Abstract
Background
Impulsivity has been proposed as an important factor in the initiation and maintenance of addiction. Indirect evidence suggests that some methamphetamine users report less impulsivity when they are using methamphetamine compared to when abstinent, but this hypothesis has not been directly tested.
Objectives/Methods
In this study, self-reports of impulsivity were obtained from 32 methamphetamine-dependent (DSM-IV) research participants and 41 healthy control subjects, using the Barratt Impulsiveness Scale-11. The methamphetamine users were assessed during an active period of methamphetamine use, as determined through urinalysis, and again after approximately one week of confirmed abstinence. Control subjects likewise completed two assessments. A subset of participants also completed serial assessments of the Beck Depression Inventory (Methamphetamine Group, N = 17, Control Group, N = 38) and the Methamphetamine Withdrawal Questionnaire (Methamphetamine Group, N = 12).
Results
There was a significant interaction of group with time on impulsivity (p = 0.044), reflecting a significant increase from the first to the second assessment in the methamphetamine users (p=0.013), but no change among healthy control subjects. In contrast, depressive and withdrawal symptoms significantly decreased between the first and second assessment in the methamphetamine users (p’s ≤ 0.01). Change in impulsivity in methamphetamine users was not significantly correlated with change in withdrawal or depression (p’s > 0.05).
Conclusions
These findings suggest that methamphetamine users report more impulsivity when abstaining from drug use, an effect that is not significantly related to methamphetamine withdrawal. Attenuation of impulsivity may reinforce continued methamphetamine use in these individuals.
Keywords: Drug abuse, impulsivity, methamphetamine, withdrawal, self-medication, abstinence, stimulant
Introduction
Substantial evidence points to a link between substance abuse and impulsivity (1–3), with impulsivity theorized to be both a predisposing factor for substance use and a consequence of it (4–6). Indeed, in the DSM-5, substance use disorder is characterized by the failure to inhibit substance use despite recurrent negative consequences (7). In animals exposed to stimulants, impulsivity, inferred from performance on a delay-discounting task, predicts the degree of sensitivity to drug-induced reward (i.e., conditioned place preference) and proclivity for drug self-administration (8, 9). In human methamphetamine users, self-reported impulsivity predicts poor response to treatment (10).
When compared to healthy control subjects, methamphetamine users report higher levels of impulsivity. For example, using the Barratt Impulsiveness Scale-11 (BIS-11), briefly abstinent (i.e., clean for two days) methamphetamine-dependent subjects obtained significantly higher scores than age and gender-matched control subjects (3). Some evidence additionally suggests that treatment-seeking methamphetamine users have higher BIS-11 scores than treatment-seeking cocaine users (10), although cocaine users also have elevated scores relative to healthy comparison subjects (11).
Although impulsivity is typically regarded as a multi-factorial personality trait, research has indicated that impulsivity may vary over time as a function of state-dependent and contextual factors, including drug intoxication, recency of drug use and withdrawal (4). Studies have found, for example, that recent stimulant use reduces impulsivity on behavioral measures tapping various aspects of the impulsivity construct. Acute administration of either d-amphetamine or methamphetamine reduces the discounting of delayed rewards by human subjects who do not use drugs (12) and by rats (13). Administration of methylphenidate to cocaine users reduces their errors of commission on a color-identification task, suggestive of improved inhibitory control (14). Cocaine users also exhibit better performance on tests of attention and working memory, which require self-control, after recent cocaine administration relative to assessments taken during abstinence (15–17), although non-significant effects of recent cocaine use on cognition have also been noted (18).
While the above findings may suggest that methamphetamine users would also self-report lower levels of impulsivity when they were actively using methamphetamine compared to when abstinent, relatively little research has examined this issue. In a cross-sectional study of methamphetamine-dependent participants abstinent for various durations, subjects who were abstinent for 30 days reported non-significantly higher scores on the BIS-11 than those who were abstinent for 6 days, possibly suggesting a subtle reduction in impulsivity from recent use (19). On the other hand, a longitudinal study of cocaine users showed that those who decreased their cocaine use over one year tended to report decreased impulsivity on the BIS-11, while those who increased their use tended to report increased impulsivity (20). These longitudinal results are contrary to the aforementioned literature, and may reflect differences in the acute versus chronic effects of stimulant use on impulsivity.
The goal of the current longitudinal study was to evaluate whether methamphetamine-dependent subjects’ (N = 32) self-reported impulsivity changes between the period of recent methamphetamine use (i.e., demonstrated by urine toxicology) and a week of confirmed abstinence (6.78 ± 2.95 days). Based on the aforementioned literature, we hypothesized that impulsivity would increase with abstinence. Healthy control participants (N = 41) were also evaluated at similar timeframes to control for effects of repeated test exposure. Impulsivity was assessed with the BIS-11; although this measure is often used to measure trait impulsivity, recent evidence suggests that it is sensitive to changes in impulsivity induced by behavioral intervention (21). Finally, symptoms of methamphetamine withdrawal and depression were also longitudinally assessed to determine whether withdrawal symptoms were associated with changes in impulsivity. During early abstinence (e.g., the first two weeks), methamphetamine users commonly show elevated scores on measures of withdrawal and depression (i.e., Methamphetamine Withdrawal Questionnaire; Beck Depression Inventory; 22), so we sought to evaluate whether these changes were coincident with potential alterations in impulsivity.
Method
Participants and Procedure
Thirty-two methamphetamine-dependent subjects, who were not seeking treatment, and 41 healthy control subjects participated (Table 1). Participants were initially recruited for studies of brain structure and cognition (e.g., 23, 24). Nine subjects participated in a previous study of methamphetamine withdrawal symptoms (22). All participants were fluent in English, received a thorough description of study procedures, and provided written informed consent, consistent with UCLA Institutional Review Board guidelines. Twenty-two of the methamphetamine-dependent subjects completed the study as inpatients at the UCLA General Clinical Research Center (GCRC), and the other participants completed the study on a nonresidential basis after closure of the GCRC. All participants completed medical and psychiatric screening procedures, including the Structured Clinical Interview for DSM-IV (SCID-IV) for Axis I diagnoses, and were medically healthy, unmedicated and without major comorbid psychiatric conditions. For a more detailed description of exclusionary criteria see previous manuscripts (22, 23).
Table 1.
Characteristics of Research Participants
| Healthy Control | Methamphetamine-Dependent | |
|---|---|---|
| Sample size | 41 | 32 |
| Age | 34.2 ± 8.7 | 34.6 ± 10.8 |
| Education (yr.) | 13.0 ± 1.6 | 12.4 ± 1.3 |
| Mother’s Education (yr.) | 12.6 ± 2.9 | 12.3 ± 3.2 |
| Ethnicity | ||
| Caucasian | 15 | 16 |
| African Am. | 6 | 1 |
| Hispanic | 14 | 10 |
| Other | 6 | 5 |
| Gender | ||
| Male | 23 | 17 |
| Female | 18 | 15 |
| Cigarette Smokers (yes/no) | 8/41 | 30/32*** |
| Cigarettes/day (smokers only) | 9.7 ± 7.3 | 12.5 ± 12.7 |
| Days Alcohol/Past 30 | 1.9 ± 3.3 | 3.8 ± 6.6 |
| Days Marijuana/Past 30 | 1.6 ± 5.2 | 3.3 ± 7.7 |
| Days Methamphetamine/Past 30 | -- | 20.7 ± 8.4 |
| Duration of Methamphetamine abuse (yr.) | -- | 7.6 ± 6.0 |
| Grams Methamphetamine/week | -- | 2.8 ± 3.0 |
| Days between BIS Administrations | 16.2 ± 7.2 | 10.5 ± 6.5**† |
| BIS 1 Total Score | 56.1 ± 10.1 | 67.2 ± 11.2*** |
| BIS 2 Total Score | 56.6 ± 11.0 | 70.7 ± 11.9*** |
Note. Values are means ± SDs, where appropriate. The symbols, ** and ***, indicate significant differences from the respective control group at p < 0.01 and p < 0.001, respectively.
For outpatient methamphetamine users, the days between BIS administrations were not always equivalent to the days of abstinence at retesting.
Participants in the Methamphetamine Group tested positive for methamphetamine at the first assessment, and tested negative for methamphetamine and other illicit substances (amphetamine, opiates, cocaine, benzodiazepines) at the second assessment (control participants tested negative at all time points). Given the long duration in which marijuana can be detected through urinalysis, brief abstinence from marijuana for nonresidential participants was verified through saliva testing (Oratect, Grapevine, TX), with all participants endorsing at least 4 days of abstinence at re-testing (however, rates of marijuana use for both groups were low; see Table 1). Abstinence for residential participants was supervised. Both residential and non-residential subjects were allowed to smoke cigarettes ad libitum.
Methamphetamine Group subjects reported a mean of 0.87 days since last methamphetamine use at first administration of BIS-11 (SD = 1.01; Range 0 to 3) and 6.78 days of abstinence from methamphetamine when re-tested (SD =2.95; Range 3 to 16). A subset of participants were also twice administered the Beck Depression Inventory (BDI; 38 Control and 17 Methamphetamine) and the Methamphetamine Withdrawal Questionnaire (MAWQ; 12 Methamphetamine), reporting an average of 0.69 and 0.50 days since last methamphetamine use at the first administration (SDs = 0.79 and 0.67; respectively, Ranges 0 to 2), and an average of 8.01 and 5.00 days of abstinence at retesting, respectively (SD = 1.57; Range 6 to 12; SD = 1.48; Range 3 to 7, respectively).
Measures
Barratt Impulsiveness Scale-11 (25)
The Barratt Impulsiveness Scale-11 is a 30-item measure with a four-point Likert-type response style that is commonly used to assess impulsivity. Original research suggested that it was comprised of three primary subscales—attentional impulsiveness, motor impulsiveness and non-planning impulsiveness (25). However, more recent psychometric analysis has failed to support the dimensionality of the original subscales, both in community samples (26) and methamphetamine users (27). Based on factor analysis, a new bifactor model has been proposed with subscales reflecting “cognitive impulsivity” and “behavioral impulsivity” (see 26). We used as our primary dependent measure the total BIS-11 score, with subscales analyzed post-hoc using the bifactor model. Test-retest reliability of the BIS in our study was adequate (r = 0.874).
Beck Depression Inventory (28)
The BDI is a 21-item multiple-choice measure of recent depression-related symptoms that is widely used as a screener for depression.
Methamphetamine Withdrawal Questionnaire
The MAWQ is a 30-item measure with a four-point Likert-type response style that assesses methamphetamine withdrawal symptoms. The MAWQ is an adapted version of the Amphetamine Withdrawal Questionnaire (29), which includes additional symptoms that are common in methamphetamine withdrawal (e.g., anger, headache) (22).
Statistical Analyses
Demographic differences between methamphetamine and control subjects were tested using chi-square or t-tests, as appropriate. Changes in impulsivity over time by group were analyzed with mixed-design repeated-measure ANOVAs. Post-hoc paired t-tests were used to evaluate within group changes in impulsivity.
Results
Demographic Analyses
The Methamphetamine and Control Groups did not significantly differ in age, education, mother’s education, ethnicity, gender, recent alcohol or marijuana use, all ps >.05 (Table 1). The Control Group had fewer cigarette smokers (p<0.001) and a longer time interval between BIS-11 administrations (p=0.001) than the Methamphetamine subjects (e.g., control subjects tended to reschedule their appointments). However, smoking status, cigarettes smoked per day, and the time interval between BIS-11 assessments were not significantly related to change in BIS-11 scores over time, ps>0.05. Days of methamphetamine use in the last 30 days, years of methamphetamine use, and grams of methamphetamine used in the week before the study were not associated with BIS-11 change scores, nor BIS-11 scores at either time point, ps>0.05.
Impulsivity Analyses
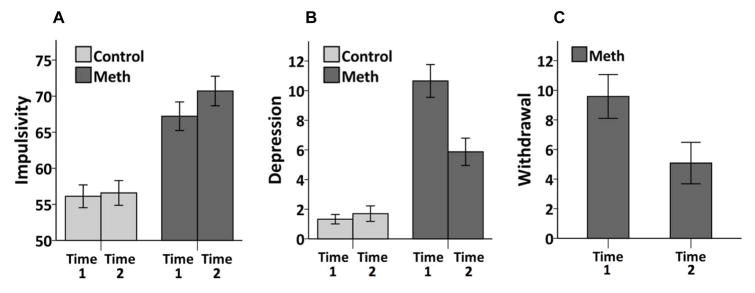
A significant interaction was observed between group and time on BIS-11 scores (F(1, 71)=4.19, p=0.044), in addition to significant main effects of group (F(1, 71)=26.10, p<.001; Methamphetamine mean=68.97, SE=1.85; Control mean=56.35, SE=1.64), and time (F(1, 71)=7.14, p=0.009; Time 1 mean=61.67, SE=1.25; Time 2 mean=63.65, SE=1.33). Post hoc analysis of the interaction revealed that Methamphetamine Group subjects significantly increased in impulsivity from Time 1 (mean=67.22, SD=11.21) to Time 2 (mean=70.72, SD=11.59; t(31)=−2.63, p=0.013; Cohen’s d = 0.463), while Control Group scores remained stable between Time 1 (mean=56.12, SD=10.12) and Time 2 (mean=56.59, SD=10.97; t(40)= −0.58, p=0.566; Cohen’s d=0.095; see Figure 1A).
Figure 1. Changes in impulsivity, depression and withdrawal over time in methamphetamine users and control subjects.
A. Methamphetamine users (Meth) (N = 32) show increased impulsivity over time (p = 0.013), while control subjects (N = 41) remain unchanged (p = 0.566). B. Methamphetamine users (N = 17) show decrease in depressive symptoms over time (p < 0.001), while control subjects (N = 38) show no change (p = 0.467). C. Methamphetamine users (N = 12) exhibit decrease in withdrawal symptoms over time (p = 0.010). Error bars represent +/− 1 SEM.
Post-hoc analysis of the revised BIS-11 subscales (26) revealed no statistically significant interactions between group and time on the “cognitive impulsivity” subscale (F(1, 71)=0.87, p=0.355) or the “behavioral impulsivity” subscale (F(1, 71)=1.85, p=0.178). On the original BIS-11 subscales, a significant group by time interaction was observed on the attentional impulsivity subscale (F(1, 71)= 8.91, p=0.004), while no significant interactions were observed on the motor or nonplanning subscales (ps>.40). As before, the interaction on the attentional impulsivity subscale reflected the Methamphetamine Group subjects significantly increasing in impulsivity from Time 1 to Time 2 (p=0.005), while the Control Group remained stable over time (p=0.904).
Analyses of Depression and Withdrawal
A significant interaction was observed between group and time on BDI scores, (F(1, 53)=49.73, p<0.001). Main effects were also observed for group (F(1, 53)=46.33, p<0.001; Methamphetamine mean=8.35, SE=0.82; Control mean=1.53, SE=0.54) and time (F(1, 53)=42.21, p <0.001; Time 1 mean=6.94, SE=0.59; Time 2 mean=2.93, SE=0.57). Post hoc analysis revealed a significant decrease in BDI scores for Methamphetamine Group subjects from Time 1 (mean=12.53, SD=6.76) to Time 2 (mean=4.18, SD=5.02; t(17)=5.44, p< 0.001; Cohen’s d = 1.355), while BDI scores for Control Group subjects remained unchanged from Time 1 (mean=1.39, SD=2.05) to Time 2 (mean=1.74, SD=3.38; t(37)= −0.74, p=0.467; Cohen’s d=0.133; see Figure 1B).
In the Methamphetamine Group alone, methamphetamine withdrawal symptoms significantly decreased over time (MAWQ; Time 1 mean=9.58, SD=5.13; Time 2 mean=5.08, SD=4.85; t(11)=3.13, p=0.010; Cohen’s d= 0.902; see Figure 1C). In the Methamphetamine Group, change in withdrawal and depression scores were not significantly correlated with change in BIS-11 scores (ps>0.05).
Discussion
Compared to an assessment taken after recent use of methamphetamine (verified by positive urine toxicology), impulsivity self-reported by methamphetamine-dependent subjects was significantly higher following approximately a week of abstinence from drugs. In contrast, impulsivity among control subjects remained stable over time. In approximately the same testing timeframe, methamphetamine subjects also reported significant decreases in depression and methamphetamine withdrawal during early abstinence, suggesting that the increase in impulsivity observed during early abstinence was not due to the acute emotional or physical effects of methamphetamine withdrawal.
These findings provide tentative support for the hypothesis that, in dependent individuals, recent stimulant use may reduce impulsivity, acting as a negative reinforcer to maintain drug use. This view is consistent with reports that recent stimulant use may attenuate pre-existing cognitive deficits (14–17), while nonetheless contributing to increased cognitive dysfunction with chronic use (5, 30, 31). Similarly, given that chronic stimulant use appears to exacerbate impulsivity (20), reductions in impulsivity associated with recent use are likely to be temporary and counterproductive in the long-term. Since BIS-11 scores predict poor treatment response in methamphetamine users (10), the current findings suggest that increases in impulsivity in early abstinence may also contribute to treatment non-response.
If recent methamphetamine use increases positive affect, it is possible that reductions in positive affect associated with abstinence increase methamphetamine users’ proclivity toward rash action, including drug relapse. Although not specifically targeted by the BIS-11, more recent conceptualizations of the impulsivity construct include the measurement of “urgency”, or impulsive behavior elicited to alleviate negative emotion (e.g., see the Urgency, Premeditation, Perseverance, and Sensation Seeking (UPPS) Impulsive Behavior Scale, 32). Recent evidence, for example, suggests that negative urgency moderates the relationship between negative emotion and symptoms of alcohol dependence (33). Although speculative, it is possible that negative urgency increases in the early stages of abstinence. Trends have been identified, for example, between urgency and the tendency to relapse in polysubstance users undergoing inpatient detoxification (34). In the current study, although depressive symptoms and withdrawal did not increase over the assessment period, it is possible that some other, unidentified form of negative affect was increased at the second assessment and facilitated the rise in impulsivity.
Recent psychometric evaluation of the original BIS-11 subscales has not supported their dimensionality (25, 26), and a new bifactor model of the BIS-11 has been proposed with “cognitive impulsivity” and “behavioral impulsivity” subscales (25). These subscales are comprised of 6 and 7 items, respectively, and exclude some of the 30 BIS items to eliminate redundancy and heterogeneity within the subscales (25). The present analysis, however, did not show significant group by time interactions for either of these subscales (p’s > 0.10). A significant group by time interaction was observed on the original attentional impulsivity subscale (24) (p = 0.004), but not the motor or nonplanning impulsivity subscales (p’s > 0.40). Because the attentional subscale is likely not unidimensional (25), it is unclear precisely what aspects of impulsivity are driving the current findings. Additional research is needed to clarify how the factorial structure of the BIS-11 is related to real-world impulsive behavior.
Limitations of this study should be noted. Given that the current results were obtained using relatively small sample sizes, replication is recommended for confirmation of the findings, potentially using multiple measures for enhanced assessment of the multi-dimensional impulsivity construct. Further, it should be noted that the period of active methamphetamine use evaluated in this study is not synonymous with methamphetamine intoxication, as a positive urinalysis at baseline has a detection window of 72–120 hours (Alpha Scientific Designs, Poway, CA), with some subjects reporting using hours before the testing and others up to 3 days prior. As such, the potential reduction in impulsivity observed at baseline may not reflect an acute drug effect. In addition, because the methamphetamine users studied here were only abstinent for approximately one week at retesting, it will be important to determine if impulsivity remains elevated with longer periods of abstinence. Further, depressive symptoms and withdrawal were not always assessed on the same day as impulsivity (although usually within one or two days); therefore, it cannot be conclusively stated that these symptoms were unrelated to the increases in impulsiveness, although clear divergent trends were noted. Similarly, the duration of abstinence reported by outpatients (N = 10) was not directly observed, although it was generally consistent with the duration between their first assessment (when positive for methamphetamine on urinalysis) and their re-test date (when negative on urinalysis). Finally, because methamphetamine use was not experimentally manipulated, a causal link to reduction in impulsivity cannot be assumed, although this appears to be a plausible explanation.
The average increase in BIS-11 scores exhibited by methamphetamine users in the current study was approximately 3 points between the first and second assessments, although some individual subjects increased by 15 to 20 points. This average increase corresponds to a medium-range effect size (Cohen’s d = 0.463, adjusted for dependent samples, 35). For reference, the difference in BIS-11 scores between methamphetamine users and control subjects at the first assessment was approximately 11 points (67.2 ± 11.2; 56.1 ± 10.1, respectively). Given these data, the increase in impulsivity shown in the current study is, on average, likely to be of modest clinical significance, although a subset of users probably does exhibit meaningful alterations in impulsivity in abstinence. More data is needed regarding the correspondence between raw score differences on the BIS-11 and functional outcomes.
If the current data are replicated, it may be useful to target impulsivity in treatment not only in individual stages, but particularly as abstinence persists. Novel behavioral strategies are beginning to be explored for impulsivity reduction. For example, mindfulness meditation has shown some success in treating the disinhibition characteristic of adulthood attention-deficit hyperactivity disorder (ADHD, 36). Controlled breathing techniques and yoga-based strategies have been shown to reduce BIS-11 scores in adolescents (21). Goal management training, a type of cognitive rehabilitation which targets executive functions, has been shown to improve decision-making and response inhibition in patients with frontal lobe damage (37), but also in polysubstance users (38). Further exploring these strategies as adjuncts to treatment for methamphetamine use may prove fruitful.
Acknowledgments
Funding
The research described here was funded in part by NIH grants P20 DA022539, R01 DA020726 (EDL), K23DA027734 (ACD), R21DA034928 (ACD), UL1TR000124 (UCLA CTSI), and endowments from the Thomas P. and Katherine P. Pike Chair in Addiction Studies, and the Marjorie Greene Trust. None of the sponsors were involved with the design, collection, analysis or interpretation of data, writing the manuscript or the decision to submit the manuscript for publications.
Footnotes
Declaration of Interest
The investigators have no conflicts of interest or financial disclosures to report.
References
- 1.Perry JL, Carroll ME. The role of impulsive behavior in drug abuse. Psychopharmacology. 2008;200(1):1–26. doi: 10.1007/s00213-008-1173-0. [DOI] [PubMed] [Google Scholar]
- 2.Verdejo-García A, Lawrence AJ, Clark L. Impulsivity as a vulnerability marker for substance-use disorders: review of findings from high-risk research, problem gamblers and genetic association studies. Neuroscience & Biobehavioral Reviews. 2008;32(4):777–810. doi: 10.1016/j.neubiorev.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Lee B, London ED, Poldrack RA, Farahi J, Nacca A, Monterosso JR, Mumford JA, Bokarius AV, Dahlbom M, Mukherjee J. Striatal dopamine d2/d3 receptor availability is reduced in methamphetamine dependence and is linked to impulsivity. The Journal of Neuroscience. 2009;29(47):14734–14740. doi: 10.1523/JNEUROSCI.3765-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Wit H. Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addiction biology. 2009;14(1):22–31. doi: 10.1111/j.1369-1600.2008.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology. 1999;146(4):373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- 6.Ivanov I, Schulz KP, London ED, Newcorn JH. Inhibitory control deficits in childhood and risk for substance use disorders: a review. The American journal of drug and alcohol abuse. 2008;34(3):239–258. doi: 10.1080/00952990802013334. [DOI] [PubMed] [Google Scholar]
- 7.Diagnostic and statistical manual of mental disorders DSM-5. American Psychiatric Association; Arlington, Va: 2013. [DOI] [PubMed] [Google Scholar]
- 8.Perry JL, Larson EB, German JP, Madden GJ, Carroll ME. Impulsivity (delay discounting) as a predictor of acquisition of IV cocaine self-administration in female rats. Psychopharmacology. 2005;178(2–3):193–201. doi: 10.1007/s00213-004-1994-4. [DOI] [PubMed] [Google Scholar]
- 9.Yates JR, Marusich JA, Gipson CD, Beckmann JS, Bardo MT. Amphetamine Conditioned Place Preference in High and Low Impulsive Rats. Pharmacology, biochemistry, and behavior. 2012;100(3):370. doi: 10.1016/j.pbb.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Winhusen T, Lewis D, Adinoff B, Brigham G, Kropp F, Donovan DM, Seamans CL, Hodgkins CC, Dicenzo JC, Botero CL, Jones DR, Somoza E. Impulsivity is associated with treatment non-completion in cocaine- and methamphetamine-dependent patients but differs in nature as a function of stimulant-dependence diagnosis. J Subst Abuse Treat. 2013;44(5):541–7. doi: 10.1016/j.jsat.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moeller FG, Hasan KM, Steinberg JL, Kramer LA, Dougherty DM, Santos RM, Valdes I, Swann AC, Barratt ES, Narayana PA. Reduced anterior corpus callosum white matter integrity is related to increased impulsivity and reduced discriminability in cocaine-dependent subjects: diffusion tensor imaging. Neuropsychopharmacology. 2005;30(3):610–7. doi: 10.1038/sj.npp.1300617. [DOI] [PubMed] [Google Scholar]
- 12.de Wit H, Enggasser JL, Richards JB. Acute administration of d-amphetamine decreases impulsivity in healthy volunteers. Neuropsychopharmacology. 2002;27(5):813–825. doi: 10.1016/S0893-133X(02)00343-3. [DOI] [PubMed] [Google Scholar]
- 13.Richards JB, Sabol KE, de Wit H. Effects of methamphetamine on the adjusting amount procedure, a model of impulsive behavior in rats. Psychopharmacology. 1999;146(4):432–439. doi: 10.1007/pl00005488. [DOI] [PubMed] [Google Scholar]
- 14.Goldstein RZ, Woicik PA, Maloney T, Tomasi D, Alia-Klein N, Shan J, Honorio J, Samaras D, Wang R, Telang F, Wang GJ, Volkow ND. Oral methylphenidate normalizes cingulate activity in cocaine addiction during a salient cognitive task. Proc Natl Acad Sci U S A. 2010;107(38):16667–72. doi: 10.1073/pnas.1011455107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pace-Schott EF, Morgan PT, Malison RT, Hart CL, Edgar C, Walker M, Stickgold R. Cocaine users differ from normals on cognitive tasks which show poorer performance during drug abstinence. The American journal of drug and alcohol abuse. 2008;34(1):109–121. doi: 10.1080/00952990701764821. [DOI] [PubMed] [Google Scholar]
- 16.Pace-Schott EF, Stickgold R, Muzur A, Wigren PE, Ward AS, Hart CL, Walker M, Edgar C, Hobson JA. Cognitive performance by humans during a smoked cocaine binge-abstinence cycle. The American journal of drug and alcohol abuse. 2005;31(4):571–591. doi: 10.1081/ada-200068120. [DOI] [PubMed] [Google Scholar]
- 17.Woicik PA, Moeller SJ, Alia-Klein N, Maloney T, Lukasik TM, Yeliosof O, Wang GJ, Volkow ND, Goldstein RZ. The neuropsychology of cocaine addiction: recent cocaine use masks impairment. Neuropsychopharmacology. 2009;34(5):1112–1122. doi: 10.1038/npp.2008.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vonmoos M, Hulka LM, Preller KH, Jenni D, Baumgartner MR, Stohler R, Bolla KI, Quednow BB. Cognitive dysfunctions in recreational and dependent cocaine users: role of attention-deficit hyperactivity disorder, craving and early age at onset. Br J Psychiatry. 2013;203(1):35–43. doi: 10.1192/bjp.bp.112.118091. [DOI] [PubMed] [Google Scholar]
- 19.Wang G, Shi J, Chen N, Xu L, Li J, Li P, Sun Y, Lu L. Effects of length of abstinence on decision-making and craving in methamphetamine abusers. PLoS One. 2013;8(7):e68791. doi: 10.1371/journal.pone.0068791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hulka LM, Vonmoos M, Preller KH, Baumgartner MR, Seifritz E, Gamma A, Quednow BB. Changes in cocaine consumption are associated with fluctuations in self-reported impulsivity and gambling decision-making. Psychol Med. 2015;45(14):3097–110. doi: 10.1017/S0033291715001063. [DOI] [PubMed] [Google Scholar]
- 21.Ghahremani DG, Oh EY, Dean AC, Mouzakis K, Wilson KD, London ED. Effects of the Youth Empowerment Seminar on impulsive behavior in adolescents. Journal of Adolescent Health. 2013;53(1):139–141. doi: 10.1016/j.jadohealth.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 22.Zorick T, Nestor L, Miotto K, Sugar C, Hellemann G, Scanlon G, Rawson R, London ED. Withdrawal symptoms in abstinent methamphetamine-dependent subjects. Addiction. 2010;105(10):1809–18. doi: 10.1111/j.1360-0443.2010.03066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dean AC, Kohno M, Morales AM, Ghahremani DG, London ED. Denial in methamphetamine users: Associations with cognition and functional connectivity in brain. Drug and alcohol dependence. 2015 doi: 10.1016/j.drugalcdep.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morales AM, Lee B, Hellemann G, O’Neill J, London ED. Gray-matter volume in methamphetamine dependence: cigarette smoking and changes with abstinence from methamphetamine. Drug Alcohol Depend. 2012;125(3):230–8. doi: 10.1016/j.drugalcdep.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patton JH, Stanford MS. Factor structure of the Barratt impulsiveness scale. Journal of clinical psychology. 1995;51(6):768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 26.Reise SP, Moore TM, Sabb FW, Brown AK, London ED. The Barratt Impulsiveness Scale-11: reassessment of its structure in a community sample. Psychol Assess. 2013;25(2):631–42. doi: 10.1037/a0032161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reid RC, Cyders MA, Moghaddam JF, Fong TW. Psychometric properties of the Barratt Impulsiveness Scale in patients with gambling disorders, hypersexuality, and methamphetamine dependence. Addict Behav. 2014;39(11):1640–5. doi: 10.1016/j.addbeh.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 28.Beck AT, Steer RA, Ball R, Ranieri WF. Comparison of Beck Depression Inventories-IA and-II in Psychiatric Outpatients. Journal of Personality Assessment. 1996;67(3):588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- 29.Srisurapanont M, Jarusuraisin N, Jittiwutikan J. Amphetamine withdrawal: I. Reliability, validity and factor structure of a measure Australian and New Zealand Journal of Psychiatry. 1999;33(1):89–93. doi: 10.1046/j.1440-1614.1999.00517.x. [DOI] [PubMed] [Google Scholar]
- 30.Dean AC, Groman SM, Morales AM, London ED. An evaluation of the evidence that methamphetamine abuse causes cognitive decline in humans. Neuropsychopharmacology. 2013;38(2):259–274. doi: 10.1038/npp.2012.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scott JC, Woods SP, Matt GE, Meyer RA, Heaton RK, Atkinson JH, Grant I. Neurocognitive effects of methamphetamine: a critical review and meta-analysis. Neuropsychology review. 2007;17(3):275–297. doi: 10.1007/s11065-007-9031-0. [DOI] [PubMed] [Google Scholar]
- 32.Whiteside SP, Lynam DR. The five factor model and impulsivity: Using a structural model of personality to understand impulsivity. Personality and Individual Differences. 2001;30:669–689. [Google Scholar]
- 33.Menary KR, Corbin WR, Leeman RF, Fucito LM, Toll BA, DeMartini K, O’Malley SS. Interactive and Indirect Effects of Anxiety and Negative Urgency on Alcohol-Related Problems. Alcohol Clin Exp Res. 2015;39(7):1267–74. doi: 10.1111/acer.12762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stevens L, Goudriaan AE, Verdejo-Garcia A, Dom G, Roeyers H, Vanderplasschen W. Impulsive choice predicts short-term relapse in substance-dependent individuals attending an in-patient detoxification programme. Psychol Med. 2015;45(10):2083–93. doi: 10.1017/S003329171500001X. [DOI] [PubMed] [Google Scholar]
- 35.Morris SB, DeShon RP. Combining effect size estimates in meta-analysis with repeated measures and independent-groups designs. Psychol Methods. 2002;7(1):105–25. doi: 10.1037/1082-989x.7.1.105. [DOI] [PubMed] [Google Scholar]
- 36.Mitchell JT, McIntyre EM, English JS, Dennis MF, Beckham JC, Kollins SH. A Pilot Trial of Mindfulness Meditation Training for ADHD in Adulthood: Impact on Core Symptoms, Executive Functioning, and Emotion Dysregulation. J Atten Disord. 2013 doi: 10.1177/1087054713513328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levine B, Schweizer TA, O’Connor C, Turner G, Gillingham S, Stuss DT, Manly T, Robertson IH. Rehabilitation of executive functioning in patients with frontal lobe brain damage with goal management training. Front Hum Neurosci. 2011;5:9. doi: 10.3389/fnhum.2011.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alfonso JP, Caracuel A, Delgado-Pastor LC, Verdejo-Garcia A. Combined Goal Management Training and Mindfulness meditation improve executive functions and decision-making performance in abstinent polysubstance abusers. Drug Alcohol Depend. 2011;117(1):78–81. doi: 10.1016/j.drugalcdep.2010.12.025. [DOI] [PubMed] [Google Scholar]