Abstract
Background and Aims Phosphoenolpyruvate carboxylase (PEPC) is a tightly regulated enzyme that controls carbohydrate partitioning to organic acid anions (malate, citrate) excreted in copious amounts by cluster roots of inorganic phosphate (Pi)-deprived white lupin plants. Excreted malate and citrate solubilize otherwise inaccessible sources of mineralized soil Pi for plant uptake. The aim of this study was to test the hypotheses that (1) PEPC is post-translationally activated by reversible phosphorylation in cluster roots of illuminated white lupin plants, and (2) light-dependent phosphorylation of cluster root PEPC is associated with elevated intracellular levels of sucrose and its signalling metabolite, trehalose-6-phosphate.
Methods White lupin plants were cultivated hydroponically at low Pi levels (≤1 µm) and subjected to various light/dark pretreatments. Cluster root PEPC activity and in vivo phosphorylation status were analysed to assess the enzyme’s diurnal, post-translational control in response to light and dark. Levels of various metabolites, including sucrose and trehalose-6-phosphate, were also quantified in cluster root extracts using enzymatic and spectrometric methods.
Key Results During the daytime the cluster root PEPC was activated by phosphorylation at its conserved N-terminal seryl residue. Darkness triggered a progressive reduction in PEPC phosphorylation to undetectable levels, and this was correlated with 75–80 % decreases in concentrations of sucrose and trehalose-6- phosphate.
Conclusions Reversible, light-dependent regulatory PEPC phosphorylation occurs in cluster roots of Pi-deprived white lupin plants. This likely facilitates the well-documented light- and sucrose-dependent exudation of Pi-solubilizing organic acid anions by the cluster roots. PEPC’s in vivo phosphorylation status appears to be modulated by sucrose translocated from CO2-fixing leaves into the non-photosynthetic cluster roots.
Keywords: Cluster (proteoid) roots, Lupinus albus L. (white lupin), phosphate limitation, phosphoenolpyruvate carboxylase, protein phosphorylation, sucrose signalling, trehalose-6-phosphate
INTRODUCTION
Phosphoenolpyruvate (PEP) carboxylase (PEPC; EC 4.1.1.31) is an important cytosolic enzyme of primary plant metabolism that catalyses the irreversible β-carboxylation of PEP to yield oxaloacetate and inorganic phosphate (Pi). It is the major anaplerotic plant enzyme that controls PEP partitioning into tricarboxylic acid cycle C-skeletons needed for biosynthesis and N assimilation (O’Leary et al., 2011). Phosphorylation by a dedicated Ca2+-independent PEPC protein kinase (PPCK) at a conserved N-terminal seryl residue activates PEPC in many plant tissues by relieving its allosteric inhibition by malate while enhancing activation by glucose-6-phosphate (O’Leary et al., 2011). Amongst its numerous and diverse physiological functions, PEPC plays a critical role during plant acclimation to nutritional Pi deprivation, a common abiotic stress that frequently limits plant growth in natural ecosystems (Vance et al., 2003; Plaxton and Tran, 2011). Activation of the PEPC isozyme AtPPC1 by in vivo phosphorylation in response to Pi deprivation of the model plant arabidopsis (Arabidopsis thaliana) has been well documented (Gregory et al., 2009). AtPPCK1 and AtPPCK2 are amongst the most strongly Pi-starvation-inducible genes of arabidopsis (Gregory et al., 2009; Plaxton and Tran, 2011), and Pi deprivation of arabidopsis cell cultures caused a striking 3-fold enhancement of in vivo flux through the PEPC reaction (Masakapalli et al., 2014). This provides a metabolic bypass with malate dehydrogenase (MDH) and NAD-malic enzyme to cytosolic pyruvate kinase (which is substrate (ADP)-limited in low-Pi plants) to facilitate continued pyruvate supply to the mitochondrial tricarboxylic acid cycle (Duff et al., 1989; Masakapalli et al., 2014). At the same time Pi, a by-product of the PEPC reaction, is recycled for its rapid re-assimilation into the metabolism of the Pi-deficient (−Pi) cells (Duff et al., 1989; Masakapalli et al., 2014). The upregulation of PEPC during Pi starvation has also been linked to the synthesis and exudation of large quantities of organic acid anions (e.g. malate, citrate) by roots of various −Pi plants (Neumann et al., 2000; Vance et al., 2003; Shane and Lambers, 2005; Plaxton and Tran, 2011; Cheng et al., 2011; Lambers et al., 2011). This greatly increases root Pi acquisition by solubilizing otherwise inaccessible sources of mineralized soil Pi, thus increasing soluble Pi concentrations by up to 1000-fold (Vance et al., 2003). This adaptive strategy is especially prevalent in highly specialized cluster (proteoid) roots (CRs), which mediate efficient Pi acquisition by certain plant species acclimating to soluble Pi-impoverished soils (Keerthisinghe et al., 1998; Neumann et al., 2000; Watt and Evans, 1999; Shane and Lambers, 2005; Cheng et al., 2011; Lambers et al., 2011). Of the various plants that form CRs, white lupin (Lupinus albus) stands out as an important agricultural crop, and is the species that has been most intensively investigated with respect to Pi nutrition (Cheng et al., 2011).
Despite numerous physiological, biochemical and transcriptomic studies of white lupin Pi-starvation responses (Uhde-Stone et al., 2003; Vance et al., 2003; Liu et al., 2005; Cheng et al., 2011, 2014), there is little information about regulatory PEPC phosphorylation in this species. PEPC was suggested to be activated by phosphorylation in CRs of −Pi white lupin since the partially purified enzyme from this tissue displayed decreased sensitivity to malate inhibition following its incubation with ATP (Uhde-Stone et al., 2003). Diurnal control of citrate exudation and Pi uptake by mature CRs of −Pi lupin has also been reported; i.e. citrate release and Pi uptake were significantly reduced during darkness, then showed marked increases during the daytime (Watt and Evans, 1999; Hocking and Jeffery, 2004; Liu et al., 2005; Cheng et al., 2014). Transcriptional expression of various Pi-responsive genes in white lupin CRs (including that encoding the dominant PEPC isozyme LaPEPC3 (PEPC isozyme-3 from L. albus)) also increased in the light and decreased in the dark, suggesting that light-driven changes in the supply of photosynthate (sucrose) from the leaves to the CRs regulated expression of these genes in the latter (Liu et al., 2005; Cheng et al., 2011, 2014). The data support the view that shoot carbohydrate supply plays a crucial role in orchestrating root metabolic responses to Pi limitation, including white lupin CRs (Liu et al., 2005; Hammond and White, 2008; Cheng et al., 2011, 2014).
The aim of the present study was to test the hypothesis that PEPC is reversibly activated by phosphorylation in CRs of illuminated, −Pi white lupin plants, and that this is closely correlated with intracellular levels of sucrose and trehalose-6-phosphate (Tre6P). Tre6P is an essential signal metabolite linking plant growth and development to carbon and nitrogen metabolism, and the sucrose–Tre6P nexus model postulates that Tre6P functions as both a signal and regulator of intracellular sucrose levels (Lunn et al., 2006; Yadav et al., 2014; Figueroa et al., 2016). Our results demonstrate that Pi limitation alone cannot completely account for the phosphorylation status of CR PEPC, and to the best of our knowledge provide the first evidence for the diurnal post-translational control of any CR protein by reversible phosphorylation.
MATERIALS AND METHODS
Plant material
White lupin (Lupinus albus L. ‘Kiev’) plants were cultivated in a greenhouse under natural light supplemented with 16 h of artificial light (high-pressure sodium lamps), 70 % relative humidity, and 21/16 °C day/night temperatures. Roots of 1-week-old seedlings germinated in standard potting mix were washed free of soil and transferred to 100-L hydroponic tanks. Four seedlings per tank were supported by foam plugs in the lid. Each tank contained a continuously aerated nutrient solution (Shane et al., 2003) supplemented with 1 µm K2HPO4, which was replaced every third day. Cluster roots of 6-week-old plants were classified according to previous studies (Neumann et al., 2000), whereas the youngest regions of lateral roots (∼3 cm including tips) were classified as non-CRs. All tissues to be analysed were rapidly excised, frozen in liquid N2 and stored at −80 °C until used.
Preparation of clarified extracts
Quick-frozen tissues were ground to a powder under liquid N2 using a mortar and pestle and extracted (1:1·5, w/v) using a PT-3100 Polytron homogenizer and ice-cold 50·mm imidazole–HCl (pH 7·0) containing 0·1 % (v/v) Triton X-100, 10 % (v/v) glycerol, 10 mm thiourea, 2 mm MgCl2, 2 % (w/v) PEG 8000, 25 mm NaF, 1 mm Na2MoO4, 1 mm Na3VO3, 1 % (w/v) polyvinyl(polypyrrolidone) (PVPP), 1 % (w/v) polyvinylpyrrolidone (PVP) and 2·5 µL mL−1 ProteCEASE-100 (G-Biosciences). Homogenates were centrifuged at 17 500 g for 15 min at 4 °C. Supernatants (0·5 ml) were desalted through 3-mL Sephadex G-25 spin columns equilibrated with extraction buffer (lacking PVPP and PVP) prior to enzymatic analysis.
PEPC activity and protein concentration assays
PEPC activity assays were conducted at 25 °C by coupling to the MDH reaction and monitoring NADH oxidation at 340 nm. All assays were conducted using a kinetics microplate spectrophotometer (Spectramax Plus, Molecular Devices), optimized with respect to pH, substrate and cofactor concentration, and were linear with respect to time and amount of enzyme assayed. One unit of activity was defined as the amount of PEPC resulting in the production of 1 µmol oxaloacetate min−1. Optimized assay conditions for PEPC were: 50 mm Hepes–KOH (pH 8·2) containing 15 % (v/v) glycerol, 2·5 mm PEP, 2 mm KHCO3, 5 mm MgCl2, 1 mm DTT, 0·2 mm NADH and 5 units mL−1 of desalted porcine muscle MDH. I50(malate) values (concentration of malate producing 50 % inhibition) were determined at pH 7·2 with 0·2 mm PEP and calculated using enzyme kinetics software as previously described (Gregory et al., 2009). All PEPC activity and I50 determinations represent the means of triplicate determinations of at least n = 5 biological replicates. Kinetic data were analysed using Student’s t-test and deemed significant if P < 0·05. Stock solutions of metabolites were prepared with an equivalent concentration of MgCl2 and adjusted to pH 7·2. Protein concentrations were determined using the Coomassie Blue G-250 dye binding method as previously described (Gregory et al., 2009) with bovine γ-globulin as the protein standard.
Electrophoresis and immunoblotting
Non-denaturing PAGE and SDS–PAGE using a Bio-Rad mini-gel apparatus (7 and 10 % separating gels, respectively), in-gel PEPC activity staining and immunoblotting were as previously described (Gregory et al., 2009). Antigenic polypeptides were visualized using an alkaline phosphatase-conjugated secondary antibody and chromogenic detection (Gregory et al., 2009). All gel and immunoblot experiments were replicated a minimum of three times with representative results shown in the various figures. Preparation of rabbit anti-(developing castor bean PEPC) IgG (anti-PEPC) and the corresponding anti-(phospho-Ser11 site-specific) IgG (anti-pSer11; raised against a synthetic phosphopeptide corresponding to castor bean PEPC’s conserved Ser11 phosphorylation site) has been described (Tripodi et al., 2005). This antibody is expected to cross-react with the phosphorylated form of LaPEPC3 (AY663387), the dominant PEPC isozyme expressed in CRs of −Pi white lupin and whose putative Ser-12 phosphorylation site and flanking residues closely align with the conserved N-terminal phosphorylation domain characteristic of all known plant-type PEPCs (Penaloza et al., 2005; O'Leary et al., 2011). For anti-pSer11 immunoblots, 10 µg mL−1 of the corresponding dephosphopeptide (Tripodi et al., 2005) was used to block any non-specific cross-reaction with non-phosphorylated PEPC. The relative amount of PEPC protein in clarified extracts was estimated by quantification of immunoreactive PEPC polypeptides on scanned immunoblots using ImageJ (http://imagej.nih.gov/ij/). Derived values were proportional to the amount of immunoblotted extract.
Metabolite extraction and assays
Trehalose-6-phosphate, phosphorylated intermediates and organic acids were assayed in chloroform/methanol tissue extracts as described (Lunn et al., 2006) with modifications (Figueroa et al., 2016) using an AB Sciex QTrap 5500 triple quadrupole mass spectrometer (http://www.absciex.com). Calibration was done using enzymatically verified Tre6P standards and samples were spiked with a [6,6-2H]Tre6P internal standard to correct for ion suppression (Lunn et al., 2006). Sucrose, glucose and fructose were measured enzymatically in the same chloroform–methanol tissue extracts (Lunn et al., 2006).
RESULTS AND DISCUSSION
Cluster root PEPC of illuminated white lupin plants is phosphorylated in vivo
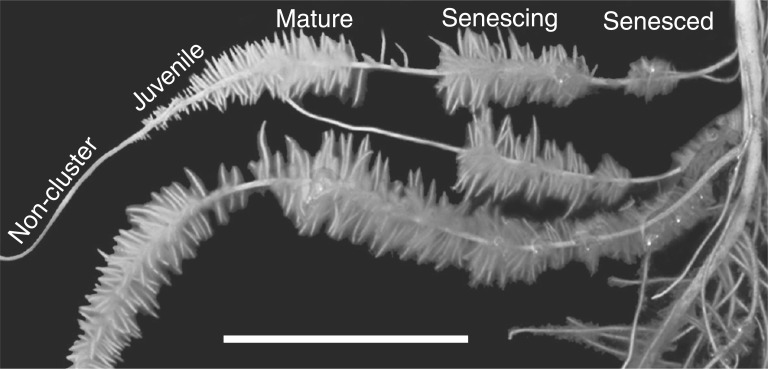
Consistent with previous studies (Shane et al., 2003; Uhde-Stone et al., 2003; Lambers et al., 2011; Wang et al., 2014): (1) 6-week-old, Pi-deprived white lupin plants formed abundant CRs (>50 % of total root biomass, data not shown) with a developmental sequence of juvenile, mature, senescing and senesced CRs visible along the lateral root axis (Fig. 1); (2) a significant increase in PEPC specific activity occurred during CR development that peaked at maturity (4–6 d) then decreased during senescence (∼10 d) (Fig. 2A); and (3) this developmental pattern largely parallels the relative abundance of 107-kDa immunoreactive PEPC polypeptides (Fig. 2B), as well as rates of organic acid anion exudation by CRs of illuminated plants (Johnson et al., 1996; Watt and Evans, 1999; Neumann et al., 2000).
Fig. 1.
Representative image of normal and cluster roots in hydroponically cultivated Pi-deficient white lupin plants. The tips of the secondary lateral roots and unbranched proximal root denote the normal roots. Developmental stages of CRs are labelled according to the number of days following rootlet emergence: juvenile (1–3 d), mature (4–6 d), senescing (7–9 d) and senesced (≥10 d). Scale bar = 35 mm.
Fig. 2.
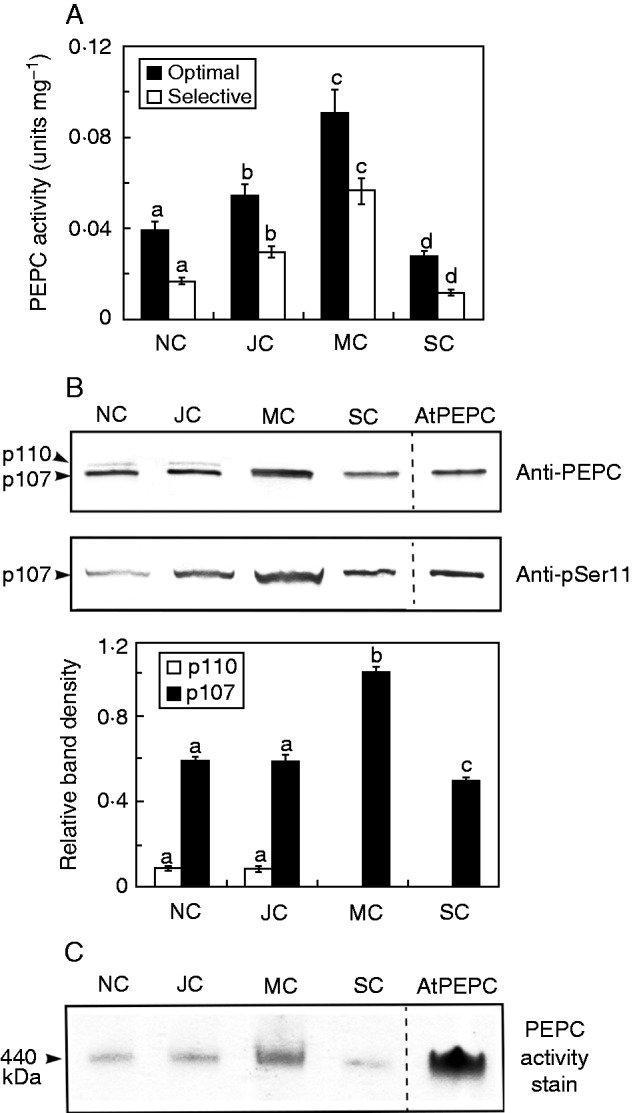
Developmental profiles for cluster root PEPC activity, abundance, subunit structure and phosphorylation status. NC, JC, MC and SC denote non-cluster, juvenile cluster, mature cluster and senescing cluster roots, respectively, as outlined in Fig. 1. (A) All values represent the mean (± s.e.m.) of PEPC activity from triplicate determinations of desalted extracts from n = 5 biological replicates determined under optimal (pH 8·2, 2·5 mm PEP) and selective (pH 7·2, 0·2 mm PEP, 0·125 mm malate) assay conditions. Different letters denote significant differences (Student’s t-test, P < 0·05) from the respective maximal value. (B) Extracts were subjected to SDS–PAGE, electroblotted onto a PVDF membrane and probed with anti-PEPC or anti-pSer11 antibodies (1 and 20 µg CR protein/lane, respectively). The lanes of the anti-PEPC and anti-pSer11 blots labelled ‘AtPEPC’ contained 50 and 100 ng, respectively, of in vivo phosphorylated AtPPC1 purified from −Pi arabidopsis suspension cells (Gregory et al., 2009). Relative amounts of immunoreactive p110 and p107 were quantified from scanned anti-PEPC immunoblots of n = 5 biological replicates using ImageJ. Different letters denote significant differences (P < 0·05) from the respective initial value. (C) In-gel PEPC activity staining was performed following non-denaturing-PAGE (8 µg CR protein/lane). The lane labelled ‘AtPEPC’ contained 0·25 µg of native AtPPC1 purified from −Pi arabidopsis suspension cells (Gregory et al., 2009).
The availability of anti-PEPC and anti-pSer11 antibodies allowed us to assess PEPC’s subunit composition and phosphorylation status by SDS–PAGE followed by immunoblotting of root extracts. Anti-PEPC immunoblots of non-cluster and immature CR extracts revealed 110- and 107-kDa immunoreactive polypeptides (p110 and p107, respectively); the p107 co-migrated with purified PEPC (AtPPC1) from −Pi arabidopsis cell cultures (Gregory et al., 2009) while displaying an approximately 6-fold greater abundance than the p110 (Fig. 2B). Maturation of CRs was accompanied by disappearance of p110 and an approximate 70 % increase in the amount of p107. These results are reminiscent of studies of PEPC in germinating castor (Ricinus communis) and sorghum (Sorghum bicolor) seeds, as well as CRs of harsh hakea (Hakea prostrata, an Australian member of the Proteaceae), in which p110 was shown to be a monoubiquitinated form of the p107 (Uhrig et al., 2008; Shane et al., 2013; Ruiz-Ballesta et al., 2014). Further experiments are necessary to confirm whether the p110 represents a monoubiquitinated version of the p107 PEPC subunits in white lupin roots, and if this post-translational modification might be coordinated with phosphorylation to fine-tune anaplerotic carbon flux according to cellular demands for citrate and malate.
Anti-pSer11 immunoblots demonstrated that the p107 of illuminated plants was in vivo-phosphorylated at a conserved N-terminal seryl residue in normal roots, as well as at all stages of CR development (Fig. 2B). Anti-pSer11 specifically cross-reacted with phospho-p107 as the signal was quenched when the immunoblot was probed with anti-pSer11 in the presence of the corresponding blocking phosphopeptide (results not shown). The anti-pSer11 immunoreactive signal of p107 in mature CRs was approximately 50 % higher than in immature CRs, and matched the parallel increase in total p107 abundance as the CRs matured (Fig. 2B). The ratio of PEPC specific activity under selective (physiological) versus optimal assay conditions (Fig. 2A) was 0·58 ± 0·09 and 0·65 ± 0·10 for the juvenile and mature CRs, respectively. These values are not significantly different (Student’s t-test, P > 0·05) and corroborate the results in Fig. 2B indicating that PEPC’s phosphorylation status was not markedly altered as the CRs matured. These findings agree with our study of PEPC in immature CRs of harsh hakea showing that only p107 (and not the monoubiquitinated p110 subunit) is phosphorylated (Shane et al., 2013). Non-denaturing PAGE followed by in-gel activity staining yielded a single PEPC activity band at each stage of CR development that co-migrated with homogeneous 440-kDa PEPC purified from −Pi arabidopsis suspension cells (Fig. 2C). This corroborates an earlier report (Uhde-Stone et al., 2003) and indicates that, as with many other PEPCs, the native enzyme from white lupin CRs exists as a tetramer.
Activation of PEPC by in vivo phosphorylation in mature cluster roots is up-/downregulated by photosynthate supply from shoots of intact plants
The kinetic properties, subunit structure and phosphorylation status of PEPC were examined in freshly prepared, desalted extracts of mature CRs collected from intact white lupin plants that had been subjected to various light/dark pretreatments. The specific activity of PEPC under optimal assay conditions (i.e. pH 8·2, 2·5 mm PEP) (Fig. 3A), which is not influenced by the phosphorylation state of the target enzyme (O’Leary et al., 2011) and the levels of PEPC protein (i.e. immunoreactive p107 subunits) (Fig. 3B) as well as total soluble protein (data not shown), remained unchanged irrespective of light or darkness. Thus, these various pretreatments did not cause global effects on this cytosolic CR enzyme but, as outlined below, specifically perturbed its phosphorylation status. Under selective, physiologically relevant, assay conditions (i.e. pH 7·2, 0·2 mm PEP, 0·125 mm malate), PEPC-specific activities were progressively and significantly reduced after 6–24 h of darkness (Fig. 3A), which implies that darkness triggered PEPC dephosphorylation (O’Leary et al., 2011). However, re-illumination of 12-h darkened plants for 8 h resulted in a recovery of selective PEPC activity to initial levels (Fig. 3A). This was paralleled by an approximate 3-fold greater I50(malate) for PEPC extracted from CRs of 8-h re-illuminated plants (0·41±0·02 mm) relative to 12-h darkened plants (0·15 ± 0.01 mm). This striking, light-induced reduction in PEPC’s sensitivity to feedback inhibition by malate would be expected to make the enzyme more active invivo, particularly since the intracellular malate concentration of the CRs was significantly elevated during the daytime (Table 1).
Fig. 3.
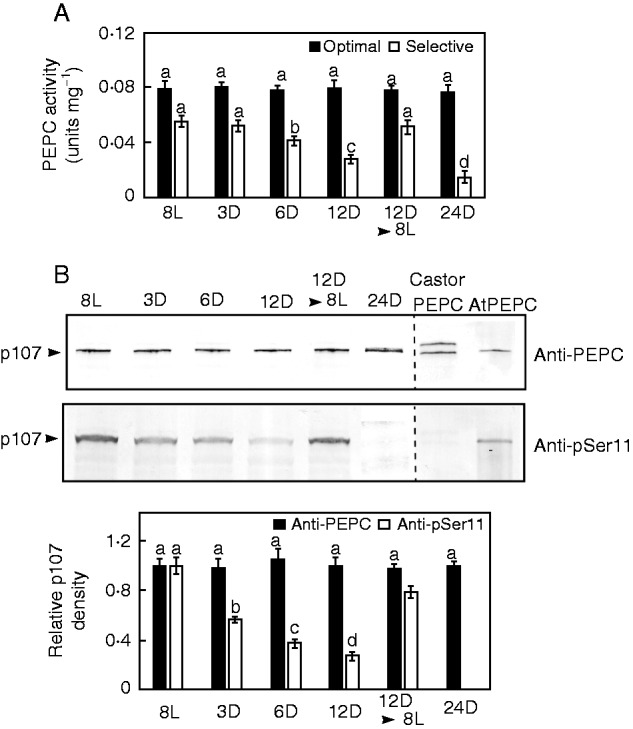
Influence of various light and dark pretreatments on cluster root PEPC activity, abundance and phosphorylation status. For panels (A–C), 8L, 3D, 6D, 12D, 8L and 24D denote the number of hours plants were exposed to constant light (L) or darkness (D), whereas 12D▸8L indicates plants that were subjected to darkness for 12 h and then reilluminated for an additional 8 h. (A) All values represent the mean (± s.e.m.) PEPC activity from triplicate determinations of desalted extracts from n = 5 biological replicates determined under optimal (pH 8·2, 2·5 mm PEP) and selective (pH 7·2, 0·2 mm PEP, 0·125 mm malate) conditions. Different letters denote significant differences (Student’s t-test, P < 0·05) from the respective initial, 8L value. (B) Extracts were subjected to SDS–PAGE, electroblotted onto a PVDF membrane and probed with anti-PEPC or anti-pSer11 antibodies (0·5 and 20 µg CR protein/lane, respectively). Relative band intensities (± s.e.m.) were quantified on n = 5 biological replicates from scanned immunoblots using ImageJ. Different letters denote significant differences (P < 0·05) from the respective initial, 8L value. The lanes of the anti-PEPC and anti-pSer11 blots labelled ‘AtPEPC’ contained 50 and 100 ng, respectively, of purified phosphorylated AtPPC1 from −Pi arabidopsis suspension cells (Gregory et al., 2009), whereas those labelled ‘Castor PEPC’ contained 50 and 250 ng, respectively, of dephosphorylated, partially monoubiquitinated PEPC purified from germinating castor beans (Uhrig et al., 2008).
Table 1.
Concentrations of various metabolites in mature cluster roots of intact Pi-deprived L. albus during mid-photoperiod or following extended darkness, determined by LC-MS
| Metabolite | Mid-photoperiod (8 h light) | Extended darkness (24 h dark) | Fold change, darkness:light |
|---|---|---|---|
| µmol g−1 fresh weight | |||
| Sucrose | 7·6 ± 0·2 | 1·5 ± 0·1 | −5·1 ± 0·4† |
| Glucose | 0·39 ± 0·02 | 0·30 ± 0·03 | −1·3 ± 0·2† |
| Fructose | 0·28 ± 0·01 | 0·15 ± 0·02 | −1·8 ± 0·2† |
| Citrate | 24·5 ± 0·6 | 22·9 ± 0·9 | −1·1 ± 0·02 |
| Malate | 10·2 ± 0·3 | 6·5 ± 0·3 | −1·5 ± 0·04† |
| nmol g−1 fresh weight | |||
| Sucrose-6-phosphate | 0·063 ± 0·004 | 0·015 ± 0·001 | −4·2 ± 0·3† |
| Trehalose-6-phosphate | 0·016 ± 0·001 | 0·004 ± 0·0003 | −4·0 ± 0·3† |
| Galactose-1-phosphate | 1·1 ± 0·1 | 0·56 ± 0·03 | −1·9 ± 0·2† |
| Glucose-1-phosphate | 2·8 ± 0·3 | 1·2 ± 0·1 | −2·3 ± 0·3† |
| UDP-glucose | 8·6 ± 1·4 | 3·8 ± 0·5 | −2·2 ± 0·4† |
| ADP-glucose | 0·027 ± 0·003 | 0·023 ± 0·001 | −1·1 ± 0·1 |
| Glycerol-3-phosphate | 0·24 ± 0·02 | 0·20 ± 0·01 | −1·2 ± 0·1 |
| Glycerate | 60 ± 3·7 | 47 ± 1·9 | −1·2 ± 0·1 |
| Pyruvate | 53·6 ± 1·8 | 34·7 ± 1·8 | −1·5 ± 0·1† |
| Shikimate | 7·3 ± 0·30 | 4·0 ± 0·2 | −1·8 ± 0·1† |
| Isocitrate | 162 ± 5 | 144 ± 7 | −1·1 ± 0·03 |
| Aconitate | 75·1 ± 2·5 | 66·7 ± 3·6 | −1·1 ± 0·04 |
| 2-Oxoglutarate | 57·6 ± 1·7 | 23·0 ± 1·1 | −2·5 ± 0·1† |
| Succinate | 168 ± 5 | 103 ± 4 | −1·6 ± 0·04† |
| Fumarate | 126 ± 3 | 111 ± 4 | −1·1 ± 0·02 |
All values represent the means (± s.e.m.) of n = 10 biological replicates.
Significant change (Student’s t-test, P < 0·05).
SDS–PAGE of clarified extracts from mature CRs followed by immunoblotting with anti-pSer11 confirmed that ≥3 h of darkness exerted an obvious negative impact on PEPC’s phosphorylation status such that p107 phosphorylation steadily declined to undetectable levels by 24 h (Fig. 3B). By contrast, PEPC phosphorylation recovered to near maximal levels (∼80 % of initial) following 8 h of re-illumination of plants that had been subjected to 12 h of darkness (Fig. 3B). Interestingly, complete dephosphorylation of the p107 subunits of CR PEPC in 24-h darkened plants (Fig. 3B) was correlated with a 75–80 % decrease in the concentrations of sucrose, sucrose-6-phosphate and Tre6P (Table 1). These results indicate that prolonged darkness greatly reduced or abolished photosynthate delivery from leaves to the CRs. Furthermore, CR levels of several metabolites downstream of the PEPC reaction were also significantly attenuated, suggesting that in vivo anaplerotic flux was also curtailed following the darkness treatment (Table 1).
Conclusions
Results of the current study demonstrate that reversible light-dependent regulatory PEPC phosphorylation occurs in CRs of −Pi white lupin plants. This has implications not only for the control of cytosolic carbon metabolism in CRs, but also for the role of photosynthate in the post-translational regulation of this ubiquitous anaplerotic enzyme in non-green tissues. Our results also corroborate investigations of soybean root nodule and developing castor bean PEPCs indicating that up-/downregulation of PEPC phosphorylation by photosynthate supply from source leaves occurs in diverse heterotrophic sink tissues (Zhang and Chollet, 1997; Tripodi et al., 2005; Murmu and Plaxton, 2007). Up to 25 % of photosynthetically fixed CO2 of −Pi white lupin plants is translocated as sucrose to its CRs to support organic acid synthesis and exudation into the rhizosphere, whereas anaplerotic CO2 fixation by PEPC in the CRs contributes about 25 % and 34 % of the carbon excreted as citrate and malate, respectively (Johnson et al., 1996; Neumann et al., 2000; Vance et al., 2003). Furthermore, a strong relationship exists between light intensity, sucrose levels and carboxylate exudation with transcriptional expression of the dominant PEPC isozyme gene, LaPEPC3, in white lupin CRs (Wang et al., 2014; Cheng et al., 2014). It was suggested that any excess photosynthate is translocated to the roots as sucrose, which serves as both a nutritional signal and a C substrate for carboxylate exudation. Sucrose translocated from source leaves is part of a systemic signalling system that leads to Pi deficiency-induced alterations in root morphology and metabolism in white lupin and other plant species, including arabidopsis (Liu et al., 2005; Hammond and White, 2008; Cheng et al., 2011, 2014; Wang et al., 2014).
Here, we have established a novel mechanistic link between reversible in vivo PEPC phosphorylation and the concentrations of sucrose and its signalling molecule Tre6P (Yadav et al., 2014), while correlating this interesting phenomenon with the diurnal control of organic anion exudation and Pi uptake by CRs of −Pi white lupin plants (Watt and Evans, 1999; Hocking and Jeffery, 2004; Liu et al., 2005; Cheng et al., 2014). It is notable that a direct relationship between Tre6P levels, anaplerotic carbon flux and PEPC's in vivo phosphorylation status was recently established in transgenic arabidopsis plants overexpressing Escherichia coli Tre6P synthase (Figueroa et al., 2016). Increased Tre6P led to PEPC activation by in vivo phosphorylation at its conserved N-terminal seryl phosphosite, coupled with a corresponding enhancement in anaplerotic photosynthate partitioning to organic acids. It was concluded that high Tre6P stimulated anaplerotic flux via PPCK-mediated PEPC phosphorylation. PPCK and phospho-PEPC phosphatase were therefore suggested to be potential primary targets of Tre6P (Figueroa et al., 2016). The current results demonstrate that the PPCK and corresponding phosphatase respectively required to catalyse in vivo phosphorylation and dephosphorylation of PEPC’s p107 subunits are clearly present, functional and reciprocally regulated in white lupin CRs. Future studies are needed to test the intriguing hypothesis that Tre6P serves to directly control PPCK or phospho-PEPC phosphatase activities and thus PEPC’s phosphorylation status in non-green sink tissues, such as CRs, legume root nodules and developing oil seeds, in response to altered sucrose translocation from CO2-fixing source leaves.
ACKNOWLEDGEMENTS
This work was supported by grants from the Australian Research Council (to M.W.S.), the Max Planck Society (to R.F. and J.E.L.) and the Natural Sciences and Engineering Research Council of Canada and Queen’s Research Chairs programme (to W.C.P.). In memory of Mike Shane who unexpectedly passed away on April 3, 2016 (http://www.barkerfh.com/fh/obituaries/obituary.cfm?o_id=3656132&fh_id=11496). Mike was a gifted plant scientist and thoughtful colleague who will be greatly missed.
LITERATURE CITED
- Cheng L, Bucciarelli B, Shen J, et al. 2011. Update on white lupin cluster root acclimation to phosphorus deficiency. Plant Physiology 156: 1025–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, Tang X, Vance CP, et al. 2014. Interactions between light intensity and phosphorus nutrition affect the phosphate-mining capacity of white lupin (Lupinus albus L.). Journal of Experimental Botany 65: 2995–3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff SMG, Moorhead GB, Lefebvre DD, et al. 1989. Phosphate starvation inducible “bypasses” of adenylate and phosphate dependent glycolytic enzymes in Brassica nigra suspension cells. Plant Physiology 90: 1275–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa CM, Feil R, Ishihara H, et al. 2016. Trehalose 6-phosphate coordinates organic and amino acid metabolism with carbon availability. The Plant Journal 85: 410–423. [DOI] [PubMed] [Google Scholar]
- Gregory AL, Hurley BA, Tran HT, et al. 2009. In vivo regulatory phosphorylation of the phosphoenolpyruvate carboxylase AtPPC1 in phosphate-starved Arabidopsis thaliana. Biochemical Journal 420: 57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond JP, White PJ. 2008. Sucrose transport in the phloem: integrating root responses to phosphorus starvation. Journal of Experimental Botany 59: 93–109. [DOI] [PubMed] [Google Scholar]
- Hocking PJ, Jeffery S. 2004. Cluster-root production and organic anion exudation in a group of old-world lupins and a new-world lupin. Plant and Soil 258: 135–150. [Google Scholar]
- Johnson JF, Allan DL, Vance CP, et al. 1996. Root carbon dioxide fixation by phosphorus-deficient Lupinus albus (contribution to organic acid exudation by proteoid roots). Plant Physiology 112: 19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keerthisinghe G, Hocking PJ, Ryan PR, et al. 1998. Effect of phosphorus supply on the formation and function of proteoid roots of white lupin (Lupinus albus L.). Plant, Cell and Environment 21: 467–478. [Google Scholar]
- Lambers H, Finnegan PM, Laliberte E, et al. 2011. Phosphorus nutrition of Proteaceae in severely phosphorus-impoverished soils: are there lessons to be learned for future crops? Plant Physiology 156: 1058–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JQ, Samac DA, Bucciarelli B, et al. 2005. Signaling of phosphorus deficiency-induced gene expression in white lupin requires sugar and phloem transport. The Plant Journal 41: 257–268. [DOI] [PubMed] [Google Scholar]
- Lunn JE, Feil R, Hendriks JHM, et al. 2006. Sugar-induced increases in trehalose 6-phosphate are correlated with redox activation of ADPglucose pyrophosphorylase and higher rates of starch synthesis in Arabidopsis thaliana. Biochemical Journal 397: 139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masakapalli SK, Bryant FM, Kruger NJ, et al. 2014. The metabolic flux phenotype of heterotrophic Arabidopsis cells reveals a flexible balance between the cytosolic and plastidic contributions to carbohydrate oxidation in response to phosphate limitation. The Plant Journal 78: 964–977. [DOI] [PubMed] [Google Scholar]
- Murmu J, Plaxton WC. 2007. Phosphoenolpyruvate carboxylase protein kinase from developing castor oil seeds: partial purification, characterization, and reversible control by photosynthate supply. Planta 226: 1299–1310. [DOI] [PubMed] [Google Scholar]
- Neumann G, Massonneau A, Langlade N, et al. 2000. Physiological aspects of cluster root function and development in phosphorus-deficient white lupin (Lupinus albus L.). Annals of Botany 85: 909–919. [Google Scholar]
- O’Leary B, Park J, Plaxton WC. 2011. The remarkable diversity of plant PEPC (phosphoenolpyruvate carboxylase): recent insights into the physiological functions and post-translational controls of non-photosynthetic PEPCs. Biochemical Journal 436: 15–34. [DOI] [PubMed] [Google Scholar]
- Penaloza E, Munoz G, Salvo-Garrido H, et al. 2005. Phosphate deficiency regulates phosphoenolpyruvate carboxylase expression in proteoid root clusters of white lupin. Journal of Experimental Botany 56: 145–153. [DOI] [PubMed] [Google Scholar]
- Plaxton WC, Tran HT. 2011. Metabolic adaptations of phosphate-starved plants. Plant Physiology 156: 1006–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Ballesta I, Feria A-B, Ni H, et al. 2014. In vivo monoubiquitination of anaplerotic phosphoenolpyruvate carboxylase occurs at Lys624 in germinating sorghum seeds. Journal of Experimental Botany 65: 443–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shane MW, Lambers H. 2005. Cluster roots: a curiosity in context. Plant and Soil 274: 101–125. [Google Scholar]
- Shane MW, De Vos M, De Roock S, et al. 2003. Shoot P status regulates cluster-root growth and citrate exudation in Lupinus albus grown with a divided root system. Plant, Cell and Environment 26: 265–273. [Google Scholar]
- Shane MW, Fedosejevs ET, Plaxton WC. 2013. Reciprocal control of anaplerotic phosphoenolpyruvate carboxylase by in vivo monoubiquitination and phosphorylation in developing proteoid roots of phosphate-deficient harsh hakea. Plant Physiology 161: 1634–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripodi KE, Turner WL, Gennidakis S, et al. 2005. In vivo regulatory phosphorylation of novel phosphoenolpyruvate carboxylase isoforms in endosperm of developing castor oil seeds. Plant Physiology 139: 969–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhde-Stone C, Gilbert G, Johnson JM-F, et al. 2003. Acclimation of white lupin to phosphorus deficiency involves enhanced expression of genes related to organic acid metabolism. Plant and Soil 248: 99–116. [Google Scholar]
- Uhrig RG, She YM, Leach CA, Plaxton WC. 2008. Regulatory monoubiquitination of phosphoenolpyruvate carboxylase in germinating castor oil seeds. Journal of Biological Chemistry 283: 29650–29657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance CP, Uhde-Stone C, Allan DL. 2003. Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource. New Phytologist 157: 427–447. [DOI] [PubMed] [Google Scholar]
- Wang Z, Straub D, Yang H, et al. 2014. The regulatory network of cluster-root function and development in phosphate-deficient white lupin (Lupinus albus) identified by transcriptome sequencing. Physiologia Plantarum 151: 323–338. [DOI] [PubMed] [Google Scholar]
- Watt M, Evans JR. 1999. Linking development and determinacy with organic acid efflux from proteoid roots of white lupin grown with low phosphorus and ambient or elevated atmospheric CO2 concentration. Plant Physiology 120: 705–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav UP, Ivakov A, Feil R, et al. 2014. The sucrose trehalose 6-phosphate (Tre6P) nexus: specificity and mechanisms of sucrose signalling by Tre6P. Journal of Experimental Botany 65: 1051–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XQ, Chollet R. 1997. Phosphoenolpyruvate carboxylase protein kinase from soybean root nodules: partial purification, characterization, and up/down-regulation by photosynthate supply from the shoots. Archives of Biochemistry and Biophysics 343: 260–268. [DOI] [PubMed] [Google Scholar]