Abstract
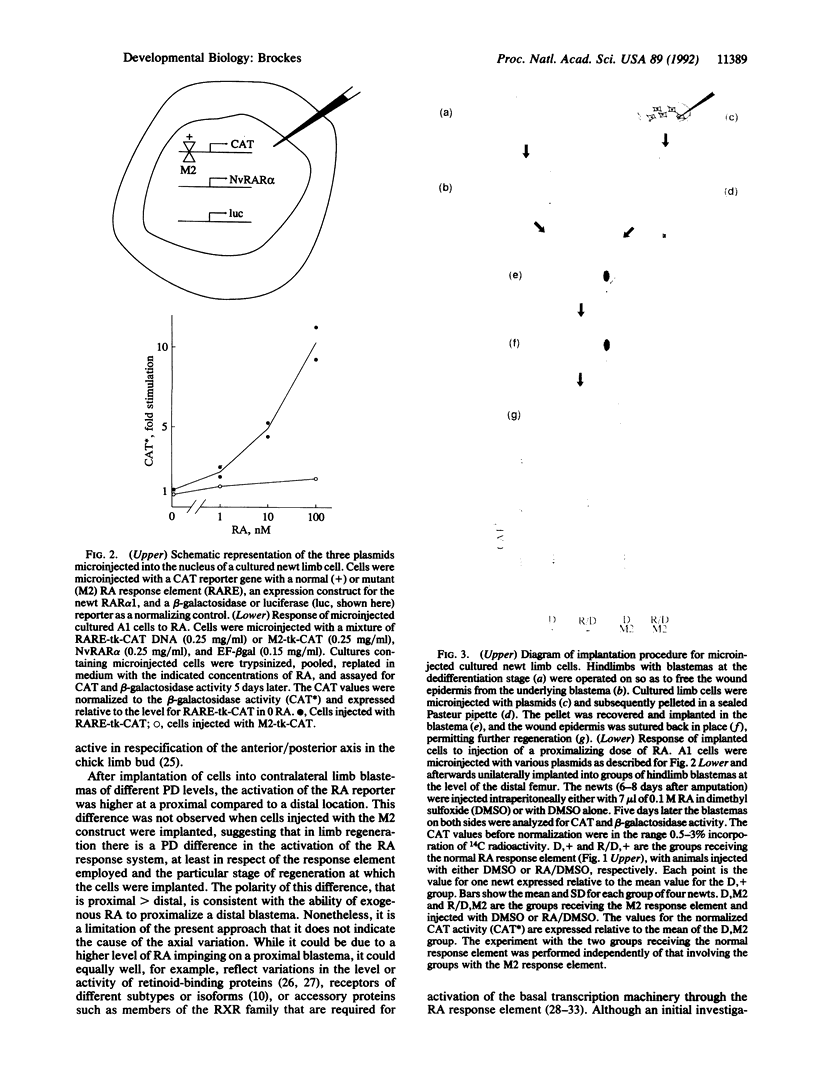
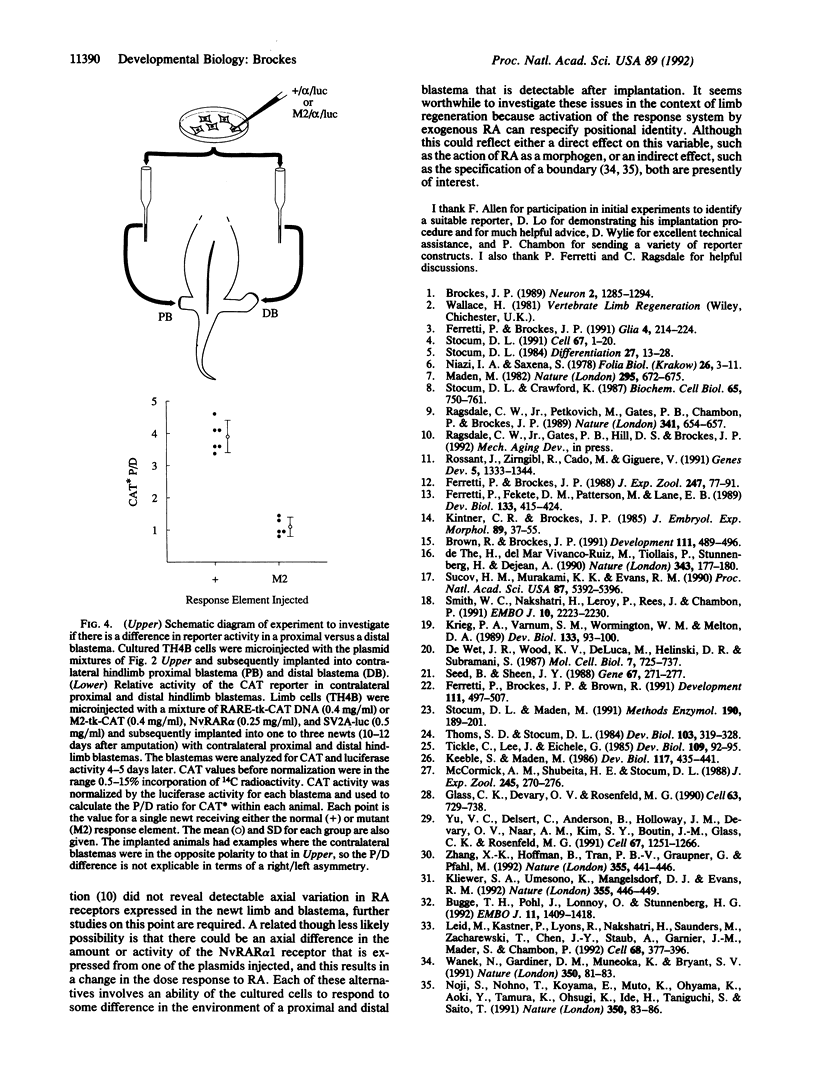
After amputation of the limb of an adult urodele amphibian at any point along the proximodistal axis, blastemal cells (the progenitor cells of the regenerate) give rise only to the missing structures. Retinoic acid (RA) is able to respecify the positional identity of the blastema to a more proximal value, thus raising the possibility that the RA response system is activated during limb regeneration. Cultured newt (Notophthalmus viridescens) limb cells were transfected by nuclear microinjection of plasmids which provided RA-sensitive reporter activity that could be normalized for differences in cell recovery and transfection efficiency. Such cells showed a dose-dependent response to RA in culture, and this required a functional RA response element. The cells were implanted under the wound epidermis of newt hindlimb blastemas. After injection of a proximalizing dose of RA there was a significant difference in the level of reporter activity dependent on a functional response element. When cells were implanted into contralateral proximal and distal hindlimb blastemas the proximal-to-distal ratio for activation of the reporter through the response element was approximately 3.5-fold, suggesting that a gene whose expression is regulated by RA could be differentially activated along the proximodistal axis during limb regeneration.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brockes J. P. Retinoids, homeobox genes, and limb morphogenesis. Neuron. 1989 Apr;2(4):1285–1294. doi: 10.1016/0896-6273(89)90066-4. [DOI] [PubMed] [Google Scholar]
- Brown R., Brockes J. P. Identification and expression of a regeneration-specific homeobox gene in the newt limb blastema. Development. 1991 Feb;111(2):489–496. doi: 10.1242/dev.111.2.489. [DOI] [PubMed] [Google Scholar]
- Bugge T. H., Pohl J., Lonnoy O., Stunnenberg H. G. RXR alpha, a promiscuous partner of retinoic acid and thyroid hormone receptors. EMBO J. 1992 Apr;11(4):1409–1418. doi: 10.1002/j.1460-2075.1992.tb05186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferretti P., Brockes J. P., Brown R. A newt type II keratin restricted to normal and regenerating limbs and tails is responsive to retinoic acid. Development. 1991 Feb;111(2):497–507. doi: 10.1242/dev.111.2.497. [DOI] [PubMed] [Google Scholar]
- Ferretti P., Brockes J. P. Cell origin and identity in limb regeneration and development. Glia. 1991;4(2):214–224. doi: 10.1002/glia.440040213. [DOI] [PubMed] [Google Scholar]
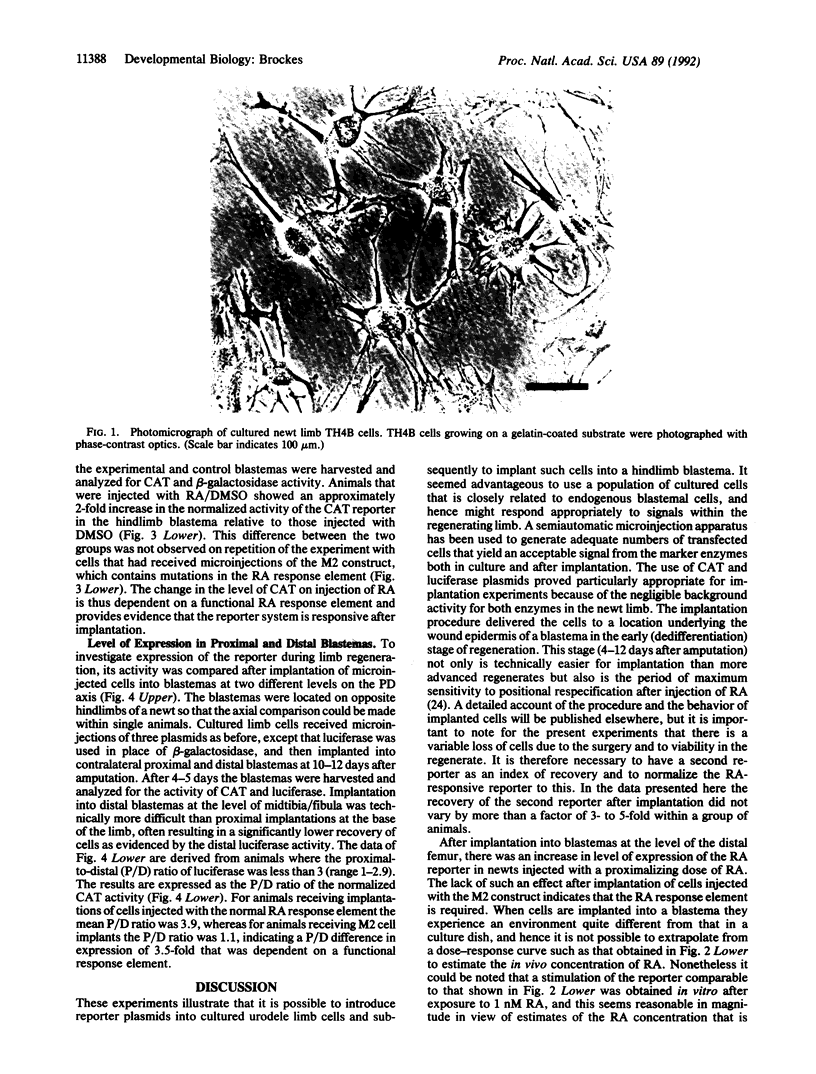
- Ferretti P., Brockes J. P. Culture of newt cells from different tissues and their expression of a regeneration-associated antigen. J Exp Zool. 1988 Jul;247(1):77–91. doi: 10.1002/jez.1402470111. [DOI] [PubMed] [Google Scholar]
- Ferretti P., Fekete D. M., Patterson M., Lane E. B. Transient expression of simple epithelial keratins by mesenchymal cells of regenerating newt limb. Dev Biol. 1989 Jun;133(2):415–424. doi: 10.1016/0012-1606(89)90045-6. [DOI] [PubMed] [Google Scholar]
- Glass C. K., Devary O. V., Rosenfeld M. G. Multiple cell type-specific proteins differentially regulate target sequence recognition by the alpha retinoic acid receptor. Cell. 1990 Nov 16;63(4):729–738. doi: 10.1016/0092-8674(90)90139-6. [DOI] [PubMed] [Google Scholar]
- Keeble S., Maden M. Retinoic acid-binding protein in the axolotl: distribution in mature tissues and time of appearance during limb regeneration. Dev Biol. 1986 Oct;117(2):435–441. doi: 10.1016/0012-1606(86)90312-x. [DOI] [PubMed] [Google Scholar]
- Kintner C. R., Brockes J. P. Monoclonal antibodies to the cells of a regenerating limb. J Embryol Exp Morphol. 1985 Oct;89:37–55. [PubMed] [Google Scholar]
- Kliewer S. A., Umesono K., Mangelsdorf D. J., Evans R. M. Retinoid X receptor interacts with nuclear receptors in retinoic acid, thyroid hormone and vitamin D3 signalling. Nature. 1992 Jan 30;355(6359):446–449. doi: 10.1038/355446a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg P. A., Varnum S. M., Wormington W. M., Melton D. A. The mRNA encoding elongation factor 1-alpha (EF-1 alpha) is a major transcript at the midblastula transition in Xenopus. Dev Biol. 1989 May;133(1):93–100. doi: 10.1016/0012-1606(89)90300-x. [DOI] [PubMed] [Google Scholar]
- Leid M., Kastner P., Lyons R., Nakshatri H., Saunders M., Zacharewski T., Chen J. Y., Staub A., Garnier J. M., Mader S. Purification, cloning, and RXR identity of the HeLa cell factor with which RAR or TR heterodimerizes to bind target sequences efficiently. Cell. 1992 Jan 24;68(2):377–395. doi: 10.1016/0092-8674(92)90478-u. [DOI] [PubMed] [Google Scholar]
- Maden M. Vitamin A and pattern formation in the regenerating limb. Nature. 1982 Feb 25;295(5851):672–675. doi: 10.1038/295672a0. [DOI] [PubMed] [Google Scholar]
- McCormick A. M., Shubeita H. E., Stocum D. L. Cellular retinoic acid binding protein: detection and quantitation in regenerating axolotl limbs. J Exp Zool. 1988 Mar;245(3):270–276. doi: 10.1002/jez.1402450307. [DOI] [PubMed] [Google Scholar]
- Niazi I. A., Saxena S. Abnormal hind limb regeneration in tadpoles of the toad, Bufo andersoni, exposed to excess vitamin A. Folia Biol (Krakow) 1978;26(1):3–8. [PubMed] [Google Scholar]
- Nicola N. A., Metcalf D. Subunit promiscuity among hemopoietic growth factor receptors. Cell. 1991 Oct 4;67(1):1–4. doi: 10.1016/0092-8674(91)90564-f. [DOI] [PubMed] [Google Scholar]
- Noji S., Nohno T., Koyama E., Muto K., Ohyama K., Aoki Y., Tamura K., Ohsugi K., Ide H., Taniguchi S. Retinoic acid induces polarizing activity but is unlikely to be a morphogen in the chick limb bud. Nature. 1991 Mar 7;350(6313):83–86. doi: 10.1038/350083a0. [DOI] [PubMed] [Google Scholar]
- Ragsdale C. W., Jr, Petkovich M., Gates P. B., Chambon P., Brockes J. P. Identification of a novel retinoic acid receptor in regenerative tissues of the newt. Nature. 1989 Oct 19;341(6243):654–657. doi: 10.1038/341654a0. [DOI] [PubMed] [Google Scholar]
- Rossant J., Zirngibl R., Cado D., Shago M., Giguère V. Expression of a retinoic acid response element-hsplacZ transgene defines specific domains of transcriptional activity during mouse embryogenesis. Genes Dev. 1991 Aug;5(8):1333–1344. doi: 10.1101/gad.5.8.1333. [DOI] [PubMed] [Google Scholar]
- Seed B., Sheen J. Y. A simple phase-extraction assay for chloramphenicol acyltransferase activity. Gene. 1988 Jul 30;67(2):271–277. doi: 10.1016/0378-1119(88)90403-9. [DOI] [PubMed] [Google Scholar]
- Smith W. C., Nakshatri H., Leroy P., Rees J., Chambon P. A retinoic acid response element is present in the mouse cellular retinol binding protein I (mCRBPI) promoter. EMBO J. 1991 Aug;10(8):2223–2230. doi: 10.1002/j.1460-2075.1991.tb07758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocum D. L., Crawford K. Use of retinoids to analyze the cellular basis of positional memory in regenerating amphibian limbs. Biochem Cell Biol. 1987 Aug;65(8):750–761. doi: 10.1139/o87-098. [DOI] [PubMed] [Google Scholar]
- Stocum D. L., Maden M. Regenerating limbs. Methods Enzymol. 1990;190:189–201. doi: 10.1016/0076-6879(90)90023-t. [DOI] [PubMed] [Google Scholar]
- Stocum D. L. The urodele limb regeneration blastema. Determination and organization of the morphogenetic field. Differentiation. 1984;27(1):13–28. doi: 10.1111/j.1432-0436.1984.tb01403.x. [DOI] [PubMed] [Google Scholar]
- Sucov H. M., Murakami K. K., Evans R. M. Characterization of an autoregulated response element in the mouse retinoic acid receptor type beta gene. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5392–5396. doi: 10.1073/pnas.87.14.5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoms S. D., Stocum D. L. Retinoic acid-induced pattern duplication in regenerating urodele limbs. Dev Biol. 1984 Jun;103(2):319–328. doi: 10.1016/0012-1606(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Tickle C., Lee J., Eichele G. A quantitative analysis of the effect of all-trans-retinoic acid on the pattern of chick wing development. Dev Biol. 1985 May;109(1):82–95. doi: 10.1016/0012-1606(85)90348-3. [DOI] [PubMed] [Google Scholar]
- Wanek N., Gardiner D. M., Muneoka K., Bryant S. V. Conversion by retinoic acid of anterior cells into ZPA cells in the chick wing bud. Nature. 1991 Mar 7;350(6313):81–83. doi: 10.1038/350081a0. [DOI] [PubMed] [Google Scholar]
- Yu V. C., Delsert C., Andersen B., Holloway J. M., Devary O. V., När A. M., Kim S. Y., Boutin J. M., Glass C. K., Rosenfeld M. G. RXR beta: a coregulator that enhances binding of retinoic acid, thyroid hormone, and vitamin D receptors to their cognate response elements. Cell. 1991 Dec 20;67(6):1251–1266. doi: 10.1016/0092-8674(91)90301-e. [DOI] [PubMed] [Google Scholar]
- Zhang X. K., Hoffmann B., Tran P. B., Graupner G., Pfahl M. Retinoid X receptor is an auxiliary protein for thyroid hormone and retinoic acid receptors. Nature. 1992 Jan 30;355(6359):441–446. doi: 10.1038/355441a0. [DOI] [PubMed] [Google Scholar]
- de Thé H., Vivanco-Ruiz M. M., Tiollais P., Stunnenberg H., Dejean A. Identification of a retinoic acid responsive element in the retinoic acid receptor beta gene. Nature. 1990 Jan 11;343(6254):177–180. doi: 10.1038/343177a0. [DOI] [PubMed] [Google Scholar]
- de Wet J. R., Wood K. V., DeLuca M., Helinski D. R., Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987 Feb;7(2):725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]