1007/s10803-015-2467-9. http://doi.org/10.1007/s10803-015-2467-9. [DOI] [PMC free article] [PubMed]
- Travers BG, Powell PS, Klinger LG, Klinger MR. Motor difficulties in autism spectrum disorder: linking symptom severity and postural stability. Journal of Autism and Developmental Disorders. 2013;43(7):1568–1583. doi: 10.1007/s10803-012-1702-x. http://doi.org/10.1007/s10803-012-1702-x. [DOI] [PubMed] [Google Scholar]
- Tseng MH, Chen KL, Shieh JY, Lu L, Huang CY. The determinants of daily function in children with cerebral palsy. Research in Developmental Disabilities. 2011;32(1):235–245. doi: 10.1016/j.ridd.2010.09.024. http://doi.org/10.1016/j.ridd.2010.09.024. [DOI] [PubMed] [Google Scholar]
- Tyler K, MacDonald M, Menear K. Physical activity and physical fitness of school-aged children and youth with autism spectrum disorders. Autism Research and Treatment. 2014;2014:312163. doi: 10.1155/2014/312163. http://doi.org/10.1155/2014/312163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. The Wechsler intelligence scale for children—third edition. San Antonio: The Psychological Corporation; 1991. [Google Scholar]
- Wechsler D. Wechsler Adult Iintelligence Scale-III (WAIS-III) San Antonio: Psychological Corporation; 1997. [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. New York: The Psychological Corporation: Harcourt Brace & Company; 1999. [Google Scholar]
- Weimer AK, Schatz AM, Lincoln A, Ballantyne AO, Trauner DA. “Motor” impairment in Asperger syndrome: evidence for a deficit in proprioception. Journal of Developmental and Behavioral Pediatrics: JDBP. 2001;22(2):92–101. doi: 10.1097/00004703-200104000-00002. [DOI] [PubMed] [Google Scholar]
- Woodman AC, Smith LE, Greenberg JS, Mailick MR. Change in autism symptoms and maladaptive behaviors in adolescence and adulthood: the role of positive family processes. Journal of Autism and Developmental Disorders. 2015;45(1):111–126. doi: 10.1007/s10803-014-2199-2. http://doi.org/10.1007/s10803-014-2199-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood S. Stable and efficient multiple smoothing parameter estimation for generalized additive models. Journal of the American Statistical Association. 2004;99(467) [Google Scholar]
- Wood S. Generalized additive models: an introduction with R. Boca Raton, FL: Chapman & Hall/CRC; 2006. [Google Scholar]
- Wood S, Scheipl F. gamm4: Generalized additive mixed models using mgcv and lme4. 2014 Retrieved from http://CRAN.R-project.org/package=gamm4.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.