ABSTRACT
Thermosensors detect temperature changes and trigger cellular responses crucial for survival at different temperatures. The thermosensor DesK is a transmembrane (TM) histidine kinase which detects a decrease in temperature through its TM segments (TMS). Here, we address a key issue: how a physical stimulus such as temperature can be converted into a cellular response. We show that the thickness of Bacillus lipid membranes varies with temperature and that such variations can be detected by DesK with great precision. On the basis of genetic studies and measurements of in vitro activity of a DesK construct with a single TMS (minimal sensor DesK [MS-DesK]), reconstituted in liposomes, we propose an interplay mechanism directed by a conserved dyad, phenylalanine 8-lysine 10. This dyad is critical to anchor the only transmembrane segment of the MS-DesK construct to the extracellular water-lipid interphase and is required for the transmembrane segment of MS-DesK to function as a caliper for precise measurement of membrane thickness. The data suggest that positively charged lysine 10, which is located in the hydrophobic core of the membrane but is close to the water-lipid interface, pulls the transmembrane region toward the water phase to localize its charge at the interface. Nevertheless, the hydrophobic residue phenylalanine 8, located at the N-terminal extreme of the TMS, has a strong tendency to remain in the lipid phase, impairing access of lysine 10 to the water phase. The outcome of this interplay is a fine-tuned sensitivity to membrane thickness that elicits conformational changes that favor different signaling states of the protein.
IMPORTANCE The ability to sense and respond to extracellular signals is essential for cell survival. One example is the cellular response to temperature variation. How do cells “sense” temperature changes? It has been proposed that the bacterial thermosensor DesK acts as a molecular caliper measuring membrane thickness variations that would occur as a consequence of temperature changes and activates a pathway to restore membrane fluidity at low temperature. Here, we demonstrated that membrane thickness variations do occur at physiological temperatures by directly measuring Bacillus lipid membrane thickness. We also dissected the N-terminal sensing motif of MS-DesK at the molecular-biophysical level and found that the dyad phenylalanine-lysine at the water-lipid phase is critical for achievement of a fine-tuned sensitivity to temperature.
INTRODUCTION
The ability to sense and respond to subtle variations in environmental temperature is critical for all kingdoms of life. Temperature changes modify protein activity directly but also indirectly, by modifying biophysical properties of membrane lipids, thereby affecting the activity of membrane-embedded or membrane-associated proteins and threatening the viability of the cell (1–3).
DesK is a transmembrane (TM) histidine kinase which, together with its cognate response regulator, DesR, operates in Bacillus subtilis to maintain membrane fluidity at low temperature. Upon cooling, DesK phosphorylates the response regulator DesR, which induces transcription of the fatty acid desaturase Δ5-Des, which is encoded by the des gene (4, 5). This DesK-dependent introduction of unsaturated fatty acids into the bacterial membrane enhances survival at low temperatures (Fig. 1) (6–8).
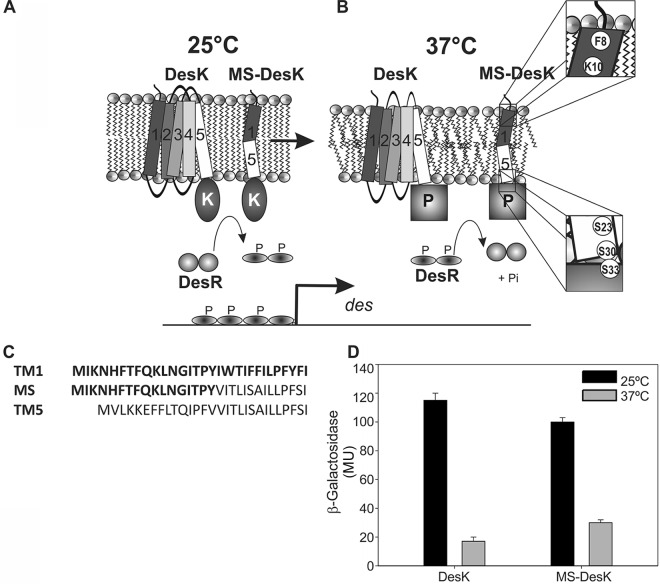
FIG 1.
Cold detection in B. subtilis. (A) The histidine kinase DesK (five TM-spanning segments) and the synthetic MS-DesK protein localize at the lipid bilayer. At low temperature (25°C), the level of order in the structures of the acyl chains of lipids increases, promoting a kinase-active state. DesK autophosphorylates and transfers the phosphoryl group to Asp54 of the DesR dimeric response regulator (two gray circles), inducing a conformational change that results in exposure of the helix-turn-helix (HTH)–DNA-binding domain. Two dimers of phosphorylated DesR bind two adjacent, nonidentical binding sites within the des promoter (40), leading to recruitment of the RNA polymerase to activate expression of the desaturase gene. (B) At higher temperature (37°C), the disordered lipids favor the phosphatase-active state of DesK, leading to dephosphorylation of phospho-DesR; thus, des transcription is turned off. The dyad Phe8-Lys10 and the serine zipper are highlighted in the insets to show their locations at the water-lipid interphase. (C) Sequence of the functional synthetic TMS of minimal sensor DesK and the nonfunctional TM1 and TM5. (D) DesK and MS kinase activities at 25°C and 37°C. MU, Miller units. (Adapted from reference 10 with permission of the publisher.)
Experimental evidence indicates that the transmembrane domain of the protein is in charge of sensing temperature variations, because a truncated version of DesK that lacks the TM region is insensitive to temperature (9, 10). In addition, previous studies demonstrated that DesK can be activated at high temperatures if the fluidity of the plasma membrane is decreased by restricting the incorporation of low-melting-point anteiso fatty acids, giving support to the idea that DesK directly responds to a physical property of the plasma membrane rather than to temperature itself (7). How could the activity of DesK be regulated by a physical environmental cue such as temperature? The five-pass transmembrane domain from DesK could be simplified into a minimal sensor containing a single membrane-spanning segment (minimal sensor DesK [MS-DesK]). In this construct, the first 17 residues of the N-terminal domain of TM1 are peptidically linked to the last 14 residues of the C-terminal domain of TM5. To complete the sensor, the catalytic cytoplasmic region is fused to the artificial transmembrane segments (TMS). MS-DesK retains, in vivo and in vitro, the complete sensing and transmission properties of the intact DesK protein (Fig. 1) (10). Given its simplicity, it was named minimal sensor DesK (MS-DesK). A multidisciplinary approach applied to this construct has identified hydrophilic motifs at each end of the MS-DesK TMS that are involved in thermosensing by DesK. It has been proposed that a lysine residue (K10) located near the N terminus of the TMS and a serine zipper located at the C terminus of the TMS, which is connected to the linker region at the intracellular membrane-water interface, act together as a molecular caliper to detect changes in membrane thickness that occur as a consequence of temperature variations (Fig. 1) (10–12). It has been reported that the thickness of lipid bilayers formed by pure synthetic phospholipids increases when temperature decreases (13, 14). Our hypothesis is that Bacillus membrane lipids behave in a similar way such that the membrane thickens at low temperature and K10 buries deeper into the hydrophobic core of the membrane, an energetically unfavorable situation that results in a kinase-activated state (10). This proposal is supported by several lines of genetic evidence. For example, a mutation that introduces an extra lysine deeper inside the transmembrane domain (MS-DesK variant L11K) results in constitutive activation of the des gene regardless of temperature. This is probably because—even at higher temperature, when the membrane is thinner—a lysine at position 11 cannot hydrate at the water-lipid interphase and therefore continues pulling the TMS outward. In contrast, if lysine 10 is replaced by leucine (K10L), a hydrophobic residue, des transcriptional activity is null. A likely explanation is that this mutant protein lacks the driving force that pulls the N terminus of the TMS to the water phase. The replacement of lysine by leucine elongates the hydrophobic stretch of the TMS, which can easily match the increase in membrane thickness upon a temperature downshift (10). These studies have suggested that regulation of DesK is modulated by the position of K10 with respect to the water-lipid interphase and that the activity therefore depends on changes in membrane thickness. This straightforward hypothesis requires the support of biochemical and biophysical experiments. In this study, small-angle X-ray scattering (SAXS) assays were performed to measure variations in the membrane thickness of B. subtilis lipid samples to test our hypothesis that the temperature-dependent change in membrane thickness is sensed by the MS-DesK. We also developed a reconstitution protocol to integrate the MS-DesK construct, as well as several of its variants, into proteoliposomes in order to characterize them biochemically. This procedure enabled us to directly correlate the in vitro assays with in vivo measurements, which utilize a reporter gene, the gene encoding β-galactosidase. The present work validates the use of in vivo β-galactosidase assays as a simple and reliable method to study the DesK signaling pathway. Mutational studies of the N terminus of the TMS suggest that lysine 10 works together with phenylalanine 8 to anchor the TMS at the external water-lipid phase, which is key to achieving a fine-tuned sensitivity to membrane thickness variations.
The molecular-caliper mechanism proposed here for DesK thermosensing extends the conceptual framework suggesting that ruler-like mechanisms control different processes in nature, such as the length of very-long-chain fatty acids (VLCFAs) (15), the needle length of type III secretion machines (16), and the length of the bacteriophage lambda tail (17).
MATERIALS AND METHODS
Lipid purification and SAXS measurements.
Bacillus subtilis wild-type cells were grown at 37°C to an optical density (OD) of 0.8. Cells were harvested and lipids were prepared following the method of Bligh and Dyer (18). Lipids were hydrated in 10 mM HEPES–145 mM NaCl (pH 8). The resulting suspensions of large multilamellar vesicles were disrupted by 10 freeze-thaw cycles. For unilamellar liposome generation, lipid dispersions were extruded 20 times through a Whatman polycarbonate filter (100-nm pore size) using a hand-held extrusion device (Avanti).
The SAXS assays were performed at the SAXS2 beamline at the Brazilian Synchrotron Light Laboratory (LNLS) at Campinas, Brazil. Data were collected by a MarCCD detector system and radially integrated using FIT2D V 12.077 from Andy Hammersley at the European Synchrotron Radiation Facility (ESRF). The data were fitted to the commonly used three-layer model, in which the three layers represent (i) the end methyl group of the acyl chains (low electron density), (ii) the central core of the acyl chains (medium electron density), and (iii) the head groups (high electron density). This method allows estimation of lengths perpendicular to the membrane, electron density, and roughness for each slab (19, 20). The roughness or smearing at each slab interface, thickness, and electron density were fitted with starting values for diacylphosphyatidylglycerol (19), which is the major lipid of the ensemble. Hydrophobic thickness was calculated from the addition of the thicknesses from layers corresponding to the methyl and core acyl-chain layers. The absence of diffraction peaks in the raw SAXS data indicates that the membranes are unilamellar.
We found that the thermal-expansion coefficient—which measures the fractional increment in thickness (∂L/liter per Celsius degree) was 3.1 10−3 K−1, which is in the range of common values for lipid membranes (13).
Plasmid and strain constructions.
The MS-desK gene and its variants were PCR amplified from plasmid TM1/5-DesKCpHPKS (10). To purify MS-DesK variants, mutations were introduced using the overlap PCR method and amplicons cloned into the NdeI-SalI sites of expression vector pET22 (Novagen), which places the coding regions under the control of the T7 promoter, including a His6 tag at the C terminus. The resulting plasmids were used to transform BL21 cells for protein overexpression. QuikChange mutagenesis (Stratagene) was performed using plasmid pHPKS-MS (10) to introduce the mutations (single mutations K10E, K10R, F8A, F8L, and F8W and double mutations K10L-F8A and F8A-L11K). The resulting plasmids were used to transform CM21 B. subtilis cells (4, 9). This strain lacks DesK and contains a transcriptional fusion between the β-galactosidase reporter gene and the promoter of the des gene that is upregulated by DesK-DesR at low temperature to allow monitoring of DesK activity. To induce the expression of DesK variants, 0.1% xylose was added to the growth medium.
All mutations were confirmed by DNA sequence analysis. Descriptions of the oligonucleotides used for mutagenesis, strains, full sequences, and detailed construction methods are available upon request.
Bacterial strains and growth conditions.
For β-galactosidase measurements, the B. subtilis JH642- CM21 cells complemented with plasmids encoding MS-DesK variants were grown at 25°C or 37°C, with 250-rpm gyration in Spizizen salts supplemented with 0.1% glycerol, a 50 μg/ml concentration of tryptophan and a 50 μg/ml concentration of phenylalanine, 0.05% Casamino Acids, and trace elements (21, 22). The results shown represent averages from three independent assays for β-galactosidase activity and correspond to 4 h after the shift from 37°C to 25°C. Standard deviations were calculated from the results of at least three independent experiments performed in duplicate. They are represented by error bars (see Fig. 5).
FIG 5.
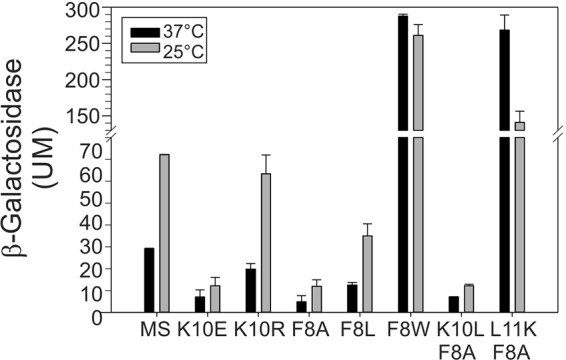
The dyad F8-K10 is critical for determining the signaling state of MS-DesK. Different MS-DesK variants with mutations in the dyad F8-K10 were analyzed for in vivo activity. The conservative replacement K10R does not eliminate thermoregulation of MS-DesK activity; however, the replacement of K10L and K10E abolished high kinase activity at low temperatures, suggesting that a positive charge is required at position 10. The conservative replacement F8L also maintains regulation, as expected, because leucine and phenylalanine have the same hydrophobic profile. F8A behaves much like K10E, probably because of the loss of hydrophobicity at residue 8. F8W is constitutively active due to the strong preference of Trp for the water-lipid interphase. The double mutant F8A-K10L lacks kinase activity, since both components of the thermosensitive dyad are eliminated. The double mutant F8A-L11K is also constitutively active; since the presence of an extra lysine residue located deeper (1.5 Å) inside the membrane exerts an outward pull on the TMS even at higher temperatures, when the membrane is thinner. β-Galactosidase activity was determined as described for Fig. 4B.
Protein overexpression and purification.
Protein overexpression and purification were performed following the Studier method for autoinduction of protein expression in the T7 system (23). The pelleted cells were resuspended in TNP buffer (50 mM Tris [pH 8], 200 mM NaCl, 1 mM phenylmethylsulfonyl fluoride) supplemented with 20 mM imidazole and a mixture of solubilizing detergents (Triton X-100 and Brij 58 [both at 0.5%]) in order to get mixed lipid-protein-detergent micelles. After a treatment with lysozyme at 1 mg/ml, cells were disrupted by sonication and then centrifuged at 37,000 × g for 15 min. MS-DesK was purified from the supernatant by affinity chromatography using a Ni2+-nitrilotriacetic acid-agarose resin (Qiagen). The His6-tagged MS-DesK eluted mainly at 500 mM imidazole and was dialyzed against a mixture containing 50 mM Tris (pH 8), 200 mM NaCl, 10% glycerol, and 1 mM dithiothreitol. The MS-DesK protein concentration was determined by densitometry. The His6-tagged cytoplasmic domain of DesK (His6-DesKC) and fusion protein DesR–glutathione S-transferase (DesR-GST) were overexpressed in Escherichia coli and purified as previously described (9).
Preparations of preformed liposomes and reconstitution of membrane proteins.
To obtain small unilamellar vesicles, 8-mg amounts of lipids (E. coli polar lipids from Avanti) were hydrated and subjected to vortex mixing in hydration buffer (20 mM Tris-HCl [pH 8], 250 mM sucrose, 100 mM K2SO4). Unilamellar liposomes were prepared as described above.
To disrupt the ordered structure of the liposomes, 0.24% Triton X-100 was added to the preformed liposomes and the mixture was incubated for 20 min with continuous stirring at 25°C. Purified MS-DesK was added to the destabilized liposomes (80/1 [wt/wt] lipid-to-protein ratio) and incubated with continuous stirring for 1 h at 4°C. The detergent was removed by incubating the sample three times with SM2 Bio-Beads (Bio-Rad) using gentle agitation for 8 to 12 h at 4°C.
Proteoliposome purification step.
To separate MS-DesK-containing proteoliposomes from free liposomes, sucrose gradient ultracentrifugation was performed. The supernatant collected from the Bio-Beads treatment (1 ml) was placed on the bottom of a step sucrose gradient (1.6, 1.2, and 0.2 M sucrose) and centrifuged overnight at 30,000 rpm in a SW40 rotor at 4°C. After centrifugation, the proteoliposome band, which floated on top of the gradient, was harvested, washed with 30 mM Tris–HCl (pH 8.0), and ultracentrifuged in a Ti90 rotor at 45,000 rpm for 1 h at 4°C. Finally, proteoliposomes containing MS-DesK were resuspended in 200 μl of hydration buffer containing 10% glycerol–1 mM dithiothreitol (DTT). The protein concentration was determined by the Lowry assay, and the quality of the sample and efficiency of protein integration were determined by SDS-PAGE followed by Western blotting using anti-His antibodies (Qiagen) and anti-DesKC antibodies. The amount of protein integrated into liposomes was determined by staining SDS-PAGE gels with Coomassie blue followed by densitometry. This quantification allows loading equal amounts of proteins regardless of variations in the efficiency of protein integration.
In vitro phosphorylation, phosphotransfer, and dephosphorylation assays.
These reactions were performed essentially as previously described (24). Briefly, for the autokinase assay, proteoliposomes containing 0.3 mg of MS-DesK protein were incubated at 25°C or 37°C with radioactive ATP (50 mM Tris-HCl [pH 8], 200 mM NaCl, 1 mM dithiothreitol, 20% [vol/vol] glycerol, 50 mM KCl, 1 mM MgCl2, 25 mM ATP, 0.25 mCi/ml [γ-32P]ATP); at different times, aliquots were removed and subjected to SDS-PAGE on 12% polyacrylamide gels.
To purify phosphorylated GST-DesR (GST-DesR-P), GST-DesR was bound to glutathione-agarose resin for 1 h at 4°C. Autophosphorylated DesKC was added to the column containing GST-DesR to allow the phosphor transfer from DesKC-P to GST-DesR. The reaction was stopped by the addition of 10 mM EDTA. The column was washed with reaction buffer, and GST-DesR-P was eluted with glutathione. Purified GST-DesR-P was immediately used for stability or phosphatase assays. For evaluation of GST-DesR-P stability, aliquots were taken at various time points, and the reaction was stopped with 5× SDS-PAGE loading buffer containing 50 mM EDTA and subjected to SDS-PAGE on 12% polyacrylamide gels. To test phosphatase activity, GST-DesR-P was mixed with proteoliposomes containing DesK variants. Samples were withdrawn at various time points, and the reaction was stopped as described above. The radioactivity of phosphorylated proteins in gels was visualized using a Typhoon 9200 PhosphorImager screen (STORM840; GE Healthcare) and quantified using ImageQuant software (version 5.2). The values obtained were expressed as percentages of total GST-DesR protein. Coomassie blue loading controls were run to confirm the amount of protein loaded in each lane.
Protocol for ADP-Glo kinase assay.
The kinase activity of each DesK variant reconstituted in liposomes was measured using the ADP-Glo kinase assay following the protocol described by the provider (Promega).The advantage of this method is that it allows performing precise measurements in a very short time by quantifying the amount of ADP produced during the kinase reaction. It requires only a small amount of sample and can be performed at high substrate concentrations (up to 1 mM ATP) so that the maximum catalytic velocity can be measured. All results shown are representative of at least three independent experiments.
RESULTS
Determination of B. subtilis membrane thickness as a function of temperature.
In order to test whether variations in membrane thickness occur in Bacillus lipid membranes when temperature changes within the physiological range, we measured membrane thickness in the range of 20 to 40°C, by using small-angle X-ray scattering (SAXS). B. subtilis lipids were purified from cells grown at 37°C, and unilamellar liposomes were prepared using the protocol described in Materials and Methods. The calculated SAXS curves are similar because the structure of the bilayered membrane is maintained at different temperatures. Nevertheless, the widening of the curves and the consequent shift to higher q values for q = >2 nm−1 indicates a thinning of the membrane upon heating (19, 20). This analysis showed that when Bacillus membranes are cooled from 37 to 25°C, the hydrophobic thickness increases by 1 Å. This result supports our hypothesis that the thickness of Bacillus membrane is modulated in a temperature-dependent manner in the physiological range (Fig. 2).
FIG 2.
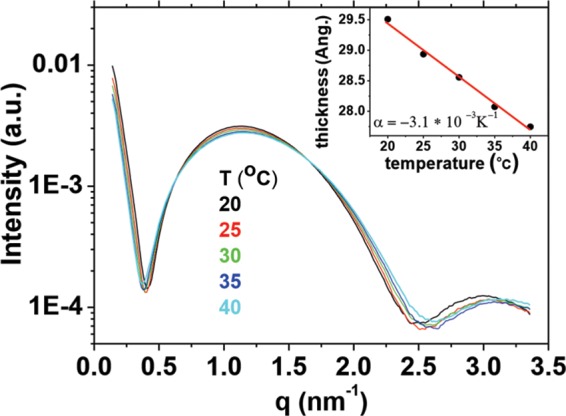
Bacillus membranes are thicker at lower temperature. Bacillus cells were grown at 37°C to an OD of 1, and lipids were extracted using the method of Bligh and Dyer (18). Liposomes prepared with these lipids were used to determine the total hydrophobic thickness (inset) across the bilayer by modeling the small-angle X-ray scattering raw data in the main graph as a function of temperature. The subtle shift of the curves upon heating indicates the change of the thickness of the membrane. The associated coefficient for thermal expansion perpendicular to the membrane (α⊥) was found to be −3.1 × 10−3 K−1, a value expected for bilayer membranes (13). The negative sign reflects the thinning of the membrane that occurs upon heating. Hydrophobic thickness changes about 1 Å over the physiological range studied (25 to 37°C). a.u., arbitrary units.
Reconstitution of MS-DesK into liposomes and functional characterization.
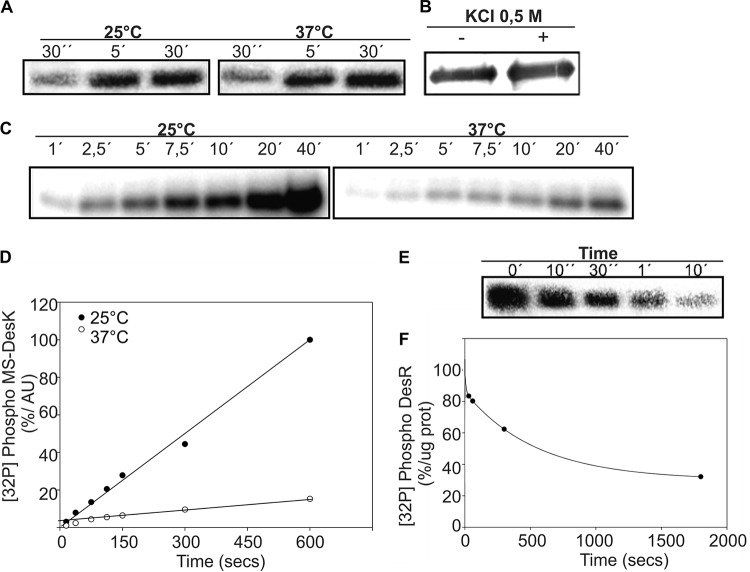
To analyze the biochemical behavior of the single TMS MS-DesK, it was first expressed as a His-tagged protein in E. coli BL21 cells and purified in the presence of the detergent Brij 58. Upon solubilization in this detergent, the protein maintained kinase activity, but the activity was temperature independent (Fig. 3A). Therefore, to study thermoregulation and to directly correlate the effect of point mutations with the activity of the protein, we developed a method to reconstitute MS-DesK protein in lipid bilayers (see Materials and Methods). To confirm that the protein was inserted into liposomes and not simply associated with the membrane, ultracentrifugation was performed in the presence of 0.5 M KCl. Figure 3B shows that MS-DesK was indeed integrated into the phospholipid vesicles rather than simply associated with the surface of the vesicles by electrostatic interaction, because a high salt concentration did not dissociate the protein from the liposomes (25, 26). MS-DesK kinase activity was assayed to investigate whether MS-DesK embedded into a lipid bilayer was able to efficiently sense and respond to a temperature downshift. MS-DesK reconstituted in liposomes was incubated in the presence of [γ-32P]ATP at 25°C and 37°C, and the levels of autophosphorylation were measured at different time points. As shown in Fig. 3C and D, MS-DesK was highly phosphorylated at 25°C. However, when the autokinase activity was assayed at 37°C, the rate of autophosphorylation was very low. Taken together, these results indicate that the in vitro autokinase activity of MS-DesK can be triggered by a decrease in temperature and that MS-DesK must be associated with a lipid bilayer for regulation of its activity.
FIG 3.
MS-DesK reconstituted in liposomes displays autokinase and phosphatase activities. (A) The autokinase activity of detergent-dissolved MS-DesK is not regulated by temperature. MS-DesK solubilized in 0.5% Brij 58 was incubated with [γ-32P]ATP at either 25 or 37°C, and the autokinase activity was analyzed at different interval points by SDS-PAGE followed by autoradiography. (B) DesK is integrated into liposomes. Proteoliposomes were purified by a sucrose gradient centrifugation in the absence (–) or presence (+) of 0.5 M KCl and later analyzed by Western blotting using anti-His antibodies. (C) MS-DesK integrated into liposomes was incubated with [γ-32P]ATP at either 25 or 37°C, and the autokinase activity was measured by taking samples at different time points. The autokinase activity of proteoliposomes is regulated by temperature, showing that MS-DesK must be integrated into a lipid bilayer to be thermoregulated. (D) Quantification of the MS-DesK kinase activity shown in panel C by the use of Image Quant software (version 5.2). AU, arbitrary units. (E) MS-DesK phosphatase activity. Proteoliposomes containing MS-DesKC were incubated at 37°C with purified DesR-P. The dephosphorylation reactions were analyzed by SDS-PAGE followed by autoradiography. (F) The total amount of DesR-P (expressed in arbitrary units) present in each well was determined by densitometry as described for panel D; the total labeling of DesR-P at the beginning of the reaction (0 min) was considered 100%. The graph shows the percentage of DesR-P protein (prot) remaining as a function of time.
We also analyzed whether MS-DesK reconstituted in liposomes retained the antagonistic phosphatase activity of full-length DesK (25). The purified response regulator DesR, tagged with GST (DesR-GST), was therefore phosphorylated using DesKC-His in a GST column. After elution of DesR-P-GST with glutathione, its stability was checked for 30 min, and no loss of radioactivity was observed (25).
Upon addition of MS-DesK incorporated into liposomes, DesR-P was actively dephosphorylated (Fig. 3E). Thus, the MS-DesK proteoliposomes displayed both autokinase and DesR-P phosphatase activities, a behavior already observed in the full-length DesK protein (24).
The reconstitution method using detergent-destabilized liposomes for MS-DesK, which we report here for the first time, thus can be used to measure both kinase and phosphatase activities in response to the natural stimulus.
The role of K10 in controlling the activity of MS-DesK in vitro.
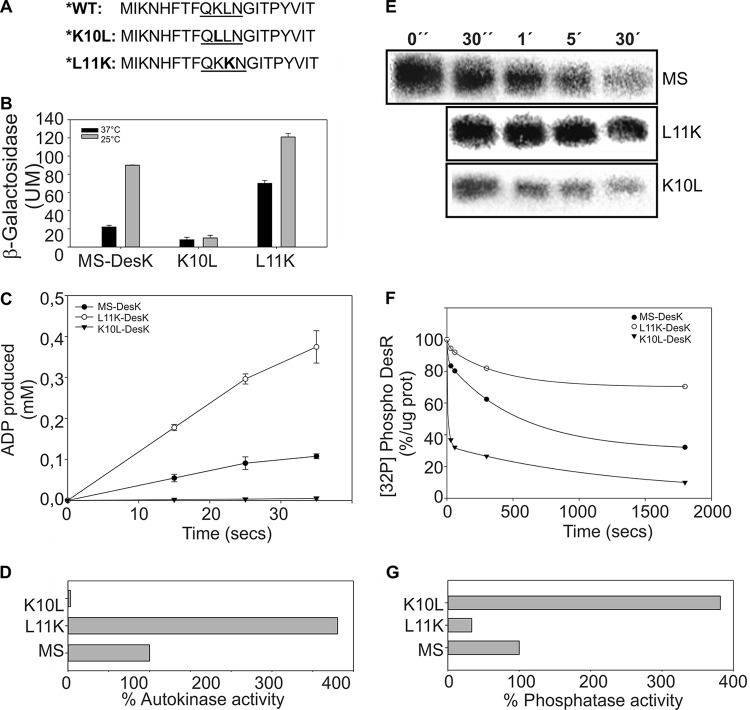
The role of K10 in signaling has been demonstrated genetically by showing that substitutions at this residue modify the levels of expression of the pdes-lacZ reporter gene (10). To confirm this finding, and to directly correlate the effect of point mutations with the activity of the protein, we analyzed the autokinase activity of MS-DesK variants reconstituted in liposomes. We cloned, expressed, and reconstituted into liposomes the two MS-DesK variants described above: (i) MS-K10L, in which K10 was replaced by Leu, and (ii) MS-L11K, which contains an extra lysine at the N terminus of the MS-DesK TMS. These two variants were shown to have decreased and increased kinase activity, respectively, in the in vivo reporter assay (Fig. 4A and B) (10).
FIG 4.
Activity of MS-DesK mutants. (A) Sequences of the N terminus of MS-DesK variants, with point mutations that eliminate (K10L) or duplicate (L11K) the positive charge. WT, wild type. (B) Cells expressing MS-DesK variants were grown at 37°C and transferred to 25°C at an OD at 525 nm (OD525) of 0.3. β-Galactosidase activity was assayed every hour in independent triplicates. The data shown are expressed as averages of the results from three independent experiments and correspond to 4 h after the cold shock; error bars represent the standard deviations for each experimental repetition (10). (C) Proteoliposomes containing MS-DesK or its variants were incubated with [γ-32P]ATP at 25°C, and the autokinase activity was determined using an ADP-Glo kinase assay. The total amount of each MS-DesK variant was determined by densitometry; equal amounts of protein were used for each reaction. (D) The corresponding relative initial velocities were calculated from the slopes of the curves shown in panel C and compared, considering MS-DesK as 100%. (E and F) The phosphatase activity of MS-DesK variants was determined as described for Fig. 3E. (G) Relative initial velocities were calculated from the slope of the curves shown in panel F, taking the activity of MS-DesK as 100%.
To compare the kinetics of these variants, and to determine whether the widely used reporter system correlates with the in vitro assays, the kinase activity of different proteoliposomes was measured with the ADP-Glo kinase assay, an accurate luminescent assay for the detection of ADP that allows rapid kinetic measurements (Fig. 4C and D). Using this assay, we found that the in vitro kinase activity of MS-K10L is greatly diminished compared to that of MS-DesK, giving support to the idea that K10 is critical for the kinase-active conformation of the protein. In contrast, the kinase activity of MS-L11K was shown to be considerably higher than that of MS-DesK, showing that another positively charged residue in the hydrophobic region of the TMS strengthens the driving force that favors kinase activity.
Because DesK can adopt two opposing activities (kinase and phosphatase), we speculated that MS-DesK variants whose kinase activity is reduced would have increased phosphatase activity. To test this idea, the phosphatase activities of the MS-DesK variants were compared by the use of the in vitro assay used with MS-DesK. We found that the phosphatase activity of MS-L11K was diminished, confirming that this variant is locked in the kinase-dominant state, regardless of temperature. In contrast, mutant MS-K10L had higher phosphatase activity than MS-DesK (Fig. 4E to G). The results of the in vitro experiments thus lend support to our reporter system approach, confirming that, in our system, β-galactosidase activities correlate well with in vitro kinase activities.
Why is K10 critical for DesK activity?
It has been previously shown that the single substitution K10L, which replaces a positively charged residue that possesses snorkeling properties with a typical hydrophobic amino acid, results in complete loss of kinase activity (10). Which physicochemical property of lysine is responsible for conferring the temperature-sensing ability? Can any charged residue replace lysine at position 10? K10 was replaced by glutamate, which is hydrophilic but with the opposite charge, or by arginine, which is hydrophilic, keeps the positive charge, and retains the snorkeling capability. MS-DesK mutants were cloned in plasmid pHPKS and introduced into strain CM21 (lacking DesK), which allowed measurement of the activation of DesK using the activity of the β-galactosidase reporter gene (Fig. 5) (9, 10). MS-K10E showed diminished kinase activity (Fig. 5), whereas the K10R replacement did not significantly affect activation of the sensor (Fig. 5). This result suggests that the positive charge and the snorkeling ability of the lateral chain of residue 10 play critical roles in DesK sensing.
What is the role of the hydrophobic F8 residue?
Phenylalanine 8, located at the N-terminal end of the TMS, could also play a role in the DesK sensing mechanism. Due to its location on top of K10 (inset in Fig. 1A) and strong preference for the lipid phase, F8 could serve as a lock by hampering neutralization of K10 by decreasing its exposure to the aqueous phase. We thus constructed mutant F8A and analyzed its sensing ability. MS-DesK F8A showed very low kinase activity, suggesting that K10 can be neutralized in the absence of phenylalanine 8, regardless of temperature, thereby losing the force that pulls K10 toward the water lipid interface and therefore leads to a kinase-off state. To confirm this hypothesis, we performed combinatorial mutagenesis of K10 and F8. The double mutant F8A-K10L showed null kinase activity at both temperatures, as expected, since both the pulling driving force and the hydrophobic restoring force were eliminated, destroying the whole thermosensing mechanism (Fig. 5). In contrast, double mutant F8A-L11K MS-DesK showed kinase-biased activation (Fig. 5). A likely explanation is that this protein, which has tandem lysine residues (K10 and K11), had duplicated the pulling force. In addition, this double mutant has a shorter hydrophobic transmembrane segment due to the L11K replacement while also losing the restoring force provided by F8. The outcome is a strong pulling of the TMS toward the extracellular water-lipid interface, regardless of temperature. To evaluate whether phenylalanine at position 8 is essential or if another aromatic or hydrophobic residue suffices, phenylalanine 8 was replaced by either leucine or tryptophan. The F8L replacement decreased the levels of kinase activity at 25°C, but temperature regulation was maintained (Fig. 5). In contrast, the F8W replacement led to a fully active kinase in a manner that was independent of temperature, suggesting that tryptophan may also pull the TMS toward the water-lipid phase because of its known strong preference for this region (Fig. 5) (27).
DISCUSSION
In previous work, we demonstrated that the MS-DesK construct and intact full-length DesK exhibit increased kinase activity when reconstituted into vesicles containing longer acyl chains (10, 28). Thus, we suggested that DesK is activated by an increase in membrane thickness when the growth temperature drops (10). Here, we confirmed this hypothesis by directly demonstrating that the thickness of purified lipids from B. subtilis increases when the temperature is decreased, as determined with SAXS analysis (Fig. 2). Considering that, in an alpha helical structure, each amino acid contributes 1.5 Å to the length of the peptide backbone, the incremental increase of 1.0 Å in thickness seen when the cells were shifted to cold temperature is compatible with temperature-independent activation of mutant L11K, in which lysine is introduced one position deeper into the hydrophobic core. SAXS measurements confirm our hypothesis and give support to the idea of a molecular-caliper system that is very sensitive to small changes in membrane thickness. In real membranes, in the presence of membrane-spanning proteins, the extent of the thickness variation may change. Nevertheless, our results allow us to propose that temperature variations, which constitute the physical signal, are sensed through subtle modifications of lipid properties that result in variations in membrane thickness. We propose that conversion of a physical stimulus into a hydrophobic mismatch represents the basis of DesK thermosensing. It is tempting to speculate that temperature-associated changes in bilayer thickness drive conformational changes by subtly altering the relative free energies of each signaling state to overcome the barrier in free energy between the conformations of the kinase-active and phosphatase-active forms of DesK. Conversion of physical stimulus into an intracellular chemical response has been a matter of intense interest for a long time. However, for most systems, the molecular mechanisms of signal transduction across the membrane have not been elucidated. Historically, most studies of two-component systems have been based on the output of transcriptional reporters. However, transcription measurements may not precisely report the actual activation/inactivation of the kinase, the first component of the signaling cascade, and may be obscured by downstream factors, such as saturated binding of response regulators to promoters. Thus, direct measurement of in vitro kinase activity is required when the focus of a study is signal recognition, and it is a necessary method for validation of reporter assays to avoid potential misinterpretations. Until now, the attempts to study DesK signal transduction in vitro have been hampered by the difficulties common to most studies of integral membrane proteins involving overexpression, solubilization, and integration of the protein into liposomes in a functional form. Although an in vitro strategy for liposome-assisted cell-free synthesis of full-length DesK has been published (25), this technique has the substantial drawback of high cost. The reconstitution technique used in this work (Fig. 3) is based on conventional purification of His-tagged proteins from bacterial extracts and reconstitution in detergent-destabilized liposomes. The proteoliposomes produced with this technique are pure and functionally active and show high levels of protein integration (60% efficiency). This simple and inexpensive method for reconstitution of the MS-DesK transmembrane sensor opened the way for the first time for determination, in molecular detail, of how the TM segments of a signaling protein act as sensors and how they are able to transmit information to the cytoplasmic portion of the receptor. The finding that MS-DesK retains its function when reconstituted into pure vesicles reveals that no other protein components other than a single TMS are involved in either sensing or signaling. We are aware that the signaling process could be more complex in full-length DesK, which has 5 TMS. However, our minimalistic approach affords a model system by which to understand the biophysical basis of thermosensing that can also be of interest in bioengineering. The dyad F8-K10 is key to the thermosensing mechanism. The physicochemical properties of lysine and the position of K10 inside the hydrophobic core of the membrane, yet close to the water-lipid interface, make the TMS sensitive to variations in membrane thickness. K10 provides the driving force for the conformational changes that occur when temperature decreases because lysine is hydrophilic and pulls toward the water phase. Both its positive charge and its snorkeling properties contribute to the function of K10. Its dyad partner, phenylalanine 8, is a large hydrophobic residue that localizes within the lipid phase but is closer to the water phase than lysine 10 and therefore functions as a “greasy cap” (Fig. 1A). We propose that the interplay of these two residues imposes a positioning of the helix such that phenylalanine is buried in the hydrophobic core and the epsilon amino group of K10 can reach the aqueous/lipid interface. If a nonconservative replacement is made at either of the two residues of the dyad, the ability to respond to changes in temperature is lost. That K10E lacks kinase activity (Fig. 5) is probably a consequence of the fact the negatively charged side-chain carboxyl of the glutamyl residue is repelled by the phosphate groups of lipids. However, because the proton concentration is higher at the interface of negatively charged membranes (29), it is possible that a glutamyl residue gets protonated—and a neutralized glutamate simply does not pull. The K10R replacement (Fig. 5) results in a partially active enzyme. It has been demonstrated that the guanidinium group of arginine and the ammonium group of lysine both interact strongly with phosphate head groups (30). The efficacy of arginine at position 10 supports the idea that the driving force of basic residues being pulled to the lipid-water interface, where the negatively charged phosphate groups are located, is critical for activation of DesK. The fact that the K10R replacement leads to slightly lower DesK activity could be because the energetic cost required to bury arginine in the lipid phase, which has a diffuse positive charge on the guanidinium group, is less than the cost of burying the more focused charge in an amino group of lysine (31), such that arginine may not pull the TMS helix of MS-DesK outward as strongly as lysine does.
The F8A variant does not respond to temperature (Fig. 5). Alanine has a small hydrophobic side chain, and therefore its hydrophobicity is very low and is comparable to those of histidine and serine in the experimentally determined Wimley-White scale—a free-energy scale for measuring amino acid partitioning at the interphases of lipid bilayers (32). The F8A replacement results in a loss of the greasy cap and may allow hydration of lysine 10. In this variant, the pull of F8 toward the hydrophobic core is lost, which may result in a change of position or tilt of the TMS.
The F8L variant responds to temperature (Fig. 5). This result is expected because leucine and phenylalanine are hydrophobic and localize at closely spaced positions in the Wimley-White scale. The replacement F8L lowers the activity, probably because leucine is less hydrophobic than phenylalanine (32). In contrast, a tryptophan at position 8 (F8W) strongly activates the kinase conformation of DesK at both temperatures. This effect can be explained by considering that tryptophan has the highest energy in the Wimley-White hydrophobicity scale because of its strong preference for the aqueous/lipid interface. The tryptophan side chain localizes at the interface because of the amphipathic character of the indole group, which directs its polar nitrogen toward the interphase to hydrogen bond with carbonyl groups of the ester linkages of the fatty acid chains of the phospholipids whereas the aromatic ring of indole embeds in the membrane core (33). This interplay results in robust localization of Trp at the water-lipid interface, as has been determined by nuclear magnetic resonance (NMR) measurements (27). In contrast, the Phe side chain can enter deeper into the hydrophobic core of the membrane because it is apolar. This explains why the presence of Trp at position 8 in MS-DesK results in a strong pulling of the TMS helix upward, which triggers the activation of DesK at all temperatures.
The analysis confirms that phenylalanine works as a greasy cap, hampering hydration of lysine 10. The hierarchy of hydrophobicity of the amino acids employed in this study is alanine<leucine<phenylalanine<tryptophan in the Wimley-White scale, suggesting that the higher the hydrophobicity of the residue at position 8 is, the higher the activity of MS-DesK.
Interestingly, similarly to the DesK mechanism, the piston-like movement involved in activation of the aspartate chemoreceptor has been shown to be susceptible to the position of transmembrane residues, such as phenylalanine, tryptophan, and arginine, located at the water-lipid interphase. Substitutions that result in pulling the TMS toward the periplasm activate the receptor, while substitutions that pull the TMS toward the cytoplasm inactivate it (34, 35).
We propose the following model for MS-DesK thermosensing. At high temperature, the membrane is thin and hydrated (36). The epsilon amino group of K10 reaches the aqueous phase, and F8 is buried in the hydrophobic core of the membrane. The TMS would adopt a more “relaxed,” tilted conformation which would correspond to the phosphatase state. At low temperature, the membrane thickens, and K10 would be located in a more hydrophobic environment. This is energetically expensive, but because F8 hampers hydration of K10, the lateral chain of K10 can snorkel to the hydrophilic aqueous phase, pulling the whole transmembrane helix outward. Serine residues located at the C terminus of the TMS penetrate deeper into the hydrophobic core of the membrane, which results in dehydration and repositioning of these residues to establish H-bonds with homologous serine residues on the other monomer (12). The pulling force of K10 triggered by the thickening of the membrane at low temperatures promotes the incorporation of an extra helical turn at the C terminus of the TMS (11). We propose that this reorganization involves a rotation of both the TMS and the contiguous cytoplasmic coiled-coil, which in turn results in a kinase-competent state (36). Our model fits well with the conformational changes proposed by Saita et al. (37), who suggested that interconversion of DesK between its two functional states implies a rotation of the TMS and the contiguous cytoplasmic coiled-coil, although their model invokes a different a different dimer interface. Similar rearrangements in response to changes in membrane thickness were found for the M13 coat protein: upon decreasing the membrane thickness, the only TMS of the protein increases its tilt angle and rotates to optimize hydrophobic interactions of phenylalanine residues and electrostatic interactions of lysine residues (38, 39). In summary, our results suggest that dyads of charged and aromatic residues, which are widespread at the water-lipid interfaces of TM α-helices of transmembrane kinases, receptors, channels, and transporters, can play important roles in modulating transitions among signaling and conformational states.
ACKNOWLEDGMENTS
R.G.O., L.E.C., and D.D.M. are career investigators at CONICET. M.E.I. is supported by a fellowship from CONICET.
We thank Ariel Fernandez for discussions and the Brazilian Synchrotron Light Laboratory (CNPEM/MCT) for X-ray beam time at the SAXS2 beamline under project D11A-SAXS1-10716.
We declare that we have no conflicts of interest with the contents of this article.
M.E.I. conducted most of the experiments, analyzed results, and wrote the paper. R.G.O. conducted SAXS experiments. D.D.M. designed experiments and wrote the paper. L.E.C. designed experiments, coordinated the study, and wrote the paper.
Funding Statement
This work was funded by grants from the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET; PIP2012-0144 to Larisa E. Cybulski) and the Agencia Nacional de Promoción Científica y Tecnológica (PICT2014-1552 to Larisa E. Cybulski and PICT2010-2678 to Diego de Mendoza).
REFERENCES
- 1.Perozo E, Kloda A, Cortes DM, Martinac B. 2002. Physical principles underlying the transduction of bilayer deformation forces during mechanosensitive channel gating. Nat Struct Biol 9:696–703. doi: 10.1038/nsb827. [DOI] [PubMed] [Google Scholar]
- 2.Nyholm TK, Ozdirekcan S, Killian JA. 2007. How protein transmembrane segments sense the lipid environment. Biochemistry 46:1457–1465. doi: 10.1021/bi061941c. [DOI] [PubMed] [Google Scholar]
- 3.Cybulski LE, de Mendoza D. 2011. Bilayer hydrophobic thickness and integral membrane protein function. Curr Protein Pept Sci 12:760–766. doi: 10.2174/138920311798841681. [DOI] [PubMed] [Google Scholar]
- 4.Aguilar PS, Hernandez-Arriaga AM, Cybulski LE, Erazo AC, de Mendoza D. 2001. Molecular basis of thermosensing: a two-component signal transduction thermometer in Bacillus subtilis. EMBO J 20:1681–1691. doi: 10.1093/emboj/20.7.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Altabe SG, Aguilar P, Caballero GM, de Mendoza D. 2003. The Bacillus subtilis acyl lipid desaturase is a delta5 desaturase. J Bacteriol 185:3228–3231. doi: 10.1128/JB.185.10.3228-3231.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klein W, Weber MH, Marahiel MA. 1999. Cold shock response of Bacillus subtilis: isoleucine-dependent switch in the fatty acid branching pattern for membrane adaptation to low temperatures. J Bacteriol 181:5341–5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cybulski LE, Albanesi D, Mansilla MC, Altabe S, Aguilar P, de Mendoza D. 2002. Mechanism of membrane fluidity optimization: isothermal control of the Bacillus subtilis acyl-lipid desaturase. Mol Microbiol 45:1379–1388. doi: 10.1046/j.1365-2958.2002.03103.x. [DOI] [PubMed] [Google Scholar]
- 8.Saita EA, de Mendoza D. 2015. Thermosensing via transmembrane protein-lipid interactions. Biochim Biophys Acta 1848:1757–1764. doi: 10.1016/j.bbamem.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Albanesi D, Mansilla MC, de Mendoza D. 2004. The membrane fluidity sensor DesK of Bacillus subtilis controls the signal decay of its cognate response regulator. J Bacteriol 186:2655–2663. doi: 10.1128/JB.186.9.2655-2663.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cybulski LE, Martin M, Mansilla MC, Fernandez A, de Mendoza D. 2010. Membrane thickness cue for cold sensing in a bacterium. Curr Biol 20:1539–1544. doi: 10.1016/j.cub.2010.06.074. [DOI] [PubMed] [Google Scholar]
- 11.Inda ME, Vandenbranden M, de Mendoza D, Russchaert JM, Cybulski LE. 2014. A lipid-mediated conformational switch modulates the thermosensing activity of DesK. Proc Natl Acad Sci U S A 111:3579–3584. doi: 10.1073/pnas.1317147111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cybulski L, Ballering J, Moussatova A, Inda ME, Vazquez D, Wassenaar T, de Mendoza D, Tieleman DP, Killian JA. 2015. Activation of the bacterial thermosensor DesK involves a serine zipper dimerization motif that is modulated by membrane thickness. Proc Natl Acad Sci U S A 112:6353–6358. doi: 10.1073/pnas.1422446112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sackmann E. 1995. Physical basis of self-organization and function of membranes: physics of vesicles, p 213–303, chapter 5 In Lipowsky R, Sackmann E (ed), Handbook of biological physics, vol 1A: structure and dynamics of membranes, from cells to vesicles. Elsevier Science BV, Amsterdam, Netherlands. [Google Scholar]
- 14.Pan J, Tristram-Nagle S, Kucerka N, Nagle JF. 2008. Temperature dependence of structure, bending rigidity, and bilayer interactions of dioleoylphosphatidylcholine bilayers. Biophys J 94:117–124. doi: 10.1529/biophysj.107.115691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Denic V, Weissman JS. 2007. A molecular caliper mechanism for determining very long-chain fatty acid length. Cell 130:663–677. doi: 10.1016/j.cell.2007.06.031. [DOI] [PubMed] [Google Scholar]
- 16.Katsura I. 1987. Determination of bacteriophage lambda tail length by a protein ruler. Nature 327:73–75. doi: 10.1038/327073a0. [DOI] [PubMed] [Google Scholar]
- 17.Journet L, Agrain C, Broz P, Cornelis GR. 2003. The needle length of bacterial injectisomes is determined by a molecular ruler. Science 302:1757–1760. doi: 10.1126/science.1091422. [DOI] [PubMed] [Google Scholar]
- 18.Bligh EG, Dyer WJ. 1959. A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 19.Riske KA, Amaral LQ, Lamy-Freund MT. 2001. Thermal transitions of DMPG bilayers in aqueous solution: SAXS structural studies. Biochim Biophys Acta 1511:297–308. doi: 10.1016/S0005-2736(01)00287-5. [DOI] [PubMed] [Google Scholar]
- 20.Bouwstra JA, Gooris GS, Bras W, Talsma H. 1993. Small angle X-ray scattering: possibilities and limitations in characterization of vesicles. Chem Phys Lipids 64:83–98. doi: 10.1016/0009-3084(93)90059-C. [DOI] [PubMed] [Google Scholar]
- 21.Spizizen J. 1958. Transformation of biochemically deficient strains of Bacillus subtilis by deoxyribonucleate. Proc Natl Acad Sci U S A 44:1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harwood CR, Cutting SM. 1990. Molecular biological methods for Bacillus. John Wiley & Sons, Chichester, United Kingdom. [Google Scholar]
- 23.Studier FW, Moffatt BA. 1986. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol 189:113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- 24.Albanesi D, Martín M, Trajtenberg F, Mansilla MC, Haouz A, Alzari PM, de Mendoza D, Buschiazzo A. 2009. Structural plasticity and catalysis regulation of a thermosensor histidine kinase. Proc Natl Acad Sci U S A 106:16185–16190. doi: 10.1073/pnas.0906699106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martín M, Albanesi D, Alzari PM, de Mendoza D. 2009. Functional in vitro assembly of the integral membrane bacterial thermosensor DesK. Protein Expr Purif 66:39–45. doi: 10.1016/j.pep.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 26.Bollag DM, Rozycki MD, Edelstein SJ. 1996. Protein methods, 2nd ed Wiley Inc., New York, NY. [Google Scholar]
- 27.Johansson A, Lindahl E. 2006. Amino-acid solvation structure in transmembrane helices from molecular dynamics simulations. Biophys J 91:4450–4463. doi: 10.1529/biophysj.106.092767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martín M, de Mendoza D. 2013. Regulation of Bacillus subtilis DesK thermosensor by lipids. Biochem J 451:269–275. doi: 10.1042/BJ20121825. [DOI] [PubMed] [Google Scholar]
- 29.Kundu A, Yamaguchi S, Tahara T. 2014. Evaluation of pH at charged lipid/water interfaces by heterodyne-detected electronic sum frequency generation. J Phys Chem Lett 5:762–766. doi: 10.1021/jz500107e. [DOI] [PubMed] [Google Scholar]
- 30.Fischer R, Fotin-Mleczek M, Hufnagel H, Brock R. 2005. Break on through to the other side—biophysics and cell biology shed light on cell-penetrating peptides. Chembiochem 6:2126–2142. doi: 10.1002/cbic.200500044. [DOI] [PubMed] [Google Scholar]
- 31.White SH, Wimley WC. 1999. Membrane protein folding and stability: physical principles. Annu Rev Biophys Biomol Struct 28:319–365. doi: 10.1146/annurev.biophys.28.1.319. [DOI] [PubMed] [Google Scholar]
- 32.Wimley WC, White SH. 1996. Experimentally determined hydrophobicity scale for proteins at membrane interfaces. Nat Struct Biol 3:842–848. doi: 10.1038/nsb1096-842. [DOI] [PubMed] [Google Scholar]
- 33.de Planque M, Bonev B, Demmers J, Greathouse D, Koeppe RII, Separovic F, Watts A, Killian A. 2003. Interfacial anchor properties of tryptophan residues in transmembrane peptides can dominate over hydrophobic matching effects in peptide-lipid interactions. Biochemistry 42:5341–5348. doi: 10.1021/bi027000r. [DOI] [PubMed] [Google Scholar]
- 34.Miller AS, Falke JJ. 2004. Side chains at the membrane-water interface modulate the signaling state of a transmembrane receptor. Biochemistry 43:1763–1770. doi: 10.1021/bi0360206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Draheim RR, Bormans AF, Lai R, Manson MD. 2005. Tuning a bacterial chemoreceptor with protein-membrane interactions. Biochemistry 44:1268–1277. doi: 10.1021/bi048969d. [DOI] [PubMed] [Google Scholar]
- 36.de Mendoza D. 2014. Temperature sensing by membranes. Annu Rev Microbiol 68:101–116. doi: 10.1146/annurev-micro-091313-103612. [DOI] [PubMed] [Google Scholar]
- 37.Saita E, Abriata LA, Tsai YT, Trajtenberg F, Lemmin T, Buschiazzo A, Dal Peraro M, de Mendoza D, Albanesi D. 2015. A coiled coil switch mediates cold sensing by the thermosensory protein DesK. Mol Microbiol 98:258–271. doi: 10.1111/mmi.13118. [DOI] [PubMed] [Google Scholar]
- 38.Stopar D, Spruijt RB, Wolfs CJA, Hemminga M. 1996. Local dynamics of the M13 major coat protein in different membrane-mimicking systems. Biochemistry 35:15467–15473. doi: 10.1021/bi961770j. [DOI] [PubMed] [Google Scholar]
- 39.Koehorst RBM, Spruijt RB, Vergeldt FJ, Hemminga MA. 2004. Lipid bilayer topology of the transmembrane a-helix of M13 major coat protein and bilayer polarity profile by site-directed fluorescence spectroscopy. Biophys J 87:1445–1455. doi: 10.1529/biophysj.104.043208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Najle SR, Inda ME, de Mendoza D, Cybulski LE. 2009. Oligomerization of Bacillus subtilis DesR is required for fine tuning regulation of membrane fluidity. Biochim Biophys Acta 1790:1238–1243. doi: 10.1016/j.bbagen.2009.07.002. [DOI] [PubMed] [Google Scholar]