Abstract
Background and aims Traits related to root depth distribution were examined in Trifolium repens × T. uniflorum backcross 1 (BC1) hybrids to determine whether root characteristics of white clover could be improved by interspecific hybridization.
Methods Two white clover cultivars, two T. uniflorum accessions and two BC1 populations were grown in 1 -m deep tubes of sand culture. Maximum rooting depth and root mass distribution were measured at four harvests over time, and root distribution data were fitted with a regression model to provide measures of root system shape. Morphological traits were measured at two depths at harvest 3.
Key Results Root system shape of the hybrids was more similar to T. uniflorum than to white clover. The hybrids and T. uniflorum had a higher rate of decrease in root mass with depth than white clover, which would result in higher proportions of root mass in the upper profile. Percentage total root mass at 100–200 mm depth was higher for T. uniflorum than white clover, and for Crusader BC1 than ‘Crusader’. Roots of the hybrids and T. uniflorum also penetrated deeper than those of white clover. T. uniflorum had thicker roots at 50–100 mm deep than the other entries, and more of its fine root mass at 400–500 mm. The hybrids and white clover had more of their fine root mass higher in the profile. Consequently, T. uniflorum had a higher root length density at 400–500 mm than most entries, and a smaller decrease in root length density with depth.
Conclusions These results demonstrate that rooting characteristics of white clover can be altered by hybridization with T. uniflorum, potentially improving water and nutrient acquisition and drought resistance. Root traits of T. uniflorum are likely to be adaptations to soil moisture and fertility in its natural environment.
Keywords: Interspecific hybrids, roots, rooting depth, root distribution, Trifolium repens, Trifolium uniflorum, white clover
INTRODUCTION
White clover (Trifolium repens) (2n = 4x = 32) is an important perennial legume component of temperate pasture systems but its desirable characteristics are offset by a number of limitations. These include a relatively small, shallow root system, poor drought resistance and poor competitiveness for soil phosphorus in mixed swards (Mouat and Walker, 1959; Jackman and Mouat, 1970; Thomas, 1984; Knowles et al., 2003). Interspecific hybridization with other Trifolium species has potential to improve white clover through the introduction of traits outside its existing genetic variation (Widdup et al., 2003; Abberton, 2007; Williams and Nichols, 2011). Recurrent backcrossing to white clover results in plants whose genes are predominantly from white clover, with some genetic material introgressed from the second parent (Williams et al., 2008; Williams and Hussain, 2008). As a result, it is possible to maintain the agronomically and economically important characteristics of white clover while introducing new traits from related species.
Rooting depth, root morphology and root architecture all influence the ability of plants to access water and nutrients. Higher proportions of root mass in deeper soil layers, or a greater maximum rooting depth, could increase access to subsoil water (Grieu et al., 2001). It could also increase interception of mobile nutrients such as nitrate, which leach down the profile (Dunbabin et al., 2003). Studies on the root depth distribution of white clover are limited, but have found up to 70 % of the total root mass occurs in the top 100–150 mm of the profile (Caradus, 1981; Nichols et al., 2007). Uptake of water and nutrients is also influenced by root diameter, with greater efficiency of uptake in fine roots due to a higher ratio of root surface area to soil volume (Eissenstat, 1992; Jungk, 1996).
Trifolium uniflorum (2n = 32) is a perennial, wild species found in Greece, Turkey, southern France, southern Italy and Libya (Zohary and Heller, 1984), occurring in naturally dry and nutrient-poor environments (Snogerup and Snogerup, 2004; Tela Botanica, http://www.tela-botanica.org/bdtfx-nn-69461-synthese). Using molecular techniques, Ellison et al. (2006) classified T. uniflorum in the ‘white clover complex’. This group comprises close relatives of white clover, considered to provide the greatest potential for successful interspecific hybridization. There are relatively few published studies on T. uniflorum, but various authors have noted its thick and deep roots, woody tap root and a relatively large seed (Chen and Gibson, 1971; Pandey and Petterson, 1978; Pandey et al., 1987). Introgression of these root characteristics could improve white clover performance under drought stress. Pandey et al. (1987) reported that some T. repens × T. uniflorum hybrid plants did show transgressive segregation, with strong, deep nodal roots. More recently, Hussain et al. (2012) described new T. repens × T. uniflorum F1 and BC1 hybrid combinations and particularly noted potential for superior root characteristics, with more thick roots in BC1 hybrids than in white clover plants. In a rainshelter experiment, Nichols et al. (2014b) also observed a greater overall root cross-sectional area (i.e. basal diameter) for the largest nodal root of plants in a T. repens × T. uniflorum BC1 family, compared with a related BC2 family and their white clover cultivar parent. Such tap root-like nodal roots are considered to penetrate to greater depths than finer roots (Caradus, 1977; Caradus and Woodfield, 1998), and this may also increase the hybrid’s access to deep soil water.
If the root distribution and rooting depth of white clover could be altered, it is possible that access to water and nutrients could be improved. Although T. uniflorum is reported to have deep roots (Pandey et al., 1987), there are no data on the rooting depth or root distribution of this species compared with white clover. The effects of hybridization with white clover on these traits are also unknown. This study reports, for the first time, a comparison of the rooting depth, root depth distribution, root diameter and root length density of T. uniflorum, white clover and T. repens × T. uniflorum interspecific hybrids. The functional implications of observed differences are discussed.
MATERIALS AND METHODS
Experimental design
Plants were grown in 1-m deep × 0·15 -m diameter tubes of sand culture, irrigated with a low ionic strength nutrient solution based on the typical soil solution of New Zealand pasture topsoils (Edmeades et al., 1985; Blamey et al., 1991). Full details of the experimental system are given in Nichols et al. (2014c), where plant growth results are reported. The design was based on a split-plot arrangement with ten replicates and four blocks within each replicate. Blocks corresponded to four harvests over time, and each contained one plant from each clover entry described in the following section.
Plant material
The six clover entries included two Trifolium uniflorum L. accessions, two white clover cultivars and two BC1 populations generated by backcrossing F1 hybrids to each of the white clover cultivar parents (Table 1). For T. uniflorum accession 4382, sufficient seed germinated for five replicates only, and the remaining replicates were planted with extra seed of accession 4383. Each BC1 entry contained two family bulks derived using two F1 hybrids (80-2 and 900-4), which each had a different T. uniflorum accession as the male parent. These family bulks will be referred to as the 80-2 and 900-4 families.
Table 1.
The white clover, T. uniflorum and T. repens × T. uniflorum BC1 entries used in the experiment. Each BC1 entry was made up of two family bulks with different F1 parents (80-2 and 900-4). The background of the backcross and F1 parents is shown
| Notes | Backcross parent |
F1 parentage |
||
|---|---|---|---|---|
| ♀ | ♀ | ♂ | ||
| Grasslands ‘Kopu II’1 | Large leaf type | |||
| ‘Crusader’1 | Medium leaf type | |||
| AZ43822 | Greek origin | |||
| AZ43832 | Turkish origin | |||
| Kopu II BC1 | Kopu II × 80-2 | ‘Kopu II’ | Kopu II-23 | T66-64 |
| Kopu II × 900-4 | ‘Kopu II’ | Kopu II-23 | AZ4383-115 | |
| Crusader BC1 | Crusader × 80-2 | ‘Crusader’ | Kopu II-23 | T66-64 |
| Crusader × 900-4 | ‘Crusader’ | Kopu II-23 | AZ4383-115 | |
1White clover cultivar.
2T. uniflorum accession number (Margot Forde Forage Germplasm Center, New Zealand), seed from open pollinated plants.
3Specific genotype of ‘Kopu II’ used as maternal F1 parent.
4Specific genotype of AZ4382 used as paternal F1 parent.
5Specific genotype of AZ4383 used as paternal F1 parent.
Plant harvests
Plants were harvested 70 (harvest 1), 119 (harvest 2), 170 (harvest 3) and 237 (harvest 4) d after sowing (DAS). Harvests were conducted following the methods of Crush et al. (2010) to determine root dry weight (DW) in 50-mm depth increments to 200 mm, and then in 100-mm increments down to 1 m. At harvest 3, the roots between 50–100 and 400–500 mm were also scanned on a flatbed scanner and analysed for total root length and root diameter distribution using WinRhizo™ image analysis software (Regent Instruments Inc., Quebec, Canada). In combination with the dry weight data, root length values were used to calculate root length density (RLD, km m−3) and specific root length (SRL, m g−1 root DW). Root volume and surface area were calculated in root diameter classes of 0·5-mm increments. Due to the small amount of root that was >2·0 mm in diameter, all root volume and area data above this value were combined into one class >2·0 mm. The maximum diameter class measured was >4·75 mm. Root samples at 50–100 mm depth were also divided into the primary tap root and nodal roots to determine whether diameter class distribution differed between the two root types.
Statistical analysis
Statistical comparisons were based on clover entries or families. Prior to root depth distribution analysis, root DW data in the four 50-mm deep sections at the top of each tube were combined into two 100-mm deep sections at 0–100 and 100–200 mm. All depth sections were then of equal size. At each harvest, root depth distribution (root DW by depth) was analysed using an exponential model (1) in SAS version 9.1 (SAS Institute):
where j denotes the six clover entries, βj = DW at 0–100 mm and Rj = the rate at which root DW decreases with depth.
To estimate the values of βj and Rj in the exponential model (1), this equation was transformed to an equivalent regression model (2) by taking natural logarithms using the general linear model (GLM) procedure:
where C1j = ln βj and C2j = ln Rj
The results for C1j and C2j were then back-transformed to βj and Rj in the exponential model (1), using βj = exp(C1j) and Rj = exp(C2j).
Differences between clover entries were determined by pairwise comparisons of the resulting values for βj and Rj using Tukey’s pairwise comparison method to account for unbalanced data and multiple comparisons (Milliken and Johnson, 2009).
In addition to the model, root distribution was also assessed at harvest 4 by comparing the percentage of total root mass at 0–100, 100–200 and 400–500 mm. Maximum rooting depth, based on the deepest section in which roots were present, was compared among clover entries at each harvest.
Maximum rooting depth, percentage root mass, RLD, SRL and diameter class data were analysed using analysis of variance (ANOVA) in Minitab version 15 or 16 (Minitab Inc.). The ANOVA of maximum rooting depth consisted of clover entry and harvest and their interaction. Values at harvest 4 were excluded from analysis since roots of some plants had reached the bottom of the tubes, but the mean and standard error of the mean are presented. All other ANOVAs consisted of only the clover entry factor. Differences between clover entries were determined using Tukey’s pairwise comparison method as above. Changes in RLD and SRL with depth were calculated on a comparative basis as RLD400–500/RLD50–100 and SRL400–500/SRL50–100, and data were analysed using the two-sample Wilcoxon test (Conover, 1980) for non-normally distributed data.
Differences in variability among clover entries for RLD and SRL were also analysed using a test for equal variances in Minitab version 15. This compares two variances using the F-test or Levene’s test, depending on the normality distribution of the data. Standard deviations are presented to indicate the relative size of the variance for each entry.
Within the BC1 populations, differences in root distribution between the 80-2 and 900-4 families at harvest 4 were assessed by comparing βj and Rj using t-tests. Differences in RLD and SRL between the two families were also examined using ANOVA in Mintab version 15. We combined RLD and SRL data for each family across populations (e.g. Kopu II × 80-2 plus Crusader × 80-2) to increase replication for ANOVA.
RESULTS
Root system shape
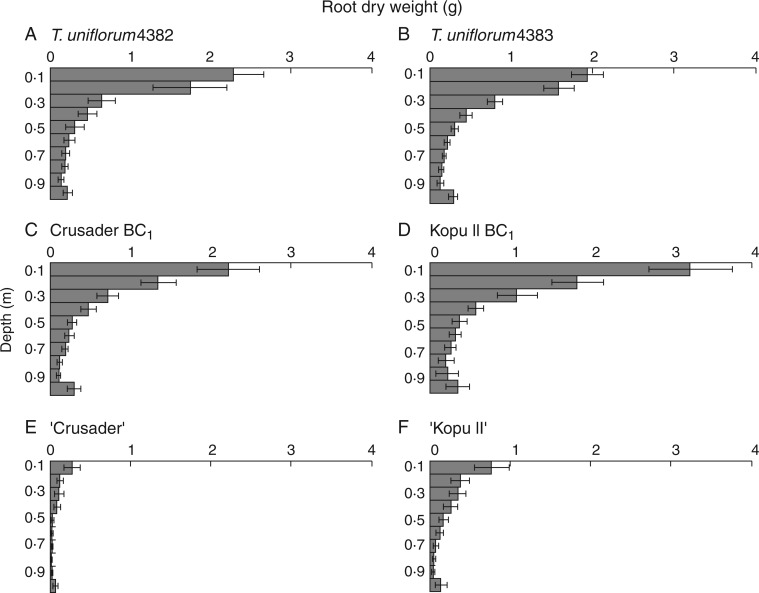
Changes over time in differences among clover entries for total shoot and root DW, which were reported by Nichols et al. (2014c), suggested that the entries differed in growth rate, particularly at the earlier harvests. However, the presence or absence of differences among entries were similar at harvests 3 and 4, indicating they had stabilized by this time. Root dry weight distribution with depth at harvest 4 is presented in Fig. 1. Differences in root system shape occurred either through differences in root mass in the top 100 mm (βj) or differences in the rate at which root mass decreased with depth (Rj) (Table 2). Significant differences among clover entries changed from harvest to harvest. In well-established plants at harvest 4, there was no difference in the root system shape of T. uniflorum accession 4382, accession 4383, Crusader BC1 and Kopu II BC1, but these entries were all different from both the white clover cultivars (Table 2). For ‘Kopu II’ this was due to a lower βj value (P < 0·001), but Rj did not differ from that of T. uniflorum accessions 4382 or 4383, Crusader BC1 (P < 0·05) or Kopu BC1 (P = 0·056). In contrast, ‘Crusader’ had both a lower βj value (P < 0·001) and a higher Rj value (i.e. slower rate of decrease in root mass with depth) (P < 0·05) than the T. uniflorum and hybrid entries. The rate of decrease did not differ between the white clover cultivars, but βj (root mass at 0–100 mm) was lower for ‘Crusader’ (P < 0·001).
Fig. 1.
Root depth distribution (g root dry weight by depth) (± s.e.m.) at harvest 4 (237 days after sowing) for (A) T. uniflorum 4382, (B) T. uniflorum 4383, (C) Crusader BC1, (D), Kopu II BC1, (E) ‘Crusader’ and (F) ‘Kopu II’.
Table 2.
Model values for root shape parameters of the six clover entries at each harvest
| Harvest | Parameter | T. uniflorum 4382 | T. uniflorum 4383 | Crusader BC1 | Kopu II BC1 | ‘Crusader’ | ‘Kopu II’ |
|---|---|---|---|---|---|---|---|
| 1 | βj | 0·042a | 0·044a | 0·048a | 0·026a | 0·036a | 0·034a |
| 2 | βj | 0·068a | 0·077a | 0·110a | 0·067a | 0·049a | 0·066a |
| 3 | βj | 0·483a | 0·373ab | 0·115c | 0·211bc | 0·037d | 0·047d |
| 4 | βj | 1·894a | 1·759a | 1·480a | 2·620a | 0·065b | 0·274c |
| 1 | Rj | 2·8E-05ab | 0·00047b | 1·4E-07a | 2·7E-05ab | 2·0E-09a | 4·1E-06ab |
| 2 | Rj | 0·00463ab | 0·00986b | 0·00004c | 0·00169a | 0·00030ac | 0·00004c |
| 3 | Rj | 0·0145ab | 0·0105ab | 0·0172ab | 0·0077a | 0·0314bc | 0·0647c |
| 4 | Rj | 0·0415a | 0·0435a | 0·0432a | 0·0293a | 0·1777b | 0·0935ab |
βj, root mass at 0–100 mm; Rj, rate of decrease with depth.
Harvest 1, 70 d after sowing (DAS); harvest 2, 119 DAS; harvest 3, 170 DAS; harvest 4, 237 DAS.
At each harvest, values with the same letter were not significantly different at the 5 % level.
There were no significant differences among entries in the percentage of roots at 0–100 mm at harvest 4 (Table 3), despite the white clover cultivars having significantly lower βj values than T. uniflorum and the hybrids. However, the T. uniflorum parents had ∼10 % more of their total root mass at 100–200 mm than the white clover cultivars (P < 0·015) (Table 3). Crusader BC1 also had a higher percentage of roots at 100–200 mm than the ‘Crusader’ parent (P = 0·044). Percentages of total root mass at 400–500 mm did not differ significantly among T. uniflorum and the hybrids or between the hybrids and their respective white clover parents (Table 3).
Table 3.
Percentage (± s.e.) of total root mass at 0–100, 100–200 and 400–500 mm depth at harvest 4 (237 d after sowing) for the six clover entries
| 0–100 mm | 100–200 mm | 400–500 mm | |
|---|---|---|---|
| T. uniflorum 4382 | 37·5a ± 3·81 | 26·4a ± 1·4 | 4·4ab ± 0·85 |
| T. uniflorum 4383 | 33·4a ± 1·82 | 26·3a ± 1·27 | 4·8ab ± 0·36 |
| Crusader BC1 | 38·6a ± 1·54 | 23·1ab ± 1·52 | 4·3a ± 0·41 |
| Kopu II BC1 | 40·7a ± 2·14 | 21·9abc ± 1·15 | 4·4ab ± 0·3 |
| ‘Crusader’ | 40·3a ± 6·33 | 16·2c ± 2·37 | 3·0a ± 0·71 |
| ‘Kopu II’ | 39·7a ± 3·76 | 16·8bc ± 1·48 | 6·5b ± 0·75 |
Within sampling depths, means with the same letter were not significantly different at the 5 % level.
Root depth penetration
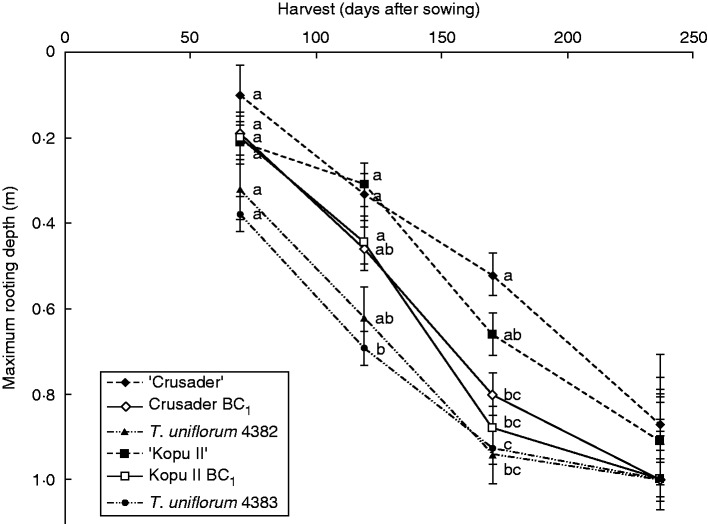
Roots of some individual T. uniflorum plants first reached 1 m by harvest 2, compared with harvest 3 for the hybrid and white clover plants. At harvest 3, 35 % of hybrid plants had reached 1 m, compared with 15 % for the white clover cultivars. There were no significant differences in mean maximum rooting depth among entries for very young plants (harvest 1), or for established plants (harvest 4) when roots were accumulating at the bottom of the tubes (Fig. 2).
Fig. 2.
Root penetration (mean maximum rooting depth ± s.e.m.) over time for the six clover entries. At each harvest, means with the same letter were not significantly different at the 5 % level.
Rooting depth differed among both clover entries and harvests (P < 0·001) but there was no significant interaction. The roots of the two T. uniflorum accessions were generally the deepest out of all the clover entries, and roots of the two hybrid entries were also deeper than those of the white clover cultivars (Fig. 2). Means taken over all harvests were consistent with these general patterns. Overall, the mean maximum rooting depths of ‘Kopu II’ (0.52 m) and ‘Crusader’ (0.46 m) were shallower (P < 0·001) than both T. uniflorum accession 4382 (0.72 m) and 4383 (0·75 m). ‘Kopu II’ also penetrated less (P < 0·03) than Kopu II BC1 (0.63 m), and the roots of ‘Crusader’ were shallower (P < 0·001) than Crusader BC1 (0.61 m). Rooting depth of the hybrid entries did not differ to those of T. uniflorum accession 4382, but both were shallower than that of accession 4383 (P = 0·005 and P < 0·001).
Root length density
At harvest 3 there was no significant difference in RLD (km m−3) at 50–100 mm depth for Kopu II BC1 compared with ‘Kopu II’ (P = 0·061) or for ‘Crusader’ compared with Crusader BC1 (P = 0·557) (Table 4). The T. uniflorum accessions also did not differ in RLD, nor did the two hybrid populations or the white clover cultivars (Table 4). As measured by the variance test, the RLD of Crusader BC1 at 50–100 mm was more variable than that of the ‘Crusader’ cultivar (P = 0·001), but the variability of Kopu II BC1 did not differ from the ‘Kopu II’ parent (Table 5). Root length density of Kopu II BC1 was more variable than that of Crusader BC1 (P = 0·024), and ‘Kopu II’ was also more variable than ‘Crusader’ (P < 0·001).
Table 4.
Mean ( ± s.e.) specific root length (SRL) and root length density (RLD) at 50–100 and 400–500 mm, plus RLD and SRL at 400–500 mm deep relative to 50–100 mm deep, for the six clover entries. Measurements were made at harvest 3 (170 d after sowing)
| RLD 50–100 mm (km m−3) | RLD 400–500 mm (km m−3) | SRL 50–100 mm (m g−1) | SRL 400–500 mm (m g−1) | RLD400–500/50–100 (%) | SRL400–500/50–100 (%) | |
|---|---|---|---|---|---|---|
| T. uniflorum 4382 | 16·1ab ± 5·8 | 2·0ab ± 0·8 | 53·9a ± 10·2 | 83·0a ± 9·9 | 22·1ab ± 10·5 | 208ab ± 76 |
| T. uniflorum 4383 | 11·9a ± 1·9 | 1·7a ± 0·4 | 65·7a ± 5·6 | 96·9a ± 9·8 | 17·3a ± 3·8 | 163a ± 20 |
| Crusader BC1 | 17·3ab ± 4·8 | 0·5bc ± 0·1 | 115·2bc ± 12·6 | 96·1a ± 17·4 | 3·7c ± 1·0 | 81c ± 15 |
| Kopu II BC1 | 37·5b ± 10·8 | 0·5bc ± 0·1 | 144·7c ± 7·5 | 107·8a ± 12·7 | 2·4c ± 0·7 | 77c ± 10 |
| ‘Crusader’ | 13·0a ± 1·3 | 0·2c ± 0·1 | 82·5ab ± 11·8 | 57·3a ± 20·3 | 4·3bc ± 1·4 | 66bc ± 22 |
| ‘Kopu II’ | 14·3ab ± 6·1 | 0·5bc ± 0·2 | 125·4c ± 12·2 | 82·5 ± 17·7 | 2·7c ± 0·7 | 62c ± 14 |
Within parameters, means with the same letter were not significantly different at the 5 % level.
Table 5.
Standard deviations for root morphological parameters at 50–100 mm depth for the six clover entries at harvest 3 (170 d after sowing)
| Root length density (km m−3) | Specific root length (m g−1) | |
|---|---|---|
| T. uniflorum 4382 | 12·90abc | 22·80a |
| T. uniflorum 4383 | 7·72a | 22·39a |
| Crusader BC1 | 14·44b | 37·67a |
| Kopu II BC1 | 34·12c | 23·70a |
| ‘Crusader’ | 3·77d | 35·50a |
| ‘Kopu II’ | 19·27bc | 28·66a |
Within parameters, variability of entries with the same letter was not significantly different at the 5 % level.
At 400–500 mm depth, there were no significant differences in RLD between the hybrids and their respective parental cultivars. However, RLD of the T. uniflorum accessions was generally higher than that of the other clover entries (Table 4). For example, RLD of T. uniflorum accession 4383 was 3·2–3·7 times higher than those of Crusader BC1 (P = 0·04), Kopu II BC1 (P = 0·039) and ‘Kopu II’ (P = 0·026), and 9·1 times higher than that of ‘Crusader’ (P = 0·005). Root length density of T. uniflorum accession 4382 at 400–500 mm was 10·4 times higher than that of the ‘Crusader’ cultivar (P = 0·019), and there was also a pattern for higher RLD compared with ‘Kopu II’ (P = 0·065), Crusader BC1 (P = 0·083) and Kopu II BC1 (P = 0·085) (Table 4). The latter may have been influenced by the standard error of the mean of accession 4382 at this depth, which was very large relative to the other clover entries (Table 4).
Root length density decreased with depth for all entries, but the change was not as pronounced for the T. uniflorum accessions (Table 4). As a consequence the RLD400–500/RLD50–100 for T. uniflorum accession 4383 was higher than those for Crusader BC1, Kopu II BC1, ‘Crusader’ and ‘Kopu II’ (P < 0·005). For T. uniflorum accession 4382, it was also higher than for Crusader BC1, Kopu II BC1 and ‘Kopu II’ (P < 0·017), but not ‘Crusader’ (P = 0·061).
Specific root length
At 50–100 mm, the SRLs of the two T. uniflorum accessions were significantly lower than those of most other clover entries (Table 4). Variability of SRL at 50–100 mm did not differ among entries (Table 5). At 400–500 mm there were no differences in SRL (Table 4).
Values of SRL400–500/SRL50–100 over 100 % indicated increases in SRL with depth, and values less than 100 % indicated decreases with depth. Data showed that SRL increased with depth for the T. uniflorum accessions and decreased for white clover and the hybrids (Table 4). The SRL400–500/SRL50–100 of T. uniflorum accession 4382 was higher than those of Crusader BC1 (P = 0·046), Kopu II BC1 (P = 0·032) and ‘Kopu II’ (P = 0·012), but not ‘Crusader’ (P = 0·061) (Table 4). It was higher for T. uniflorum accession 4383 than for the hybrid populations and white clover cultivars (P < 0·015).
Differences among families
There was a significant difference in root shape within both hybrid populations, with family 80-2 having higher βj values (root mass at 0–100 mm) (Table 6). Based on the combined data of the Crusader BC1 and Kopu II BC1 populations, RLD and SRL at 50–100 mm deep were also higher for the 80-2 family than for the 900-4 family (Table 7). There were no differences between the families for RLD or SRL at 400–500 mm.
Table 6.
Model values for βj (root mass at 0–100 mm) and Rj (decrease in root mass with depth) for families within BC1 populations at harvest 4 (237 d after sowing)
| Kopu II BC1 |
Crusader BC1 |
|||||
|---|---|---|---|---|---|---|
| Model value | 80-2 | 900-4 | P value | 80-2 | 900-4 | P value |
| βj | 3·289 | 2·086 | 0·010 | 2·588 | 0·847 | <0·001 |
| Rj | 0·0293 | 0·0293 | 1·000 | 0·0432 | 0·0432 | 1·000 |
Table 7.
Mean root length density (RLD) and specific root length (SRL) (± s.e.) for the 80-2 and 900-4 families at 50–100 and 400–500 mm depths. Measurements were made at harvest 3 (170 d after sowing)
| Depth (mm) | 80-2 | 900-4 | P value | |
|---|---|---|---|---|
| RLD (km m−3) | 50–100 | 41·04 ± 8·526 | 16·09 ± 8·088 | 0·049 |
| 400–500 | 0·48 ± 0·125 | 0·54 ± 0·119 | 0·696 | |
| SRL (m g−1) | 50–100 | 155·5 ± 8·10 | 108·4 ± 7·68 | 0·001 |
| 400–500 | 105·6 ± 15·52 | 99·2 ± 14·72 | 0·767 |
Diameter class distribution
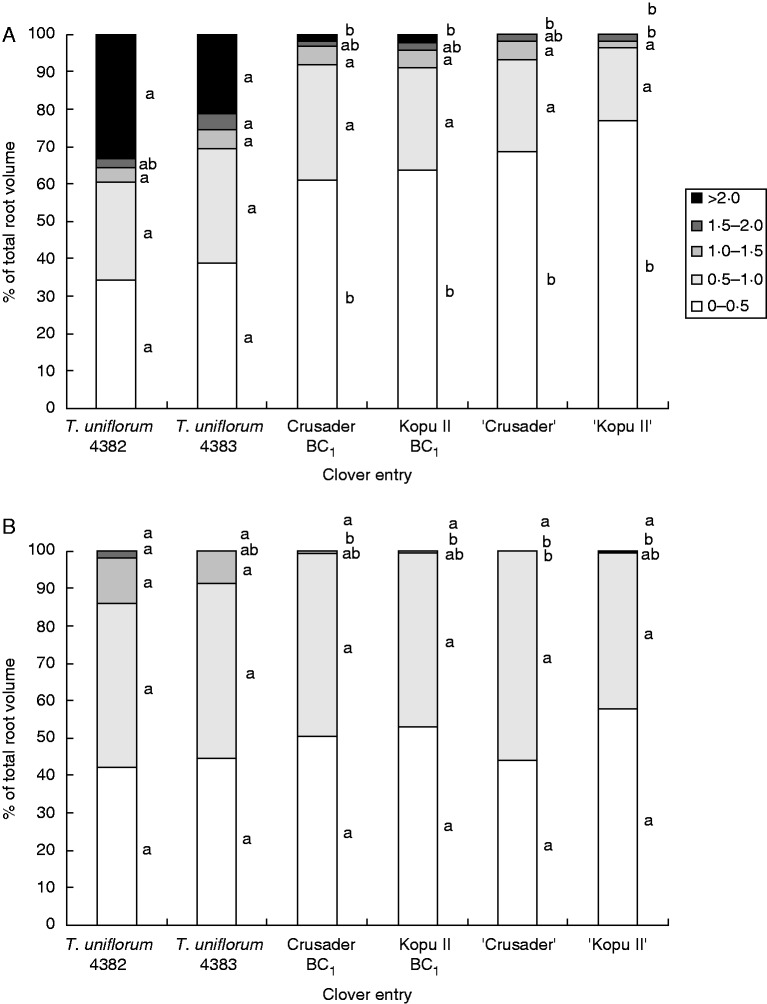
At the 50–100 mm depth the 0–0·5 mm diameter class accounted for 34–39 % of total root volume in the T. uniflorum accessions compared with 69–77 % for white clover. In contrast, total root volume in diameter classes >1 mm decreased from T. uniflorum to white clover (Fig. 3A). This was particularly pronounced for root volume >2 mm in diameter, which accounted for 21–33 % of total volume for T. uniflorum, compared with 2 % for the two hybrid populations and <1 % for the white clover cultivars. Statistically, the T. uniflorum accessions had a lower (P ≤ 0·006) percentage of root volume in the 0–0·5-mm diameter class and a higher (P < 0·001) percentage >2 mm in diameter, compared with the hybrid and white clover entries (Fig. 3A).
Fig. 3.
Mean percentage of total root volume in root diameter classes from 0–0·5 to >2·0 mm at harvest 3 (170 d after sowing). (A) 50–100 mm depth. (B) 400–500 mm depth. Within diameter classes, means with the same letter were not significantly different at the 5 % level.
Relative differences among clover entries for the diameter class distribution of total root surface area at 50–100 mm followed very similar patterns to root volume, although the contribution of finer roots (0–0.5 mm) increased while thicker classes decreased (data not shown). For example, roots in the 0–0·5-mm diameter class represented 34 % of root volume but 65 % of root surface area for T. uniflorum accession 4382, while roots > 2.0 mm diameter were 33 % of root volume but only 9 % of root surface area. Any differences between the diameter class distribution of surface area and volume usually involved the T. uniflorum accessions and the white clover cultivars, which had the most contrasting mean values.
Generally, there were no roots >2 mm in diameter at 400–500 mm depth. However, the T. uniflorum accessions still had 8–14 % of root volume >1 mm diameter compared with just 1 % for the hybrid populations and ‘Kopu II’ and 0 % for ‘Crusader’ (Fig. 3B). For the highest root diameter classes present at this depth, T. uniflorum accessions 4382 and 4383 had higher (P = 0·008 and P < 0·001, respectively) percentages of root volume in the 1·0–1·5-mm diameter class than ‘Crusader’, and T. uniflorum accession 4382 also had a higher (P = 0·034) percentage of root volume in the 1·5–2·0-mm class than the hybrids and white clover cultivars.
Between the 50–100 and 400–500 mm depths, the pattern of changes in volume in the lower diameter classes mirrored the observed changes in SRL (Fig. 3). At 400–500 mm the absolute percentage of root volume in the 0–0·5-mm class was 6–8 % higher than at 50–100 mm for the T. uniflorum accessions, compared with 10 % lower for the hybrid populations, 25 % lower for ‘Crusader’ and 19 % lower for ‘Kopu II’.
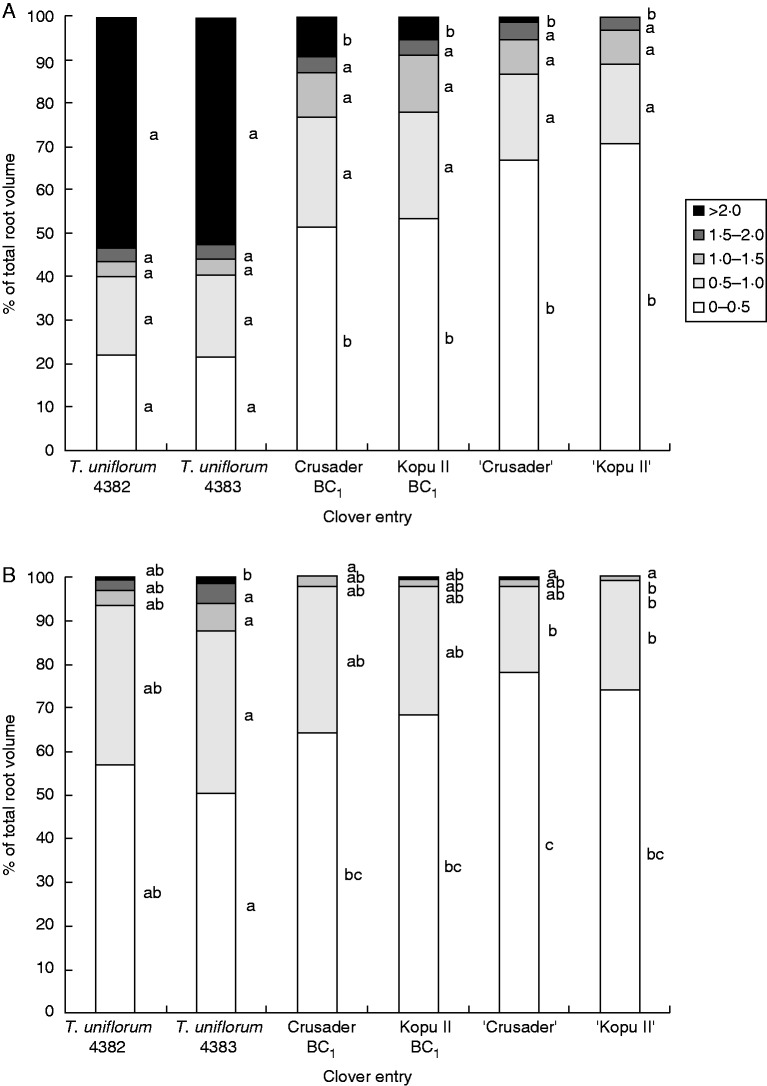
When divided into tap root and nodal roots, the diameter class distribution of root volume of both root types at 50–100 mm depth was very similar to the pattern seen with the overall data (Fig. 4). Compared with the other clover entries, both the T. uniflorum accessions had a lower (P ≤ 0·04) percentage of tap root volume in the 0–0·5-mm diameter class and a higher (P<0·001) percentage in the >2·0-mm diameter class. For nodal roots, the pattern was also similar in T. uniflorum accession 4383, which had a lower (P ≤ 0·041) percentage of root volume in the 0–0·5-mm diameter class than the hybrid and white clover entries. It also had a higher percentage of root volume in all diameter classes >1.0 mm compared with ‘Kopu II’ (P ≤ 0·048), in the 0·5–1·0-mm class (P = 0·003) compared with ‘Crusader’, and in the >2·0-mm class (P = 0·011) compared with ‘Crusader’ and Crusader BC1.
Fig. 4.
Mean percentage of root volume at 50–100 mm depth in root diameter classes from 0–0·5 to >2·0 mm at harvest 3 (170 d after sowing). (A) Tap root. (B) Nodal roots. Within diameter classes, means with the same letter were not significantly different at the 5 % level.
DISCUSSION
Root distribution
Root system shape and root depth distribution characteristics of the hybrids were more similar to the T. uniflorum parent than to white clover, despite the BC1 hybrid genomes being, on average, 75 % white clover. In particular, T. uniflorum and the hybrids had a higher proportion of their total root mass high in the profile, shown by lower Rj values than white clover and, in the case of T. uniflorum and Crusader BC1, by a higher percentage of root mass at 100–200 mm. van Wijk (2011) modelled the effects of rooting strategies, soil type and rainfall on transpiration and suggested that as rainfall decreases, evaporation is a more important source of water loss than drainage. As a result, roots are distributed higher in the profile to maximize water uptake. Shallower roots may also provide some adaptation for acquisition of water from intermittent rain or dew under dry conditions. This suggests that adaptation of T. uniflorum to low soil moisture may be a determining factor in root depth distribution of T. repens × T. uniflorum hybrids. Trifolium uniflorum occurs naturally in dry environments (Tela Botanica, 2015), and hybrids with white clover have been found to have increased drought resistance compared with white clover cultivars (Nichols et al., 2014b, 2015a).
Shallow rooting is also advantageous for phosphorus (P) acquisition (Ge et al., 2000; Lynch and Brown, 2001) as most soil P is concentrated near the soil surface (Haynes and Williams, 1992). The root distribution of T. uniflorum and the T. repens × T. uniflorum hybrids may therefore also reflect the soil fertility status in T. uniflorum’s natural environment. The degree of root system plasticity in response to drying conditions is unknown for T. repens × T. uniflorum hybrids, and could alter the root distribution observed here (Pang et al., 2011). Given the positive effects of P on drought stress in white clover (Singh et al., 1997; Singh and Sale, 1998) and evidence for drought resistance and low P tolerance in the hybrids (Nichols et al., 2014a, b, c, 2015a), the potential for drought stress to be ameliorated by low soil P tolerance in this material requires further investigation.
Differences in total DM production among the clover entries in the current experiment, reported by Nichols et al. (2014c), were likely to reflect differences in tolerance to low external P supply. Shoot P concentrations of the entries tested were well below reported critical internal levels for white clover growth. The impacts of nutrient availability and potential differences in critical internal P levels on the observed patterns of root shape and distribution in the current experiment are unknown. Differences in the values of root mass at 0–100 mm, derived from the model, reflect these differences in plant size and were likely to have been influenced by the effects of nutrient supply on growth. Although the percentage of roots at 0–100 mm did not differ among entries, the higher absolute root mass of T. uniflorum and the hybrids in the upper profile of this growth system would increase their ability to capture P in the field. Similarly, while the higher RLDs at 50–100 mm for the hybrid populations were not significant they may still have a functional effect on the hybrids’ ability to acquire P in the topsoil compared with white clover. However, the degree to which root traits must change in order to produce these functional effects is unknown in white clover.
Rooting depth
The deeper mean maximum rooting depths of the hybrid populations appear to have been inherited from the T. uniflorum parent, which had the greatest maximum rooting depth. To determine whether this results in deeper rooting and improved access to deep soil moisture in the field requires further testing under those conditions. This would remove the barrier to rooting depth posed by a limited pot size, while incorporating the physical effects of field soil on root penetration. The faster penetration by roots of T. uniflorum and the hybrids, compared with the white clover parents, could aid establishment under dry soil conditions or enable rapid early growth to access deeper water before seasonal decreases in soil moisture occur. The larger seed of T. uniflorum, compared with white clover (Gibson et al., 1971), may also aid establishment as this is a trait that has been associated with larger, more vigorous seedlings in temperate pasture species (Moot et al., 2000).
Root mass in the current study was predominantly from primary tap root systems, although nodal roots were also present, particularly at later harvests. Pederson (1989) found that cultivar differences for root characteristics were similar in primary roots and nodal roots of white clover. Therefore, it is likely that root traits observed in tap root systems in the current study would also be present in clonal plants reliant solely on nodal root systems following the loss of the tap root. This was confirmed to some extent by the diameter class distribution data, which showed similar patterns among entries in the relative proportion of finer and thicker roots for both the tap root and nodal roots. For example, T. uniflorum had a higher percentage of thick roots and a lower percentage of fine roots than white clover, in both root types.
Root morphology
Generally, higher SRL values indicate thinner roots, assuming equal tissue densities (Eissenstat, 1992). Specific root length data in this experiment indicate that the T. uniflorum accessions had thicker roots at 50–100 mm than white clover and the hybrids. The diameter class distribution of root volume also showed that T. uniflorum had more root volume in thicker diameter classes and less volume in the finest diameter class, compared with white clover and the hybrid populations. Therefore, it is unlikely that tissue density has confounded the interpretation of SRL data. The changes in SRL at 400–500 mm (increasing for the T. uniflorum accessions and decreasing for the hybrids and white clover) suggest that T. uniflorum had more of its fine root mass at depth, while the hybrids and white clover had more of their fine root mass higher in the profile. Again, the diameter class distribution data showed changes with depth in the percentage of root volume in the 0–0·5-mm diameter class that reflect changes in SRL.
Specific root length data did not indicate any differences in root diameter between the hybrids and their white clover parents. However, SRL is strongly influenced by finer roots (Boot, 1989), which accounted for a considerable proportion of both root volume and surface area in the current experiment. The diameter class data may therefore provide a more useful indication of the contribution of thicker roots to the root mass of white clover compared with the hybrids. Although the proportion of total root volume in the thickest diameter class at 50–100 mm depth was not significantly higher for the hybrid entries (2·0–2·4 %) than for white clover (0·2–0·3 %), this variation may still be sufficient, functionally, to impact drought resistance. For instance, Kirkegaard et al. (2007) found that small increases in availability of subsoil water could have considerable impacts on grain yield in wheat. In hybrids this could operate through increasing the plant’s access to deeper subsoil moisture, via greater penetration of thicker roots to depth, rather than by experiencing an increase in soil water per se. Thicker roots were not present in T. repens × T. uniflorum hybrids at 400–500 mm deep, but as diameter distribution was not assessed between the 100 - and 400-mm depths we cannot comment on where the relative depth limits may be for thicker roots of hybrids versus white clover cultivars.
Ekanayake et al. (1985) found significant correlations between root characteristics (such as thickness, length and density) and drought stress symptoms and recovery in rice. Babu et al. (2003) also reported positive correlations between quantitative trait loci for root traits and rice yield under drought. In contrast, Annicchiarico and Piano (2004) found no correlation between root traits and drought tolerance in white clover, but suggested this was due to its poor physiological adaptation to water stress. Improved physiological tolerance of T. repens × T. uniflorum BC1 hybrids (Nichols et al., 2015a) may enable the beneficial effects of root characteristics on drought resistance to be expressed. Annicchiarico and Piano (2004) also speculated on the possible importance of fibrous roots in white clover during drought, and it seems likely that both fine roots and thicker, deeply penetrating roots play a role in drought resistance. It is worth noting that a combination of fine and thick roots may be particularly important in the presence of multiple soil limiting factors, such as drought and low soil fertility. For example, thicker roots for access to deeper soil moisture in addition to finer roots with high surface area for absorption of both water and nutrients.
While potentially advantageous under water stress, the thicker roots of T. uniflorum at 50–100 mm could be a disadvantage for nutrient uptake due to a lower ratio of root surface area to soil volume (Eissenstat, 1992). This was also reflected by the differences in the relative contributions of thicker versus finer roots for surface area and volume. However, other factors could increase the capture of subsoil water and leaching nutrients in the field, such as the increase in finer roots (higher root surface area:soil or root volume) and greater RLD [increased exploration of the growth media (Dunbabin et al., 2003)] at 400–500 mm. At 50–100 mm depth, higher RLD and SRL in the 80-2 population could also have increased the nutrient uptake of these plants, which may have contributed to their larger root and shoot DWs relative to the 900-4 population (Nichols et al., 2014c). Further study is required on the functional impacts of hybridization on the root morphological traits that affect nutrient interception, water uptake and responses to drought stress.
Selection for root traits
Despite moderate heritability for responses to P application under controlled conditions (Caradus et al., 1992), white clover genotypes selected for improved phosphorus use efficiency (PUE) demonstrated no advantage under field conditions (Caradus, 1994; Caradus and Dunn, 2000), possibly due to a large genotype × environment interaction on the expression of PUE traits in the field. Therefore, root characteristics may be a more achievable target for improvement of white clover growth at low soil P levels. Our data show that changes to root traits are possible through interspecific hybridization, and the limited literature on the heritability of root traits in white clover suggests there should be a good response to selection (Caradus and Woodfield, 1998). Assessment of a wider range of hybrid populations and white clover cultivars would be useful to confirm the differences in root characteristics observed in the current experiment and help inform the potential advantages of these traits for water and nutrient interception.
As different hybrid genotypes contain different combinations of T. uniflorum genes, it is expected that the hybrids will be very variable for some traits. For example, the variance of RLD at 50–100 mm was significantly higher for Crusader BC1 compared with ‘Crusader’ and for Kopu II BC1 compared with Crusader BC1. This may be responsible for the absence of statistically significant differences in RLD between these entries. We have also observed variation among hybrid families for growth at low soil P levels, and for root traits such as branching frequency, root:shoot ratio and nodal root thickness (Nichols et al., 2014a, b). The variability present within and among hybrid families provides a valuable genetic resource for selection. Wide screening of hybrid families and genotypes is therefore necessary in order to incorporate the best hybrid material into breeding populations.
Conclusions
Root system shape, root distribution and maximum rooting depth of T. repens × T. uniflorum interspecific hybrids were strongly influenced by the T. uniflorum parent. These results confirm a previous observation, under field conditions, that some root characteristics of white clover can be altered by hybridization with T. uniflorum (Nichols et al., 2015b). In contrast, the hybrids were more similar to white clover than to T. uniflorum for the limited number of root morphological traits that were measured. Differences in the root traits of T. uniflorum and the BC1 hybrids compared with those of white clover may reflect soil moisture and soil fertility conditions in the natural environment of T. uniflorum. These traits may influence factors such as access to deep soil water, drought resistance, acquisition of water and nutrients, and seedling establishment in the hybrids compared with white clover, but further work is required to confirm these functional and ecological links. The use of a wider range of germplasm in future studies would incorporate a greater extent of the potential genetic variation, and help to confirm both the observed results and their functional implications, as well as determining the potential for selection within or among hybrid populations.
ACKNOWLEDGEMENTS
We would like to thank Isabelle Verry (AgResearch) for creating and supplying the hybrid material; Lily Ouyang (AgResearch) for technical assistance; Dr John Waller (AgResearch) for the experimental design; and Dr Jim Crush (AgResearch) for comments on the manuscript. This work was supported by the New Zealand Ministry of Business, Innovation and Employment (contract C02X0810) and Dairy New Zealand (contract FD617).
LITERATURE CITED
- Abberton MT. 2007. Interspecific hybridization in the genus Trifolium. Plant Breeding 126: 337–342. [Google Scholar]
- Annicchiarico P, Piano E. 2004. Indirect selection for root development of white clover and implications for drought tolerance. Journal of Agronomy and Crop Science 190: 28–34. [Google Scholar]
- Babu RC, Nguyen BD, Chamarerk V, et al. 2003. Genetic analysis of drought resistance in rice by molecular markers: association between secondary traits and field performance. Crop Science 43: 1457–1469. [Google Scholar]
- Blamey FPC, Edmeades DC, Asher CJ, Edwards DG, Wheeler DM. 1991. Evaluation of solution culture techniques for studying aluminium toxicity in plants. In: RJ Wright, VC Baligar, RP Murrmann, eds. Plant-soil interactions at low pH. Dordrecht: Kluwer Academic Press, 905–912. [Google Scholar]
- Boot RGA. 1989. The significance of size and morphology of root systems for nutrient acquisition and competition. In: H Lambers, ML Cambridge, H Konings, TL Pons, eds. Causes and consequences of variation in growth rate and productivity of higher plants. The Hague: SPB Academic Publishing, 299–311. [Google Scholar]
- Caradus J. 1994. Selection for improved adaptation of white clover to low phosphorus and acid soils. Euphytica 77: 243–250. [Google Scholar]
- Caradus JR. 1977. Structural variation of white clover root systems. New Zealand Journal of Agricultural Research 20: 213–219. [Google Scholar]
- Caradus JR. 1981. Root growth of white clover (Trifolium repens L.) lines in glass-fronted containers. New Zealand Journal of Agricultural Research 24: 43–54. [Google Scholar]
- Caradus JR, Dunn A. 2000. Adaptation to low fertility hill country of white clover lines selected for differences in response to phosphorus. New Zealand Journal of Agricultural Research 43: 63–69. [Google Scholar]
- Caradus JR, Woodfield DR. 1998. Genetic control of adaptive root characteristics in white clover. Plant and Soil 200: 63–69. [Google Scholar]
- Caradus JR, Mackay AD, Wewala S, et al. 1992. Inheritance of phosphorus response in white clover (Trifolium repens L.). Plant and Soil 146: 199–208. [Google Scholar]
- Chen C-C, Gibson PB. 1971. Seed development following the mating of Trifolium repens × T. uniflorum. Crop Science 11: 667–672. [Google Scholar]
- Conover WJ. 1980. Practical nonparametric statistics. New York: John Wiley and Sons. [Google Scholar]
- Crush JR, Nichols SN, Ouyang L. 2010. Adventitious root mass distribution in progeny of four perennial ryegrass (Lolium perenne L.) groups selected for root shape. New Zealand Journal of Agricultural Research 53: 193–200. [Google Scholar]
- Dunbabin V, Diggle A, Rengel Z. 2003. Is there an optimal root architecture for nitrate capture in leaching environments? Plant, Cell and Environment. 26: 835–844. [DOI] [PubMed] [Google Scholar]
- Edmeades DC, Wheeler DM, Clinton OE. 1985. The chemical composition and ionic strength of soil solutions from New Zealand topsoils. Australian Journal of Soil Research 23: 151–165. [Google Scholar]
- Eissenstat DM. 1992. Costs and benefits of constructing roots of small diameter. Journal of Plant Nutrition 15: 763–782. [Google Scholar]
- Ekanayake IJ, O'Toole JC, Garrity DP, Masajo TM. 1985. Inheritance of root characters and their relations to drought resistance in rice. Crop Science 25: 927–933. [Google Scholar]
- Ellison NW, Liston A, Steiner JJ, Williams WM, Taylor NL. 2006. Molecular phylogenetics of the clover genus (Trifolium - Leguminosae). Molecular Phylogenetics and Evolution 39: 688–705. [DOI] [PubMed] [Google Scholar]
- Ge Z, Rubio G, Lynch J. 2000. The importance of root gravitropism for inter-root competition and phosphorus acquisition efficiency: results from a geometric simulation model. Plant and Soil 218: 159–171. [DOI] [PubMed] [Google Scholar]
- Gibson PB, Chen C-C, Gillingham JT, Barnett OW. 1971. Interspecific hybridization of Trifolium uniflorum L. Crop Science 11: 895–899. [Google Scholar]
- Grieu P, Lucero DW, Ardiani R, Ehleringer JR. 2001. The mean depth of soil water uptake by two temperate grassland species over time subjected to mild soil water deficit and competitive association. Plant and Soil 230: 197–209. [Google Scholar]
- Haynes RJ, Williams PH. 1992. Long-term effect of superphosphate on accumulation of soil phosphorus and exchangeable cations on a grazed, irrigated pasture site. Plant and Soil 142: 123–133. [Google Scholar]
- Hussain SW, Williams WM, Verry IM, Jahufer MZZ. 2012. A morphological and cytological analysis of interspecific hybrids: Trifolium repens L. × T. uniflorum L. In: C Harris, ed. Australian Legume Symposium. Tooborac, Victoria: Australian Grasslands Association, 67–69. [Google Scholar]
- Jackman RH, Mouat MCH. 1970. The effect of browntop (Agrostis tenuis (Sibth.)) and increasing phosphorus deficiency on the growth of white clover (Trifolium repens L.). In: Proceedings of the XI International Grassland Congress , 354–357. [Google Scholar]
- Jungk AO. 1996. Dynamics of nutrient movement at the soil-root interface. In: Y Waisel, A Eshel, U Kafkafi, eds. Plant roots: the hidden half. New York: Marcel Dekker, 581–605. [Google Scholar]
- Kirkegaard JA, Lilley JM, Howe GN, Graham JM. 2007. Impact of subsoil water use on wheat yield. Australian Journal of Agricultural Research 58: 303–315. [Google Scholar]
- Knowles IM, Fraser TJ, Daly MJ. 2003. White clover: loss in drought and subsequent recovery. In: DJ Moot, ed. Legumes for dryland pastures. Proceedings of a New Zealand Grassland Association (Inc.) Symposium. Grassland Research and Practice Series No. 11. Wellington: New Zealand Grassland Association, 37–42. [Google Scholar]
- Lynch JP, Brown KM. 2001. Topsoil foraging – an architectural adaptation of plants to low phosphorus availability. Plant and Soil 237: 225–237. [Google Scholar]
- Milliken GA, Johnson DE. 2009. Analysis of messy data. Vol. 1. Designed experiments. Boca Raton: Chapman & Hall/CRC. [Google Scholar]
- Moot DJ, Scott WR, Roy AM, Nicholls AC. 2000. Base temperature and thermal time requirements for germination and emergence of temperate pasture species. New Zealand Journal of Agricultural Research 43: 15–25. [Google Scholar]
- Mouat MCH, Walker TW. 1959. Competition for nutrients between grasses and white clover. I. Effect of grass species and nitrogen supply. Plant and Soil 11: 30–40. [Google Scholar]
- Nichols SN, Crush JR, Woodfield DR. 2007. Effects of inbreeding on nodal root system morphology and architecture of white clover (Trifolium repens L.). Euphytica 156: 365–373. [Google Scholar]
- Nichols SN, Crush JR, Ouyang L. 2014a. Phosphate responses of some Trifolium repens × Trifolium uniflorum interspecific hybrids grown in soil. Crop and Pasture Science 65: 382–387. [Google Scholar]
- Nichols SN, Hofmann RW, Williams WM. 2014b. Drought resistance of Trifolium repens × Trifolium uniflorum interspecific hybrids. Crop and Pasture Science 65: 911–921. [Google Scholar]
- Nichols SN, Hofmann RW, Williams WM, Crush JR. 2014c. Nutrient responses and macronutrient composition of some Trifolium repens × Trifolium uniflorum interspecific hybrids. Crop and Pasture Science 65: 370–381. [Google Scholar]
- Nichols SN, Hofmann RW, Williams WM. 2015a. Physiological drought resistance and accumulation of leaf phenolics in white clover interspecific hybrids. Environmental and Experimental Botany 119: 40–47. [Google Scholar]
- Nichols SN, Hofmann RW, Williams WM. 2015b. The effect of hybridisation with Trifolium uniflorum on tap root survival in white clover. New Zealand Journal of Agricultural Research 58: 371–383. [Google Scholar]
- Pandey KK, Petterson GB. 1978. Fertile interspecific hybrids between Trifolium repens and T. uniflorum: prospects for grasslands white clover improvement. Australian Plant Breeding and Genetics Newsletter 28: 114–116. [Google Scholar]
- Pandey KK, Grant JE, Williams EG. 1987. Interspecific hybridisation between Trifolium repens and T. uniflorum. Australian Journal of Botany 35: 171–182. [Google Scholar]
- Pang J, Yang J, Ward P, et al. 2011. Contrasting responses to drought stress in herbaceous perennial legumes. Plant and Soil 348: 299–314. [Google Scholar]
- Pederson GA. 1989. Taproot and adventitious root growth of white clover as influenced by nitrogen nutrition. Crop Science 29: 764–768. [Google Scholar]
- Singh DK, Sale PWG. 1998. Phosphorus supply and the growth of frequently defoliated white clover (Trifolium repens L.) in dry soil. Plant and Soil 205: 155–162. [Google Scholar]
- Singh DK, Sale PWG, McKenzie BM. 1997. Water relations of white clover (Trifolium repens L.) in a drying soil, as a function of phosphorus supply and defoliation frequency. Australian Journal of Agricultural Research 48: 675–682. [Google Scholar]
- Snogerup S, Snogerup B. 2004. Changes in the flora of some Aegean islets 1968–2000. Plant Systematics and Evolution 245: 169–213. [Google Scholar]
- Thomas H. 1984. Effects of drought on growth and competitive ability of perennial ryegrass and white clover. Journal of Applied Ecology 21: 591–602. [Google Scholar]
- Widdup KH, Hussain SW, Williams WM, Lowther WL, Pryor HN, Sutherland BL. 2003. The development and plant characteristics of interspecific hybrids between white and caucasian clover. In: DJ Moot, ed. Legumes for dryland pastures. Proceedings of a New Zealand Grassland Association (Inc.) Symposium. Grassland Research and Practice Series No. 11. Wellington: New Zealand Grassland Association, 143–148. [Google Scholar]
- van Wijk MT. 2011. Understanding plant rooting patterns in semi-arid systems: an integrated model analysis of climate, soil type and plant biomass. Global Ecology and Biogeography 20: 331–342. [Google Scholar]
- Williams WM, Hussain SW. 2008. Development of a breeding strategy for interspecific hybrids between Caucasian clover and white clover. New Zealand Journal of Agricultural Research 51: 115–126. [Google Scholar]
- Williams WM, Nichols SN. 2011. Trifolium. In: C Kole, ed. Wild crop relatives: genomic and breeding resources. Legume crops and forages. Berlin: Springer, 249–272. [Google Scholar]
- Williams WM, Ansari HA, Hussain SW, Ellison NW, Williamson ML, Verry IM. 2008. Hybridization and introgression between two diploid wild relatives of white clover, Trifolium nigrescens Viv. and T. occidentale Coombe. Crop Science 48: 139–148. [Google Scholar]
- Zohary M, Heller D. 1984. The genus Trifolium. Jerusalem: Israel Academy of Sciences and Humanities. [Google Scholar]