Abstract
Decades of basic and translational studies have identified the mechanisms by which pancreatic cancer cells use molecular pathways to hijack the normal homeostasis of the pancreas, promoting pancreatic cancer initiation, progression, and metastasis, as well as drug resistance. These molecular pathways were explored to develop targeted therapies to prevent or cure this fatal disease. Regrettably, the studies found that majority of the molecular events that dictate carcinogenic growth in the pancreas are non-actionable (potential non-responder groups of targeted therapy). In this review we discuss exciting discoveries on CCN-siblings that reveal how CCN-family members contribute to the different aspects of the development of pancreatic cancer with special emphasis on therapy.
Keywords: CCN1, CCN2, CCN3, CCN4, CCN5, Pancreatic cancer, Patient derived xenograft, Genetically engineered mice model
Introduction
Human pancreatic ductal adenocarcinoma (PDAC) is a common form of human pancreatic cancer (PC) with a 5-year survival rate of less than 5 % after diagnosis and treatment, indicating the dire need for improved therapy (Grote and Logsdon 2007; Han and Von Hoff 2013; Hidalgo 2010). PDAC develops through the accumulation of multiple genetic lesions, which lead to sequential, atypical, histological, preneoplastic changes, also known as pancreatic intraepithelial neoplasms (PanINs) and ultimately invasive/metastatic PDAC (Abraham et al. 2003; Abramson et al. 2007; Aguirre et al. 2003; Aguirre et al. 2004; Banerjee et al. 2000; Caldas et al. 1994; Goggins 2005; Griffin et al. 1994; Keleg et al. 2003; Kern et al. 2002; Maitra et al. 2003; Maitra et al. 2002; Ottenhof et al. 2011; Reichert and Rustgi 2011; Stromnes and Greenberg 2016; Wilentz et al. 1998; Zhang et al. 1997). The earliest detectable mutations identified in precursor lesions are activating mutations of K-Ras, which is detected in ~95 % of PDACs (Banerjee et al. 2000; Eser et al. 2014; Huang et al. 2014; Kanda et al. 2012). The genetically engineered mouse models (GEMMs) such as KC (LSL-KrasG12D/Pdx-1-Cre) indicates that activated K-Ras mutations are sufficient to induce acinar-to-ductal metaplasia (ADM) (Perera and Bardeesy 2012; Reichert and Rustgi 2011) followed by PanIN lesions, but these abnormalities rarely develop into invasive cancer (Abramson et al. 2007; Hingorani et al. 2005; Ying et al. 2012). In contrast, KPC (LSL-KrasG12D/LSL-Trp53R172H/+/Pdx-1-Cre) GEMM harboring missense point mutations in p53 (Mutp53), along with K-Ras mutants, develop invasive and metastatic PDAC with drug resistance via gain-of-function activities compared to loss of heterozygosity or null mutations (Hanel et al. 2013; Lang et al. 2004; Olive et al. 2004; Weissmueller et al. 2014). These findings recapitulate the cognate human condition (Kanda et al. 2013; Masciarelli et al. 2014; Olive and Tuveson 2006; Olivier et al. 2006) and thus, initially, Mutp53 was considered an attractive target to treat the PC progression and metastasis. However, efforts to target Mutp53 therapeutically have been challenging and have yet to make an impact on patient care (Lehmann and Pietenpol 2012; Levine and Oren 2009). Therefore, efforts need to be focused on identifying the underlying novel mechanism(s) that suppress the downstream molecules of Mutp53 in PC cells or other pathways involved in the genesis of PDAC.
Despite multiple studies and clinical trials, the current therapeutic regimens for PDAC are (i) gemcitabine (Voutsadakis 2011), (ii) combination therapy of nab-paclitaxel plus gemcitabine (Von Hoff et al. 2013), (iii) Folfirinox (a combination chemotherapy regimen consisting of oxaliplatin, irinotecan, fluorouracil and leucovorin) (Conroy et al. 2011) and (iv) Onivyde (an irinotecan liposome injection) (Wang-Gillam et al. 2016). The median overall survival (OS) in these therapeutic groups remains modest, varying from 4 to 12 months with degradation of quality of life (Conroy et al. 2011; Mohammed et al. 2015; Von Hoff et al. 2013; Wang-Gillam et al. 2016). The limited efficacies of these drugs are due to the acquisition of chemo-resistant characteristics of PDAC. Multiple studies evaluating the mechanism of resistance to gemcitabine have been documented, and several genes’ expressions and physiological changes (desmoplastic reaction) have been shown to correlate with the resistance mechanism (Blackstock et al. 2001; Damaraju et al. 2003; Duxbury et al. 2004; Galmarini et al. 2004; Merika et al. 2012; Nakahira et al. 2007). However, the modus operandi of drug resistance is still a mystery.
As the PDAC progresses from PanINs to invasive capacity, members of the CCN (Cyr61-CTGF-NOV) family of regulatory elements unveil malfunctions, thereby creating anomalous signaling circuitries that ultimately assist in promoting cancer initiation and progression, as well as drug resistance, culminating in a fatal disease. In this review, we propose to specifically address the role of CCN-family proteins in regulation of PDAC.
The molecular skeleton of the CCN family and their biological significance
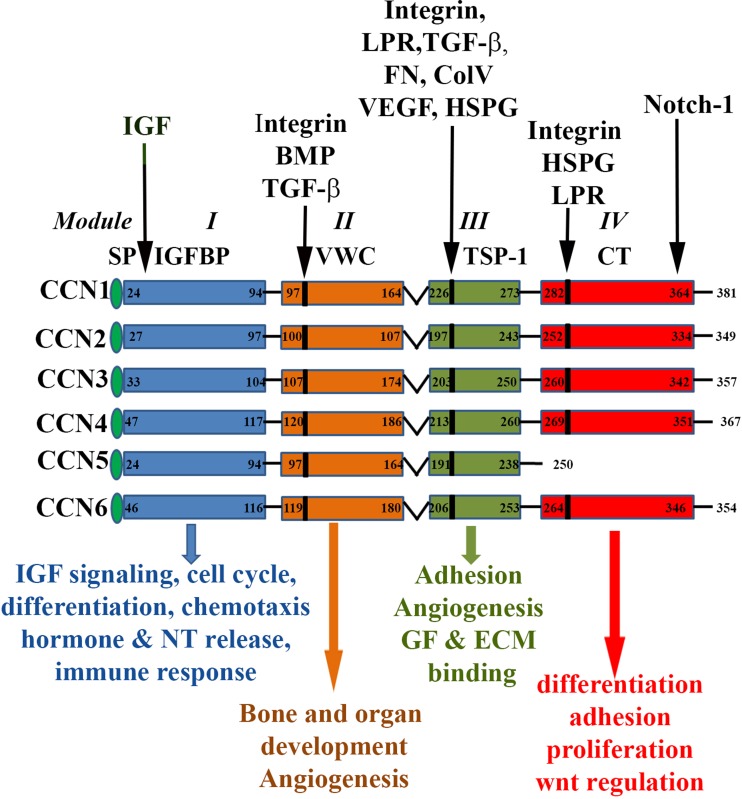
CCN is a small family of matricellular proteins with different homologs in different vertebrate species (Banerjee and Banerjee 2012; Brigstock 2003; Jun and Lau 2011; Lau 2016; Perbal 2001; Perbal 2004). In human, these siblings are Cyr61/CCN1 (Cysteine rich 61), CTGF/CCN2 (Connective tissue growth Factor), CCN3, WISP-1/CCN4 (WNT1 inducible signaling protein 1), WISP-2/CCN5 (WNT1 inducible signaling protein 2) and WISP-3/CCN6 (WNT1 inducible signaling protein 3) with various synonyms (Li et al. 2015). The CCN-siblings share four conserved, multimodular domains in all except CCN5, in which the C-terminal Cysteine-knot (CT) domain is absent (Banerjee and Banerjee 2012) (Fig. 1 ). Each module has unique functions in CCN proteins but their presence is not necessarily critical for their functions. For example, the absence of module 4 in CCN5 does not revoke its ability to perform adhesion function in osteoblasts (Ball et al. 2003; Perbal 2004; Takigawa 2003). CCN proteins have multifunction potencies that can be mediated through the interaction of each module with various proteins in different cells and tissues (Perbal 2001; Perbal 2004). Moreover, the CCN family of proteins has many transcription variants though their biological implication is still unknown (Perbal 2001; Perbal 2004).
Fig. 1.
The molecular domains of CCN-siblings and their functions. The CCN family members (i.e., CCN1, CCN2, CCN3, CCN4, CCN5 and CCN6) share common structure and regulatory domains, consisting of a secretory signal peptide (SP), an IGF-binding domain (Module I), a von Willebrand type C domain (VWC, Module II), a thrombospondin-1 domain (TSP-1, Module III) and a cysteine knot carboxy terminal (CT, Module IV) domain. Domains are linked by hinge regions, susceptible to protease cleavage. Numbers in each module refer to the amino acid (a.a.) sequence for each CCN protein. Integrins binding sites are in the different domains are indicated in the figure. The locations of some important binding partners of each module are indicated by arrow on the top of the diagram: IGF (Insulin like Growth factor, BMP (Bone Morphogenic Protein, TGF-β (Transforming Growth Factor), Integrin, LPR (Lipoprotein Receptor related protein), FN (Fibronectin), Col V (Collagen V), VEGF (Vascular Endothelial Growth Factor), HSPG (Heparin Sulfate Proteoglycan), Notch1 and GF, Growth factor Individual modular domain mediated some cellular functions are listed at the bottom of schematic diagram with respective color coded arrows
The biological significances of CCN-siblings are multifaceted and can be mediated through their cognate receptors integrins with various combinations (Banerjee and Banerjee 2012; Haque et al. 2012; Jun and Lau 2011; Lau 2012). The CCN-siblings are either positive or negative regulators of various biological and pathobiological events, depending on the cellular context (Perbal 2001; Perbal 2004). These contexts include cell proliferation, differentiation, migration, angiogenesis, wound-healing and cancers (Banerjee and Banerjee 2012; Brigstock 1999; Brigstock 2002; Brigstock 2003; Ji et al. 2014; Lake and Castellot 2003; Leask 2010; Lin et al. 2003; Perbal 2001; Perbal 2004). The unique biology of CCN-siblings now places them in an area of great interest in understanding the mechanisms of disease-progression and aiding in the development of mechanism-driven biomarkers that may lead to the discovery of new drug designs.
Why CCN-siblings are targetable in pancreatic cancer?
Like other cancers, pancreatic cancer (PC) is a “mixed-bag” of cells with heterogeneous behavior of cancer cells. Despite having disagreement about the role of cancer stem cells in tumorigenesis (Kong et al. 2009), studies from our laboratories and others strongly suggest a small subpopulation of malignant PC cells, usually termed “PC-initiating cells/stem cells (PICs),” have true tumorigenic potential in a xenograft model as compared to the counterpart (non-side population) or parental cells (Haque et al. 2011; Hermann et al. 2007; Ji et al. 2009; Lee et al. 2008a; Lee et al. 2008b; Mimeault and Batra 2014; Vaz et al. 2013). Like other cancer stem cells, PICs can be identified and separated from rest of the cancer cells based on specific features and protein markers, such as self-renewal, differentiation, sphere (pancosphere) formation ability under non-adherent conditions, tumorigenic potency in a xenograft model, resistance to chemotherapies and expression of CD44, CD24, ESA, CD133 and CXCR4 (surface markers) depending on the functional skills of PICs. CD133 and CXCR4 positive PICs are not only highly tumorigenic but these cells are highly resistant to gemcitabine and have a predisposition to metastasize (Hermann et al. 2007; Li et al. 2007; Simeone 2008). Moreover, multiple studies, have demonstrated that PICs display upregulation of certain genes needed to maintain self-renewal, epithelial-mesenchymal transition (EMT) and stemness. Sonic hedgehog (SHh) is one such gene (Berman et al. 2003; Haque et al. 2011; Lee et al. 2008a; Thayer et al. 2003). Despite substantial studies of the vital roles of PICs in PDAC progression and drug resistance, driver genes or signaling molecules that regulate PICs survival and maintenance have not been fully defined and, thus, it is difficult to develop drug target(s) to prevent, delay or cure the disease.
The studies on the role of CCN-siblings in regulation of pancreatic cancer development, differentiation, progression and drug resistance open a new window of research for understanding the mechanism of pancreatic carcinogenesis and highlighting the fine balance between the activities of CCN-siblings (Dhar et al. 2007; Haque et al. 2012; Haque et al. 2011; Leask 2011; Leask 2013; Maity et al. 2014; Neesse et al. 2013).
CCN1 overexpression increases PDAC progression
In an orthotopic mouse model of pancreatic cancer, CCN1 showed increased expression in metastatic lesions. This study suggested that interaction between CCN1 and integrins (αvβ3) may promote formation of peritoneal metastases (Holloway et al. 2005); yet, its importance in PC was not clearly understood until we uncovered the precise role of CCN1 in PC development (Fig. 2 ). Our studies demonstrate that when overexpressed in PC and its precursor lesions, CCN1 promotes proliferation, epithelial to mesenchymal transition (EMT), stemness, migration and the growth of xenograft tumors of PC cells (Haque et al. 2011; Leask 2011). PC cell-secreted CCN1 enhances abnormal neovascularization (tumor angiogenesis) under in vitro and in vivo conditions (Maity et al. 2014). Moreover, we found that CCN1 is a potent regulator of SHh expression via the integrins/Notch-1-signaling pathway. CCN1 activity was mediated, at least in part, through altering proteasome activity (Haque et al. 2012) (Leask 2011). Finally, we found that the sensitivity of GEMCITABINE was increased in CCN1 silenced pancreatic cancer cells through unknown mechanisms (Banerjee, Personal communication). Despite having functional dichotomy of CCN1 in some cancers (Chen et al. 2016; Jun and Lau 2011), targeting CCN1 could be an ideal approach for PDAC therapy.
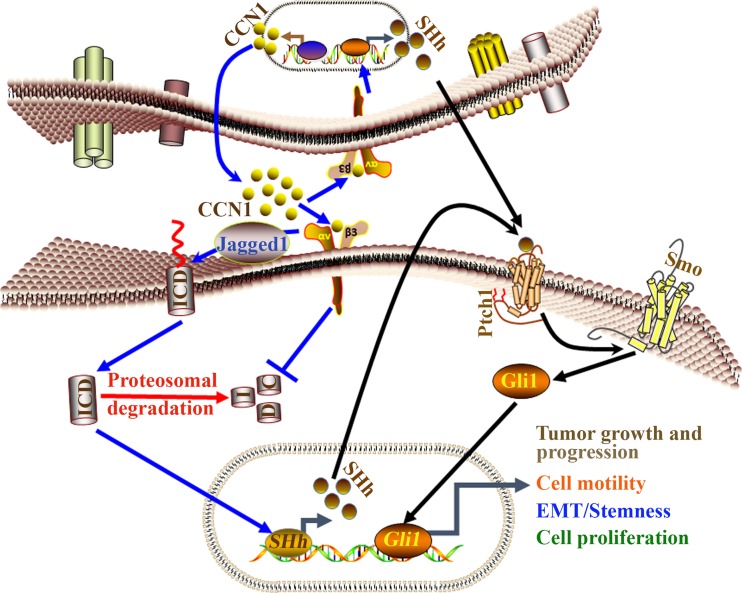
Fig. 2.
Diagrammatic illustrations of CCN1 signaling involved pancreatic cancer progression via CCN1-Notch1-SHh axes. Our studies postulated that CCN1-mediated pancreatic carcinogenesis is regulated via integrin-αvβ3-Notch-1-SHh signaling pathway in autocrine-paracrine manner. Firstly, Jagged1 is activated by CCN1 which in turn releases an intracellular domain of Notch (ICD) from the membrane into the cytoplasm. CCN1 inhibits the proteasomal degradation process to keep Notch-1(ICD) stable and active in pancreatic cancer cells. CCN1 recruits active ICD into the nucleus to regulate transcriptional complex (TC) for the induction of SHh. SHh binds with its receptor 12-span-transmembrane protein Patched (Ptch1) and relieves another 7-span-transmembrane protein receptor, Smoothened (Smo) inhibition, which in turn induces the signal transduction pathway by activating the nuclear translocation of transcription factor Gli1 protein. Finally, the SHh-Ptch1-Smo-Gli1 signaling pathway promotes pancreatic cancer growth and progression, cell motility, EMT/Stemness and cell proliferation.
CCN2 is an important factor that promotes drug resistance in PC
Although the location of the pancreas is one of the vital impediments for treatment and leads to late diagnosis and unresectability (Han and Von Hoff 2013), a major contributor to poor clinical outcomes in PC is chemoresistance. Currently, there are a few FDA-approved efficacious therapies for chemo-resistant patients that help improve survival for 4–11 months compared to the old standard of care (gemcitabine therapy) (Conroy et al. 2011; Han and Von Hoff 2013; Von Hoff et al. 2013; Von Hoff et al. 2009; Wang-Gillam et al. 2016). Thereby, chemoresistance is still a challenging task for PC therapy. In PC, the therapeutic obstacle is due to PICs because these cells are resistant to radiation and chemotherapy (Donnenberg et al. 2009; Zhou et al. 2009). In addition, desmoplastic reaction (DR)/desmoplasia, a dynamic process regulated by a cross-talk of tumor cells and/or PICs and surrounding stroma, plays a critical role in chemo-resistance in human PC (Han and Von Hoff 2013; Mahadevan and Von Hoff 2007; Merika et al. 2012; Whatcott et al. 2012). Desmoplasia leads to a significant increase in the production of dense and fibrous connective tissues, extracellular matrix proteins and proliferation of myofibroblast cells/active stellates cells (PSCs) (Whatcott et al. 2012). This reaction contributes to enhancing the tumor fluid pressure and chemoresistance (Mahadevan and Von Hoff 2007; Olive et al. 2009). As with human samples, desmoplasia is an easily recognizable event in orthotopic samples or spontaneous cancer in mice models (Mazur and Siveke 2012) (Banerjee, Personal communication and see in Fig. 3A). Therefore, several preclinical studies in animal models, as well as clinical trials, have been undertaken to develop a novel therapy against desmoplasia in an attempt to overcome the chemoresistance. Since SHh, a secreted ligand of the Patched1 receptor and activator of Gli-family of transcription factors (Marigo et al. 1996), is known to be a prime regulator of desmoplasia in pancreatic cancer (Bailey et al. 2008; Spivak-Kroizman et al. 2013), an inhibitor of SHh was evaluated in both animal models and clinical trials with huge expectations. Although there were promising outcomes in animal studies (Olive et al. 2009), the Phase II human trial was terminated due to the ineffectiveness of the drug (Merika et al. 2012). A recent study found that SHh-deficiency in PC may reduce desmoplastic burden, but surprisingly, this deficiency caused aggressive tumor growth (Rhim et al. 2014). Thus, the SHh-inhibitors are still being investigated as possible treatment options.
Fig. 3.
(A) Detection of desmoplasia in orthotopic human PC xenograft mouse model using an ultrasound (Vevo2100)-guided injection (USGI) non-invasive technique, without surgery: Panc-1 aggressive pancreatic cancer cells (1x106) were orthotopically implanted into the mouse pancreas using USGI technique (Huynh et al. 2011). After 2.5 months, tumor growths in the pancreas were detected by ultrasound and then documented after sacrificing the animals. For the detection of DR in PC samples, tissues were fixed in formalin, and H&E staining was carried out in tissue sections. (B) Diagrammatic illustration of Autocrine-paracrine regulation of CCN2 during desmoplasia and chemoresistance
Several other small molecules and agents were also evaluated either alone or in different combinations for translational research and clinical trials. Thus far, these therapies either have shown significant toxicity or proven ineffective in abolishing desmoplasia. Thus, it’s our opinion that new drug development approaches are necessary to target desmoplasia. Nevertheless, this cannot be done outright; several factors are accountable and thus cannot be ignored. These factors include, but are not limited to, mutant p53 and tumor initiating cells/cancer stem cells because they are responsible for disease progression and resistance to therapy (Bullock and Fersht 2001; Fiorini et al. 2015; Lane and Hupp 2003; Masciarelli et al. 2014; Merika et al. 2012). Targeting mutant p53 or cancer stem cells or both is a challenging task and not yet possible. These limitations emphasize the need to identify new targets that are common to all important factors.
CCN2/CTGF could be a novel target to destroy the desmoplastic reaction as CCN2 is a profibrotic secretory growth factor that plays a vital role in tumor-stromal interaction and desmoplasia in PC (Charrier and Brigstock 2013; Neesse et al. 2013) (Banerjee, unpublished data) (Fig. 3B). Moreover, studies found that treatment with a monoclonal antibody against CCN2 enhances drug delivery in the KPC (KrasLSL-G12D/+/p53LSL-R172H/Pdx1-Cre) spontaneous pancreatic cancer mouse model (Hingorani et al. 2005), indicating CCN2 plays vital role within the tumor microenvironment mediating gemcitabine resistance (Neesse et al. 2013) and thereby CCN2 can be considered as an important string of the desmoplastic bow.
CCN3 regulation of PDAC growth and progression
CCN3 is one of the founding members of the CCN-family and plays vital roles in tumorigenesis (Li et al. 2015; Planque and Perbal 2003). The function of CCN3 in carcinogenesis is different in the context of tissue types and organs. In Wilm’s tumor, glioma, rhabdomyosarcoma, chondrosarcoma, breast and prostate cancers, CCN3 acts as a tumor suppressor gene (Dobson et al. 2014; Gupta et al. 2001; Montero et al. 2012; Wu et al. 2014; Yu et al. 2003), while CCN3 promotes cancer cell proliferation and tumor progression in bladder cancer and pancreatic cancer (Chen et al. 2014; Cui et al. 2014) and correlates with poor overall survival of colon cancer (Ueda et al. 2015). Although the mechanism of CCN3 action is unclear, recent studies found that CCN3 is a positive regulator of EMT in pancreatic cancer (Cui et al. 2014).
CCN4 expression correlates with PDAC progression
CCN4 appears to be a factor stimulating aggressive behaviors of various cancers (Chen et al. 2007; Davies et al. 2010; Davies et al. 2007; Gurbuz and Chiquet-Ehrismann 2015; Tian et al. 2007; Wang et al. 2006) except in lung cancer and prostate cancer cells where CCN4 exhibits contrast behavior (Ono et al. 2013; Soon et al. 2003). A univariate and multivariate correlative analysis demonstrates that CCN4 may serve as a potential biomarker for PDA progression (Yang et al. 2015).
PDAC progression is accompanied by killing CCN5
CCN5, which is a ~ 29-35 kDa protein, could be considered as tumor suppressor gene in PDAC (Fig. 4 ). Like colon and breast cancers, the CCN5 protein is overexpressed in normal and preneoplastic cells, but is mostly undetected or minimally detected in various pancreatic cancer cell lines and tissue samples (Banerjee and Banerjee 2012; Dhar et al. 2007; Russo and Castellot 2010; Sabbah et al. 2011; Saxena et al. 2001). Functional studies demonstrate that the exposure of pancreatic cancer cells to CCN5 recombinant protein reverses the EMT process (Dhar et al. 2007), thereby suggesting that CCN5 silencing may promote tumor progression and drug resistance in PDAC. However, our knowledge about the mechanism of suppression of CCN5 expression in pancreatic cancer cells is very limited. Previously, we established that CCN5 expression can be blocked at the transcription level by gain-of-function of mutant p53 in non-invasive breast cancer epithelial cells (Dhar et al. 2008). In parallel, we also demonstrated that CCN5 expression is inversely correlated with the expression of mutant p53 in pancreatic cancer cells (Dhar et al. 2007), though no link was detected with the K-Ras mutations in pancreatic cancer cells (Banerjee, unpublished data). Collectively, we anticipate that mutant p53-mediated suppression of CCN5 is required to promote invasion, metastasis and drug resistance. Further studies are urgently needed to establish the role of CCN5 in prevention of PDAC progression and drug resistance, which help in classifying CCN5 protein as an actionable element (Carr et al. 2016) with practical overtone for targeted therapy.
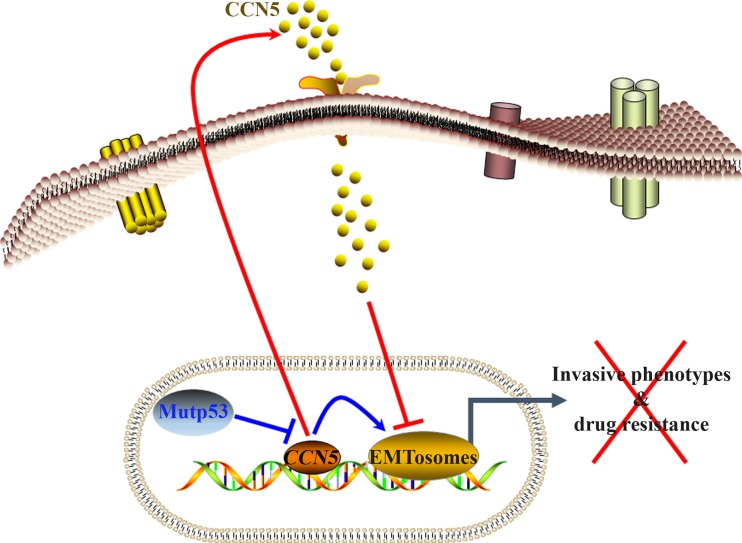
Fig. 4.
Diagrammatic representation of CCN5 regulation of invasive phenotypes of pancreatic cancer cells. CCN5 is overexpressed and functionally active in normal microenvironment of pancreatic epithelial cells and its expression could be suppressed in PC cells by mutant p53 gain-of-function. Therapeutic application of CCN5 or ectopic overexpression of CCN5 suppresses EMTosomes (regulators of EMT) activity resulted reverses invasive phenotypes of PC cells
Conclusions and future perspectives
Pancreatic ductal adenocarcinoma is the most common malignant disease in the human pancreas and is a deadly disease across all ethnic backgrounds. One of the main reasons it has such a poor prognosis is that it is almost always detected at an advanced stage in the majority of patients already have invasive phenotypes (i.e., multiple genomic aberrations, desmoplasia and metastatic growth in distant organs) at the time of diagnosis. These invasive phenotypes make pancreatic cancer cells insensitive to many chemotherapeutic drugs including gemcitabine, folfirinox and nab-paclitaxel. Thus, new ways to combat this disease are urgently needed.
Over a decade of studies indicates that the dysregulated expression of CCN-siblings (e.g. CCN1, CCN2, CCN3, CCN4 and CCN5) play key roles in pancreatic cancer progression and drug resistance (Dhar et al. 2007; Haque et al. 2012; Haque et al. 2011; Neesse et al. 2013). Thereby, anti-CCN strategies notably anti-CCN2 therapeutics should be considered as potentially viable strategies to block pancreatic cancer. However, a complete map and understanding of the regulatory landscape is lacking. In particular, a major gap exists in our understanding of how CCN-siblings regulate cancer cells and their microenvironments. Do they regulate and cross-talk to each other, resulting in cancer progression? If so then what oncogenic signaling is required for this anarchy? These findings, along with the existing body of work, will open new doors into our understanding of cancer etiology and future targeted therapy.
Acknowledgments
We would like to thank other members of our cancer research unit for valuable and helpful comments on this manuscript. We would also like to thank LaCoiya Harris for editing and organizing this manuscript. This work was supported by the Kansas City Area Life Science grant award (SKB), Merit review grant from Department of Veterans Affairs (SKB, 5I01BX001989-03 and SB,1I01BX001002-04), and KUMC Van Goethem Family Endowed Funds (SKB).
Author contributions
Snigdha Banerjee and Sushanta K. Banerjee performed the literature search and wrote the manuscript. Inamul Haque, Gargi Maity, Vijayalaxmi Gupta, Sandipto Sarkar and Arnab Ghosh revised it critically for important intellectual content. Donald Campbell and Daniel Von Hoff provided expert comments and editing.
Compliance with ethical standards
Conflicts of interest
The authors declare no conflict of interest.
Contributor Information
Sushanta K. Banerjee, Phone: 816-861-4700, Email: sbanerjee2@kumc.edu, Email: cancerresearchunit@icloud.com
Snigdha Banerjee, Phone: 816-861-4700, Email: sbanerjee@kumc.edu.
References
- Abraham SC, Wilentz RE, Yeo CJ, Sohn TA, Cameron JL, Boitnott JK, Hruban RH. Pancreaticoduodenectomy (Whipple resections) in patients without malignancy: are they all 'chronic pancreatitis'? Am J Surg Pathol. 2003;27:110–120. doi: 10.1097/00000478-200301000-00012. [DOI] [PubMed] [Google Scholar]
- Abramson MA, Jazag A, van der Zee JA, Whang EE. ThMahadevane molecular biology of pancreatic cancer. Gastrointestinal Cancer Res: GCR. 2007;1:S7–S12. [PMC free article] [PubMed] [Google Scholar]
- Aguirre AJ, et al. Activated Kras and Ink4a/Arf deficiency cooperate to produce metastatic pancreatic ductal adenocarcinoma. Genes Dev. 2003;17:3112–3126. doi: 10.1101/gad.1158703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre AJ, et al. High-resolution characterization of the pancreatic adenocarcinoma genome. Proc Natl Acad Sci U S A. 2004;101:9067–9072. doi: 10.1073/pnas.0402932101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey JM, et al. Sonic hedgehog promotes desmoplasia in pancreatic cancer. Clin Cancer Res. 2008;14:5995–6004. doi: 10.1158/1078-0432.CCR-08-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball DK, Rachfal AW, Kemper SA, Brigstock DR. The heparin-binding 10 kDa fragment of connective tissue growth factor (CTGF) containing module 4 alone stimulates cell adhesion. J Endocrinol. 2003;176:R1–R7. doi: 10.1677/joe.0.176R001. [DOI] [PubMed] [Google Scholar]
- Banerjee SK, Banerjee S. CCN5/WISP-2: A micromanager of breast cancer progression. JCell CommunSignal. 2012;6:63–71. doi: 10.1007/s12079-012-0158-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee SK, Zoubine MN, Mullick M, Weston AP, Cherian R, Campbell DR. Tumor angiogenesis in chronic pancreatitis and pancreatic adenocarcinoma: impact of K-ras mutations. Pancreas. 2000;20:248–255. doi: 10.1097/00006676-200004000-00005. [DOI] [PubMed] [Google Scholar]
- Berman DM, et al. Widespread requirement for hedgehog ligand stimulation in growth of digestive tract tumours. Nature. 2003;425:846–851. doi: 10.1038/nature01972. [DOI] [PubMed] [Google Scholar]
- Blackstock AW, et al. Tumor uptake and elimination of 2',2'-difluoro-2'-deoxycytidine (gemcitabine) after deoxycytidine kinase gene transfer: correlation with in vivo tumor response. Clin Cancer Res. 2001;7:3263–3268. [PubMed] [Google Scholar]
- Brigstock DR. The connective tissue growth factor/cysteine-rich 61/nephroblastoma overexpressed (CCN) family. Endocr Rev. 1999;20:189–206. doi: 10.1210/edrv.20.2.0360. [DOI] [PubMed] [Google Scholar]
- Brigstock DR. Regulation of angiogenesis and endothelial cell function by connective tissue growth factor (CTGF) and cysteine-rich 61 (CYR61. Angiogenesis. 2002;5:153–165. doi: 10.1023/A:1023823803510. [DOI] [PubMed] [Google Scholar]
- Brigstock DR. The CCN family: a new stimulus package. J Endocrinol. 2003;178:169–175. doi: 10.1677/joe.0.1780169. [DOI] [PubMed] [Google Scholar]
- Bullock AN, Fersht AR. Rescuing the function of mutant p53 nature reviews. Cancer. 2001;1:68–76. doi: 10.1038/35094077. [DOI] [PubMed] [Google Scholar]
- Caldas C, Hahn SA, Hruban RH, Redston MS, Yeo CJ, Kern SE. Detection of K-ras mutations in the stool of patients with pancreatic adenocarcinoma and pancreatic ductal hyperplasia. Cancer Res. 1994;54:3568–3573. [PubMed] [Google Scholar]
- Carr TH, et al. Defining actionable mutations for oncology therapeutic development nature reviews. Cancer. 2016;16:319–329. doi: 10.1038/nrc.2016.35. [DOI] [PubMed] [Google Scholar]
- Charrier A, Brigstock DR. Regulation of pancreatic function by connective tissue growth factor (CTGF, CCN2) Cytokine Growth Factor Rev. 2013;24:59–68. doi: 10.1016/j.cytogfr.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen PP, et al. Expression of Cyr61, CTGF, and WISP-1 correlates with clinical features of lung cancer. PLoS One. 2007;2:e534. doi: 10.1371/journal.pone.0000534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Gao Y, Xu B, Cui X, Xu D. NOV is upregulated and promotes migration and invasion in bladder cancer tumour biology: the journal of the international society for. Oncodevelopmental Biology and Medicine. 2014;35:6749–6755. doi: 10.1007/s13277-014-1919-8. [DOI] [PubMed] [Google Scholar]
- Chen CC, Kim KH, Lau LF. The matricellular protein CCN1 suppresses hepatocarcinogenesis by inhibiting compensatory proliferation. Oncogene. 2016;35:1314–1323. doi: 10.1038/onc.2015.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conroy T, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- Cui L, et al. NOV promoted the growth and migration of pancreatic cancer cells. Tumour Biology : the Journal of the International Society for Oncodevelopmental Biology and Medicine. 2014;35:3195–3201. doi: 10.1007/s13277-013-1418-3. [DOI] [PubMed] [Google Scholar]
- Damaraju VL, Damaraju S, Young JD, Baldwin SA, Mackey J, Sawyer MB, Cass CE. Nucleoside anticancer drugs: the role of nucleoside transporters in resistance to cancer chemotherapy. Oncogene. 2003;22:7524–7536. doi: 10.1038/sj.onc.1206952. [DOI] [PubMed] [Google Scholar]
- Davies SR, Watkins G, Mansel RE, Jiang WG. Differential expression and prognostic implications of the CCN family members WISP-1, WISP-2, and WISP-3 in human breast cancer. Ann Surg Oncol. 2007;14:1909–1918. doi: 10.1245/s10434-007-9376-x. [DOI] [PubMed] [Google Scholar]
- Davies SR, Davies ML, Sanders A, Parr C, Torkington J, Jiang WG. Differential expression of the CCN family member WISP-1, WISP-2 and WISP-3 in human colorectal cancer and the prognostic implications. Int J Oncol. 2010;36:1129–1136. doi: 10.3892/ijo_00000595. [DOI] [PubMed] [Google Scholar]
- Dhar G, et al. Loss of WISP-2/CCN5 signaling in human pancreatic cancer: a potential mechanism for epithelial-mesenchymal-transition. Cancer Lett. 2007;254:63–70. doi: 10.1016/j.canlet.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Dhar G, Banerjee S, Dhar K, Tawfik O, Mayo MS, Vanveldhuizen PJ, Banerjee SK. Gain of oncogenic function of p53 mutants induces invasive phenotypes in human breast cancer cells by silencing CCN5/WISP-2. Cancer Res. 2008;68:4580–4587. doi: 10.1158/0008-5472.CAN-08-0316. [DOI] [PubMed] [Google Scholar]
- Dobson JR, et al. hsa-mir-30c promotes the invasive phenotype of metastatic breast cancer cells by targeting NOV/CCN3. Cancer Cell Int. 2014;14:73. doi: 10.1186/s12935-014-0073-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnenberg VS, Meyer EM, Donnenberg AD. Measurement of multiple drug resistance transporter activity in putative cancer stem/progenitor cells. Methods Mol Biol. 2009;568:261–279. doi: 10.1007/978-1-59745-280-9_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duxbury MS, Ito H, Zinner MJ, Ashley SW, Whang EE. RNA interference targeting the M2 subunit of ribonucleotide reductase enhances pancreatic adenocarcinoma chemosensitivity to gemcitabine. Oncogene. 2004;23:1539–1548. doi: 10.1038/sj.onc.1207272. [DOI] [PubMed] [Google Scholar]
- Eser S, Schnieke A, Schneider G, Saur D. Oncogenic KRAS signalling in pancreatic cancer. Br J Cancer. 2014 doi: 10.1038/bjc.2014.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorini C, Cordani M, Padroni C, Blandino G, Di Agostino S, Donadelli M. Mutant p53 stimulates chemoresistance of pancreatic adenocarcinoma cells to gemcitabine. Biochim Biophys Acta. 2015;1853:89–100. doi: 10.1016/j.bbamcr.2014.10.003. [DOI] [PubMed] [Google Scholar]
- Galmarini CM, Clarke ML, Jordheim L, Santos CL, Cros E, Mackey JR, Dumontet C. Resistance to gemcitabine in a human follicular lymphoma cell line is due to partial deletion of the deoxycytidine kinase gene. BMC Pharmacol. 2004;4:8. doi: 10.1186/1471-2210-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goggins M. Molecular markers of early pancreatic cancer. J Clin Oncol. 2005;23:4524–4531. doi: 10.1200/JCO.2005.19.711. [DOI] [PubMed] [Google Scholar]
- Griffin CA, Hruban RH, Long PP, Morsberger LA, Douna-Issa F, Yeo CJ (1994) Chromosome abnormalities in pancreatic adenocarcinoma Genes Chromosom Cancer 9:93-100 [DOI] [PubMed]
- Grote T, Logsdon CD. Progress on molecular markers of pancreatic cancer. Curr Opin Gastroenterol. 2007;23:508–514. doi: 10.1097/MOG.0b013e3282ba5724. [DOI] [PubMed] [Google Scholar]
- Gupta N, et al. Inhibition of glioma cell growth and tumorigenic potential by CCN3 (NOV) Mol Pathol: MP. 2001;54:293–299. doi: 10.1136/mp.54.5.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurbuz I, Chiquet-Ehrismann R. CCN4/WISP1 (WNT1 inducible signaling pathway protein 1): a focus on its role in cancer. Int J Biochem Cell Biol. 2015;62:142–146. doi: 10.1016/j.biocel.2015.03.007. [DOI] [PubMed] [Google Scholar]
- Han H, Von Hoff DD. SnapShot: pancreatic cancer. Cancer Cell. 2013;23:424–424e421. doi: 10.1016/j.ccr.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanel W, Marchenko N, Xu S, Yu SX, Weng W, Moll U. Two hot spot mutant p53 mouse models display differential gain of function in tumorigenesis. Cell Death Differ. 2013;20:898–909. doi: 10.1038/cdd.2013.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque I, et al. Cyr61/CCN1 signaling is critical for epithelial-mesenchymal transition and stemness and promotes pancreatic carcinogenesis. Mol Cancer. 2011;10:8. doi: 10.1186/1476-4598-10-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque I, et al. The matricellular protein CCN1/Cyr61 is a critical regulator of Sonic Hedgehog in pancreatic carcinogenesis. J Biol Chem. 2012;287:38569–38579. doi: 10.1074/jbc.M112.389064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann PC, et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1:313–323. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605–1617. doi: 10.1056/NEJMra0901557. [DOI] [PubMed] [Google Scholar]
- Hingorani SR, et al. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005;7:469–483. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- Holloway SE, Beck AW, Girard L, Jaber MR, Barnett CC, Jr, Brekken RA, Fleming JB. Increased expression of Cyr61 (CCN1) identified in peritoneal metastases from human pancreatic cancer. J Am Coll Surg. 2005;200:371–377. doi: 10.1016/j.jamcollsurg.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Huang H, Daniluk J, Liu Y, Chu J, Li Z, Ji B, Logsdon CD. Oncogenic K-ras requires activation for enhanced activity. Oncogene. 2014;33:532–535. doi: 10.1038/onc.2012.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh AS, Abrahams DF, Torres MS, Baldwin MK, Gillies RJ, Morse DL. Development of an orthotopic human pancreatic cancer xenograft model using ultrasound guided injection of cells. PLoS One. 2011;6:e20330. doi: 10.1371/journal.pone.0020330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Q, et al. MicroRNA miR-34 inhibits human pancreatic cancer tumor-initiating cells. PLoS One. 2009;4:e6816. doi: 10.1371/journal.pone.0006816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji J, Jia S, Ji K, Jiang WG. Wnt1 inducible signalling pathway protein-2 (WISP2/CCN5): roles and regulation in human cancers (review) Oncol Rep. 2014;31:533–539. doi: 10.3892/or.2013.2909. [DOI] [PubMed] [Google Scholar]
- Jun JI, Lau LF. Taking aim at the extracellular matrix: CCN proteins as emerging therapeutic targets. Nat Rev Drug Discov. 2011;10:945–963. doi: 10.1038/nrd3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda M, et al. Presence of somatic mutations in most early-stage pancreatic intraepithelial neoplasia. Gastroenterology. 2012;142:730–733e739. doi: 10.1053/j.gastro.2011.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda M, et al. Mutant TP53 in duodenal samples of pancreatic juice from patients with pancreatic cancer or high-grade dysplasia. Clinical Gastroenterology and Hepatology : the Official Clinical Practice Journal of the American Gastroenterological Association. 2013;11:719–730e715. doi: 10.1016/j.cgh.2012.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keleg S, Buchler P, Ludwig R, Buchler MW, Friess H. Invasion and metastasis in pancreatic cancer. Mol Cancer. 2003;2:14. doi: 10.1186/1476-4598-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern SE, Hruban RH, Hidalgo M, Yeo CJ. An introduction to pancreatic adenocarcinoma genetics, pathology and therapy. Cancer Biol Ther. 2002;1:607–613. doi: 10.4161/cbt.307. [DOI] [PubMed] [Google Scholar]
- Kong B, Michalski CW, Kleeff J. Tumor initiating cells in pancreatic cancer: a critical view. World Journal of Stem Cells. 2009;1:8–10. doi: 10.4252/wjsc.v1.i1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake AC, Castellot JJ Jr (2003) CCN5 modulates the antiproliferative effect of heparin and regulates cell motility in vascular smooth muscle cells. Cell Communication and Signaling : CCS 1:5. doi:10.1186/1478-811X-1-5 [DOI] [PMC free article] [PubMed]
- Lane DP, Hupp TR. Drug discovery and p53. Drug Discov Today. 2003;8:347–355. doi: 10.1016/S1359-6446(03)02669-2. [DOI] [PubMed] [Google Scholar]
- Lang GA, et al. Gain of function of a p53 hot spot mutation in a mouse model of Li-Fraumeni syndrome. Cell. 2004;119:861–872. doi: 10.1016/j.cell.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Lau LF. CCN1 and CCN2: blood brothers in angiogenic action. Journal of Cell Communication and Signaling. 2012;6:121–123. doi: 10.1007/s12079-012-0169-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau LF. Cell surface receptors for CCN proteins. Journal of Cell Communication and Signaling. 2016;10:121–127. doi: 10.1007/s12079-016-0324-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leask A. Yin and Yang part Deux: CCN5 inhibits the pro-fibrotic effects of CCN2. Journal of Cell Communication and Signaling. 2010;4:155–156. doi: 10.1007/s12079-010-0092-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leask A. CCN1: a novel target for pancreatic cancer. Journal of Cell Communication and Signaling. 2011;5:123–124. doi: 10.1007/s12079-011-0127-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leask A. Sonic advance: CCN1 regulates sonic hedgehog in pancreatic cancer. Journal of Cell Communication and Signaling. 2013;7:61–62. doi: 10.1007/s12079-012-0187-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CJ, Dosch J, Simeone DM. Pancreatic cancer stem cells. J Clin Oncol. 2008;26:2806–2812. doi: 10.1200/JCO.2008.16.6702. [DOI] [PubMed] [Google Scholar]
- Lee CJ, Li C, Simeone DM. Human pancreatic cancer stem cells: implications for how we treat pancreatic cancer. Transl Oncol. 2008;1:14–18. doi: 10.1593/tlo.08013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann BD, Pietenpol JA. Targeting mutant p53 in human tumors. J Clin Oncol. 2012;30:3648–3650. doi: 10.1200/JCO.2012.44.0412. [DOI] [PubMed] [Google Scholar]
- Levine AJ, Oren M. The first 30 years of p53: growing ever more complex nature reviews. Cancer. 2009;9:749–758. doi: 10.1038/nrc2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, et al. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- Li J, Ye L, Owen S, Weeks HP, Zhang Z, Jiang WG. Emerging Role of CCN Family Proteins in Tumorigenesis and Cancer Metastasis (Review) International Journal of Molecular Medicine. 2015;36:1451–1463. doi: 10.3892/ijmm.2015.2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CG, Leu SJ, Chen N, Tebeau CM, Lin SX, Yeung CY, Lau LF. CCN3 (NOV) is a novel angiogenic regulator of the CCN protein family. J Biol Chem. 2003;278:24200–24208. doi: 10.1074/jbc.M302028200. [DOI] [PubMed] [Google Scholar]
- Mahadevan D, Von Hoff DD. Tumor-stroma interactions in pancreatic ductal adenocarcinoma. Mol Cancer Ther. 2007;6:1186–1197. doi: 10.1158/1535-7163.MCT-06-0686. [DOI] [PubMed] [Google Scholar]
- Maitra A, et al. Cyclooxygenase 2 expression in pancreatic adenocarcinoma and pancreatic intraepithelial neoplasia: an immunohistochemical analysis with automated cellular imaging. Am J Clin Pathol. 2002;118:194–201. doi: 10.1309/TPG4-CK1C-9V8V-8AWC. [DOI] [PubMed] [Google Scholar]
- Maitra A, et al. Multicomponent analysis of the pancreatic adenocarcinoma progression model using a pancreatic intraepithelial neoplasia tissue microarray. Modern Pathology : an Official Journal of the United States and Canadian Academy of Pathology, Inc. 2003;16:902–912. doi: 10.1097/01.MP.0000086072.56290.FB. [DOI] [PubMed] [Google Scholar]
- Maity G, Mehta S, Haque I, Dhar K, Sarkar S, Banerjee SK, Banerjee S. Pancreatic tumor cell secreted CCN1/Cyr61 promotes endothelial cell migration and aberrant neovascularization. Sci Report. 2014;4:4995. doi: 10.1038/srep04995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marigo V, Johnson RL, Vortkamp A, Tabin CJ. Sonic hedgehog differentially regulates expression of GLI and GLI3 during limb development. Dev Biol. 1996;180:273–283. doi: 10.1006/dbio.1996.0300. [DOI] [PubMed] [Google Scholar]
- Masciarelli S, Fontemaggi G, Di Agostino S, Donzelli S, Carcarino E, Strano S, Blandino G. Gain-of-function mutant p53 downregulates miR-223 contributing to chemoresistance of cultured tumor cells. Oncogene. 2014;33:1601–1608. doi: 10.1038/onc.2013.106. [DOI] [PubMed] [Google Scholar]
- Mazur PK, Siveke JT. Genetically engineered mouse models of pancreatic cancer: unravelling tumour biology and progressing translational oncology. Gut. 2012;61:1488–1500. doi: 10.1136/gutjnl-2011-300756. [DOI] [PubMed] [Google Scholar]
- Merika EE, Syrigos KN, Saif MW. Desmoplasia in pancreatic cancer. Can we fight it? Gastroenterol Res Pract. 2012;2012:781765. doi: 10.1155/2012/781765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimeault M, Batra SK. Molecular biomarkers of cancer stem/progenitor cells associated with progression, metastases, and treatment resistance of aggressive cancers Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer. Research, Cosponsored by the American Society of Preventive Oncology. 2014;23:234–254. doi: 10.1158/1055-9965.EPI-13-0785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed A, Janakiram NB, Pant S, Rao CV. Molecular targeted intervention for pancreatic cancer. Cancer. 2015;7:1499–1542. doi: 10.3390/cancers7030850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero AJ, et al. Phase 2 study of neoadjuvant treatment with NOV-002 in combination with doxorubicin and cyclophosphamide followed by docetaxel in patients with HER-2 negative clinical stage II-IIIc breast cancer. Breast Cancer Res Treat. 2012;132:215–223. doi: 10.1007/s10549-011-1889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahira S, et al. Involvement of ribonucleotide reductase M1 subunit overexpression in gemcitabine resistance of human pancreatic cancer. International Journal of Cancer Journal International Du Cancer. 2007;120:1355–1363. doi: 10.1002/ijc.22390. [DOI] [PubMed] [Google Scholar]
- Neesse A, et al. CTGF antagonism with mAb FG-3019 enhances chemotherapy response without increasing drug delivery in murine ductal pancreas cancer. Proc Natl Acad Sci U S A. 2013;110:12325–12330. doi: 10.1073/pnas.1300415110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive KP, Tuveson DA. The use of targeted mouse models for preclinical testing of novel cancer therapeutics. Clin Cancer Res. 2006;12:5277–5287. doi: 10.1158/1078-0432.CCR-06-0436. [DOI] [PubMed] [Google Scholar]
- Olive KP, et al. Mutant p53 gain of function in two mouse models of Li-Fraumeni syndrome. Cell. 2004;119:847–860. doi: 10.1016/j.cell.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Olive KP, et al. Inhibition of hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivier M, et al. The clinical value of somatic TP53 gene mutations in 1,794 patients with breast cancer. Clin Cancer Res. 2006;12:1157–1167. doi: 10.1158/1078-0432.CCR-05-1029. [DOI] [PubMed] [Google Scholar]
- Ono M, et al. WISP1/CCN4: a potential target for inhibiting prostate cancer growth and spread to bone. PLoS One. 2013;8:e71709. doi: 10.1371/journal.pone.0071709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottenhof NA, de Wilde RF, Maitra A, Hruban RH, Offerhaus GJ. Molecular characteristics of pancreatic ductal adenocarcinoma. Pathol Res Int. 2011;2011:620601. doi: 10.4061/2011/620601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perbal B. The CCN family of genes: a brief history. Molecular Pathol: MP. 2001;54:103–104. doi: 10.1136/mp.54.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perbal B. CCN proteins: multifunctional signalling regulators. Lancet. 2004;363:62–64. doi: 10.1016/S0140-6736(03)15172-0. [DOI] [PubMed] [Google Scholar]
- Perera RM, Bardeesy N. Ready, set, go: the EGF receptor at the pancreatic cancer starting line. Cancer Cell. 2012;22:281–282. doi: 10.1016/j.ccr.2012.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planque N, Perbal B. A structural approach to the role of CCN (CYR61/CTGF/NOV) proteins in tumourigenesis. Cancer Cell Int. 2003;3:15. doi: 10.1186/1475-2867-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichert M, Rustgi AK. Pancreatic ductal cells in development, regeneration, and neoplasia. J Clin Invest. 2011;121:4572–4578. doi: 10.1172/JCI57131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhim AD, et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell. 2014;25:735–747. doi: 10.1016/j.ccr.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo JW, Castellot JJ. CCN5: biology and pathophysiology. Journal of Cell Communication and Signaling. 2010;4:119–130. doi: 10.1007/s12079-010-0098-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbah M, et al. CCN5, a novel transcriptional repressor of the transforming growth factor beta signaling pathway. Mol Cell Biol. 2011;31:1459–1469. doi: 10.1128/MCB.01316-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena N, Banerjee S, Sengupta K, Zoubine MN, Banerjee SK. Differential expression of WISP-1 and WISP-2 genes in normal and transformed human breast cell lines. Mol Cell Biochem. 2001;228:99–104. doi: 10.1023/A:1013338912642. [DOI] [PubMed] [Google Scholar]
- Simeone DM. Pancreatic cancer stem cells: implications for the treatment of pancreatic cancer. Clin Cancer Res. 2008;14:5646–5648. doi: 10.1158/1078-0432.CCR-08-0584. [DOI] [PubMed] [Google Scholar]
- Soon LL, Yie TA, Shvarts A, Levine AJ, Su F, Tchou-Wong KM. Overexpression of WISP-1 down-regulated motility and invasion of lung cancer cells through inhibition of Rac activation. J Biol Chem. 2003;278:11465–11470. doi: 10.1074/jbc.M210945200. [DOI] [PubMed] [Google Scholar]
- Spivak-Kroizman TR, et al. Hypoxia triggers hedgehog-mediated tumor-stromal interactions in pancreatic cancer. Cancer Res. 2013;73:3235–3247. doi: 10.1158/0008-5472.CAN-11-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromnes IM, Greenberg PD. Pancreatic cancer: planning ahead for metastatic spread. Cancer Cell. 2016;29:774–776. doi: 10.1016/j.ccell.2016.05.013. [DOI] [PubMed] [Google Scholar]
- Takigawa M. CTGF/Hcs24 as a multifunctional growth factor for fibroblasts, chondrocytes and vascular endothelial cells. Drug News & Perspectives. 2003;16:11–21. doi: 10.1358/dnp.2003.16.1.829302. [DOI] [PubMed] [Google Scholar]
- Thayer SP, et al. Hedgehog is an early and late mediator of pancreatic cancer tumorigenesis. Nature. 2003;425:851–856. doi: 10.1038/nature02009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian C, et al. Overexpression of connective tissue growth factor WISP-1 in Chinese primary rectal cancer patients. World J Gastroenterol: WJG. 2007;13:3878–3882. doi: 10.3748/wjg.v13.i28.3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda M, et al. Clinical significance of expression of nephroblastoma overexpressed (NOV) in patients with colorectal cancer. Anticancer Res. 2015;35:6591–6597. [PubMed] [Google Scholar]
- Vaz AP, Ponnusamy MP, Batra SK. Cancer stem cells and therapeutic targets: an emerging field for cancer treatment. Drug Delivery and Translational Research. 2013;3:113–120. doi: 10.1007/s13346-012-0095-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Hoff DD, Korn R, Mousses S. Pancreatic cancer--could it be that simple? A different context of vulnerability. Cancer Cell. 2009;16:7–8. doi: 10.1016/j.ccr.2009.06.011. [DOI] [PubMed] [Google Scholar]
- Von Hoff DD, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voutsadakis IA. Molecular predictors of gemcitabine response in pancreatic cancer. World J Gastrointest Oncol. 2011;3:153–164. doi: 10.4251/wjgo.v3.i11.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Zhang GY, Li XH. Effect of indomethacin on Bfl-1, WISP-1 and proliferating cell nuclear antigen in colon cancer cell line HCT116 cells. Chin J Dig Dis. 2006;7:219–224. doi: 10.1111/j.1443-9573.2006.00272.x. [DOI] [PubMed] [Google Scholar]
- Wang-Gillam A, et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, phase 3 trial. Lancet. 2016;387:545–557. doi: 10.1016/S0140-6736(15)00986-1. [DOI] [PubMed] [Google Scholar]
- Weissmueller S, et al. Mutant p53 drives pancreatic cancer metastasis through cell-autonomous PDGF receptor beta signaling. Cell. 2014;157:382–394. doi: 10.1016/j.cell.2014.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whatcott CJ, Posner RG, Von Hoff DD, Han H. Desmoplasia and chemoresistance in pancreatic cancer. In: PJ G, HG M, editors. Pancreatic Cancer and Tumor Microenvironment. India: Trivandrum; 2012. [PubMed] [Google Scholar]
- Wilentz RE, et al. Inactivation of the p16 (INK4A) tumor-suppressor gene in pancreatic duct lesions: loss of intranuclear expression. Cancer Res. 1998;58:4740–4744. [PubMed] [Google Scholar]
- Wu L, et al. CCN3/NOV gene expression in human prostate cancer is directly suppressed by the androgen receptor. Oncogene. 2014;33:504–513. doi: 10.1038/onc.2012.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JY, et al. High expression of WISP-1 correlates with poor prognosis in pancreatic ductal adenocarcinoma. Am J Transl Res. 2015;7:1621–1628. [PMC free article] [PubMed] [Google Scholar]
- Ying H, et al. Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell. 2012;149:656–670. doi: 10.1016/j.cell.2012.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C, Le AT, Yeger H, Perbal B, Alman BA. NOV (CCN3) regulation in the growth plate and CCN family member expression in cartilage neoplasia. J Pathol. 2003;201:609–615. doi: 10.1002/path.1468. [DOI] [PubMed] [Google Scholar]
- Zhang L, et al. Gene expression profiles in normal and cancer cells. Science. 1997;276:1268–1272. doi: 10.1126/science.276.5316.1268. [DOI] [PubMed] [Google Scholar]
- Zhou BB, Zhang H, Damelin M, Geles KG, Grindley JC, Dirks PB. Tumour-initiating cells: challenges and opportunities for anticancer drug discovery. Nat Rev Drug Discov. 2009;8:806–823. doi: 10.1038/nrd2137. [DOI] [PubMed] [Google Scholar]