Abstract
Purpose of review
The modern antiretroviral therapy (ART) era has seen substantial reductions in mortality among people living with HIV. However, HIV-positive people who inject drugs (PWIDs) continue to experience high rates of suboptimal HIV-related outcomes. We review recent findings regarding factors contributing to premature and preventable mortality among HIV-positive PWID, and describe the promise of interventions to improve survival in this group.
Recent findings
The current leading causes of death among HIV-positive PWID are HIV/AIDS-related causes, overdose, and liver-related causes, including infection with hepatitis C virus. Elevated mortality levels in this population are driven by social–structural barriers to ART access and adherence, particularly criminalization and stigmatization of drug use. In contexts where opioid substitution therapy and ART adherence support programs are widely accessible, evidence highlights comparable levels of survival among HIV-positive PWID and people living with HIV who do not inject drugs.
Summary
The life-saving benefits of ART can be realized among HIV-positive PWID when it is paired with strategies that address barriers to evidence-based medical care. Joint administration of ART and opioid substitution therapy, as well as repeal of punitive laws that criminalize drug users, are urgently needed to reduce HIV and injection-related mortality among PWID.
Keywords: antiretroviral therapy, HIV, injection drug use, mortality, survival
INTRODUCTION
The widespread rollout of combination antiretro- viral therapy (ART) has markedly altered the natural history of HIV infection. Improved tolerability, safety, and efficacy of therapies, in addition to scale-up of strategies to promote access and adherence to ART, have led to dramatic declines in all-cause mortality of people living with HIV (PLHIV) across a variety of settings and among diverse populations [1**,2,3**,4].
Unfortunately, the gains made in the modern ART era have not fully extended to all PLHIV, including many of the estimated 1.7 million HIV- positive people who inject drugs (PWIDs) worldwide [5]. Despite advances in availability and tolerability of treatment, mortality remains high among HIV- positive PWID when compared with other key populations of PLHIV, in low-, middle-, and high-income settings [6–9,10*,11,12,13*,14,15,16*,17–23]. For example, across the 16 European and North American cohorts in the Antiretroviral Therapy Cohort Collaboration, which comprise 32 703 HIV-positive adults who started ART between 2000 and 2009, all- cause mortality among people with a history of injection drug use (IDU) was more than twice that of individuals with no history of IDU [10*]. Such stark inequities are especially troubling in the context of healthcare settings with well established ART delivery systems, programming, and supports for individuals who experience barriers to treatment adherence.
As the estimates of lower life expectancy among PWID are frequently grounded in individual-level analyses, most research cites IDU as a risk factor for premature mortality because of higher levels of various health and social harms (e.g., higher prevalence of comorbidities [24*] and injection-related illnesses, greater socioeconomic disadvantage [25*], inferior contact with the healthcare system [9,11,26]). However, the influence of sociostructural factors on mortality of PWID in the era of modern ART remains relatively under-researched. We review recent research that identifies factors contributing to premature and preventable mortality of HIV- positive PWID, drawing attention to the dearth of research on the social, systemic, and structural barriers that drive these inequities, and describe the promise of interventions to remove such obstacles and improve survival in this group.
HIV/AIDS-RELATED MORTALITY IN THE MODERN TREATMENT ERA
Given high levels of adherence, ART suppresses HIV to undetectable levels in plasma, restoring immune function and preventing progression to death [27], as well as secondarily preventing HIV transmission [28]. Accordingly, the expansion of ART has led to important shifts in the causes of death in comparison with deaths preceding the widespread availability of ART. Recent data from The Joint United Nations Programme on HIV/AIDS described a 19% decline in AIDS-related deaths from 2011 to 2014, and a 35% decline from 2005 to 2014 [1**]. Among HIV-positive PWID however, the leading cause of death remains HIV/AIDS-related causes [2,3**,7,29,30,31*,32–34,35*,36**,37], reflecting suboptimal levels of ART access, and adherence in this population.
Low levels of ART coverage among PWID populations is a concern in both low and high-income settings worldwide, with only an estimated one in 10 HIV-positive PWID on ART [1**]. A review of care and treatment targets for HIV-positive PWID in six countries that account for half of the global PWID population demonstrated staggering short- falls in ART access among this population. The review noted increasing ART coverage in Malaysia, China, Ukraine, and Vietnam; however, policies governing care and treatment of HIVpositive PWID in the United States and Russia have not shifted. For example, Russia’s most recent update demonstrates approximately 1% of all HIV-positive PWID were accessing ART in 2010. The deficiencies in ART coverage across these countries were driven largely by macrolevel barriers, such as funding gaps and policies, deterring effective HIV prevention, and treatment for PWID [38]. Additionally, criminalization of drug use in these and other settings [39] continues to clash with public health policies aimed at improving access to HIV prevention and treatment coverage.
Moreover, many studies describe suboptimal ART adherence among PWID [40] despite wide- spread evidence that PWID are able to achieve the same levels of adherence as PLHIV who do not inject drugs [41], suggesting that both social and policy- level barriers to optimal ART adherence among this group persist. For example, research has shown that physicians often defer ART initiation for some PWID because of concerns of a so-called unstable lifestyle and competing priorities that could lead to noncompliance, and potentially the emergence and transmission of drug-resistant HIV [38]. Such concerns have subsequently contributed to lower levels of access to treatment among HIV-positive PWID [42], thus contributing to higher mortality rates in this group.
Additionally, PWID systemically experience disproportionate levels of homelessness [9,25*,29], untreated mental health comorbidities [37], polysubstance use [32] (including alcohol use [33]), and lower levels of healthcare engagement [9], all of which shape access and adherence to ART, as well as progression to AIDS and death. Additionally, incarceration [1**,43] and personal violence [3**,7,10*] remain persistent issues among HIV-positive PWID, with research demonstrating increased rates of suicides [7,44] being independently associated with a history of IDU (adjusted hazard ratio: 3.95; confidence interval: 1.99 – 7.86) [44]. Studies conducted in settings without universal healthcare may not be able to distinguish the effect of the aforementioned factors independent of the influence of financial need. However, Joseph and colleagues [45] demonstrated that even in a setting with free access to HIV care, becoming non-adherent was associated with periods of homelessness, active IDU, and incarceration. In the same setting, Zivanovic and colleagues [25*] found that after adjusting for confounders such as HIV infection and drug use patterns, housing instability was independently associated with all-cause mortality in a cohort of PWID. This research highlights the need to consider contextual factors in the design of HIV care and treatment to PWID beyond treatment access, extending to the provision of basic subsistence needs, including supportive housing.
Additionally, studies undertaken in healthcare settings with programs that address barriers to ART access and adherence through low-threshold approaches such as maximally assisted therapy and directly administered ART programs provide an opportunity to observe the impact of treatment on survival of PWID. A study of PLHIV on treatment in British Columbia, Canada, where ART treatment and care are provided free of charge, highlighted that cumulative non-accidental mortality rate was not statistically different between individuals with a history of injection compared with non-injectors [46]. These results were echoed in a study from Melbourne, Australia, where PWID on HIV treatment were no more likely to experience AIDS or death than non-injectors [47]. More recently, Hayashi and colleagues [3**] reported rates and predictors of death among HIVpositive PWID in the context of a province-wide treatment as prevention (TasP) initiative aimed at improving access and adherence to ART in British Columbia. The 18-year cohort study of com- munity recruited HIV-positive PWID showed slow declines in HIV-related death in the late 1990s to early 2000s with high overall levels of mortality among PWID. Significant reductions in HIV-related mortality rates were observed among PWID from 2010 onward, coinciding with TasP-based efforts to scale-up treatment in this population [3**]. Such studies add to the growing consensus that where care is taken to address ART access and adherence barriers faced by HIV-positive PWID, this population can experience the same outcomes as non-injecting PLHIV, including improved life expectancy.
DRUG-RELATED FATALITIES
A review examining non-AIDS mortality among HIV-positive and HIV-negative PWID across 42 cohort studies in 18 countries, found that HIV- positive PWID experience greater levels of mortality from non-AIDS-related causes than their HIV- negative counterparts. The authors suggest the need for further research to understand if this finding is driven by HIV-positive PWID’s poorer physical health, increased social disadvantage, or greater likelihood of engaging in higher risk behaviors that may contribute to fatal outcomes, namely, drug overdose [24*]. Indeed, overdose and accidental poisoning have been highlighted in recent scholar- ship as a leading cause of death for HIV-positive PWID [3**,7,11,31*,32–34,35*,36**]. In a prospective multicenter study of HIV and HCV co-infected patients across six Canadian provinces, 18% of the cohort died because of drug overdose during the study period [11].
Evidence-based harm reduction may mitigate risk of death among HIV-positive PWID by reducing fatal overdose, as well as facilitating adherence to life-saving ART [48]. Studies undertaken in Vancouver, Canada, between 1996 and 2011 report a marked decline in rates of all-cause mortality among PWID [3**,29]. For example, Lappalainen and colleagues [29] found that rates of accidental death, inclusive of overdose, fell from 12.79 deaths per 1000 person-years from 1996 to 1999 to 8.56 deaths per 1000 person-years in 2008 – 2011. The shift is credited to significant investments in harm reduction approaches in this setting, including North America’s first legal supervised injection site. Researchers have also hypothesized that positive interactions between clients and staff at harm reduction facilities and shifts in type of drug use might partially explain the declines in drug overdose during the study period [29].
Additionally, several studies have demonstrated the efficacy of pharmacological interventions, such as provision of opioid substitution therapy (OST) [24*,36**], including methadone maintenance therapy [37]. Notably, in a systematic review examining non-AIDS mortality among PWID, non-AIDS crude mortality rates were more than three times greater during periods without OST than during periods with OST, although the authors caution that data on OST coverage are limited [24*]. Nosyk and colleagues showed a significant reduction in risk of death associated with both ART and OST, although the greatest reduction in risk of death was observed when both OST and ART were used together. This study provided compelling evidence of the benefit of combined ART and OST services to protect against HIV-related and drug-related death and death from other causes [36**]. However, the World Drug Report shows that only 79 of 192 countries providing data offered OST [5]. Such evidence highlights the sociostructural factors – including punitive laws, severe human rights abuse, inadequate prevention services, and stigma associated with IDU – impeding access to harm reduction worldwide [1**]. It is important to note that even in settings with access to OST, barriers to initiate OST (e.g., rigid intake criteria and lengthy waitlists) and maintain treatment (e.g., no individualization of treatment and no patient choice on medication and dose) [49] remain, which impacts ART adherence for HIV-positive PWID [48]. In light of the evidence of the efficacy of OST reducing drug-related fatalities, and facilitating access to life-saving ART, these impediments urgently need to be addressed.
ADDRESSING HEPATITIS C VIRUS- RELATED MORTALITY
Research has underscored the impact of liver-related diseases [2,11,30,31*,50], including hepatitis C virus (HCV) [7,34,51], on premature mortality among HIV-positive PWID, with HIV – HCV co-infected individuals having a significantly increased risk of death [11]. A 15-year prospective cohort study investigating predictors of liver-related death in a cohort of PWID in Vancouver showed that HIV seropositivity was independently and positively associated with liver-related mortality [31*]. Notably, individuals who were HIV – HCV co-infected had a 2.53 (95% confidence interval 1.18 – 5.46) times hazard of liverrelated death compared with individuals who were HCV mono-infected. These findings add to the wealth of studies [52] calling for expansion of HCV testing and treatment, specifically among HIV-positive PWID [31*].
In light of the new direct-acting antivirals for HCV treatment, which have the potential to reduce incidence of liver-related disease, the research reviewed here, in addition to lessons leveraged from the early history of ART, may prove pivotal in addressing HCV-related mortality among PWID. First, the various barriers to adherence described above are modifiable because of the development of evidence-based interventions. When these barriers are addressed, PWID – presumed to be ‘hard to reach’ – are able to achieve similar outcomes to people who do not inject drugs. Second, where possible, service integration (e.g., HCV and ART treatment with addictions treatment) facilitates treatment success. Third, addressing stigma in all of its manifestations (e.g., in healthcare settings and in public policy) is critical as individuals who are marginalized are far less likely to access care and treatment.
Although various lessons learned from HIV treatment interventions may be suitable to mitigate HCV-related mortality, possible HCV reinfection often adds to provider concerns around prescribing HCV treatment to PWID; particularly, patients who are likely to continue injecting drugs after clearing HCV. Research on the likelihood of reinfection for PWID is inconclusive, with some studies suggesting reinfection is low among PWID [53], whereas others reporting high reinfection rates among patients who continue to inject during and following HCV treatment [54]. Taken together, this research suggests that in addition to provision of harm reduction programming and OST, there is an urgent need to apply a TasP approach to HCV treatment (i.e., scale-up of prevention and treatment services) to reduce HCV-related mortality among PWID.
FUTURE DIRECTIONS
Understanding the factors that contribute to higher levels of mortality among HIVpositive PWID is instrumental to both the design of targeted interventions that address modifiable barriers to treatment success and to treatment parity with non-injecting PLHIV. This review suggests that recent research on mortality among HIV-positive PWID has not focused on the underlying social– structural drivers of these harms, although these factors have been well characterized in previous research [55]. For example, it is well established that HIV-positive Indigenous PWID are disproportionately impacted by HIV in a variety of settings [56,57]. However, with the exception of one study that observed significant improvements in Indigenous HIV-positive PWID’s HIV treatment and care [58], issues impacting survival in Indigenous communities were not extensively addressed in recent research.
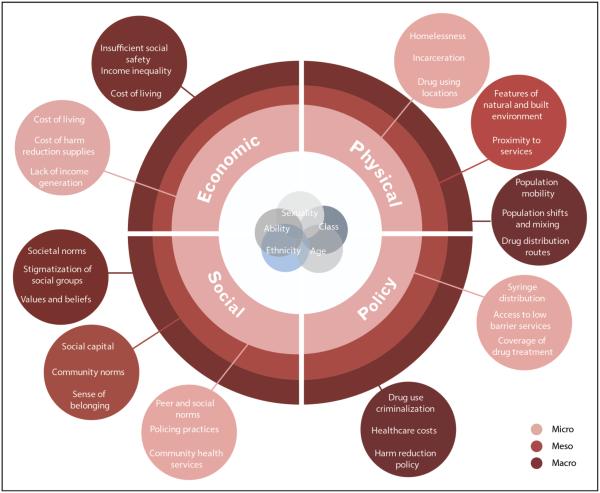
Having identified a need for a social–structural approach to understanding survival of PWID in the modern ART era, we designed a conceptual model adapted from Rhodes’ seminal ‘risk environment’ framework. Rhodes [59] conceptualizes the physical and social spaces in which a multitude of factors exogenous to the individual interact to produce or reduce drug-related harm. He calls for a recognition of the interplay between factors that operate within and across physical, social, economic, and policy environments at the macro, meso, and microlevels [60]. Our proposed framework (Fig. 1) situates the observed elevated levels of mortality among HIV-positive PWID within the various contexts that shape health outcomes, and aims to explain the pervasive inequities that underpin these outcomes. As such, this framework provides a more critical perspective of the broader structural determinants shaping access to ART, harm reduction programming, and basic necessities for individuals to safeguard health and quality of life. Reporting elevated levels of mortality among HIV-positive PWID compared with non-injecting PLHIV without addressing the social – structural factors that shape health outcomes will fail to uncover the root of these inequities. Future research must include improved methods of measuring and reporting the impact of social – structural barriers to treatment, as well as interventions that address basic material needs and rights (e.g., housing support, employment initiatives, nutrition programs, and social sup- port). Moreover, we must continue to examine cause of death, which accurately characterizes the failures of health, social, and legal systems, thus resisting the tendency to downgrade blame to the individual.
FIGURE 1.
Sociostructural factors impacting survival of HIV-positive people who inject drugs.
CONCLUSION
The potential for ART to have far-reaching benefits on the longevity and health for PWID is within our rasp. However, if treatment barriers are left unchecked as ART expansion continues, disparities between HIV-positive PWID and PLHIV who do not inject will be exacerbated, undermining the advances made in the modern ART era. Now is not the time to consider health inequities and treatment barriers as insurmountable, but to document and scale-up evidence-based treatment modalities that are effective in facilitating access and adherence to ART.
KEY POINTS.
Elevated mortality levels among HIV-positive PWID are driven by social–structural barriers to ART access and adherence.
Recent research suggests that ART expansion, paired with evidence-based harm reduction approaches, is associated with markedly improved life expectancy among HIV-positive PWID.
In order for the advances of the modern ART era to benefit HIV-positive PWID, efforts to mitigate social– structural barriers to treatment and harm reduction programming must be expanded, particularly strategies to address criminalization and stigmatization of drug use.
Acknowledgements
We thank Katrina Koehn for research assistance and Leanne Kelly for editorial assistance.
Financial support and sponsorship
M.J.M. is supported, in part, by the NIH [grant number R01-DA021525]. The University of British Columbia has received unstructured funding from NG Biomed, Ltd., to support M.J.M.’s research. J.M.’s TasP research has received support from the BC-Ministry of Health, US NIH (NIDA R01DA036307), UNAIDS, ANRS, and MAC AIDS Fund. Institutional grants have been provided by Abbvie, BMS, Gilead Sciences, J&J, Merck, and ViiV Healthcare. J.M. has served on Advisory Boards for Teva, Gilead Sciences, and InnaVirVax.
Footnotes
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as: * of special interest; ** of outstanding interest
- 1**.Joint United Nations Programme on HIV/AIDS . The Gap Report. UNAIDS; Geneva: 2014. The report presents the sociostructural factors and policy obstacles that impede HIVpositive PWIDs from access to harm reduction, treatment, and prevention services worldwide. [Google Scholar]
- 2.Van Santen DK, vander Helm JJ, Grady BP, et al. Temporal trends in mortality among people who use drugs compared with the general Dutch population differ by hepatitis C virus and HIV infection status. AIDS. 2014;28:2589–2599. doi: 10.1097/QAD.0000000000000450. [DOI] [PubMed] [Google Scholar]
- 3**.Hayashi K, Dong H, Marshall BD, et al. Sex-based differences in rates, causes, and predictors of death among injection drug users in Vancouver, Canada. Am J Epidemiol. 2016;183:544–552. doi: 10.1093/aje/kwv207. Hayashi et al. demonstrated the impact of expanded public health interventions on reducing HIV-related mortality rates, highlighting the need for sex-specific interventions for female injection drug users in an urban Canadian setting. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antiretroviral Therapy Cohort Collaboration Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet. 2008;372:293–299. doi: 10.1016/S0140-6736(08)61113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lemahieu J-L, Me A. World Drug Report. UNODC; Vienna: 2014. [Google Scholar]
- 6.Garriga C, de Olalla Garcia P, Miro JM, et al. Mortality, causes of death and associated factors relate to a large HIV population-based cohort. PLoS One. 2015;10:e0145701. doi: 10.1371/journal.pone.0145701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nambiar D, Weir A, Aspinall EJ, et al. Mortality and cause of death in a cohort of people who had ever injected drugs in Glasgow: 1982–2012. Drug Alcohol Depend. 2015;147:215–221. doi: 10.1016/j.drugalcdep.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 8.Lesko CR, Moore RD, Tong W, Lau B. Association of injection drug use with incidence of HIV-associated non-AIDS-related morbidity by age, 1995–2014. AIDS. 2016 doi: 10.1097/QAD.0000000000001087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liappis AP, Laake AM, Delman M. Active injection drug-abuse offsets health-care engagement in HIV-infected patients. AIDS Behav. 2015;19:81–84. doi: 10.1007/s10461-014-0757-4. [DOI] [PubMed] [Google Scholar]
- 10*.May MT, Justice AC, Birnie K, et al. Injection drug use and hepatitis C as risk factors for mortality in HIV-infected individuals: the antiretroviral therapy cohort collaboration. J Acquir Immune Defic Syndr. 2015;69:348–354. doi: 10.1097/QAI.0000000000000603. The cross-cohort analysis demonstrated that excess mortality in HIV-infected injection drug users is largely explained by hepatitis C infection, and highlight the potential of hepatitis C treatment on mortality rates in HIV–HCV coinfected individuals. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klein MB, Rollet-Kurhajec KC, Moodie EE, et al. Mortality in HIV-hepatitis C co-infected patients in Canada compared to the general Canadian population (2003–2013) AIDS. 2014;28:1957–1965. doi: 10.1097/QAD.0000000000000377. [DOI] [PubMed] [Google Scholar]
- 12.Sheehan DM, Trepka MJ, Fennie KP, et al. Individual and neighborhood predictors of mortality among HIV-positive Latinos with history of injection drug use, Florida, 2000–2011. Drug Alcohol Depend. 2015;154:243–250. doi: 10.1016/j.drugalcdep.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13*.Weber R, Huber M, Battegay M, et al. Influence of non-injecting and injecting drug use on mortality, retention in the cohort, and antiretroviral therapy, in participants in the Swiss HIV Cohort Study. HIV Med. 2015;16:137–151. doi: 10.1111/hiv.12184. The cohort analysis demonstrated how non-injection and IDU are modifiable risks for death, and highlights the need for comprehensive HIV care to prevent and treat these modifiable risk factors. [DOI] [PubMed] [Google Scholar]
- 14.Patterson S, Cescon A, Samji H, et al. Life expectancy of HIV-positive individuals on combination antiretroviral therapy in Canada. BMC Infect Dis. 2015;15:274. doi: 10.1186/s12879-015-0969-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suligoi B, Zucchetto A, Grande E, et al. Risk factors for early mortality after AIDS in the cART era: a population-based cohort study in Italy. BMC Infect Dis. 2015;15:229. doi: 10.1186/s12879-015-0960-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16*.Marcus JL, Chao CR, Leyden WA, et al. Narrowing the gap in life expectancy between HIV-infected and HIV-uninfected individuals with access to care. J Acquir Immune Defic Syndr. 2016 doi: 10.1097/QAI.0000000000001014. Marcus et al. highlight that a gap in survival remains for PLHIV compared with HIVnegative persons, underscoring the need for timely ART initiation and risk-reduction interventions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murray M, Hogg RS, Lima VD, et al. The effect of injecting drug use history on disease progression and death among HIV-positive individuals initiating combination antiretroviral therapy: collaborative cohort analysis. HIV Med. 2012;13:89–97. doi: 10.1111/j.1468-1293.2011.00940.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lima VD, Eyawo O, Ma H, et al. The impact of scaling-up combination antiretroviral therapy on patterns of mortality among HIV-positive persons in British Columbia, Canada. J Int AIDS Soc. 2015;18:20261. doi: 10.7448/IAS.18.1.20261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.An Q, Song R, Hernandez A, Hall HI. Trends and differences among three new indicators of HIV infection progression. Public Health Rep. 2015;130:468–474. doi: 10.1177/003335491513000510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Solomon SS, Mehta SH, Srikrishnan AK, et al. Burden of hepatitis C virus disease and access to hepatitis C virus services in people who inject drugs in India: a cross-sectional study. Lancet Infect Dis. 2015;15:36–45. doi: 10.1016/S1473-3099(14)71045-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tancredi MV, Waldman EA. Survival of AIDS patients in Sao Paulo-Brazil in the pre- and post-HAART eras: a cohort study. BMC Infect Dis. 2014;14:599. doi: 10.1186/s12879-014-0599-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Su S, Chen X, Mao L, et al. Superior effects of antiretroviral treatment among men who have sex with men compared to other HIV at-risk populations in a large cohort study in Hunan, China. Int J Environ Res Publ Health. 2016;13:E283. doi: 10.3390/ijerph13030283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thao VP, Quang VM, Wolbers M, et al. Second-line HIV therapy outcomes and determinants of mortality at the largest HIV referral center in Southern Vietnam. Medicine (Baltimore) 2015;94:e1715. doi: 10.1097/MD.0000000000001715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24*.Mathers BM, Degenhardt L. Examining non-AIDS mortality among people who inject drugs. AIDS. 2014;28(Suppl 4):S435–S444. doi: 10.1097/QAD.0000000000000435. The meta-analysis reviews literature from 42 cohorts of PWID and highlights the need for both a comprehensive response to addressing non-AIDS mortality, as well as additional research on non-AIDS mortality among this population. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25*.Zivanovic R, Milloy MJ, Hayashi K, et al. Impact of unstable housing on all- & cause mortality among persons who inject drugs. BMC Public Health. 2015;15:106. doi: 10.1186/s12889-015-1479-x. Zivanovic et al.’s study of injection drug users in Vancouver, Canada, demonstrated that unstable housing is an important and preventable risk factor for mortality, independent of HIV infection and drug use patterns. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richardson LA, Milloy MJ, Kerr TH, et al. Employment predicts decreased mortality among HIV-seropositive illicit drug users in a setting of universal HIV care. J Epidemiol Community Health. 2014;68:93–96. doi: 10.1136/jech-2013-202918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hogg RS, Heath KV, Yip B, et al. Improved survival among HIV-infected individuals following initiation of antiretroviral therapy. JAMA. 1998;279:450–454. doi: 10.1001/jama.279.6.450. [DOI] [PubMed] [Google Scholar]
- 28.Montaner JSG, Lima VD, Harrigan PR, et al. Expansion of HAART coverage is associated with sustained decreases in HIV/AIDS morbidity, mortality and HIV transmission: the ‘HIV Treatment as Prevention’ experience in a Canadian setting. PLoS One. 2014;9:e87872. doi: 10.1371/journal.pone.0087872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lappalainen L, Hayashi K, Dong H, et al. Ongoing impact of HIV infection on mortality among people who inject drugs despite free antiretroviral therapy. Addiction. 2015;110:111–119. doi: 10.1111/add.12736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suarez-Garcia I, Sobrino-Vegas P, Dalmau D, et al. Clinical outcomes of patients infected with HIV through use of injected drugs compared to patients infected through sexual transmission: late presentation, delayed antiretroviral treatment, and higher mortality. Addiction. 2016 doi: 10.1111/add.13348. doi:10.1111/add.13348. [DOI] [PubMed] [Google Scholar]
- 31*.Hayashi K, Milloy MJ, Wood E, et al. Predictors of liver-related death among people who inject drugs in Vancouver, Canada: a 15-year prospective cohort study. J Int AIDS Soc. 2014;17:19296. doi: 10.7448/IAS.17.1.19296. The study highlighted how HIV mono and co-infection (HIV–HCV) predicted liverrelated mortality among PWID in Vancouver, Canada, underscoring the need for expanded access to HCV testing and treatment for HIV-positive injection drug users. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hayden A, Hayashi K, Dong H, et al. The impact of drug use patterns on mortality among polysubstance users in a Canadian setting: a prospective cohort study. BMC Public Health. 2014;14:1153. doi: 10.1186/1471-2458-14-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson C, Dong H, Ahamad K, et al. Impact of binge alcohol on mortality among people who inject drugs. Addict Behav Rep. 2015;2:28–32. doi: 10.1016/j.abrep.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hickman M, Vickerman P, Degenhardt L. Editorial commentary: the impact of opiate substitution therapy and highly active antiretroviral therapy on mortality risk among people who inject drugs. Clin Infect Dis. 2015;61:1166–1168. doi: 10.1093/cid/civ481. [DOI] [PubMed] [Google Scholar]
- 35*.Thomas FK, Melinda C, Jinhee L, et al. Reducing mortality of people who use opioids through medication assisted treatment for opioid dependence. J HIV Retrovirus. 2015;1:5. The review highlights how medication-assisted treatment for opioid dependence can substantially reduce mortality in HIV-positive PWID. [Google Scholar]
- 36**.Nosyk B, Min JE, Evans E, et al. The effects of opioid substitution treatment && and highly active antiretroviral therapy on the cause-specific risk of mortality among HIVpositive people who inject drugs. Clin Infect Dis. 2015;61:1157–1165. doi: 10.1093/cid/civ476. The study provides strong evidence from a linked population-level database in British Columbia, Canada, of the benefit of integrated ART and OST services to prevent HIV-related and drug-related deaths. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muga R, Rivas I, Faure E, et al. Sex-specific disease outcomes of HIV-positive and HIVnegative drug users admitted to an opioid substitution therapy program in Spain: a cohort study. BMC Infect Dis. 2014;14:504. doi: 10.1186/1471-2334-14-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Degenhardt L, Mathers BM, Wirtz AL, et al. What has been achieved in HIV prevention, treatment and care for people who inject drugs, 2010–2012? A review of the six highest burden countries. Int J Drug Policy. 2014;25:53–60. doi: 10.1016/j.drugpo.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 39.Joseph B, Wood E, Hayashi K, et al. Factors associated with initiation of antiretroviral therapy among HIV-positive people who use injection drugs in a Canadian setting. AIDS. 2016;30:925–932. doi: 10.1097/QAD.0000000000000989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feelemyer J, Des Jarlais D, Arasteh K, Uusküla A. Adherence to antiretroviral medications among persons who inject drugs in transitional, low and middle income countries: an international systematic review. AIDS Behav. 2015;19:575–583. doi: 10.1007/s10461-014-0928-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Malta M, Magnanini MM, Strathdee SA, Bastos FI. Adherence to antiretroviral therapy among HIV-infected drug users: a meta-analysis. AIDS Behav. 2010;14:731–747. doi: 10.1007/s10461-008-9489-7. [DOI] [PubMed] [Google Scholar]
- 42.Rebeiro PF, Gange SJ, Horberg MA, et al. Geographic variations in retention in care among HIV-infected adults in the United States. PloS One. 2016;11:e0146119. doi: 10.1371/journal.pone.0146119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cescon A, Chan K, Raboud JM, et al. Significant differences in clinical outcomes between HIV-hepatitis C virus co-infected individuals with and without injection drug use history. AIDS. 2014;28:121–127. doi: 10.1097/QAD.0000000000000020. [DOI] [PubMed] [Google Scholar]
- 44.Gurm J, Samji H, Nophal A, et al. Suicide mortality among people accessing highly active antiretroviral therapy for HIV/AIDS in British Columbia: a retrospective analysis. CMAJ Open. 2015;3:e140. doi: 10.9778/cmajo.20140101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Joseph B, Kerr T, Puskas CM, et al. Factors linked to transitions in adherence to antiretroviral therapy among HIV-infected illicit drug users in a Canadian setting. AIDS Care. 2015;27:1128–1136. doi: 10.1080/09540121.2015.1032205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wood E, Hogg RS, Lima VD, et al. Highly active antiretroviral therapy and survival in HIV-infected injection drug users. JAMA. 2008;300:550–554. doi: 10.1001/jama.300.5.550. [DOI] [PubMed] [Google Scholar]
- 47.Walsh N, Mijch A, Watson K, et al. HIV treatment outcomes among people who inject drugs in Victoria, Australia. BMC Infect Dis. 2014;14:707. doi: 10.1186/s12879-014-0707-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lappalainen L, Nolan S, Dobrer S, et al. Dose – response relationship between methadone dose and adherence to antiretroviral therapy among HIV-positive people who use illicit opioids. Addiction. 2015;110:1330–1339. doi: 10.1111/add.12970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kourounis G, Richards BD, Kyprianou E, et al. Opioid substitution therapy: lowering the treatment thresholds. Drug Alcohol Depend. 2016;161:1–8. doi: 10.1016/j.drugalcdep.2015.12.021. [DOI] [PubMed] [Google Scholar]
- 50.Ingle SM, May MT, Gill MJ, et al. Impact of risk factors for specific causes of death in the first and subsequent years of antiretroviral therapy among HIV- infected patients. Clin Infect Dis. 2014;59:287–297. doi: 10.1093/cid/ciu261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Basnayake SK, Easterbrook PJ. Wide variation in estimates of global pre- valence and burden and death estimates from chronic HBV, HCV and coinfection with HIV in literature. J Viral Hepat. 2016 doi: 10.1111/jvh.12519. doi:10.1111/ jvh.12519. [DOI] [PubMed] [Google Scholar]
- 52.Peters L, Klein MB. Epidemiology of hepatitis C virus in HIV-infected patients. Curr Opin HIV AIDS. 2015;10:297–302. doi: 10.1097/COH.0000000000000183. [DOI] [PubMed] [Google Scholar]
- 53.Grady BP, Schinkel J, Thomas XV, Dalgard O. Hepatitis C virus reinfection following treatment among people who use drugs. Clin Infect Dis. 2013;57(Suppl 2):S105–S110. doi: 10.1093/cid/cit301. [DOI] [PubMed] [Google Scholar]
- 54.Peters L, Mocroft A, Soriano V, et al. High rate of hepatitis C virus (HCV) recurrence in HIV-infected individuals with spontaneous HCV RNA clearance. HIV Med. 2014;15:615–620. doi: 10.1111/hiv.12160. [DOI] [PubMed] [Google Scholar]
- 55.Wood E, Kerr T, Tyndall MW, Montaner JS. A review of barriers and facilitators of HIV treatment among injection drug users. AIDS. 2008;22:1247–1256. doi: 10.1097/QAD.0b013e3282fbd1ed. [DOI] [PubMed] [Google Scholar]
- 56.Martin LJ, Houston S, Yasui Y, et al. All-cause and HIV-related mortality rates among HIV-infected patients after initiating highly active antiretroviral therapy: the impact of aboriginal ethnicity and injection drug use. Can J Public Health. 2011;102:90–96. doi: 10.1007/BF03404154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gracey M, King M. Indigenous health part 1: determinants and disease patterns. Lancet. 2009;374:65–75. doi: 10.1016/S0140-6736(09)60914-4. [DOI] [PubMed] [Google Scholar]
- 58.Milloy MJ, King A, Kerr T, et al. Improvements in HIV treatment outcomes among indigenous and non-Indigenous people who use illicit drugs in a Canadian setting. J Int AIDS Soc. 2016;19:20617. doi: 10.7448/IAS.19.1.20617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rhodes T. The ‘risk environment’: a framework for understanding and reducing drugrelated harm. Int J Drug Policy. 2002;13:85–94. [Google Scholar]
- 60.Rhodes T, Singer M, Bourgois P, et al. The social structural production of HIV risk among injecting drug users. Soc Sci Med. 2005;61:1026–1044. doi: 10.1016/j.socscimed.2004.12.024. [DOI] [PubMed] [Google Scholar]