Abstract
Background
Chryseobacterium indologenes is an uncommon organism that has been documented to cause a variety of invasive infections mostly in hospitalized patients with severe underlying diseases.
Case presentation
A three-month-old female infant born at term by caesarean section with meningomyelocele and congenital diaphragmatic hernia had two surgeries for the repair of meningomyelocele and diaphragmatic hernia on her 3rd and 14th day, respectively. On the 3rd month of her life, she deteriorated clinically with fever, leukocytosis and increase of acute-phase reactants. Gas exchange condition became worse than it was before. Respiratory secretions, oxygen requirements and ventilator demand increased. Chest X-ray showed bilateral pulmonary infiltrates. Bacteriological blood, urine and cerebrospinal fluid culture test results were negative. C. indologenes was isolated from tracheobronchial secretion sample obtained by endotracheal aspiration. Although susceptible to ciprofloxacin (MIC:0.5 gr/L), levofloxacin and piperacillin–tazobactam, the isolate was resistant to meropenem, imipenem and colistin. She was treated with ciprofloxacin successfully. Her fever resolved and gas exchange condition improved after 72 h of the treatment. The antibiotic treatment was given for a course of 14 days.
Conclusion
Chryseobacterium indologenes may emerge as a potential pathogen in infants with the factors such as invasive equipment, having underlying diseases and prolonged hospitalization.
Keywords: Chryseobacterium indologenes, Infant case, Ventilator-associated pneumonia
Background
Chryseobacterium indologenes is a Gram-negative, aerobic, non-fermenting, non-motile, catalase-, oxidase-, and indole positive bacillus. It is widely distributed in environmental sources including water, soil and plants (Omar et al. 2014). It is possible that physicians may encounter this pathogenic microorganism in hospital environment such as mechanical ventilator circuits. C. indologenes is a very rare pathogen in human that has been reported to cause infections mostly in hospitalized patient with immunocompromised conditions or infants. C. indologenes is inherently resistant to many antimicrobial agents including carbapenems (Omar et al. 2014).
In our case, C. indologenes was isolated from a tracheobronchial secretion sample in a 3-month-old infant diagnosed with ventilator-associated pneumonia.
Case description
A three-month-old female infant born at term by caesarean section was prenatally diagnosed with meningomyelocele and congenital diaphragmatic hernia and was transferred to the neonatal intensive care unit (NICU) for further management. Because of severe dyspnea, she was intubated and given mechanical ventilatory support. She had two surgeries for the repair of meningomyelocele and congenital diaphragmatic hernia on the 3rd and 14th days of life, respectively. VP shunt was inserted when she was one month old because of hydrocephalus. The patient had a bacteremia caused by Stenotrophomonas maltophilia. The pathogen was susceptible to ceftazidime and ciprofloxacin and treated with ceftazidime. After the completion of treatment period, the patient remained antibiotic-free for 7 days. While she was monitored on mechanical ventilation on the 3rd month of life, she clinically deteriorated with fever (38.5 °C). Gas exchange condition became worse than it was before. Respiratory secretions, oxygen requirements and ventilator demand increased. Her laboratory findings showed leukocytosis with increased number of neutrophils (WBC: 14,500/mm3, neutrophils: 8600/mm3) and high levels of acute phase reactant (C-reactive protein: 19.2 mg/dl). Chest X-ray showed bilateral pulmonary infiltrates compatible with pneumonia. Blood, cerebrospinal fluid, urine and tracheobronchial secretion specimen obtained by sterile endotracheal aspiration were sent to microbiology laboratory for bacterial culture. Increased leukocytes were observed on smear of tracheobronchial secretion sample. Previous infection history caused by Stenotrophomonas maltophilia bacteremia was considered and empiric antibiotic therapy with vancomycin, ceftazidime and ciprofloxacin were started. Tracheobronchial secretion obtained by sterile endotracheal aspiration yielded yellow-colored colonies after 24 h incubation on sheep blood agar (Fig. 1a). Similar yellow-pigmented colonies were also observed on Müller-Hinton Agar (Fig. 1b). C. indologenes was identified by conventional methods, VITEK 2 ID-AST (bioM´erieux, France) fully automatized system and Matrix-Assisted Laser Desorption/Ionization time-of-flight, Mass Spectrometry(MALDI-TOF MS). Based on sequencing result of partial 16S rRNA gene, the isolate matched 99 % identities with the region from 852 to 860 bp of the 16S rRNA sequence of C. indologenes strain (GenBank sequence ID: LN681561.1). There was no other co-pathogen. Antimicrobial susceptibility testing was performed by both determining the minimal inhibitory concentration (MIC) value using microdilution method and measuring the inhibition zone diameter onto Mueller–Hinton agar (Oxoid Ltd., Basingstoke, UK) medium aerobically at 35 ± 2 °C for 18–24 h using Kirby-Bauer’s disk diffusion method according to Clinical and Laboratory Standards Institute (CLSI) guidelines for non-fermenting microorganisms. Antimicrobial susceptibility testing of the organism revealed resistance to aminoglycosides, ceftazidime, meropenem, imipenem, colistin and was susceptible to ciprofloxacin, levofloxacin, piperacillin–tazobactam and cefepime.
Fig. 1.
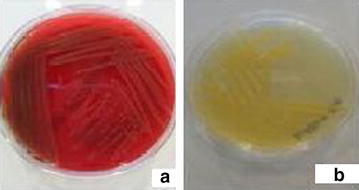
Yellow colonies of Chryseobacterium indologenes on sheep blood agar (a) and Müller-Hinton agar (b)
Her fever resolved and gas exchange condition improved after 72 h of treatment. The patient gave a good clinical response with the empiric treatment. For this reason, we did not want to change ciprofloxacin, and the treatment was continued with ciprofloxacin monotherapy. Blood, urine and cerebrospinal fluid culture test results were negative. Repeated endotracheal aspiration specimen culture was also negative after 72 h of antibiotherapy. The antibiotic treatment was given for a course of 14 days.
Discussion
Chryseobacterium genus is a group of Gram-negative, aerobic bacilli that belong to Flavobacteriaceae family. C. indologenes is the most common species and was first described by Vandamme et al. in 1994 (Vandamme et al. 1994). However C. indologenes is not a part of the human microflora, it can be found in water supplies in the hospital environment. Contamination of the medical devices containing water (intubation tubes, respirators, humidifiers, etc.) in hospital settings may lead to severe infections in hospitalized patients. Both long-term colonization with C. indologenes of medical devices and invasive infections have been reported Hsueh et al. (1996). It is known that the production of biofilm and protease activity by C. indologenes is an important mechanism involved in its virulence although the exact mechanism of pathogenicity is not well determined Hsueh et al. (1996).
Chryseobacterium indologenes infections in children are very rare and usually associated with the presence of invasive medical equipment as in our case. It has been reported to cause a variety of invasive infections such as ventilator-associated pneumonia, bacteremia, catheter-related bloodstream infection, lumboperitoneal shunt infection, pyelonephritis, biliary tract infections, peritonitis, ocular infections, surgical site infection, wound infection, endocarditis, and keratitis (Hsueh et al. 1996; Deng et al. 2015; Bayraktar et al. 2007; Douvoyiannis et al. 2010; Al-Tatari et al. 2007). Besides the use of invasive medical devices, other important risk factors for C. indologenes infection are use of broad-spectrum antibiotics, underlying diseases and primary or acquired immunosuppressive conditions. Infections caused by C. indologenes are associated with a high mortality rate (Nemli et al. 2015).
Chryseobacterium indologenes is a rare pathogen isolated from clinical specimens, and its antimicrobial susceptibility pattern is not well defined. The organism has a limited antimicrobial sensitivity. The choice of an effective antibiotic for the empirical treatment is difficult. Chryseobacterium organisms produce class A -lactamase and class B carbapenem-hydrolyzing -lactamase molecules that cause intrinsic carbapenem and cephalosporin resistance. C. indologenes is usually resistant to aminoglycosides, other -lactams, chloramphenicol, linezolid, and glycopeptides and is usually susceptible to ciprofloxacin, levofloxacin, trimethoprim-sulfamethoxazole (TMP–SMX), and piperacillin–tazobactam (Nemli et al. 2015; Lin et al. 2010). According to the results of the SENTRY Antimicrobial Surveillance Program, the most active antimicrobials against C. indologenes are quinolones (≥95 % susceptibility) and trimethoprim–sulfamethoxazole (95 % susceptibility), followed by piperacillin–tazobactam (90 % susceptibility). Ciprofloxacin, cefepime, ceftazidime, piperacillin, and rifampin showed reasonable activity (85 % susceptibility) (Kirby et al. 2004). Due to the limited data in the pediatric age group, a standard and effective treatment for C. indologenes infections is still not clear. Our case was ventilator-associated pneumonia caused by C. indologenes, which was successfully treated with ciprofloxacin monotherapy.
Chryseobacterium indologenes is a widespread bacterium in the environment, in particular on the wet surfaces of hospitals and water systems. Although there is not any outbreak report in pediatric wards, a distillate water tank was shown to be the source of C. indologenes that caused a blood stream infection (Bayraktar et al. 2007). The organism may spread because of limited education of the healthcare personnel and incomplete adherence to infection control measures. The physician should report this rare pathogen to infection control department. If necessary, environmental cultures should be performed to identify the source. Healthcare personnel have to be careful and they should be educated about the implementation of infection control measures, especially hand hygiene compliance. We reported this case to our hospital infection control committee. Environmental cultures such as the respiratory circuit, humidifier, etc. were not performed. Contact isolation precautions were applied to the patient, and healthcare workers were educated and reinforced about infection control measures. Outbreak did not occur.
Review of the literature about C. indologenes infections in pediatric age groups
We searched for information about C. indologenes infections in the MEDLINE (PubMed, Ovid) database and could able to suitable 24 pediatric cases. Patients were excluded if they were an adult (>18 years) case from this review. The most important characteristics of cases were presented in Table 1. Gender was reported for 23 patients, 12 (52 %) of them were female, and 16 (66.6 %) patients were ≤1 year of age. Most of the patients (n = 21, 87.5 %) had underlying conditions and only 6 (25 %) patients had no medical device. Five patients (1, 18, 20, 21, 23rd patients in Table 1) had co-infections, including Escherichia coli, Morganella morganii, Acinetobacter baumannii, vancomycin resistant enterococcus, Stenotrophomonas maltophilia and Burkholderia cepacia. The most commonly used antibiotics were ciprofloxacin and TMP-SMX. Four patients died and the mortality rate was found 16.6 % in this series.
Table 1.
Characteristics of pediatric cases caused by Chryseobacterium indologenes
| No | Age/sex | Underlying condition | Medical device | Infection type | Treatment | Outcome | Year/reference |
|---|---|---|---|---|---|---|---|
| 1 | 1 year/M | Burn | Ventilator | VAP | Ciprofloxacin, cefoxitin, amikacin | Died | 1996/Hsueh et al. (1996) |
| 2 | 5 year/F | Neuroblastoma | CVC | Bacteremia | NR | Survived | 1996/Hsueh et al. (1996) |
| 3 | 1 year/F | Hepatoblastoma | CVC | Bacteremia | NR | Survived | 1996/Hsueh et al. (1996) |
| 4 | 2 year/M | Diabetes mellitus (Type 1) | Peripheral catheter | Bacteremia | Ceftriaxone | Survived | 2005/(Cascio et al. 2005) |
| 5 | 5 month/M | Down syndrome, diaphragmatic hernia, ASD | Ventilator | Bacteremia | Vancomycin, ofloxacin | Died | 2007/(Bayraktar et al. 2007) |
| 6 | 13 year/M | Congenital hydrocephalus | Lumboperitoneal shunt | Lumboperitoneal shunt infection | TMP–SMX, Rifampin | Survived | 2007/(Al-Tatari et al. 2007) |
| 7 | 33 day/F | None | None | Bacteremia | Cefepime | Survived | 2010/(Douvoyiannis et al. 2010) |
| 8 | 2 month/M | Hydrocephaly | External shunt | Meningitis, sepsis | Ampicillin–sulbactam, levofloxacin | Died | 2011/(Ceylan et al. 2011) |
| 9 | 36 week newborn/NR | Prematurity | Ventilator | Bacteremia | Cefoperazone–sulbactam | Survived | 2011/(Sudharani and Asiya Saxena 2011) |
| 10 | 20 day/M | Complex congenital heart disease | Ventilator | VAP | Piperacillin–tazobactam | Survived | 2011/(Calderón et al. 2011) |
| 11 | 8 day/F | None | None | Meningitis | Cefepime | Survived | 2013/(Hendaus and Zahraldin 2013) |
| 12 | 3 year/F | Acute myeloid leukemia | CVC | CRBSI | Ciprofloxacin, minocycline | Survived | 2013/(Kodama et al. 2013) |
| 13 | 6 month/M | Congenital hydrocephalus, prematurity | Ventriculoperitoneal shunt | Meningitis | TMP–SMX, cefoperazone–sulbactam | Survived | 2013/(Ozcan et al. 2013) |
| 14 | 11 month/M | Holoprosencephaly, obstructive hydrocephalus | Ventriculoperitoneal shunt | Meningitis | TMP–SMX, ceftazidime | Survived | 2014/(Olbrich et al. 2014) |
| 15 | 6 day/F | SGA | None | Meningitis, sepsis | Ciprofloxacin, TMP–SMX | Survived | 2014/(Eshwara et al. 2014) |
| 16 | 3 month/F | ASD | CVC | Bacteremia | TMP–SMX | Survived | 2014/(Aydin et al. 2014) |
| 17 | 27 week newborn/F | Complex congenital heart disease | Central catheter, arterial and venous line | Bacteremia | Ciprofloxacin, imipenem | Survived | 2014/(Alford and Shelton 2014) |
| 18 | 3 month/M | Metabolic disease | CVC, ventilator | CRBSI | Ciprofloxacin, imipenem, colimycin, linezolid | Died | 2016/(Aykac et al. 2016) |
| 19 | 2 year/F | Congenital hydrocephalus | External shunt | Meningitis | Ciprofloxacin, TMP–SMX | Survived | 2016/(Aykac et al. 2016) |
| 20 | 8 year/M | Cystic fibrosis, nephrotic syndrome | None | Pneumonia | Ceftriaxone | Survived | 2016/(Aykac et al. 2016) |
| 21 | 8 month/M | Ileus | CVC | Bacteremia | Ciprofloxacin, meropenem, vancomycin | Survived | 2016/(Aykac et al. 2016) |
| 22 | 16 month/F | ITP, immunosuppressive therapy | None | Bacteremia | Ceftriaxone | Survived | 2016/(Aykac et al. 2016) |
| 23 | 3 year/F | Cerebral palsy | CVC | CRBSI | Meropenem, amikacin | Survived | 2016/(Aykac et al. 2016) |
| 24 | 11 year/F | None | None | Soft tissue infection | Ceftazidime, metronidazole | Survived | 2016/(Srinivasan et al. 2016) |
| 25 | 3 month/F | Meningomyelocele, congenital diaphragmatic hernia | Ventilator | VAP | Ciprofloxacin | Survived | Our case |
M male, F female, NR not reported, TMP–SMX Trimethoprim–Sulfamethoxazole, VAP ventilator-associated pneumonia, CVC central venous catheter, CRBSI catheter-related blood stream infection, SGA small for gestational age, ASD atrial septal defect, ITP immune thrombocytopenic purpura
Conclusion
Chryseobacterium indologenes may emerge as a potential pathogen in infants with risk factors such as invasive medical equipment, underlying diseases, broad-spectrum antibiotics usage and prolonged hospitalization. Physicians should consider this pathogen in the etiology of medical device-associated infections. C. indologenes may have resistance to empirically administered antimicrobial treatment for nosocomial infections and antimicrobial susceptibility test results are important to guide the antibiotic treatment.
Authors’ contributions
All authors interested in management, treatment and outcome the patient. All authors have been involved in drafting the manuscript or revising critically for important intellectual content. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent
Written informed consent was obtained from the patient parent for the publication of this report.
Contributor Information
Serkan Atıcı, Phone: +90 506 154 7667, Email: aticiserkan@yahoo.com.
Zeynep Alp Ünkar, Email: md.zeynepalp@gmail.com.
Kübra Erdem, Email: kubraerdem@yandex.com.
Eda Kepenekli Kadayifci, Email: ekepenekli@yahoo.com.
Ayşe Karaaslan, Email: akaraaslan78@gmail.com.
Aslı Çınar Memişoğlu, Email: acinarmemisoglu@gmail.com.
Ahmet Soysal, Email: ahsoysal@yahoo.com.
Nurver Ülger Toprak, Email: nurverulger@yahoo.com.
Güner Söyletir, Email: gsoyletir@yahoo.com.
Eren Özek, Email: ozekeren@gmail.com.
Mustafa Bakır, Email: mustafabakir65@gmail.com.
References
- Alford EL, Shelton CM. Ciprofloxacin and imipenem for Chryseobacterium indologenes presumed meningitis in a preterm neonate. Pediatric Infect Dis. 2014;6(3):94–96. doi: 10.1016/j.pid.2014.05.003. [DOI] [Google Scholar]
- Al-Tatari H, Asmar BI, Ang JY. Lumboperitonial shunt infection due to Chryseobacterium indologenes. Pediatr Infect Dis J. 2007;26(7):657–659. doi: 10.1097/INF.0b013e3180616d25. [DOI] [PubMed] [Google Scholar]
- Aydin TT, Oz FN, Metin O, Bayhan GI, Gayretli ZG, Oguz M, Tanir G. Chryseobacterium indologenes Septicemia in an Infant. Case Rep Infect Dis. 2014 doi: 10.1155/2014/270521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aykac K, Ozsurekci Y, Tuncer O, Sancak B, Cengiz AB, Kara A, Ceyhan M. Six cases during 2012-2015 and literature review of Chryseobacterium indologenes infections in pediatric patients. Can J Microbiol. 2016;17:1–8. doi: 10.1139/cjm-2015-0800. [DOI] [PubMed] [Google Scholar]
- Bayraktar MR, Aktaş E, Ersoy Y, Cicek A, Durmaz R. Postoperative Chryseobacterium indologenes bloodstream infection caused by contamination of distillate water. Infect Control Hosp Epidemiol. 2007;28(3):368–369. doi: 10.1086/508839. [DOI] [PubMed] [Google Scholar]
- Calderón G, García E, Rojas P, García E, Rosso M, Losada A. Chryseobacterium indologenes infection in a newborn: a case report. J Med Case Rep. 2011;14(5):10. doi: 10.1186/1752-1947-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascio A, Stassi G, Costa GB, Crisafulli G, Rulli I, Ruggeri C, Iaria C. Chryseobacterium indologenes bacteraemia in a diabetic child. J Med Microbiol. 2005;54(Pt 7):677–680. doi: 10.1099/jmm.0.46036-0. [DOI] [PubMed] [Google Scholar]
- Ceylan A, Güdücüoğlu H, Akbayram S, Bektaş A, Berktaş M. Sepsis caused by Chryseobacterium indologenes in a patient with hydrocephalus. Mikrobiyol Bulteni. 2011;45(4):735–740. [PubMed] [Google Scholar]
- Deng L, Li MF, Li YH, Yang JL, Zhou X. Chryseobacterium indologenes in four patients with leukemia. Transpl Infect Dis. 2015;17(4):583–587. doi: 10.1111/tid.12400. [DOI] [PubMed] [Google Scholar]
- Douvoyiannis M, Kalyoussef S, Philip G, Mayers MM. Chryseobacterium indologenes bacteremia in an infant. Int J Infect Dis. 2010;14(6):531–532. doi: 10.1016/j.ijid.2009.06.015. [DOI] [PubMed] [Google Scholar]
- Eshwara VK, Sasi A, Munim F, Purkayastha J, Lewis LE, Mukhopadhyay C. Neonatal meningitis and sepsis by Chryseobacterium indologenes: a rare and resistant bacterium. Indian J Pediatr. 2014;81(6):611–613. doi: 10.1007/s12098-013-1040-9. [DOI] [PubMed] [Google Scholar]
- Hendaus MA, Zahraldin K. Chryseobacterium indologenes meningitis in a healthy newborn: a case report. Oman Med J. 2013;28(2):133–134. doi: 10.5001/omj.2013.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsueh PR, Teng LJ, Yang PC, Ho SW, Hsieh WC, Luh KT. Clinical and microbiological characteristics of Flavobacterium indologenes infections associated with indwelling devices. J Clin Microbiol. 1996;34:1908–1913. doi: 10.1128/jcm.34.8.1908-1913.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsueh PR, Hsiue TR, Wu JJ, Teng LJ, Ho SW, Hsieh WC, Luh KT. Flavobacterium indologenes bacteremia: clinical and microbiological characteristics. Clin Infect Dis. 1996;23(3):550–555. doi: 10.1093/clinids/23.3.550. [DOI] [PubMed] [Google Scholar]
- Kirby JT, Sader HS, Walsh TR, Jones RN. Anti microbial susceptibility and epidemiology of a worldwide collection of Chryseobacterium spp: report from the SENTRY Antimicrobial Surveillance Program (1997–2001) J Clin Microbiol. 2004;42(1):445–448. doi: 10.1128/JCM.42.1.445-448.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama Y, Nishimura M, Nakashima K, Ito N, Fukano R, Okamura J, Inagaki J. Central intravenous catheter-related bacteremia due to Chryseobacterium indologenes after cord blood transplantation. Rinsho Ketsueki. 2013;54(3):305–310. [PubMed] [Google Scholar]
- Lin YT, Jeng YY, Lin ML, Yu KW, Wang FD, Liu CY. Clinical and microbiological characteristics of Chryseobacterium indologenes bacteremia. J Microbiol Immunol Infect. 2010;43(6):498–505. doi: 10.1016/S1684-1182(10)60077-1. [DOI] [PubMed] [Google Scholar]
- Nemli SA, Demirdal T, Ural S. A case of healthcare associated Pneumonia Caused by Chryseobacterium indologenes in an immunocompetent patient. Infect Dis: Case Rep; 2015. pp. 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olbrich P, Rivero-Garvía M, Falcón-Neyra MD, Lepe JA, Cisneros JM, Marquez-Rivas J, Neth O. Chryseobacterium indologenes central nervous system infection in infancy: an emergent pathogen? Infection. 2014;42(1):179–183. doi: 10.1007/s15010-013-0479-y. [DOI] [PubMed] [Google Scholar]
- Omar A, Camara M, Fall S, Ngom-Cisse S, Fall B, Ba-Diallo A, Diop-Ndiaye H, Toure-Kane C, Mboup S, Gaye-Diallo A. Chryseobacterium indologenes in a woman with acute leukemia in Senegal: a case report. J Med Case Rep. 2014;8(138):1–5. doi: 10.1155/2014/842872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozcan N, Dal T, Tekin A, Kelekci S, Can S, Ezin O, Kandemir I, Gul K. Is Chryseobacterium indologenes a shunt-lover bacterium? a case report and review of the literature. Infez Med. 2013;21(4):312–316. [PubMed] [Google Scholar]
- Srinivasan G, Muthusamy S, Raveendran V, Joseph NM, Easow JM. Unforeseeable presentation of Chryseobacterium indologenes infection in a paediatric patient. BMC Res Notes. 2016;9(212):1–5. doi: 10.1186/s13104-016-2022-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudharani V, Asiya Saxena NK. Chryseobacterium indologenes bacteraemia in a preterm baby. Indian J Med Microbiol. 2011;29(2):196–198. doi: 10.4103/0255-0857.81783. [DOI] [PubMed] [Google Scholar]
- Vandamme P, Bernardet JF, Segers P, Kersters K, Holmes B. New perspectives in the classification of the flavobacteria: description of Chryseobacterium gen. nov., Bergeyella gen. nov., and Empedobacter nom. rev. Int J Syst Microb. 1994;44(4):827–831. [Google Scholar]


