Abstract
The northern pig-tailed macaque (Macaca leonina) has been identified as an independent species of Old World monkey, and we previously found that PBMCs from M. leonina were susceptible to human immunodeficiency virus type 1 (HIV-1), which may be due to the absence of a TRIM5 protein restricting HIV-1 replication. Here we investigated the infection potentials of six laboratory adapted HIV-1 strains and three primary HIV-1 isolates in PBMCs from M. leonina. The results indicate that these strains are characterized by various but low replication levels, and among which, HIV-1NL4-3 shows the highest replication ability. Based on the abundant evidence of species-specific interactions between restriction factors APOBEC3 and HIV/SIV-derived Vif protein, we subsequently examined the replication potentials of vif-substituted HIV-1 (HSIV) in M. leonina PBMCs. Notably, HSIV-vifmac and stHIV-1SV chimeras, two HIV-1NL4-3-derived viruses encoding the viral infectivity factor (Vif) protein from SIVmac239, replicated robustly in cells from M. leonina, which suggests that HSIV could effectively antagonize the antiviral activity of APOBEC3 proteins expressed in cells of M. leonina. Therefore, our data demonstrate that M. leonina has the potential to be developed into a promising animal model for human AIDS.
Keywords: HIV-1, HSIV, Replication, Replication, Northern pig-tailed macaque (Macaca leonina)
The lack of animal models that can be efficiently infected by HIV-1 has been a major impediment to the study of AIDS, anti-HIV-1 drugs and vaccines (Hatziioannou & Evans, 2012; Zhang et al, 2007). Presently, the most widely used non-human primate models for HIV/AIDS research are rhesus (Macaca mulatta), pig-tailed and cynomolgus macaques (M. fascicularis) infected with simian immunodeficiency viruses (SIVs) or SIV/HIV chimeric viruses (SHIVs) encompassing the HIV-1 env, tat, rev and vpu genes or reverse transcriptase gene (Baroncelli et al, 2008; Hatziioannou & Evans, 2012). Although these models have offered us with abundant information on immunopathogenesis and antiretroviral strategies (Evans & Silvestri, 2013; Lackner & Veazey, 2007), they have limitations largely due to the significant genetic differences between HIV-1 and SIV (Ambrose et al, 2007). SHIVs contain certain HIV-1 genes, but the absence of other HIV-1 genes has restricted their functional evaluation in viral pathogenesis or as targets for antiretroviral therapies in vivo.
Among Old World monkeys, the pig-tailed macaque is the only primate that can be infected by HIV-1, though the infection is transient and limited (Agy et al, 1992; Bosch et al, 2000; Hu, 2005; Kent et al, 1995). Of note, some HIV-2 strains have been adapted in pig-tailed macaques and can result in persistent infection, CD4+ T cell depletion, and AIDS (Kuller et al, 2001; Otten et al, 1999). Additionally, in contrast to rhesus macaques, the course of SIV infection in pig-tailed macaques more closely resemble that of HIV-1 infection in humans (Batten et al, 2006). Consequently, there has been increasing interest in the use of pig-tailed macaques in HIV/AIDS research (Lei et al, 2013). Based on morphological characteristics and phylogeographic studies, pig-tailed macaques have been classified into three species: Sunda pig-tailed macaques or Southern pig-tailed macaques (M. nemestrina), locating in Borneo, Bangka, Sumatra and the Malay peninsula; Northern pig-tailed macaques (M. leonina), mainly occurring in China (southwestern Yunnan), eastern Bangladesh, Cambodia, Laos, Myanmar, Thailand, southern Vietnam and India; and Mentawai macaques (M. pagensis), living in the Mentawai islands (Groves, 2001; Gippoliti, 2001; Kuang et al, 2009; Rosenblum et al, 1997). So far, the majority of pig-tailed macaques used in SIV/SHIV infection is M. nemestrina.
In recent years, it has been reported that the restriction factors, TRIM5α and APOBEC3, are the major barriers for HIV-1 to infect non-human primate cells (Huthoff & Towers, 2008; Liu et al, 2005; Thippeshappa et al, 2012). The TRIM5α protein mediates a post-entry block to retroviral infection by binding to incoming viral capsids through its C-terminal domain (Stremlau et al, 2004, 2006). The cytidine deaminases APOBEC3, especially APOBEC3G/3F, can be packaged into progeny virions, which can then inhibit viral replication largely by causing lethal hypermutations in viral genomes during reverse transcription. However, this restriction can be counteracted by HIV/ SIV-derived Vif protein in a species-specific manner (Mariani et al, 2003; Sheehy et al, 2002; Stopak et al, 2003; Thippeshappa et al, 2012; Zennou & Bieniasz, 2006). Interestingly, our laboratory previously found that M. leonina lacks a TRIM5α, and its novel TRIM5-Cyclophilin A (TRIM5-CypA) fusion protein is dysfunctional in blocking HIV-1 infection, which may explain why pig-tailed macaques are susceptible to HIV-1 (Kuang et al, 2009; Liao et al, 2007). These findings are consistent with previous studies in M. nemestrina (Brennan et al, 2007, 2008; Newman et al, 2008; Virgen et al, 2008). To overcome barriers imposed by APOBEC3, functional substitution of the vif gene with that from pathogenic SIV enables persistent infection of HIV-1 in M. nemestrina both in vitro and in vivo (Hatziioannou et al, 2009; Thippeshappa et al, 2011). Therefore, pig-tailed macaques appear to be a promising animal model for HIV-1 infection.
Here, to identify an isolate that can replicate efficiently in M. leonina cells, we investigated the replication potentials of six laboratory-adapted HIV-1 strains and three primary HIV-1 isolates in M. leonina peripheral blood mononuclear cells (PBMCs). The results showed that the replications in these HIV-1 strains are various and transient, whereas, constructed HSIV strains based on HIV-1NL4-3 with a substitutional vif gene from SIVmac239 replicate robustly. These results suggest that HSIV strains are resistant to APOBEC3G/3F proteins in M. leonina cells and can be applied to infect M. leonina, in vivo.
MATERIALS AND METHODS
HIV-1 strains and HSIV proviral plasmids
A panel of six lab-adapted subtype B HIV-1 strains, including HIV-1ⅢB, HIV-1RF, HIV-1MN, HIV-1SF2, HIV-1NL4-3, and HIV-1SF162, were obtained from the NIH AIDS Research and Reference Reagent Program (USA) or MRC AIDS Research Project (UK). Primary isolates HIV-1KM018, HIV-1TC2 and HIV-1WAN were isolated from HIV-1 infected individual in Yunnan Province, China (Huang et al, 2013). All the above-mentioned HIV-1 strains are X4-tropic except that HIV-1SF162 and HIV-1KM018 are R5 tropism. Viral stocks were stored at -80 ℃.
The infectious molecular clone of SIVmac239 (Shibata et al, 1991) has been previously described (Li et al, 2007). Proviral plasmids of HIV-1NL4-3 (Adachi et al, 1986), stHIV-1SV (Hatziioannou et al, 2009) and HSIV-vifmac were kindly contributed by Prof. Guang-Xia GAO of Institute of Biophysics from Chinese Academy of sciences (Figure 1). stHIV-1SV, a simian-tropic(st) HIV-1, containing a macaque-adapted HIV-1 env gene (from SHIVKB9) and its vif gene from SIVmac239 has been described in detail previously (Hatziioannou et al, 2009). HSIV-vifmac chimera differs from HIV-1NL4-3 only in the vif gene.
Figure 1.
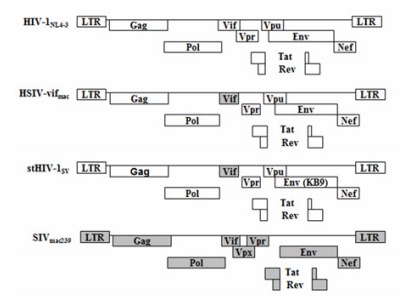
Schematic representation of viral clones used in this study
Cell culture
H9 (human lymphoblastoma) and CEM×174 suspension cells were cultured in RPMI-1640 medium (Gibco) supplemented with 10% heat-inactivated fetal bovine serum (FBS), 100 U/mL penicillin, 100 μg/mL streptomycin and 2 mM L-glutamine. The adherent cell lines TZM-bl and 293T were maintained in complete DMEM containing 10% FBS.
PBMCs were isolated from adult healthy M. leonina, Chinese rhesus macaques, or human peripheral blood by Ficoll-Paque gradient centrifugation as described previously (Dai et al, 2013). All PBMCs were cultured in RPMI-1640 medium with 10% FBS and 50 U/mL IL-2 (Sigma) before infection and thereafter. Human PBMCs were activated with 5 μg/mL phytohemagglutinin (PHA; Sigma) for 72 h. PBMCs from M. leonina and Chinese rhesus were activated for 72 h by 10 μg/mL and 5 μg/mL Concanavalin A (ConA; Sigma), respectively.
Transfection and replication assays
To obtain HIV-1/HSIV stocks, 293T cells in a 6-well plate were transfected with infectious molecular clones by using Lipofectamine 2000 (Invitrogen). After 48 h, culture supernatants were harvested and stored at -80 ℃ until use. Infectious virus titers were determined by serial 5-fold dilutions of the virus stock using TZM-bl reporter cells in a 96-well plate. After 48 h, cells were lysed and treated with Bright-GloTM Reagent to read relative luminescence units (RLU) in the luminometer (Molecular Devices). TCID50 of SIVmac239 was determined by infecting CEM×174 cells with serial dilutions of the stock as described previously (Aldovini & Walker, 1990).
For replication assays, 1×107 activated M. leonina PBMCs were infected with each of the nine HIV-1 strains (40 pg p24) mentioned above in duplicates for 3 h at 37 ℃. Then the cells were rinsed three times with PBS to remove cell-free virus and resuspended in fresh medium. To monitor viral replication, supernatants were harvested and replaced every three days for p24 antigen quantification using an enzyme-linked immunosorbent assay kit (ELISA; ZeptoMetrix, Bufflaflo, NY). To compare HIV-1NL4-3 and HSIV replication in H9 and stimulated PBMCs, viral stocks were mixed with 5×105 H9 cells or 1×106 activated PBMCs at a multiplicity of infection (MOI) of 0.01. Cells were rinsed three times after incubation, and supernatants were collected with the half of the medium being replaced at 3-4 day intervals postinfection for p24 analysis.
qRT-PCR assay
PBS-rinsed H9 and PBMCs (from human, M. leonina and Chinese rhesus macaque) were prepared for RNA isolation. Total RNA was extracted with TRIzol (Invitrogen) according to the manufacturer's protocols. cDNA was synthesized using the PrimeScript® RT reagent Kit with gDNA Eraser (Takara, Dalian, China). To examine TRIM5 and APOBEC3G/3F mRNA expression, qRT-PCR was performed in triplicate with SYBR® Premix Ex TaqTM II (Tli RNase H Plus) kit as described by the manufacturer (Takara, Dalian, China) in the ABI 7500 Fast Real-Time PCR System (Applied Biosystems). Human and macaque target gene expression were normalized to the endogenous GAPDH mRNA level and ribosomal protein L13A (RPL13A) mRNA level, respectively (Ahn et al, 2008). The mRNA expression levels of target genes in different cells are calculated using the 2-△Ct×100% (△Ct=Ct(Target gene)-Ct(Internal reference gene)) method. Chinese rhesus macaque TRIM5α primers (Arhel et al, 2008) and other gene-specific primers used in our study are presented in Table 1.
Table 1.
Primers used for qRT-PCR
| Gene | Primera | Sequence |
| Human-A3G | F | 5'-CACGTGAGCCTGTGCATCTTC-3' |
| R | 5'-AAAGGTGTCCCAGCAGTGCTTA-3' | |
| Human-A3F | F | 5'-GTCCTGAAACCTGGAGCCT-3' |
| R | 5'-AGACGGTATTCCGACGAGA-3' | |
| Human-TRIM5α | F | 5'-ATGTCCGACGCTACTGGGTTGATGT-3' |
| R | 5'-TGTCTGGTATCTTGTCCCTCGTGCC-3' | |
| Human-GAPDH | F | 5'-GAAATCCCATCACCATCTTCCAGG-3' |
| R | 5'-GAGCCCCAGCCTTCTCCATG-3' | |
| NPMb-A3G | F | 5'-TACCACCCAGAGATGAGATT-3' |
| R | 5'-GTTTCCAGAAGTAGTAGAGG-3' | |
| NPM-TRIM5-CypA | F | 5'-CAAAGTCTGAAACGAAGATGGT-3' |
| R | 5'-GCGGCAGCGTCTCTAAACA-3' | |
| Macaque-A3F | F | 5'-CTTTAATAACAGACCCATCCTT-3' |
| R | 5'-GTTGCCACAGAACCGAGA-3' | |
| ChRMc-A3G | F | 5'-AACCTTGGGTCAGTGGACAGC-3' |
| R | 5'-TGGAGCCTGGTTGCGTAGA-3' | |
| ChRM-A3F | F | 5'-CTTTAATAACAGACCCATCCTT-3' |
| R | 5'-GTTGCCACAGAACCGAGA-3' | |
| ChRM-TRIM5α | F | 5'-TTGGATCCTGGGGGTATGTGCTGG-3' |
| R | 5'-TGATATTGAAGAATGAGACAGTGCAAG-3' | |
| Macaque-RPL13A | F | 5'-AAGGTGTTTGACGGCATCCC-3' |
| R | 5'-CTTCTCCTCCAAGGTGGCTGT-3' |
a: The primers are presented as forward (F) and reverse (R); b: denotes northern pig-tailed macaque; c: denotes Chinese rhesus macaques.
RESULTS
Replication of HIV-1 strains in M. leonina PBMCs
Our laboratory has previously reported that in contrast to Chinese rhesus macaque cells, M. leonina cells are susceptible to HIV-1, which may be due to the dysfunctional TRIM5-CypA in the TRIM5 locus (Kuang et al, 2009; Liao et al, 2007). To examine the replication potentials of different HIV-1 strains in M. leonina PBMCs, six lab-adapted HIV-1 strains and three primary HIV-1 isolates were used to infect M. leonina cells in vitro. Lab-adapted subtype B HIV-1NL4-3 and HIV-1ⅢB were chosen because of their ability in infecting T cells of pig-tailed macaques in previous studies (Agy et al, 1992, 1997; Gartner et al, 1994). HIV-1MN, HIV-1RF, HIV-1SF162 and HIV-1SF2 were used due to their close genetic similarity with HIV-1NL4-3 and HIV-1ⅢB. The primary isolates HIV-1KM018 (Wang et al, 2011), HIV-1TC2 (Zhang et al, 2010) and HIV-1WAN, which were often used in studies of anti-HIV-1 drugs in our lab, were also chosen to assess their replication potentials in M. leonina cells.
As shown in Figure 2, all the HIV-1 strains replicated transiently with different susceptibility in M. leonina PBMCs from four different donors and there was no significant increasing trend after day 3 post-infection. In lab-adapted HIV-1 strains, HIV-1NL4-3, and to a lesser extent, HIV-1ⅢB, HIV-1MN and HIV-1SF162 were all able to replicate productively in M. leonina cells. Nevertheless, the replication levels of HIV-1RF and HIV-1SF2 were low, which suggested that they were not adapted well in M. leonina cells. Meanwhile, primary isolates HIV-1WAN and HIV-1KM018 were unable to replicate productively in M. leonina PBMCs. In contrast, the replication level of clinical isolated HIV-1TC2 in M. leonina PBMCs was much higher than that of HIV-1WAN and HIV-1KM018, though was little lower than that of HIV-1NL4-3. Taken together, our results indicate that although lab-adapted HIV-1 strains and primary HIV-1 isolates replicate differently in M. leonina cells, their replication levels are low.
Figure 2.
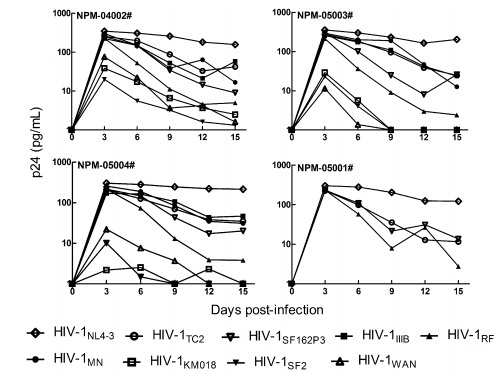
Replication of HIV-1 strains in M. leonina PBMCs
HIV-1 with vif substitution replicates robustly in M. leonina cells
The fact that HIV-1 replicates transiently in M. leonina cells despite the absence of a post-entry block to viral infection prompted us to consider other factors restricting HIV-1 replication. Several studies have demonstrated that APOBEC3 proteins in cells from rhesus macaque and African green macaque, which are resistant to HIV-1 Vif protein, can effectively inhibit HIV-1 infection (Mariani et al, 2003; Virgen & Hatziioannou, 2007). Therefore, we determined the mRNA expressions of APOBEC3G/3F, the two potent antiviral proteins among APOBEC3 family members (Albin & Harris, 2010), in M. leonina PBMCs, human and Chinese rhesus macaque cells (Figure 3). As expected, TRIM5α mRNA was expressed in H9, human PBMCs and Chinese rhesus macaque PBMCs, whereas, M. leonina cells expressed TRIM5-CypA mRNA rather than TRIM5α mRNA (Fiugre 3). Accordingly, we hypothesized that the reason why HIV-1 failed to infect M. leonina cells may be due to the potent anti-HIV-1 activity imposed by APOBEC3 proteins.
Figure 3.
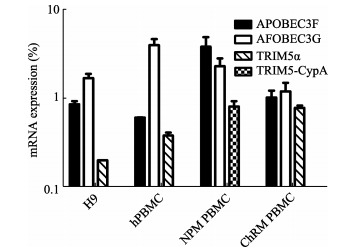
TRIM5 and APOBEC3G/3F mRNA expression in human and macaque cells
To overcome the antiretroviral activity of APOBEC3 in M. leonina cells, we subsequently investigated the replication potentials of two HIV-1-derived infectious clones in M. leonina PBMCs, stHIV-1SV and HSIV-vifmac chimeras containing the vif gene from SIVmac239 (Figure 1). Of note, stHIV-1SV, encompassing a macaque-adapted HIV-1 env gene from SHIVKB9, replicates efficiently in cells of M. nemestrina as described previously (Hatziioannou et al, 2009). Additionally, it has been previously reported that SIVmac-derived Vif proteins could potently counteract the antiviral activity of APOBEC3 proteins in rhesus and pig-tailed macaques (Hatziioannou et al, 2009; Kamada et al, 2006). In our experiment, we used HIV-1NL4-3 and SIVmac239 to infect M. leonina PBMCs, meanwhile, took assays of HIV-1/HSIV infection in human T cell line H9, as well as human and Chinese rhesus macaque PBMCs as controls. We found that the replication levels of wild-type HIV-1NL4-3 and HSIV-vifmac in H9 cell were higher than those of stHIV-1SV (Figure 4A), suggesting that the inclusion of the env gene from SHIVKB9 might, at least to some extent, affect HIV-1 infection. However, stHIV-1SV and HSIV-vifmac replicated as efficiently as HIV-1NL4-3 in human PBMCs from different donors (Figure 4A), demonstrating that the vif substitution could not influence HIV-1 infection. Notably, we observed that the replication levels of stHIV-1SV and HSIV-vifmac in M. leonina PBMCs were almost as high as those of SIVmac239 (Figure 4B), a pathogenic virus that can result in AIDS in pig-tailed macaques (Klatt et al, 2012). However, the replication level of HIV-1NL4-3 was much lower than that of HSIV, indicating that the vif replacement was sufficient for HIV-1 to robustly infect M. leonina cells in vitro. Meanwhile, stHIV-1SV replicated better than HSIV-vifmac in M. leonina cells, suggesting the incorporation of macaque-adapted HIV-1 envelope proteins might be conducive to vif-substituted HIV-1 replication in M. leonina cells. More importantly, based on the well-studied interactions between Vif protein and APOBEC3 proteins, HSIV-derived Vif protein might effectively antagonize the antiretroviral activity of APOBEC3G/3F proteins in M. leonina cells.
Figure 4.
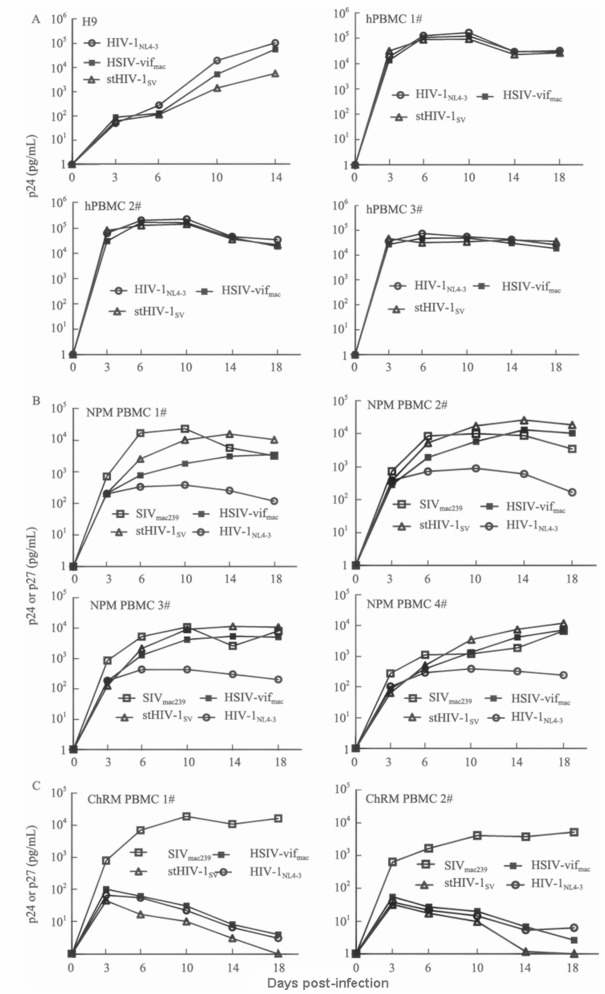
Replication of HIV and HSIV chimeras in vitro
We next examined the replication potentials of HIV-1/HSIV in Chinese rhesus macaque PBMCs, which were resistent to HIV-1 infection as observed previously (Agy et al, 1992). As we expected, the replication level of HIV-1NL4-3 in Chinese rhesus macaque PBMCs was rather low compared with that of the pathogenic SIVmac239 (Figure 4C). M. leonina cells were more susceptible than Chinese rhesus macaque cells to HIV-1 (Figure 4B). Surprisingly, we found no difference between HIV-1NL4-3 and HSIV in their ability to infect Chinese rhesus macaque PBMCs, indicating that the vif substitution was insufficient for HIV-1 to replicate robustly in Chinese rhesus macaque PBMCs. Collectively, our results reveal that APOBEC3 proteins in M. leonina cells may function as an important barrier for HIV-1 infection and by replacing HIV-1 vif gene with that from pathogenic SIV can overcome this potent block.
DISCUSSION
According to primate taxonomy, M. leonina and M. nemestrina are two independent species in Old World monkeys (Groves, 2001; Malaivijitnond et al, 2012). Our lab has previously reported that M. leonina cells are prone to HIV-1 infection, which may be due to the absence of a post-entry restriction imposed by a TRIM5 protein (Kuang et al, 2009; Liao et al, 2007). In this study, we showed that the replication levels of six lab-adapted HIV-1 strains and three primary HIV-1 isolates were various but low in M. leonina cells. Among the HIV-1 strains, HIV-1NL4-3 replicated best in M. leonina cells, which was consistent with the previous studies (Agy et al, 1997). However, HIV-1RF and HIV-1SF2, the two HIV-1 strains possessing similar biological properties with HIV-1NL4-3 (X4-tropic and lab-adapted subtype B virus), replicated poorly in M. leonina cells. Primary isolate HIV-1TC2, rather than HIV-1KM018 and HIV-1WAN, could replicate in M. leonina cells. A similar phenomenon has also been reported regarding HIV-1 infection in cells of pig-tailed macaques, which suggested that the limited permissivity of macaque cells for HIV-1 may account for certain HIV-1 strains’ failing in infecting pig-tailed macaque cells productively (Gartner et al, 1994). Moreover, a recent study suggested that the adaptation of HIV-1-derived envelope protein, which is responsible for viral entry, was necessary for the virus to infect cells from pig-tailed macaques (Humes & Overbaugh, 2011). However, whether the inability of certain HIV-1 isolates to infect M. leonina cells is related with viral entry or some other factors remains to be elucidated.
It is well known that APOBEC3 proteins in macaque cells is a major impediment in HIV-1 replication in Old World monkeys and their antiviral activity can be abrogated by some SIV Vif proteins (Huthoff & Towers, 2008; Thippeshappa et al, 2012). Recently, some studies showed that SIV vif substitution is sufficient for HIV-1 to persistently infect M.nemestrina cells both in vitro and in vivo (Thippeshappa et al, 2012). Thus, we subsequently examined the replication potentials of stHIV-1SV and HSIV-vifmac (two chimeras encoding Vif protein from SIVmac239) in PBMCs from human and M. leonina. Consistent with previous studies, we found that these two chimeras could replicate productively in human PBMCs, implying that SIVmac Vif protein could inactivate human APOBEC3 proteins (Gaddis et al, 2004; Thippeshappa et al, 2011).
Importantly, we showed that stHIV-1SV and HSIV-vifmac are able to replicate robustly in M. leonina cells in vitro, suggesting that APOBEC3 proteins expressed by M. leonina cells are a major barrier to HIV-1 infection in this primate species. We also showed that the replication level of stHIV-1SV expressing the SHIVKB9-derived envelope protein is higher than that of HSIV-vifmac in M. leonina PBMCs, implying that the modified env gene may be conducive for HSIV to infect M. leonina cells, which is consistent with previous reports that stHIV-1 can replicate efficiently in cells from M. nemestrina both in vitro and in vivo (Hatziioannou et al, 2009). A more recent study also showed that HSIV-vif, in which the HIV-1NL4-3 vif gene was functionally substituted by the vif gene from SIVmne027, can replicate as efficiently as SIVmne027 in cells from M. Nemestrina (Thippeshappa et al, 2012). By contrast, HIV-1NL-DT5R, a virus containing a part of the Gag CA sequence (corresponding to the HIV-1 CypA-binding loop) and a vif gene from SIVmac239, is unable to achieve the replication level of SIVmac239 in pig-tailed macaque cells in vitro (Kamada et al, 2006), which may result in its transient infection in pig-tailed macaques (Igarashi et al, 2007). Although further modification or passaging in vitro of HIV-1NL-DT5R to some extent had enhanced its replication in cells from cynomolgus macaques, a macaque-tropic virus that can replicate as efficiently as SIVmac239 in macaque cells could not be obtained (Kamada et al, 2009; Kuroishi et al, 2009; Nomaguchi et al, 2013a, 2013b; Saito et al, 2011). In comparison, the HSIV-vifmac we used seemed to replicate better than HIV-1NL-DT5R with SIVmac239 serving as a control, which may be either due to HIV-1NL-DT5R expressing modified Gag protein that was unnecessary for high- level of HIV-1 infection in cells of pig-tailed macaques or because the cells we used in this study was from M. leonina instead of M. nemestrina. However, whether there are significant differences between M. nemestrina and M. leonina after HSIV infection needs to be determined.
We also observed that HIV-1 and HSIV replication in Chinese rhesus macaque PBMCs were potently inhibited, which was consistent with a previous study (Hatziioannou et al, 2006). However, it has also been demonstrated that stHIV-1 containing both Gag CA (expressing viral capsid) sequence and a vif gene from SIVmac, after serial passage in vitro, could replicate robustly in rhesus macaque lymphocytes, whereas, the replication of HIV(SCA) carrying only SIVmac CA was low and transient (Hatziioannou et al, 2006). Additionally, HIV-1NL-DT5R as described above could also replicate in CD8-depleted PBMCs from a rhesus macaque (Kamada et al, 2006). It is known that TRIM5α protein mediates the early block to HIV-1 infection in Old World monkey cells (Blanco-Melo et al, 2012). Thus, we conclude that TRIM5α, a potent retrovirus inhibitor, may function as a major impediment in Chinese rhesus macaque cells to HIV-1/HSIV replication. Comparatively, the absence of a TRIM5 blocking HIV-1 replication in M. leonina cells may partly explain why M. leonina cells are more sensitive than Chinese rhesus macaque cells to HIV-1 infection.
In summary, our results indicate that the abilities of HIV-1 strains to persistently infect M. leonina cells in vitro are various and limited. Notably, HSIV chimeras based on HIV-1NL4-3 encoding the SIVmac239 Vif protein can achieve the SIVmac239 replication level in M. leonina cells. Thus, HSIV infection in M. leonina may be developed into a promising animal model for human AIDS.
Acknowledgements
We thank Prof. Guang-Xia GAO (Institute of Biophysics, Chinese Academy of Sciences) for kindly providing HSIV proviral plasmids.We also thank Long-Bao LV, Gui LI and Dong-Ti HUANG of Kunming Primate Research Center for their assistance in obtaining blood samples from northern pig-tailed macaques (M. leonina) and Chinese rhesus macaques.
Funding Statement
This work was supported by the National Basic Research Program (2012CBA01305); the National Natural Science Foundation of China (81172876, U0832601, 81273251 and U1202228); the Knowledge Innovation Program of CAS (KSCX2-EW-R-13, Y206A- 71181), and the Key Scientific and Technological Program of China (2012ZX10001-007, 2013ZX10001-002)
REFERENCES
- 1. Adachi A, Gendelman HE, Koenig S, Folks T, Willey R, Rabson A, Martin MA. 1986. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. Journal of Virology, 59 (2): 284- 291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Agy MB, Frumkin LR, Corey L, Coombs RW, Wolinsky SM, Koehler J, Morton WR, Katze MG. 1992. Infection of Macaca nemestrina by human immunodeficiency virus type-1. Science, 257 (5066): 103- 106. [DOI] [PubMed] [Google Scholar]
- 3. Agy MB, Schmidt A, Florey MJ, Kennedy BJ, Schaefer G, Katze MG, Corey L, Morton WR, Bosch ML. 1997. Serial in vivo passage of HIV-1 infection in Macaca nemestrina. Virology, 238 (2): 336- 343. [DOI] [PubMed] [Google Scholar]
- 4. Ahn K, Huh JW, Park SJ, Kim DS, Ha HS, Kim YJ, Lee JR, Chang KT, Kim HS. 2008. Selection of internal reference genes for SYBR green qRT-PCR studies of rhesus monkey (Macaca mulatta) tissues. BMC Molecular Biology, 9 78- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Albin JS, Harris RS. 2010. Interactions of host APOBEC3 restriction factors with HIV-1 in vivo: implications for therapeutics. Expert Reviews in Molecular Medicine, 12 e4- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aldovini A, Walker BD. 1990. Techniques in HIV Research. New York: Stockton Press, [Google Scholar]
- 7. Ambrose Z, KewalRamani VN, Bieniasz PD, Hatziioannou T. 2007. HIV/AIDS: in search of an animal model. Trends in Biotechnology, 25 (8): 333- 337. [DOI] [PubMed] [Google Scholar]
- 8. Arhel NJ, Nisole S, Carthagena L, Coutant F, Souque P, Brussel A, Estaquier J, Charneau P. 2008. Lack of endogenous TRIM5α-mediated restriction in rhesus macaque dendritic cells. Blood, 112 (9): 3772- 3776. [DOI] [PubMed] [Google Scholar]
- 9. Baroncelli S, Negri DR, Michelini Z, Cara A. 2008. Macaca mulatta, fascicularis and nemestrina in AIDS vaccine development. Expert Review of Vaccines, 7 (9): 1419- 1434. [DOI] [PubMed] [Google Scholar]
- 10. Batten CJ, De Rose R, Wilson KM, Agy MB, Chea S, Stratov I, Montefiori DC, Kent SJ. 2006. Comparative evaluation of simian, simian-human, and human immunodeficiency virus infections in the pigtail macaque (Macaca nemestrina) model. AIDS Research and Human Retroviruses, 22 (6): 580- 588. [DOI] [PubMed] [Google Scholar]
- 11. Blanco-Melo D, Venkatesh S, Bieniasz PD. 2012. Intrinsic cellular defenses against human immunodeficiency viruses. Immunity, 37 (3): 399- 411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bosch ML, Schmidt A, Chen JL, Florey MJ, Agy M, Morton WR. 2000. Enhanced replication of HIV-1 in vivo in pigtailed macaques (Macaca nemestrina). Journal of Medical Primatology, 29 (3-4): 107- 113. [DOI] [PubMed] [Google Scholar]
- 13. Brennan G, Kozyrev Y, Hu SL. 2008. TRIMCyp expression in Old World primates Macaca nemestrina and Macaca fascicularis. Proceedings of the National Academy of Sciences of the United States of America, 105 (9): 3569- 3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brennan G, Kozyrev Y, Kodama T, Hu SL. 2007. Novel TRIM5 isoforms expressed by Macaca nemestrina. Journal of Virology, 81 (22): 12210- 12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dai ZX, Zhang GH, Zhang XH, Zheng YT. 2013. Identification and characterization of a novel splice variant of rhesus macaque MHC IA. Molecular Immunology, 53 (3): 206- 213. [DOI] [PubMed] [Google Scholar]
- 16. Groves CP. 2001. Primate Taxonomy. Washington DC: Smithsonian Institution Press, 222- 224. [Google Scholar]
- 17. Evans DT, Silvestri G. 2013. Nonhuman primate models in AIDS research. Current Opinion in HIV and AIDS, 8 (4): 255- 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gaddis NC, Sheehy AM, Ahmad KM, Swanson CM, Bishop KN, Beer BE, Marx PA, Gao F, Bibollet-Ruche F, Hahn BH, Malim MH. 2004. Further investigation of simian immunodeficiency virus Vif function in human cells. Journal of Virology, 78 (21): 12041- 12046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gartner S, Liu YL, Polonis V, Lewis MG, Elkins WR, Hunter EA, Miao J, Corts KJ, Eddy GA. 1994. Adaptation of HIV-1 to Pigtailed Macaques. Journal of Medical Primatology, 23 (2-3): 155- 163. [DOI] [PubMed] [Google Scholar]
- 20. Gippoliti S. 2001. Notes on the taxonomy of Macaca nemestrina leonina blyth, 1863 (Primates: Cercopithecidae). Hystrix-Italian Journal of Mammalogy, 12 (1): 51- 54. [Google Scholar]
- 21. Hatziioannou T, Evans DT. 2012. Animal models for HIV/AIDS research. Nature Reviews Microbiology, 10 (12): 852- 867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hatziioannou T, Princiotta M, Piatak M Jr., Yuan F, Zhang FW, Lifson JD, Bieniasz PD. 2006. Generation of simian-tropic HIV-1 by restriction factor evasion. Science, 314 (5796): 95- [DOI] [PubMed] [Google Scholar]
- 23. Hatziioannou T, Ambrose Z, Chung NPY, Piatak M Jr, Yuan F, Trubey CM, Coalter V, Kiser R, Schneider D, Smedley J, Pung R, Gathuka M, Estes JD, Veazey RS, KewalRamani VN, Lifson JD, Bieniasz PD. 2009. A macaque model of HIV-1 infection. Proceedings of the National Academy of Sciences of the United States of America, 106 (11): 4425- 4429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hu SL. 2005. Non-human primate models for AIDS vaccine research. Current Drug Targets-Infectious Disorders, 5 (2): 193- 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Huang N, Wu MY, Zheng CB, Zhu L, Zhao JH, Zheng YT. 2013. The depolymerized fucosylated chondroitin sulfate from sea cucumber potently inhibits HIV replication via interfering with virus entry. Carbohydrate Research, 380 64- 69. [DOI] [PubMed] [Google Scholar]
- 26. Humes D, Overbaugh J. 2011. Adaptation of Subtype A Human Immunodeficiency Virus Type 1 Envelope to Pig-Tailed Macaque Cells. Journal of Virology, 85 (9): 4409- 4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Huthoff H, Towers GJ. 2008. Restriction of retroviral replication by APOBEC3G/F and TRIM5α. Trends in Microbiology, 16 (12): 612- 619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Igarashi T, Iyengar R, Byrum RA, Buckler-White A, Dewar RL, Buckler CE, Lane HC, Kamada K, Adachi A, Martin MA. 2007. Human immunodeficiency virus type 1 derivative with 7% simian immunodeficiency virus genetic content is able to establish infections in pig-tailed macaques. Journal of Virology, 81 (20): 11549- 11552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kamada K, Yamashita T, Hatcho K, Adachi A, Nomaguchi M. 2009. Evasion from CypA-and APOBEC-mediated restrictions is insufficient for HIV-1 to efficiently grow in simian cells. Microbes and Infection, 11 (2): 164- 171. [DOI] [PubMed] [Google Scholar]
- 30. Kamada K, Igarashi T, Martin MA, Khamsri B, Hatcho K, Yamashita T, Fujita M, Uchiyama T, Adachi A. 2006. Generation of HIV-1 derivatives that productively infect macaque monkey lymphoid cells. Proceedings of the National Academy of Sciences of the United States of America, 103 (45): 16959- 16964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kent SJ, Corey L, Agy MB, Morton WR, McElrath MJ, Greenberg PD. 1995. Cytotoxic and proliferative T cell responses in HIV-1-infected Macaca nemestrina. The Journal of Clinical Investigation, 95 (1): 248- 256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Klatt NR, Canary LA, Vanderford TH, Vinton CL, Engram JC, Dunham RM, Cronise HE, Swerczek JM, Lafont BA, Picker LJ, Silvestri G, Brenchley JM. 2012. Dynamics of simian immunodeficiency virus SIVmac239 infection in pigtail macaques. Journal of Virology, 86 (2): 1203- 1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kuang YQ, Tang X, Liu FL, Jiang XL, Zhang YP, Gao GX, Zheng YT. 2009. Genotyping of TRIM5 locus in northern pig-tailed macaques (Macaca leonina), a primate species susceptible to Human Immunodeficiency Virus type 1 infection. Retrovirology, 6 (1): 58- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kuller L, Schmidt A, Mack H, Durning M, Birkebak T, Reiner MT, Anderson DM, Morton WR, Agy MB. 2001. Systemic and intestinal immune responses to HIV-2287 infection in Macaca nemestrina. AIDS Research and Human Retroviruses, 17 (12): 1191- 1204. [DOI] [PubMed] [Google Scholar]
- 35. Kuroishi A, Saito A, Shingai Y, Shioda T, Nomaguchi M, Adachi A, Akari H, Nakayama EE. 2009. Modification of a loop sequence between α-helices 6 and 7 of virus capsid (CA) protein in a human immunodeficiency virus type 1 (HIV-1) derivative that has simian immunodeficiency virus (SIVmac239) vif and CA α-helices 4 and 5 loop improves replication in cynomolgus monkey cells. Retrovirology, 6 (1): 70- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lackner AA, Veazey RS. 2007. Current concepts in AIDS pathogenesis: insights from the SIV/macaque model. Annual Review of Medicine, 58 461- 476. [DOI] [PubMed] [Google Scholar]
- 37. Lei AH, Pang W, Zhang GH, Zheng YT. 2013. Use and research of pigtailed macaques in nonhuman primate HIV/AIDS Models. Zoological Research, 34 (2): 77- 88. [DOI] [PubMed] [Google Scholar]
- 38. Li MH, Li SY, Xia HJ, Wang L, Wang YY, Zhang GH, Zheng YT. 2007. Establishment of AIDS animal model with SIVmac239 infected Chinese rhesus monkey. Virologica Sinica, 22 (6): 509- 516. [Google Scholar]
- 39. Liao CH, Kuang YQ, Liu HL, Zheng YT, Su B. 2007. A novel fusion gene, TRIM5-Cyclophilin A in the pig-tailed macaque determines its susceptibility to HIV-1 infection. AIDS, 21 (Suppl 8): S19- S26. [DOI] [PubMed] [Google Scholar]
- 40. Liu HL, Wang YQ, Liao CH, Kuang YQ, Zheng YT, Su B. 2005. Adaptive evolution of primate TRIM5α, a gene restricting HIV-1 infection. Gene, 362 109- 116. [DOI] [PubMed] [Google Scholar]
- 41. Malaivijitnond S, Arsaithamkul V, Tanaka H, Pomchote P, Jaroenporn S, Suryobroto B, Hamada Y. 2012. Boundary zone between northern and southern pig-tailed macaques and their morphological differences. Primates, 53 (4): 377- 389. [DOI] [PubMed] [Google Scholar]
- 42. Mariani R, Chen D, Schröfelbauer B, Navarro F, König R, Bollman B, Münk C, Nymark-McMahon H, Landau NR. 2003. Species-specific exclusion of APOBEC3G from HIV-1 virions by Vif. Cell, 114 (1): 21- 31. [DOI] [PubMed] [Google Scholar]
- 43. Newman RM, Hall L, Kirmaier A, Pozzi LA, Pery E, Farzan M, O'Neil SP, Johnson W. 2008. Evolution of a TRIM5-CypA splice isoform in old world monkeys. PLoS Pathogens, 4 (2): e1000003- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nomaguchi M, Doi N, Fujiwara S, Saito A, Akari H, Nakayama EE, Shioda T, Yokoyama M, Sato H, Adachi A. 2013. a. Systemic biological analysis of the mutations in two distinct HIV-1mt genomes occurred during replication in macaque cells. Microbes and Infection, 15 (4): 319- 328. [DOI] [PubMed] [Google Scholar]
- 45. Nomaguchi M, Yokoyama M, Kono K, Nakayama EE, Shioda T, Saito A, Akari H, Yasutomi Y, Matano T, Sato H, Adachi A. 2013. b. Gag-CA Q110D mutation elicits TRIM5-independent enhancement of HIV-1mt replication in macaque cells. Microbes and Infection, 15 (1): 56- 65. [DOI] [PubMed] [Google Scholar]
- 46. Otten RA, Ellenberger DL, Adams DR, Fridlund CA, Jackson E, Pieniazek D, Rayfield MA. 1999. Identification of a window period for susceptibility to dual infection with two distinct human immunodeficiency virus type 2 isolates in a Macaca nemestrina (pig-tailed macaque) model. The Journal of Infectious Diseases, 180 (3): 673- 684. [DOI] [PubMed] [Google Scholar]
- 47. Rosenblum LL, Supriatna J, Melnick DJ. 1997. Phylogeographic analysis of pigtail macaque populations (Macaca nemestrina) inferred from mitochondrial DNA. American Journal of Physical Anthropology, 104 (1): 35- 45. [DOI] [PubMed] [Google Scholar]
- 48. Saito A, Nomaguchi M, Iijima S, Kuroishi A, Yoshida T, Lee YJ, Hayakawa T, Kono K, Nakayama EE, Shioda T, Yasutomi Y, Adachi A, Matano T, Akari H. 2011. Improved capacity of a monkey-tropic HIV-1 derivative to replicate in cynomolgus monkeys with minimal modifications. Microbes and Infection, 13 (1): 58- 64. [DOI] [PubMed] [Google Scholar]
- 49. Sheehy AM, Gaddis NC, Choi JD, Malim MH. 2002. Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein. Nature, 418 (6898): 646- 650. [DOI] [PubMed] [Google Scholar]
- 50. Shibata R, Kawamura M, Sakai H, Hayami M, Ishimoto A, Adachi A. 1991. Generation of a chimeric human and simian immunodeficiency virus infectious to monkey peripheral blood mononuclear cells. Journal of Virology, 65 (7): 3514- 3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Stopak K, de Noronha C, Yonemoto W, Greene WC. 2003. HIV-1 Vif blocks the antiviral activity of APOBEC3G by impairing both its translation and intracellular stability. Molecular Cell, 12 (3): 591- 601. [DOI] [PubMed] [Google Scholar]
- 52. Stremlau M, Owens CM, Perron MJ, Kiessling M, Autissier P, Sodroski J. 2004. The cytoplasmic body component TRIM5α restricts HIV-1 infection in Old World monkeys. Nature, 427 (6977): 848- 853. [DOI] [PubMed] [Google Scholar]
- 53. Stremlau M, Perron M, Lee M, Li Y, Song B, Javanbakht H, Diaz-Griffero F, Anderson DJ, Sundquist WI, Sodroski J. 2006. Specific recognition and accelerated uncoating of retroviral capsids by the TRIM5α restriction factor. Proceedings of the National Academy of Sciences of the United States of America, 103 (14): 5514- 5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Thippeshappa R, Ruan H, Kimata JT. 2012. Breaking barriers to an AIDS model with macaque-tropic HIV-1 derivatives. Biology (Basel), 1 (2): 134- 164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Thippeshappa R, Polacino P, Kimata MTY, Siwak EB, Anderson D, Wang WM, Sherwood L, Arora R, Wen M, Zhou P, Hu SL, Kimata JT. 2011. Vif substitution enables persistent infection of pig-tailed macaques by human immunodeficiency virus type 1. Journal of Virology, 85 (8): 3767- 3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Virgen CA, Hatziioannou T. 2007. Antiretroviral activity and Vif sensitivity of rhesus macaque APOBEC3 proteins. Journal of Virology, 81 (24): 13932- 13937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Virgen CA, Kratovac Z, Bieniasz PD, Hatziioannou T. 2008. Independent genesis of chimeric TRIM5-cyclophilin proteins in two primate species. Proceedings of the National Academy of Sciences of the United States of America, 105 (9): 3563- 3568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wang RR, Gao YD, Ma CH, Zhang XJ, Huang CG, Huang JF, Zheng YT. 2011. Mangiferin, an anti-HIV-1 agent targeting protease and effective against resistant strains. Molecules, 16 (5): 4264- 4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zennou V, Bieniasz PD. 2006. Comparative analysis of the antiretroviral activity of APOBEC3G and APOBEC3F from primates. Virology, 349 (1): 31- 40. [DOI] [PubMed] [Google Scholar]
- 60. Zhang GH, Li MH, Zheng YT. 2007. Application of AIDS macaque animal model in HIV vaccine research. Zoological Research, 28 (5): 556- 562. [Google Scholar]
- 61. Zhang XJ, Yang GY, Wang RR, Pu JX, Sun HD, Xiao WL, Zheng YT. 2010. 7,8-secolignans from Schisandra wilsoniana and their anti-HIV-1 activities. Chemistry & Biodiversity, 7 (11): 2692- 2701. [DOI] [PubMed] [Google Scholar]


