Abstract
Postpartum depression (PPD) is a modified form of major depressive disorders (MDD) that can exert profound negative effects on both mothers and infants than MDD. Within the postpartum period, both mothers and infants are susceptible; but because PPD typically occurs for short durations and has moderate symptoms, there exists challenges in exploring and addressing the underlying cause of the depression. This fact highlights the need for relevant animal models. In the present study, postpartum adult female cynomolgus monkeys (Macaca fascicularis) living in breeding groups were observed for typical depressive behavior. The huddle posture behavior was utilized as an indicator of behavioral depression postpartum (BDP) as it has been established as the core depressive-like behavior in primates. Monkeys were divided into two groups: A BDP group (n=6), which were found to spend more time huddling over the first two weeks postpartum than other individuals that formed a non-depression control group (n=4). The two groups were then further analyzed for locomotive activity, stressful events, hair cortisol levels and for maternal interactive behaviors. No differences were found between the BDP and control groups in locomotive activity, in the frequencies of stressful events experienced and in hair cortisol levels. These findings suggested that the postpartum depression witnessed in the monkeys was not related to external factors other than puerperium period. Interestingly, the BDP monkeys displayed an abnormal maternal relationship consisting of increased infant grooming. Taken together, these findings suggest that the adult female cynomolgus monkeys provide a natural model of behavioral postpartum depression that holds a number of advantages over commonly used rodent systems in PPD modeling. The cynomolgus monkeys have a highly-organized social hierarchy and reproductive characteristics without seasonal restriction—similar to humans—as well as much greater homology to humans than rodents. As such, this model may provide a greater translational efficiency and research platform for systematically investigating the etiology, treatment, prevention of PPD.
Keywords: Postpartum depression, Cynomolgus monkeys, Huddle behavior, Locomotion activity, Stressful events, Hair cortisol, Maternal relationship
Postpartum depression (PPD) is commonly identified as a subtype of major depressive disorder (MDD) with the specification of depression onset within two months after delivery (Friedman & Resnick, 2009). It is estimated that 15% of mothers overall suffer from PPD, with symptoms that last at least a few weeks to months (England, 1994; Flores & Hendrick, 2002; Ghubash & Abou-Saleh, 1997; OHara, 1987; Sit & Wisner, 2009). In general, PPD primarily exerts profound adverse effects on mothers and their infants through disturbing the maternal relationship, which may be a disabling or life-threatening disruption (Epperson & Ballew, 2004). However, the cause of PPD is not understood and the exact mechanisms remain unclear (O'Hara & McCabe, 2013).
Still, a further confounding factor to understanding PPD development is the crucial and susceptible period, including the time over pregnancy and puerperium, which affects the mental health of mothers and the maturation of infants. This limited window is a difficult period to perform scientific investigation and ultimately slows down the pace of PPD research. The fact highlights the importance of animal models in studying and developing treatments for PPD. However, most models of PPD have been adopted in rodents, and have focused on exploring only a few biological factors involved in PPD, such as ovarian hormones withdrawal, stress and corticosterone exposures, etc. (Brummelte & Galea, 2010). No PPD rodent model fully contains the disorders full spectrum, as there are large differences in rodent and human homology (Willner, 1991), and there are, thus, translational limitations to using rodent models to investigate PPD. Conversely, non-human primates possess physiological, behavioral, and genetic characteristics similar to humans (Sibley & Ahlquist, 1987; Kalin & Shelton, 2003), which make them potential tools in the development of natural models of PPD.
In the present study, adult female cynomolgus monkeys in puerperium were selected as subjects to investigate their use as a natural model of PPD because they have very similar reproductive behaviors, without seasonal restrictions, as humans and a well-organized hierarchical society similar to humans (Sapolsky, 2005; Van Esch et al, 2008) in addition to the biologically similar characteristics between monkeys and humans mentioned above. Furthermore, depression-associated behaviors in non-human primates have long been established by studying maternal separation (Bowlby, 1962; Spitz & Wolf, 1946), and are now widely used in the definition of primate models of mood-related disorders (Shively et al, 2002, 2005, 2006; Strome et al, 2002). As such, researchers have observed that primates demonstrate, along with depressive behaviors, a series of behavioral and neurophysiological abnormalities which are analogous to relevant changes in patients with MDD and PPD, including an increased responsiveness in the sympathetic nervous system (Pryce et al, 2004), dysfunction of the HPA axis (Shively, 1998; Shively et al, 1997), monoamine transmitter deficits (Shively, 1998; Willard & Shively, 2012), and anhedonia (Pryce et al, 2004). These characteristics make the adult female cynomolgus monkey a potentially useful system to explore the etiology and clinical interventions of PPD. And the huddle posture behavior, generally considered to be the core depressive-associated behaviors in non-human primates (Chilton et al, 2011; Shively et al, 2005), was accordingly used as the behavioral indicator to differentiate between monkeys displaying depression postpartum from monkeys who were not in the study.
Numerous stressors, including stressful life events, have been identified as the predictors of PPD (Liu & Tronick, 2013; O'Hara & Swain, 1996). Therefore, the monkeys displaying PPD-like behaviors in this study were then evaluated for stress related indicators. In primates, most sources of stress originate from two main types of conflict events: the receipt of aggression and the display of submission. These stressful events are opposed by two other conflict events or non-stressful events: the receipt of submission and the display of aggression (Koolhaas et al, 1999). Stressful events are often regarded as alternative strategies of coping with stress and non-stressful events are ways of alleviating stress (Folkman et al, 1986; Koolhaas et al, 1999). Therefore, the numbers of times receipt of aggression and submission displays by the monkeys were used to reflect the intensity of stress the monkeys experienced. Previous studies have shown that conflict events have a link with hyperactivity of the HPA axis in both animals and humans, but that non-stressful events did not (Koolhaas et al, 1999). The continuous hypersecretions of the glucocorticoids, such as cortisol, by the HPA axis provides a crucial link between chronic stress exposure and mental disorders in humans (Carroll et al, 1976; McClure, 1966; Parker et al, 2003; Schüle, 2007) and has been commonly utilized in assessing stress conditions experienced in other species (Abbott et al, 2003; Burke et al, 2005; Cattet et al, 2003; Constable et al, 2006; Keay et al, 2006; Millspaugh et al, 2002; Touma & Palme, 2005; Whitten et al, 1998). However, the relevant relationship of stress in PPD patients is still an unclear issue (Gard et al, 1986; Groer & Morgan, 2007; Harris et al, 1989, 1996; Jolley et al, 2007; Nierop et al, 2006; Okano & Nomura, 1992). Therefore, this study further evaluated hair cortisol levels in the subjects from hair samples taken on the first day of the third week postpartum, and were used as an approximate reflection of the accumulated stress experienced by the monkeys over last trimester before delivery until sampling (Russell et al, 2012). A substantial body of evidence indicates that cortisol in hair provides a functional instrument in qualifying the degree of long-term stress in both humans and primates (Davenport et al, 2006; Feng et al, 2011; Stalder et al, 2012) and that hair cortisol is a stable biomarker to assess the state of stress over time, whereas cortisol levels evaluated in plasma, saliva, urine, or feces only reflect cortisol levels at a single point in time (Wennig, 2000; Russell et al, 2012).
Monkeys displaying PPD-like behaviors in this study were then evaluated for the quality of the mother-infant relationships formed after birth. A poor mother-infant relationship is the prominent outcome in PPD patients (Field, 2010). As such, the monkeys displaying PPD-like behaviors in a breeding group provided a unique and controlled environment to assess the nature of the maternal-child relationship as the mother-infant bond forms the fundamental foundation of well-organized social groups. In breeding groups of cynomolgus monkeys, there are two predominantly observable forms of mother-infant interactions: (1) mothers holding infants and (2) mothers grooming infants (Nakamichi et al, 1990). Conversely, poor maternal relationships have been demonstrated where the maternal monkeys abuse and neglect their babies, which resulted in decreased times in holding and grooming their infants (Maestripieri & Carroll, 2000; Nakamichi et al, 1990). Moreover, grooming interactions have been correlated with levels of stress. While, rodent studies have shown that grooming positively correlated with the state of stress (Kalueff & Tuohimaa, 2005), grooming by maternal primates paralleled with reduced levels of stress (Nakamichi, 2003; Shutt et al, 2007). Thus, by observing behavioral depression postpartum in a breeding group of adult female cynomolgus monkeys, new knowledge can be gained into abnormal parental behaviors and physiological stress states that will provide vital clues for investigating PPD in the future.
METHODS
Subjects
Ten healthy adult female cynomolgus monkeys (5.80±0.79 years, mean±SE) were chosen which selected randomly from 62 healthy adult female cynomolgus monkeys we observed in the preliminary study, and were distributing in 8 breeding groups (population numbers ranked from 22 to 29 in each group, where two males were included). Each of the subjects was multiparous with only one infant being reared with their colonies. The colonies were housed in a cage with a roof, which was divided equally into three connected quarters (each of the quarters measured 3.0 m×3.3 m×2.9 m). All the subjects were provided commercial biscuits twice a day and fruit & vegetables once daily. The animals had tap water available ad libitum. All animal procedures were carried out in accordance with the Kunming Institute of Zoology Animal Care and Use Committee and with the National Institute of Health's (USA) Guide for the Care and Use of Laboratory Animals.
Group classification and experimental design
All animals were identified for their exhibition of huddle behavior. The huddle behavior is a typical depressive-like behavior that is commonly used as an indicator of behavioral depression (Chilton et al, 2011). Behavior recordings of all subjects were performed immediately on the first day after giving birth and then for 14 consecutive days of postpartum observation in order to calculate the score of the huddling behavior and other behaviors, e.g., locomotive activity, stressful events, and parental behaviors. As a result, subjects were classified into two groups: (1) a behavioral depression postpartum (BDP) group (n=6) and (2) a non-behavioral depression postpartum control (control) group (n=4). On To evaluate cortisol levels, on the 15th postpartum day, hair samples were obtained.
Behavior sampling
Behavior data were collected using a high-resolution portable digital video camera fixed on a tripod. For habituating the animals to the recording activity, the observer entered the monkey farm at least two weeks before recording began; and while videotaping, the observation site was set up on a platform as far away as possible from the cage to prevent disturbing the monkeys. Two 25-minute recordings were collected on each day of observation during two separate time windows: 0900h-1230h and 1400h-1730h, respectively. All data recordings were stored on a hard disk before being analyzed blindly by two trained technicians; the two viewers reached a consensus to the behavioral classification, and all their analyses were done on the computer.
Behavioral categories and definitions
In the present study, four behavioral categories were measured in the subjects: huddling, locomotive activity, stressful events, and parental behavior. First, both depressed macaques and MDD patients have been reported to exhibit an increased huddle time (Canales et al, 2010; Harlow and Suomi, 1974) and the huddle behavior, specifically, predicted behavioral depression in cynomolgus monkeys. The huddle behavior is characteristic of head flexion and thoracic kyphosis (Shively et al, 2005). Second, locomotive activity was appointed to evaluate general physiological functions of the subjects after child birth. Lastly, stressful events and parental behavior were analyzed in both BDP group and control group as incremental stressful life events and disrupted maternal relationships have been associated with the onset of PPD (Field, 2010; Hammen, 2005). In general, stressful events were classified as either aggressive behaviors in the cynomolgus monkeys, which included stare, threat, open-mouth threat, chase, displace, bite, and slap/grab; or submissive behaviors, which included lip smack, grimace, submissive present, move away, scream, scream threat, crouch, flee (Shively et al, 2005). The maternal parental relationship was monitored by counting mother to infant grooming and holding times.
Hair sampling and cortisol RIA assay
Hair sampling was conducted on the first day of the third postpartum week, following the completion of the behavioral recording. The animals were captured by an experienced technician with a net and removed from the colonies. Then, hair samples were taken from their backs of the subjects using a pair of scissors under manual restraint. The hair samples were placed into a small punch of aluminum foil for protection and storage (Davenport et al, 2006; Wennig, 2000). As mentioned before, the cortisol levels in the hair samples would be used to evaluate approximate amounts of accumulated stress experienced by the monkeys in the study over last three months prior to delivery (Russell et al, 2012).
Before being assayed, cortisol was extracted from the hair sample as detailed previously (Davenport et al, 2006; Feng et al, 2011). In brief, approximately 500 mg of hair was washed twice in 10 mL isopropanol for 3 minutes to remove surface contaminants, dried at 37 ℃ for 8 hours, and then pulverized using a Retsch ball mill (Retsch M400) at 26 Hz for 2.5 minutes. Afterwards, 400 mg of the powderized hair was weighed precisely and incubated in 8 mL of methanol at room temperature for 24 h with a slow rotation to extract the cortisol. The samples were then centrifuged at 8 000 rpm for 5 minutes and 4 mL of the supernatant was pipetted into a centrifuge tube and dried under a stream of nitrogen gas. The precipitate was reconstituted in 0.25 mL of PBS and stored at -20 ℃.
All measurements of cortisol were performed with a commercially available radioimmunoassay kit (Cortisol RIA DSL-2000, America) at the Radioimmuno-laboratory of Yunnan Second People's Hospital. The cortisol extraction and RIA assay were performed under a double-blind procedure, and each hair sample was tested three times with the mean of the three hair cortisol values used to diminish measurement error.
Data analysis
Huddle behavior, locomotive activity, stressful events, and parental behaviors were all quantified for duration (in seconds) and/or frequency of occurrence. The data was analyzed with the Mann-Whitney U (Wilcoxon rank-sum) test to compare differences between the BDP and control group for each behavioral measure. For all analyses, significance was set at P < 0.05 and determined via two-tailed tests. All data analysis was done using SPSS 16.0 (SPSS Inc, Chicago, IL, USA).
RESULTS
Depressive-like behavior
The animals were grouped into a BDP group (n=6) and control group (n=4) based on their time spent in the huddle posture during the first two weeks postpartum. As shown in Figure 1A, the values of huddle time of the BDP monkeys were higher than the control monkeys over the first 6 days, but persistently dropped down over time. Despite this drop, the BDP monkeys still had higher huddle times than those of the control monkeys at each time point; until the last two time points (day13-14 postpartum) where the behaviors of both groups started to match and had relatively low huddle times (Figure 1A). On average, the BDP monkeys had significantly higher huddle times in comparison with the control monkeys (Figure 1B; 108.40±19.14 vs. 11.44±4.57, P < 0.001).
Figure 1.
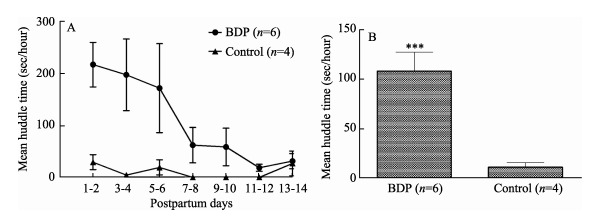
Time being spent in the huddle posture over the first two weeks postpartum in BDP monkeys (n=6) and control monkeys (n=4)
Locomotive activity
No significant differences were discovered for both locomotion duration (Figure 2A; 140.31±7.52 vs. 146.85± 9.077, P=0.23) and frequency (Figure 2B; 39.86±2.16 vs. 45.54±3.26, P=0.49) between BDP and control monkeys, suggesting that monkeys in the BDP group behaved as normally as their counterparts in the control group.
Figure 2.
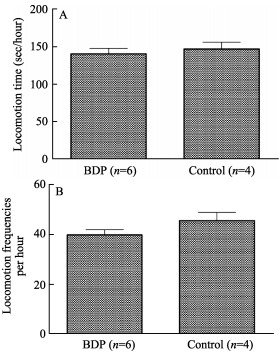
Comparison of locomotive activity between the BDP group (n=6) and the control group (n=4)
Stressful events and stress hormone levels
There were no statistically significant differences found between the BDP group and the control group for both stressful events experienced (Figure 3A; 14.71±1.40 vs. 15.56±1.75, P=0.58) and hair cortisol levels (Figure 3B; 28.62±4.66 vs. 32.01±4.57, P=0.52). This suggests that the social stress suffered by both the BDP monkeys and the control monkeys were at similar levels following child birth and during pregnancy.
Figure 3.

Measures of stressful events experienced (A) and hair cortisol levels (stress hormone) (B) in the BDP group (n=6) and control group (n=4) monkeys
Maternal relationship
Two kinds of recognizable behaviors were used to evaluate the maternal relationship: grooming infants and holding infants. Observations revealed that there were no differences in the "holding" between the BPD monkeys and the control monkeys (Figure 4A; 59.49±0.30 vs. 59.85±0.09, P=0.50). However, the BPD monkeys demonstrated a significantly higher amount of time performing the "grooming" behavior compared with control monkeys (Figure 4B; 91.81±12.17 vs. 65.64±15.56, P < 0.001). Specifically, as shown in Figure 4C, the values of groom time of both groups stayed even in total before the third time point (day 5-6 postpartum), and were both slightly declining over this period. Afterwards, the BDP monkeys started to spend more time grooming than those of the control monkeys over the last 8 days, in spite of almost having the same values of groom time with the control monkeys at the fifth time point (day 9-10 postpartum) (Figure 4C). Overall, this suggested there was an abnormal mother-infant relationship in the BPD monkeys.
Figure 4.
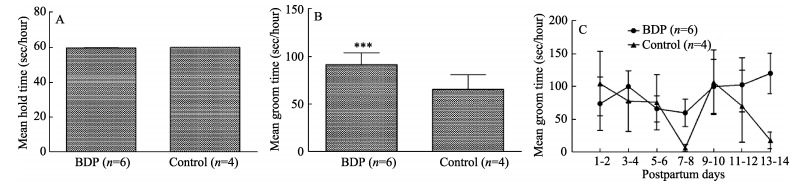
Analyses of the maternal relationship in the BDP monkeys (n=6) and control monkeys (n=4)
DISCUSSION
To the best our knowledge, this report is the first on a natural model of behavioral postpartum depression in non-human primates which is similar to PPD in humans. The BDP-like patterns in adult female cynomolgus monkeys reflected a miniature version of PPD (Epperson & Ballew, 2006) with onset occurring on the first day of delivery and lasting a two-week duration. This study used the huddle behavior as the core indicator of behavioral depression in primates. While this behavior is not the only candidate depression measure available, due to the recognition and effectiveness of the huddle posture measurement provides adequate utilization for measuring behavioral depression in the development of primate depression models (Chilton et al, 2011; Shively et al, 2002, 2005, 2006; Strome et al, 2002). Furthermore, the BDP monkeys demonstrated no differences in locomotive activity in comparison with the control monkeys. The locomotive activity of animals has been found to be affected when parturition is accompanied by loss of blood. The fact that BDP monkeys and control monkeys had similar locomotive behaviors suggested that blood hemorrhaging during delivery was not a confounding factor in the occurrence of the behavioral depression in the postpartum adult female cynomolgus monkeys observed here. All told, these findings represent the first demonstration of behavioral postpartum depression in primates.
In a stable society of most macaques, the behavioral and physiological stress levels of individuals in groups can be predicted by their social status, meaning that subordinate animals experience more stressors such as stressful events and stress hormone responsiveness (Michopoulos et al, 2012; Sapolsky, 2005). In the present study, no associations were found in the BDP group with stressful events and hair cortisol levels, suggesting that stress played little role in the behavioral postpartum depression we observed. By extension, if the stressful events were not an external biological factor in the development of the BDP-like patterns in the adult female cynomolgus monkeys, they may instead be an indicator of a balanced social status in both the BDP and control groups, which corresponded to no differences in stress hormone levels as well (Michopoulos et al, 2012; Shively et al, 2005). This finding contradicts several previous studies in which the presumptive manifestation of behavioral depression in the adult female cynomolgus monkeys positively correlated with stressful events and stress hormone responsiveness (Shively et al, 2005). Therefore, it is plausible that the underlying mechanisms of the behavioral depression phenomena related to either postpartum or stress experiences are significantly different, with wide implications on the elucidation of the basis of PPD and MDD, respectively (Douma et al, 2005; Hammen, 2005).
In addition, the BDP monkeys in this study were found to spend more time grooming their infants than the control monkeys, despite no significant differences being found in the time spent holding infants in both groups. These findings may suggest that "grooming" behavior was more vulnerable to the occurrence of behavioral depression in postpartum adult female cynomolgus monkeys, while the "holding" behavior might reflect a manner of infant abuse and neglect that is separate from the BDP. In fact, infant abuse and neglect by the adult female cynomolgus monkeys is considered a severe form of maltreatment, where as the "grooming" infant behavior represents a mild form of maternal behaviors (Carroll & Maestripieri, 1998; Tsuchida et al, 2008). In a common sense, "grooming" has been understood as a socially affiliative behavior, and has been considered in correlation with lower physiological stress levels (Nakamichi, 2003; Shutt et al, 2007). However, the increased "grooming" behavior in the BDP monkeys does not explicitly correspond to the null differences in stressful events and stress hormone levels in the monkeys as referred to above. The "infant grooming" observed here may be different than the normalized "social grooming" observed in monkey social hierarchies, where the "infant grooming" may have reflected a state of compensation for a certain kind of psychopathological condition associated with the behavioral postpartum depression. In any case, the abnormal parenting relationship in the BDP monkey may be an important behavioral dysfunction in this natural model that may be related to PPD in humans. Cumulative evidence has shown that most PPD patients have poor maternal relationships with their children, which can lead to adverse effects on the behavioral, cognitive, emotional development of their infants (Field, 2010; Goodman et al, 2011), such that this model may be furthered to investigate both physiological and behavioral changes to infant monkeys in response to BDP in maternal monkeys.
Furthermore, this natural model to in the adult female cynomolgus monkeys can be expanded to examine other precipitating factors contributing to behavioral postpartum depression in the monkeys. Previous studies in PPD patients and in rodent systems have provided many clues to what underlying physiological factors are associated with BDP symptoms, such as ovarian hormone withdrawal (Brummelte & Galea, 2010; Douma et al, 2005; Osterlund, 2010). Human clinical trials have found that the ovarian hormone withdrawal can trigger depressive symptoms in susceptible women with a history of PPD (Bloch et al, 2000). Meanwhile, in rodents a hormone-stimulated pregnancy/hormone withdrawal protocol was developed to investigate this issue. The research found that behavioral depression was predicted in the withdrawal phase (Galea et al, 2001; Green et al, 2009; Stoffel and Craft, 2004; Suda et al, 2008). Similar postpartum hormonal changes have been found in cynomolgus macaques. Goodman & Hodgen (1978) reported that progesterone levels dropped over the first two weeks postpartum and then leveled off following this period. Likewise, estradiol levels followed a similar pattern to progesterone, except the lower levels lasted less than one week postpartum. It then stands to reason that the BDP-like patterns witnessed during this study on adult female cynomolgus monkeys may be associated pathologically with ovarian hormones withdrawal. Going forward, this natural PPD model will provide an adequate system to investigate the correlation between ovarian hormone withdrawal in depressed and non-depressed monkeys, as well as investigate the uses of hormone replacement therapy and/or novel drug therapeutics in the treatment of postpartum depression.
In summary, this novel natural model is attempted to define PPD in non-human primates. The BDP-like patterns occurring spontaneously in the postpartum adult female cynomolgus macaque show a much closer relationship to PPD than any other existing model (Brummelte & Galea, 2010). This suggests that it will be a very useful tool to systematically explore the etiology, treatment, and prevention of PPD in future.
Funding Statement
This study was supported by National Natural Science Foundation of China (31271167, 81271495, 31070963, 30921064), the Yunnan Provincial Project to attract ore-hundred exceptional talents from Overseas
REFERENCES
- 1. Abbott DH, Keverne EB, Bercovitch FB, Shively CA, Mendoza SP, Saltzman W, Snowdon CT, Ziegler TE, Banjevic M, Garland T, Sapolsky RM. 2003. Are subordinates always stressed? A comparative analysis of rank differences in cortisol levels among primates. Hormones and Behavior, 43 (1): 67- 82. [DOI] [PubMed] [Google Scholar]
- 2. Bloch M, Schmidt PJ, Danaceau M, Murphy J, Nieman L, Rubinow DR. 2000. Effects of gonadal steroids in women with a history of postpartum depression. American Journal of Psychiatry, 157 (6): 924- 930. [DOI] [PubMed] [Google Scholar]
- 3. Bowlby J. 1962. Attachment and Loss. New York: Basic Books, [Google Scholar]
- 4. Brummelte S, Galea LAM. 2010. Depression during pregnancy and postpartum: contribution of stress and ovarian hormones. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 34 (5): 766- 776. [DOI] [PubMed] [Google Scholar]
- 5. Burke HM, Davis MC, Otte C, Mohr DC. 2005. Depression and cortisol responses to psychological stress: a meta-analysis. Psychoneuroendocrinology, 30 (9): 846- 856. [DOI] [PubMed] [Google Scholar]
- 6. Canales JZ, Cordas TA, Fiquer JT, Cavalcante AF, Moreno RA. 2010. Posture and body image in individuals with major depressive disorder: a controlled study. Revista Brasileira de Psiquiatria, 32 (4): 375- 380. [DOI] [PubMed] [Google Scholar]
- 7. Carroll BJ, Curtis GC, Davies BM, Mendels J, Sugerman AA. 1976. Urinary free cortisol excretion in depression. Psychological Medicine, 6 (1): 43- 50. [DOI] [PubMed] [Google Scholar]
- 8. Carroll KA, Maestripieri D. 1998. Infant abuse and neglect in monkeys-A discussion of definitions, epidemiology, etiology, and implications for child maltreatment: reply to Cicchetti (1998) and Mason (1998). Psychological Bulletin, 123 (3): 234- 237. [DOI] [PubMed] [Google Scholar]
- 9. Cattet MRL, Christison K, Caulkett NA, Stenhouse GB. 2003. Physiologic responses of grizzly bears to different methods of capture. Journal of Wildlife Diseases, 39 (3): 649- 654. [DOI] [PubMed] [Google Scholar]
- 10. Chilton FH, Lee TC, Wilard SL, Ivester P, Sergeant S, Register TC, Shively CA. 2011. Depression and altered serum lipids in cynomolgus monkeys consuming a Western diet. Physiology & Behavior, 104 (2): 222- 227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Constable S, Parslow A, Dutton G, Rogers T, Hogg C. 2006. Urinary cortisol sampling: a non invasive technique for examining cortisol-concentrations in the Weddell seal, Leptonychotes weddellii. Zoo Biology, 25 (2): 137- 144. [Google Scholar]
- 12. Davenport MD, Tiefenbacher S, Lutz CK, Novak MA, Meyer JS. 2006. Analysis of endogenous cortisol concentrations in the hair of rhesus macaques. General and Comparative Endocrinology, 147 (3): 255- 261. [DOI] [PubMed] [Google Scholar]
- 13. Douma SL, Husband C, O'Donnell ME, Barwin BN, Woodend AK. 2005. Estrogen-related mood disorders. Advances in Nursing Science, 28 (4): 364- 375. [DOI] [PubMed] [Google Scholar]
- 14. England R. 1994. Infant development and management of infant problems in a family setting. Australian Family Physician, 23 (10): 1877- 1882. [PubMed] [Google Scholar]
- 15. Epperson CN, Ballew J. 2006. Postpartum depression. In: Current Clinical Practice: Psychiatric Disorders in Pregnancy and the Postpartum: Principles and Treatment. Totowa: Humana Press, 41- 81. [Google Scholar]
- 16. Feng XL, Wang L, Yang SC, Qin DD, Wang JH, Li CL, Lü LB, Ma YY, Hu XT. 2011. Maternal separation produces lasting changes in cortisol and behavior in rhesus monkeys. Proceedings of the National Academy of Sciences of the United States of America, 108 (34): 14312- 14317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Field T. 2010. Postpartum depression effects on early interactions, parenting, and safety practices: a review. Infant Behavior and Development, 33 (1): 1- 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Flores DL, Hendrick VC. 2002. Etiology and treatment of postpartum depression. Current Psychiatry Reports, 4 (6): 461- 466. [DOI] [PubMed] [Google Scholar]
- 19. Folkman S, Lazarus RS, Dunkel-Schetter C, Delongis A, Gruen RJ. 1986. Dynamics of a stressful encounter: Cognitive appraisal, coping, and encounter outcomes. Journal of Personality and Social Psychology, 50 (5): 992- 1003. [DOI] [PubMed] [Google Scholar]
- 20. Friedman SH, Resnick PJ. 2009. Postpartum depression: an update. Women's Health, 5 (3): 287- 295. [DOI] [PubMed] [Google Scholar]
- 21. Galea LAM, Wide JK, Barr AM. 2001. Estradiol alleviates depressive-like symptoms in a novel animal model of post-partum depression. Behavioural Brain Research, 122 (1): 1- 9. [DOI] [PubMed] [Google Scholar]
- 22. Gard PR, Handley SL, Parsons AD, Waldron G. 1986. A multivariate investigation of postpartum mood disturbance. The British Journal of Psychiatry, 148 (5): 567- 575. [DOI] [PubMed] [Google Scholar]
- 23. Ghubash R, Abou-Saleh MT. 1997. Postpartum psychiatric illness in Arab culture: prevalence and psychosocial correlates. The British Journal of Psychiatry, 171 (1): 65- 68. [DOI] [PubMed] [Google Scholar]
- 24. Goodman RL, Hodgen GD. 1978. Post partum patterns of circulating FSH, LH, prolactin, estradiol, and progesterone in nonsuckling cynomolgus monkeys. Steroids, 31 (5): 731- 744. [DOI] [PubMed] [Google Scholar]
- 25. Goodman SH, Rouse MH, Connell AM, Broth MR, Hall CM, Heyward D. 2011. Maternal depression and child psychopathology: A meta-analytic review. Clinical Child and Family Psychology Review, 14 (1): 1- 27. [DOI] [PubMed] [Google Scholar]
- 26. Green AD, Barr AM, Galea LAM. 2009. Role of estradiol withdrawal in 'anhedonic' sucrose consumption: a model of postpartum depression. Physiology & Behavior, 97 (2): 259- 265. [DOI] [PubMed] [Google Scholar]
- 27. Groer MW, Morgan K. 2007. Immune, health and endocrine characteristics of depressed postpartum mothers. Psychoneuroendocrinology, 32 (2): 133- 139. [DOI] [PubMed] [Google Scholar]
- 28. Hammen C. 2005. Stress and depression. Annual Review of Clinical Psychology, 1 293- 319. [DOI] [PubMed] [Google Scholar]
- 29. Harlow HF, Suomi SJ. 1974. Induced depression in monkeys. Behavioral Biology, 12 (3): 273- 296. [DOI] [PubMed] [Google Scholar]
- 30. Harris B, Johns S, Fung H, Thomas R, Walker R, Read G, Riad-Fahmy D. 1989. The hormonal environment of post-natal depression. The British Journal of Psychiatry, 154 (5): 660- 667. [DOI] [PubMed] [Google Scholar]
- 31. Harris B, Lovett L, Smith J, Read G, Walker R, Newcombe R. 1996. Cardiff puerperal mood and hormone study. III. Postnatal depression at 5 to 6 weeks postpartum, and its hormonal correlates across the peripartum period. The British Journal of Psychiatry, 168 (6): 739- 744. [DOI] [PubMed] [Google Scholar]
- 32. Ising M, Kunzel HE, Binder EB, Nickel T, Modell S, Holsboer F. 2005. The combined dexamethasone/CRH test as a potential surrogate marker in depression. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 29 (6): 1085- 1093. [DOI] [PubMed] [Google Scholar]
- 33. Jolley SN, Elmore S, Barnard KE, Carr DB. 2007. Dysregulation of the hypothalamic-pituitary-adrenal axis in postpartum depression. Biological Research for Nursing, 8 (3): 210- 222. [DOI] [PubMed] [Google Scholar]
- 34. Keay JM, Singh J, Gaunt MC, Kaur T. 2006. Fecal glucocorticoids and their metabolites as indicators of stress in various mammalian species: a literature review. Journal of Zoo and Wildlife Medicine, 37 (3): 234- 244. [DOI] [PubMed] [Google Scholar]
- 35. Kalin NH, Shelton SE. 2003. Nonhuman primate models to study anxiety, emotion regulation, and psychopathology. Annals of the New York Academy of Sciences, 1008 189- 200. [DOI] [PubMed] [Google Scholar]
- 36. Koolhaas JM, Korte SM, De Boer SF, Van Der Vegt BJ, Van Reenen CG, Hopster H, De Jong IC, Ruis MAW, Blokhuis HJ. 1999. Coping styles in animals: current status in behavior and stress-physiology. Neuroscience and Biobehavioral Reviews, 23 (7): 925- 936. [DOI] [PubMed] [Google Scholar]
- 37. Liu CH, Tronick E. 2013. Rates and predictors of postpartum depression by race and ethnicity: Results from the 2004 to 2007 New York City PRAMS survey (Pregnancy Risk Assessment Monitoring System). Maternal and Child Health Journal, 17 (9): 1599- 1610. [DOI] [PubMed] [Google Scholar]
- 38. Maestripieri D, Carroll KA. 2000. Causes and consequences of infant abuse and neglect in monkeys. Aggression and Violent Behavior, 5 (3): 245- 254. [Google Scholar]
- 39. McClure DJ. 1966. The diurnal variation of plasma cortisol levels in depression. Journal of Psychosomatic Research, 10 (2): 189- 195. [DOI] [PubMed] [Google Scholar]
- 40. Michopoulos V, Higgins M, Toufexis D, Wilson ME. 2012. Social subordination produces distinct stress-related phenotypes in female rhesus monkeys. Psychoneuroendocrinology, 37 (7): 1071- 1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Millspaugh JJ, Washburn BE, Milanick MA, Beringer J, Hansen LP, Meyer TM. 2002. Non-invasive techniques for stress assessment in white-tailed deer. Wildlife Society Bulletin, 30 (3): 899- 907. [Google Scholar]
- 42. Nakamichi M. 2003. Age-related differences in social grooming among adult female Japanese monkeys (Macaca fuscata). Primates, 44 (3): 239- 246. [DOI] [PubMed] [Google Scholar]
- 43. Nakamichi M, Cho F, Minami T. 1990. Mother-infant interactions of wild-born, individually-caged cynomolgus monkeys (Macaca fascicularis) during the first 14 weeks of infant life. Primates, 31 (2): 213- 224. [Google Scholar]
- 44. Nierop A, Bratsikas A, Zimmermann R, Ehlert U. 2006. Are stress-induced cortisol changes during pregnancy associated with postpartum depressive symptoms. Psychosomatic Medicine, 68 (6): 931- 937. [DOI] [PubMed] [Google Scholar]
- 45. O'Hara MW. 1987. Post-partum "blues," depression, and psychosis: A review. Journal of Psychosomatic Obstetrics and Gynecology, 7 (3): 205- 227. [Google Scholar]
- 46. O'Hara MW, Swain AM. 1996. Rates and risk of postpartum depression: a meta-analysis. International Review of Psychiatry, 8 (1): 37- 54. [Google Scholar]
- 47. O'Hara MW, McCabe JE. 2013. Postpartum depression: current status and future directions. Annual Review of Clinical Psychology, 9 379- 407. [DOI] [PubMed] [Google Scholar]
- 48. Okano T, Nomura J. 1992. Endocrine study of the maternity blues. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 16 (6): 921- 932. [DOI] [PubMed] [Google Scholar]
- 49. Osterlund MK. 2010. Underlying mechanisms mediating the antidepressant effects of estrogens. Biochimica et Biophysica Acta, 1800 (10): 1136- 1144. [DOI] [PubMed] [Google Scholar]
- 50. Parker KJ, Schatzberg AF, Lyons DM. 2003. Neuroendocrine aspects of hypercortisolism in major depression. Hormones and Behavior, 43 (1): 60- 66. [DOI] [PubMed] [Google Scholar]
- 51. Pryce CR, Dettling AC, Spengler M, Schnell CR, Feldon J. 2004. Deprivation of parenting disrupts development of homeostatic and reward systems in marmoset monkey offspring. Biological Psychiatry, 56 (2): 72- 79. [DOI] [PubMed] [Google Scholar]
- 52. Russell E, Koren G, Rieder M, Van Uum S. 2012. Hair cortisol as a biological marker of chronic stress: Current status, future directions and unanswered questions. Psychoneuroendocrinology, 37 (5): 589- 601. [DOI] [PubMed] [Google Scholar]
- 53. Sapolsky RM. 2005. The influence of social hierarchy on primate health. Science, 308 (5722): 648- 652. [DOI] [PubMed] [Google Scholar]
- 54. Schüle C. 2007. Neuroendocrinological mechanisms of actions of antidepressant drugs. Journal of Neuroendocrinology, 19 (3): 213- 226. [DOI] [PubMed] [Google Scholar]
- 55. Shively CA. 1998. Social subordination stress, behavior, and central monoaminergic function in female cynomolgus monkeys. Biological Psychiatry, 44 (9): 882- 891. [DOI] [PubMed] [Google Scholar]
- 56. Shively CA, Laber-Laird K, Anton RF. 1997. Behavior and physiology of social stress and depression in female cynomolgus monkeys. Biological Psychiatry, 41 (8): 871- 882. [DOI] [PubMed] [Google Scholar]
- 57. Shively CA, Williams JK, Laber-Laird K, Anton RF. 2002. Depression and coronary artery atherosclerosis and reactivity in female cynomolgus monkeys. Psychosomatic Medicine, 64 (5): 699- 706. [DOI] [PubMed] [Google Scholar]
- 58. Shively CA, Register TC, Friedman DP, Morgan TM, Thompson J, Lanier T. 2005. Social stress-associated depression in adult female cynomolgus monkeys (Macaca fascicularis). Biological Psychology, 69 (1): 67- 84. [DOI] [PubMed] [Google Scholar]
- 59. Shively CA, Friedman DP, Gage HD, Bounds MC, Brown-Proctor C, Blair JB, Henderson JA, Smith MA, Buchheimer N. 2006. Behavioral depression and positron emission tomography-determined serotonin 1A receptor binding potential in cynomolgus monkeys. Archives of General Psychiatry, 63 (4): 396- 403. [DOI] [PubMed] [Google Scholar]
- 60. Shutt K, MacLarnon A, Heistermann M, Semple S. 2007. Grooming in Barbary macaques: better to give than to receive. Biology Letters, 3 (3): 231- 233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sibley CG, Ahlquist JE. 1987. DNA hybridization evidence of hominoid phylogeny: results from an expanded data set. Journal of Molecular Evolution, 26 (1-2): 99- 121. [DOI] [PubMed] [Google Scholar]
- 62. Sit DK, Wisner KL. 2009. Identification of postpartum depression. Clinical Obstetrics and Gynecology, 52 (3): 456- 468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Spitz RA, Wolf KA. 1946. Anaclitic depression: An inquiry into the genesis of psychiatric conditions in early childhood. The Psychoanalytic Study of the Child, 2 313- 342. [PubMed] [Google Scholar]
- 64. Stalder T, Steudte S, Alexander N, Miller R, Gao W, Dettenborn L, Kirschbaum C. 2012. Cortisol in hair, body mass index and stress-related measures. Biological Psychology, 90 (3): 218- 223. [DOI] [PubMed] [Google Scholar]
- 65. Stoffel EC, Craft RM. 2004. Ovarian hormone withdrawal-induced "depression" in female rats. Physiology & Behavior, 83 (3): 505- 513. [DOI] [PubMed] [Google Scholar]
- 66. Strome EM, Wheler GHT, Higley JD, Loriaux DL, Suomi SJ, Doudet DJ. 2002. Intracerebroventricular corticotropin-releasing factor increasees limbic glucose metabolism and has social context-dependent behavioral effects in nonhuman primates. Proceedings of the National Academy of Sciences of the United States of America, 99 (24): 15749- 15754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Suda S, Segi-Nishida E, Newton SS, Duman RS. 2008. A postpartum model in rat: behavioral and gene expression changes induced by ovarian steroid deprivation. Biological Psychiatry, 64 (4): 311- 319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Touma C, Palme R. 2005. Measuring fecal glucocorticoid metabolites in mammals and birds: the importance of validation. Annals of the New York Academy of Sciences, 1046 54- 74. [DOI] [PubMed] [Google Scholar]
- 69. Tsuchida J, Yoshida T, Sankai T, Yasutomi Y. 2008. Maternal behavior of laboratory-born, individually reared long-tailed macaques (Macaca fascicularis). American Association for Laboratory Animal Science, 47 (5): 29- 34. [PMC free article] [PubMed] [Google Scholar]
- 70. Van Esch E, Cline MJ, Buse E, Wood CE, De Rijk EPCT, Weinbauer GF. 2008. Summary comparison of female reproductive system in human and the cynomolgus monkey (Macaca fascicularis). Toxicologic Pathology, 36 171S- 172S. [Google Scholar]
- 71. Wennig R. 2000. Potential problems with the interpretation of hair analysis results. Forensic Science International, 107 (1-3): 5- 12. [DOI] [PubMed] [Google Scholar]
- 72. Whitten PL, Stavisky R, Aureli F, Rusell E. 1998. Response of fecal cortisol to stress in captive chimpanzees (Pan troglodytes). American Journal of Primatology, 44 (1): 57- 69. [DOI] [PubMed] [Google Scholar]
- 73. Willard SL, Shively CA. 2012. Modeling depression in adult female cynomolgus monkeys (Macaca fascicularis). American Journal of Primatology, 74 (6): 528- 542. [DOI] [PubMed] [Google Scholar]
- 74. Willner P. 1991. Animal models as simulations of depression. Trends in Pharmacological Sciences, 12 (4): 131- 136. [DOI] [PubMed] [Google Scholar]


