Abstract
Background and Aims Flooding imposes stress upon terrestrial plants because it results in oxygen deficiency, which is considered a major problem for submerged plants. A common response of terrestrial plants to flooding is the formation of aquatic adventitious roots. Some studies have shown that adventitious roots on submerged plants are capable of absorbing water and nutrients. However, there is no experimental evidence for the possible oxygen uptake function of adventitious roots or for how important this function might be for the survival of plants during prolonged submergence. This study aims to investigate whether adventitious roots absorb oxygen from the water column, and whether this new function is beneficial to the survival of completely submerged plants.
Methods Taking Alternanthera philoxeroides (Mart.) Griseb. as a representative species, the profiling of the underwater oxygen gradient towards living and dead adventitious roots on completely submerged plants was conducted, the oxygen concentration in stem nodes with and without adventitious roots was measured, and the growth, survival and non-structural carbohydrate content of completely submerged plants with and without adventitious roots was investigated.
Key Results Oxygen profiles in the water column of adventitious roots showed that adventitious roots absorbed oxygen from water. It is found that the oxygen concentration in stem nodes having adventitious roots was higher than that in stem nodes without adventitious roots, which implies that the oxygen absorbed by adventitious roots from water was subsequently transported from the roots to other plant tissues. Compared with plants whose adventitious roots had been pruned, those with intact adventitious roots had slower leaf shedding, slower plant mass reduction, more efficient carbohydrate economy and prolonged survival when completely submerged.
Conclusions The adventitious roots of A. philoxeroides formed upon submergence can absorb oxygen from ambient water, thereby alleviating the adverse effects of oxygen deficiency, enabling efficient utilization of carbohydrates and delaying the death of completely submerged plants.
Keywords: Alternanthera philoxeroides (Mart.) Griseb., aquatic adventitious roots, microelectrode, oxygen uptake, submergence tolerance
INTRODUCTION
Flooding profoundly affects a wide range of ecosystems, from river forelands to farmlands, and it is one of the main causes of natural disasters worldwide (Smith, 2013). Moreover, as a consequence of climate change, the frequencies and intensities of floods are expected to increase in the future (Hirabayashi et al., 2013; Stocker et al., 2013). Flooding is detrimental to many terrestrial plants as it generally hampers their growth and may ultimately cause their death (Voesenek et al., 2006; van Bodegom et al., 2008). However, some terrestrial plant clades have evolved a number of adaptive mechanisms that are considered to reduce the negative effects of submergence (Mommer and Visser, 2005). Such adaptations may constitute an important source of plant diversity in areas that have had a flooding regime for a long time and where adapted species have had the time to establish (Silvertown et al., 1999).
The adaptations to flooding generally relate to coping with oxygen limitations, given that, when plants become submerged, oxygen is an important limiting resource for metabolic performance and survival (Vartapetian and Jackson, 1997). The sources of O2 potentially available to plants when completely submerged are internal, i.e. from their own photosynthesis, or external from the water column. Light availability is an important factor for photosynthesis; compared with the conditions above water, aquatic environments are generally considered to be shaded environments, since light is attenuated by surface reflection, back-scattering, and absorption by water and suspended particles (Sand-Jensen, 1989). This particularly applies to river water, in which the load of suspended sediment is often very high, and thus light transmission is poor. For example, median transmission in floodplains of the river Rhine is <1 % when flooded to 1 m depth, even at the lowest levels of suspended loads observed during flooding (Vervuren et al., 2003). Similarly, seasonally flooded rice fields may also suffer from turbid conditions, with light penetrating to a depth of <0·4 m into floodwater (Ram et al., 2002). Such low light conditions result in a particularly unfavourable environment for photosynthesis. Additionally, not only will photosynthesis be limited by light during flooding, but the availability of carbon dioxide is also severely limited (Maberly and Madsen, 1998; Sand-Jensen and Frost-Christensen, 1999), as the slow diffusion rate in water will greatly hamper uptake rates by the leaves compared with those in air (Bowes, 1987; Madsen and Sand-Jensen, 1994). Boundary layers around the leaves and stem are likely to be several orders greater in water than in air, particularly in stagnant or slow flowing water (Smith and Walker, 1980). These factors together indicate that, at these low photosynthesis rates, the oxygen dissolved in water becomes the main oxygen source for completely submerged plants, and plants must take up oxygen through their submerged tissues.
The supply of O2 to plants in the water column diminishes because water contains less gas than the atmosphere, as diffusion of dissolved gases in water is 10 000-fold slower than that in air (Jackson, 1985). Oxygen deficiency inhibits respiration, causing an ‘energy crisis’ in anoxic cells or tissues, and ultimately results in the death of plants (Crawford and Brändle, 1996). Plants submerged due to the low availability of internal O2 usually switch from aerobic to anaerobic respiration for energy conservation (Perata and Alpi, 1993). The level of carbohydrate storage in plant tissues greatly affects the survival during submergence. The low internal O2 concentration induces more consumption of stored carbohydrates, accelerates the process of cell death and leads to low survival rates (Bailey-Serres and Chang, 2005).
Some terrestrial plants have evolved particular adaptive mechanisms to reduce the negative effects of submergence via low oxygen availability. For instance, leaves with gas films are able to improve their gas exchange with the water column and improve the internal aeration during complete submergence, because the gas films on submerged leaves enlarge the water–gas interface and also facilitate the uptake of oxygen via stomata, thereby bypassing the resistance of foliar cuticles (Pedersen et al., 2009; Verboven et al., 2014; Winkel et al., 2014). Also, when submerged, terrestrial plants may acclimate to submerged conditions by developing new leaves with thin blades and undeveloped cuticles, so as to reduce diffusion resistance and take up oxygen from the surrounding water column more efficiently (Mommer et al., 2004; Colmer et al., 2011).
In addition to forming these gas films and acclimated leaves, a more common response of most terrestrial plants to flooding is the formation of an aquatic adventitious root system (Jackson and Drew, 1984). Aquatic adventitious roots contain very thin or no cuticles and have a large surface area per unit root biomass because of the small root diameter and numerous root hairs. Some literature has shown that aquatic adventitious roots on submerged plants are capable of absorbing water and nutrients, as substitutes for sediment roots which become dysfunctional or die upon submergence (Končalová, 1990; Polthanee and Changdee, 2008; Sauter, 2013). It has also been suggested that adventitious roots may absorb oxygen from the surrounding water column (Rich et al., 2012). However, to our knowledge, there is no experimental evidence for this possible oxygen uptake function of aquatic adventitious roots; or for how important this function might be for the survival of plants during prolonged submergence. Here we hypothesize that adventitious root uptake is an important O2 source for plant survival under completely submerged and dark conditions.
To test our hypothesis, we applied a new methodology of experimentally pruning adventitious roots of submerged terrestrial plants and measured its impacts on oxygen uptake and on plant survival. We used Alternanthera philoxeroides, a submergence-tolerant terrestrial species as a model, as it is known to produce a number of adventitious roots de novo on the stem nodes in a short time upon submergence (Gao et al., 2008). We tested our hypothesis in three steps. First, we monitored the oxygen uptake by adventitious roots during submergence in the dark; secondly, we measured the oxygen concentration in stem nodes with adventitious roots vs. in stem nodes with all adventitious roots removed; and, thirdly, we quantified the survival status of plants with or without adventitious roots (due to pruning treatment) during prolonged complete submergence in the dark.
MATERIALS AND METHODS
Species and plant preparation
Alternanthera philoxeroides (Mart.) Griseb., a perennial terrestrial plant of the Amaranthaceae family, originates in South America, but has spread to many parts of the world and is considered an invasive species in Australia, China, New Zealand, Thailand and the USA. This species possesses a good tolerance to submergence, upon which it usually produces adventitious roots on the nodes of the submerged stem. Under normal conditions, A. philoxeroides grows to a height of 50–120 cm, with a long, single or sparsely branched stem.
The A. philoxeroides plants used in the experiment were cultivated from cuttings that had been obtained from plants naturally growing on the banks of the Jialing River in the sub-tropical Chongqing District, south-west of China (29°49′N, 106°25′E). Unbranched plants with a stem length of approx. 35 cm were selected and cut at the stem base. Each cutting was planted in a 15 cm diameter and 15 cm deep plastic pot containing a soil mixture of 40 % clay, 40 % humus soil and 20 % sand; two stem nodes of the cutting were buried in the soil for rooting. All these plantlets were kept in an open field of the experimental garden of the Key Laboratory of Eco-environments in Three Gorges Reservoir Region (Ministry of Education) at Southwest University, Chongqing (i.e. close to the collection site), and cultivated under the same conditions of temperature (approx, 10–15 °C in the daytime), relative humidity (75–85 %), light (daily maximum 600–800 μmol m–2 s–1) and water provision (approx. 80–90 % of soil water-holding capacity). After growing for about 1 month, all plants had rooted and were in good health, around 40 cm tall, ready for the submergence experiments.
Experimental set-up
Before complete submergence, 24 plants were harvested as the initial harvest group for measurement of the total leaf number, length and width of each leaf, the leaf area and the stem diameter of each plant. The dry mass of leaves, stem, rhizome, roots and adventitious roots was also quantified. Regression equations between the width, length and dry mass of leaves, and between the volume and dry mass of the stem were constructed; these equations were used to estimate the initial dry mass of the remaining plants [for analysis of the relative growth rate (RGR); see below] before complete submergence. In this study, leaf area was measured by a Leaf Image Analysis System (WD3 WinDIAS, Delta-T), and the dry mass of each tissue was determined by weighing after oven-drying at 60 °C for 72 h. For the complete submergence experiment, 44 rooted plants were used, of which 24 plants comprised the control group with adventitious roots intact and 20 plants constituted the pruning group whose adventitious roots were to be pruned (see below). These potted plants were subsequently placed in 1·5 × 0·8 × 1·0 m (length × width × depth) water tanks, which were placed in a growth room, and submerged by adding tap water to the tank until the water level was 10 cm above the top of the stems. During the experiment, all plants were completely submerged, in complete darkness, and the water temperature was kept at around 23–25 °C. In order to maintain continuous oxygen availability, the water column was gently bubbled with air during the submergence treatment. Adventitious roots emerged from the stem nodes after 7–8 d of submergence. The control plants were kept intact, but, once the plants of the pruning group started to develop any adventitious roots on the nodes, these adventitious roots were carefully pruned from the attachment point to the stem under water with a small knife, preventing any visual damage on the stem and leaves. After 15 d of complete submergence, ten plants (seven plants with intact adventitious roots and three with adventitious roots removed) were chosen for O2 measurements, and the others were kept in the tanks for survival status testing (see below).
O2 measurements
Profiling of the oxygen gradient towards submerged adventitious roots under water.
To clarify whether adventitious roots on submerged plants are able to take up oxygen from water, we investigated underwater profiling of the O2 gradient towards: (a) living adventitious roots; (b) dead adventitious roots (as a control for adventitious roots in the absence of metabolic activity); and (c) background (i.e. no adventitious roots present).
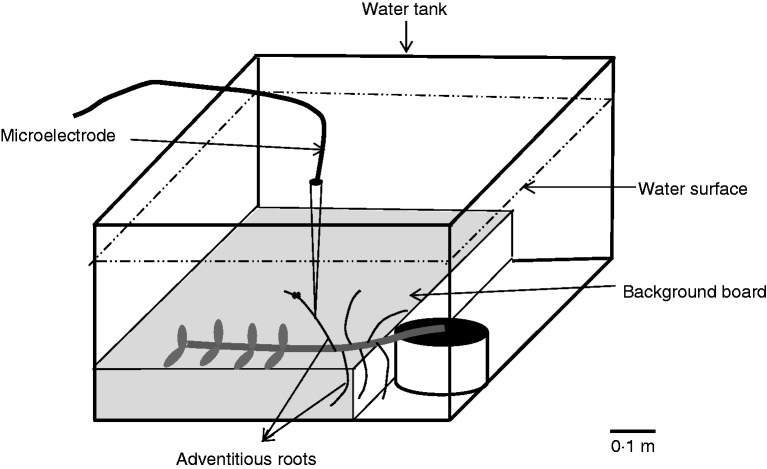
Living adventitious roots: each selected completely submerged plant (in a pot) with adventitious roots was transferred under water to a PVC tank (length × width × height: 0·8 × 0·5 × 0·4 m) filled with air-saturated tap water, and stem, leaves and adventitious roots were fixed onto a background board (smooth foam board) which was positioned at the bottom of the tank (Fig. 1). One adventitious root below the foliated stem part, 10–12 cm in length and 0·3–0·5 cm in diameter, was randomly chosen to conduct oxygen profiling and was fixed onto the background board far from the stem and other adventitious roots, thus preventing them from disturbing the oxygen gradient in the water column. The water level of the air-saturated tap water in the tank was maintained at approx. 15 cm above the background board. A microelectrode (tip diameter = 10 μm, OX10, Unisense) mounted on a motor-driven micromanipulator controlled by microprofiling software (SensorTrace Pro, Unisense, Denmark) was used to determine the O2 concentration profiles. These profiles were taken at a position of the adventitious root which was approx. 10 mm away from the attachment point of the root to the stem, by advancing the electrode vertically downward over a distance of approx. 1200 μm in steps of 50 μm every 5 s, moving towards the adventitious root surface. The signal from the microelectrode was amplified using a picoamperometer with an analogue/digital converter. Electrode signals were converted into O2 concentration using calibration between 0 and 100 % of air equilibrium. The transfer of plants and microelectrode positioning were done in green light; all measurements were performed in darkness at 25 °C. Four plants were used for oxygen profiling, and for each of these plants, one adventitious root was chosen for the profiling.
Dead adventitious roots: when the underwater oxygen profiling of the living adventitious root of each of four plants was finished in procedure (a), the water in the tank was drained to a suitable water level so as to de-submerge only the target living adventitious root but to leave the whole plant and other adventitious roots still submerged; the target living adventitious root was detached from the background board and then treated with hot steam for 2 min. This was sufficient to kill the living adventitious root and stop metabolic activities, but did not affect the stem attached. Steaming treatment was done by boiling de-ionized water and conveying the steam through a thin glass pipette to the adventitious root. We checked the effectiveness of this method to kill the adventitious roots in pilot experiments, in which we used Evans blue to stain dead adventitious roots. After the steaming treatment, the dead adventitious root was fixed onto the background board again and the tank was refilled with air-saturated tap water to the same water level; the profiling of oxygen gradient towards the dead root under water was investigated in the same way as described in procedure (a).
Background board: in order to check and preclude any artefacts caused by the background board on the O2 profiles of the water column submerging adventitious roots, O2 profiles of the water column above the background board without any plant tissues was investigated in the same way as described in procedure (a). Four replicates were measured.
Fig. 1.
Schematic representation of the experimental set-up for profiling of the oxygen gradient towards an adventitious root surface under water. The plant with adventitious roots was placed at the bottom of the water tank and the target adventitious root was fixed onto the background board. The whole potted Alternanthera philoxeroides plant was submerged with air-saturated water for about 1 h. The microelectrode was fixed on a micromanipulator, and used to measure the underwater oxygen profile over a distance of approx. 1200 μm by advancing a step of 50 μm every 5 s, moving towards the root surface.
Oxygen concentration in submerged nodes.
The node tissue O2 concentration of A. philoxeroides plants with intact adventitious roots vs. with adventitious roots removed was measured under the condition of complete submergence in darkness. Each potted plant was placed in the tank with a background board (Fig. 1); plants were mounted onto the background board and submerged 15 cm deep in air-saturated tap water. A 25 μm tip diameter O2 microelectrode (OX25, Unisense) was mounted on a motor-driven micromanipulator and inserted 300 μm deep into the node. Three replicate plants with and without adventitious roots, respectively, were measured.
Survival status testing
During submergence, the number of shed leaves was recorded every day as an indicator of decline in plant vitality. The complete submergence experiment lasted for 38 d, after which all plants in the tanks were harvested. The total leaf number, leaf area, stem diameter, and dry mass of each tissue of each plant were measured in the same ways as for the initial harvest. The firmness of the stem tip tissues of each plant was recorded as another measure of its vitality, where we interpreted a firm tip as belonging to a still surviving plant and a soft tip to a dying plant. Also, the non-structural carbohydrates (NSCs), including both sugars and starch, in stem and leaves of final harvested plants were analysed as a measure of anaerobic vs. aerobic respiration (see the Introduction). For methodological details on the NSC assay see Wong (1980) and Hoch et al. (2003).
Data analysis
The RGR of dry mass of each individual plant was calculated as:
where A1 is the estimated dry mass of each plant before complete submergence. A1 was the sum of the estimated dry mass of leaves, stem, rhizome, roots and adventitious roots, in which the initial dry mass of leaves was estimated from regression equations using leaf length and width, and initial dry mass of stem was from regression equations using stem volume (see above); the initial dry masses of roots and rhizome were the mean values obtained from the initial harvest group (see the Experimental set-up). A2 was obtained from the final harvest of each plant after complete submergence, while t1 and t2 represent the start and end date of complete submergence, respectively.
The leaf area ratio (LAR) at final harvest was determined as:
A repeated measure analysis of variance (ANOVA) was run for testing the differences in the oxygen profile between living adventitious roots, dead adventitious roots and the background board without adventitious roots over the interval of 600 μm to 0 μm from the adventitious root surface. A χ2 test was run for testing the difference in numbers of plants with firm vs. soft apical tissues. An independent t-test was carried out to test for differences in oxygen content in nodes, final recorded leaf number, LAR and RGR between control and pruned plants. In addition, a repeated measure ANOVA was run to determine the differences in dry mass with treatment as the between-subjects factor and tissue as the within-subject factor; an independent t-test was also carried out to test the differences between treatments in dry mass of each tissue. Data transformation was performed to equalize variances when necessary in repeated measure ANOVA; significant differences were reported at P < 0·05. All analyses were performed using SPSS 21.
RESULTS
It was found in the study that oxygen profiles in the water column differed between experimental treatments, i.e. background board, living adventitious roots and dead adventitious roots. In this study, it appeared that 600 μm was the distance range within which the dissolved oxygen concentration in the water column was affected by the presence of living adventitious roots (Fig. 2). From a distance of 600 μm to 0 μm, oxygen profiles in the water column adjacent to living adventitious roots were significantly different from those adjacent to the background board and dead adventitious roots; the oxygen profiles in the water column adjacent to living adventitious roots had a smaller slope than that in the water column adjacent to dead adventitious roots (Fig. 2). Furthermore, the oxygen concentration in water at the surface (at distance 0 μm) of living adventitious roots was lower than that of dead adventitious roots. In this study, we also investigated the oxygen concentration in stem nodes of completely submerged plants and we found that the oxygen concentration in the stem nodes was significantly higher (P = 0·034) in the plants with adventitious roots than in those without (Fig. 3).
Fig. 2.
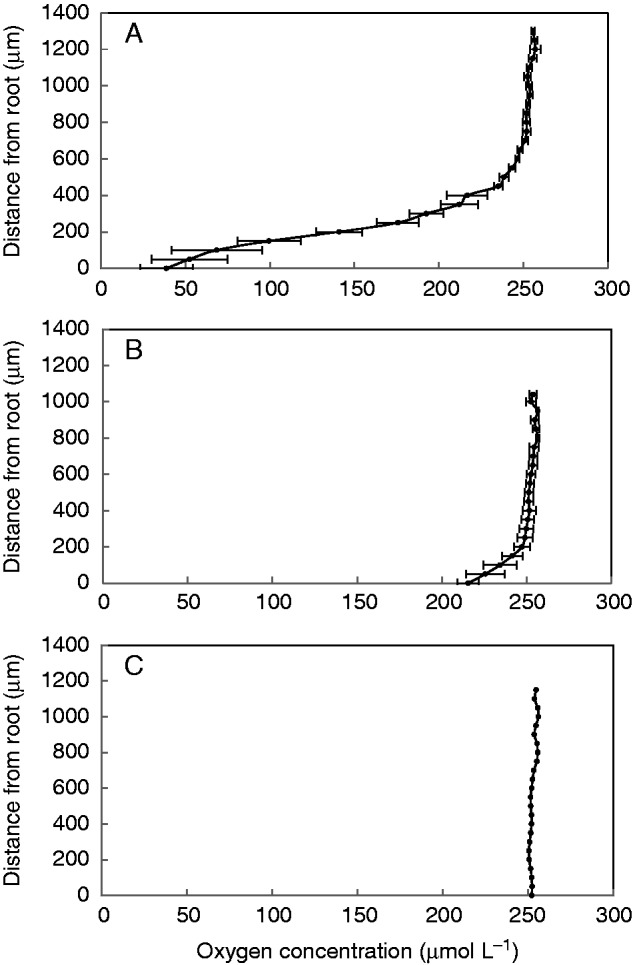
Oxygen profiles in the water column adjacent to (A) living adventitious roots (n = 4), (B) dead adventitious roots (n = 3) and (C) the background board without roots (n = 4) in darkness at 25 °C. Values are given as the mean ± s.e. By using an O2 microelectrode (tip diameter 10 μm), underwater profiling was conducted towards a point of the adventitious root; the point was approx. 10 mm away from the point of attachment of the adventitious root to the stem of Alternanthera philoxeroides.
Fig. 3.
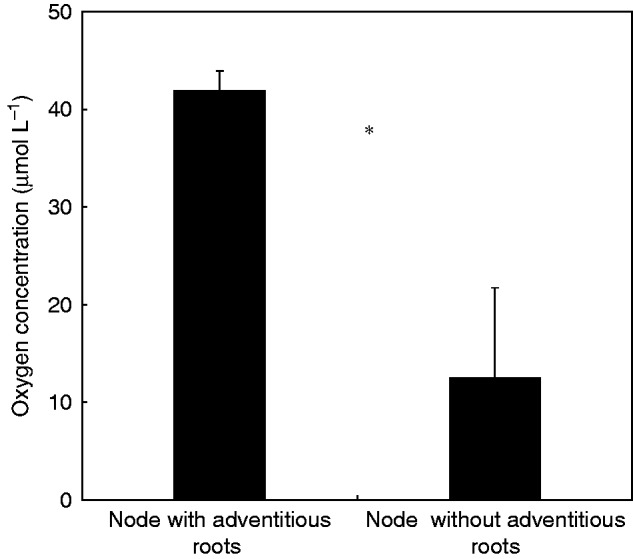
O2 concentration (mean ± s.e., n = 3) in stem nodes with or without adventitious roots in darkness at 25 °C under complete submergence. During the measurement, Alternanthera philoxeroides plants were completely submerged to a depth of 15 cm. An asterisk indicates a significant difference at P < 0·05 between treatments (unpaired t-test).
In the experiment, it was shown that in the first 14 d of complete submergence, no leaves were shed from the plants either with or without adventitious roots (Fig. 4A). However, after 26 d of submergence, the plants started to lose some leaves; the plants without adventitious roots always lost more leaves compared with plants with adventitious roots. It is clear that the presence or absence of adventitious roots affected the tolerance of plants to complete submergence; at the end of the study, the percentage of plants with soft stem apical tissues (Fig. 4B) was significantly larger in the adventitious root pruning group than that in the group with intact adventitious roots (χ2 test, P = 0·039).
Fig. 4.
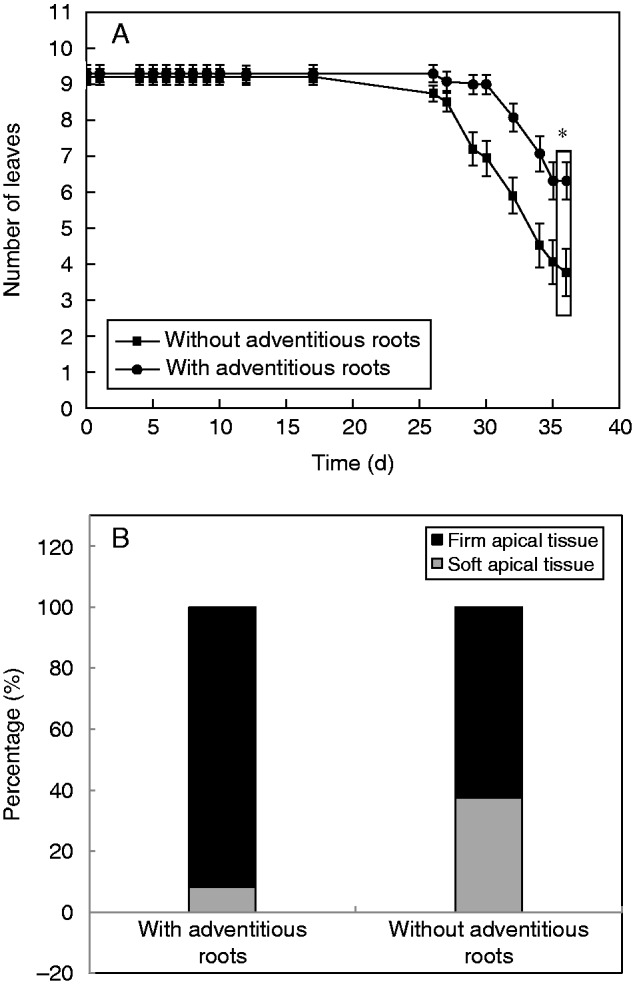
Indicators of plant vitality. Dynamics of leaf shedding (A) and stem apical tissue vitality (B) of Alternanthera philoxeroides plants with and without adventitious roots during complete submergence in darkness at 25 °C. In (A), values are given as the mean ± s.e. (n = 17) and an asterisk indicates that the number of leaves on plants between the two treatments was significantly different (P < 0·05, unpaired t-test) on the last day of recording. In (B), the pruning of adventitious roots had negative effects on the stem apical tissue vitality of plants at final harvest. (χ2 test, d.f. = 1, χ2 = 4·246, P = 0·039; 17 plants used in each treatment).
Under complete submergence in the dark, plants cannot carry out photosynthesis to accumulate carbohydrates, so they can only consume carbohydrates already stored in tissues before submergence, and, consequently, the experimental plants generally had a negative RGR (Fig. 5A). The plants without adventitious roots had a significantly higher consumption of biomass, i.e. a more negative RGR, than plants with intact adventitious roots during submergence (P = 0·041). Due to the slower leaf shedding, the LAR of plants with adventitious roots was significantly higher than that of plants without adventitious roots at final harvest (P = 0·044) (Fig. 5B). It was revealed in this study that adventitious roots had an impact on the consumption of carbohydrates. The concentration of leaf soluble sugars showed no significant difference between the two treatments (P = 0·421, Fig. 6), but the starch concentration of leaves in plants with adventitious roots was significantly higher than that in plants without adventitious roots (P = 0·015). The concentration of soluble sugars and starch in stems had the same trend as that in leaves; only the starch concentration of the stem in plants with adventitious roots was significantly higher than that in plants without adventitious roots (P = 0·026).
Fig. 5.
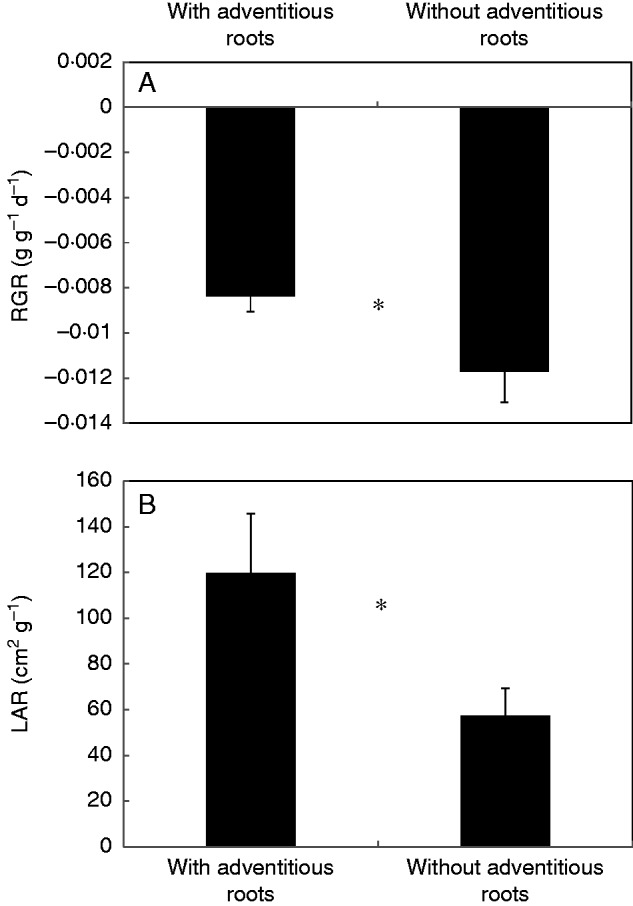
Relative growth rate (RGR) (g g–1 d–1) (means ± s.e., n = 17) of dry mass (A) and leaf area ratio (LAR) (B) of Alternanthera philoxeroides plants with and without adventitious roots after 38 d complete submergence in darkness at 25 °C. Asterisk indicates significant differences between the two treatments (P < 0·05, unpaired t-test).
Fig. 6.
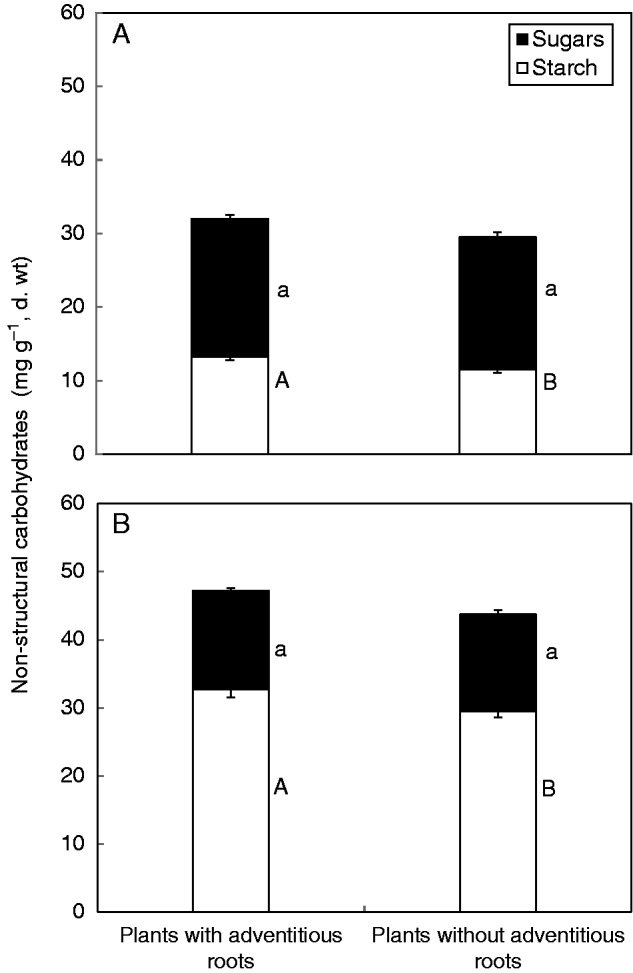
Content (means ± s.e.) of non-structural carbohydrates (including starch and soluble sugars) in leaves (A) and stems (B) of completely submerged Alternanthera philoxeroides plants with and without adventitious roots. For either soluble sugars or starch, different letters indicate a significant difference (P < 0·05) between treatments. Stem non-structural carbohydrate contents were determined with 20 replicates each for plants with and without adventitious roots; leaf non-structural carbohydrate contents were determined with eight replicates for plants with adventitious roots and six replicates for plants without adventitious roots.
In the study, we found that, after a period of complete submergence, stem mass comprised the largest proportion of the whole plant mass in all plants. In plants either with or without adventitious roots, the stem had a higher mass than other organs (Supplementary Data Fig. S1). Plants with intact adventitious roots had a significantly higher stem mass and rhizome mass than plants with pruned adventitious roots. However, no significant differences were found between plants with or without adventitious roots in terms of leaf and root mass (Fig. S1).
DISCUSSION
Oxygen uptake and transport through aquatic adventitious roots
A common response of plants to flooding is the formation of an adventitious root system, which usually forms on their stem or branches (Etherington, 1984; Naidoo and Naidoo, 1992; Rich et al., 2008, 2012). Aquatic adventitious roots are adapted to the flooded environment and support or replace the primary soil roots, which usually die and lose their water and nutrient uptake functions during prolonged flooding (Sauter, 2013). Recent research revealed a new function for aquatic adventitious roots: production of internal oxygen and carbohydrate by photosynthesis in chloroplasts in root cells (Rich et al., 2008, 2011). In our study, we have provided experimental evidence for another function for aquatic adventitious roots, i.e. uptake of external oxygen from the ambient water column. By comparing the oxygen profiles in the water column from the surface of living adventitious roots outwards with those of dead adventitious roots (Fig. 2), we found clear evidence that the living adventitious roots depleted the oxygen much more strongly and over a larger distance than dead roots, which resulted in a shallower slope of the oxygen profile close to the living adventitious roots. The reason for this stronger oxygen depletion and resultant shallower slope is that, compared with the dead adventitious roots, the living adventitious roots had a higher demand for oxygen; part of the oxygen was consumed by the respiration of living root cells, and the other part was transported from the adventitious roots to other parts of plants. Therefore, the living adventitious roots had a larger oxygen flux than dead adventitious roots. Strikingly, and consistent with the depletion outside the adventitious roots, the internal oxygen concentration of stem nodes with intact adventitious roots was considerably higher than that of stem nodes from which the adventitious roots had been cut off (Fig. 3). These findings show clearly that the oxygen taken up from the water column by adventitious roots can be transported to other part of the plant. Uptake of oxygen from the water column into the plant has been observed for leaves (Mommer et al., 2004) but, to our knowledge, our study is the first time that this process has been shown in aquatic adventitious roots.
Benefit of oxygen uptake by aquatic adventitious roots to submerged plants
When the completely submerged plants do not obtain internal oxygen from photosynthesis because of poor illumination due to turbid water or deep submergence, the water column functions as the only possible source of oxygen for the plant. Many studies have shown that these floodplain species usually employ some adaptive responses to increase the gas exchange under water to overcome the negative effects of submergence, which include both metabolic and morphological plasticity, such as by enhanced elongation and reorientation of the leaves, by an increase of the amount of air space (aerenchyma) within the shoot and the roots, or by switching to anaerobic metabolic pathways to prevent energy deficits. (Perata and Alpi, 1993; Armstrong et al., 1994; Blom, 1999; Colmer, 2003; Voesenek et al., 2003). Furthermore, it has been hypothesized that gas films on leaves (Pedersen et al., 2009) enable stomata to remain open; uptake of O2 via stomata would bypass the resistance of the cuticle and epidermal cells to O2 entry into submerged leaves (Verboven et al., 2014). Therefore, the gas films improve gas exchange under water, with benefits to whole-plant internal aeration and growth of completely submerged plants. However, not all flooded plant species can produce leaf gas films under water, so many or most species have to deal with the diffusion resistance to O2. Some plant species can produce new acclimated leaves under water, with lower cuticle and epidermal cell wall thickness, promoting oxygen diffusion (Frost-Christensen et al., 2003; Mommer et al., 2004, 2005; Mommer and Visser, 2005). However, completely submerged plants take several weeks to produce new acclimated leaves under water; before that, the adventitious roots may already have formed and played an important role in O2 uptake from the water column. Moreover, adventitious roots may be the only important source of absorbing oxygen from ambient water for most flooded plant species, especially under deep submergence, where they probably do not grow any new acclimated leaves. Hence, the function of aquatic adventitious roots to absorb O2 from the water column, thereby improving the plant internal O2 status, may be especially important in dark conditions, such as in turbid water or under deep submergence. Further, even in more sunlit water, these adventitious roots may also improve aeration of plants during the night.
There may be two more reasons why adventitious roots may be more effective at improving the oxygen supply to plants during prolonged submergence. First, the ratio of total surface area to total volume of adventitious roots is higher than that of leaves. Moreover, some researchers have found that the percentage of aerenchyma in adventitious roots is larger than that in leaves of flooding-tolerant plants (Visser et al., 2000; Mommer et al., 2007; Rich et al., 2012), which means that adventitious roots use less oxygen for cell respiration compared with leaves, while they can transport relatively more O2 to other plant parts. Secondly, compared with uptake through leaves, the oxygen taken up by adventitious roots can easily reach the sediment root system to improve the functioning of sediment roots under the flooded conditions, due to the short distance from adventitious roots to soil roots (Sauter, 2013).
Enhancement of submergence tolerance of plants due to oxygen uptake by aquatic adventitious roots
Complete submergence imposes considerable stress on plant functioning, predominantly by way of oxygen deprivation. Low oxygen levels in plants limit aerobic respiration and other essential oxygen-dependent processes (Armstrong and Gaynard, 1976; Laan et al., 1990). Anaerobic metabolic pathways, such as fermentation, may partly compensate the low ATP yield from impaired aerobic metabolism (Perata and Alpi, 1993; Gibbs and Greenway, 2003), but these pathways are far less efficient than aerobic respiration and thus reduce the pool of carbohydrate reserves relatively rapidly (Laan and Blom, 1990; Guglielminetti et al., 1997). Submergence-induced oxygen deficiency in plants is therefore inevitably accompanied by carbohydrate and energy deficits. During a long period of submergence, the increasing consumption of carbohydrates, and possibly the accumulation of toxic substances in plants [e.g. sulphide, reactive oxygen species (ROS), Fe2+ and Mn2+], results in loss of biomass and ultimately in death of many plant species. In our study, the amount of starch in plants with intact adventitious roots was significantly higher than that in plants with adventitious roots experimentally removed after a period of complete submergence (Supplementary Data Fig. S1). Moreover, the rates of leaf shedding, loss of plant and stem biomass and decrease of LAR in plants with adventitious roots were lower than those in plants without adventitious roots. These experimental results support our expectation that O2 absorbed by adventitious roots improves the O2 availability in the plants, enabling them to use their carbohydrate reserves more efficiently, thereby enhancing their flooding tolerance. It is clearly shown in our study that oxygen uptake by adventitious roots prolonged plants’ survival during complete submergence, which was indicated by a lower proportion of plants having soft apical tissues (a symptom of dying) in plants with adventitious roots compared with those without adventitious roots.
In summary, the aquatic adventitious roots of A. philoxeroides that develop during prolonged complete submergence can absorb O2 from the surrounding water column, thereby alleviating the adverse effects of oxygen deficiency, enabling efficient utilization of carbohydrate reserves and delaying the process of plant death. The next research challenge will be to study the occurrence and relative importance of plant O2 uptake by aquatic adventitious roots in other plant species in different biomes of the world, and under different flooding regimes (e.g. partial submergence), and to explore how the O2 uptake capacity of aquatic adventitious roots affects species’ competitiveness and ecological adaptation to environments.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of Figure S1: organ dry mass (g) of completely submerged Alternanthera philoxeroides plants with adventitious roots and without adventitious roots.
ACKNOWLEDGEMENTS
We thank Songping Liu for his assistance in plant preparation and sample collection. The work was supported by the National Natural Science Foundation of China (grant nos 31400480, 31370443 and 31070474), Ministry of Finance & State Council Executive Office of Three Gorges Project Construction Committee of China (grant nos 5000002013BB5200001 and 5000002013BB5200002), Fundamental Research Funds for the Central Universities (grant no. XDJK2013A003) and Research Grant for Disciplines Conferring Doctorate in China (grant no. 20100182110022). Research trips by J.H.C.C. to China were supported by the Royal Netherlands Academy of Arts and Sciences (KNAW, CEP grant 12CDP007).
LITERATURE CITED
- Armstrong W, Gaynard TJ. 1976. The critical oxygen pressures for respiration in higher plants. Physiologia Plantarum 37: 200–206. [DOI] [PubMed] [Google Scholar]
- Armstrong W, Brändle R, Jackson MB. 1994. Mechanisms of flood tolerance in plants. Acta Botanica Neerlandica 43: 307–358. [Google Scholar]
- Bailey-Serres J, Chang R. 2005. Sensing and signaling in response to oxygen deprivation in plants and other organisms. Annals of Botany 96: 507–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blom CWPM. 1999. Adaptations to flooding stress from plant community to molecule. Plant Biology 1: 261–273. [Google Scholar]
- van Bodegom PM, Sorrell BK, Oosthoek A, Bakker C, Aerts R. 2008. Separating the effects of partial submergence and soil oxygen demand on plant physiology. Ecology 89: 193–204. [DOI] [PubMed] [Google Scholar]
- Bowes G. 1987. Aquatic plant photosynthesis: strategies that enhance carbon gain. In: Crawford RMM, ed. Plant life in aquatic and amphibious habitats. Oxford: Blackwell Scientific Press, 79–98. [Google Scholar]
- Colmer TD. 2003. Long distance transport of gases in plants a perspective on internal aeration and radial oxygen loss from roots. Plant, Cell and Environment 26: 17–36. [Google Scholar]
- Colmer TD, Winkel A, Pedersen O. 2011. A perspective on underwater photosynthesis in submerged terrestrial wetland plants. AoB Plants plr030, doi:10.1093/aobpla/plr030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford RMM, Bräendle R. 1996. Oxygen deprivation stress in a changing environment. Journal of Experimental Botany 47: 145–159. [Google Scholar]
- Etherington JR. 1984. Comparative studies of plant growth and distribution in relation to waterlogging: X. Differential formation of adventitious roots and their experimental excision in Epilobium hirsutum and Chamerion angustifolium. Journal of Ecology 72: 389–404. [Google Scholar]
- Frost-Christensen H, Bolt Jørgensen L, Floto F. 2003. Species specificity of resistance to oxygen diffusion in thin cuticular membranes from amphibious plants. Plant, Cell and Environment 26: 561–569. [Google Scholar]
- Gao J, Xiao Q, Yin L. 2008. Isolation of cDNA clones for genes up-regulated in drought-treated Alternanthera philoxeroides root. Molecular Biology Reports 35: 485–488. [DOI] [PubMed] [Google Scholar]
- Gibbs J, Greenway H. 2003. Mechanisms of anoxia tolerance in plants. I. Growth, survival and anaerobic catabolism. Functional Plant Biology 30: 1–47. [DOI] [PubMed] [Google Scholar]
- Guglielminetti L, Wu Y, Boschi E, et al. 1997. Effects of anoxia on sucrose degrading enzymes in cereal seeds. Journal of Plant Physiology 150: 251–258. [Google Scholar]
- Hirabayashi Y, Mahendran R, Koirala S, et al. 2013. Global flood risk under climate change. Nature Climate Change 3: 816–821. [Google Scholar]
- Hoch G, Richter A, Körner Ch. 2003. Non-structure carbon compounds in temperate forest trees. Plant, Cell and Environment 26: 1067–1081. [Google Scholar]
- Jackson MB. 1985. Ethylene and responses of plants to soil waterlogging and submergence. Annual Review of Plant Physiology 36: 145–174. [Google Scholar]
- Jackson MB, Drew MC. 1984. Effects of flooding on growth and metabolism of herbaceous plants. In: Kozlowski TT, ed. Flooding and plant growth. London: Academic Press, 47–128. [Google Scholar]
- Končalová H. 1990. Anatomical adaptation to waterlogging in roots of wetland graminoids: limitation and drawbacks. Aquatic Botany 38: 127–134. [Google Scholar]
- Laan P, Blom CWPM. 1990. Growth and survival responses of Rumex species to flooded and submerged conditions: the importance of shoot elongation, underwater photosynthesis and reserve carbohydrates. Journal of Experimental Botany 41: 775–783. [Google Scholar]
- Laan P, Tosserams M, Blom CWPM, Veen BW. 1990. Internal oxygen transport in Rumex species and its significance for respiration under hypoxic conditions . Plant and Soil 122: 39–46. [Google Scholar]
- Maberly SC, Madsen TV. 1998. Affinity for CO2 in relation to the ability of freshwater macrophytes to use HCO3–. Functional Ecology 12: 99–106. [Google Scholar]
- Madsen TV, Sand-Jensen K. 1994. The interactive effects of light and inorganic carbon on aquatic plant growth. Plant, Cell and Environment 17: 955–962. [Google Scholar]
- Mommer L, Visser EJ. 2005. Underwater photosynthesis in flooded terrestrial plants: a matter of leaf plasticity. Annals of Botany 96: 581–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mommer L, Pedersen O, Visser EJW. 2004. Acclimation of a terrestrial plant to submergence facilitates gas exchange under water. Plant, Cell and Environment 27: 1281–1287. [Google Scholar]
- Mommer L, Pons TL, Wolters-Arts M, Venema JH, Visser EJ. 2005. Submergence-induced morphological, anatomical, and biochemical responses in a terrestrial species affect gas diffusion resistance and photosynthetic performance. Plant Physiology 139: 497–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mommer L, Wolters-Arts M, Andersen C, Visser EJ, Pedersen O. 2007. Submergence-induced leaf acclimation in terrestrial species varying in flooding tolerance. New Phytologist 176: 337–345. [DOI] [PubMed] [Google Scholar]
- Naidoo G, Naidoo S. 1992. Waterlogging responses of Sporobolus virginicus L. Kunth. Oecologia 90: 445–450. [DOI] [PubMed] [Google Scholar]
- Pedersen O, Rich SM, Colmer TD. 2009. Surviving floods: leaf gas films improve O2 and CO2 exchange, root aeration, and growth of completely submerged rice. The Plant Journal 58: 147–156. [DOI] [PubMed] [Google Scholar]
- Perata P, Alpi A. 1993. Plant responses to anaerobiosis. Plant Science 93: 1–17. [Google Scholar]
- Polthanee A, Changdee T. 2008. Influence of adventitious root removing and timing of fertilizer application in flooded soil on growth, yield and N, P, K uptake of kenaf (Hibiscus cannabinus L.) under greenhouse and field conditions. Asian Journal of Plant Sciences 7: 352–359. [Google Scholar]
- Ram PC, Singh BB, Singh AK, et al. 2002. Submergence tolerance in rainfed lowland rice: physiological basis and prospects for cultivar improvement through marker-aided breeding. Field Crops Research 76: 131–152. [Google Scholar]
- Rich SM, Ludwig M, Colmer TD. 2008. Photosynthesis in aquatic adventitious roots of the halophytic stem-succulent Tecticornia pergranulata (formerly Halosarcia pergranulata). Plant, Cell and Environment 31: 1007–1016. [DOI] [PubMed] [Google Scholar]
- Rich SM, Ludwig M, Pedersen O, Colmer TD. 2011. Aquatic adventitious roots of the wetland plant Meionectes brownii can photosynthesize: implications for root function during flooding. New Phytologist 190: 311–319. [DOI] [PubMed] [Google Scholar]
- Rich SM, Ludwig M, Colmer TD. 2012. Aquatic adventitious root development in partially and completely submerged wetland plants Cotula coronopifolia and Meionectes brownii. Annals of Botany 110: 405–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sand-Jensen K. 1989. Environmental variables and their effect on photosynthesis of aquatic plant communities. Aquatic Botany 34: 5–25. [Google Scholar]
- Sand-Jensen K, Frost-Christensen H. 1999. Plant growth and photosynthesis in the transition zone between land and stream. Aquatic Botany 63: 23–35. [Google Scholar]
- Sauter M. 2013. Root responses to flooding. Current Opinion in Plant Biology 16: 282–286. [DOI] [PubMed] [Google Scholar]
- Silvertown J, Dodd ME, Gowing DJG, Mountford JO. 1999. Hydrologically defined niches reveal a basis for species richness in plant communities. Nature 400: 61–63. [Google Scholar]
- Smith FA, Walker NA. 1980. Photosynthesis by aquatic plants: effects of unstirred layers in relation to assimilation of CO2 and HCO3– and to carbon isotopic discrimination. New Phytologist 86: 245–259. [Google Scholar]
- Smith K. 2013. Hydrological hazards: floods. In: Environmental hazards: assessing risk and reducing disaster. London & New York: Routledge, 299–329. [Google Scholar]
- Stocker TF, Qin D, Plattner GK, et al. 2013. Technical summary. In: Stocker TF, Qin D, Plattner GK, et al., eds. IPCC (2013): Climate Change 2013: the physical science basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge: Cambridge University Press, 33–109. [Google Scholar]
- Vartapetian BB, Jackson MB. 1997. Plant adaptations to anaerobic stress. Annals of Botany 79: 3–20. [Google Scholar]
- Verboven P, Pedersen O, Ho QT, Nicolai BM, Colmer TD. 2014. The mechanism of improved aeration due to gas films on leaves of submerged rice. Plant, Cell and Environment 37: 2433–2452. [DOI] [PubMed] [Google Scholar]
- Vervuren PJA, Blom CWPM, De Kroon H. 2003. Extreme flooding events on the Rhine and the survivial and distribution of riparian plant species. Journal of Ecology 91: 135–146. [Google Scholar]
- Visser EJ, Colmer TD, Blom CWPM, Voesenek LACJ. 2000. Changes in growth, porosity, and radial oxygen loss from adventitious roots of selected mono- and dicotyledonous wetland species with contrasting types of aerenchyma. Plant, Cell and Environment 23: 1237–1245. [Google Scholar]
- Voesenek LACJ, Benschop JJ, Bou J, et al. 2003. Interactions between plant hormones regulate submergence-induced shoot elongation in flooding-tolerant dicot Rumex palustris. Annals of Botany 91: 205–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voesenek LACJ, Colmer TD, Pierik R, Millenaar FF, Peeters AJM. 2006. How plants cope with complete submergence. New Phytologist 170: 213–226. [DOI] [PubMed] [Google Scholar]
- Winkel A, Pedersen O, Ella E, Ismail AM, Colmer TD. 2014. Gas film retention and underwater photosynthesis during field submergence of four contrasting rice genotypes. Journal of Experimental Botany 65: 3225–3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SC. 1980. Elevated atmospheric partial pressure of CO2 and plant growth. Ι. Interactions of nitrogen nutrition and photosynthetic capacity in C3 and C4 plants. Oecologia 44: 68–74. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.