Abstract
Hair loss is common in macaque colonies. Very little is known about the relationship between psychological stress and hair loss. We initially examined alopecia and hair cortisol concentrations in 198 (89 male) rhesus macaques from three primate centers and demonstrated replicability of our previous finding that extensive alopecia (> 30% hair loss) is associated with increased chronic cortisol concentrations and significantly affected by facility. A subset of these monkeys (142 of which 67 were males) were sampled twice approximately 8 months apart allowing us to examine the hypotheses that gaining hair should be associated with decreases in cortisol concentrations and vice versa. Hair loss was digitally scored using ImageJ software for the first sample. Then visual assessment was used to examine the second sample, resulting in 3 categories of coat condition: 1) monkeys that remained fully haired, 2) monkeys that remained alopecic (with more than 30% hair loss), or 3) monkeys that showed more than a 15% increase in hair. The sample size for the group that lost hair was too small to be analyzed. Consistent with our hypothesis, monkeys that gained hair showed a significant reduction in hair cortisol concentrations but this effect only held for females. Coat condition changed little across sampling periods with only 25 (11 male) monkeys showing a greater than 15% gain of hair. Twenty (7 male) monkeys remained alopecic, whereas 97 (49 males) remained fully haired. Hair cortisol was highly correlated across samples for the monkeys that retained their status (remained alopecic or retained their hair).
Keywords: alopecia, hypothalamic-pituitary-adrenal axis, cortisol, stress
INTRODUCTION
Alopecia or hair loss is a common concern in nonhuman primates living both in natural environments and laboratory facilities. In the latter instance, the percentage of monkeys showing hair loss, regardless of amount, is quite high. In a recent study, hair loss prevalence in 1,258 rhesus monkeys at four national primate centers ranged from 34.3% to 86.5% (mean, 49.3%); hair pulling behavior was only a small predictor of hair loss in this population [Lutz et al., 2013]. Hair loss also varies considerably across individuals both under natural and laboratory conditions. Some rhesus monkeys show extreme hair loss, encompassing most of their body, others have bald patches or thin hair coats; however, some monkeys consistently have high quality coats. This variation is present in all facilities examined to date [Novak et al., 2014]. Additionally, female rhesus monkeys housed in laboratory settings are more susceptible to hair loss compared to males, an effect which is independent of pregnancy status [Lutz et al., 2013; Kroeker et al., 2014].
Many factors are known to contribute to poor coat quality [Novak and Meyer, 2009]. Hair loss can be associated with naturally occurring phenomena, e.g., seasonal changes in day length in which hair coat quality waxes and wanes (Isbell, 1995), and aging in which hair coat quality declines in elderly primates [Steinmetz et al., 2006]. Hair loss can also result from endocrine diseases [Lair et al., 1999], immunologic disorders [Malinow et al., 1982], atopic dermatitis [Kramer et al., 2010], mineral imbalances [Swenerton and Hurley, 1980], and pregnancy [Novak and Meyer, 2009]. Additionally, hair loss may result from friction with certain physical structures in the environment. For example, monkeys that sit in their hammocks may present with hair loss on the underside of their lower legs [Novak et al., 2014]. Some of the conditions described above can be treated and/or prevented. However, others cannot be resolved without having to invoke a cost/benefit analysis. Seasonal influences can be reduced if monkeys are relocated to indoor housing under constant day/night cycles, but both environmental and social complexity are likely to be reduced. Hammocks can be eliminated from the enrichment program; however, for monkeys that sit in them for long durations, removal may affect animal comfort. Even with this information in hand, a substantial number of monkeys show hair loss which cannot be explained by the factors listed above. In these cases, increased exposure to stressors has been suggested as a possible cause.
Recently, there has been renewed regulatory focus on hair loss in laboratory housed macaques because of the possibility that hair loss might be stress related and thus serve as a biomarker for stress exposure. Although there is direct, experimental evidence linking stress exposure to hair loss in rodents [Aoki et al., 2003; Katayama et al., 2007; Wikramanayake et al., 2010], no such experimental evidence exists for rhesus macaques. Most research on alopecia in laboratory housed macaques is retrospective inasmuch as subjects are identified only after they lose their hair. In these cases, researchers look for relevant physiological and behavioral correlates of the stress response system and compare these measures in monkeys with and without hair loss.
The hypothalamic-pituitary-adrenal (HPA) axis is one arm of the stress response system that is activated when rhesus macaques are exposed to stressors. Activation of this system increases the glucocorticoid, cortisol, and typically cortisol concentration is used as an index of stress [O'Connor et al., 2000]. In the past, cortisol was measured primarily in blood or saliva. However, these measures were limited in that they were point sample estimates of cortisol concentrations at a single moment in time which were also impacted by circadian variation and the stress of the sample collection procedure itself (e.g., venipuncture). A more recent approach involves measuring cortisol in hair [Davenport et al., 2006]. Cortisol is deposited in the hair shaft and most likely reflects chronic HPA axis activation averaged across the highs and lows over several months [Meyer and Novak, 2012]. Furthermore, hair cortisol concentrations are not subject to circadian variation or the stress of sample collection. Most importantly, stress responses can be discerned in hair. In rhesus monkeys, hair cortisol was significantly elevated in response to the major stressor of relocation [Davenport et al., 2006; Davenport et al., 2008]. Recent research also suggests that a retrospective calendar of HPA axis activation can be obtained through a segmental analysis of the hair in orangutans [Carlitz et al., 2014] and humans [Thomson et al., 2009]. Subsequent work on a variety of species [dogs: Accorsi et al., 2008; humans: Kirshbaum et al., 2009; polar bears: Bechshoft et al., 2011; and monkeys: Fairbanks et al., 2011] continues to support hair cortisol as an important biomarker of chronic stress across a wide range of conditions, both biomedical and environmental.
To determine whether hair loss was a biomarker for stress in rhesus monkeys, we examined hair cortisol concentrations in alopecic monkeys and normally haired monkeys at three national primate facilities across the United States [Novak et al., 2014]. This initial study provided some evidence for an association between HPA axis activity and hair loss. Overall, monkeys with 30% or more hair loss (alopecic) had significantly higher hair cortisol levels than their normally haired counterparts (5% or less hair loss). Furthermore, in the alopecic group, hair loss was positively correlated with hair cortisol concentrations. However, the effect was large in only one facility, modest in another, and not significant in a third facility. These facility differences could not be explained by variations in day-night cycles, enrichment strategies, chow manufacturer, feeding schedule, or prevalence rates of alopecia.
One limitation of this study was that only one assessment of hair loss was made and only one hair sample was collected. Very little information is available on the persistence of hair loss in laboratory housed monkeys across months. In the present study, we first examined whether the findings from Novak et al., 2014 were replicated when the sample was doubled in size from 99 subjects to 198 subjects with a particular focus on whether alopecic monkeys (>30% hair loss) showed higher levels of hair CORT compared to fully haired monkeys (<5% hair loss) and whether this effect varied across facility. Then, we examined the stability of coat condition across two sample periods 6-8 months apart. If hair loss is a biomarker for stress exposure, substantial regrowth of hair should be associated with a reduction in hair cortisol concentrations. In this sample, we lacked sufficient numbers of subjects to test the reverse prediction that monkeys losing hair should show an increase in hair cortisol.
METHODS
Subjects
For the initial part of the project, the subjects were 198 rhesus monkeys, Macaca mulatta, (89 males) maintained at 3 national primate research centers: Washington National Primate Research Center, Oregon National Primate Research Center, and Southwest Primate Research Center. The subjects ranged in age from 2 – 24 years (mean = 8.9 years). Because biological samples were required for this project, we had access to a convenience sample based on unassigned monkeys and some assigned monkeys with investigator approval. This convenience sample was considerably smaller than the sample of 1258 monkeys in which we established prevalence rates of alopecia at the three facilities [Lutz et al., 2013]. For the convenience sample, we needed both alopecic monkeys (>30% hair loss) and fully haired monkeys (<5% hair loss) to discern effects; thus this sample was not representative of the alopecia prevalence at these facilities (see Table I for a listing by facility, sex, and alopecia status)
TABLE I.
Distribution by Facility, Sex and Alopecia Condition for the Replication
| Alopecic |
Fully Haired |
||||
|---|---|---|---|---|---|
| Facility | Males | Females | Males | Females | Total |
| A | 8 | 10 | 11 | 10 | 39 |
| B | 13 | 11 | 19 | 28 | 71 |
| C | 3 | 12 | 35 | 38 | 88 |
| Total | 24 | 33 | 65 | 76 | 198 |
For the subsequent part of the study involving a comparison of two samples, the available subject population was reduced to 142 monkeys (67 males). The subjects ranged in age from 4 to 22 y (mean, 9.8 y), and none of the females was pregnant. This reduction in subject numbers was associated with the following: 1) monkeys no longer available for a second sample (n = 45; 13 of which were alopecic), 2) monkeys excluded for blurry photos in the second set (n=1), and 3) monkeys that lost hair because the sample size was too small to analyze (n=10, 7 females and 3 males with at least one from each facility). Table II contains a listing of monkeys as a function of sex and change in coat status from sample 1 to sample 2 (stayed alopecic, stayed fully haired, remained alopecic, or gained hair). All facilities were accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC), and these facilities were assigned the designation of A, B, or C used previously in Novak et al., 2014. All monkeys were housed in size appropriate cages in rooms where they had visual, auditory, and olfactory contact with other monkeys. Most of the monkeys were individually housed, a small number were pair housed or housed in grooming contact. In the second sample, 109 (57 males) were individually housed, 24 (10 males) were pair housed, and 9 (0 males) were housed in grooming contact.
TABLE II.
Distribution by Sex and Change in Alopecia Status for Assessing Relationships
| Sex |
Stayed Alopecic |
Stayed Fully Haired |
Gained Hair |
Total |
|---|---|---|---|---|
| Males | 7 | 49 | 11 | 67 |
| Females | 13 | 48 | 14 | 75 |
| Total | 20 | 97 | 25 | 142 |
Rhesus macaques at all three facilities received commercial chow (LabDiet 5038, PMI, St Louis, MO) provided twice daily, supplemented with produce, fruits, and grains. Additionally these centers used the same day/night cycle (12/12) with little across center variation in room temperatures (Facility A = 19-25°C; Facility B = 18-27°C; and Facility C = 22-25°C). The macaques were maintained in accordance with the Guide for the Care and Use of Laboratory Animals and the research complied with protocols approved by the appropriate Institutional Animal Care Committee (Oregon Health Sciences IACUC, University of Washington IACUC, and Southwest Foundation for Biomedical Research IACUC). In addition, this research adhered to the American Society of Primatologists (ASP) Principles for the Ethical Treatment of Non-Human Primates (see https://www.asp.org/society/resolutions/EthicalTreatmentOfNonHumanPrimates.cfm.)
Procedure
Both procedures (photography and hair sample collection) took place during routine health exams. Animals were anesthetized (ketamine 10mg/kg) for the exam. The monkeys were initially photographed on a white mat background in three positions (left side, right side, and prone). After the photos were taken, a small 2 cm2 patch of hair was gently cut with clippers as close to the skin surface at the nape of the neck as possible. In general, even in severe cases of alopecia, a sufficient hair sample can be obtained from the nape of the neck or the top of the head. Hair was then packaged in aluminum foil and stored at 4 degrees Celsius. Subject photos and hair samples were shipped to the University of Massachusetts where the hair samples were analyzed for cortisol concentrations in our Assay Core, and the photos were scored for amount of alopecia. A total of two samples per monkey was collected 6-8 months apart.
Alopecia Scoring
Photos collected from the first sample were digitally scored in order to obtain a percent alopecia score using a process previously described in Novak et al., 2014. During the sample collection procedure, the monkeys were laid gently on a light background and the same kind of ruler was placed next to the monkey. The distance between the camera and the monkey was standardized across facilities. Blurry photographs were not scored, and those subjects were eliminated from the data set from the beginning (1 case). In this process, we obtained 3 photographs of each monkey lying on its left side, right side, or in the prone position. Alopecia scoring was restricted to the dark coat eliminating the inner white coat of the chest and abdomen, inner thighs and inner upper arm as well as the hands and feet. Photoshop CS6 (Adobe) was used to outline the body perimeter and exclude these non-scoring areas. The most important positions were the left and right side because the outer arms and legs could be visualized clearly, and there was no possible overlap in scores derived from the right and left side. When the animal was in the prone position, the legs and arms were not scored as they could be captured fully from the left and right side scoring.
Subsequently, open source ImageJ software (National Institutes of Health) was used to calculate body area and score regions of alopecia. We examined areas of alopecia by using the threshold image adjuster in ImageJ, thus highlighting areas of alopecia in red. The threshold was set to identify any patches of alopecia that were 0.5 cm2 or larger in the photograph. A ruler contained in each photograph ensured that this criterion was applied equally across all subjects. When the cursor clicked on a particular patch, the program automatically calculated its area and maintained a sum for each animal as the cursor was moved from patch to patch, yielding a total area score for each position which was divided by the total dark coat area derived for that position. The process was repeated with the remaining 2 positions and the 3 scores were summed to create an overall alopecia score. The only part of this process that involved possible subjectivity was outlining the body area. A rescoring of 15 randomly selected images balanced across facilities yielded an average percentage agreement score of 96.5%, with a range across images of 93.8% to 99.0%.
To determine whether alopecia status changed, the photos from each sampling period were compared visually by two highly trained digitizers. The three position photos (left, right, and prone) were placed on a single page and then were sorted by the digitizers into 3 coat change categories: 1) subjects who remained alopecic (gained or lost hair by less than 15%), 2) subjects who remained fully haired across both sample collections, and 3) subjects who gained hair by 15% or more. Each coder sorted the photographs independently, and then the results were compared. The observers were blind to the individual monkey ID and to the facility. The two primary coders agreed on 134 cases, yielding an interobserver reliability between the two coders of greater than 94%. A third reviewer scored approximately 20% of the images including the 8 cases in which there was disagreement. Although this sorting procedure did not allow us to determine the actual amount of hair loss, we were only interested in creating a categorical variable (change) and not in determining the precise amount of the change.
Cortisol Extraction and Assay
Coded hair samples were sent to the UMass Hormone Assay Core and assayed by technicians who were blind to the alopecia status, facility, and sex of the monkeys. The hair was assayed according to the method described in Davenport et al., 2006 and depicted on a published video [Meyer et al., 2014]. Hair samples were washed twice in isopropanol (5ml) in order to remove any surface contaminants. Hair was allowed to dry for several days at room temperature. Once dry, each sample was ground into a fine powder using a Retsch ball mill. Fifty milligrams (+/− .5mg) of finely ground hair was weighed and transferred to a microcentrifuge tube. Methanol (1.5ml) was added and rotated overnight at room temperature. Samples were then centrifuged and the supernatant was dried down in a vacuum evaporator. Dried extracts were reconstituted using an assay buffer. Hair cortisol concentrations were determined using the Salimetrics assay kit (State College, PA). The intra-assay and inter-assay coefficient of variation was 1.6% and 7.0% respectively.
Data Analysis
For the first sample (198 subjects), we examined the replicability of a relationship between the first hair sample (hair CORT1) and alopecia in a larger sample of monkeys as compared to the data reported in Novak et al., 2014. The data were subjected to two Analyses of Variance (ANOVA) with condition (alopecic or fully haired) and facility as between subject variables, one for females and one for males. Because the hair CORT1 data were not normally distributed for females (Shapiro-Wilk Test = 0.96, p< 0.01) or males (Shapiro-Wilk Test = 0.85, p< 0.01), the data were transformed using a log transformation. This resulted in normalizing the data for both females (Shapiro-Wilk Test = 0.99, p = 0.36) and males (Shapiro-Wilk Test = 0.98, p<0.08). In the non-transformed data, heterogeneity of variance was detected only for females (Levene's Test = 6.85, p< 0.01), this effect was reduced in the transformed data (Levene's Test = 3.00, p <0.07). The log transformation yielded similar significance effects to the non-transformed data, most likely because the residuals were normally distributed, and therefore the non-transformed data are conveyed statistically and portrayed in the figures. Subsequent significant effects in the ANOVA were evaluated using Bonferroni pairwise comparisons.
For subjects with both samples (142 subjects), the correlation between the first and second hair CORT sample was analyzed for sex and for each coat change category using Pearson correlations with Bonferroni probabilities. A difference score was then created (hair CORT2 – hair CORT1). A negative score indicated that hair CORT concentrations decreased across the two samples whereas a positive score indicated that hair CORT concentrations increased across the two sampling periods. The difference score was then subjected to ANOVA with Coat Change (stayed alopecic, stayed fully haired, or gained hair) and sex as the between subject variables. Bonferroni pairwise comparisons were used to compare different groups. Subsequent inspection revealed that the data lacked normality and homogeneity of variance. No transformation solved this problem. The data were then subject to a nonparametric Kruskall-Wallace one-way ANOVA across six groups (three coat conditions in females and three coat conditions in males). This procedure allowed us to subsequently compare specific pairs of male and female groups using the Mann-Whitney U Test. Because the findings were identical, both the parametric ANOVA on the non-transformed data and the Kruskall-Wallace Test and Mann Whitney U tests are reported here. Data reported in the text represent means and standard errors of the mean (± SEM).
To examine the possible influence of other factors on the hair cortisol data, we also examined the timing of the sample collection which varied across facilities and subjects as a function of the schedule of routine health exams. The 6-8 month interval between samples was maintained for the individual subjects. To determine whether there was an effect of time of year, the hair cortisol results were initially subjected to an analysis of variance (ANOVA) using Systat Software (Systat Software, San Jose, CA) with season (spring, summer, fall, and winter) as the between subjects variable. There was no effect of season on either hair CORT1 with 198 subjects (F = 1.48(3,194); p =0.22) or hair CORT2 with 142 subjects (F = 0.90(3,138); p = 0.40), and thus this variable was not considered further. The lack of seasonal variation is not surprising given that monkeys at the three facilities were maintained under the same constant day/night cycle. There was also no effect of housing condition (individual, pair, or grooming contact) on hair CORT2 with 142 subjects (F = 1.41(2,139); p = 0.25).
RESULTS
Replicability in Females
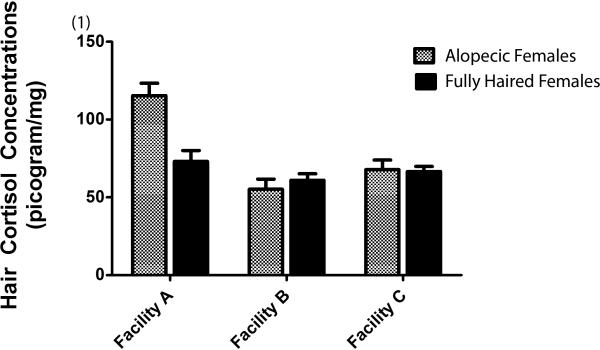
A comparison of a much larger sample of female monkeys yielded essentially the same effects as reported in Novak et al., 2014. Alopecic female monkeys had significantly higher hair cortisol levels than their fully haired counterparts (alopecic mean = 79.49 ± 4.00 vs. fully haired mean = 66.87 ± 3.01; F = 6.35(1,103); p = 0.01). Hair CORT levels also varied by facility (Facility A = 94.23 ± 5.33; Facility B = 58.11 ± 3.88; Facility C = 67.21; ± 3.61; F = 15.28(2,103) = 17.48; p <0.01). Pairwise Bonferroni comparisons revealed that female monkeys at Facility A had higher hair CORT than monkeys at Facility B (p< 0.01) and at Facility C (p< 0.01) whereas hair CORT levels in monkeys at Facilities B and C did not differ (p = 0.27). A significant interaction of coat condition with facility (F = 7.17(2,103); p <0.01) revealed that the largest difference in hair CORT as a function of alopecia status was observed in Facility A (see Figure 1). Bonferroni pairwise comparisons revealed that the alopecic female monkeys at Facility A had significantly higher hair CORT concentrations than all other groups, i.e., alopecic monkeys at Facility B (p<0.01) and Facility C (p< 0.01) as well as fully haired monkeys at all three facilities (A, p< 0.01; B, p< 0.01, and C, p < 0.01).
Fig. 1.
Average hair cortisol concentrations (± SEM) in alopecic and fully haired females as a function of facility
Replicability in Males
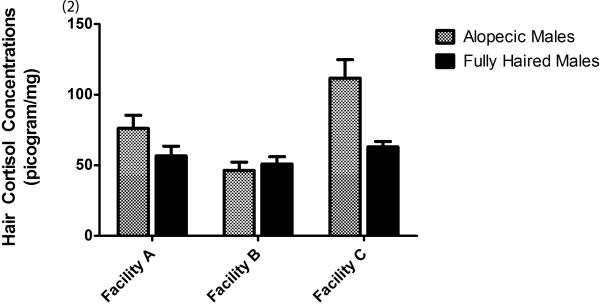
In males, alopecic male monkeys had had significantly higher hair cortisol levels than fully haired monkeys (alopecic mean = 77.88 ± 5.68 vs. fully haired mean = 56.86 ± 3.13; F = 10.50(1,83); p = 0.01). Hair CORT also varied significantly by facility (Facility A = 66.39 ± 5.74; Facility B = 48.61 ± 3.91; Facility C = 87.11; ± 6.81; F = 12.78(2,83) = 12.78; p <0.01). Pairwise Bonferroni comparisons showed that males at facility A had higher hair CORT concentrations than males at Facility B (p <0.04), an effect reported in Novak et al., 2014. A significant interaction of Hair CORT with facility (F = 5.96(2.83); p <0.01) revealed that hair CORT concentrations in male alopecic monkeys at Facility C were significantly higher than male fully haired monkeys at all three facilities (Facility A, p <0.01; Facility B, p<0.01, and Facility C, p = 0.01) and alopecic monkeys at Facility B; see Figure 2). It should be noted that there were only 3 alopecic monkeys at Facility C. If Facility C is eliminated, then hair CORT concentrations in all of the male monkeys were significantly elevated in Facility A compared to Facility B (F = 9.68(1.47); p <0.01).
Fig. 2.
Average hair cortisol concentrations (± SEM) in alopecic and fully haired males as a function of facility.
Proportion of Severely Alopecic Monkeys
Although each facility had monkeys with relatively severe alopecia, Facility A had a higher proportion of monkeys that were severely alopecic than Facilities B and C. Using a criterion of 50% or more hair loss, derived from the digitized scores, Facility A had 8 monkeys in this category. In contrast, Facilities B and C had only one monkey each that met the criterion.
Change in Alopecia Status
The aim of this analysis was to examine changes in hair CORT concentrations across two samples in association with changes in alopecia status. Even though the timing of sampling collection varied widely across subjects, alopecia status remained stable across the two sampling periods in this convenience sample of 142 monkeys. Only 17% of the subjects showed 15% or more hair regrowth (11 males, 14 females). Fifteen percent remained alopecic (7 males, 14 females) whereas 68% remained fully haired (49 males, 48 females). Because this is a convenience sample wherein we attempted to get as many hair samples as possible from both alopecic and fully haired monkeys, the actual percentages in the convenience sample should not be taken to reflect the prevalence of hair loss at these facilities. Hair cortisol concentrations were highly correlated across sampling periods in the two groups that either remained alopecic (r=0.87, p<0.01) or fully haired (r=0.43, p<0.01). Not surprisingly, hair cortisol concentrations were uncorrelated in the monkeys that showed a significant gain of hair (r=0.09, p = 0.66).
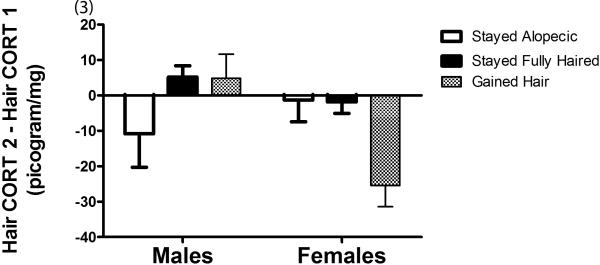
The hypothesis that changes in hair cortisol were tightly linked to changes in hair coat condition was then examined. An ANOVA revealed a marginally significant main effect of sex (F(1.136) = 4.03, p = 0.051) with females showing a decline in hair CORT concentrations as compared to males; mean difference score for females −9.00 ± SEM 3.09 vs. males +0.81 ± SEM 3.79). This finding was further explained by a significant interaction of change by sex (F(2.136) = 4.45, p = 0.01). Subsequent Bonferroni pairwise comparisons revealed that females that gained hair showed a significant reduction in hair CORT from sample one to sample two compared to females that remained fully haired (p< 0.01), males that remained fully haired (p< 0.01) and males than gained hair (p< 0.01; see Figure 3). The data were also significant using a non-parametric test. A Kruskall-Wallace Test was significant across the six test groups (χ2 = 12.60, p <0.05). Subsequent pairwise comparisons using the Mann Whitney U Test statistic revealed that females that gained hair showed a significant reduction in hair CORT from sample one to sample two compared to females that remained fully haired (U= 197.00, p <0.02), males that remained fully haired (U = 130.00. p <0.02, and males that gained hair (U = 28.00, p <0.01). Other pairwise comparisons were not significant. Thus the nonparametric data yielded exactly the same findings as ANOVA.
Fig. 3.
Difference in hair cortisol concentrations (sample 2 – sample 1; ± SEM) for males and females who gained hair, stayed alopecic, and stayed fully haired between sampling periods.
DISCUSSION
This study extends our previous findings of a relationship between HPA axis activity and hair loss in monkeys at some facilities and further suggests that coat condition tends to remain relatively stable over long periods of time. We replicated previous findings with a larger sample showing that extensive hair loss was associated with increased hair CORT concentrations and that the effect was facility dependent. In females, this effect was most pronounced in Facility A as previously reported [Novak et al., 2014]. In males, this effect was strongest in Facility C. This latter finding is new because in the previous article, no alopecic males were sampled from Facility C. However, we were able to obtain samples from only 3 males at this facility; thus caution should be used in interpreting this effect. On the other hand, if Facility C is excluded from the analysis, the effects of facility remain with males at Facility A showing significantly higher hair CORT concentrations than males at Facility B.
In this study, many monkeys in the convenience sample retained their coat condition status (alopecic or full haired) across 8 months or more. Although this finding suggests that coat condition may be relatively stable across long periods of time, we did not have access to a randomly derived sample of monkeys at these facilities to provide verification of this effect. At the very least, we can conclude that for some monkeys, alopecia is not a temporary phenomenon lasting only a few months.
In assessing monkeys with two samples six to eight months apart, hair CORT concentrations were significantly correlated from across samples for monkeys that remained alopecic and for monkeys that maintained a fully haired coat. The prediction that regrowth of hair should be associated with a reduction in hair CORT concentrations was confirmed for females, but not for males. It is not clear why this effect was present only in females. However, it should be noted that prevalence rates for alopecia are considerably higher in females than males [Lutz et al., 2013], and females are more likely to show extreme amounts of hair loss [Novak et al., 2014].
This research was focused on the question, is hair loss a biomarker for stress? Our answer to the question is not definitive for two reasons. First, we did not manipulate hair condition experimentally, and indeed it might be difficult to replicate the gradual hair loss process in macaques. Although we added some information to this relationship, namely that regrowth of hair is associated with a reduction in hair cortisol concentrations, we cannot assert cause and effect. There are at least two possible interpretations of the relationship between hair loss and stress. Monkeys are exposed to stressors which elevate hair cortisol concentrations which in turn lead to hair loss, or monkeys experience hair loss for an entirely different reason and elevations in hair cortisol concentration are possibly related to changes in other systems (e.g., thermoregulation or development of disease states such as hypercortisolemia).
Importantly, our research also suggests that the relationship between hair loss and cortisol concentrations is dependent on the severity of hair loss. Only monkeys with greater than 30% hair loss showed significant elevations in hair cortisol concentrations. Furthermore, the most severely alopecic monkeys (> 50% hair loss) were disproportionately located at Facility A, which may, in part, explain the facility effect. Thus, hair loss may be a biomarker for stress only in monkeys with severe hair loss. This is consistent with our previous findings suggesting that milder forms of hair loss may be related to non-stress related factors such as friction with cage or enrichment surfaces [Novak et al., 2014]. From a regulatory perspective, the mere presence of hair loss is probably not a biomarker for stress; other factors such as the severity of hair loss and health information should be taken into account.
These findings reveal the value of conducting research across multiple facilities. Although the expectation is that research findings at one facility should be “replicated” at another facility, this is frequently not the case and can result in disagreements about who is “right.” But that is essentially the wrong issue. As noted by Warren Burggren [2014], far too often “our inability to recognize and control for variation has, to some extent, contributed to an unfortunate disciplinary mindset of what might be called ‘my data versus your data’ rather than simply ‘the data’.” Our findings differed as a function of facility. Had the research been conducted only at facility B, the conclusions would be starkly different than those drawn from facilities A. Thus a multiple facility study allows us to examine the possible conditions in which relationships seem to develop and to rule out factors that are common across facilities. While all 3 facilities were similar on a number of parameters (day/night cycles), cage housing, chow manufacturer, and enrichment programs, there are obviously a number of other variables that might contribute to psychological stress-based model of hair loss (e.g., caretaker turnover) or to a possible non-stress based model of hair loss (e.g., genetic and epigenetic differences, Cushing's Syndrome). Moving forward, the challenge is to understand the totality of the data set (e.g., facility effects) in answering the questions, is hair loss a possible biomarker for stress, and if so what factors are more likely to elicit hair loss?
ACKNOWLEDGEMENTS
This research was supported by grant nos. R24OD01180-15 (to MAN), P51OD011133 to Texas Biomedical Research Institute (SNPRC), 8P51OD011092-53 to the Oregon National Primate Research Center (ONPRC), and P51OD010425 to the Washington National Primate Research Center (WaNPRC). We thank the technicians who assisted in data collection and analysis: Kim Linsenbardt (SNPRC), Nicola D Robertson (ONPRC), and Grace Lee and Rose Kroeker (WaNPRC).
REFERENCES
- Accorsi PA, Carloni E, Valsecchi P. Cortisol determination in hair and feces from domestic cats and dogs. General and Comparative Endocrinology. 2008;155:398–402. doi: 10.1016/j.ygcen.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Aoki E, Shibasaki T, Kawana S. Intermittent foot-shock stress prolongs the telogen stage in the hair cycle of mice. Experimental Dermatology. 2003;12:371–377. doi: 10.1034/j.1600-0625.2002.120403.x. [DOI] [PubMed] [Google Scholar]
- Bechshoft TO, Sonne C, Dietz R, et al. Cortisol levels in hair of East Greenland polar bears. Science of the Total Environment. 2011;409:831–834.s. doi: 10.1016/j.scitotenv.2010.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burggren W. Epigenetics as a source of variation in comparative animal physiology - or - Lamarck is lookin' pretty good these days. Journal of Experimental Biology. 2014;217:682–689. doi: 10.1242/jeb.086132. [DOI] [PubMed] [Google Scholar]
- Carlitz EHD, Kirschbaum C, Stalder T, van Schaik CP. Hair as a long-term retrospective cortisol calendar in orangutans (Pongo spp): new perspectives for stress monitoring in captive management and conservation. General and Comparative Endocrinology. 2014;195:151–156. doi: 10.1016/j.ygcen.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Davenport MD, Lutz CK, Tiefenbacher S, Novak MA, Meyer JS. A rhesus monkey model of self-injury: effects of relocation stress on behavior and neuroendocrine function. Biological Psychiatry. 2008;63:990–996. doi: 10.1016/j.biopsych.2007.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport MD, Tiefenbacher ST, Lutz CK, Novak MA, Meyer JS. Analysis of endogenous cortisol concentrations in the hair of rhesus macaques. General and Comparative Endocrinology. 2006;147:255–261. doi: 10.1016/j.ygcen.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Fairbanks LA, Jorgensen MJ, Bailey JN, et al. Heritability and genetic correlation of hair cortisol in vervet monkeys in low and high stress environments. Psychoneuroendocrinology. 2011;36:1201–1209. doi: 10.1016/j.psyneuen.2011.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isbell LA. Seasonal and social correlates of changes in hair, skin, and scrotal condition in vervet monkeys (Cercopithecus aethiops) of Amboseli National Park, Kenya. American Journal of Primatology. 1995;36:61–70. doi: 10.1002/ajp.1350360105. [DOI] [PubMed] [Google Scholar]
- Katayama M, Aoki E, Suzuki H, Kawana S. Foot-shock stress prolongs the telogen stage of the spontaneous hair cycle in a nondepilated mouse model. Experimental Dermatology. 2007;16:553–560. doi: 10.1111/j.1600-0625.2007.00558.x. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Tietze A, Skoluda N, Dettenborn L. Hair as a retrospective calendar of cortisol production-increased cortisol incorporation into hair in the third trimester of pregnancy. Psychoneuroendocrinology. 2009;34:32–37. doi: 10.1016/j.psyneuen.2008.08.024. [DOI] [PubMed] [Google Scholar]
- Kramer J, Fahey M, Santos R, et al. Alopecia in rhesus macaques correlates with immunophenotypic alterations in dermal inflammatory infiltrates consistent with hypersensitivity etiology. Journal of Medical Primatology. 2010;39:112–122. doi: 10.1111/j.1600-0684.2010.00402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroeker R, Bellanca RU, Lee GH, Thom JP, Worlein JM. Alopecia in 3 macaque species housed in a laboratory environment. American Journal of Primatology. 2014;76:325–334. doi: 10.1002/ajp.22236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lair S, Crawshaw GJ, Mehren KG, Perrone MA. Diagnosis of hypothyroidism in a Western lowland gorilla (Gorilla gorilla gorilla) using human thyroid-stimulating hormone assay. Journal of Zoo and Wildlife Medicine. 1999;30:537–540. [PubMed] [Google Scholar]
- Lutz CK, Coleman K, Worlein J, Novak MA. Hair loss and hair pulling in rhesus macaques. Journal of the American Association for Laboratory Animal Science. 2013;52:454–457. [PMC free article] [PubMed] [Google Scholar]
- Malinow MR, Bardana EJ, Pirofsky B, Craig S, McLaughlin P. Systemic lupus erythematosus-like syndrome in monkeys fed alfalfa sprouts: role of a nonprotein amino acid. Science. 1982;216:415–417. doi: 10.1126/science.7071589. [DOI] [PubMed] [Google Scholar]
- Meyer JS, Novak MA. Minireview: Hair Cortisol: A Novel Biomarker of Hypothalamic-Pituitary-Adrenocortical Activity. Endocrinology. 2012;153:4120–4127. doi: 10.1210/en.2012-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JS, Novak MA, Hamel AF, Rosenberg K. Extraction and analysis of cortisol from human and monkey hair. Journal of Visualized Experiments. 2014;83:e50882. doi: 10.3791/50882. doi:10.3791/50882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak MA, Hamel AF, Coleman K, et al. Hair loss and hypothalamic–pituitary–adrenocortical axis activity in captive rhesus macaques (Macaca mulatta). Journal of the American Association for Laboratory Animal Science. 2014;53:261–266. [PMC free article] [PubMed] [Google Scholar]
- Novak MA, Meyer JM. Alopecia: possible causes and treatments with an emphasis on captive nonhuman primates. Comparative Medicine. 2009;59:18–26. [PMC free article] [PubMed] [Google Scholar]
- O'Connor TM, O'Halloran DJ, Shanahan F. The stress response and the hypothalamic-pituitary-adrenal axis: from molecule to melancholia. QJM: An International Journal of Medicine. 2000;93:323–333. doi: 10.1093/qjmed/93.6.323. [DOI] [PubMed] [Google Scholar]
- Steinmetz HW, Kaumanns W, Dix I, et al. Coat condition, housing condition and measurement of faecal cortisol metabolites—a noninvasive study about alopecia in captive rhesus macaques (Macaca mulatta). Journal of Medical Primatology. 2006;35:3–11. doi: 10.1111/j.1600-0684.2005.00141.x. [DOI] [PubMed] [Google Scholar]
- Swenerton H, Hurley LS. Zinc deficiency in rhesus and bonnet monkeys, including effects on reproduction. Journal of Nutrition. 1980;110:575–583. doi: 10.1093/jn/110.3.575. [DOI] [PubMed] [Google Scholar]
- Thomson S, Koren G, Fraser L-A, Rieder M, Friedman TC, Van Uum SHM. Hair analysis provides a historical record of cortisol levels in cushing's syndrome. Experimental and Clinical Endocrinology and Diabetes. 2009;118:133–138. doi: 10.1055/s-0029-1220771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikramanayake TC, Alvarez-Connelly E, Simon J, et al. Heat treatment increases the incidence of alopecia aureate in the C3H/HeJ mouse model. Cell Stress and Chaperones. 2010;15:985–991. doi: 10.1007/s12192-010-0209-7. [DOI] [PMC free article] [PubMed] [Google Scholar]