Abstract
Exosomes, which act as biological cargo vessels, are cell-released, phospholipid-enclosed vesicles. In eukaryotic cells, exosomes carry and exchange biological materials or signals for the benefit or detriment to the cells. Thereby, we consider exosomes to be molecular Palkis (carriers). Although exosomes are currently one of the most popularly researched cellular entities, they have remained largely enigmatic and warrant continued investigation into their structure and functions. These membraned vesicles are between 30 and 150 nm in diameter and are actively secreted by all cell types. While initially considered cellular “trash bags,” recent years have revealed exosomes to be dynamic and multi-functional vesicles that may play a crucial role in cancer development, progression and metastasis. Thereby, they have the potential to be used in development of therapeutic modalities for cancer and other diseases. As more research studies emerge, it’s becoming evident that exosomes are released by cells with a purpose and are representatives of certain cell types and disease conditions. Hence, they may also be used as biomarkers for the detection of cancer initiation, progression and organotropic metastatic growth of cancer cells. This review will focus on the recent developments achieved in identifying the role of exosomes in cancer development and progression as well as therapeutic implications. The review will also discuss the pitfalls of methodologies used for the extraction of exosomes.
Keywords: Exosome, Cancer, Vesicle, Progression and metastasis
Introduction
Cells periodically release extracellular vesicles as a part of their normal physiological processes (Chargaff and West 1946). These vesicles, released from both eukaryotic and prokaryotic cells with diameters ranging from 30 nm to 1 μm, have dedicated functions (van der Pol et al. 2012). Based on size, morphology and origin, eukaryotic-cell-derived vesicles are divided into five categories (Brinton et al. 2015; Colombo et al. 2014; van der Pol et al. 2012). These categories include extracellular vesicles, vacuoles, lysosomes, transport vesicles and secretory vesicles. Exosomes are considered extracellular vesicles with endocytic origins (Kalluri 2016; Raposo and Stoorvogel 2013; van der Pol et al. 2012). Another larger vesicle created as a direct result of endocytosis from the plasma membrane is the microvesicle. Like microvesicles, exosomes are surrounded by a phospholipid bilayer membrane and are enclosed with genetic material and protein (Raposo and Stoorvogel 2013; van der Pol et al. 2012). Although both exosomes and microvesicles are cell-derived extracellular vesicles with similar enclosing membranes, there are many differences that distinguish them by structure, origin and function. This review highlights how exosomes promote cancer progression and metastasis as well as identifies the underlying mechanisms that could be potential efficacious targets for prevention and therapy. The pitfalls and limitations of exosome research are also discussed in this review.
Exosome biogenesis and beyond
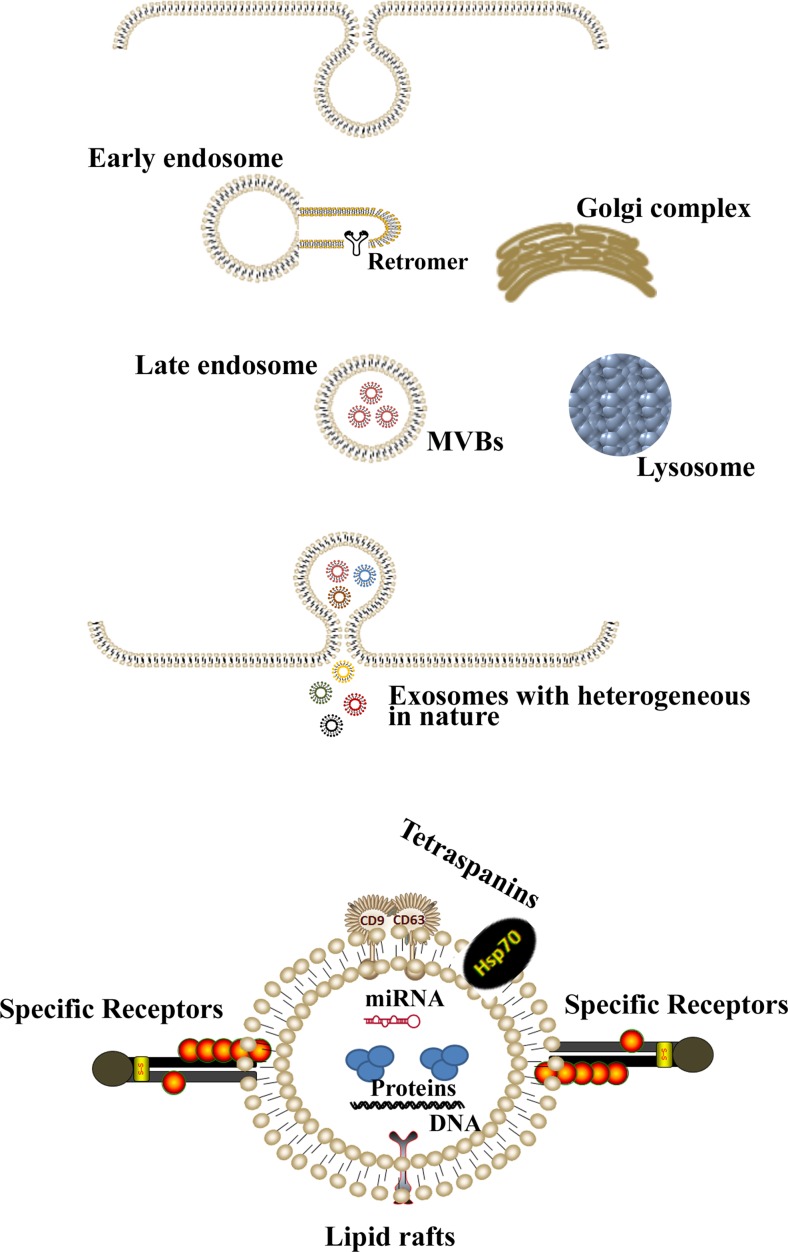
Exosomes and microvesicles originate in different ways. They’re first conceived when endocytic vesicles on the plasma membrane are fused to form early endosomes, and mature into late endosomes (Brinton et al. 2015; Lee et al. 2012; Raposo and Stoorvogel 2013). The late endosomes then undergo inward budding, in which vesicles form on the membrane of the endosome and are internalized (Brinton et al. 2015; Lee et al. 2012; Raposo and Stoorvogel 2013). At this point, the endosome contains many small vesicles referred to as multivesicular bodies (MVBs), while the vesicles within the MVB are called intraluminal endosomal vesicles (ILVs) (Lee et al. 2012). Rather than fusing with the lysosome, the MVBs fuse with the plasma membrane, releasing their internalized ILVs within extracellular space (Lee et al. 2012). Once the ILVs are released into extracellular space, they are considered exosomes (Figs. 1 and 2 ). Although the biogenesis, secretion and delivery of exosomes are still unknown phenomena, recent studies suggest that the syndecan heparan sulphate proteoglycans and their cytoplasmic adaptor, syntenin, control the formation of exosomes (Baietti et al. 2012; Roucourt et al. 2015), while secretion and delivery to their correct destinations are regulated by Rab GTPases pathways (Ostrowski et al. 2010; Stenmark 2009). Additionally, endosomal sorting complexes required for transport (ESCRTs), intra- and intercellular pH and Ca2+ channels or H+ pumps are also known to play critical roles in exosome secretion and delivery to the recipient cells (Bobrie et al. 2011; Iero et al. 2008; Michelet et al. 2010; Parolini et al. 2009; Ramachandran and Palanisamy 2012; Savina et al. 2003; Zhang et al. 2015). The uptake of the exosomes by recipient cells, which can be accomplished by endocytosis, receptor-ligand interaction or fusion (Zhang et al. 2015), is dependent on microenvironmental pH (Parolini et al. 2009). At a low pH, the exosomes show increased release and uptake of recipient cells via fusion (Parolini et al. 2009) (Fig. 3 ). Conversely, microvesicles are formed in a less complex manner. They are formed simply by the shedding of a cell’s plasma membrane through exocytosis (Zomer et al. 2010). Interestingly, the term “exosomes” was initially used to classify microvesicles on the nano-scale (Raposo and Stoorvogel 2013). However, the discovery of the exosomes’ intracellular origin prompted a transfer of nomenclature of vesicles. Exosomes range in size between 30 and 150 nm in diameter (Kahlert et al. 2014; Kalluri 2016; Patel et al. 2016; Raposo and Stoorvogel 2013; van der Pol et al. 2012), and morphologically, they look like a cup or dish under an electron microscope and express several identifiable molecular markers (Kalluri 2016; Patel et al. 2016; Zhang et al. 2015). The size and morphology of exosomes extracted from two different breast cancer cell lines are depicted in Fig. 2. Microvesicles, on the other hand, can range between 50 and 1000 nm in diameter (Lee et al. 2012). However, it is important to note that the majority of microvesicles will most likely be >1000 nm (Zomer et al. 2010). Exosomes also differ from microvesicles in the genetic information they carry.
Fig. 1.
A schematic diagram depicting the exosome biogenesis in cells. Endocytosis leads to the formation of early endosomes which collect miRNA, proteins, DNA, RNA and forms membrane-bound intraluminal vesicles by budding within the lumen. At this stage, the structure is called late endosome or multi-vesicular bodies (MVBs) and contains a heterogeneous population of exosomes, which are either destroyed if fused with lysosomes or released into the extracellular milieu via fusion with the plasma membrane
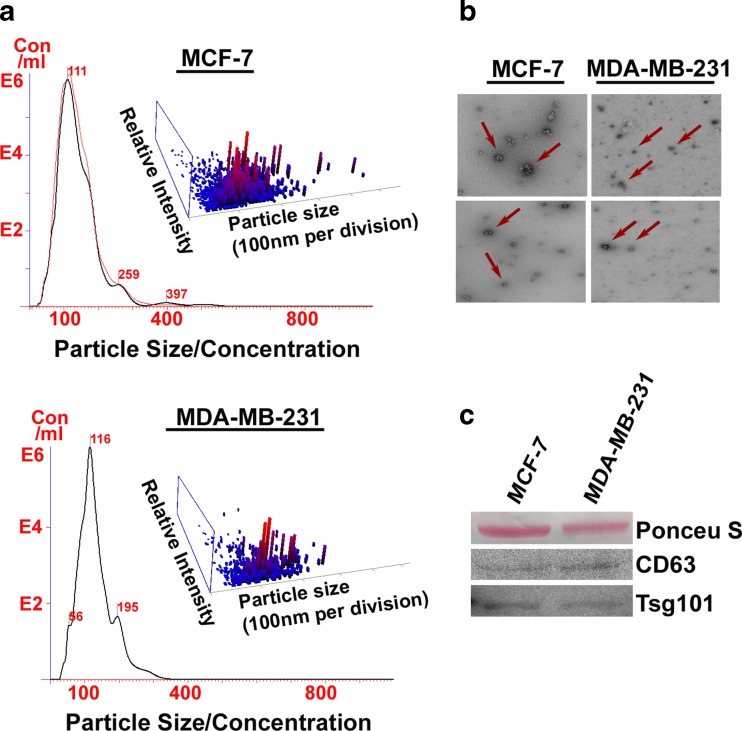
Fig. 2.
Qualitative and quantitative analysis of exosome in MCF-7 and MDA-MB-231 breast cancer cells using standard methods applied in exosome analysis. a Nano sight analysis showed different particle sizes with a peak in the desired range (exosomes). b Electron microscopy (TEM) confirmed the presence of exosomes based on morphology and size (Red arrows). c CD63 and TSG-101, markers for exosomes were expressed in the protein extract
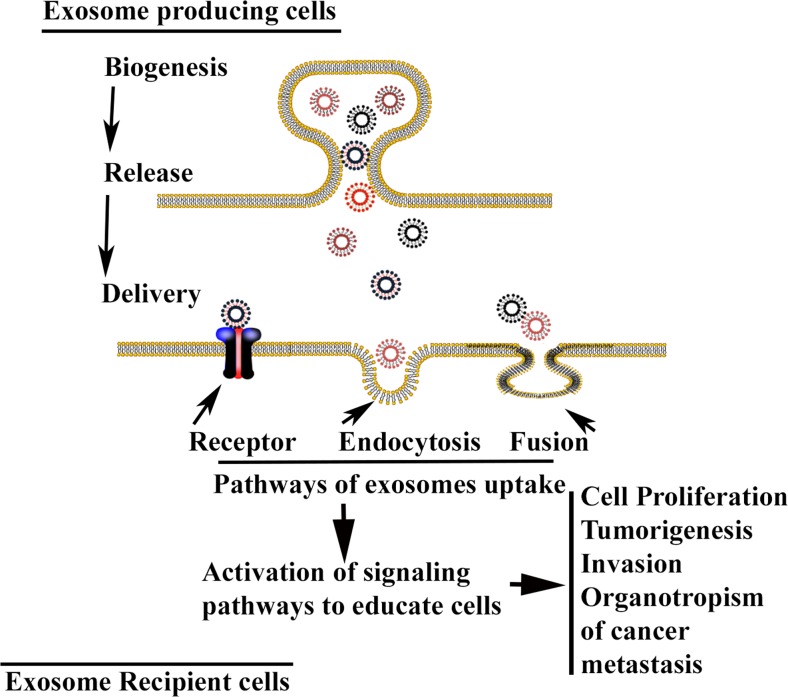
Fig. 3.
A schematic diagram illustrating the exosome release and delivery from exosome producing cells to the recipient cells. Exosome uptake by recipient cells are mediated through receptors, endocytosis or membrane fusion. Upon gaining entry into the cells, they manifest their effects by altering the physiological processes like cell proliferation, tumorigenesis, invasion and metastasis
Exosomes have emerged as important extracellular signalosomal vesicles that could play a vital role in intercellular communication (Syn et al. 2016; Thery et al. 2002a; Thery et al. 2002b), and thus, could be referred to as communicasomes (Zhang et al. 2015) or “molecular Palkis” (we designated the exosome as a Palki because historically a palki is an ancient cargo carrier that was used for communications individuals or kingdoms). Exosomes were believed to be homogenous, but as research advanced, it became evident that exosomes are heterogeneous based on the cell of origin and the status of the tissue or cells (Kalluri 2016). Before the discovery of exosomes, two possible methods of intercellular communication were known, direct contact between cells or transfer of molecules secreted by the cells. However, it is now, understood that there is a third process of intercellular communication mediated by exosomes under a wide range of physiological and pathophysiological contexts (Kalluri 2016; Raposo and Stoorvogel 2013; Syn et al. 2016; van der Pol et al. 2012). These include malignant transformation and metastasis.
Molecular markers for the detection of exosomes
Exosomes carry several cytoplasmic and membrane proteins that may serve as molecular markers (Labani-Motlagh et al. 2016; Mathivanan et al. 2012; Mincheva-Nilsson and Baranov 2010; Suetsugu et al. 2013) . These include chaperon or heat shock cytoplasmic proteins such as heat shock proteins 70 and 90 (Hsp70 and Hsp90), the ESCRT-associated protein Alix and Tsg101 and membrane proteins such as tetraspanins (e.g. CD9, CD63 and CD81). In addition, several ligands (i.e., EGFR ligands including EGF, TGF-α and amphiregulin and Fas ligand) are differentially expressed in exosomes for various functions (Abusamra et al. 2005; Higginbotham et al. 2011; Labani-Motlagh et al. 2016). These ligands can be used as ideal markers for exosomes.
Exosomes instigate cancer initiation and progression
Exosomes are capable of altering the characteristics of the recipient cells for cancer initiation and progression. Exosomes have recently been shown to play a role in angiogenesis, immunosuppression and other physiognomies associated with cancer initiation and progression. They mainly alter the microenvironment and create a niche conducive to cancer initiation and progression (Ge et al. 2012; Kahlert and Kalluri 2013).
After the discovery of an exosomal mode of intercellular communication, the exosomal role in tumor initiation and progression has been studied by multiple scientific groups. A tumor initiates with incidence of single or multiple genetic mutations as well as epigenetic changes in one or more cells in a tissue resulting in transformation of the cells. The transformed cells gain the hallmarks of cancer, paving the tumor’s path to malignancy (Hanahan and Weinberg 2011). During this course, the language of the intercellular cross-talks changes owing to the major alterations in the transcriptomic and proteomic landscape of the communicating cells. Studies have shown that exosomes released by the tumor cells can be co-opted by the neighboring cells in the microenvironment including fibroblasts, endothelial cells, immune cells and other epithelial cells to stimulate cancer progression and metastasis to the distant organs (Gu et al. 2012; Park et al. 2010; Skog et al. 2008; Webber et al. 2010; Webber et al. 2014; Webber et al. 2015; You et al. 2015; Zhou et al. 2014). The stromal cells also shed exosomes which are assimilated by the tumor cells (Atula and Grenman 1997; Luga et al. 2012), thus the exosome-mediated influence is bi-directional. There has been direct evidence of transformed tumor cells transferring oncoproteins like mutated EGFR and K-Ras to adjacent tumor cells lacking their expression which enables the recipient cells to achieve unrestrained proliferation (Al-Nedawi et al. 2008; Demory Beckler et al. 2013). It has also been shown that exosomes might carry oncogenic viral proteins, capable of transforming normal epithelial cells to neoplastic ones (Aga et al. 2014). Recent studies of Melo et al. suggest that glypican-1 (GPC1) positive circulating exosomes may serve as a diagnostic tool for the detection of early stages of pancreatic cancer and expedite curative surgical therapy (Melo et al. 2015), which are direly needed for prevention or cure of diseases like pancreatic cancer.
The most crucial pathological step to malignancy is the invasion of cancer cells where the disease literally crosses all boundaries into the surrounding stromal tissue. The widely studied cell biological phenomenon of epithelial to mesenchymal transition (EMT) holds the key to this step (Thiery et al. 2009). Tumor-derived exosomes have been shown to carry a pro-EMT load of proteins comprised of transcription factors like HIF-1α, β-catenin and signaling factors like TGFβ, TNF-α, PDGF along with various MMPs which have been shown to play a significant role in remodeling of the tumor-adjacent stroma (Aga et al. 2014; Syn et al. 2016; You et al. 2015). As a result, stromal fibroblasts have been shown to be “activated” to cancer associated fibroblasts (CAFs) (Gu et al. 2012; Webber et al. 2010), which play an important agonistic role in tumor progression (Brentnall 2012; Camps et al. 1990; Orimo et al. 2005). Moreover, cancer exosome-mediated TGFβ/Smad signaling induces differentiation of myeloid derived suppressor cells, mesenchymal stem cells and normal stromal fibroblasts to pro-angiogenic myofibroblasts (Cho et al. 2012; Vong and Kalluri 2011; Webber et al. 2010; Webber et al. 2014). One important local target of the exosomes shed by the tumor cells is the vascular endothelial cells for the activation of pro-angiogenic switch to access the host vasculature for their nutrition (Park et al. 2010; Skog et al. 2008; Zhou et al. 2014). The tumor cells thriving in a hypoxic environment shed exosomes that are functionally different from the exosomes released in normoxic conditions from the same cancer cells (Kucharzewska et al. 2013). The hypoxic-cancer cell-shed exosomes hijack the host’s angiogenic program inducing formation of new blood vessels for the tumor. The endothelial cells are affected by the tumor exosomes that release growth factors to stimulate pericytes via the AKT pathway (Kucharzewska et al. 2013).
The tumor derived exosomes, host immune system and cancer progression
The exchange of tumor-derived exosomes and the immune cells’ exosomes between the immune and tumor cells plays a major part in the tug-of-war between the tumor and the host immune system. Multiple studies indicate that the exosomes secreted by the tumor cells have an important role in outwitting the host’s immunosurveillance system by numerous diverse mechanisms, allowing the tumor to evade the immune responses (Kahlert and Kalluri 2013). The manner in which exosomes transfer information from one cell to another is very dynamic. As previously mentioned in this review, the majority of cells internalize the exosomes, after which the cargo is released into the cells. However, a recent report showed that tumor-derived exosomes can communicate with T-cells without becoming internalized. This is achieved by interacting with the receptors present on the T-cells and regulating the gene expression (Muller et al. 2016). Thus, exosomes have emerged as an entity that was, for many years, covered in a cloak of invisibility but have now stepped into the spotlight in the field of cancer as a ray of hope in understanding and eradicating the disease.
The tumor derived exosomes, microRNA biogenesis and cancer progression
Multiple micro-RNAs (e.g. miR-10b, miR-29 and others) shown to have direct effect on cancer progression and metastasis are also exchanged between the cancer cells (Table 1 ). The cancer cells’ secreted exosomes facilitates the initiation, progression or both via inducing EMT and creating a stromal environment favorable for tumor cell invasion (Brinton et al. 2015; Kahlert and Kalluri 2013; Suchorska, and Lach, 2016; Syn et al. 2016). Moreover, recent studies found that the exosomes from breast cancer cells can promote cell-independent microRNA biogenesis and initiate tumorigenesis in non-tumor epithelial cells via Dicer-dependent pathways (Melo et al. 2014). Collectively, these unique findings suggest an opportunity for development of microRNA-based bio-markers and therapies.
Table 1.
Functional miRNA in exosomes associated with tumorigenesis
| miRNA | Function of miRNA | Pathological Levels | miRNA target | Source of exosomes | References |
|---|---|---|---|---|---|
| miR-451 | Promotes tumor growth, role in signaling in cancer and stroma cells | Upregulated | Not Known | MCF-7, Bt-20, SK-BR-3, MDA-MB-231 | (Guduric-Fuchs et al. 2012; Pigati et al. 2010) |
| miR-146a | Increase Risk of BC | Upregulated | 3′-UTR of Numb (Notch signaling inhibitor) | Multiple Myeloma Cells | (Dai et al. 2015; De Veirman et al. 2016; Guduric-Fuchs et al. 2012) |
| miR-10a | Associated with tumor progression | Upregulated | MDM2 and P53 | Mesenchymal Stromal Cells from Myelodysplastic Syndrome | (Melo et al. 2014; Muntion et al. 2016) |
| miR-10b | Associated with breast cancer progression | Upregulated | HOXD10 and 3′-UTR of KLF4 | MDA-MB-231 | (Melo et al. 2014; Singh et al. 2014) |
| miR-21 | Associated with breast and cervical cancer progression | Upregulated | PDCD4 | Cervicovaginal Lavage Specimen | (Liu et al. 2014; Melo et al. 2014) |
| miR-1059 | Destroys endothelial barrier and targets ZO-1 tight junction protein | Upregulated | ZO-1 | MDA-MB-231 | (Zhou et al. 2014) |
| miR-34a | Associated with prostate cancer progression | Upregulated | BCL-2 | 22Rv1, DU145, PC3 | (Corcoran et al. 2014) |
| miR-1225-5p | Associated with peritoneal dissemination of gastric cancer | Upregulated | Polycystic Kidney Disease Gene (PKD1) | Stage T4 Gastric Cancer cells | (Tokuhisa et al. 2015) |
| miR-15a | Associated with tumor progression | Upregulated | MDM2 and P53 | Mesenchymal Stromal Cells from Myelodysplastic Syndrome | (Muntion et al. 2016) |
| miR-128 | Associated with breast cancer progression | Upregulated | Bax | MCF-7 | (Tokuhisa et al. 2015) |
Proteome-signatures of cancer exosomes dictate cancer cells for organ-specific metastasis
Organotropic (organ-specific) metastatic growth of cancer cells from a primary site (Lu and Kang 2007) is still an unsolved phenomenon more than a century after the “seed and soil” hypothesis, which was first proposed in 1889 (Paget 1889). Although metastasis is the major contributor of cancer-related death, the metastatic process is greatly inefficient and only few progressive cancer cells have the skill to form a colony in the distant organs (Fidler 1973; Mehlen and Puisieux 2006; Steeg 2016). Studies have proposed several mechanisms to explain why only a few cancer cells gain metastatic growth and the others are restricted from this growth (Hess et al. 2006; Mehlen and Puisieux 2006; Steeg 2016). A recent review article by Patricia Steeg proposed that specific genomic instability may be the fuel used to complete the metastasis to the distant organs, which is lacking in other cellular phenotypes (Steeg 2016). Thereby, therapeutic control of those instabilities may improve patient outcomes and decrease death associated with metastasis. However, it is undeniable that no such mechanisms or biomarkers for earlier intervention are still obtainable to target cancers.
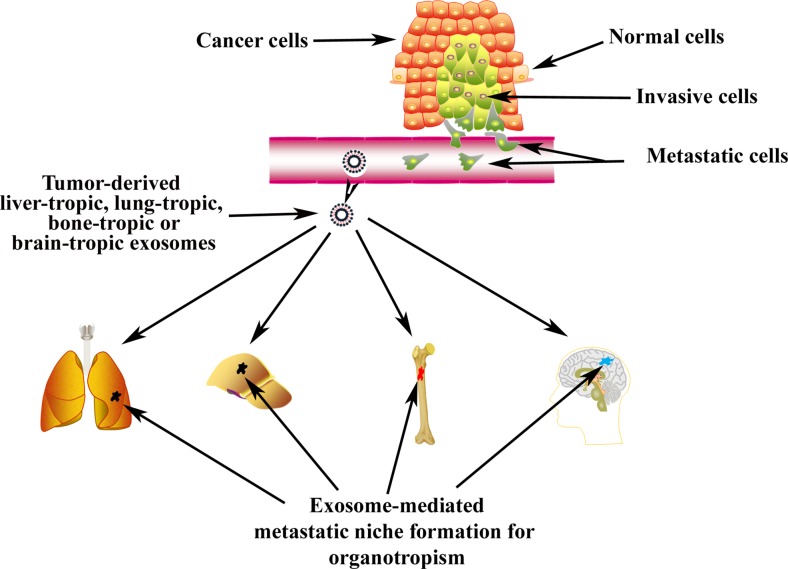
Cancer cells are able to secrete factors that promote metastasis. For example, breast cancer-cell-secreted microRNA-105 (miR-105) abolishes vascular endothelial barriers via regulating the tight junction protein ZO-1 and promotes metastasis (Zhou et al. 2014). Interleukin-6 (IL-6), produced by breast cancer cells, promotes tumor growth and metastasis via IL-6/Janus kinase (JAK)/signal transducer and is an activator of transcription 3 (Stat3) (Chang et al. 2013). Moreover, an interaction of the intrinsic properties of cancer cells and the microenvironment of the host organ are critical in determining the effectiveness of organ-specific metastasis (Lu and Kang 2007). Consistent with previous perceptions, recent studies proposed that exosomes are those tumor-derived factors that educate cancer cells and host-stromal cells to gain metastatic phenotypes and determine organ-specific metastasis (Fig. 4 ) (Costa-Silva et al. 2015; Hoshino et al. 2015; Peinado et al. 2012; Zomer et al. 2015). Costa-Silva et.al found that the uptake of exosomes by liver host-Kupffer cells (stellate macrophages- phagocytic cells, KCs) promotes liver fibrosis via activating several canonical molecular signatures, including connective tissue growth factor (CTGF/CCN2), insulin-like growth factor (IGF), PDGF and transforming growth factor-β (TGF-β) (Costa-Silva et al. 2015). The exosome-KC-mediated activation of liver fibrosis pathways creates a pro-inflammatory environment that ultimately supports metastasis (Costa-Silva et al. 2015). Specifically, macrophage migration inhibitory factor (MIF), which is highly expressed in pancreatic cancer cell-derived exosomes, attacks KCs in the liver and induces secretion of TGF-β. The secreted TGF-β attacks neighboring hepatic stellate cells for the production and deposition of fibronectin, which, in turn, arrests bone-marrow-derived macrophages and neutrophils in the liver for metastatic niche formation (Costa-Silva et al. 2015).
Fig. 4.
Cancer-derived exosomes act as messengers of metastasis. Exosomes exhibit tissue/organ specificity and act by “priming” the site to create a microenvironment that is conducive for cancer metastasis
Recent studies of Hoshino et.al proposed a novel role of breast tumor cell secreted exosomal integrins in the formation of metastasis (Hoshino et al. 2015). They found that lung metastasis can be induced by exosomal integrins α6β4 and α6β1, while exosomal integrin αvβ5 can promote liver metastasis (Hoshino et al. 2015). Moreover, the studies demonstrate that the tumor-derived exosomal integrins may selectively adhere to cell-associated extracellular matrix (ECM)-enriched cellular areas of specific organs and promoting Src phosphorylation and pro-inflammatory S100 gene expression for organ specific metastasis (Hoshino et al. 2015). Therefore, they suggest that exosomal integrins could be ideal markers to predict organ-specific metastasis.
Metastatic melanoma derived exosomes, which are produced by Ras-related proteins 27a (Rab27a), promote the metastatic behavior of primary melanoma by educating bone marrow progenitor cells (Peinado et al. 2012). Melanoma-specific molecular signatures of exosomes have been detected in association with metastatic niche formation. These signatures include tyrosinase-related protein-2 (TYRP2), very late antigen 4 (VLA-4), heat shock protein 70 (HSP70), an HSP90 isoform, and MET oncoprotein. Education of bone marrow progenitor cells is achieved through MET protooncogene receptor/ hepatocyte growth factor receptor (HGFR) (Peinado et al. 2012).
Collectively, these studies demonstrate that exosomes of various cancers mediate metastatic niche formation in a very organ-specific manner. Various cell types such as endothelial cells, fibroblasts, bone marrow and other stromal cells are participants in the formation of metastatic niche via the unique influence of horizontal transfer of molecules (Colombo et al. 2014; Costa-Silva et al. 2015; Hoshino et al. 2015; Kalluri 2016; Peinado et al. 2012). Some regulation mechanisms have been uncovered but there still much to learn.
Pitfalls of exosome research
While many important molecular targets and pathways are expected to link with exosomes, isolation methods have proved a major obstacle in exosome research (Safdar et al. 2016). Because standardized isolation methods are still being developed, exosome yield, quality and reproducibility is a continuous concern. Differential centrifugation, a process involving a series of centrifugations steps of increasing speeds, is currently the most common method for isolating exosomes. However, there are several disadvantages to ultracentrifugation (Lobb et al. 2015). The process often results in lost or damaged exosomes, rendering them undesirable for experimentation (Lobb et al. 2015). In addition, the yield of exosomes is low and variable as they are prone to clumping, which makes it difficult to separate them based on size in density-dependent ultracentrifugation (Greening et al. 2015; Tauro et al. 2012). Another problem with differential centrifugation is that other particles of similar weight and density to exosomes are also pelleted down, including protein aggregates and lipoproteins (Tauro et al. 2012; Witwer et al. 2013). Exosome cargo is also affected by the ultracentrifugation process. For example, exosomes isolated from ultracentrifugation generally yield lower levels of protein and RNA (Taylor and Shah 2015). Furthermore, ultracentrifugation concentrates albumin and IgG when used to isolate exosomes from serum, thereby contaminating the exosome pellet (Gyorgy et al. 2011). More effective methods, like immunoaffinity, are available, but limited due to paucity of antibodies and exosome markers (Greening et al. 2015). Several exosome isolation kits, which were found to be more efficient and reproducible than ultracentrifugation, are also available (Gyorgy et al. 2011). However, these kits are expensive, which is why ultracentrifugation remains a popular method for isolating exosomes. Our lab routinely uses ultracentrifugation and has obtained very consistent results (see Fig. 2 ).
Conclusion and future perspectives
Exosomes, nano-vesicles with an endocytic origin, are excreted by nearly all cells and contain genetic materials and various molecular signatures. The transfer of exosomes between cells allows for intercellular communication - an essential component for biological and pathobiological functions. Based on their functions, exosomes are considered molecular cargo (Kalluri 2016), signalosomes (Syn et al. 2016) or molecular palkis. Exosomes promote invasive phenotypes, angiogenesis and metastatic growth to the distant organs and, as a result, aid in therapeutics by acting as biomarkers and vehicles for genetic therapy; they can be potentially beneficial since exosomes are non-invasive bioavailable vehicles (Tickner et al. 2014). On the other hand, the destruction/inactivation of cancer-derived exosomes itself may lead to inhibition of angiogenesis and metastasis, thus controlling the tumor. As a result, exosomes can be beneficial or destructive depending upon their environment. Their multi-faceted and dynamic role in cancer initiation, progression and metastasis offers a fertile ground to determine the cause, effect and treatment of various cancers. Ultimately, as research continues to decode the regulatory languages of exosomes’ signals, this will pave the way for new strategies for cancer therapies. For example, exosomal MIF regulates TGF-β-signaling for metastatic niche formation but it is unclear whether CTGF/CCN2, which is a downstream mediator of TGF-β-induced fibrosis (Ruiz-Ortega et al. 2007), plays any role in the formation of metastatic niche. Uncovering this molecular link between MIF and CTGF may open a new therapeutic window. Moreover, the involvement of other members of the CCN-family cannot be ignored as their participation via cross-talking/side-talking with TGF-β and integrins signaling are well-documented (Banerjee and Banerjee 2012; Jun and Lau 2011). Thus, targeting CCN-family proteins’ interactions can be effective, foreshadowing a promising future in our understanding of the underlying mechanisms of metastasis and developing prognostic markers for clinical studies.
Acknowledgments
We would like to thank other members of our cancer research unit for valuable and helpful comments on this manuscript. We would also like to thank LaCoiya Harris for editing and organizing this manuscript. This work was supported by the Kansas City Area Life Science grant award (SKB), Merit review grant from Department of Veterans Affairs (SKB, 5I01BX001989-03 and SB,1I01BX001002-04), and KUMC Van Goethem Family Endowed Funds (SKB).
Author contributions
Arvind Subramanian, Gargi Maity, Vijayalaxmi Gupta and Sandipto Sarkar performed the literature search and wrote the manuscript. Arnab Ghosh, LaCoiya Harris and Ajay Bansal revised it critically for important intellectual content. Lane Christenson and WeiTing Hung help in generating valuable data. Snigdha Banerjee and Sushanta K. Banerjee provided expert comments and editing.
Compliance with ethical standards
Conflicts of interest
The authors declare no conflict of interest.
Footnotes
Arvind Subramanian, Vijayalaxmi Gupta and Sandipto Sarkar are authors have equal contribution to prepare this article
Arvind Subramanian is a High school (Olathe North High School, Kansas) student joined the Cancer Research Unit as a summer student in 2015 and 2016.
Contributor Information
Snigdha Banerjee, Phone: 816-861-4700, Email: sbanerjee@kumc.edu, Email: cancerresearchunit@icloud.com.
Sushanta K. Banerjee, Phone: 816-861-4700, Email: sbanerjee2@kumc.edu, Email: cancerresearchunit@icloud.com
References
- Abusamra AJ, Zhong Z, Zheng X, Li M, Ichim TE, Chin JL, Min WP. Tumor exosomes expressing Fas ligand mediate CD8+ T-cell apoptosis Blood cells. Molecules & diseases. 2005;35:169–173. doi: 10.1016/j.bcmd.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Aga M, et al. Exosomal HIF1alpha supports invasive potential of nasopharyngeal carcinoma-associated LMP1-positive exosomes. Oncogene. 2014;33:4613–4622. doi: 10.1038/onc.2014.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Nedawi K, Meehan B, Micallef J, Lhotak V, May L, Guha A, Rak J. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol. 2008;10:619–624. doi: 10.1038/ncb1725. [DOI] [PubMed] [Google Scholar]
- Atula S, Grenman R, Syrjanen S (1997) Fibroblasts can modulate the phenotype of malignant epithelial cells in vitro Exp Cell Res 235:180–187 doi:10.1006/excr.1997.3676 [DOI] [PubMed]
- Baietti MF, et al. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat Cell Biol. 2012;14:677–685. doi: 10.1038/ncb2502. [DOI] [PubMed] [Google Scholar]
- Banerjee SK, Banerjee S (2012) CCN5/WISP-2: A micromanager of breast cancer progression J Cell Commun Signal 6:63–71 doi:10.1007/s12079-012-0158-2 [DOI] [PMC free article] [PubMed]
- Bobrie A, Colombo M, Raposo G, Thery C. Exosome secretion: molecular mechanisms and roles in immune responses. Traffic. 2011;12:1659–1668. doi: 10.1111/j.1600-0854.2011.01225.x. [DOI] [PubMed] [Google Scholar]
- Brentnall TA. Arousal of cancer-associated stromal fibroblasts: palladin-activated fibroblasts promote tumor invasion. Cell Adhes Migr. 2012;6:488–494. doi: 10.4161/cam.21453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton LT, Sloane HS, Kester M, KA K. Formation and role of exosomes in cancer. Cellular and molecular life sciences : CMLS. 2015;72:659–671. doi: 10.1007/s00018-014-1764-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camps JL et al. (1990) Fibroblast-mediated acceleration of human epithelial tumor growth in vivo Proceedings of the National Academy of Sciences of the United States of America 87:75–79 [DOI] [PMC free article] [PubMed]
- Chang Q, et al. The IL-6/JAK/Stat3 feed-forward loop drives tumorigenesis and metastasis. Neoplasia. 2013;15:848–862. doi: 10.1593/neo.13706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chargaff E, West R. The biological significance of the thromboplastic protein of blood. J Biol Chem. 1946;166:189–197. [PubMed] [Google Scholar]
- Cho JA, Park H, Lim EH, KW L. Exosomes from breast cancer cells can convert adipose tissue-derived mesenchymal stem cells into myofibroblast-like cells. Int J Oncol. 2012;40:130–138. doi: 10.3892/ijo.2011.1193. [DOI] [PubMed] [Google Scholar]
- Colombo M, Raposo G, Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–289. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- Corcoran C, Rani S, O'Driscoll L. miR-34a is an intracellular and exosomal predictive biomarker for response to docetaxel with clinical relevance to prostate cancer progression. Prostate. 2014;74:1320–1334. doi: 10.1002/pros.22848. [DOI] [PubMed] [Google Scholar]
- Costa-Silva B, et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol. 2015;17:816–826. doi: 10.1038/ncb3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai ZJ, et al. Five common functional polymorphisms in microRNAs (rs2910164, rs2292832, rs11614913, rs3746444, rs895819) and the susceptibility to breast cancer: evidence from 8361 cancer cases and 8504 controls. Curr Pharm Des. 2015;21:1455–1463. doi: 10.2174/1381612821666141208143533. [DOI] [PubMed] [Google Scholar]
- De Veirman K, et al. Induction of miR-146a by multiple myeloma cells in mesenchymal stromal cells stimulates their pro-tumoral activity. Cancer Lett. 2016;377:17–24. doi: 10.1016/j.canlet.2016.04.024. [DOI] [PubMed] [Google Scholar]
- Demory Beckler M, et al. Proteomic analysis of exosomes from mutant KRAS colon cancer cells identifies intercellular transfer of mutant. KRAS Molecular & cellular proteomics : MCP. 2013;12:343–355. doi: 10.1074/mcp.M112.022806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidler IJ. Selection of successive tumour lines for metastasis. Nat New Biol. 1973;242:148–149. doi: 10.1038/newbio242148a0. [DOI] [PubMed] [Google Scholar]
- Ge R, Tan E, Sharghi-Namini S, Asada HH. Exosomes in Cancer Microenvironment and Beyond: have we Overlooked these Extracellular Messengers? Cancer Microenviron : official journal of the International Cancer Microenvironment Society. 2012;5:323–332. doi: 10.1007/s12307-012-0110-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greening DW, Xu R, Ji H, Tauro BJ, Simpson RJ. A protocol for exosome isolation and characterization: evaluation of ultracentrifugation, density-gradient separation, and immunoaffinity capture methods. Methods Mol Biol. 2015;1295:179–209. doi: 10.1007/978-1-4939-2550-6_15. [DOI] [PubMed] [Google Scholar]
- Gu J et al. (2012) Gastric cancer exosomes trigger differentiation of umbilical cord derived mesenchymal stem cells to carcinoma-associated fibroblasts through TGF-beta/Smad pathway PloS one 7:e52465 doi:10.1371/journal.pone.0052465 [DOI] [PMC free article] [PubMed]
- Guduric-Fuchs J, O'Connor A, Camp B, O'Neill CL, Medina RJ, Simpson DA (2012) Selective extracellular vesicle-mediated export of an overlapping set of microRNAs from multiple cell types BMC genomics 13:357 doi:10.1186/1471-2164-13-357 [DOI] [PMC free article] [PubMed]
- Gyorgy B, et al. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cellular and molecular life sciences : CMLS. 2011;68:2667–2688. doi: 10.1007/s00018-011-0689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Hess KR, Varadhachary GR, Taylor SH, Wei W, Raber MN, Lenzi R, Abbruzzese JL. Metastatic patterns in adenocarcinoma. Cancer. 2006;106:1624–1633. doi: 10.1002/cncr.21778. [DOI] [PubMed] [Google Scholar]
- Higginbotham JN, et al. Amphiregulin exosomes increase cancer cell invasion. Current biology : CB. 2011;21:779–786. doi: 10.1016/j.cub.2011.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino A, et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527:329–335. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iero M, Valenti R, Huber V, Filipazzi P, Parmiani G, Fais S, Rivoltini L. Tumour-released exosomes and their implications in cancer immunity. Cell Death Differ. 2008;15:80–88. doi: 10.1038/sj.cdd.4402237. [DOI] [PubMed] [Google Scholar]
- Jun JI, Lau LF. Taking aim at the extracellular matrix: CCN proteins as emerging therapeutic targets. Nat Rev Drug Discov. 2011;10:945–963. doi: 10.1038/nrd3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahlert C, Kalluri R. Exosomes in tumor microenvironment influence cancer progression and metastasis. J Mol Med. 2013;91:431–437. doi: 10.1007/s00109-013-1020-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahlert C, et al. Identification of double-stranded genomic DNA spanning all chromosomes with mutated KRAS and p53 DNA in the serum exosomes of patients with pancreatic cancer. J Biol Chem. 2014;289:3869–3875. doi: 10.1074/jbc.C113.532267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri R. The biology and function of exosomes in cancer. J Clin Invest. 2016;126:1208–1215. doi: 10.1172/JCI81135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucharzewska P, et al. Exosomes reflect the hypoxic status of glioma cells and mediate hypoxia-dependent activation of vascular cells during tumor development. Proc Natl Acad Sci U S A. 2013;110:7312–7317. doi: 10.1073/pnas.1220998110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labani-Motlagh A, et al. Differential expression of ligands for NKG2D and DNAM-1 receptors by epithelial ovarian cancer-derived exosomes and its influence on NK cell cytotoxicity Tumour biology. The Journal of the International Society for Oncodevelopmental Biology and Medicine. 2016;37:5455–5466. doi: 10.1007/s13277-015-4313-2. [DOI] [PubMed] [Google Scholar]
- Lee Y, El Andaloussi S, Wood MJ. Exosomes and microvesicles: extracellular vesicles for genetic information transfer and gene therapy. Hum Mol Genet. 2012;21:R125–R134. doi: 10.1093/hmg/dds317. [DOI] [PubMed] [Google Scholar]
- Liu J, Sun H, Wang X, Yu Q, Li S, Yu X, Gong W. Increased exosomal microRNA-21 and microRNA-146a levels in the cervicovaginal lavage specimens of patients with cervical cancer. Int J Mol Sci. 2014;15:758–773. doi: 10.3390/ijms15010758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobb RJ, Becker M, Wen SW, Wong CS, Wiegmans AP, Leimgruber A, Moller A (2015) Optimized exosome isolation protocol for cell culture supernatant and human plasma Journal of extracellular vesicles 4:27031 doi:10.3402/jev.v4.27031 [DOI] [PMC free article] [PubMed]
- Lu X, Kang Y. Organotropism of breast cancer metastasis. J Mammary Gland Biol Neoplasia. 2007;12:153–162. doi: 10.1007/s10911-007-9047-3. [DOI] [PubMed] [Google Scholar]
- Luga V, et al. Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration. Cell. 2012;151:1542–1556. doi: 10.1016/j.cell.2012.11.024. [DOI] [PubMed] [Google Scholar]
- Mathivanan S, Fahner CJ, Reid GE, Simpson RJ. ExoCarta 2012: database of exosomal proteins. RNA and lipids Nucleic acids research. 2012;40:D1241–D1244. doi: 10.1093/nar/gkr828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlen P, Puisieux A. Metastasis: a question of life or death. Nat Rev Cancer. 2006;6:449–458. doi: 10.1038/nrc1886. [DOI] [PubMed] [Google Scholar]
- Melo SA, et al. Cancer exosomes perform cell-independent microRNA biogenesis and promote tumorigenesis. Cancer Cell. 2014;26:707–721. doi: 10.1016/j.ccell.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo SA, et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature. 2015;523:177–182. doi: 10.1038/nature14581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelet X, Djeddi A, Legouis R. Developmental and cellular functions of the ESCRT machinery in pluricellular organisms. Biology of the Cell / Under the Auspices of the European Cell Biology Organization. 2010;102:191–202. doi: 10.1042/BC20090145. [DOI] [PubMed] [Google Scholar]
- Mincheva-Nilsson L, Baranov V. The role of placental exosomes in reproduction. Am J Reprod Immunol. 2010;63:520–533. doi: 10.1111/j.1600-0897.2010.00822.x. [DOI] [PubMed] [Google Scholar]
- Muller L, Mitsuhashi M, Simms P, Gooding WE, Whiteside TL (2016) Tumor-derived exosomes regulate expression of immune function-related genes in human T cell subsets Scientific reports 6:20254 doi:10.1038/srep20254 [DOI] [PMC free article] [PubMed]
- Muntion S et al. (2016) Microvesicles from Mesenchymal Stromal Cells Are Involved in HPC-Microenvironment Crosstalk in Myelodysplastic Patients PloS one 11:e0146722 doi:10.1371/journal.pone.0146722 [DOI] [PMC free article] [PubMed]
- Orimo A, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- Ostrowski M et al. (2010) Rab27a and Rab27b control different steps of the exosome secretion pathway Nat Cell Biol 12:19–30; sup pp 11–13 doi:10.1038/ncb2000 [DOI] [PubMed]
- Paget S. The distribution of secondary growths in cancer of the breast. Lancet. 1889;1:3. [PubMed] [Google Scholar]
- Park JE, et al. Hypoxic tumor cell modulates its microenvironment to enhance angiogenic and metastatic potential by secretion of proteins and exosomes. Molecular & Cellular Proteomics : MCP. 2010;9:1085–1099. doi: 10.1074/mcp.M900381-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parolini I, et al. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J Biol Chem. 2009;284:34211–34222. doi: 10.1074/jbc.M109.041152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel GK, Patton MC, Singh S, Khushman M, Singh AP (2016) Pancreatic Cancer Exosomes: Shedding Off for a Meaningful Journey Pancreatic disorders & Therapy 6:e148 doi:10.4172/2165-7092.1000e148 [DOI] [PMC free article] [PubMed]
- Peinado H, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012;18:883–891. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigati L, et al. Selective release of microRNA species from normal and malignant mammary epithelial cells. PLoS One. 2010;5 doi: 10.1371/journal.pone.0013515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran S, Palanisamy V (2012) Horizontal transfer of RNAs: exosomes as mediators of intercellular communication Wiley interdisciplinary reviews RNA 3:286–293 doi:10.1002/wrna.115 [DOI] [PMC free article] [PubMed]
- Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roucourt B, Meeussen S, Bao J, Zimmermann P, David G. Heparanase activates the syndecan-syntenin-ALIX exosome pathway. Cell Res. 2015;25:412–428. doi: 10.1038/cr.2015.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Ortega M, Rodriguez-Vita J, Sanchez-Lopez E, Carvajal G, Egido J. TGF-beta signaling in vascular fibrosis. Cardiovasc Res. 2007;74:196–206. doi: 10.1016/j.cardiores.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Safdar A, Saleem A, Tarnopolsky MA (2016) The potential of endurance exercise-derived exosomes to treat metabolic diseases Nature reviews Endocrinology doi:10.1038/nrendo.2016.76 [DOI] [PubMed]
- Savina A, Furlan M, Vidal M, Colombo MI. Exosome release is regulated by a calcium-dependent mechanism in K562 cells. J Biol Chem. 2003;278:20083–20090. doi: 10.1074/jbc.M301642200. [DOI] [PubMed] [Google Scholar]
- Singh R, Pochampally R, Watabe K, Lu Z, Mo YY (2014) Exosome-mediated transfer of miR-10b promotes cell invasion in breast cancer Molecular cancer 13:256 doi:10.1186/1476-4598-13-256 [DOI] [PMC free article] [PubMed]
- Skog J, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10:1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steeg PS. Targeting metastasis. Nat Rev Cancer. 2016;16:201–218. doi: 10.1038/nrc.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. 2009;10:513–525. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- Suchorska WM, Lach MS. The role of exosomes in tumor progression and metastasis (Review) Oncol Rep. 2016;35:1237–1244. doi: 10.3892/or.2015.4507. [DOI] [PubMed] [Google Scholar]
- Suetsugu A, Honma K, Saji S, Moriwaki H, Ochiya T, Hoffman RM. Imaging exosome transfer from breast cancer cells to stroma at metastatic sites in orthotopic nude-mouse models. Adv Drug Deliv Rev. 2013;65:383–390. doi: 10.1016/j.addr.2012.08.007. [DOI] [PubMed] [Google Scholar]
- Syn N, Wang L, Sethi G, Thiery JP, Goh BC (2016) Exosome-Mediated Metastasis: From Epithelial-Mesenchymal Transition to Escape from Immunosurveillance Trends in pharmacological sciences doi:10.1016/j.tips.2016.04.006 [DOI] [PubMed]
- Tauro BJ, Greening DW, Mathias RA, Ji H, Mathivanan S, Scott AM, Simpson RJ. Comparison of ultracentrifugation, density gradient separation, and immunoaffinity capture methods for isolating human colon cancer cell line LIM1863-derived exosomes. Methods. 2012;56:293–304. doi: 10.1016/j.ymeth.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Taylor DD, Shah S. Methods of isolating extracellular vesicles impact down-stream analyses of their cargoes. Methods. 2015;87:3–10. doi: 10.1016/j.ymeth.2015.02.019. [DOI] [PubMed] [Google Scholar]
- Thery C, Duban L, Segura E, Veron P, Lantz O, Amigorena S. Indirect activation of naive CD4+ T cells by dendritic cell-derived exosomes. Nat Immunol. 2002;3:1156–1162. doi: 10.1038/ni854. [DOI] [PubMed] [Google Scholar]
- Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2:569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Tickner JA, Urquhart AJ, Stephenson SA, Richard DJ, O'Byrne KJ (2014) Functions and therapeutic roles of exosomes in cancer Frontiers in oncology 4:127 doi:10.3389/fonc.2014.00127 [DOI] [PMC free article] [PubMed]
- Tokuhisa M, et al. Exosomal miRNAs from Peritoneum Lavage Fluid as Potential Prognostic Biomarkers of Peritoneal Metastasis in Gastric Cancer. PLoS One. 2015;10 doi: 10.1371/journal.pone.0130472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Pol E, Boing AN, Harrison P, Sturk A, Nieuwland R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol Rev. 2012;64:676–705. doi: 10.1124/pr.112.005983. [DOI] [PubMed] [Google Scholar]
- Vong S, Kalluri R. The role of stromal myofibroblast and extracellular matrix in tumor angiogenesis. Genes & Cancer. 2011;2:1139–1145. doi: 10.1177/1947601911423940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webber J, Steadman R, Mason MD, Tabi Z, Clayton A. Cancer exosomes trigger fibroblast to myofibroblast differentiation. Cancer Res. 2010;70:9621–9630. doi: 10.1158/0008-5472.CAN-10-1722. [DOI] [PubMed] [Google Scholar]
- Webber J, et al. Proteomics analysis of cancer exosomes using a novel modified aptamer-based array (SOMAscan) platform. Molecular & cellular proteomics : MCP. 2014;13:1050–1064. doi: 10.1074/mcp.M113.032136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webber JP, et al. Differentiation of tumour-promoting stromal myofibroblasts by cancer exosomes. Oncogene. 2015;34:290–302. doi: 10.1038/onc.2013.560. [DOI] [PubMed] [Google Scholar]
- Witwer KW et al. (2013) Standardization of sample collection, isolation and analysis methods in extracellular vesicle research Journal of Extracellular Vesicles 2 doi:10.3402/jev.v2i0.20360 [DOI] [PMC free article] [PubMed]
- You Y, et al. Matrix metalloproteinase 13-containing exosomes promote nasopharyngeal carcinoma metastasis. Cancer Sci. 2015;106:1669–1677. doi: 10.1111/cas.12818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Yuan X, Shi H, Wu L, Qian H, Xu W (2015) Exosomes in cancer: small particle, big player Journal of hematology & Oncology 8:83 doi:10.1186/s13045-015-0181-x [DOI] [PMC free article] [PubMed]
- Zhou W, et al. Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell. 2014;25:501–515. doi: 10.1016/j.ccr.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zomer A, Vendrig T, Hopmans ES, van Eijndhoven M, Middeldorp JM, Pegtel DM. Exosomes: Fit to deliver small RNA. Communicative & Integr Biol. 2010;3:447–450. doi: 10.4161/cib.3.5.12339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zomer A, et al. In Vivo imaging reveals extracellular vesicle-mediated phenocopying of metastatic behavior. Cell. 2015;161:1046–1057. doi: 10.1016/j.cell.2015.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]