Abstract
Dopamine (DA) and acetylcholine (ACh) signals converge onto protein kinase A (PKA) in medium spiny neurons of the striatum to control cellular and synaptic activities of these neurons, although underlying molecular mechanisms are less clear. Here we measured phosphorylation of the α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor (AMPAR) at a PKA site (S845) as an indicator of AMPAR responses in adult rat brains in vivo to explore how DA and ACh interact to modulate AMPARs. We found that subtype-selective activation of DA D1 receptors (D1Rs), D2 receptors (D2Rs), or muscarinic M4 receptors (M4Rs) induced specific patterns of GluA1 S845 responses in the striatum. These defined patterns support a local multitransmitter interaction model in which D2Rs inhibited an intrinsic inhibitory element mediated by M4Rs to enhance the D1R efficacy in modulating AMPARs. Consistent with this, selective enhancement of M4R activity by a positive allosteric modulator resumed the cholinergic inhibition of D1Rs. In addition, D1R and D2R coactivation recruited GluA1 and PKA preferentially to extrasynaptic sites. In sum, our in vivo data support an existence of a dynamic DA-ACh balance in the striatum which actively modulates GluA1 AMPAR phosphorylation and trafficking.
Keywords: D1, D2, muscarinic receptor, S845, protein kinase A, caudate putamen, nucleus accumbens, positive allosteric modulator
1. Introduction
Dopamine (DA) D1 receptors (D1Rs) and D2 receptors (D2Rs) are the most abundant dopamine receptor subtypes in the striatum, a key structure in the basal ganglia and a focus of studies on various psychiatric disorders. These receptors are prominently segregated into two equally-populated subtypes of medium spiny neurons (MSNs) making up 90-95% of striatal neurons (Gerfen et al., 1990; Aubert et al., 2000; Bertran-Gonzalez et al., 2010). While D1Rs are primarily expressed in striatonigral output neurons giving rise to the direct pathway, D2Rs reside in striatopallidal output neurons triggering the polysynaptic indirect pathway (Onn et al., 2000). Both receptors are G protein-coupled receptors. Via distinct G proteins, D1Rs (Gas/Golf-coupled) and D2Rs (Gai/o-coupled) respectively stimulate and inhibit adenylyl cyclase and the downstream cAMP formation and protein kinase A (PKA) activation (Neve et al., 2004). As a result, D1Rs are usually considered to enhance glutamatergic actions in striatonigral neurons, whereas D2Rs exert the opposite effects in striatopallidal neurons (Surmeier et al., 2007).
Acetylcholine (ACh) is another key transmitter in the striatum and is mainly provided by local large aspiny cholinergic interneurons. Despite few in number (only 1-2% of the total striatal cell population) (Bolam et al., 1984; Phelps et al., 1985), cholinergic interneurons widely influence surrounding MSNs via their extremely dense and branched axonal arbors. The intrinsic cholinergic transmission is traditionally viewed as a strategic drive balancing DA signaling and maintaining MSN homeostasis. Indeed, pharmacological blockade of muscarinic ACh receptors (mAChRs) caused hyperlocomotion and augmented DA-stimulated motor activities (Chou et al., 1992; Morelli et al., 1993; Wang and McGinty, 1996a) and gene expression in the striatum (Chou et al., 1992; Bernard et al., 1993; Morelli et al., 1993; Wang and McGinty, 1996a; 1996b; 1997). Thus, ACh through mAChRs acts as an inhibitory regulator of tonic and phasic DA signaling, although detailed molecular mechanisms, especially the mAChR subtype, underlying the ACh-DA interplay remain elusive.
The α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor (AMPAR) is critical for normal glutamatergic transmission and is linked to various mental illnesses (Dingledine et al., 1999). These receptors become functional upon homo- or heterotetrameric assembly of four subunits, GluA1-4 (formerly GluR1-4) (Greger et al., 2007). AMPARs are knowingly regulated by posttranslational phosphorylation (Lu and Roche, 2012; Wang et al., 2014). Two well-characterized phosphorylation sites are serine 831 (S831) and serine 845 (S845) located in intracellular C-terminal tails of GluA1 subunits (Roche et al., 1996; Barria et al., 1997; Mammen et al., 1997; Serulle et al., 2007). The former is phosphorylated by protein kinase C and Ca2+/calmodulin-dependent protein kinase II, while the latter is phosphorylated by PKA. As a labile, inducible and reversible process sensitive to changing synaptic input, phosphorylation of S831 and S845 controls synaptosomal delivery of GluA1 and physiological properties of GluA1-containing AMPARs (Lu and Roche, 2012; Wang et al., 2014).
AMPARs are enriched in DA-innervated forebrain regions, including the striatum (Bernard et al., 1997; Kondo et al., 2000; Reimers et al., 2011). In the striatum, GluA1-containg AMPARs exist in all MSNs (Bernard et al., 1997). These AMPARs are believed to be tightly regulated by DA given that D1R agonists readily elevated GluA1 phosphorylation usually at S845 in striatal neurons (Price et al., 1999; Snyder et al., 2000; Chao et al., 2002; Swayze et al., 2004). However, how the D1R-D2R interaction and DA-ACh interplay regulate GluA1 phosphorylation is unclear. Here we investigated the cooperative role of D1Rs and D2Rs in regulating GluA1 AMPAR phosphorylation in the rat striatum and evaluated the detailed contribution of the DA-ACh balance to controlling AMPAR trafficking in vivo.
2. Materials and Methods
2.1. Animals
Adult male Wistar rats (210-300 g; Charles River, New York, NY) were housed in pairs in a controlled environment at a constant temperature of 23°C and humidity of 50 ± 10% with food and water available ad libitum. The animal room was on a 12-h/12-h light/dark cycle. Rats were allowed 6-7 days of habituation to the facility. All animal use and procedures were in strict accordance with the US National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee.
2.2. Drug administration and subsynaptic protein fractionation
Rats received an intraperitoneal (i.p.) injection of systemically active agents in a volume of 1 ml/kg. Doses of agents were calculated as the salt. An effective dose (3 mg/kg, i.p.) of the D1R agonist SKF81297 and the D2R agonist quinpirole for a systemic injection was determined by literatures and our previous studies (Wang and McGinty, 1995). A positive allosteric modulator (PAM) of M4R, VU0152100, was used. Its doses for a systemic administration (6 and 60 mg/kg, i.p.) were chosen from early studies (Brady et al., 2008; Byun et al., 2014) in which VU0152100 at 56.6 mg/kg blocked motor responses to DA stimulation in wild type rats and mice but not in M4R knockout mice. Age-matched rats that received physiological saline or vehicle injections served as controls. After drug injection, rats were anesthetized and decapitated. Rat brains were removed and coronal slices were cut. The entire striatum, including the caudate putamen and nucleus accumbens, was dissected from slices for following fractionation procedures at 4°C. Striatal tissue was homogenized in isotonic sucrose homogenization buffer (SHB) containing 0.32 M sucrose, 10 mM HEPES, pH 7.4, 2 mM EDTA, and a protease/phosphatase inhibitor cocktail (Thermo Scientific, Rochester, NY). Homogenates were centrifuged at 800 g (10 min). The supernatant was collected and centrifuged at 10,000 g (30 min) to obtain the pellet 2 (P2) containing crude synaptosomal plasma membranes. To enrich synaptic and extrasynaptic membranes as described previously (Mao et al., 2013), washed P2 was resuspended in SHB containing Triton X-100 (0.5%, v/v) and incubated for 20 min with gentle rotation. Samples were centrifuged (32,000 g, 20 min). The supernatant enriched with Triton X-100-soluable extrasynaptic membranes and the pellet enriched with Triton X-100-insoluable synaptic membranes were yielded. P2, synaptic, and extrasynaptic membranes were solubilized in SHB containing 0.5% Triton X-100, 1% sodium dodecyl sulfate (SDS), and a protease/phosphatase inhibitor cocktail with gentle rotation (1 h, 4°C). Protein concentrations were determined. Samples were stored at −80°C until use.
2.3. Western blot
As described previously (Van Dolah et al., 2011; Jin et al., 2013), proteins were separated on SDS NuPAGE Novex 4-12% gels (Invitrogen, Carlsbad, CA) and transferred to polyvinylidene fluoride membranes (Millipore, Bedford, MA). Membranes after blocking were incubated in a solution containing a primary rabbit or mouse antibody overnight at 4°C. This was followed by incubation in a horseradish peroxidase-linked secondary antibody. Immunoblots were developed with an enhanced chemiluminescence reagent (GE Healthcare Life Sciences, Piscataway, NJ). Optical density of immunoblots was measured using NIH gel analysis software.
2.4. Antibodies and pharmacological agents
Primary antibodies used in this study include rabbit antibodies against phospho-S831 (pS831, PhosphoSolutions, Aurora, CO), phospho-S845 (pS845, PhosphoSolutions), GluA1 (Millipore), PKA catalytic subunit a (PKA Cα; Cell Signaling Technology, Danvers, MA), or PKA regulatory subunit IIβ (PKA RIIβ; Abcam, Cambridge, MA), or mouse antibodies against GluA2 (Millipore), GluA3 (Millipore), or tubulin (Millipore). Pharmacological agents, including (±)-6-chloro-PB hydrobromide (SKF81297), (-)-quinpirole hydrochloride, R(+)-SCH23390 hydrochloride, S-(-)-eticlopride hydrochloride and (-)-scopolamine hydrobromide, were purchased from Sigma-Aldrich. VU0152100 [3-amino-N-(4-methoxybenzyl)-4,6-dimethylthieno[2,3-b]pyridine carboxamide] was purchased from Axon Medchem (Reston, VA). All agents were freshly prepared at the day of experiments. VU0152100 was dissolved in 10% Tween 80 and dH2O with the pH adjusted to approximately 7.0 using 1 N NaOH. Other agents were dissolved in physiological saline.
2.6. Statistics
The results are presented as means ± SEM. These results were statistically analyzed using a oneway analysis of variance followed by a Bonferroni (Dunn) comparison of groups using least squares-adjusted means or two-tailed unpaired Student's t-test. Probability levels of < 0.05 were considered statistically significant.
3. Results
3.1. D1Rs and D2Rs differentially regulate GluA1 phosphorylation
We first investigated the role of D1Rs and D2Rs in regulating GluA1 phosphorylation. To this end, we subjected rats to a single dose of the D1R agonist SKF81297 (3 mg/kg, i.p.) or the D2R agonist quinpirole (3 mg/kg, i.p.) or both. We then sacrificed rats 20 min after drug injection to detect changes in GluA1 phosphorylation at S845 and S831 in the striatum using immunoblots. SKF81297 increased pS845 proteins, while quinpirole did not (Fig. 1A and 1B).
Figure 1. Effects of the D1R and D2R agonists on basal GluA1 phosphorylation in the rat striatum.

(A) Representative immunoblots illustrating effects of SKF81297 and quinpirole on GluA1 phosphorylation in the striatum. (B-D) Quantification of effects of SKF81297 and quinpirole on GluA1 phosphorylation at S845 (B) and S831 (C) and total GluA1 expression (D). Note that SKF81297 increased S845 phosphorylation, while quinpirole did not. Co-administration of the two agonists induced a greater increase in pS845 levels than that induced by the D1R agonist alone. Rats were given an i.p. injection of SKF81297 (SKF, 3 mg/kg), quinpirole (Quin, 3 mg/kg) or both drugs and were sacrificed 20 min after drug injection for immunoblot analysis. Data are presented as means ± SEM (n = 4 per group). *P < 0.05 versus saline.
Coadministration of the two agonists produced an even greater increase in pS845 than that induced by SKF81297 alone. In contrast to pS845, pS831 levels were not altered by either agonist alone or by the coadministered agonists (Fig. 1A and 1C). A total amount of GluA1 proteins remained unchanged following all drug treatments (Fig. 1A and 1D). These data demonstrate that D1Rs upregulate GluA1 phosphorylation at a PKA site (S845), presumably in D1R-bearing projection neurons (striatonigral MSNs). Noticeably, the D2R agonist that inhibits the cAMP-PKA pathway augmented the D1R agonist-induced S845 phosphorylation, which was probably mediated via an indirect mechanism involving local ACh (see below).
We next tested effects of D1R and D2R antagonists on basal and SKF81297/quinpirole-stimulated S845 phosphorylation. Rats received an i.p. injection of a D1R antagonist SCH23390 (0.1-0.5 mg/kg) or a D2R antagonist eticlopride (0.5 mg/kg) alone or 15 min prior to coadministered SKF81297/quinpirole (both at 3 mg/kg) and were sacrificed 20 min after the final drug injection. SCH23390 reduced basal levels of pS845 and blocked the S845 phosphorylation induced by SKF81297/quinpirole in the striatum (Fig. 2A and 2B). In sharp contrast to SCH23390, eticlopride itself elevated pS845 levels. When coinjected with SKF81297/quinpirole, eticlopride did not alter the S845 phosphorylation induced by the two agonists. S831 again was insensitive to all drug treatments. These data support the positive linkage from D1Rs to S845 in the striatum. As to D2Rs, blocking them with eticlopride may release the D2R inhibition of PKA and thus enhance S845 phosphorylation.
Figure 2. Effects of the D1R and D2R antagonists on basal and SKF81297/quinpirole-stimulated GluA1 phosphorylation in the rat striatum.

(A) Representative immunoblots showing effects of SCH23390 and eticlopride on basal and stimulated GluA1 phosphorylation in the striatum. (B-D) Quantification of effects of SCH23390 and eticlopride on basal and stimulated GluA1 phosphorylation at S845 (B) and S831 (C) and total GluA1 expression (D). Note that eticlopride elevated pS845 levels and did not affect the S845 phosphorylation induced by coinjected SKF81297 and quinpirole. Rats were given an i.p. injection of SCH23390 (SCH, 0.1-0.5 mg/kg) or eticlopride (Eti, 0.5 mg/kg) 15 min prior to saline (sal) or SKF81297 (SKF)/quinpirole (Quin) at 3 mg/kg and were sacrificed 20 min after the final drug injection for immunoblot analysis. Data are presented as means ± SEM (n = 4 per group). *P < 0.05 versus saline + saline. +P < 0.05 versus saline + SKF81297/quinpirole.
3.2. Muscarinic receptors inhibit GluA1 phosphorylation
The muscarinic M4 receptor (M4R), a major mAChR subtype in the striatum, is predominantly coexpressed with D1Rs in striatonigral MSNs (Ince et al., 1997; Santiago and Potter, 2001). The receptor is negatively coupled to adenylyl cyclase via Gαi/o proteins (Wess, 1996) and thus mediates ACh inhibition of the cAMP-PKA pathway. To determine the role of M4Rs in regulating GluA1 phosphorylation, we monitored the impact of enhanced M4R activity on GluA1 phosphorylation. To selectively enhance M4R activity, we used a PAM in the light of an increasing interest in exploring PAMs as a novel approach for treating schizophrenia and other neuropsychiatric disorders. VU0152100 is a recently developed, systemically active and M4R selective PAM (Brady et al., 2008). We thus investigated its effect on S845 and S831 phosphorylation. We first examined the effect of the PAM on basal GluA1 phosphorylation. A single injection of VU0152100 at 6 or 60 mg/kg (i.p.) did not alter basal pS845 levels in the striatum (Fig. 3A and 3B). No significant effect of VU0152100 on S831 phosphorylation was observed at either dose (Fig. 3A and 3C). The total GluA1 abundance remained unchanged following VU0152100 administration (Fig. 3A and 3D). The high M4R subtype-selective property of VU0152100 was demonstrated in early studies in which the PAM at 56.6 mg/kg (i.p.) potently blocked motor responses to the DA indirect agonist amphetamine in wild type rats and mice but not in M4R knockout mice (Brady et al., 2008; Byun et al., 2014). Thus, the M4R PAM has a limited impact on constitutive GluA1 phosphorylation in striatal neurons under normal conditions.
Figure 3. Effects of the M4R PAM on basal GluA1 phosphorylation in the rat striatum.

(A) Representative immunoblots showing effects of VU0152100 on basal GluA1 phosphorylation in the striatum. (B-D) Quantification of effects of VU0152100 on basal GluA1 phosphorylation at S845 (B) and S831 (C) and total GluA1 expression (D). Rats were given an i.p. injection of vehicle or VU0152100 (VU) at 6 or 60 mg/kg and were sacrificed 20 min after drug injection for immunoblot analysis. Data are presented as means ± SEM (n = 4 per group).
We next evaluated the impact of the PAM on phasic GluA1 phosphorylation. To do this, we subjected rats to an injection of VU0152100 (60 mg/kg) 20 min prior to SKF81297 (3 mg/kg) and sacrificed rats 20 min after SKF81297 administration. Remarkably, VU0152100 significantly reduced pS845 levels induced by SKF81297 in the striatum (Fig. 4A and 4B). Neither VU0152100 nor SKF81297 injected alone or together altered S831 phosphorylation (Fig. 4A and 4C) or total GluA1 expression (Fig. 4A and 4D). Thus, enhancing the cholinergic tone on M4Rs limits the S845 response to D1R signals.
Figure 4. Effects of the M4R PAM on the D1R agonist-stimulated GluA1 phosphorylation in the rat striatum.

(A) Representative immunoblots showing effects of VU0152100 on SKF81297-stimulated GluA1 phosphorylation in the striatum. (B-D) Quantification of effects of VU0152100 on SKF81297-stimulated GluA1 phosphorylation. Note that VU0152100 significantly reduced the SKF81297-stimulated S845 phosphorylation. Rats were given an i.p. injection of vehicle (Veh) or VU0152100 (VU, 60 mg/kg) 20 min prior to saline (sal) or SKF81297 (SKF, 3 mg/kg) and were sacrificed 20 min after the final drug injection for immunoblot analysis. Data are presented as means ± SEM (n = 4 per group). *P < 0.05 versus vehicle + saline. +P < 0.05 versus vehicle + SKF81297.
Coadministered quinpirole was hypothesized to reduce the degree of an increase in the D1R agonist-stimulated release of the endogenous ligand of M4Rs (i.e., ACh) and thereby reduce the cholinergic inhibition of D1R signaling, leading to an augmented efficacy of D1Rs in stimulating S845 phosphorylation (see above Fig. 1). To clarify this hypothesis and to explore the role of M4Rs in this process, we tested the effect of selective enhancement of M4R activity on the D1R/D2R synergy. Remarkably, adding an exogenous PAM of M4Rs resumed the cholinergic inhibition of D1Rs. As shown in Fig. 5A and 5B, in rats pretreated with VU0152100 (60 mg/kg), SKF81297/quinpirole coadministration (3 mg/kg, 20 min after VU0152100) produced a less increase in pS845 levels in the striatum. No change in pS831 and GluA1 levels was observed after all drug treatment (Fig. 5C and 5D).
Figure 5. Effects of the M4R PAM on the D1R/D2R agonists-stimulated GluA1 phosphorylation in the rat striatum.

(A) Representative immunoblots showing effects of VU0152100 on SKF81297/quinpirole-stimulated GluA1 phosphorylation in the striatum. (B-D) Quantification of effects of VU0152100 on SKF81297/quinpirole-stimulated GluA1 phosphorylation. Note that VU0152100 markedly reduced the SKF81297/quinpirole-stimulated S845 phosphorylation. Rats were given an i.p. injection of vehicle (Veh) or VU0152100 (VU, 60 mg/kg) 20 min prior to SKF81297 (SKF)/quinpirole (Quin) at 3 mg/kg and were sacrificed 20 min after the final drug injection for immunoblot analysis. Data are presented as means ± SEM (n = 4 per group). *P < 0.05 versus vehicle + saline. +P < 0.05 versus vehicle + SKF81297/quinpirole.
We then examined whether a mAChR antagonist could augment the efficacy of D1Rs in stimulating S845 phosphorylation in the striatum. Administration of the mAChR antagonist scopolamine (5 mg/kg, i.p.) increased the basal level of pS845 (Fig. 6A and 6B). Scopolamine also significantly enhanced the elevation of pS845 induced by SKF81297 (1 mg/kg, i.p.). Moreover, in the presence of scopolamine, quinpirole did not further enhance the S845 phosphorylation response to SKF81297 (scopolamine + SKF81297 versus scopolamine + SKF81297/quinpirole). All drug treatments did not alter S831 phosphorylation (Fig. 6A and 6C) and total GluA1 expression (Fig. 6A and 6D). Thus, blocking the cholinergic tone on mAChRs by scopolamine potentiates the D1R-regulated S845 responses. Meanwhile, since quinpirole no longer augmented the SKF81297-stimulated S845 phosphorylation after mAChRs are blocked by scopolamine, the D2R agonist is believed to inhibit the scopolamine-sensitive and mAChR-mediated cholinergic transmission to exert its effect.
Figure 6. Effects of scopolamine on GluA1 S845 phosphorylation induced by SKF81297 and by co-administration of SKF81297 and quinpirole in the rat striatum.
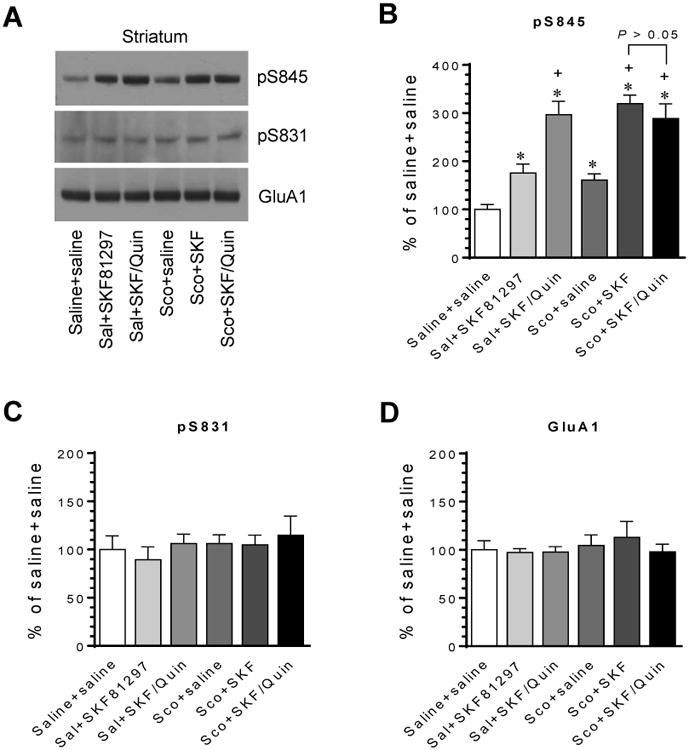
(A) Representative immunoblots showing effects of scopolamine on SKF81297- and SKF81297/quinpirole-stimulated GluA1 phosphorylation in the striatum. (B-D) Quantification of effects of scopolamine on SKF81297- and SKF81297/quinpirole-stimulated GluA1 phosphorylation. Note that either scopolamine or quinpirole significantly augmented the SKF81297-stimulated S845 phosphorylation. Moreover, in the presence of scopolamine, quinpirole did not further augment the response of S845 phosphorylation to SKF81297. Rats were given an i.p. injection of saline (Sal) or scopolamine (Sco, 5 mg/kg) 10 min prior to SKF81297 (SKF, 1 mg/kg) or co-administration of SKF81297 (1 mg/kg) and quinpirole (Quin, 1.5 mg/kg) and were sacrificed 20 min after the final drug injection for immunoblot analysis. Data are presented as means ± SEM (n = 5 per group). *P < 0.05 versus saline + saline. +P < 0.05 versus saline + SKF81297.
3.3. Dopamine and ACh regulate trafficking of AMPARs and PKA in vivo
S845 phosphorylation may trigger GluA1 trafficking and redistribute the modified receptors in synaptic and extrasynaptic locations (Lu and Roche, 2012; Wang et al., 2014). We have observed that D1R and D2R coactivation induced robust S845 phosphorylation. To determine whether elevated S845 phosphorylation can translate to subsequent recruitments of AMPARs into synaptic and/or extrasynaptic compartments, we analyzed changes in the abundance of AMPARs in synaptic versus extrasynaptic locations following coadministration of D1R and D2R agonists. In the striatum, coinjected SKF81297 and quinpirole (3 mg/kg, 90 min prior to tissue collection) did not alter the amount of GluA1, GluA2, and GluA3 in the synaptic fraction (Fig. 7A). A moderate increase in synaptic pS845 signals was seen in drug-treated rats compared to saline-treated rats, while pS831 levels were not altered. In the extrasynaptic fraction, GluA1 was significantly elevated after SKF81297 and quinpirole coadministration (Fig. 7B). GluA2 showed an insignificant change. GluA3 seemed to show an increase, although it did not reach a significant level. A parallel increase in pS845 was seen in the extrasynaptic fraction. The fact that the magnitude of this increase is much greater than that seen in the synaptic location is noteworthy. There was no change in pS831 in the extrasynaptic location. These results reveal a model that D1R and D2R activation recruits S845-phosphorylated and total GluA1 proteins to the extrasynaptic region in striatal neurons in vivo.
Figure 7. Effects of coactivation of D1Rs and D2Rs on subsynaptic redistribution of AMPARs and PKA in the rat striatum.

(A) Effects of SKF81297/quinpirole on GluA1 and PKA expression in the synaptic fraction. (B) Effects of SKF81297/quinpirole on GluA1 and PKA expression in the extrasynaptic fraction. Representative immunoblots are shown left to the quantified data. Rats were given an injection of saline or a coinjection of SKF81297 (SKF) and quinpirole (Quin) at 3 mg/kg (i.p.) and were sacrificed 90 min after drug injection for enriching synaptic and extrasynaptic membranes. Note that SKF81297/quinpirole increased the abundance of pS845 proteins in the synaptic fraction and pS845, GluA1, PKA Cα, and PKA RIIβ proteins in the extrasynaptic fraction, while two agonists had no significant effect on GluA2, GluA3, and pS831 levels in either subsynaptic region. Data are presented as means ± SEM (n = 5 per group). *P < 0.05 versus saline.
Since S845 is a PKA site, we monitored the abundance of PKA in confined synaptic and extrasynaptic pools. Two catalytic (C) and two regulatory (R) subunits are known to form a functional PKA tetramer (Brandon et al., 1997). Among PKA subunits, PKA Cα and RIIβ are the prime subunits present within synapses of cortical neurons (Qiu et al., 2014). We then focused on these two subunits. SKF81297 and quinpirole (3 mg/kg, 90 min) did not alter the abundance of the two subunits in the synaptic fraction of striatal neurons (Fig. 7A). However, extrasynaptic levels of both were elevated (Fig. 7B). Thus, PKA Cα and RIIβ, like their substrate GluA1, are accumulated to the extrasynaptic site following dopamine stimulation.
4. Discussion
In this study, important findings include: 1) the D1R agonist and antagonist increased and decreased S845 phosphorylation, respectively, 2) the D2R agonist had a minimal effect on, while the D2R antagonist elevated, S845 phosphorylation, 3) D1R and D2R coactivation synergistically increased S845 phosphorylation, 4) the D1R antagonist blocked the effect of D1R and D2R coactivation on S845 phosphorylation, while the D2R antagonist did not, 5) the M4R PAM reduced the D1R-upregulated S845 phosphorylation, 6) the M4R PAM also reduced the synergistic S845 phosphorylation induced by D1R and D2R coactivation, 7) the mAChR antagonist potentiated the efficacy of D1Rs in stimulating S845 phosphorylation, and 8) the D2R agonist lost its ability to augment the D1R agonist-stimulated S845 phosphorylation after mAChRs are blocked. These results provide a set of solid evidence collectively supporting a model of D1R-D2R segregation (Albin et al., 1989) and an existence of an intrinsic ACh-M4R component in balancing DA (Di Chiara et al., 1994). Specifically, D1Rs and D2Rs are primarily separated onto two subpopulations of MSNs with M4Rs coexpressed in striatonigral neurons (Ince et al., 1997; Santiago and Potter, 2001). Under normal conditions, antagonistic D1Rs and M4Rs converge onto PKA to form a dynamic balance controlling PKA-mediated S845 phosphorylation in striatonigral neurons. In response to SKF81297, D1Rs outweigh M4Rs and shift the balance towards to a higher level of PKA activity and thus enhanced S845 phosphorylation. Meanwhile, as a compensatory mechanism, SKF81297 increases local Ach release from an internal source, i.e., cholinergic interneurons (DeBoer and Abercrombie, 1996; Acquas and Di Chiara, 2001), to form an activity-dependent and M4R-mediated inhibition of D1R-mediated responses. Quinpirole, on the other hand, activates presynaptic D2R heteroreceptors on cholinergic terminals to reduce the degree of the D1R agonist-stimulated ACh release from cholinergic interneurons (Bertorelli and Consolo, 1990; Robertson et al., 1993; Brooks et al., 2007). This reduces the M4R-mediated inhibitory drive and enables D1Rs to phosphorylate S845 to a greater extent, establishing an M4R-dependent mechanism underlying the D1R/D2R synergy in stimulating S845 phosphorylation. As a result, exogenously applying an M4R potentiator VU0152100 could enhance the M4R inhibition and reduce the D1R/D2R synergy. In addition, D2R blockade increased S845 phosphorylation. This may occur primarily in striatopallidal neurons due to releasing D2R inhibition of PKA. Of note, the responsive pattern of S845 phosphorylation remarkably resembles the response of immediate early gene c-fos/c-Fos (often used as a functional marker of neuronal activation) and opioid peptides to dopamine and ACh (Wang and McGinty, 1996a; 1996b; Robertson et al., 1992; Bernard et al., 1993; Gerfen et al., 1995; Keefe and Gerfen, 1995; Steiner and Gerfen, 1998). Thus, S845 phosphorylation, like c-Fos expression, may serve as a biochemical marker of MSN activation (see below). Nevertheless, the cell-type specific events described above need to be experimentally proven through use of a cell-type-specific approach in the future.
MSNs are excited by incoming glutamatergic projections and, at excitatory synapses, postsynaptic AMPARs in MSNs mediate the fast synaptic transmission. DA and ACh, on the other hand, are modulatory in nature and act indirectly by setting the excitability of MSNs to incoming glutamatergic input (Di Chiara et al., 1994). According to the most widely circulated model, D1Rs set striatonigral neurons in a more depolarized ‘UP’ state (i.e., more excitable), whereas D2Rs promote striatopallidal neurons in a more hyperpolarized ‘DOWN’ state (less excitable) (Albin et al., 1989; Surmeier et al., 2007). ACh in these activities acts as a central balancer antagonistically modulating D1R and D2R signaling (Di Chiara et al., 1994). At the postreceptor level, PKA serves as a key mediator for the DA-ACh interplay. Through PKA and its downstream effectors, DA and ACh modulate the MSN excitability in a cell type-specific manner. For instance, PKA phosphorylates GluA1 S845, a posttranslational modification that increases the responsiveness of GluA1 AMPARs (Lu and Roche, 2012; Wang et al., 2014). Thus, DA and ACh through PKA modulate and determine the responsiveness of AMPARs at excitatory synapses to incoming phasic excitatory input. As such, the S845 phosphorylation level seems to closely reflect the degree of the excitability of neurons. Thus, S845 phosphorylation could serve as a sensitive, reliable, and easily measurable marker for inducible changes in the MSN excitability in adult rat brains in vivo.
Protein phosphorylation represents an important posttranslational modification of subcellular and subsynaptic distribution of glutamate receptors (Wang et al., 2014). S845 phosphorylation may affect subsynaptic redistribution of GluA1 in an activity-dependent manner (Lu and Roche, 2012; Wang et al., 2014). Indeed, we found that coactivation of D1Rs and D2Rs increased the number of GluA1 at extrasynaptic sites in striatal neurons. This change may constitute a biochemical basis for the dopamine regulation of GluA1 homomeric receptors and for long-term changes in AMPAR plasticity in response to dopamine stimulation.
A dopamine (DA) D1 receptor agonist elevates GluA1 phosphorylation in the striatum.
DA D2 receptors augment efficacy of the D1 receptor agonist.
A positive modulator of muscarinic M4 receptors (M4R) inhibits D1/D2 synergy.
D1/D2 synergy determines the number of GluA1 and PKA at extrasynaptic sites.
Acknowledgments
This work was supported by NIH grants R01DA10355 (JQW) and R01MH61469 (JQW). Authors thank Chad W. Kaplan for his assistance.
Footnotes
Chemical compounds studied in this article: SKF81297 (PubChem CID: 1218); (-)-quinpirole hydrochloride (PubChem CID: 55397); SCH23390 (PubChem CID: 5018); S-(-)-eticlopride hydrochloride (PubChem CID: 11973707); VU0152100 (PubChem CID: 864492); (-)-scopolamine hydrobromide (PubChem CID: 517999)
Conflict of interest: The authors declare that they have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acquas E, Di Chiara G. Role of dopamine D1 receptors in the control of striatal acetylcholine release by endogenous dopamine. Neurol Sci. 2001;22:41–42. doi: 10.1007/s100720170037. [DOI] [PubMed] [Google Scholar]
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- Aubert I, Ghorayeb I, Normand E, Bloch B. Phenotypical characterization of the neurons expressing the D1 and D2 dopamine receptors in the monkey striatum. J Comp Neurol. 2000;418:22–32. [PubMed] [Google Scholar]
- Barria A, Derkach V, Soderling T. Identification of the Ca2+/calmodulin-dependent protein kinase II regulatory phosphorylation site in the α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate-type glutamate receptor. J Biol Chem. 1997;272:32727–32730. doi: 10.1074/jbc.272.52.32727. [DOI] [PubMed] [Google Scholar]
- Bernard V, Dumartin B, Lamy E, Bloch B. Fos immunoreactivity after stimulation or inhibition of muscarinic receptors indicates anatomical specificity for cholinergic control of striatal efferent neurons and cortical neurons in the rat. Eur J Neurosci. 1993;5:1218–1225. doi: 10.1111/j.1460-9568.1993.tb00976.x. [DOI] [PubMed] [Google Scholar]
- Bernard V, Somogyi P, Bolam JP. Cellular, subcellular, and subsynaptic distribution of AMPA-type glutamate receptor subunits in the neostriatum of the rats. J Neurosci. 1997;17:819–833. doi: 10.1523/JNEUROSCI.17-02-00819.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertorelli R, Consolo S. D1 and D2 dopaminergic regulation of acetylcholine release from striata of freely moving rats. J Neurochem. 1990;54:2145–2148. doi: 10.1111/j.1471-4159.1990.tb04922.x. [DOI] [PubMed] [Google Scholar]
- Bertran-Gonzalez J, Herve D, Girault JA, Valjent E. What is the degree of segregation between striatonigral and striatopallidal projections? Front Neuroanat. 2010;4:136. doi: 10.3389/fnana.2010.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolam JP, Wainer BH, Smith AD. Characterization of cholinergic neurons in the rat neostriatum. A combination of choline acetyltransferase immunocytochemistry, Golgiimpregnation and electron microscopy. Neuroscience. 1984;12:711–718. doi: 10.1016/0306-4522(84)90165-9. [DOI] [PubMed] [Google Scholar]
- Brady AE, Jones CK, Bridges TM, Kennedy JP, Thompson AD, Heiman JU, Breininger ML, Gentry PR, Yin H, Jadhav SB, Shirey JK, Conn PJ, Lindsley CW. Centrally active allosteric potentiators of the M4 muscarnic acetylcholine receptor reverse amphetamine-induced hyperlocomotor activity in rats. J Pharmacol Exp Ther. 2008;327:941–953. doi: 10.1124/jpet.108.140350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon EP, Idzerda RL, McKnight GS. PKA isoforms, neural pathways, and behaviour: making the connection. Curr Opin Neurobiol. 1997;7:397–403. doi: 10.1016/s0959-4388(97)80069-4. [DOI] [PubMed] [Google Scholar]
- Brooks J, Sarter M, Bruno JP. D2-like receptors in nucleus accumbens negatively modulate acetylcholine release in prefrontal cortex. Neuropharmacology. 2007;53:455–463. doi: 10.1016/j.neuropharm.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byun NE, Grannan M, Bubser M, Barry RL, Thompson A, Rosanelli J, Gowrishankar R, Kelm ND, Damon S, Bridges TM, Melancon BJ, Tarr JC, Brogan JT, Avison MJ, Deutch AY, Wess J, Wood MR, Lindsley CW, Gore JC, Conn PJ, Jones CK. Antipsychotic drug-like effects of the selective M4 muscarinic acetylcholine receptor positive allosteric modulator VU0152100. Neuropsychopharmacology. 2014;39:1578–1593. doi: 10.1038/npp.2014.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao SZ, Lu W, Lee HK, Huganir RL, Wolf ME. D(1) dopamine receptor stimulation increases GluR1 phosphorylation in postnatal nucleus accumbens cultures. J Neurochem. 2002;81:984–992. doi: 10.1046/j.1471-4159.2002.00877.x. [DOI] [PubMed] [Google Scholar]
- Chou H, Ogawa N, Asanuma M, Hirata H, Mori A. Muscarinic cholinergic receptor-mediated modulation on striatal c-fos mRNA expression induced by levodopa in rat brain. J Neural Transm. 1992;90:171–181. doi: 10.1007/BF01250959. [DOI] [PubMed] [Google Scholar]
- DeBoer P, Abercrombie ED. Physiological release of striatal acetylcholine in vivo: modulation by D1 and D2 dopamine receptor subtypes. J Pharmacol Exp Ther. 1996;277:775–783. [PubMed] [Google Scholar]
- Di Chiara G, Morelli M, Consolo S. Modulatory functions of neurotransmitters in the striatum: ACh/dopamine/NMDA interactions. Trends Neurosci. 1994;17:228–233. doi: 10.1016/0166-2236(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ, Jr, Sibley DR. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250:1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Keefe KA, Gauda EB. D1 and D2 dopamine receptor function in the striatum: coactivation of D1- and D2-dopamine receptors on separate populations of neurons results in potentiated immediate early gene response in D1-containing neurons. J Neurosci. 1995;15:8167–8176. doi: 10.1523/JNEUROSCI.15-12-08167.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greger IH, Ziff EB, Penn AC. Molecular determinants of AMPA receptor subunit assembly. Trends Neurosci. 2007;30:407–416. doi: 10.1016/j.tins.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Ince E, Ciliax BJ, Levey AI. Differential expression of D1 and D2 dopamine and m4 muscarinic acetylcholine receptor proteins in identified striatonigral neurons. Synapse. 1997;27:357–366. doi: 10.1002/(SICI)1098-2396(199712)27:4<357::AID-SYN9>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Jin DZ, Guo ML, Xue B, Fibuch EE, Choe ES, Mao LM, Wang JQ. Phosphorylation and feedback regulation of metabotropic glutamate receptor 1 by calcium/calmodulin-dependent protein kinase II. J Neurosci. 2013;33:3402–3412. doi: 10.1523/JNEUROSCI.3192-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe KA, Gerfen CR. D1-D2 dopamine receptor synergy in striatum: effects of intrastriatal infusions of dopamine agonists and antagonists on immediate early gene expression. Neuroscience. 1995;66:903–913. doi: 10.1016/0306-4522(95)00024-d. [DOI] [PubMed] [Google Scholar]
- Kondo M, Okabe S, Sumino R, Okado H. A high GluR1:GluR2 expression ratio is correlated with expression of Ca2+-binding proteins in rat forebrain neurons. Eur J Neurosci. 2000;12:2812–2822. doi: 10.1046/j.1460-9568.2000.00167.x. [DOI] [PubMed] [Google Scholar]
- Lu W, Roche KW. Posttranslational regulation of AMPA receptor trafficking and function. Curr Opin Neurobiol. 2012;22:470–479. doi: 10.1016/j.conb.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao LM, Reusch JM, Fibuch EE, Liu Z, Wang JQ. Amphetamine increases phosphorylation of MAPK/ERK at synaptic sites in the rat striatum and medial prefrontal cortex. Brain Res. 2013;1494:101–108. doi: 10.1016/j.brainres.2012.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammen AL, Kameyama K, Roche KW, Huganir RL. Phosphorylation of the alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor GluR1 subunit by calcium/calmodulin-dependent kinase II. J Biol Chem. 1997;272:32528–32533. doi: 10.1074/jbc.272.51.32528. [DOI] [PubMed] [Google Scholar]
- Morelli M, Fenu S, Cozzolino A, Pinna A, Carta A, Di Chiara G. Blockade of muscarinic receptors potentiates D1 dependent turning behavior and c-fos expression in 6-hydroxydopamine-lesioned rats but does not influence D2 mediated response. Neuroscience. 1993;53:673–678. doi: 10.1016/0306-4522(93)90615-m. [DOI] [PubMed] [Google Scholar]
- Neve KA, Seamans JK, Trantham-Davidson H. Dopamine receptor signaling. J Recept Signal Transduct Res. 2004;24:165–205. doi: 10.1081/rrs-200029981. [DOI] [PubMed] [Google Scholar]
- Onn SP, West AR, Grace AA. Dopamine-mediated regulation of striatal neuronal and network interactions. Trends Neurosci. 2000;23:S48–56. doi: 10.1016/s1471-1931(00)00020-3. [DOI] [PubMed] [Google Scholar]
- Phelps PE, Houser CR, Vaughn JE. Immunocytochemical localization of choline acetyltransferase within the rat neostriatum: a correlated light and electron microscopic study of cholinergic neurons and synapses. J Comp Neurol. 1985;238:286–307. doi: 10.1002/cne.902380305. [DOI] [PubMed] [Google Scholar]
- Price CJ, Kim P, Raymond LA. D1 dopamine receptor-induced cyclic AMP dependent protein kinase phosphorylation and potentiation of striatal glutamate receptors. J Neurochem. 1999;73:2441–2446. doi: 10.1046/j.1471-4159.1999.0732441.x. [DOI] [PubMed] [Google Scholar]
- Qiu S, Zhang M, Liu Y, Guo Y, Zhao H, Song Q, Zhao M, Huganir RL, Luo J, Xu H, Zhuo M. GluA1 phosphorylation contributes to postsynaptic amplification of neuropathic pain in the insular cortex. J Neurosci. 2014;34:13505–13515. doi: 10.1523/JNEUROSCI.1431-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimers JM, Milovanovic M, Wolf ME. Quantitative analysis of AMPA receptor subunit composition in addiction-related brain regions. Brain Res. 2011;1367:223–233. doi: 10.1016/j.brainres.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson GS, Tham CS, Wilson C, Jakubovic A, Fibiger HC. In vivo comparison of the effects of quinpirole and putative presynaptic dopaminergic agonists G-HT 920 and SND 919 on striatal dopamine and acetylcholine release. J Pharmacol Exp Ther. 1993;264:1344–1351. [PubMed] [Google Scholar]
- Robertson GS, Vincent SR, Fibiger HC. D1 and D2 dopamine receptors differentially regulate c-fos expression in striatonigral and striatopallidal neurons. Neuroscience. 1992;49:285–296. doi: 10.1016/0306-4522(92)90096-k. [DOI] [PubMed] [Google Scholar]
- Roche KW, O'Brien RJ, Mammen AL, Bernhardt J, Huganir RL. Characterization of multiple phosphorylation sites on the AMPA receptor GluR1 subunit. Neuron. 1996;16:1179–1188. doi: 10.1016/s0896-6273(00)80144-0. [DOI] [PubMed] [Google Scholar]
- Santiago MP, Potter LT. Biotinylated m4-toxin demonstrates more M4 muscarinic receptor protein on direct than indirect striatal projection neurons. Brain Res. 2001;894:12–20. doi: 10.1016/s0006-8993(00)03170-x. [DOI] [PubMed] [Google Scholar]
- Serulle Y, Zhang S, Ninan I, Puzzo D, McCarthy M, Khatri L, Arancio O, Ziff EB. A GluR1-cGKII interaction regulates AMPA receptor trafficking. Neuron. 2007;56:670–688. doi: 10.1016/j.neuron.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder GL, Allen PB, Fienberg AA, Valle CG, Huganir RL, Nairn AC, Greengard P. Regulation of phosphorylation of the GluR1 AMPA receptor in the neostriatum by dopamine and psychostimulants in vivo. J Neurosci. 2000;20:4480–4488. doi: 10.1523/JNEUROSCI.20-12-04480.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner H, Gerfen CR. Role of dynorphin and encephalin in the regulation of striatal output pathways and behavior. Exp Brain Res. 1998;123:60–76. doi: 10.1007/s002210050545. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Ding J, Day M, Wang Z, Shen W. D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci. 2007;30:228–235. doi: 10.1016/j.tins.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Swayze RD, Lise MF, Levinson JN, Phillips A, El-Husseini A. Modulation of dopamine mediated phosphorylation of AMPA receptors by PSD-95 and AKAP79/150. Neuropharmacology. 2004;47:764–778. doi: 10.1016/j.neuropharm.2004.07.014. [DOI] [PubMed] [Google Scholar]
- Van Dolah DK, Mao LM, Shaffer C, Guo ML, Fibuch EE, Chu XP, Buch S, Wang JQ. Reversible palmitoylation regulates surface stability of AMPA receptors in the nucleus accumbens in response to cocaine in vivo. Biol Psychiatry. 2011;69:1035–1042. doi: 10.1016/j.biopsych.2010.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JQ, McGinty JF. Differential effects of D1 and D2 dopamine receptor antagonists on acute amphetamine- or methamphetamine-induced up-regulation of zif/268 mRNA expression in rat forebrain. J Neurochem. 1995;65:2706–2715. doi: 10.1046/j.1471-4159.1995.65062706.x. [DOI] [PubMed] [Google Scholar]
- Wang JQ, Guo ML, Jin DZ, Xue B, Fibuch EE, Mao LM. Roles of subunit phosphorylation in regulating glutamate receptor function. Eur J Pharmacol. 2014;728:183–187. doi: 10.1016/j.ejphar.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JQ, McGinty JF. Scopolamine augments c-fos and zif/268 messenger RNA expression induced by the full D1 dopamine receptor agonist SKF-82958 in the intact rat striatum. Neuroscience. 1996a;72:601–616. doi: 10.1016/0306-4522(95)00597-8. [DOI] [PubMed] [Google Scholar]
- Wang JQ, McGinty JF. Muscarinic receptors regulate striatal neuropeptide gene expression in normal and amphetamine-treated rats. Neuroscience. 1996b;75:43–56. doi: 10.1016/0306-4522(96)00277-1. [DOI] [PubMed] [Google Scholar]
- Wang JQ, McGinty JF. Intrastriatal injection of a muscarinic receptor agonist and antagonist regulates striatal neuropeptide mRNA expression in normal and amphetamine-treated rats. Brain Res. 1997;748:62–70. doi: 10.1016/s0006-8993(96)01244-9. [DOI] [PubMed] [Google Scholar]
- Wess J. Molecular biology of muscarinic acetylcholine receptors. Crit Rev Neurobiol. 1996;10:69–99. doi: 10.1615/critrevneurobiol.v10.i1.40. [DOI] [PubMed] [Google Scholar]


