Abstract
Resting state functional magnetic resonance imaging (rsfMRI) has been widely used to measure functional connectivity between cortical regions of the brain. However, there have been minimal reports of bold oxygenation level dependent (BOLD) signals in white matter, and even fewer attempts to detect resting state connectivity. Recently, there has been growing evidence that suggests reliable detection of white matter BOLD signals may be possible. We have previously shown that nearest neighbor inter-voxel correlations of resting state BOLD signal fluctuations in white matter are anisotropic and can be represented by a functional correlation tensor, but the biophysical origins of these signal variations are not clear. We aimed to assess whether MRI signal fluctuations in white matter vary for different baseline levels of neural activity. We performed imaging studies on live squirrel monkeys under different levels of isoflurane anesthesia at 9.4T. We found 1) the fractional power (0.01 – 0.08 Hz) in white matter was between 60 to 75% of the level in gray matter; 2) the power in both gray and white matter low frequencies decreased monotonically in similar manner with increasing levels of anesthesia; 3) the distribution of fractional anisotropy values of the functional tensors in white matter were significantly higher than those in gray matter; and 4) the functional tensor eigenvalues decreased with increasing level of anesthesia. Our results suggest that as anesthesia level changes baseline neural activity, white matter signal fluctuations behave similarly to those in gray matter, and functional tensors in white matter are affected in parallel.
Keywords: fMRI, white matter, monkeys, anesthesia
1. Introduction
Functional magnetic resonance imaging (fMRI) records bold oxygenation level dependent (BOLD) magnetic resonance (MR) signals, and has been widely exploited by the neuroscience community for identifying patterns of neural activation in the brain. The magnitude of BOLD hemodynamic effects has previously been directly related to the level of underlying electrical activity [1]. Resting state fMRI has been used to quantify inter-voxel and inter-areal correlations of fluctuations in BOLD signals, which are then interpreted as indications of functional connectivity between cortical regions [2]. White matter possesses a sparse vasculature compared to gray matter with an approximately four-fold decrease in blood flow, so in general there have been very few investigations of BOLD signals in white matter, and even fewer attempts to detect resting state connectivity. In addition, the presence of action potentials in white matter instead of post-synaptic potentials, which are believed to be the primary source of fMRI signals, further complicates and questions the possibility of detecting BOLD signals in white matter [3]. However, BOLD fMRI has also been found to correlate with mostly spiking activity [4]. Indeed, there is growing evidence that suggests reliable detection of white matter BOLD signals may be possible [5]. For example, fMRI activation in the genu of the corpus callosum has been well-established when subjects are exposed to the Poffenberger paradigm [6]. This finding has been confirmed in other studies [7,8], providing strong evidence that fMRI activations may be detectable in white matter. Moreover, Fabri et al. investigated functional topological mapping of the corpus callosum with fMRI data, and found that activation at discrete regions of the corpus callosum were consistently detected [9]. Beyond the corpus callosum, several studies have also found BOLD fMRI activation in the internal capsule associated with swallowing [10] as well as finger tapping [11,12]. More recently, Astafiev et al. found that the most consistent BOLD signal changes between healthy controls and chronic mild traumatic brain injury patients were localized in white matter while performing visual tasks [13]. However, it should be emphasized that the production of focal task-derived activation is neither a necessary nor sufficient condition for detecting steady state correlations in fluctuations in a baseline signal.
We have previously reported our observations that variations in T2* weighted MRI signals in a resting state show comparable intensity and temporal variability profiles in both white and gray matter. The voxel-averaged temporal variations of MRI signals in white matter are ~80% those in gray matter [14]; analyses of the power spectra of BOLD signals from both white matter and gray matter show that the ratio of signal power in the low frequency range of 0.01 to 0.08 Hz to total variance are comparable [14]. These indicate that resting state variations that potentially reflect neural activity in gray matter may also be detectable in white matter. Moreover, we have shown that inter-voxel correlations of resting state signals from white matter exhibit spatial anisotropy and reveal distinct underlying structures [14,15]. Based on these observations, we have proposed that appropriate analysis of resting state acquisitions may reveal signal variations within white matter that reflect functional activity. However, the basis for this hypothesis demands further investigation.
It has been recognized that anesthesia reduces spontaneous neural electrical activity in the brain [16]. Indeed, the effects of increased anesthesia on fMRI signals have been demonstrated in both human and animal studies. For example, it has been shown that with increased anesthesia levels, functional connectivity decreases between cortices in macaque monkeys [17]. Similarly, we have also reported diminishing contributions of low frequency fluctuations and correlation strengths between regions in the primary somatosensory cortex of squirrel monkeys as anesthesia levels were increased [18]. A recent observational study in rhesus monkeys on dose-dependent effects of anesthesia on regional activity also demonstrated a similar phenomenon [19]. However, these previous studies did not assess possible changes within white matter. Thus, by carrying out imaging studies on live squirrel monkeys under different levels of anesthesia, we aimed to assess changes in MRI signals from white matter for different baseline levels of neural activity. In particular, we aimed to compare how different anesthesia levels modulate fractional power and spatio-temporal correlation tensors in white matter in a resting state.
2. Materials and Method
2.1 Animals and Preparation
Four adult male squirrel monkeys were studied for functional MRI scans at decreasing isoflurane levels (1.25%, 0.875% and 0.5%). Each animal was sedated with ketamine hydrochloride (10mg/kg)/atropine (0.05 mg/kg) before it was maintained on mechanical ventilation (40 bpm) with isoflurane anesthesia (0.5%–1.25%) delivered in a 70:30 N2O/O2 mixture. After intubation, each animal was placed in a custom-designed MR cradle where the head was secured with ear and head bars to prevent any motion related artifacts. A solution of 2.5% dextrose in saline solution was also infused intravenously (3ml/kg/hr) throughout the imaging session. Vital signs of the animal including peripheral oxygen saturation and heart rate, EKG, end-tidal CO2, and respiratory pattern were continuously monitored. Temperature of the animal was kept between 37.5 and 38.5°C with a circulating water blanket. All procedures were in compliance with the Society for Neuroscience guidelines for animal use in research and were approved by the Institutional Animal Care and Use Committee (IACUC) at Vanderbilt University.
2.2 Variation of Isoflurane Levels
Multiple functional MRI scans (runs) at each decreasing isoflurane level (1.25%, 0.875% and 0.5%) were acquired. Before image acquisition at each isoflurane level, at least 10 minutes were allocated for the stabilization of anesthesia and animal’s physiological condition through monitoring of vital signals such as the heart rate, end tidal CO2 and respiration patterns.
2.3 MRI Data acquisitions
All scans were acquired on a 9.4T magnet with a quadrature birdcage volume coil (inner diameter = 85mm) and Varian/Agilent MR spectrometer. Structural T1-weighted images were collected using a fast inversion recovery gradient echo sequence (TR/TE=3000/2.8 ms, echo train length ETL=4, inversion recovery time TI=600 ms, flip angle=80 degrees, resolution of 0.5 × 0.5 × 0.5 mm3), while structural T2*-weighted images were collected using a gradient echo sequence (TR/TE=500/10 ms, NEX=2, flip angle=35 degrees, resolution of 0.125 × 0.125 × 0.125 mm3). Resting state BOLD-sensitive axial images were acquired using a T2*-weighted GE-EPI sequence (TR/TE=750/16ms, 2 shots, resolution of 1×1×1 mm3, 1.5s/volume). Each imaging run contained 300 image volumes.
2.4 fMRI Data Pre-processing and Power Analyses
Slice timing correction was performed with spm8 in Matlab followed by motion correction (three translations and three rotations) and isotropic smoothing with a full width at half maximum of 1.5 mm. A temporal 128 second high pass filter along with linear detrending was applied before resting state power analyses were performed. RETROICOR [20] was then applied in spm8 with custom scripts [21] to correct for cardiac and respiratory interferences. Fractional power maps were computed by transforming each voxel time series into its power spectral density via a Fourier Transform, and dividing the integrated power amplitudes in the low frequency range (0.01–0.08 Hz) by the sum over the entire frequency range (0.01–0.33 Hz). Power measures were subsequently normalized within each monkey by computing relative z-scores before combining the runs for group analyses.
2.5 Region of Interest Selection/Segmentation
White and gray matter voxels in the brain were segmented using high resolution T2*-weighted anatomical images. Intensity histogram thresholding was first used for segmenting gray matter before manually selecting and removing voxels subject to partial volume effects. Similarly, manual selection of white matter was performed. The full segmentation of white matter for each monkey is presented in Supplementary Information I. The segmented white matter masks were further subjected to a morphological erosion algorithm using the Matlab function imerode with 2 × 2 structuring element (Supplementary Information III). This was performed to remove outermost voxels and further ensure the absence of partial volume effects. As control reference regions, adjacent muscle voxels were also selected with the functional images; care was taken to avoid any gray matter partial volumes (Supplementary Information II).
2.6 Calculation of Spatio-Temporal Correlation Tensors
After slice timing and motion correction, the resting state data were subjected to isotropic smoothing with a full width at half maximum of 2 mm, and a bandpass filter of 0.01–0.08 Hz was then applied to each voxel time series. Spatio-temporal correlation tensors were constructed using the method reported in Ding et al. [14]. Briefly, each voxel in the fMRI signal exhibits a series of small amplitude fluctuations. Since each voxel has 26 nearest neighbors, a total of 26 cross-correlation coefficients can be estimated that describe the degree of synchronous but anisotropic covariations with neighboring voxels. Using these values for each voxel, a 3×3 spatio-temporal correlation tensor was computed, with details analogous to those for diffusion tensor computations [22]. Spatio-temporal correlation tensors were derived for all four monkeys across the three different anesthesia levels. Finally, histograms of the values of the fractional anisotropy and principal eigenvectors of these tensors were calculated for voxels in gray and white matter.
3. Results
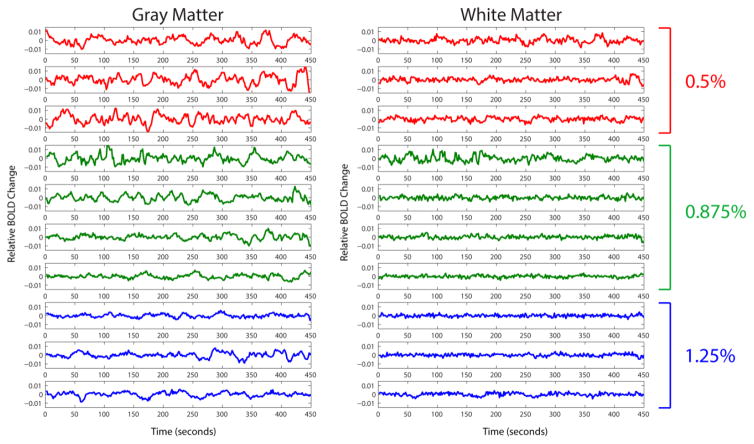
Averaged time series of multiple voxels in both gray and white matter in one representative monkey for three levels of anesthesia (0.5, 0.875, 1.25%) are shown in Figure 1. As can be seen, there is a trend of consistently diminishing spontaneous signal fluctuations from low (0.5%) to high isoflurane (1.25%) levels. This trend is visible in both gray and white matter, although not as prominent in white matter with overall lower amplitude fluctuations. This is revealed quantitatively through the averaged root-mean-square variations in Figure 1, which were 0.0049, 0.0035 and 0.0025 for gray matter and 0.0025, 0.0021 and 0.0015 for white matter time series at 0.5%, 0.875% and 1.25% isoflurane levels respectively.
Figure 1.
Multiple runs of averaged time series in gray and white matter voxels from one representative squirrel monkey. The monkey was under three different anesthesia levels: 0.5%, 0.875% and 1.25% represented by red, green and blue lines respectively. The averaged root-mean-square variations were 0.0049, 0.0035 and 0.0025 for gray matter and 0.0025, 0.0021 and 0.0015 for white matter time series at 0.5%, 0.875% and 1.25% isoflurane levels respectively.
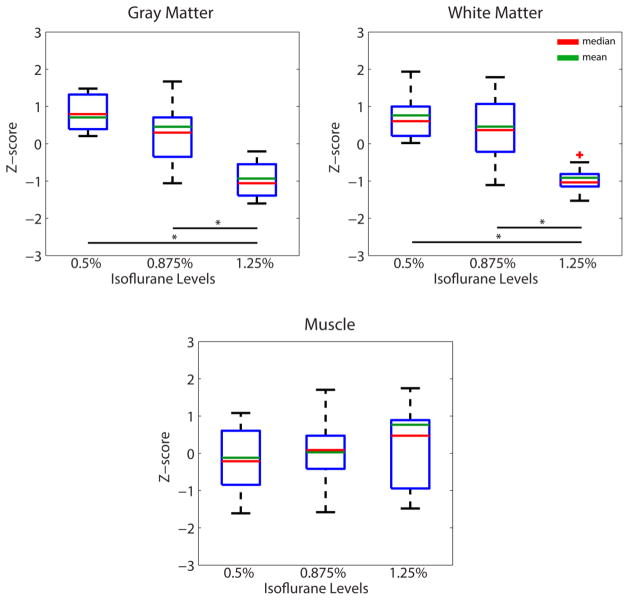
Group analyses of fractional power in white and gray matter are presented in Figure 2, in which boxplots demonstrate low frequency oscillations in white and gray matter as neural activity baseline is varied. A monotonically decreasing trend in fractional power was observed in both tissue types as we increased the isoflurane level, although the statistical difference between 0.5% and 0.875% levels did not reach statistical significance. By contrast, fractional power remained relatively constant across different anesthesia levels in the muscle region. Overall, the mean fractional power in white matter is approximately 60–75% of that in gray matter.
Figure 2.
Effects of anesthesia on fractional power in white matter, gray matter and a control muscle region. N=9, 14, 11 runs for 0.5%, 0.875% and 1.25% isoflurane levels respectively. * p <0.005 (Mann-Whitney Test).
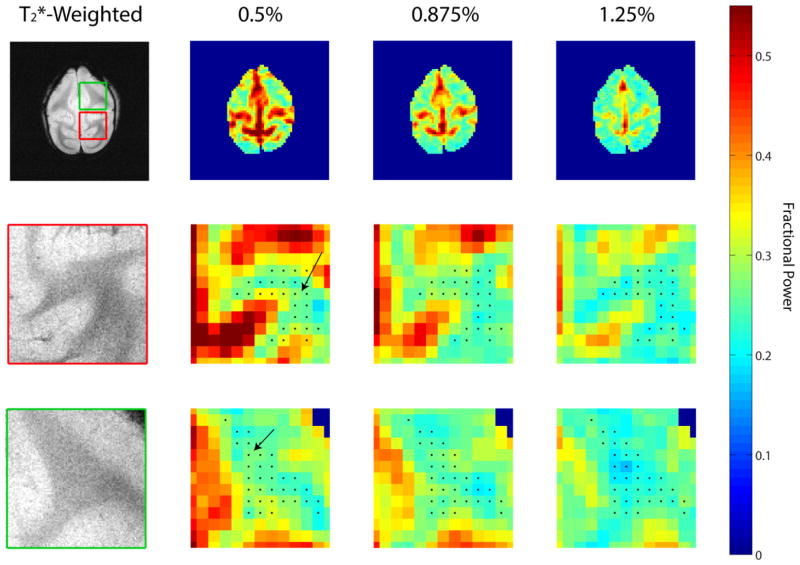
Figure 3 shows how the fractional power changed within a single slice as the isoflurane levels were altered. Black arrows in the enlarged views of the fractional power maps (rows 2 and 3 in Figure 3) point to regions of white matter with visible diminishing fractional power with increased isoflurane levels. It is clear from the axial slice that as the isoflurane levels are increased, the fractional power for both gray and certain white matter regions are also lowered.
Figure 3.
Averaged fractional power maps for a selected axial slice from one of the monkeys across different isoflurane levels. The upper row (from left to right) presents T2*-weighted anatomical image with fractional power maps with increasing isoflurane levels 0.5%, 0.875% and 1.25%. The second and third row present enlarged views of the red and green boxes indicated on the T2*-weighted anatomical image. The black dots on the enlarged fractional power maps represent segmented white matter regions while the black arrows point to white matter regions of increased fractional power with lowered isoflurane level.
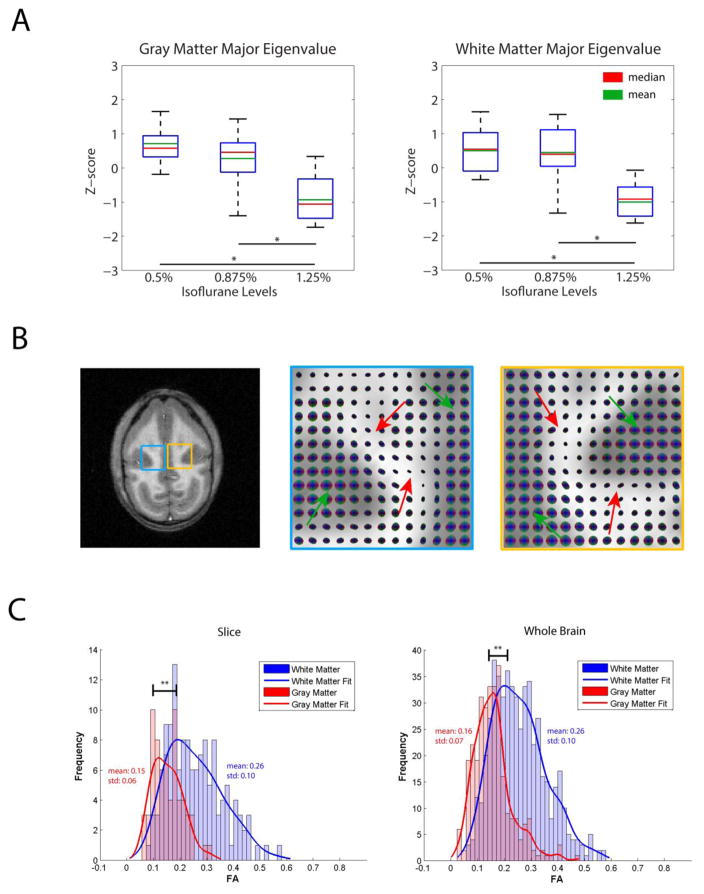
Group analyses of the major eigenvalues of the spatio-temporal correlation tensors in white and gray matters are shown for each anesthesia level in Figure 4A. It is evident that larger eigenvalues (i.e. greater nearest neighbor anisotropy of resting state correlations) are associated with lower anesthesia level. This is expected given the trends observed in the time series in Figure 1, though it also may imply changes in connectivity along tracts. Figure 4B shows a T1-weighted slice from one representative monkey, with spatio-temporal correlation maps enlarged at the blue and yellow boxed regions respectively. For each tensor map, the green arrow points to nearly isotropic tensors in gray matter while red arrows point to anisotropic tensors in white matter. Lastly, Figure 4C quantifies the fractional anisotropy of the spatio-temporal correlation tensors in the slice and the whole brain. Fractional anisotropies of white matter tensors are obviously greater than those of gray matter, providing further evidence that there are clear directions of correlational connectivity within white matter that are much less apparent in gray matter.
Figure 4.
(A) Effects of anesthesia on major eigenvalues of the functional tensors (a measure of the correlation coefficient). N=9, 14, 11 runs for 0.5%, 0.875% and 1.25% isoflurane levels respectively. * p <0.005 (Mann-Whitney Test). (B) T1-weighted anatomical image accompanied by enlarged spatio-temporal correlation tensor maps in the blue and yellow regions. Green arrows on tensor maps point to gray matter isotropic tensors while red arrows point to white matter anisotropic tensors (C) Histograms of fractional anisotropy values for the tensors in the slice as well as for the whole brain presented in (B). ** p <0.0005 (Mann-Whitney Test).
4. Discussion
4.1 White Matter Fractional Power Modulated by Anesthesia
Observation of the averaged time series and group analysis boxplots demonstrate that low frequency MR signal fluctuations behaved similarly in both gray and white regions as the level of anesthesia changed. Although fractional power in gray matter is more susceptible to changes in anesthesia level, white matter presents a similar trend. This is reflected in both the time series and fractional power analyses. Furthermore, noise scans (Supplementary Information V) and muscle control regions were immune to changes in anesthesia. This indicates that our observations were not simply a global scanner drift artifact. Also, muscle serves as a reference region that suggests the trend observed is likely driven by changes in baseline neural activity, modulated by isoflurane. A baseline fractional power in muscle, however, is quite measureable and may reflect the presence of vasomotor effects or other vascular phenomena in muscles. Liu et al. [23] recently computed a normalized quantitative mapping of cerebrovascular reactivity with BOLD MRI, and found that vasculature in white matter is capable of dilation upon appropriate stimulation. Therefore, our findings of modulated BOLD signal fluctuations in white matter with anesthesia adds another piece of evidence that BOLD signal fluctuations in white matter may be detected in a resting state.
4.2 Possible Contributing Factors to Anesthesia Changes
As claims of BOLD signal detection in white matter remain controversial, we considered the possibility that confounds such as cardiac beating, respiration and motion may have contributed to the low frequency oscillation changes as the anesthesia levels were varied. One possible artifact is whether motion of the monkey correlated with anesthesia levels, which could have affected the trend observed. First, our animals were immobilized within a special holder. Second, although only resting state fMRI BOLD images were acquired, fractional power at voxels near boundaries should still be particularly prone to motion effects. However, close inspection of our data suggests that this is not the case. For each run for the four monkeys, six motion parameters (3 translations and 3 rotations) were estimated for motion correction. With these parameters, fractional power (0.01–0.08Hz) was computed for each run at different anesthesia levels (Supplementary Information IV). No trends were observed which is indicative that motion is unlikely to have contributed to the fractional power changes observed in white matter.
Another possible confound that could have given rise to the trends observed is the increase in physiological noise as isoflurane levels were lowered. However, a human study suggested that BOLD fMRI signals in white matter are actually less subject to physiological noises than in gray matter [24]. Similarly, Birn et al. demonstrated that cardiac induced changes are most significant in gray matter and large vessels [25]. On top of that, our regression of recorded cardiac and respiratory signals with RETROICOR further excludes the contamination of physiological signals in the trends observed.
Signal contamination from adjacent subcortical regions could also have given rise to the trend observed in white matter. This effect would be further exacerbated with the isotropic smoothing of 1.5 mm full width at half maximum in order to improve the signal-to-noise ratio (SNR). Although the effects of partial volume cannot be completely eliminated, our conservative segmentation of white and gray matter minimizes this possibility. A large majority of the white matter voxels are dominated by ones with no adjacent other tissue. This was further crossed checked by applying the mask of the original EPI acquisition to further ensure regions with minimal partial volume effects were selected for analyses. Even if partial volume effects were included, they should account only for 1 to 2 pixels as outliers in our calculations. Moreover, the exact same white matter masks were subjected to an erosion algorithm before the same fractional power analyses were performed. The same diminishing trend of fractional power across increasing anesthesia levels is observed (Supplementary Information III), which further confirms minimal inclusion of partial volume effects. As pointed out in the review by Gawryluk et al [5], recent works have also provided evidence that white matter activation found cannot be fully explained due to partial volume effects. For instance, Mazerolle [12] and Fraser [26] have both shown activation in white matter under minimal partial volume effects.
Close inspection of the spatio-temporal correlation maps further negates the possibility of partial volume effects, and strengthens the argument that BOLD signal variations are observable in white matter in a resting state. If the effects of partial volume were significant with no neural signal present in white matter, spatio-temporal correlation tensors would generally be fat with their long axes perpendicular to white and gray matter boundaries, as this direction would have the largest coherences in signal fluctuations. However, we observe anisotropic white matter tensors (indicated by the red arrows in Figure 3B) that are mostly parallel to the boundaries, suggesting little influence from partial volume effects. The presence of anisotropic tensors away from the boundaries further eliminates the possibility of dominant system level fluctuations, as isotropic round tensors would be expected with no preferred directions. Therefore, given the evidence that gray matter BOLD signal fluctuations reflect neural signals, a consistent interpretation of the observed white matter signal variations and their changes with anesthesia level is that neural BOLD signals in white matter are observable with minimal partial volume effects and share a similar origin.
4.3 Future works
Although our results here present strong evidence of resting state fluctuations in BOLD signals in white matter, further work can be performed to strengthen and verify their biophysical origins. For example, we observe qualitatively through the fractional power maps that not all white matter regions appear to be modulated by changing anesthesia levels. Deeper analysis of which specific white matter tracts or pathways are more susceptible to anesthesia level changes may provide greater insight into white matter BOLD signal detection for future studies. Also, the directional consistencies of spatio-temporal correlation tensors presented here are not as robust compared to those presented in human studies [15]. Further improvements in data acquisition and processing may improve the spatio-temporal correlation tensor maps. For instance, the investigation of resting state frequencies above 0.08 Hz could improve our sensitivity to white matter BOLD signals. Recent papers have investigated and found useful connectivity above 0.1 Hz [27,28]. In fact, we have recently shown increased correlations within the center of gray matter horns in the spinal cord when using frequencies from 0.01 to 0.13 Hz [29]. Little is known about the hemodynamic nature of BOLD white matter signal, so exploring band-pass filters beyond the conventional 0.01–0.08 Hz [30] could provide better sensitivity and insights into deciphering the origins of resting state fluctuations and functional connectivity within white matter. Lastly, our findings may have further implications on white matter signal regression as a common pre-processing step in future fMRI studies. With neural activity encoded in white matter resting state signals, such practice may not be ideal as important signal variations may be removed. We believe careful examination and comparison of results with and without white matter signal regression may allow more robust and possibly new findings in future studies.
5. Conclusion
Our finding that fractional power is modulated similarly in both white and gray matter as level of anesthesia and associated neural baseline activity varies implies that neural activity is encoded in white matter resting state signals. With possible confounds carefully examined, the trends observed are deemed unlikely to be driven by noise nor artifacts. Spatio-temporal correlation tensor maps also reveal that signal fluctuations in white matter can be detected in a resting state and are consistent with underlying neural activity. Overall, our study presented here is the first reported white matter BOLD signals detected in non-human primates.
Supplementary Material
Acknowledgments
We thank Dr. Chaohui Tang and Mr. Fuxue Xin of the Vanderbilt University Institute of Imaging Science for their assistance in animal preparation and care during MRI data collection. This work was supported by NIH grants NS069909 to LMC, NS078680 and NS093669 to JCG.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–11. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 2.van den Heuvel MP, Hulshoff Pol HE. Exploring the brain network: A review on resting-state fMRI functional connectivity. Eur Neuropsychopharmacol. 2010;20:519–34. doi: 10.1016/j.euroneuro.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 3.Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann a. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–7. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- 4.Heeger DJ, Ress D. What does fMRI tell us about neuronal activity? Nat Rev Neurosci. 2002;3:142–51. doi: 10.1038/nrn730. [DOI] [PubMed] [Google Scholar]
- 5.Gawryluk JR, Mazerolle EL, D’Arcy RCN. Does functional MRI detect activation in white matter? A review of emerging evidence, issues, and future directions. Front Neurosci. 2014;8:1–12. doi: 10.3389/fnins.2014.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tettamanti M, Paulesu E, Scifo P, Maravita a, Fazio F, Perani D, et al. Interhemispheric transmission of visuomotor information in humans: fMRI evidence. J Neurophysiol. 2002;88:1051–8. doi: 10.1152/jn.00417.2001. [DOI] [PubMed] [Google Scholar]
- 7.Weber B, Treyer V, Oberholzer N, Jaermann T, Boesiger P, Brugger P, et al. Attention and interhemispheric transfer: a behavioral and fMRI study. J Cogn Neurosci. 2005;17:113–23. doi: 10.1162/0898929052880002. [DOI] [PubMed] [Google Scholar]
- 8.Gawryluk JR, Brewer KD, Beyea SD, D’Arcy RCN. Optimizing the detection of white matter fMRI using asymmetric spin echo spiral. Neuroimage. 2009;45:83–8. doi: 10.1016/j.neuroimage.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 9.Fabri M, Polonara G, Mascioli G, Salvolini U, Manzoni T. Topographical organization of human corpus callosum: An fMRI mapping study. Brain Res. 2011;1370:99–111. doi: 10.1016/j.brainres.2010.11.039. [DOI] [PubMed] [Google Scholar]
- 10.Mosier KM, Liu WC, Maldjian JA, Shah R, Modi B. Lateralization of cortical function in swallowing: A functional MR imaging study. Am J Neuroradiol. 1999;20:1520–6. [PMC free article] [PubMed] [Google Scholar]
- 11.Gawryluk JR, Mazerolle EL, Brewer KD, Beyea SD, D’Arcy RCN. Investigation of fMRI activation in the internal capsule. BMC Neurosci. 2011;12:56. doi: 10.1186/1471-2202-12-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mazerolle EL, Gawryluk JR, Dillen KNH, Patterson SA, Feindel KW, Beyea SD, et al. Sensitivity to White Matter fMRI Activation Increases with Field Strength. PLoS One. 2013:8. doi: 10.1371/journal.pone.0058130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Astafiev SV, Shulman GL, Metcalf NV, Rengachary J, MacDonald CL, Harrington DL, et al. Abnormal White Matter Blood-Oxygen-Level-Dependent Signals in Chronic Mild Traumatic Brain. Injury J Neurotrauma. 2015;32:1254–71. doi: 10.1089/neu.2014.3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ding Z, Newton AT, Xu R, Anderson AW, Morgan VL, Gore JC. Spatio-temporal correlation tensors reveal functional structure in human brain. PLoS One. 2013;8:e82107. doi: 10.1371/journal.pone.0082107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ding Z, Xu R, Bailey SK, Wu T-L, Morgan VL, Cutting LE, et al. Visualizing functional pathways in the human brain using correlation tensors and magnetic resonance imaging. Magn Reson Imaging. 2016;34:8–17. doi: 10.1016/j.mri.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heinke W, Koelsch S. The effects of anesthetics on brain activity and cognitive function. Curr Opin Anaesthesiol. 2005;18:625–31. doi: 10.1097/01.aco.0000189879.67092.12. [DOI] [PubMed] [Google Scholar]
- 17.Hutchison RM, Hutchison M, Manning KY, Menon RS, Everling S. Isoflurane induces dose-dependent alterations in the cortical connectivity profiles and dynamic properties of the brain’s functional architecture. Hum Brain Mapp. 2014;35:5754–75. doi: 10.1002/hbm.22583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu T-L, Mishra A, Wang F, Yang P-F, Gore JC, Chen LM. Effects of Isoflurane Anesthesia on Resting State fMRI signals and Functional Connectivity within Primary Somatosensory Cortex of Monkeys. doi: 10.1002/brb3.591. (Unpublished Results, Submitted for Publication) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lv P, Xiao Y, Liu B, Wang Y, Zhang X, Sun H, et al. Neuroscience Letters Dose-dependent effects of isoflurane on regional activity and neural network function3: A resting-state fMRI study of 14 rhesus monkeys. An observational study. 2016;611:116–22. doi: 10.1016/j.neulet.2015.11.037. [DOI] [PubMed] [Google Scholar]
- 20.Glover GH, Li TQ, Ress D. Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn Reson Med. 2000;44:162–7. doi: 10.1002/1522-2594(200007)44:1<162::AID-MRM23>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 21.Chen LM, Turner GH, Friedman RM, Zhang N, Gore JC, Roe AW, et al. High-resolution maps of real and illusory tactile activation in primary somatosensory cortex in individual monkeys with functional magnetic resonance imaging and optical imaging. J Neurosci. 2007;27:9181–91. doi: 10.1523/JNEUROSCI.1588-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones DK, Cercignani M. Twenty-five pitfalls in the analysis of diffusion MRI data. NMR Biomed. 2010;23:803–20. doi: 10.1002/nbm.1543. [DOI] [PubMed] [Google Scholar]
- 23.Liu P, Li Y, Lu H. Proc Intl Soc Mag Reson Med. Vol. 22. Milan: 2014. Normalizing cerebrovascular reactivity map via concomitant CO2 and O2 challenge; p. 753. [Google Scholar]
- 24.Bodurka J, Ye F, Petridou N, Murphy K, Bandettini Pa. Mapping the MRI voxel volume in which thermal noise matches physiological noise-Implications for fMRI. Neuroimage. 2007;34:542–9. doi: 10.1016/j.neuroimage.2006.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Birn RM, Murphy K, Handwerker Da, Bandettini Pa. fMRI in the presence of task-correlated breathing variations. Neuroimage. 2009;47:1092–104. doi: 10.1016/j.neuroimage.2009.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fraser LM, Stevens M, Beyea SD, D’Arcy RCN. White versus gray matter: fMRI hemodynamic responses show similar characteristics, but differ in peak amplitude. BMC Neurosci. 2012;13:91. doi: 10.1186/1471-2202-13-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen JE, Glover GH. BOLD fractional contribution to resting-state functional connectivity above 0. 1Hz. Neuroimage. 2015;107:207–18. doi: 10.1016/j.neuroimage.2014.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gohel SR, Biswal BB. Functional integration between brain regions at rest occurs in multiple-frequency bands. Brain Connect. 2015;5:23–34. doi: 10.1089/brain.2013.0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barry RL, Rogers BP, Smith SA, Gore JC. Reproducibility of resting state spinal cord networks at 7 Tesla. 2015;23:3708. doi: 10.1016/j.neuroimage.2016.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional Connectivity in the Motor Cortex of Resting. Brain. 1995;34:537–41. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.