Abstract
In this study, we analyzed diffusion tensor imaging (DTI) results of brain white matter in rhesus macaques (Macaca mulatta) with four different parameter settings and found that the sequence A (b=1 000 s/mm2, spatial resolution=1.25 mm×1.25 mm× 1.25 mm, numbers of direction=33, NSA=3) and B (b=800 s/mm2, spatial resolution=1.25 mm×1.25 mm×1.25 mm, numbers of direction=33, NSA=3) could accurately track coarse fibers. The fractional anisotropy (FA) derived from sequence C (b=1 000s/mm2, spatial resolution=0.55 mm×0.55 mm×2.5 mm, direction number=33, NSA=3) was too fuzzy to be used in tracking white matter fibers. By comparison, the high resolution and the FA with high contrast of gray matter and white matter derived from sequence D (b=800 s/mm2, spatial resolution=1.0 mm×1.0 mm ×1.0 mm, numbers of direction=33, NSA=3) qualified in its application in tracking both thick and thin fibers, making it an optimal DTI setting for rhesus macaques.
Keywords: DTI, Whiter matter, Rhesus macaque
Neuropsychiatric disorders such as Parkinson’s syndrome and senile dementia remain global health problems worldwide, and are only expected to grow. In conducting research to gain a clearer picture of these disorders and developing novel therapeutics, animal models have proved critical. Due to their phylogenetic closeness to humans and to circumvent ethical issues, nonhuman primates in particular are widely used in the pathological and etiological researches of these diseases.
In recent years, magnetic resonance imaging (MRI) has become an important tool in brain diseases study because it is non-invasive and can be used to observe the chronic changes in brain function and morphology. Among the various forms of MRI, diffusion MRI, also referred to as diffusion tensor imaging (DTI) has been extraordinarily successful, especially in the study of brain anatomical connectivity. Molecular diffusion in tissues reflects interactions with many obstacles. Therefore, water molecule diffusion patterns can reveal microscopic details about tissue architecture. The neural axons of the white matter in the brain have an internal fibrous structure analogous to the anisotropy of certain crystals. Water will then diffuse more rapidly in 1 the direction aligned with the internal structure. This means that the measured rate of diffusion will differ depending on the observing direction. The neural tract directional information derived from DTI, are sufficient to compute the diffusion tensor, and then from the diffusion tensor, diffusion anisotropy measures such as the fractional anisotropy (FA), can be computed (Kubicki et al, 2002). Thus the principal direction of the diffusion tensor can be used to infer the white-matter connectivity of the brain (Behrens et al, 2003; Schmahmann et al, 2007).
The brain volume of humans is 1, 400 mL, but is only 100 mL in monkeys. Because the brain volume is important to image quality, scanning parameters applied to humans cannot be used directly on monkeys. Yet the lack of normative descriptions has limited the application of DTI on monkeys. Currently, only a few research institutions have dedicated MRI scanners for animals and the clinical MRI is still widely in use. In this study, four different parameter settings were compared by performing the PHILIPS Achieve 3.0T MRI on rhesus macaques (Macaca mulatta) to confirm the optimal settings.
MATERIALS AND METHODS
Experimental animals
Rhesus macaques used in this study were 1.5–8 years old and weighed 3.2–6.8 kg (n=30, 24 males, 6 females). All monkeys were obtained from the Animal Center of Kunming Institute of Zoology, CAS. The animals were habituated for at least four weeks in a temperature-controlled (21) colony room with food ℃ and water available ad libitum using a 12h:12h (light : dark) schedule (white lights on: 8:00–20:00). Experimental protocols were consistent with the Society for Neuroscience and National Institute of Health guidelines for the humane use and care of animals.
Experiment procedure
Anesthesia
Subjects were deprived of water 12 hours before the experiment. Prior to the scanning, atropine (i.m, 0.2−0.5 mg/kg, Batch Number: 120502, Shanghai Harvest Pharmaceutical Co., LTD.) was injected to avoid vomiting or oral secretions blocking airways. Following with the onset of preanesthetic ketamine (i.m, 10 mg/kg, Batch Number: KH121011, Jiangsu Hengrui Medicine Co., LTD), subjects were maintained at deep anesthesia with amyl sodium pentobarbital (i.m, 35 mg/kg, Batch Number: WS20110112, Shanghai Westang Bio-Tech Co., LTD).
DTI image acquisition
All subjects were scanned on a PHILIPS 3.0T (Achieve) superconducting MRI scanner by referring the knee coil as the scan coil. The head of subject was fixed with a sponge to reduce noise and prevent head shaking. Each monkey received at least two types of the acquisition sequences. Single-shot echo planar imaging (EPI) combined with parallel acquisition techniques were used in the axial DTI data acquisition. Each monkey was scanned by four different scanning parameter settings, and all scans were conducted by a same MRI technician.
Sequence A: b=1 000 s/mm2, resolution=1.25 mm× 1.25 mm×1.25 mm, numbers of direction=33, numbers of signal acquisition (NSA)=3;
Sequence B: b=800 s/mm2, resolution=1.25 mm× 1.25 mm×1.25 mm, numbers of direction=33, NSA=3;
Sequence C: b=1 000 s/mm2, resolution= 0.55 mm× 0.55 mm×2.5 mm, numbers of direction=33, NSA=3;
Sequence D: b=800 s/mm2, resolution=1.0 mm× 1.0 mm×1.0 mm, numbers of direction=33, NSA=3.
Other parameters: frequency direction (FOV)=100, time echo (TE)=shortest, time repetition (TR)=shortest, scanning layers=48-60, flip angle=90 ℃, scanning time= 12-22 min.
Statistical analysis
All values were presented as mean±SD, with P < 0.05 was taken as statistically significant. Statistical analyses were performed by one-way ANOVA. When statistical differences were revealed by ANOVA, the statistical post-hoc analysis of LSD was applied.
RESULTS
Effects of different parameters on the image quality
By comparing the image quality and tracking data, we found that the sequence A performed well in the accurate tracking of thick fibers, e.g. white matter fibers (Figure 3). No significant difference was found between the results obtained from sequence A and B. Relative to the sequence A, sequence D was high in noise level, because this sequence has a relatively high resolution, but the contrast of FA in 1 mm is higher than that in 1.25 mm (FA of gray matter to FA of white matter). This sequence can track both the thick and the fine fibers (Figure 2), so the white matter can be more abundantly displayed. In sequence C which was high in resolution, but significantly low in the signal to noise ratio (SNR), although the b value was reduced to 700, the display of FA values was still completely fuzzy (Figure 1) and cannot be used to track white matter fiber. Thus, our results indicate that the sequence D is the optimal setting to acquire DTI data in rhesus macaques.
Figure 3.
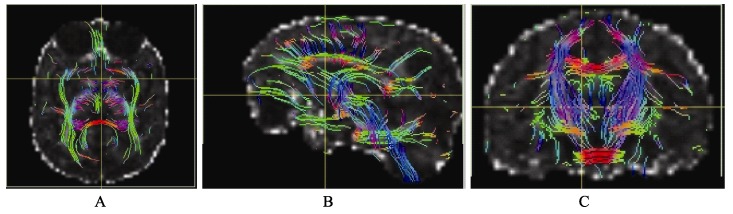
Transverse (A), sagittal (B) and coronal DTI tractography based on sequence A
Figure 2.
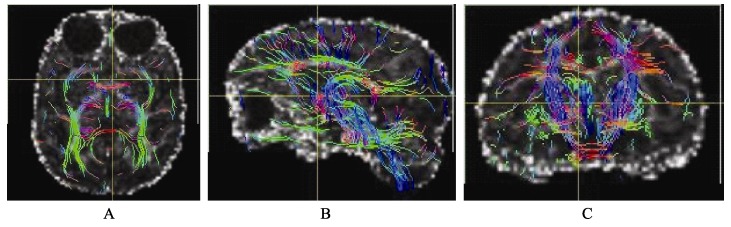
Transverse (A), sagittal (B) and coronal DTI tractography based on sequence D
Figure 1.
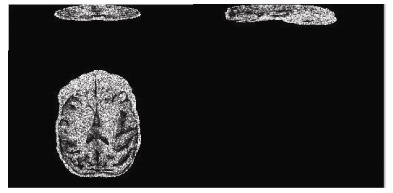
Original DTI images based on sequence C
White matter growth in baby monkeys
The DTI tractography of white matter growth in four baby monkeys (from 6 to 16 months after birth) indicates that the myelination of the corpus callosum is faster in 16-month-old subject than that in 6-month and 8-monthold subjects, whereas, the myelination of the prefrontal cortex is relatively slow (Figure 4).
Figure 4.
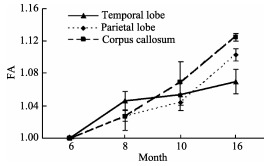
Myelination in baby monkeys
DISCUSSION
Spatial resolution, b value and motivate frequency are critical for DTI image quality and fiber tracking (Kim et al, 2006; Liu et al, 2009; Oouchi et al, 2007; Roebroeck et al, 2008). The significant difference in brain volume of monkeys and humans determines that high spatial resolution is necessary to image sophisticated brain structures in monkeys.
The resolution level of 2 mm×2 mm×2 mm or even higher commonly used in humans may induce a signifycant partial volume effect in monkeys (Alexander et al, 2001), and thereafter, increase errors in FA and MD (average diffusion coefficient) values (Kim et al, 2006; Papanikolaou et al, 2006). Thus, with a certain SNR, high spatial resolution should be applied to reduce the partial volume effect. Moreover, the higher the resolution is, the better the white matter fibers can be displayed.
In this study, with the resolution of 1.25 mm× 1.25 mm×1.25 mm, the SNR and contrast were good, the scan time was acceptable, but only the thick white matter fibers were well shown, whereas, with the resolution of 1.0 mm×1.0 mm×1.0 mm more white matter fibers were shown. However, due to the EPI sequence we used in DTI, the high resolution had remarkably reduced the SNR of the obtained image, therefore, it is necessary to keep the spatial resolution at certain level. With the resolution of 0.55 mm×0.55 mm×2.5 mm (Yundi et al, Shi et al, 2013), neither the image contrast or SNR were too low to infer FA, nor the white matter fiber direction could be tracked.
In general, the higher the b value, the more sensitive the DTI is in the differences in proton diffusion rate but meanwhile, the signal is quite reduced and less informative with lower SNR. To obtain a relatively good image quality and tracking, b=1 000 s/mm2 is the most widely used setting in DTI. Naganawa et al (2004) reported that good tracking can also be realized at b= 700 s/mm2, In our study, no significant differences in SNR and contrast were found between sequence A (b=1000 s/mm2) and B (b=800 s/mm2).
The diffusion gradient directions and image sensitivity are not always positively correlated in a linear way. Jones et al (2004) showed that qualified FA and MD values could be obtained at a direction of 20−30. In this study, within an acceptable time range, a high diffusion gradient direction (33) was used to improve SNR. Keeping subjects under anaesthetic during the whole procedure requires brief scanning time. Among all the parameters, spatial resolution is the most critical one affects scanning time, e.g. in this study the scanning time at the resolution of 1.0 mm×1.0 mm×1.0 mm almost doubled as that at a resolution of 1.25 mm×1.25 mm×1.25 mm (Table 1). Because the increasing of NSA significantly prolongs scanning time, so in this study, to get high SNR while also keeping the scanning time within an acceptable range, we set NSA=3. In this study, all the 30 monkeys were under anaesthesia during the scanning, indicating that the scanning time we used meets the requirement of anesthesia.
Table 1.
Effects of different parameters on scanning time
| b value (s/mm2) | Resolution(mm3) | NSA and the corresponding scanning time (min) | ||
| NSA=2 | NSA=3 | NSA=4 | ||
| 1 000 | 1.25×1.25×1.25 | 7.59 | 12.59 | 17.41 |
| 800 | 1.0×1.0×1.0 | 14 | 21.32 | 28.37 |
| 800 | 1.25×1.25×1.25 | 7.21 | 12.16 | 16.43 |
| 1 000 | 1.0×1.0×1.0 | 14.23 | 22.17 | 29.39 |
In summary, our results indicate that resolution= 1.0 mm×1.0 mm×1.0 mm, b=800 and NSA=3 are the optimal setting to obtain satisfactory SNR and contrast, as well as the thereafter fiber tracking and FA value calculation.
Funding Statement
This work was supported by the 973 Program (2012CBB25503, 2011CB707800)
REFERENCES
- 1. Alexander AL, Hasan KM, Lazar M, Tsuruda JS, Parker DL. 2001. Analysis of partial volume effects in diffusion-tensor MRI. Magnetic Resonance in Medicine, 45 (5): 770- 780. [DOI] [PubMed] [Google Scholar]
- 2. Behrens TEJ, Johansen-Berg H, Woolrich MW, Smith SM, Wheeler-Kingshott CAM, Boulby PA, Barker GJ, Sillery EL, Sheehan K, Ciccarelli O, Thompson AJ, Brady JM, Matthews PM. 2003. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nature Neuroscience, 6 (7): 750- 757. [DOI] [PubMed] [Google Scholar]
- 3. Jones DK. 2004. The effect of gradient sampling schemes on measures derived from diffusion tensor MRI: a Monte Carlo study. Magnetic Resonance in Medicine, 51 (4): 807- 815. [DOI] [PubMed] [Google Scholar]
- 4. Kim M, Ronen I, Ugurbil K, Kim DS. 2006. Spatial resolution dependence of DTI tractography in human occipito-callosal region. Neuroimage, 32 (3): 1243- 1249. [DOI] [PubMed] [Google Scholar]
- 5. Kubicki M, Westin CF, Maier SE, Mamata H, Frumin M, Ersner-Hershfield H, Kikinis R, Jolesz FA, Mccarley R, Shenton ME. 2002. Diffusion tensor imaging and its application to neuropsychiatric disorders. Harvard Review of Psychiatry, 10 (6): 324- 336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu XX, Zhu T, Gu TL, Zhong JH. 2009. A practical approach to in vivo high-resolution diffusion tensor imaging of rhesus monkeys on a 3-T human scanner. Magnetic Resonance Imaging, 27 (3): 335- 346. [DOI] [PubMed] [Google Scholar]
- 7. Naganawa S, Koshikawa T, Kawai H, Fukatsu H, Ishigaki T, Maruyama K, Takizawa O. 2004. Optimization of diffusion-tensor MR imaging data acquisition parameters for brain fiber tracking using parallel imaging at 3 T. European Radiology, 14 (2): 234- 238. [DOI] [PubMed] [Google Scholar]
- 8. Oouchi H, Yamada K, Sakai K, Kizu O, Kubota T, Ito H, Nishimura T. 2007. Diffusion anisotropy measurement of brain white matter is affected by voxel size: underestimation occurs in areas with crossing fibers. AJNR: American Journal of Neuroradiology, 28 (6): 1102- 1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Papanikolaou N, Karampekios S, Papadaki E, Malamas M, Maris T, Gourtsoyiannis N. 2006. Fractional anisotropy and mean diffusivity measurements on normal human brain: comparison between low- and high- resolution diffusion tensor imaging sequences. European Radiology, 16 (1): 187- 192. [DOI] [PubMed] [Google Scholar]
- 10. Roebroeck A, Galuske R, Formisano E, Chiry O, Bratzke H, Ronen I, Kim DS, Goebel R. 2008. High-resolution diffusion tensor imaging and tractography of the human optic chiasm at 9. 4 T. Neuroimage, 39 (1): 157- 168. [DOI] [PubMed] [Google Scholar]
- 11. Schmahmann JD, Pandya DN, Wang R, Dai G, D'Arceuil HE, De Crespigny AJ, Wedeen VJ. 2007. Association fibre pathways of the brain: parallel observations from diffusion spectrum imaging and autoradiography. Brain, 130 (Pt 3): 630- 653. [DOI] [PubMed] [Google Scholar]
- 12. Shi YD, Short SJ, Knickmeyer RC, Wang JP, Coe CL, Niethammer M, Gilmore JH, Zhu HT, Styner MA. 2013. Diffusion tensor imaging-based characterization of brain neurodevelopment in primates. Cerebral Cortex, 23 (1): 36- 48. [DOI] [PMC free article] [PubMed] [Google Scholar]


