Abstract
The northern pig-tailed macaque (NPM, Macaca leonina) has become a widely used animal model in biomedical research. In this study, we measured serum immunoglobulin IgG, IgM, IgA, complement C3, C4 and CRP levels in 3-11 year old captive northern pig-tailed macaques using HITACHI 7600-20 automated chemistry analyzer in order to determine the influences of age and gender on these items. The results showed that serum IgA, IgM, C3 and C4 levels were not correlated with age (P>0.05), while serum IgG levels increased progressively with age (r=0.202; P=0.045). Serum IgG, IgA, IgM and C3 levels were higher in females than in males (P<0.05). Moreover, serum C3 concentration was both positively and strongly correlated with that of C4 (r=0.700; P<0.0001). This study provides basic serum immunoglobulin and complement data of captive northern pig-tailed macaques, which may prove useful for future breeding efforts and biomedical research.
Keywords: Northern pig-tailed macaque (Macaca leonina), Immunoglobulin, Complement, C-reactive protein
Macaque species, including pig-tailed macaques (Macaca nemestrina group), rhesus macaques (M. Mulatta) and cynomolgus macaques (M. fascicularis), have been widely used in biomedical research (Yoshino et al, 2000) because of their phylogenetic proximity to humans, such as in HIV infection (Kuang et al, 2009; Lei et al, 2013; Zhu et al, 2010), chlamydial infection (Patton et al, 2001), Campylobacter infection (Flores et al, 1990), immunogenetics (Knapp et al, 1996), immune cell function (Li et al, 2012; Shaulov & Murali-Krishna, 2008), xenotransplantation (Watts et al, 2012), neurophysiology (Rausell et al, 1998) and cognitive behavioural studies (Macellini et al, 2010; Sussman & Ha, 2011). The taxonomic status of Macaque species have been reevaluated, and three subspecies have been corrected into three independent species: northern pig-tailed macaques (NPMs, M. leonina), southern pig-tailed macaques (M. nemestrina) and Mentawai macaques (M. pagensis) (Groves, 2001; Kuang et al, 2009).
While these studies provide critical information, to date that physiological and biochemical characteristics of captive NPMs have not been as well described as those of the other commonly-used macaques. We have previously reported the reference values of blood chemistry and hematology of NPMs (Pang et al, 2013), but little is known about the normal levels of its basic immunological parameters, such as serum immunoglobulins, complements and CRP levels, which may closely correlate with age, gender, breeding environment and sampling methods (Curry, 2001).
In this study, we analyzed the levels of serum IgG, IgM, IgA, C3, C4 and CRP of healthy captive NPMs with different age and gender, which will likely benefit further applications towards infectious diseases, immuneology and other biomedical researches.
MATERIALS AND METHODS
Animals
One hundred NPMs used in this study were housed in Kunming Institute of Zoology, Chinese Academy of Sciences (KIZ, CAS). Animals were free of any known virus, bacteria and parasites and visually showed with no trauma, no pregnancy and no estrus. Age, gender and body weight information were presented in Table 1. These NPMs were maintained in social groups in enclosures at room temperature and provided with a diet of fruits, monkey biscuits, and vegetables at 0800h, 1300h and 1600h, respectively. Animal care was performed according to the regulations and recommendations of the Animal Care Committee of KIZ, CAS.
Table 1.
Age, gender and body weight of NPMs
| Groups | Female | Male | ||||
| Number (n) | Age (years) | Body weight (kg) | Number (n) | Age (years) | Body weight (kg) | |
| 3-4 years old (Juveniles) | 11 | 3.4±0.5 | 2.37±0.33 | 24 | 3.5±0.5 | 2.51±0.29 |
| 5-11 years old (Adults) | 39 | 6.5±1.5 | 3.83±0.86 | 26 | 6.6±1.7 | 3.87±1.38 |
| Total | 50 | 5.8±1.9 | 3.51±0.98 | 50 | 5.1±2.0 | 3.21±1.22 |
Serum samples
Blood samples (1-2 mL) from each subject were collected sterilely from the saphenous vein without anesthesia, and allowed to clot at room temperature. Serum was further separated by centrifuging at 2, 500 r/min, 4 ℃ for 20 minutes 1-hour later after bleeding and stored at-80 ℃ until use.
Assays for serum immunoglobulins, complements and CRP
Serum specimens were delivered to Department of Clinical Laboratory, the Second Hospital of Yunnan Province, China, and then the turbidimetric immuneoassay determining levels of serum IgG, IgM, IgA, C3, C4 and CRP was performed on a HITACHI 7600-020 automated chemistry analyzer using rabbit anti-human polyclonal antibodies, protein standards and reagents from Orion Corporation according to users’ manuals. IgA, IgG, IgM, C3, C4 and CRP standards were diluted into known concentrations. Next, protein standards dilutions and serum specimens were measured simultaneously. Upon adding a serum sample, target antigen and polyclonal antibodies would form an immune complex that would precipitate and then increase the turbidity of the reaction solution. When light was shone on the sample, some were scattered, some were absorbed, and the rest passed through. The automated chemistry analyzer can measure the sample’s light absorbance, which is positively correlated with the protein concentrations in it. Accordingly, target serum protein level could be calculated by referring to this protein’s known diluted concentration series. Detailed protocols are available from the manufacturer.
Statistical analysis
All statistical analyses were conducted using GraphPad Prism 5.0. The normal distributions were tested by the Kolmogorov-Smirnov test. Spearman partial correlation coefficients were calculated by taking gender as control and taking each serum immunological parameters as dependent variables against age. When dependent variables were significantly correlated with age, then gender effects were analyzed under covariance analysis taking gender as a factor and age as a covariate. Other times, an unpaired t-test was used to compare the means between male and female groups. Pearson’s r-test was used to analyze the significance in correlations between each serum immunological variables. All analyses were two-tailed, with results expressed as mean±SD, with P=0.05 being statistically significant.
RESULTS
Levels of serum immunoglobulins, complements and CRP
The levels of serum immunoglobulins, complements and CRP in captive NPMs from different gender and age groups are shown in Table 2. Among the total serum samples, 34 samples were showed with no detectable CRP signals, and another 34 samples displayed CRP levels less than 0.1 mg/L (data not shown).
Table 2.
Levels of serum immunoglobulins and complements of NPMs
| Grouping | 3-4 years old (Juveniles) | 5-11 years old (Adults) | Total | |
| IgG (g/L) | Female | 17.236±2.900 | 19.808±3.47 | 19.242±3.491 |
| Male | 16.954±2.509 | 17.570±2.875 | 17.274±2.696 | |
| Total | 17.043±2.598 | 18.912±3.403 | 18.258±3.257 | |
| Range | 12.400-22.900 | 10.400-30.000 | 10.400-30.000 | |
| IgA (g/L) | Female | 0.924±0.377 | 1.041±0.060 | 1.015±0.375 |
| Male | 0.739±0.327 | 0.815±0.359 | 0.779±0.343 | |
| Total | 0.797±0.349 | 0.951±0.383 | 0.897±0.377 | |
| Range | 0.260-1.550 | 0.290-2.290 | 0.260-2.290 | |
| IgM (g/L) | Female | 2.025±0.652 | 1.864±0.693 | 1.886±0.682 |
| Male | 1.488±0.503 | 1.406±0.485 | 1.445±0.490 | |
| Total | 1.656±0.600 | 1.670±0.651 | 1.665±0.631 | |
| Range | 0.820-3.590 | 0.580-4.040 | 0.580-4.030 | |
| C3 (g/L) | Female | 1.851±0.419 | 1.892±0.316 | 1.883±0.337 |
| Male | 1.813±0.240 | 1.688±0.297 | 1.748±0.276 | |
| Total | 1.825±0.301 | 1.810±0.322 | 1.816±0.314 | |
| Range | 1.310-2.650 | 1.030-2.560 | 1.030-2.650 | |
| C4 (g/L) | Female | 0.315±0.097 | 0.298±0.087 | 0.302±0.089 |
| Male | 0.321±0.095 | 0.274±0.091 | 0.297±0.095 | |
| Total | 0.319±0.094 | 0.228±0.089 | 0.299±0.091 | |
| Range | 0.100-0.510 | 0.100-0.480 | 0.100-0.510 | |
Effects of age on the levels of serum immunoglobulins and complements
While the development, maturation and functional decline of immune system are age-related biological processes, we assessed the effects of age on the levels of serum immunoglobulins and complements in the 3-11 years old subjects. We found that the serum IgG levels were significantly increased with age (r=0.202; P=0.045), whereas, the serum IgA (r=0.146; P=0.149), IgM (r=-0.073; P=0.474), C3 (r=-0.055; P=0.591) and C4 (r=-0.164; P=0.105) levels showed no significant correlations with age (Figure 1).
Figure 1.
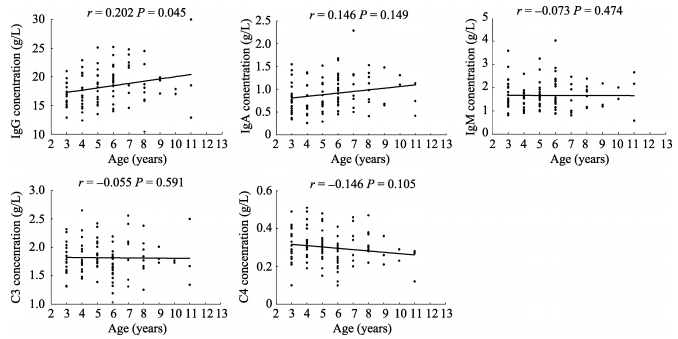
Effects of age on the levels of serum IgG, IgA, IgM, C3 and C4 in NPMs
Gendered levels of serum IgG, IgA, IgM, C3 and C4
Given the differences between female and male immune systems, we tested whether levels of serum IgA, IgG, IgM, C3 and C4 of females were significantly different with those of males. As shown in Figure 2, significant gender-related differences were observed in the levels of serum IgG (P < 0.001), IgA (P=0.001), IgM (P < 0.001) and C3 (P=0.031). All of the female serum IgA, IgG, IgM and C3 levels, especially IgM levels, were higher than those of the male. However, there was no significant gender-related difference in the serum C4 levels (P=0.777) between male and female groups.
Figure 2.

Effects of gender on the levels of serum IgG, IgA, IgM, C3 and C4 in NPMs
Correlation analysis of serum immunoglobulins and complements
The complement system is closely correlated with immunoglobulins. Certain immunoglobulins can activate the complement system, and the activated complement system can therefore enhance the biological activities of immunoglobulins. We analyzed the correlations among serum IgA, IgG, IgM, C3 and C4 levels in NPMs and the statistical analysis showed significantly positive correlations only between the following pairs of components: IgA and IgM (r=0.457; P < 0.0001), C3 and IgM (r=0.246; P=0.014), C3 and IgA (r=0.295; P=0.003), C3 and IgG (r=0.289; P=0.004), C3 and C4 (r=0.700; P < 0.0001) (Figure 3).
Figure 3.
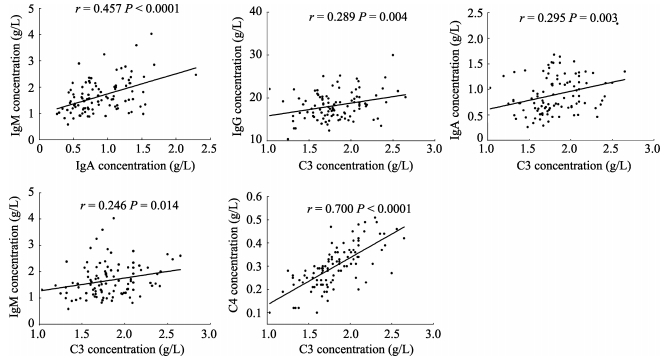
Correlations of serum immunoglobulins and complements in NPMs
DISCUSSION
Due to the high phylogenetic proximity between humans and non-human primates, proteins—such as immunoglobulins, complements and CRP—share a high degree of homology. Earlier studies evaluated the crossreactivities between anti-human antibodies and nonhuman primate antigens and demonstrated that the homology could reach to 80%-100% (Jinbo et al, 1998; Shuster et al, 1969). Therefore, lots of monkey proteins have been measured with homologous antibodies of human (Axberg et al, 1991; Chen et al, 2006; Cheng et al, 2003; Cole & Bowen, 1976; Fujimoto et al, 1982; Mayer et al, 1993). Moreover, in many previous studies, antihuman polyclonal antibodies have been successfully used in determining serum immunoglobulin, complement and CRP levels in rhesus macaques and/or cynomolgus macaques (Jinbo et al, 1998; Socha et al, 1993; Yoshino et al, 2000).
Immunoglobulins play a critical role in the immediate defense against infection and its quantitative internal concentration determination is helpful in disease diagnosis, monitoring and medication. In our study, we found no effects of age (3-11 years old) on the serum IgA and IgM levels of NPMs, which was consistent with the findings in rhesus macaques and cynomolgus macaques. However, serum IgG levels increased progressively with age, which was inconsistent with previous studies on rhesus monkeys and cynomolgus macaques (Cheng et al, 2003; Cole & Bowen, 1976). Such contradictory results are probably due to the interspecies differences in growth and development. The serum IgA, IgG and IgM levels of female NPMs were higher than those of males, which was in accordance with studies on other species. It has been documented that in general, females have better humoral immunity than their male counterparts (Ahmed et al, 1985; Ahmed & Talal, 1990; Grossman, 1984), by showing higher immunoglobulin levels in serum, particularly IgM (Allansmith et al, 1968). This gender-specific differences in serum IgA, IgG and IgM levels could mainly due to the effects of sex hormones (Ahmed et al, 1985; Ahmed & Talal, 1990). NPMs were characterized with similar levels of serum IgG and IgM, but lower level of serum IgA compared with rhesus macaques and cynomolgus macaques. However, the serum IgA levels between rhesus macaques and cynomolgus macaques was similar (Cole & Bowen, 1976; Miller et al, 1992; Patterson et al, 1976). This phenomenon could be explained by the perspectives of species evolution. Pig-tailed macaques were estimated to have evolutionarily differentiated from rhesus macaques and cynomolgus macaques approximately 5 million years ago (mya). However, the divergence between rhesus macaques and cynomolgus macaques occurred approximately 2.4 mya (Morales & Melnick, 1998). Additionally, blood chemistry and hematology parameters of captive populations of NPMs also have indicated that the differences between NPMs and rhesus macaques or cynomolgus macaques were greater than those between rhesus macaques and cynomolgus macaques (Pang et al, 2013). As the most important and richest immunoglobulin in the mucosal immune system, the low level of IgA in the mucosal immune system may result in reduced mucosal resistance to microbial infection. In the future, it may be worth exploring and then exploiting whether or not the lower serum IgA level is related with the lower IgA of the mucosal immune system. Consequently, understanding the differences of serum and mucosal IgA levels between NPMs and other experimental non-human primates will likely benefit from its application to infectious disease studies, vaccine evaluation, as well as other fields of viral immunology.
Compared with antibodies, the complement system had an earlier origin. Complements not only act as cofactors and enhancers of antibody activities, but also have other biological functions independent of antibodies. The complement system plays an important role in innate defense against common pathogens, the modulation of inflammatory response and coagulation. For example, C3 is critical in activating the whole complement system, and C4 is the major protein of classical pathways (Kasperska-Zajac et al, 2013). As such, determining the serum levels of C3 and C4 in healthy population is helpful in disease diagnosis. The levels of complements increased after delivery, so that by 3-6 months of age the mean levels of serum C3 and C4 in the infant populations are already similar to those in the normal adults (Fireman et al, 1969). Our results showed that serum C3 and C4 levels did not fluctuate with age (3-11 years old), which was consistent with Cheng et al’s (2003) study on rhesus macaques. While serum C3 levels of female NPMs were higher than those of males, no significant gender-specific differences were found in serum C4 levels. This phenomenon could be explained by sex hormone differences, since it has long been documented that sex hormones can markedly affect the immune system (Ahmed et al, 1985; Verthelyi & Klinman, 2000), and also influence pro-inflammatory cytokines IL-6 and IL-1 production (Angstwurm et al, 1997; Cannon & Dinarello, 1985). Moreover, evidence suggested that IL-1 and IL-6 have an enhancing effect on the production of C3, whereas neither IL-1 nor IL-6 affect the biosynthesis of C4 (Falus et al, 1990). Serum C3 levels of NPMs were close to those of rhesus macaques and cynomolgus macaques, whereas, serum C4 levels of NPMs were higher than those of rhesus macaques but lower than those of cynomolgus macaques (Cheng et al, 2003; Poskitt et al, 1974), which had indicated that non-human primates displayed marked interspecies variations regarding complements (Ellingsworth et al, 1983). Additionally, the changes in complement pathway activities were involved in the acute rejection after xenotransplantation and the pathogenesis of systemic autoimmune diseases (Chen et al, 2010; Saadi et al, 2004; Yu & Whitacre, 2004). Ultimately then, when non-human primate animal models are applied to studying human complement-related diseases, it is necessary to consider and understand the species-specific characteristics of the complement systems.
CRP is a classical acute-phase serum protein, which interacts with complements, neutrophils, monocytes, etc, and functions not only as an inflammation regulator, but also as a host defender against infection. CRP is primarily synthesized in livers and is simultaneously released into the bloodstream during acute phase response. CRP testing has been applied in differential diagnosis of infectious diseases (Du Clos & Mold, 2004), assessing cardiovascular risks (Albert et al, 2002) and monitoring autoimmune diseases progression. Previous studies reported that both the detection methods and many other factors may influence the quantifications of serum CRP levels (Zhang et al, 2011; Khera et al, 2005)). In our study, no detectable signals of CRP were found In 34 serum samples. We assume this is due to traces of serum CRP in NPMs had outreached the instrumental detection limit (0.01 mg/L), which is special designed for human beings.
We also analyzed correlations among serum IgA, IgG, IgM, C3 and C4 levels of NPMs. As we known, complement systems, immunoglobulins, and the related components are closely interacted with each other. Serum levels of certain complement components are highly correlated with those of certain immunoglobulins (Plebani et al, 1984), and are functioned as bridges between innate and adaptive immune responses. The classic pathway is activated by immune complexes of IgG, IgM and complements, meanwhile, IgG and IgA immune complexes are acting as activators of the alternative pathways (Roach et al, 1981; Wagner & Frank, 2009). In addition, pro-inflammatory cytokines, for example IL-6, are modulators of the levels of lgG, IgM, IgA and some complements (Maes et al, 1997; Ritchie et al, 2004). Roach et al (1981) found that serum components of certain classical pathways and their alternative pathways were significantly correlated (r > 0.537). Yilmazer et al (2003) showed that serum C3 and C4 levels were highly correlated (r > 0.6, P < 0.001). In some diseases, the correlations between serum complement components and immunoglobulins levels was markedly altered (Gewurz et al, 1968; Kohler & Muller-Eberhard, 1969). In our study, we found significant positive correlations between the following pairs: IgA and IgM, C3 and IgM, C3 and IgA, C3 and IgG, C3 and C4, all of which are consistent with the findings in other species.
In conclusion, this study shows that serum IgA, IgM, C3 and C4 levels in NPMs were irrelevant with age, whereas, IgG levels increased progressively with age. Serum IgG, IgA, IgM and C3 levels were higher in females than in males, meanwhile, C3 concentrations were positively and closely correlated with those of C4. These basic data of captive NPMs may, in the future, promote its future and more detailed application to infectious diseases, immunology and other fields of biomedical research.
Funding Statement
This work was supported by the National Natural Science Foundation of China (81172876, U0832601, 81273251, U1202228); the National Special Science Research Program of China (2012CBA01305); the National Science and Technology Major Project (2013ZX10001-002, 2012ZX10001-007) and the Knowledge Innovation Program of CAS (KSCX2-EW-R-13)
REFERENCES
- 1. Ahmed SA, Penhale W, Talal N. 1985. Sex hormones, immune responses, and autoimmune diseases. Mechanisms of sex hormone action. The American Journal of Pathology, 121 (3): 531- 551. [PMC free article] [PubMed] [Google Scholar]
- 2. Ahmed SA, Talal N. 1990. Sex hormones and the immune system-part 2. Animal data. Baillieres Clinical Rheumatology, 4 (1): 13- 31. [DOI] [PubMed] [Google Scholar]
- 3. Albert CM, Ma J, Rifai N, Stampfer MJ, Ridker PM. 2002. Prospective study of C-reactive protein, homocysteine, and plasma lipid levels as predictors of sudden cardiac death. Circulation, 105 (22): 2595- 2599. [DOI] [PubMed] [Google Scholar]
- 4. Allansmith M, Mcclellan B, Butterworth M, Maloney J. 1968. The development of immunoglobulin levels in man. The Journal of Pediatrics, 72 (2): 276- 290. [DOI] [PubMed] [Google Scholar]
- 5. Angstwurm MW, Gärtner R, Ziegler-Heitbrock H. 1997. Cyclic plasma IL-6 levels during normal menstrual cycle. Cytokine, 9 (5): 370- 374. [DOI] [PubMed] [Google Scholar]
- 6. Axberg I, Gale Jr MJ, Afar B, Clark EA. 1991. Characterization of Tcell subsets and T-cell receptor subgroups in pigtailed macaques using two-and three-color flow cytometry. Journal of Clinical Immunology, 11 (4): 193- 204. [DOI] [PubMed] [Google Scholar]
- 7. Cannon JG, Dinarello CA. 1985. Increased plasma interleukin-1 activity in women after ovulation. Science, 227 (4691): 1247- 1249. [DOI] [PubMed] [Google Scholar]
- 8. Chen M, Daha MR, Kallenberg CG. 2010. The complement system in systemic autoimmune disease. Journal of Autoimmunity, 34 (3): 276- 286. [DOI] [PubMed] [Google Scholar]
- 9. Chen Y, Li CH, Tong PF, Sun XM. 2006. Analyze on immunoglobulin IgG, IgM, IgA, Complement C3 and C4 in laboratory rhesus monkey (macaca mulatta) of different ages. Chinese Journal of Comparative Medicine, 16 (5): 257- 259. [Google Scholar]
- 10. Cheng SJ, Huang R, Huang ZY, Yu B. 2003. Antibody and complement levels in serum of rhesus monkey. Chinese Journal of Veterinary Medicine, 39 (4): 14- 15. [Google Scholar]
- 11. Cole MF, Bowen WH. 1976. Immunoglobulins A, G, and M in serum and in some secretions of monkeys (Macaca fascicularis syn. irus). Infection and Immunity, 13 (5): 1354- 1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Curry BB. 2001. Animal models used in identifying gender-related differences. International Journal of Toxicology, 20 (3): 153- 160. [DOI] [PubMed] [Google Scholar]
- 13. Ellingsworth LR, Holmberg CA, Osburn BI. 1983. Hemolytic complement measurement in eleven species of nonhuman primates. Veterinary Immunology and Immunopathology, 5 (2): 141- 149. [DOI] [PubMed] [Google Scholar]
- 14. Falus A, Rokita H, Walcz E, Brozik M, Hidvegi T, Merétey K. 1990. Hormonal regulation of complement biosynthesis in human cell lines. Ⅱ. Upregulation of the biosynthesis of complement components C3, factor B and Cl inhibitor by interleukin-6 and interleukin-1 in human hepatoma cell line. Molecular Immunology, 27 (2): 197- 201. [DOI] [PubMed] [Google Scholar]
- 15. Fireman P, Zuchowski DA, Taylor PM. 1969. Development of human complement system. The Journal of Immunology, 103 (1): 25- 31. [PubMed] [Google Scholar]
- 16. Flores BM, Fennell CL, Kuller L, Bronsdon MA, Morton WR, Stamm WE. 1990. Experimental infection of pig-tailed macaques (Macaca nemestrina) with Campylobacter cinaedi and Campylobacter fennelliae. Infection and Immunology, 58 (12): 3947- 3953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fujimoto K, Terao K, Cho F, Nakamura F, Honjo S. 1982. Age-related immunoglobulin levels in cynomolgus monkeys. Japanese Journal of Medical Science and Biology, 35 (1): 17- 23. [DOI] [PubMed] [Google Scholar]
- 18. Gewurz H, Pickering RJ, Christian CL, Snyderman R, Mergenhagen SE, Good RA. 1968. Decreased C'1q protein concentration and agglutinating activity in agammaglobulinaemia syndromes: an inborn error reflected in the complement system. Clinical and Experimental Immunology, 3 (5): 437- 445. [PMC free article] [PubMed] [Google Scholar]
- 19. Grossman CJ. 1984. Regulation of the immune system by sex steroids. Endocrine Reviews, 5 (3): 435- 455. [DOI] [PubMed] [Google Scholar]
- 20. Groves CP. 2001. Primate Taxonomy. Washington DC: Smithsonian Institution Press; 222 [Google Scholar]
- 21. Jinbo T, Hayashi S, Iguchi K, Shimizu M, Matsumoto T, Naiki M, Yamamoto S. 1998. Development of monkey C-reactive protein (CRP) assay methods. Veterinary Immunology and Immunopathology, 61 (2-4): 195- 202. [DOI] [PubMed] [Google Scholar]
- 22. Kasperska-Zajac A, Grzanka A, Machura E, Misiolek M, Mazur B, Jochem J. 2013. Increased serum complement C3 and C4 concentrations and their relation to severity of chronic spontaneous urticaria and CRP concentration. Journal of Inflammation, 10 (1): 1- 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Khera A, Mcguire DK, Murphy SA, Stanek HG, Das SR, Vongpatanasin W, Wians FH, Grundy SM, De Lemos JA. 2005. Race and gender differences in C-reactive protein levels. Journal of American College of Cardiology, 46 (3): 464- 469. [DOI] [PubMed] [Google Scholar]
- 24. Knapp LA, Ha JC, Sackett GP. 1996. Parental MHC antigen sharing and pregnancy wastage in captive pigtailed macaques. Journal of Reproductive Immunology, 32 (1): 73- 88. [DOI] [PubMed] [Google Scholar]
- 25. Kohler PF, Muller-Eberhard HJ. 1969. Complement-immunoglobulin relation: Deficiency of C'lq associated with impaired immunoglobulin G synthesis. Science, 163 (3866): 474- 475. [DOI] [PubMed] [Google Scholar]
- 26. Kuang YQ, Tang X, Liu FL, Jiang XL, Zhang YP, Gao GX, Zheng YT. 2009. Genotyping of TRIM5 locus in Northern pig-tailed macaques (Macaca leonina), a primate species susceptible to human immunodeficiency virus type 1 infection. Retrovirology, 6 (1): 58- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lei A, Pang W, Zhang GH, Zheng YT. 2013. Use and research of pigtailed macaques in nonhuman primate HIV/AIDS models. Zoological Research, 34 (2): 77- 88. [DOI] [PubMed] [Google Scholar]
- 28. Li X, Polacino P, Garcia-Navarro R, Hu SL, Tsuji M. 2012. Peripheral blood invariant natural killer T cells of pig-tailed macaques. PLoS One, 7 (10): e48166- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Macellini S, Ferrari PF, Bonini L, Fogassi L, Paukner A. 2010. A modified mark test for own-body recognition in pig-tailed macaques (Macaca nemestrina). Animal Cognition, 13 (4): 631- 639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Maes M, Hendriks D, Van Gastel A, Demedts P, Wauters A, Neels H, Janca A, Scharpé S. 1997. Effects of psychological stress on serum immunoglobulin, complement and acute phase protein concentrations in normal volunteers. Psychoneuroendocrinology, 22 (6): 397- 409. [DOI] [PubMed] [Google Scholar]
- 31. Mayer P, Geissler K, Ward M, Metcalf D. 1993. Recombinant human leukemia inhibitory factor induces acute phase proteins and raises the blood platelet counts in nonhuman primates. Blood, 81 (12): 3226- 3233. [PubMed] [Google Scholar]
- 32. Miller C, Kang D, Marthas M, Moldoveanu Z, Kiyono H, Marx P, Eldridge J, Mestecky J, Mcghee J. 1992. Genital secretory immune response to chronic simian immunodeficiency virus (SIV) infection: a comparison between intravenously and genitally inoculated rhesus macaques. Clinical and Experimental Immunology, 88 (3): 520- 526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Morales JC, Melnick DJ. 1998. Phylogenetic relationships of the macaques (Cercopithecidae: Macaca), as revealed by high resolution restriction site mapping of mitochondrial ribosomal genes. Journal of Human Evolution, 34 (1): 1- 23. [DOI] [PubMed] [Google Scholar]
- 34. Pang W, Lü LB, Wang Y, Li G, Huang DT, Lei AH, Zhang GH, Zheng YT. 2013. Measurement and analysis of hematology and blood chemistry parameters in northern pig-tailed macaques (Macaca leonina). Zoological Research, 34 (2): 89- 96. [DOI] [PubMed] [Google Scholar]
- 35. Patterson R, Harris K, Suszko I, Roberts M. 1976. Reagin-mediated asthma in rhesus monkeys and relation to bronchial cell histamine release and airway reactivity to carbocholine. The Journal of Clinlinical Investigation, 57 (3): 586- 593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Patton DL, Cosgrove-Sweeney YT, Rabe LK, Hillier SL. 2001. The pig-tailed macaque rectal model: microflora and chlamydial infection. Sexually Transmitted Diseases, 28 (7): 363- 366. [DOI] [PubMed] [Google Scholar]
- 37. Plebani A, Notarangelo L, Duse M, Avanzini A, Massa M, Ugazio A. 1984. Serum IgG levels and complement activity in hypogammaglobulinaemic patients under substitution therapy. Clinical Experimental Immunology, 58 (1): 193- 198. [PMC free article] [PubMed] [Google Scholar]
- 38. Poskitt TR, Fortwengler Jr HP, Bobrow JC, Roth GJ. 1974. Naturally occurring immune-complex glomerulonephritis in monkeys (Macaca irus): I. Light, immunofluorescence and electron microscopic studies. The American Journal of Pathology, 76 (1): 145- 164. [PMC free article] [PubMed] [Google Scholar]
- 39. Rausell E, Bickford L, Manger PR, Woods TM, Jones EG. 1998. Extensive divergence and convergence in the thalamocortical projection to monkey somatosensory cortex. The Journal of Neuroscience, 18 (11): 4216- 4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ritchie RF, Palomaki GE, Neveux LM, Navolotskaia O, Ledue TB, Craig WY. 2004. Reference distributions for complement proteins C3 and C4: a practical, simple and clinically relevant approach in a large cohort. Journal of Clinical Laboratory Analysis, 18 (1): 1- 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Roach B, Kim Y, Jerome E, Michael AF. 1981. Influence of age and sex on serum complement components in children. Archives of Pediatrics and Adolescent Medicine, 135 (10): 918- 920. [DOI] [PubMed] [Google Scholar]
- 42. Saadi S, Takahashi T, Holzknecht RA, Platt JL. 2004. Pathways to acute humoral rejection. The American Journal of Pathology, 164 (3): 1073- 1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shaulov A, Murali-Krishna K. 2008. CD8 T cell expansion and memory differentiation are facilitated by simultaneous and sustained exposure to antigenic and inflammatory milieu. The Journal of Immunology, 180 (2): 1131- 1138. [DOI] [PubMed] [Google Scholar]
- 44. Shuster J, Warner NL, Fudenberg HH. 1969. Discussion paper: crossreactivity of primate immunoglobulins. Annals of the New York Academy of Sciences, 162 (1): 195- 201. [DOI] [PubMed] [Google Scholar]
- 45. Socha W, Blancher A, Ruffie J. 1993. Comparative study of human monoclonal anti-D antibodies of IgG and IgM classes in tests with red cells of nonhuman primates. Revue Française de Transfusion et d'Hémobiologie, 36 (6): 485- 497. [DOI] [PubMed] [Google Scholar]
- 46. Sussman A, Ha J. 2011. Developmental and cross-situational stability in infant pigtailed macaque temperament. Developmental Psychology, 47 (3): 781- 791. [DOI] [PubMed] [Google Scholar]
- 47. Verthelyi D, Klinman D. 2000. Sex hormone levels correlate with the activity of cytokine - secreting cells in vivo. Immunology, 100 (3): 384- 390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wagner E, Frank MM. 2009. Therapeutic potential of complement modulation. Nature Reviews Drug Discovery, 9 (1): 43- 56. [DOI] [PubMed] [Google Scholar]
- 49. Watts KL, Nelson V, Wood BL, Trobridge GD, Beard BC, Humphries RK, Kiem HP. 2012. Hematopoietic stem cell expansion facilitates multilineage engraftment in a nonhuman primate cord blood transplantation model. Experimental Hematology, 40 (3): 187- 196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yilmazer M, Fenkci V, Fenkci S, Aktepe O, Sonmezer M, Kurtay G. 2003. Association of serum complement (C3, C4) and immunoglobulin (IgG, IgM) levels with hormone replacement therapy in healthy post-menopausal women. Human Reproduction, 18 (7): 1531- 1535. [DOI] [PubMed] [Google Scholar]
- 51. Yoshino N, Ami Y, Terao K, Tashiro F, Honda M. 2000. Upgrading of flow cytometric analysis for absolute counts, cytokines and other antigenic molecules of cynomolgus monkeys (Macaca fascicularis) by using anti-human cross-reactive antibodies. Experimental Animal, 49 (2): 97- 110. [DOI] [PubMed] [Google Scholar]
- 52. Yu CY, Whitacre CC. 2004. Sex, MHC and complement C4 in autoimmune diseases. Trends in Immunology, 25 (12): 694- 699. [DOI] [PubMed] [Google Scholar]
- 53. Zhang XH, Li GT, Zhang ZL. 2011. Clinical significances of C-reactive protein and hypersensitive C-reactive protein. Chinese Journal of Allergy and Clinical Immunology, 5 (1): 74- 79. [Google Scholar]
- 54. Zhu L, Zhang GH, Zheng YT. 2010. Application studies of animal models in evaluating safety and efficacy of HIV-1 microbicides. Zoological Research, 31 (1): 66- 76. [DOI] [PubMed] [Google Scholar]


