Abstract
Background
Patients with Duchenne muscular dystrophy (DMD) require frequent imaging to assess left ventricular (LV) function. Poor imaging windows can limit the diagnostic utility of echocardiography. Cardiac MRI (CMR) is the gold standard for assessment of LV function but has not been universally adopted in DMD patients. The study objectives were: 1) evaluate reproducibility of echocardiographic measures of LV function; 2) evaluate which echocardiographic methods correlate best with CMR LVEF; 3) evaluate whether CMR provides additional value compared with echocardiography.
Methods
28 DMD participants prospectively underwent echocardiography and CMR. Two blinded readers measured: fractional shortening (FS) from M-mode and 2-dimensional images and LV ejection fraction (LVEF) using 4-chamber, biplane Simpson, 5/6 area-length, and 3-dimensional methods. Speckle tracking echocardiography was used to analyze circumferential strain. Readers subjectively rated function and segmental wall motion. Agreement was assessed using intraclass correlation coefficient, Bland-Altman plots, Spearman correlation, and weighted Kappa.
Results
2-dimensional FS and 5/6 area-length LVEF had the best combination of reproducibility and correlation with CMR LVEF, though both misclassified approximately 20% as either normal or abnormal function. Other measures of LV function were less reproducible with worse correlation to CMR LVEF. 37% of segments not visible on echocardiography were felt to have wall motion abnormalities by CMR.
Conclusions
2-dimensional FS and 5/6 area-length LVEF represent the most accurate and reproducible echocardiographic measures of LV function in patients with DMD. CMR should be considered when neither of these techniques are measurable or when it is necessary to detect more subtle cardiovascular changes.
Keywords: Duchenne muscular dystrophy, Left ventricular dysfunction, Echocardiography, Cardiac magnetic resonance imaging, Cardiomyopathy
INTRODUCTION
Duchenne muscular dystrophy (DMD) affects 1 in 4700 live male births and leads to loss of ambulation and cardiomyopathy.[1] Boys with DMD can have rapid progression of left ventricular (LV) dysfunction without symptoms, making accurate assessment of cardiac function integral to their routine care. Current recommendations call for initial cardiac evaluation starting at 6 years of age with at least biannual evaluation until 10 years of age, then at least annual evaluation with more frequent evaluations after development of cardiac imaging abnormalities.[2, 3]
Echocardiography is the most frequently used test to assess ventricular function in patients with DMD.[4, 5] However, patients with DMD have notoriously poor acoustic windows, due in part to adiposity and scoliosis.[6] Recent evaluations in patients with DMD have demonstrated moderate reproducibility of echocardiographic measures of LV function and a correlation coefficient of only 0.67 between fractional shortening (FS) by echocardiography and left ventricular ejection fraction (LVEF) by cardiac magnetic resonance imaging (CMR).[7, 8] Echocardiography and CMR have been compared in other disease processes and, in patients with myocardial infarction, echocardiography underestimates LV volumes and LVEF and is less sensitive in the detection of focal wall motion abnormalities.[9] CMR provides superior delineation of the border between the blood pool and endocardium and image quality is not affected by external factors such as body habitus. It also allows for accurate volumetric measurements, subjective and objective assessment of wall motion abnormalities, and tissue characterization by methods such as late gadolinium enhancement (LGE). For these reasons, CMR has become the gold standard for the assessment of ventricular function in patients with cardiomyopathy.[10] Citing many of these advantages, a recent DMD cardiovascular working group emphasized the importance of CMR for evaluation of LV function in this population.[11]
Despite the advantages of CMR in patients with DMD, CMR is not available in all centers and many still support echocardiography as the standard modality for DMD functional imaging.[4] Clinical and research assessments of cardiac function require accurate and precise measures. Given the clinical and research implications of inaccurate LV functional assessment in this population and the known limitations of echocardiography in DMD, a study comparing the accuracy and reproducibility of echocardiography to CMR is essential. In addition, the increasing difficulty of obtaining insurance approval for medical testing requires that all medical testing demonstrate “value added” in order to obtain authorization. Therefore, an evaluation of the reproducibility and accuracy of echocardiographic measures of LV function, and a comparison with CMR, is integral for clinical care and research in patients with DMD. The objectives of this study were the following: 1) evaluate the reproducibility of multiple echocardiographic measures of LV function in the DMD population, 2) evaluate which echocardiographic methods correlate best with CMR LVEF, and 3) evaluate whether CMR provides additional value compared with echocardiography.
METHODS
This study was approved by the institutional review board and all participants signed the appropriate consent or assent documents. DMD participants were prospectively enrolled from the multidisciplinary Neuromuscular-Cardiology Clinic. Inclusion criteria included: 1) diagnosis of DMD by clinical phenotype and either skeletal muscle biopsy or genetic testing, 2) age 8 years or older and able to undergo CMR without sedation. Exclusion criteria were: 1) muscular dystrophy other than DMD and 2) CMR and echocardiography performed greater than 30 days apart. Consent was obtained directly from participants 18 years of age or older. Those under 18 signed age-appropriate assent forms while their parents completed the informed consent documents. Additional pertinent data were recorded.
Echocardiography
All echocardiograms were performed by 1 of 4 research sonographers with experience imaging patients with DMD. Whenever possible, echocardiograms were performed in a supine position; in a minority of participants unable to transfer to a supine position, echocardiograms were performed in a reclined wheelchair. Images were de-identified using Showcase (Trillium Technology Inc., Version 5.3.0.0) and placed into the DICOM viewer under unique study identification numbers.
Blinded analysis was performed on Xcelera workstations (Philips Medical Systems, Best, The Netherlands) by two independent readers (JS and DP). Readers were asked to grade the echocardiographic quality on a scale of 1 to 5 defined as the following: 1 inadequate (very poor image quality with inability to visualize endocardial borders or visualize most cardiac structures; no or very little objective data obtainable), 2 Poor (poor image quality with only marginal delineation of endocardial borders in some views and limited assessment of atrial sizes and valves; only some objective data obtainable), 3 Average (average image quality with adequate delineation of endocardial borders in most views and adequate visual assessment of atrial sizes and all valves but the tricuspid valve; approximately half of objective data obtainable), 4 Good (at least adequate delineation of endocardial borders in all views with good delineation of endocardial borders in most views and good visualization of atrial sizes and all valves; all objective data obtainable), 5 Excellent (excellent delineation of endocardial borders in all views and excellent visualization of all other cardiac structures assessed; all objective data obtainable). A subset of 10 echocardiograms was re-analyzed by one blinded reader (JS) 3 months after the initial analysis to assess intra-observer variability.
Echocardiographic assessments were performed as described in the American Society for Echocardiography guidelines[12, 13] and included the following: 1) FS measured from M-mode images obtained in either the short axis or long axis planes, 2) FS measured from 2-dimensional images obtained in the short axis at the level of the papillary muscles, 3) single plane LVEF measured in the apical 4 chamber view, 4) modified Simpson’s biplane LVEF measured in the apical 4 chamber view and 2 chamber view, 5) 5/6 area-length LVEF measured from the short axis and apical 4 chamber views, and 6) 3-dimensional LVEF measured using dedicated software (4D LV analysis, TomTec Imaging Systems, Unterschleissheim, Germany). When image quality allowed, measurements were averaged over multiple beats. Using the standard AHA 17-segment model, one blinded reader determined which segments were visible and, if visible, which segments had wall motion abnormalities.[14] Lastly, readers subjectively graded the global systolic function on a scale of 0–6 ranging from normal to severely depressed (Table 1).
Table 1.
Subjective Assessment of Left Ventricular Function
| Subjective Score | Corresponding LVEF1 |
|---|---|
| 0 - Normal | ≥55% |
| 1 - Borderline Depressed | <55% and ≥50% |
| 2 - Mildly Depressed | <50% and ≥45% |
| 3 - Mild-moderately Depressed | <45% and ≥40% |
| 4 - Moderately Depressed | <40% and ≥35% |
| 5 - Moderate-markedly Depressed | <35% and ≥30% |
| 6 - Markedly Depressed | <30% |
Left ventricular ejection fraction (LVEF)
Speckle tracking echocardiography was used to measure peak myocardial circumferential strain (εcc) in the short axis at the level of the papillary muscles. Analysis was performed by two blinded readers with expertise in speckle tracking analysis (JS, MS) using dedicated software (Cardiac Performance Analysis, TomTec Imaging Systems, Unterschleissheim, Germany). One reader (JS) repeated analysis of 10 subjects at least 3 months after the initial analysis to assess intra-observer variability.
Cardiac magnetic resonance imaging
CMRs were performed on a 1.5 Tesla Siemens Avanto (Siemens Healthcare Sector, Erlangen, Germany) with an 8 channel cardiac coil. Functional assessment was performed as previously described with breath-held, electrocardiogram-gated, balanced steady-state free precession (bSSFP) cine imaging obtained in 10–16 contiguous slices in the short axis.[15] Myocardial grid tagging was performed with a breath-hold in the short axis at the base, level of the papillary muscles, and apex with typical imaging parameters: FOV 340×350mm2, matrix 256×192, slice thickness 8mm, voxel size 1.3×1.8×8mm3, minimal echo and repetition times, parallel imaging (GRAPPA) with an acceleration factor of 2. LGE imaging was performed 10 minutes after injection of Gd-DTPA contrast (gadopentate dimeglumine, Magnevist®, Bayer Healthcare Pharmaceuticals, Wayne, NJ, USA) through a peripheral intravenous line (PIV) at a dose of 0.2mmol/kg. LGE was assessed using single shot and segmented inversion recovery bSSFP imaging with an inversion recovery to optimally null myocardium as well as phase sensitive inversion recovery bSSFP with an inversion time of 300ms. All patients were able to perform adequate breath-holds for functional analysis; when necessary, slices with respiratory artifact were repeated as per our standard CMR protocol.
Image processing
The endocardial and epicardial borders at end-diastole and end-systole were manually contoured and used to calculate left ventricular volumes, mass, and ejection fraction using the Leonardo Workstation (Siemens Healthcare Sector, Erlangen, Germany). One reader (JS) performed all CMR analyses. The same reader who qualitatively assessed for segmental wall motion abnormalities by echocardiography also assessed wall motion abnormalities by CMR. A random subset of 10 CMRs was re-analyzed by a second reader (DP) to evaluate inter-observer variability. One reader (JS) repeated analysis in 10 subjects at least 3 months after the initial analysis to assess intra-observer variability. Peak global εcc was measured in the short axis at the level of the papillary muscles using harmonic phase (HARP) analysis (Diagnosoft Inc., Morrisville, NC) as previously described.[16] In brief, a mesh was created by contouring the epicardial and endocardial borders of the tagged images. Peak strain values were then generated by the software program. Two readers evaluated LGE sequences independently for presence or absence of LGE in each segment. In segments where the readers did not agree, the two readers reviewed the images together and formed a consensus.
Statistical analysis
The choice of statistic used to summarize agreement depended on whether the comparison was being made for variables measured in the same units and whether the variables were continuous or categorical. Intra- and inter-observer variability of continuously-measured echocardiographic and CMR measures of function were estimated using intraclass correlation coefficients (ICC), and graphical comparisons were made using Bland-Altman plots.[17] Agreement between readers for subjective evaluation of function on a 5-point scale was estimated using a weighted Kappa. Spearman’s rho was used to estimate the correlation between measures of fractional shortening and CMR LVEF. The correlations between echocardiographic measures of LVEF and CMR LVEF were estimated using ICC and Bland-Altman plots. We used bootstrapping to estimate 95% confidence intervals for the ICC, Spearman's rho, and weighted Kappa.
Analyses were performed with the statistical programming language R, version 2.14.1 (R Development Core Team, Vienna, Austria) or IBM SPSS statistics, version 23.0 (Armonk, NY: IBM Corp). Study data were collected and managed using REDCap (Research Electronic Data Capture) electronic data capture tools hosted at Vanderbilt.[18]
RESULTS
Demographics
A total of 28 DMD participants were enrolled. The average age of participants was 14.7 years (Table 2). The mean LVEF by CMR was 51% (Table 3). Based on indexed left ventricular end diastolic volumes, the majority of participants did not have left ventricular dilation. The median number of days between echocardiograms and CMRs was 0 (all but 3 echocardiograms were performed the same day as the CMR).
Table 2.
Demographics
| N=28 | |
|---|---|
| Age (years) | 14.7 ± 4.8 (6.9–27.5) |
| Height (cm) | 149 ± 15 (114–178) |
| Weight (kg) | 54 ± 17 (28–86) |
| Body surface area (m2) | 1.49 ± 0.28 (0.99–2.0) |
| Body mass index (kg/m2) | 24 ± 6.7 (14–39) |
| Ambulatory | 4 (14%) |
| Positive pressure ventilation (nocturnal or continuous) | 7 (25%) |
| Continuous positive pressure ventilation | 3 (11%) |
| Race | |
| Caucasian | 25 (89%) |
| African American | 2 (7%) |
| Asian | 1 (4%) |
| Hispanic/Latino | 4 (14%) |
| Medical Therapy at time of CMR§ | |
| ACEi1 | 16 (57%) |
| ARB2 | 5 (18%) |
| β-blocker | 10 (36%) |
| Corticosteroids | 17 (61%) |
| Mean corticosteroid duration (years) | 4.4 ± 2.8 |
Data are mean +/− SD (range), median (range), or N (%).
Angiotensin converting enzyme inhibitor (ACEi)
Angiotensin receptor blocker (ARB)
Table 3.
Measures of Cardiac Function
| CMR1 Functional Measures | N=28 |
|---|---|
| CMR LVEF2 (%) | 51 ± 9.3 (33–66) |
| CMR indexed LVEDV3 (ml/m2) | 66 ± 14 (42–104) |
| CMR indexed LVESV4 (ml/m2) | 33 ± 12 (17–70) |
| CMR RVEF5 (%) | 54 ± 5.3 (42–66) |
| Late gadolinium enhancement (N=25) | 19 (76%) |
| CMR εcc6 (%) | −14.1 ± 3.3 (−7.8–−20.1) |
| Median Days between CMR and Echocardiogram | 0 (0–22) |
| Mean Echocardiographic Functional Measures | |
| M-mode FS7 (%) | 27.0 ± 5.3 |
| 2-Dimensional FS (%) | 26.5 ± 6.1 |
| Biplane LVEF (%) | 51.1 ± 5.4 |
| 4 chamber LVEF (%) | 47.7 ± 7.4 |
| 5/6 area-length LVEF (%) | 50.8 ± 8.4 |
| 3-Dimensional LVEF (%) | 44.5 ± 10.2 |
| Echocardiographic εcc (%) | −16.2 ± 4.4 (−5.1–−23.0) |
Cardiac magnetic resonance imaging (CMR)
Left ventricular ejection fraction (LVEF)
Left ventricular end diastolic volume (LVEDV)
Left ventricular end systolic volume (LVESV)
Right ventricular ejection fraction (RVEF)
Circumferential strain (εcc)
Fractional shortening (FS)
Image Quality and Intra- and Inter-observer Variability
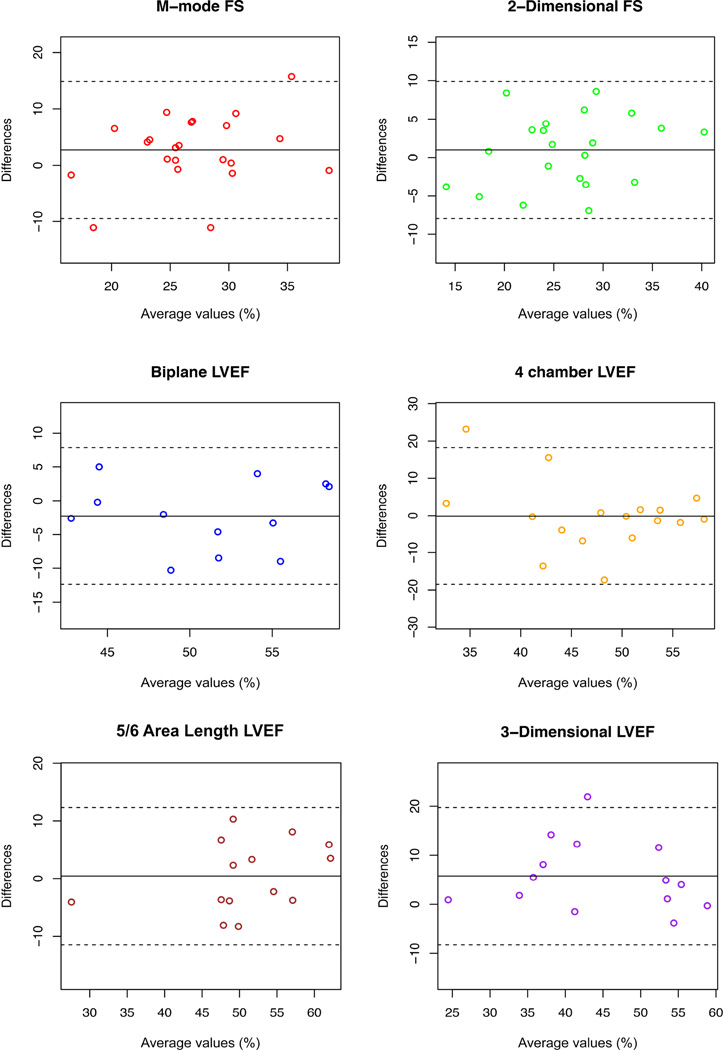
Correlation of subjective assessment of image quality was strong (r=0.84, p<0.001). Reader 1 rated 60.7% of studies as average or better while reader 2 rated 64.3% as average or better. The median rating of echocardiogram quality was average for both readers. At least 3 objective measures of function were measureable by both readers on 18 of the participants’ studies (21 for reader 1 and 20 for reader 2). Echocardiogram quality was negatively correlated to age, with a significant decrease in quality in older participants (r=−0.63, p<0.001). Intra- and inter-observer variability is demonstrated in Table 4. The most reproducible measures were 2-dimensional FS (ICC=0.86, 95% CI[0.69, 0.94]), 5/6 area-length LVEF (ICC=0.87, 95% CI[0.45, 0.97]) and 3-dimensional LVEF (ICC=0.88, 95% CI[0.61, 0.96]) (Figure 1). Notably, M-mode FS and 4-chamber LVEF both had relatively low reproducibility. Intra-observer agreement was above 0.8 for most echocardiographic measurements (Table 4). Inter-observer agreement was highest for CMR LVEF (ICC=0.94, 95% CI[0.85, 0.98]).
Table 4.
Inter-observer and Intra-observer Variability of Echocardiographic Measures of Function
| Inter-observer variability | ||
| Echocardiographic measures | ICC1 | 95% CI |
| M-mode FS2 (N=22) | 0.66 | (0.18, 0.87) |
| 2-Dimensional FS (N=22) | 0.86 | (0.69, 0.94) |
| Biplane LVEF3 (N=12) | 0.77 | (0.47, 0.94) |
| 4 chamber LVEF (N=17) | 0.60 | (0.09, 0.88) |
| 5/6 area-length LVEF (N=14) | 0.87 | (0.45, 0.97) |
| 3-Dimensional LVEF (N=14) | 0.88 | (0.61, 0.96) |
| εcc4 (N=20) | 0.84 | (0.61, 0.94) |
| CMR5 | ||
| LVEF (N=10) | 0.94 | (0.85, 0.98) |
| Intra-observer variability | ||
| Echocardiographic measures (N=10) | ICC | 95% CI |
| M-mode FS | 0.93 | (0.75, 0.98) |
| 2-Dimensional FS | 0.80 | (0.30, 0.96) |
| Biplane LVEF | 0.80 | (0.48, 0.93) |
| 4 chamber LVEF | 0.69 | (−0.18, 0.90) |
| 5/6 area-length LVEF | 0.99 | (0.96, 0.99) |
| 3-Dimensional LVEF | 0.94 | (0.53, 0.97) |
| εcc | 0.90 | (0.47, 0.98) |
| CMR (N=10) | ||
| LVEF | 0.98 | (0.92, 0.99) |
| εcc | 0.97 | (0.87, 0.99) |
Intraclass correlation coefficient (ICC)
Fractional shortening (FS)
Left ventricular ejection fraction (LVEF)
Circumferential strain (εcc)
Cardiac magnetic resonance imaging (CMR)
Figure 1.
Bland-Altman plot evaluating inter-observer variability of echocardiographic measures of left ventricular function.
Correlation Between Echocardiography and CMR
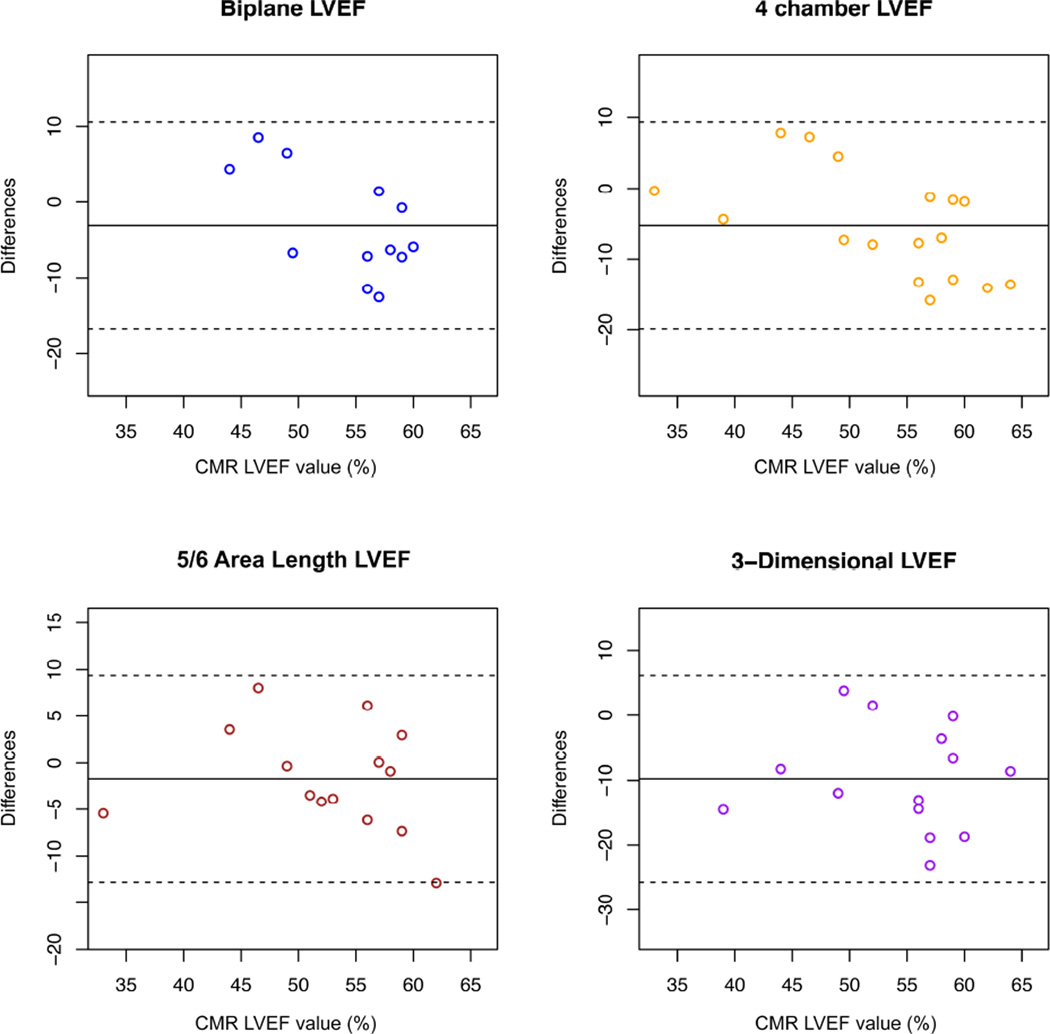
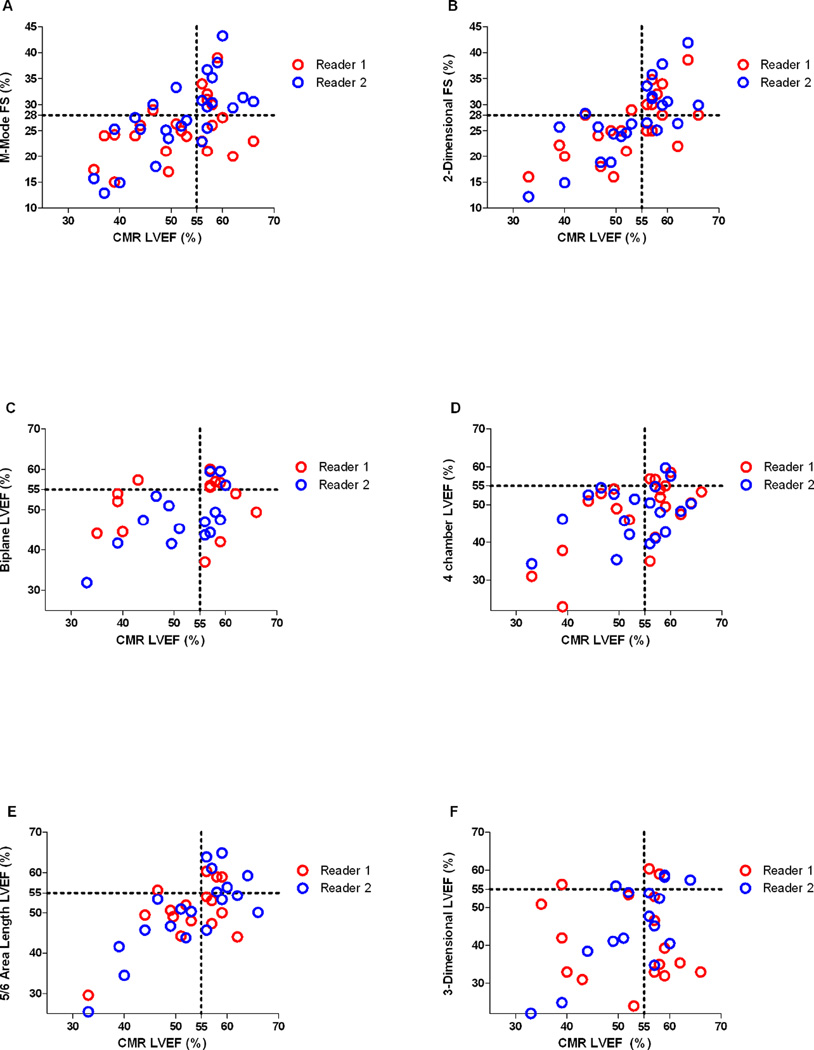
Correlation between echocardiographic and CMR measures of function are demonstrated in Table 5. 2-dimensional FS and 5/6 area-length LVEF correlated well with CMR LVEF (r=0.75, 95% CI[0.41, 0.91] and ICC=0.75, 95% CI[0.24, 0.95], respectively) (Figure 2). M-mode FS, 4-chamber LVEF, and 3-dimensional LVEF had relatively low correlation, while biplane LVEF did not correlate with CMR LVEF. 2-dimensional FS was measurable in 79% of participants while 5/6 area-length was only measurable in 50%. When using the cut-offs of <28% for abnormal FS and <55% for abnormal LVEF, both readers misclassified approximately 20% of participants with measurable 2-dimensional FS values (Figure 3). For 5/6 area-length LVEF, functional status was misclassified frequently (approximately 20% and 40%) depending on the reader. All echocardiographic measures of LV function had a tendency to underestimate function, though this was more pronounced in measures of LVEF than FS (Figure 3). There was no systematic bias to suggest that error changed with increasing or decreasing LVEF.
Table 5.
Comparison of Echocardiographic and Cardiac Magnetic Resonance Imaging (CMR) Measures of Left Ventricular function
| Echocardiographic Measures | N | Correlation | 95% CI |
|---|---|---|---|
| Average Values | CMR LVEF1 | ||
| M-mode FS2 | 22 (79%) | 0.583 | (0.09, 0.82) |
| 2-Dimensional FS | 22 (79%) | 0.753 | (0.41, 0.91) |
| Biplane LVEF | 12 (43%) | 0.174 | (−0.36, 0.60) |
| 4 chamber LVEF | 17 (61%) | 0.564 | (−0.10, 0.83) |
| 5/6 area-length LVEF | 14 (50%) | 0.754 | (0.24, 0.95) |
| 3-Dimensional LVEF | 14 (50%) | 0.564 | (0.00, 0.85) |
| εcc5 | 20 (71%) | −0.663 | (−0.29, −0.88) |
Left ventricular ejection fraction (LVEF)
Fractional shortening (FS)
Spearman Correlation
Intraclass Correlation Coefficient
Circumferential strain (εcc)
Figure 2.
Bland-Altman variant with gold-standard cardiac magnetic resonance imaging (CMR) left ventricular ejection fraction (LVEF) plotted on x-axis and difference between echocardiographic measures of LVEF and CMR LVEF plotted on y-axis.
Figure 3.
Scatter plots of measured values of echocardiographic left ventricular function for both readers plotted against cardiac magnetic resonance imaging (CMR) left ventricular ejection fraction (LVEF). A) Fractional shortening (FS) measured on M-mode, B) FS measured on 2-dimensional images, C) biplane LVEF, D) 4 chamber LVEF, E) 5/6 area-length LVEF, F) 3-dimensional LVEF. Dotted lines represent the cut-off for normal fractional shortening (28%) and LVEF (55%).
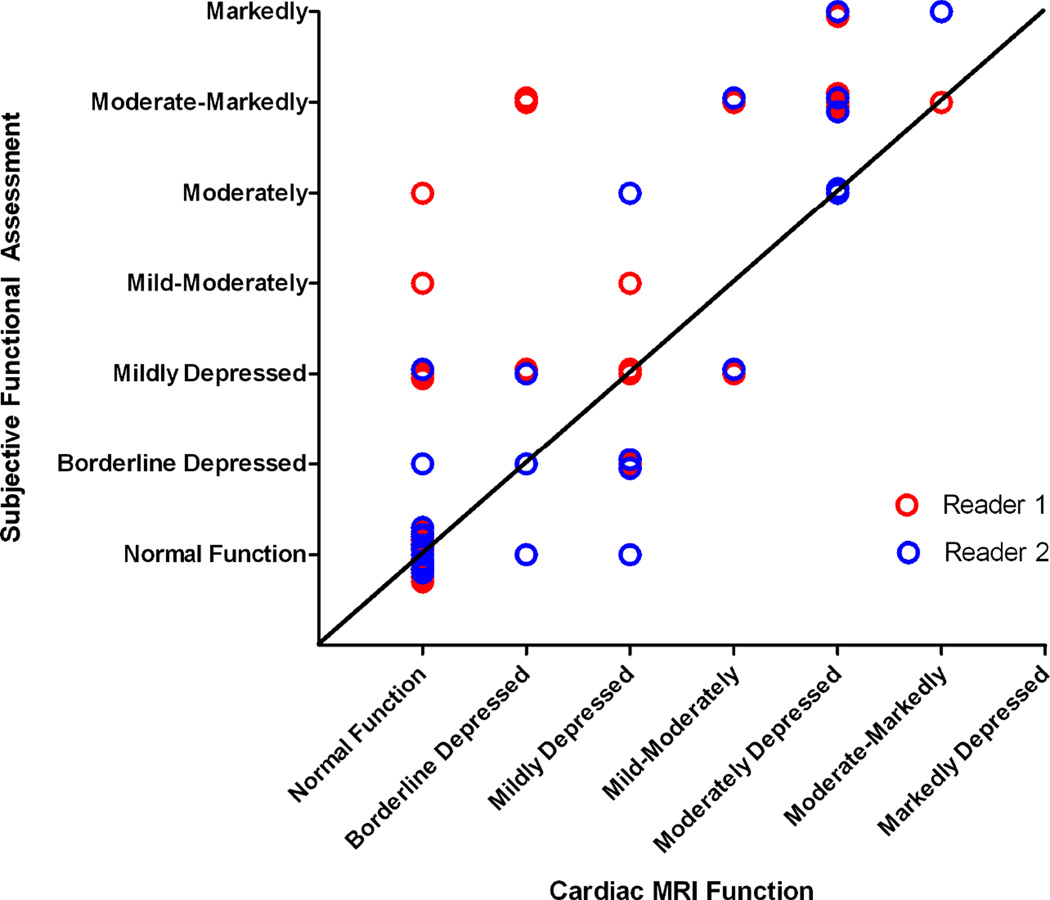
The weighted Kappa of subjective assessment of echocardiographic function to CMR was 0.65 (95% CI[0.39, 0.82]) for reader 1 and 0.86 (95% CI[0.73, 0.93]) for reader 2. Although the subjective assessment agreed well statistically, graphical assessment suggests average accuracy with a tendency to underestimate function compared with CMR (Figure 4).
Figure 4.
Scatter plot comparing the readers’ subjective assessment of left ventricular (LV) function and the objective measure based on cardiac magnetic resonance imaging (CMR). Values from CMR LVEF converted as follows: ≥55% = 0, ≥50%–<55% = 1, ≥45%–<50% = 2, ≥40%–<45% = 3, ≥35%–<40% = 4, ≥30%– <35% = 5, <30% = 6.
Wall Motion Abnormalities
A total of 471 of 476 segments were seen well by CMR. The 5 that were difficult to visualize were due to susceptibility artifact from dental braces, though endocardial borders at end systole and diastole were seen well enough to contour. Only 334 of 476 segments (70%) were visible by echocardiography. A total of 142 CMR segments (30%) were subjectively assessed as having wall motion abnormalities by CMR. By echocardiography, 87 segments (26%) were felt to have wall motion abnormalities. Of the 142 segments not visible by echocardiography, 53 (37%) were felt to have wall motion abnormalities by CMR.
The segments that were most difficult to visualize were the anterolateral and anterior basal segments, the anterolateral and anterior mid-LV segments, and the lateral and anterior apical segments. The segments most likely to have wall motion abnormalities on CMR were: mid inferolateral (53.6%), basal inferolateral (53.6%), mid inferior (50%), basal anterolateral (46.4%), basal inferior (42.9%), and mid anterolateral (39.3%).
Late Gadolinium Enhancement
Gadolinium was not administered in 3 participants. Of the 25 participants with LGE imaging, 19 (76%) had positive LGE. Seven of 13 (54%) participants with normal CMR LVEF had positive LGE. Of the 11 participants with normal 2-dimensional FS, 5 (45%) had positive LGE. Of the 4 participants with normal 5/6 area-length LVEF, 1 (25%) had positive LGE.
Strain
A total of 27 participants had CMR tagging performed at the level of the papillary muscles; only 20 participants (71%) had adequate imaging for assessment of circumferential strain by speckle tracking echocardiography. The CMR and speckle tracking echocardiographic circumferential strain correlated moderately well (r=0.64, p=0.018). Interestingly, echocardiographic strain correlated more strongly with CMR LVEF than CMR strain (r=−0.66, p=0.001 and r=−0.46, p=0.016). CMR strain was reduced in patients with LGE (CMR strain: −16.7% ± 3.4 vs −13.2% ± 3.0, p=0.042) and there was a trend towards reduced echocardiographic strain in patients with LGE (−19.4% ± 3.1 vs −15.9% ± 3.7, p=0.089).
DISCUSSION
We prospectively evaluated objective echocardiographic measures of LV function in DMD participants and found that 2-dimensional FS and 5/6 area-length LVEF have the lowest inter-observer variability and the highest correlation with CMR LVEF. The other echocardiographic methodologies evaluated were less reproducible and had a lower correlation with CMR. Subjective assessment varied significantly between readers. Objective and subjective echocardiographic measures had a tendency to underestimate LV function as compared with CMR, likely due to suboptimal delineation of endocardial borders by echocardiography. These data are integral to the care of patients with DMD, as echocardiography comprises the majority of cardiac testing in this patient population. These data also reinforce the difficulty inherent in quantitative echocardiographic assessment of patients with DMD and suggest that, when quantitative data are necessary, CMR should be considered.
Based on these data, we recommend obtaining alternate imaging modalities when either or both of these echocardiographic methods (2-dimensional FS and 5/6 area-length LVEF) are not measurable due to image quality. This is especially important in older patients, as the imaging quality correlated inversely with age. Of note, 2-dimensional FS was measurable in almost 80% of participants while 5/6 area-length LVEF was measurable in only half. While the correlation was strong for both measures (r=0.75 and ICC=0.75), it must be emphasized that it was not excellent. The clinical and research implications of small variations in cardiac function must be weighed closely when determining the best imaging modality. In the setting of satisfactory imaging windows, both methods should be more than adequate for routine functional evaluation and screening. However, in situations where more subtle changes need to be detected, especially in research studies where the primary outcome is cardiac function, CMR remains the best option.
Given the significant variability between readers and the tendency to underestimate function, we recommend against subjective assessment of ventricular function. We hypothesize that the variability between readers is due to the inherent subjectivity of “eyeballing” function in patients with limited imaging windows. Some of this variability may also have been related to one reader having more experience reading DMD echocardiograms, as this reader had a stronger correlation with CMR LVEF. This leads us to suggest that, in cases where objective data are unavailable and CMR cannot be obtained, the same reader should perform serial subjective functional assessments.
All objective echocardiographic measures in this cohort seemed to underestimate LV function compared with CMR LVEF. Interestingly, previous comparisons between echocardiography and CMR in adult patients with various disease processes have demonstrated results ranging from underestimation to overestimation of LVEF.[9, 19–21] It is possible that the underestimation in this study is due to poor visualization of the endocardial borders. Of note, there was no correlation between image quality and the difference between CMR LVEF and echocardiographic measures of LVEF, suggesting minimal bias related to image quality.
A previous study evaluated the echocardiographic reproducibility of FS and biplane LVEF in DMD patients;[7] our data are similar, though our study evaluates more echocardiographic methods to determine LV function. That study found lower intraclass correlation coefficients for both FS and 4-chamber LVEF than in our study. It is possible that the decreased variability we describe is related to the fact that both readers were from the same institution and presumably have similar methods of performing functional analysis. The inter-observer reproducibility in this study is similar to that found by Margossian et al. in a cohort of pediatric cardiomyopathy patients, which also demonstrated the highest reproducibility for 5/6 area-length LVEF.[22]
One prior study has also evaluated FS and its correlation to CMR LVEF and found a similar correlation between M-mode FS and CMR LVEF. However, this study did not assess the other echocardiographic methods of LV functional assessment.[8] In our study, we found a higher correlation between 2-dimensional FS and CMR LVEF than that of M-mode FS.
As in the prior study by Brunklaus et al, a significant number of participants in this study with normal function by echocardiography had LGE on CMR. Our study also demonstrated that a significant number of segments with abnormal wall motion by CMR were not visible by echocardiography. Finally, the correlation of strain calculated by speckle tracking echocardiography with strain by tagging was adequate. Echocardiographic strain also correlated with CMR LVEF, though not strongly. We only analyzed the short axis at the level of the papillary muscles based on previously published literature.[16] These findings suggest the potential added value of CMR in this patient population, especially CMR with contrast.
There are disadvantages to CMR, including claustrophobia, patient discomfort, and length of study. These must also be considered when evaluating the optimal methodology. While contrast provides valuable information, we have found that a non-contrast study to determine LV function in patients with poor echocardiographic windows is feasible, shorter in duration, and can limit many of these drawbacks.
Limitations
This study was performed at a single institution, though our institution has a broad catchment area of DMD patients, minimizing this limitation. Although 3-beat averages improve intra- and inter-observer variability,[22] we chose not to require 2 or 3-beat averages for measurements due to the poor imaging windows in this patient population. Requiring multiple beat averages may have improved our reproducibility, but it also would have significantly decreased the number of measurable studies and would not have accurately reflected the clinical workflow currently employed for these patients in our institution. We do not use anesthesia or sedation for CMR in patients with DMD and the minimum age for unsedated CMR in our institution is 8 years of age. Therefore, by necessity, the youngest participants were 8 years old. Although we expect that the correlation would improve in younger patients with better imaging windows, this study was designed to assess the accuracy and reproducibility in DMD participants with a higher likelihood of LV dysfunction. Many of the echocardiographic methods were not measureable in all participants, decreasing the sample size available for analysis. Evaluation of this question as part of a larger, multi-center trial would provide more adequate numbers.
Conclusions
Based on these data, 2-dimensional FS and 5/6 area-length LVEF represent the most accurate and reproducible objective echocardiographic measures of LV function in patients with DMD. In cases where neither of these techniques is measurable, or in cases where only one measure of LV function is possible due to image quality, clinicians should consider an alternate imaging modality such as CMR. In addition, CMR has added value for wall motion assessment and LGE. Based on these data, CMR should be strongly considered when quantitative assessment is necessary to monitor disease progression or evaluate the efficacy of therapy.
Highlights.
-
-
We evaluated the reproducibility of echo measures of LV function in DMD
-
-
Echo measures of LV function compared to LVEF from contemporaneous cardiac MRI (CMR)
-
-
In DMD, 2-dimensional FS and 5/6 area-length are the most accurate and reproducible
-
-
CMR has added value for wall motion assessment and late gadolinium enhancement
-
-
CMR is preferable when subtle cardiovascular changes must be detected
Acknowledgments
None
Funding: This work was supported by American Heart Association Grant 13CRP14530007 (Soslow) (Dallas, TX).
Research reported in this publication was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number K23HL123938 (Bethesda, MD) (Soslow). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The project was supported by the National Center for Research Resources, Grant UL1 RR024975-01, and is now at the National Center for Advancing Translational Sciences, Grant 2 UL1 TR000445-06 (Bethesda, MD). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
This project was supported by the Fighting Duchenne Foundation and the Fight DMD/Jonah & Emory Discovery Grant (Nashville, TN) (Markham).
The sponsors and funders had no role in the design and conduct of the study or in the collection, analysis, and interpretation of the data, or in the preparation, review, or approval of the manuscript.
Abbreviations
- bSSFP
balanced steady state free precession
- CMR
cardiac magnetic resonance imaging
- DMD
Duchenne muscular dystrophy
- FS
fractional shortening
- ICC
intraclass correlation coefficient
- LGE
late gadolinium enhancement
- LV
left ventricular
- LVEF
left ventricular ejection fraction
- εcc
circumferential strain
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jonathan H Soslow, Email: Jonathan.h.soslow@vanderbilt.edu.
Meng Xu, Email: Meng.xu@vanderbilt.edu.
James C Slaughter, Email: James.c.slaughter@vanderbilt.edu.
Kimberly Crum, Email: Kimberly.crum@vanderbilt.edu.
Larry W Markham, Email: Larry.markham@vanderbilt.edu.
David A Parra, Email: David.parra@vanderbilt.edu.
References
- 1.Dooley J, Gordon KE, Dodds L, MacSween J. Duchenne muscular dystrophy: a 30-year population-based incidence study. Clin Pediatr (Phila) 2010;49:177–179. doi: 10.1177/0009922809347777. [DOI] [PubMed] [Google Scholar]
- 2.Bushby K, Finkel R, Birnkrant DJ, Case LE, Clemens PR, Cripe L, et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and pharmacological and psychosocial management. The Lancet Neurology. 2010;9:77–93. doi: 10.1016/S1474-4422(09)70271-6. [DOI] [PubMed] [Google Scholar]
- 3.Bushby K, Finkel R, Birnkrant DJ, Case LE, Clemens PR, Cripe L, et al. Diagnosis and management of Duchenne muscular dystrophy, part 2: implementation of multidisciplinary care. The Lancet Neurology. 2010;9:177–189. doi: 10.1016/S1474-4422(09)70272-8. [DOI] [PubMed] [Google Scholar]
- 4.Allen HD, Thrush PT, Hoffman TM, Flanigan KM, Mendell JR. Cardiac management in neuromuscular diseases. Phys Med Rehabil Clin N Am. 2012;23:855–868. doi: 10.1016/j.pmr.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Spurney CF. Cardiomyopathy of Duchenne muscular dystrophy: current understanding and future directions. Muscle Nerve. 2011;44:8–19. doi: 10.1002/mus.22097. [DOI] [PubMed] [Google Scholar]
- 6.Romfh A, McNally EM. Cardiac assessment in duchenne and becker muscular dystrophies. Current heart failure reports. 2010;7:212–218. doi: 10.1007/s11897-010-0028-2. [DOI] [PubMed] [Google Scholar]
- 7.Spurney CF, McCaffrey FM, Cnaan A, Morgenroth LP, Ghelani SJ, Gordish-Dressman H, et al. Feasibility and Reproducibility of Echocardiographic Measures in Children with Muscular Dystrophies. J Am Soc Echocardiogr. 2015;28:999–1008. doi: 10.1016/j.echo.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brunklaus A, Parish E, Muntoni F, Scuplak S, Tucker SK, Fenton M, et al. The value of cardiac MRI versus echocardiography in the pre-operative assessment of patients with Duchenne muscular dystrophy. Eur J Paediatr Neurol. 2015;19:395–401. doi: 10.1016/j.ejpn.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 9.Gardner BI, Bingham SE, Allen MR, Blatter DD, Anderson JL. Cardiac magnetic resonance versus transthoracic echocardiography for the assessment of cardiac volumes and regional function after myocardial infarction: an intrasubject comparison using simultaneous intrasubject recordings. Cardiovasc Ultrasound. 2009;7:38. doi: 10.1186/1476-7120-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karamitsos TD, Francis JM, Myerson S, Selvanayagam JB, Neubauer S. The role of cardiovascular magnetic resonance imaging in heart failure. J Am Coll Cardiol. 2009;54:1407–1424. doi: 10.1016/j.jacc.2009.04.094. [DOI] [PubMed] [Google Scholar]
- 11.McNally EM, Kaltman JR, Benson DW, Canter CE, Cripe LH, Duan D, et al. Contemporary cardiac issues in Duchenne muscular dystrophy. Working Group of the National Heart, Lung, and Blood Institute in collaboration with Parent Project Muscular Dystrophy. Circulation. 2015;131:1590–1598. doi: 10.1161/CIRCULATIONAHA.114.015151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lopez L, Colan SD, Frommelt PC, Ensing GJ, Kendall K, Younoszai AK, et al. Recommendations for quantification methods during the performance of a pediatric echocardiogram: a report from the Pediatric Measurements Writing Group of the American Society of Echocardiography Pediatric and Congenital Heart Disease Council. J Am Soc Echocardiogr. 2010;23:465–495. doi: 10.1016/j.echo.2010.03.019. quiz 576-7. [DOI] [PubMed] [Google Scholar]
- 13.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39. e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105:539–542. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 15.Schulz-Menger J, Bluemke DA, Bremerich J, Flamm SD, Fogel MA, Friedrich MG, et al. Standardized image interpretation and post processing in cardiovascular magnetic resonance: Society for Cardiovascular Magnetic Resonance (SCMR) Board of Trustees Task Force on Standardized Post Processing. J Cardiovasc Magn Reson. 2013;15:35. doi: 10.1186/1532-429X-15-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hor KN, Wansapura J, Markham LW, Mazur W, Cripe LH, Fleck R, et al. Circumferential strain analysis identifies strata of cardiomyopathy in Duchenne muscular dystrophy: a cardiac magnetic resonance tagging study. J Am Coll Cardiol. 2009;53:1204–1210. doi: 10.1016/j.jacc.2008.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 18.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rijnierse MT, van der Lingen AL, Weiland MT, de Haan S, Nijveldt R, Beek AM, et al. Clinical Impact of Cardiac Magnetic Resonance Imaging Versus Echocardiography-Guided Patient Selection for Primary Prevention Implantable Cardioverter Defibrillator Therapy. Am J Cardiol. 2015;116:406–412. doi: 10.1016/j.amjcard.2015.04.059. [DOI] [PubMed] [Google Scholar]
- 20.Greupner J, Zimmermann E, Grohmann A, Dubel HP, Althoff TF, Borges AC, et al. Head-to-head comparison of left ventricular function assessment with 64-row computed tomography, biplane left cineventriculography, and both 2- and 3-dimensional transthoracic echocardiography: comparison with magnetic resonance imaging as the reference standard. J Am Coll Cardiol. 2012;59:1897–1907. doi: 10.1016/j.jacc.2012.01.046. [DOI] [PubMed] [Google Scholar]
- 21.Li C, Lossnitzer D, Katus HA, Buss SJ. Comparison of left ventricular volumes and ejection fraction by monoplane cineventriculography, unenhanced echocardiography and cardiac magnetic resonance imaging. Int J Cardiovasc Imaging. 2012;28:1003–1010. doi: 10.1007/s10554-011-9924-0. [DOI] [PubMed] [Google Scholar]
- 22.Margossian R, Chen S, Sleeper LA, Tani LY, Shirali G, Golding F, et al. The reproducibility and absolute values of echocardiographic measurements of left ventricular size and function in children are algorithm dependent. J Am Soc Echocardiogr. 2015;28:549–558. e1. doi: 10.1016/j.echo.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]