Abstract
Paraoxonase 2 (PON2) is a member of the paraoxonase gene family also comprising PON1 and PON3. PON2 functions as a lactonase and exhibits anti-bacterial as well as antioxidant properties. At the cellular level, PON2 localizes to the mitochondrial and endoplasmic reticulum membranes where it scavenges reactive oxygen species. PON2 is of particular interest as it is the only paraoxonase expressed in brain tissue and appears to play a critical role in mitigating oxidative stress in the brain. The aim of this study was to investigate the expression of PON2 at the protein and mRNA level in the brain and liver of mice through development to identify potential age windows of susceptibility to oxidative stress, as well as to compare expression of hepatic PON2 to expression of PON1 and PON3. Overall, PON2 expression in the brain was lower in neonatal mice and increased with age up to postnatal day (PND) 21, with a significant decrease observed at PND 30 and 60. In contrast, the liver showed continuously increasing levels of PON2 with age, similar to the patterns of PON1 and PON3. PON2 protein levels were also investigated in brain samples from non-human primates, with PON2 increasing with age up to the infant stage and decreasing at the juvenile stage, mirroring the results observed in the mouse brain. These variable expression levels of PON2 suggest that neonatal and young adult animals may be more susceptible to neurological insult by oxidants due to lower levels of PON2 in the brain.
Keywords: Paraoxonase 2, development, brain, liver, oxidative stress, paraoxonase 1, paraoxonase 3
1. INTRODUCTION
The paraoxonase gene family consists of three closely related genes, PON1, PON2 and PON3, aligned in tandem on mouse chromosome 6 and the long arm of human chromosome 7q21-22 [1]. Based on phylogenetic analysis, PON2 is thought to be the oldest of the PONs, from which PON1 and PON3 evolved [2]. Only PON1 exhibits esterase activity toward a number of organophosphates, such as paraoxon, the active metabolite of the insecticide parathion, from which the gene family was named. However, all three PONs possess lactonase activity, exhibiting overlapping but distinct substrate specificities for lactone hydrolysis [3]. Of the three PONs, PON2 has the highest hydrolytic activity for acyl-homoserine lactones (acyl-HCL), molecules that are important for mediating bacterial quorum-sensing signals and are key regulators of bacterial virulence factors affecting the host inflammatory response [3–6]. Beyond bacterial quorum quenching, the importance of the lactonase activity of PON2 has yet to be fully understood.
In contrast to PON1 and PON3, PON2 is expressed in most tissues examined and has been shown to exert an antioxidant effect [6–11], with subcellular localization studies suggesting it localizes primarily to the mitochondrial membrane [10, 12]. Mitochondria are a considerable source of oxidative stress [13], and the predominant localization of PON2 in mitochondria supports a role for it to prevent oxidative damage at the mitochondrial level. Additional studies have shown PON2 interacts with Coenzyme Q10 associated with Complex III in the mitochondria, likely scavenging reactive oxygen species (ROS) produced during oxidative phosphorylation [12]. Superoxide (O2−) is a common ROS produced as an incidental by-product of the electron transport chain and leads to the production of hydrogen peroxide (H2O2), another ROS, which can then lead to the production of hydroxyl radicals [14]. Increased levels of ROS are associated with numerous disorders such as cancer [15], cardiovascular disease [16], neurodegenerative diseases [17, 18], and diabetes [19], highlighting the importance of proper antioxidant control of ROS.
PON2 is necessary for properly functioning mitochondria, as PON2-deficient mice display mitochondrial dysfunction [12]. In the brain, PON2 expression has been found highest in dopaminergic regions, namely the nucleus accumbens, striatum and substantia nigra [10]. Upon examination of specific cell types, astrocytes have been found to have significantly higher PON2 levels than neurons, with the loss of PON2 in both cell types impairing their ability to recover from toxic levels of oxidative stress generated by oxidants hydrogen peroxide (H2O2) or 2,3-dimethoxy-1,4-naphthoquinone (DMNQ) [10]. Glutathione levels are identical in cells from wildtype and PON2 knockout mice, suggesting that the difference in susceptibility to oxidative stress toxicity is due to PON2 and is not the consequence of other affected pathways [10]. Accordingly, PON2 appears to be a critical antioxidant in the central nervous system (CNS), with the loss of PON2 predisposing cells to increased ROS and subsequently death.
In addition to regional differences in the brain, gender differences have been described in PON2 expression [11]. Female mice have consistently higher levels of PON2 than male mice, most likely owing to the differences in estradiol between sexes, as PON2 expression appears to be modulated by estrogens [11]. The polyphenolic compound quercetin, a phytoestrogen, also modulates PON2 expression and may utilize similar signaling pathways as estrogen to influence PON2 transcription [20]. Also of relevance is that, in addition to differential susceptibility to oxidative stress between wild-type and PON2-null mice [10], significant differences in oxidant susceptibility have been associated with different levels of PON2 expression, exemplified by male and female differences in PON2 levels [11], or after positive modulation of PON2 by estradiol [11] or quercetin [20].
Given these findings, investigation of additional modulating factors, such as age, are of interest to further understand the regulation of PON2. The aim of the present study was to determine PON2 expression during development in brain and liver and, in the latter, to compare it with that of PON1 and PON3.
2. MATERIALS AND METHODS
2.1 Materials
Anti-PON1, PON2, and PON3 antibodies were from Abcam (Cambridge, MA, USA). Anti β-actin antibody was from Sigma-Aldrich (St. Louis, MO, USA), while 10× Tris-buffered saline, Tween-20, Coomassie Brilliant Blue R-250 were from Bio-Rad Laboratories (Hercules, CA, USA). XCell II™ Blot Module, XCell SureLock™ Electrophoresis Cell, NuPAGE® MOPS SDS Running Buffer 20×, NuPAGE® LDS Sample Buffer 4×, NuPAGE® Antioxidant, NuPAGE® Sample Reducing Agent 10× and NuPAGE® 10% Bis-Tris Protein Gels were from Life Technologies (Carlsbad, CA, USA). Immobilon®-P Transfer Membrane was from Millipore Corporation (Billerica, MA, USA). Restore™ Western Blot Stripping Buffer, PageRuler™ Prestained Protein Ladder, SuperSignal® West Pico Chemiluminescent Substrate, SuperScript® III Reverse Transcriptase and TaqMan® Gene Expression Assays were from Thermo-Fisher Scientific (Waltham, MA, USA). Antirabbit IgG HRP-linked antibody and Cell Lysis Buffer 10× were from Cell Signaling Technology (Danvers, MA, USA). HRP Goat Anti-Mouse Ig was from BD Biosciences (San Jose, CA, USA). RNeasy® Mini Kit was purchased from Qiagen (Hilden, Germany). TaqMan® Gene Expression Master Mix was from Applied Biosystems (Foster City, CA, USA).
2.2 Animals and tissue samples
2.2.1 Mice
Wild-type C57BL/6J mice were used for this study. Mice were sacrificed at postnatal day (PND) 1, 7, 14, 21, 30 or 60 and brain and livers were removed and flash-frozen in liquid nitrogen. Whole brains were pulverized with a pre-chilled mortar and pestle into a fine powder, stored at −80°C and aliquoted into appropriate lysis buffers for protein and RNA extraction as needed, utilizing sonication for homogenization. Liver tissue was mechanically homogenized in appropriate lysis buffer using a Wheaton Potter-Elvehjem tissue grinder followed by sonication. Mice were housed in a specific pathogen-free facility on a 12-hour light/dark cycle with ad libitum access to food and water. All procedures were conducted in accordance with the National Institute of Health Guide for the Use and Care of Laboratory Animals and were approved by the University of Washington Institutional Animal Care and Use Committee.
2.2.2 Non-human primate samples
Flash frozen ventral caudate samples from brains of African green or vervet monkeys (Chlorocebus sabaeus) were provided by Dr. John Elsworth from Yale University School of Medicine. Animals were housed in the St. Kitts Biomedical Research Foundation, an AAALAC accredited facility. All tissue samples were from animals that were controls for other studies each with their own protocol approved by the Yale University Institutional Animal Care and Use Committee. Tissue samples were shipped on dry ice and processed as the mouse samples.
2.3 Immunoblotting
Immunoblots were carried out as previously described [21]. Briefly, 25 µg of protein was mixed with SDS running buffer and sample reducing agent and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Following electrophoresis, proteins were transferred to polyvinylidene difluoride membranes and the membrane blocked for 1 hour with 5% nonfat milk. Membranes were then probed with the following diluted primary antibodies: 1:750 for brain PON2, 1:1000 for liver PON1, PON2 and PON3. Following primary antibody incubation, membranes were washed with Tris-buffered saline with 0.1% Tween-20 (pH = 7.5) and incubated with horseradish peroxidase-conjugated anti-rabbit secondary antibody at a dilution of 1:1500 (brain PON2) or 1:1000 (liver PON2), or with horseradish peroxidase-conjugated anti-mouse secondary antibody at a dilution of 1:1000 (liver PON1 and PON3). Membranes were stripped with Restore™ Western Blot Stripping Buffer and re-probed for β-actin using a dilution of 1:1000 for the β-actin primary antibody and 1:2500 for the horseradish peroxidase-conjugated anti-mouse secondary antibody. Total protein was measured using Coomassie Brilliant Blue R-250 at a concentration of 0.025% weight/volume. Intensity of bands was measured by densitometry using ImageJ software (NIH), with the band intensity normalized to β-actin expression or total protein as noted. Monkey brain tissue was homogenized in CLB buffer (10 mM HEPES, 150 mM NaCl, 1 mM CaCl2, 0.5 mM MgCl2, 1 mM PMSF,50 mM NaF, 1 mM sodium orthovanadate) and whole homogenates were subjected to SDS-PAGE and immunoblotting using rabbit anti-PON2 (1:500), or mouse anti-β-actin (1:1000) antibodies. After electrophoresis, proteins were transferred to polyvinylidene difluoride membranes and incubated with the above antibodies. Membranes were rinsed in Tris-buffered saline with 0.1% Tween-20 and incubated with horseradish peroxidase–conjugated anti-rabbit IgG or with horseradish peroxidase–conjugated anti-mouse IgG (for actin) at the appropriate dilutions (1:2000, 1:5000, respectively).
2.4 RT-PCR
PONs mRNA levels were measured by RT-PCR. Reverse transcription was performed according to the manufacturer's established protocol using total RNA and the SuperScript® III First-Strand Synthesis System. For gene expression measurements, 4 µL of cDNA were included in a PCR reaction (20 µL final volume) that also consisted of 1 µL of appropriate TaqMan® Gene Expression Assay mixture and 10 µL TaqMan® Gene Expression Master Mix. Amplification and detection of PCR amplicons were performed with the ABI PRISM 7900 system (Applied Biosystems, Foster City, CA) with the following PCR reaction profile: 1 cycle of 95°C for 10 min, 40 cycles of 95°C for 30 sec and 62°C for 1 min. β-actin amplification plots taken from serial dilutions of an established reference sample were used to create a linear regression formula in order to calculate expression levels, and β-actin gene expression levels were utilized as an internal control to normalize the data.
2.5 Statistical Analysis
Data are expressed as the mean ± SEM of at least three independent experiments. One-way ANOVA followed by the Tukey test for multiple comparisons was utilized for statistical analysis, while Student’s t-test was utilized for comparing two groups as noted.
3. RESULTS
3.1 PON2 Developmental Expression in Brain
3.1.1 PON2 in mouse brain
In a first experiment, we determined whether PON2 sex differences would be observed in whole brain. Fig. 1 shows that levels of PON2 protein in whole of brain of female mice (PND 21) were significantly higher than in males. Using a polyclonal antibody from Abcam (ab409969), more than one band is detected (Fig. 1B). PON2 is seen at its expected MW of 43 kDa, and this band was quantified in this and all other experiments. Western blot analysis using PON2 knockout brain tissue has shown the 43kDa band to be specific to PON2 with the polyclonal antibody used in this study (data not shown).
Figure 1. PON2 protein levels in whole brain of mice.
A. Quantification of PON2 protein in whole brain of female and male PND 21 mice after normalization to β-actin. Results show the means (± SE) of 3 animals/sex. Significantly different from male, *** p < 0.001. (Student’s t-test). B. Western blots of the same brain samples. PON2 is seen at ~43kDa.
Based on the observed sex difference at PND 21, female mice were utilized for the purposes of this study, due to their higher levels of PON2. Figs. 2A and 2B show PON2 protein and mRNA expression levels, respectively, in whole mouse brain over the course of postnatal development (PND 1, 7, 14, 21, 30 and 60) capturing the developmental scale of neonatal to young adulthood. PON2 protein appears to be lowest directly after birth, steadily increasing with age up until PND 21. A significant decrease in both protein and mRNA is noted from PND 21 to 30 and continuing to PND 60.
Figure 2. PON2 protein and mRNA levels in brain of female mice during postnatal development.
A. Quantification of PON2 protein after normalization to total protein. Results are expressed as percentage of PND 1 optical density (mean ± SE, with n = 4). a, b, c: significantly different from PND 1, PND 30, and PND 60, respectively (all p < 0.01). B. Levels of PON2 mRNA normalized for β-actin. Results represent the mean (± SE) of 3–4 mice. Significantly different from PND 1, * p < 0.05.
3.1.2 PON2 in the brain of non-human primates
We also measured PON2 protein levels in the ventral caudate region of the brain from African green monkeys across development. The investigated developmental time points were mid-gestation (gestational day 89), late-gestation (gestational day 148), infant (PND 27), juvenile (1.9 years), young adult (5–7 years), and aged (21–30 years). PON2 protein levels were lower at mid-gestation and gradually increased up to infant age (Fig. 3A). This was followed by a decrease in the juvenile stage and a stabilization, in which similar levels were found in young adult and in aged monkey brains (Fig. 3A). We also investigated sex differences in PON2 expression in brains from non-human primates, with Figs. 3B and 3C showing a significant difference in PON2 protein level between male and female young adult monkeys.
Figure 3. PON2 protein levels in brain of non-human primates.
A. Quantification of PON2 protein in brain (ventral caudate) of African green monkeys, after normalization to β-actin. Results represent the mean (± SE) with n = 5 (midgestation, late-gestation, infant and aged) 8 (juvenile) and 11 (young adult). Samples are mixed gender. Infant and juvenile stage significantly different, ** p < 0.01 B. Quantification of PON2 protein in brain (ventral caudate) of male and female young-adult monkeys after normalization to β-actin. Results show the mean (± SE) of 4–6 samples for each group. Significantly different from male, * p < 0.05 (Student t-test). C. Representative Western blot of monkey brain samples. PON2 is seen at ~43kDa.
3.2 PON2 Developmental Expression in Liver
In addition to the brain, we measured PON2 protein and mRNA levels in liver of female mice of different ages. As shown in Fig. 4, PON2 levels increased with age from PND 1 to PND 60. In contrast with the brain, PON2 protein significantly increased with age, but did not exhibit a decrease after PND 21 (Fig. 4A). PON2 mRNA levels followed a similar trend of increasing with age, although the sample variability makes interpretation of the mRNA level trend between PND 21 and PND 60 uncertain (Fig. 4B).
Figure 4. Developmental expression of PON2 protein and mRNA levels in female mouse liver.
A. Quantification of PON2 protein after normalization to β-actin. Results are expressed as percentage of PND 1 optical density, and represent the mean (± SE) of 5 mice/group. Significantly different from PND 1, * p < 0.05. B. Levels of PON2 mRNA in liver from female mice of different ages, corrected for β-actin. Results are the mean (± SE) of 3 animals/group.
3.3 PON1 and PON3 Developmental Expression in the Liver
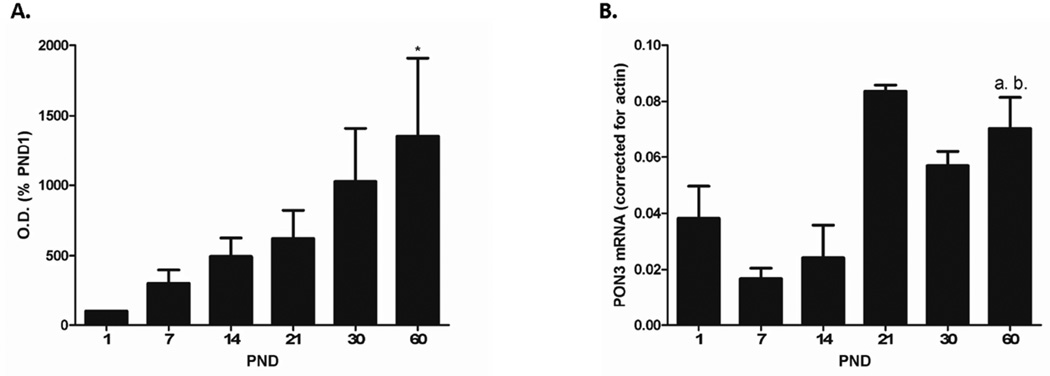
For comparison with PON2 developmental expression, PON1 and PON3 protein and mRNA levels were also investigated in this study. PON1 and PON3 are not expressed at significant levels in brain tissue [10], and were therefore only examined in the liver. Figs. 5 and 6 show PON1 and PON3 expression, respectively, in liver of female mice of different ages. Protein levels of PON1 (Fig. 5A) and PON3 (Fig. 6A) increased with age from PND 1 to PND 60. Levels of mRNA for PON1 (Fig. 5B) and PON3 (Fig. 6B) also increased with age from PND 1 to PND 21, but then appeared to stabilize from PND 21 to PND 60.
Figure 5. Developmental expression of PON1 protein and mRNA levels in female mouse liver.
A. Quantification of PON1 protein after normalization to β-actin. Results are expressed as percentage of PND 1 optical density, and represent the mean (± SE) of 5 mice/group. B. Levels of PON1 mRNA in liver from female mice of different ages, corrected for β-actin. Results are the mean (± SE) of 3–4 animals/group except for PND 21 in which n = 2 and error bar denotes range. a, b, c: significantly different from PND 1, PND 7, and PND 14 (all, p < 0.05).
Figure 6. Developmental expression of PON3 protein and mRNA levels in female mouse liver.
A. Quantification of PON3 protein after normalization to β-actin. Results are expressed as percentage of PND 1 optical density, and represent the mean (± SE) of 4 mice/group. Significantly different from PND 1,* p < 0.05. B. Levels of PON3 mRNA in liver from female mice of different ages, corrected for β-actin. Results are the mean (± SE) of 4 animals/group except for PND 21 in which n = 2 and error bar denotes range. a, b: significantly different from PND 7 (p < 0.01), and PND 14 (p < 0.05), respectively.
4. DISCUSSION
Results from this study are the first to describe the developmental expression of PON2 in brain and to examine PON2 developmental expression in liver with respect to both protein and mRNA levels. PON2 is unique among the three PONs, as it is expressed in measurable levels in brain tissue. PON2 mRNA had been found in mouse and human brain [1, 10, 11, 22, 24], while PON2 protein had been detected in mouse brain [10, 11, 22, 25], and in rat and human CNS cells [11]. In contrast, PON1 was detected at very low levels (20- to 40-fold less than PON2) in the brain, possibly resulting from residual blood in tissue homogenates while PON3 was not detected [10]. In every mouse brain region, PON2 levels were higher in female mice than in male mice, and the same was true with mouse neurons and astrocytes as well as rat and human astrocytes [11].
In the present study, we confirmed that PON2 protein levels were higher in whole brain homogenates from female mice compared with brain homogenates from male mice (Fig. 1). In addition, we found that in African green monkey brain, female animals had about twice the level of PON2 protein than males in the ventral caudate (Fig. 3B). Although the mouse and monkey sample sets were of different brain region (whole brain versus ventral caudate), it appears sex differences generally exist across species, which may be explained by the ability of estrogens to positively modulate PON2 expression [11]. The functional consequences of a higher expression of PON2 in females may have several ramifications, as several neurodegenerative diseases involve oxidative stress and neuroinflammation in their etiopathology. For example, the incidence of Parkinson’s disease is 90% higher in males [26, 27], suggesting that the higher PON2 levels in dopaminergic neurons of females may provide better protection against oxidative stress. In brain, the PON2 polyclonal antibody recognized more than one band, the lower at MW ~43 kDa, which corresponds to the reported MW of PON2 (Fig. 1) and has been determined to be specific for PON2 in this study. Higher MW bands (e.g. 55 kDa) have been reported by some investigators [6, 8, 22, 23], but not by others [7, 9], and may represent PON2 alloforms, though their exact nature has not been defined yet.
Overall, analysis of PON2 protein and mRNA in brain of female mice showed an increase in PON2 levels during early development followed by a decrease (Fig. 2). A similar developmental pattern was observed in brain of non-human primates (Fig. 3A), although species differences in rates of brain development and differences in time points used due to sample availability limit direct comparison of PON2 levels between species. However, the overall trend observed in both species reveals a possible window of susceptibility to oxidative stress in young adult mice and monkeys which may point to a change in cellular environment driving a decrease in PON2 expression. Of interest is that the developmental profile of PON2 in monkey brain seen in this study is in agreement with previous findings on susceptibility to MPTP- and methamphetamine-induced dopaminergic toxicity, with infant brain observed as the most resistant [40, 41]. Further study will be required to determine what biological changes are mediating the decrease in PON2, and whether these persist into later ages in mice as observed in monkeys. One avenue of research could be the estrus cycle, which was not accounted for in this study. As indicated earlier, estradiol has been demonstrated to increase PON2 expression in-vitro by increasing PON2 transcription [11]. In the estrus cycle of the female mouse, estrogen levels vary during different points in the cycle, peaking during pro-estrus [28], and could lead to variable levels of PON2 corresponding to circulating levels of estrogen.
While whole brain was utilized for the present study, brain region analysis of PON2 protein levels showed regional differences, with the highest levels of PON2 observed in the dopaminergic areas of the brain such as the nucleus accumbens, substantia nigra and striatum [10]. These three regions are known to sustain high levels of oxidative stress due to dopamine metabolism [29] and may have higher levels of PON2 to address the increased ROS. Further research into the regional developmental expression of PON2 should be of interest to determine if there are differences, either at the expression level or temporal level, in the various regions of the brain and potentially identify regions which may be at increased risk for ROS toxicity due to lower PON2 levels. In addition, genetic variation should also contribute to inter-individual differences in PON2 expression, would be a valuable avenue of study to determine potential susceptible populations with regards to ROS toxicity. Although not thought to influence antioxidant capacity, the Cys(311)Ser polymorphism of PON2 has been shown to affect lactonase activity [15] and could prove to be an additionally valuable avenue of study along with genetic expression differences.
As previously indicated, the lower levels of PON2 during early development and young adulthood may represent windows of susceptibility to oxidative stress in the brains of mice and monkeys, particularly when considering data from our laboratory showing that PON2 is critical for mitigating oxidative stress in the central nervous system [10]. Compensatory antioxidant mechanisms, such as ROS scavenging by glutathione, do not appear to correct the elevated oxidative stress levels in situations of decreased PON2, highlighting the potential pathological significance of lower PON2 levels [10]. These windows of susceptibility are of interest for neurodegenerative diseases, as evidence mounts for mitochondrial dysfunction and elevated oxidative stress as key mediators of diseases such as Alzheimer and Parkinson’s [17, 18, 30]. Recent data suggest that PON2 interacts directly with DJ-1 (PARK7), allowing DJ-1 to exert antioxidant properties through the regulation of PON2 [31]. Loss of function mutations in DJ-1 are associated with some familial forms of Parkinson’s disease [32], offering a potential link between PON2 and neurodegenerative disease pathogenesis. Furthermore, PON2 is known to associate with dopamine receptors in the kidney [33, 34], acting as an important antioxidant in the renal dopamine system, and whether this same relationship exists in brain dopaminergic regions needs to be investigated.
In mouse liver, PON2 protein and mRNA increased with age from PND 1 to PND 60 (Fig. 4). The findings were in accordance with the developmental profiles of other paraoxonases, PON1 and PON3, which also generally increased with age (Figs. 5 and 6). Unfortunately, non-human primate liver samples were not available for analysis, and the persistence of expression patterns between species for PON2 in the liver is unclear. Previous studies had examined the developmental expression of PON1 mRNA in liver and of paraoxonase activity in plasma of mice, and found PON1 levels to increase with age [35–37], in agreement with the present findings. PON3 temporal expression has received less attention, with limited studies showing a decrease or an increase with age [37–39]. Belteki et al. [39] found a time dependent increase of PON3 mRNA levels prenatally in rat and sheep in various tissues, including the liver. Shih et al. [38] reported lower levels of PON3 mRNA in various tissues of newborn mice compared to adult mice, while Cheng and Klaassen [37] found high levels of PON3 mRNA in prenatal mouse liver, followed by a decline at PND5 then a gradual increase in levels.
In summary, results of the present study confirm that sex is an important determinant of PON2 expression, and provides novel information indicating that age also modulates the levels of PON2. Because of the role of PON2 as a potent intracellular antioxidant and anti-inflammatory factor, strategies attempting to modulate its level of expression [20] should also consider sex and age as additional variables.
Highlights.
Paraoxonase 2 (PON2) is differentially expressed in brain of male and female mice and non-human primates
PON2 protein and mRNA increase then decrease during development in brain from mice and non-human primates
PON2 protein and mRNA, similarly to PON1 and PON3, increase with age in mice
Acknowledgments
This study was supported in part by grants P42ES004696 and P30ES07033 from the National Institute of Environmental Health Sciences. Primate research at Yale University was supported by grant AG048918 from the National Institute on Aging. We thank Zahra Afsharinejad from the Functional Genomics and Proteomics Facility in the Department of Environmental and Occupational Health Sciences at the University of Washington for measuring mRNA levels.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
REFERENCES
- 1.Primo-Parmo SL, Sorenson RC, Teiber J, La Du BN. The human serum paraoxonase/arylesterase gene (PON1) is one member of a multigene family. Genomics. 1996;33:498–507. doi: 10.1006/geno.1996.0225. PubMed PMID: 8661009. [DOI] [PubMed] [Google Scholar]
- 2.Draganov DI, La Du BN. Pharmacogenetics of paraoxonase: a brief review. Naunyn Schmiedebergs Arch. Pharmacol. 2004;369:78–88. doi: 10.1007/s00210-003-0833-1. PubMed PMID: 14579013. [DOI] [PubMed] [Google Scholar]
- 3.Draganov DI, Teiber JF, Speelman A, Osawa Y, Sunahara R, La Du BN. Human paraoxonases (PON1, PON2 and PON3) are lactonases with overlapping and distinct substrate specificities. J. Lipid Res. 2005;46:1239–1247. doi: 10.1194/jlr.M400511-JLR200. PubMed PMID: 15772423. [DOI] [PubMed] [Google Scholar]
- 4.Stoltz DA, Ozer EA, Ng CJ, Yu JM, Reddy ST, Lusis AJ, Bourquard N, Parsek MR, Zabner J, Shih DM. Paraoxonase-2 deficiency enhances Pseudomonas aeruginosa quorum sensing in murine tracheal epithelia. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007;292:L852–L860. doi: 10.1152/ajplung.00370.2006. PubMed PMID: 17122353. [DOI] [PubMed] [Google Scholar]
- 5.Teiber JF, Horke S, Haines DC, Chowdhary PK, Xiao J, Kramer GL, Haley RW, Draganov DI. Dominant role of paraoxonases in inactivation of the Pseudomonas aeruginosa quorum-sensing signal N-(3-oxododecanoyl)-L-homoserine lactone. Infect. Immun. 2008;76:2512–2519. doi: 10.1128/IAI.01606-07. PubMed PMID: 18347034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horke S, Witte I, Altenhöfer S, Wilgenbus P, Goldeck M, Förstermann U, Xiao J, Kramer GL, Haines DC, Chowdhary PK, Haley RW, Teiber JF. Paraoxonase-2 is down regulated by the Pseudomonas aeruginosa quorum-sensing signal N-(3-oxododecanyl)-L-homoserine lactone and attenuates oxidative stress induced by pyocyanin. Biochem. J. 2010;426:73–83. doi: 10.1042/BJ20091414. PubMed PMID: 19925453. [DOI] [PubMed] [Google Scholar]
- 7.Ng CJ, Wadleigh DJ, Gangopadhyay A, Hama S, Grijalva VR, Navab M, Fogelman AM, Reddy ST. Paraoxonase-2 is a ubiquitously expressed protein with antioxidant properties and is capable of preventing cell-mediated oxidative modification of low density lipoprotein. J. Biol. Chem. 2001;276:44444–44449. doi: 10.1074/jbc.M105660200. PubMed PMID: 11579088. [DOI] [PubMed] [Google Scholar]
- 8.Horke S, Witte I, Wilgenbus P, Kruger M, Strand D, Förstermann U. Paraoxonase-2 reduces oxidative stress in vascular cells and decreases endoplasmic reticulum stress-induced caspase activation. Circulation. 2007;115:2055–2064. doi: 10.1161/CIRCULATIONAHA.106.681700. PubMed PMID: 17404154. [DOI] [PubMed] [Google Scholar]
- 9.Levy E, Trudel K, Bendayan M, Seidman E, Delvin E, Elchebly M, Lavoie JC, Precourt LP, Amre D, Sinnett D. Biological role, protein expression, subcellular localization, and oxidative stress response of paraoxonase 2 in the intestine of human and rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;293:G1252–G1261. doi: 10.1152/ajpgi.00369.2007. PubMed PMID: 17916643. [DOI] [PubMed] [Google Scholar]
- 10.Giordano G, Cole TB, Furlong CE, Costa LG. Paraoxonase 2 (PON2) in the mouse central nervous system: a neuroprotective role? Toxicol. Appl. Pharmacol. 2011;256:369–378. doi: 10.1016/j.taap.2011.02.014. PubMed PMID: 21354197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giordano G, Tait L, Furlong CE, Cole TB, Kavanagh TJ, Costa LG. Gender differences in brain susceptibility to oxidative stress are mediated by levels of paraoxonase-2 expression. Free Rad. Biol. Med. 2013;58:98–108. doi: 10.1016/j.freeradbiomed.2013.01.019. PubMed PMID: 23376469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Devarajan A, Bourquard N, Hama S, Navab M, Grijalva VR, Morvardi S, Clarke C, Vergnes L, Reue K, Teiber JF, Reddy ST. Paraoxonase 2 deficiency alters mitochondrial function and exacerbates the development of atherosclerosis. Antiox. Redox Signal. 2011;14:341–351. doi: 10.1089/ars.2010.3430. PubMed PMID: 20578959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higgins GC, Beart PM, Shin YS, Chen MJ, Cheung NS, Nagley P. Oxidative stress: emerging mitochondrial and cellular themes and variations in neuronal injury. J. Alzheim. Dis. 2010;20(Suppl. 2):S453–S473. doi: 10.3233/JAD-2010-100321. PubMed PMID: 20463398. [DOI] [PubMed] [Google Scholar]
- 14.Kehrer JP, Klotz LO. Free radicals and relates reactive species as mediators of tissue injury and disease: implications for Health. Crit. Rev. Toxicol. 2015;45(9):765–798. doi: 10.3109/10408444.2015.1074159. PubMed PMID: 26610815. [DOI] [PubMed] [Google Scholar]
- 15.Altenhöfer S, Witte I, Teiber JF, Wilgenbus P, Pautz A, Li H, Daiber A, Witan H, Clement AM, Förstermann U, Horke S. One enzyme, two functions. PON2 prevents mitochondrial superoxide formation and apoptosis independent from its lactonase activity. J. Biol. Chem. 2010;285:24398–24403. doi: 10.1074/jbc.M110.118604. PubMed PMID: 20530481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Madamanchi NR, Vendrov A, Runge MS. Oxidative stress and vascular disease. Arterioscler. Thromb. Vasc. Biol. 2005;25(1):29–38. doi: 10.1161/01.ATV.0000150649.39934.13. PubMed PMID: 15539615. [DOI] [PubMed] [Google Scholar]
- 17.Aliev G, Priyadarshini M, Reddy VP, Grieg NH, Kaminsky Y, Cacabelos R, Ashraf GM, Jabir NR, Kamal MA, Nikoleno VN, Zamyatin AA, Jr, Benberin VV, Bachurin SO. Oxidative stress mediated mitochondrial and vascular lesions as markers in the pathogenesis of Alzheimer disease. Curr. Med. Chem. 2014;21(19):2208–2217. doi: 10.2174/0929867321666131227161303. PubMed PMID: 24372221. [DOI] [PubMed] [Google Scholar]
- 18.Gaki GS, Papavassiliou AG. Oxidative stress-induced signaling pathways implicated in the pathogenesis of Parkinson’s disease. Neuromolec. Med. 2014;16(2):217–230. doi: 10.1007/s12017-014-8294-x. PubMed PMID: 24522549. [DOI] [PubMed] [Google Scholar]
- 19.Landriscina M, Maddalena F, Laudiero G, Esposito F. Adaptation to oxidative stress, chemoresistance, and cell survival. Antioxid. Redox Signal. 2009;11(11):2701–2716. doi: 10.1089/ars.2009.2692. PubMed PMID: 19778285. [DOI] [PubMed] [Google Scholar]
- 20.Costa LG, Tait L, de Laat R, Dao K, Giordano G, Pellacani C, Cole TB, Furlong CE. Modulation of paraoxonase 2 (PON2) in mouse brain by the polyphenol quercetin; a mechanism of neuroprotection? Neurochem. Res. 2013;38:1809–1818. doi: 10.1007/s11064-013-1085-1. PubMed PMID: 23743621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giordano G, White CC, McConnachie LA, Fernandez C, Kavanagh TJ, Costa LG. Neurotoxicity of domoic acid in cerebellar granule neurons in a genetic model of glutathione deficiency. Mol Pharmacol. 2006;70:2116–21126. doi: 10.1124/mol.106.027748. PubMed PMID: 17000861. [DOI] [PubMed] [Google Scholar]
- 22.Ng CJ, Bourquard N, Grijalva V, Hama S, Shih DM, Navab M, Fogelman AM, Lusis AJ, Young S, Reddy ST. Paraoxonase-2 deficiency aggravates atherosclerosis in mice despite lower apolipoprotein-B-containing lipoproteins. Antiatherogenic role for paraoxonase-2. J. Biol. Chem. 2006;281:29491–29500. doi: 10.1074/jbc.M605379200. PubMed PMID: 16891303. [DOI] [PubMed] [Google Scholar]
- 23.Witte I, Altenhöfer S, Wilgenbus P, Amort J, Clement AM, Pautz A, Li H, Förstermann U, Horke S. Beyond reduction of atherosclerosis: PON2 provides apoptosis resistance and stabilizes tumor cells. Cell Death Dis. 2011;2:e112. doi: 10.1038/cddis.2010.91. PubMed PMID: 21368884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mackness B, Beltran-Debon R, Aragones G, Joven J, Camps J, Mackness M. Human tissue distribution of paraoxonases 1 and 2 mRNA. IUBMB Life. 2010;62:480–482. doi: 10.1002/iub.347. PubMed PMID: 20503442. [DOI] [PubMed] [Google Scholar]
- 25.Marsillach J, Mackness B, Mackness M, Riu F, Beltran R, Joven J, Camps J. Immunohistochemical analysis of paraoxonase-1, 2 and 3 expression in normal mouse tissues. Free Rad. Biol. Med. 2008;45:146–157. doi: 10.1016/j.freeradbiomed.2008.03.023. PubMed PMID: 18440321. [DOI] [PubMed] [Google Scholar]
- 26.Van den Eeden SK, Tanner CM, Bernstein AL, Fross RD, Leimpeter A, Bloch DA, Nelson LM. Incidence of Parkinson’s disease: variation by age, gender, and race/ethnicity. Am. J. Epidemiol. 2003;157:1015–1022. doi: 10.1093/aje/kwg068. PubMed PMID: 12777365. [DOI] [PubMed] [Google Scholar]
- 27.Wirdefeldt K, Adami HO, Cole P, Trichopoulos D, Mandel J. Epidemiology and etiology of Parkinson’s disease: a review of the evidence. Eur. J. Epidemiol. 2011;26:S1–S58. doi: 10.1007/s10654-011-9581-6. PubMed PMID: 21626386. [DOI] [PubMed] [Google Scholar]
- 28.Caligioni CS. Assessing reproductive status/stages in mice. Curr. Protoc. Neurosci. 2009 doi: 10.1002/0471142301.nsa04is48. Appendix 4: Appendix 4I, PubMed PMID: 19575469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miyazaki I, Asanuma M. Dopaminergic neuron-specific oxidative stress caused by dopamine itself. Acta Med. Okayama. 2008;62(3):141–150. doi: 10.18926/AMO/30942. PubMed PMID: 18596830. [DOI] [PubMed] [Google Scholar]
- 30.Thanan R, Oikawa S, Hiraku Y, Ohnishi S, Ma N, Pinlaor S, Yongvanit P, Kawanishi Murata M. Oxidative stress and its significant roles in neurodegenerative diseases and cancer. Int. J. Mol. Sci. 2014;16(1):193–217. doi: 10.3390/ijms16010193. PubMed PMID: 25547488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parsanejad M, Bourguard N, Qu D, Zhang Y, Huang E, Rousseaux MW, Aleyasin H, Irrcher I, Callaghan S, Vaillant DC, Kim RH, Slack RS, Mak TW, Reddy ST, Figeys D, Park DS. DJ-1 interacts with and regulates paraoxonase-2, an enzyme critical for neuronal survival response to oxidative stress. PLoS One. 2014;9(9):e106601. doi: 10.1371/journal.pone.0106601. PubMed PMID: 25210784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bonifati V, Rizzu P, van Baren MJ, Schaap O, Breedveld GJ. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science. 2003;299(5604):256–259. doi: 10.1126/science.1077209. PubMed PMID: 12446870. [DOI] [PubMed] [Google Scholar]
- 33.Yang Y, Zhang Y, Cuevas S, Villar VA, Escano C, D Asico L, Yu P, Grandy DK, Felder RA, Armando I, Jose PA. Paraoxonase 2 decreases renal reactive oxygen species production, lowers blood pressure, and mediates dopamine D2 receptor-induced inhibition of NADPH oxidase. Free Radic. Biol. Med. 2012;53(3):437–436. doi: 10.1016/j.freeradbiomed.2012.05.015. PubMed PMID: 22634053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang S, Yang Y, Yu P, Yang J, Jiang X, Villar VA, Sibley DR, Jose PA, Zeng C. Dopamine D1 an D5 receptors differentially regulate oxidative stress through paraoxonase 2 in kidney cells. Free Radic. Res. 2015;49(4):397–410. doi: 10.3109/10715762.2015.1006215. PubMed PMID: 25740199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li WF, Matthews C, Disteche CM, Costa LG, Furlong CE. Paraoxonase (Pon1) gene in mice: sequencing, chromosomal localization and developmental expression. Pharmacogenetics. 1997;7:137–144. doi: 10.1097/00008571-199704000-00007. PubMed PMID: 9170151. [DOI] [PubMed] [Google Scholar]
- 36.Cole TB, Jampsa RL, Walter BJ, Arndt TL, Richter RJ, Shih DM, Tward A, Lusis AJ, Jack RM, Costa LG, Furlong CE. Expression of human paraoxonase (PON1) during development. Pharmacogenetics. 2003;13:357–364. doi: 10.1097/00008571-200306000-00007. PubMed PMID: 12777966. [DOI] [PubMed] [Google Scholar]
- 37.Cheng X, Klaassen CD. Hormonal and chemical regulation of paraoxonases in mice. J. Pharmacol. Exp. Ther. 2012;342(3):688–695. doi: 10.1124/jpet.112.194803. PubMed PMID: 22653878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shih DM, Xia YR, Yu JM, Lusis AJ. Temporal and tissue-specific patterns of Pon3 expression in mouse: in situ hybridization analysis. Adv. Exp. Med. Biol. 2010;660:73–87. doi: 10.1007/978-1-60761-350-3_8. PubMed PMID: 20221872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Belteki G, Kempster SL, Forhead AJ, Giussani DA, Fowden AL, Curley A, Charnock-Jones DS, Smith GCS . Paraoxonase-3, a putative circulating antioxidant, is systematically upregulated in late gestation in the fetal rat, sheep and human. J. Clin. Endocrinol. Metab. 2010;95:3798–3805. doi: 10.1210/jc.2010-0037. PubMed PMID: 20463093. [DOI] [PubMed] [Google Scholar]
- 40.Morrow BA, Roth RH, Redmond DE, Jr, Diano S, Elsworth JD. Susceptibility to a parkinsonian toxin varies during primate development. Exp. Neurol. 2012;235:273–281. doi: 10.1016/j.expneurol.2012.02.005. PubMed PMID: 22366325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morrow BA, Roth RH, Redmond DE, Elsworth JD. Impact of methamphetamine on dopamine neurons in primates is dependent on age: implications for development of Parkinson's disease. Neuroscience. 2011;189:277–285. doi: 10.1016/j.neuroscience.2011.05.046. PubMed PMID: 21640165. [DOI] [PMC free article] [PubMed] [Google Scholar]