Abstract
Background and Aims Regulation of water channel aquaporins (AQPs) provides another mechanism by which abscisic acid (ABA) may influence water flow through plants. To the best of our knowledge, no studies have addressed the changes in ABA levels, the abundance of AQPs and root cell hydraulic conductivity (LpCell) in the same tissues. Thus, we followed the mechanisms by which ABA affects root hydraulics in an ABA-deficient barley mutant Az34 and its parental line ‘Steptoe’. We compared the abundance of AQPs and ABA in cells to determine spatial correlations between AQP abundance and local ABA concentrations in different root tissues. In addition, abundance of AQPs and ABA in cortex cells was related to LpCell.
Methods Root hydraulic conductivity (LpRoot) was measured by means of root exudation analyses and LpCell using a cell pressure probe. The abundance of ABA and AQPs in root tissues was assessed through immunohistochemical analyses. Isoform-specific antibodies raised against HvPIP2;1, HvPIP2;2 and HvPIP2;5 were used.
Key Results Immunolocalization revealed lower ABA levels in root tissues of Az34 compared with ‘Steptoe’. Root hydraulic conductivity (LpRoot) was lower in Az34, yet the abundance of HvPIPs in root tissues was similar in the two genotypes. Root hair formation occurred closer to the tip, while the length of the root hair zone was shorter in Az34 than in ‘Steptoe’. Application of external ABA to the root medium of Az34 and ‘Steptoe’ increased the immunostaining of root cells for ABA and for HvPIP2;1 and HvPIP2;2 especially in root epidermal cells and the cortical cell layer located beneath, parallel to an increase in LpRoot and LpCell. Treatment of roots with Fenton reagent, which inhibits AQP activity, prevented the ABA-induced increase in root hydraulic conductivity.
Conclusion Shortly after (<2 h) ABA application to the roots of ABA-deficient barley, increased tissue ABA concentrations and AQP abundance (especially the plasma-membrane localized isoforms HvPIP2;1 and HvPIP2;2) were spatially correlated in root epidermal cells and the cortical cell layer located beneath, in conjunction with increased LpCell of the cortical cells. In contrast, long-term ABA deficiency throughout seedling development affects root hydraulics through other mechanisms, in particular the developmental timing of the formation of root hairs closer to the root tip and the length of the root hair zone.
Keywords: Barley, aquaporins, abscisic acid, hydraulic conductivity, immunolocalization
INTRODUCTION
Abscisic acid (ABA) has long been known to control plant water relations by closing stomata (for a review, see Dodd 2005). The capacity of ABA to influence water-conducting paths within plants has received less attention, with data showing a positive effect of exogenous ABA on root hydraulic conductance (Ludewig et al., 1988; Zhang et al., 1995; Hose et al., 2000; Mahdieh and Mostajeran, 2009), no effect (Aroca et al., 2003), variable effect depending on the concentration of applied ABA or a negative effect in the shoot (Pantin et al., 2013). This apparently differential effect of ABA on root and shoot hydraulic conductance may simply be reconciled by a unified dose–response curve to exogenous ABA (Dodd, 2013). The discovery of water channels known as aquaporins (AQPs), capable of controlling cellular hydraulic conductivity by altering membrane permeability for water, has added a new target through which ABA may influence water flow through plants (for a review, see Maurel et al., 2008). ABA can influence activity of AQPs at different levels by either changing the expression of genes for AQPs or post-transcriptional modifications of gene products (Maurel et al., 2008, and references therein). Comparing AQP gene expression in maize plants differing in ABA concentration revealed a positive relationship between ABA concentration and either AQP expression or root hydraulic conductance (Parent et al., 2009).
Barley plants present an ideal model system to study the interplay between AQPs, ABA and root hydraulic conductivity (LpRoot) and cell hydraulic conductivity (LpCell), as the transcellular pathway where water crosses cell membranes through AQPs is more important in these plants than in other species such as maize (Steudle and Jeschke, 1983; Steudle and Peterson, 1998; Knipfer and Fricke, 2010). To the best of our knowledge, the effects of exogenous ABA on the relationship between AQPs and root hydraulics have not been studied in barley, nor are we aware of any study in which changes in ABA levels have actually been followed at root tissue level and been related to changes in LpCell. Thus, we addressed the effects of exogenous ABA on AQPs and hydraulic conductance of barley plants differing in ABA concentration. We compared the abundance of ABA and AQP isoforms in root tissues and LpRoot in the ABA-deficient (but not insensitive) barley mutant Az34 (Walker-Simmons et al., 1989, Martin-Vertedor and Dodd, 2011) and its parent ‘Steptoe’. Previous studies indicated that Az34 retained normal stomatal (Mulholland et al., 1996) and leaf growth (Martin-Vertedor and Dodd, 2011) sensitivity to addition of exogenous ABA. Moreover, we examined the effects of exogenous ABA on hydraulic characteristics in roots of Az34 plants. Root cortex LpCell was also analysed in Az34 using the cell pressure probe. We used antibodies raised against ABA (Fricke et al., 2004, Akhiyarova et al., 2006) and anti- (HvPIP2) AQPs antibodies raised specifically against those plasma membrane-localized barley AQP isoforms (HvPIP2;1, HvPIP2;2, HvPIP2;5) which are candidates to facilitate water flow through barley roots (Horie et al., 2011; Knipfer et al., 2011). Immunohistochemistry was used to determine spatial correlations between AQP abundance and local ABA concentrations. The formation of root hairs was also determined in these regions, as these cells facilitate a considerable portion of the water uptake of barley roots (Knipfer and Fricke, 2010). We hypothesized that different mechanisms could regulate the response of LpRoot and LpCell to long-term endogenous ABA concentrations (root hair development) and to transient exogenous ABA treatment (AQP abundance), respectively.
MATERIALS AND METHODS
Plant growth
Seeds were germinated for 3 d in darkness at 21–24 °С on either rafts made from sealed glass tubes tied together or on a nylon mesh floated over tap water, which were then suspended over 0·1 strength Hoagland–Arnon nutrient medium (0·5 mm KNO3, 0·5 mm Ca(NO3)2, 0·1 mm KH2PO4, 0·1 mm MgSO4, 0·5 mm CaSO4) in 3-litre containers and grown at an irradiance of 400 μmol m–2 s–1 and a 14-h photoperiod for further 4–5 d. In experiments with exogenous hormone, ABA was added to the nutrient solution to yield a concentration of 10–5 m. Twenty minutes after addition of exogenous ABA, shoots were excised for the collection of bleeding sap (representing ‘xylem exudate’) from the roots over the subsequent 1 h. For LpCell determination, plants were analysed between 20 and 90 min after addition of ABA.
As root hairs were not visible in low-magnification images of the entire root system (×1), 50 images of root portions from the zone where root hairs were present were made under the microscope (×50) from which the image of the whole root was reconstructed in Adobe Photoshop CS6.
Root exudation analyses
Bleeding sap flow from detached root systems was measured according to Carvajal et al. (1996) with the modifications described by Kudoyarova et al. (2011). In short, the aerial parts of the plant were removed leaving a cylinder of leaf bases still attached to the root system. The cylinder of leaf bases was connected to a thin pre-weighed capillary by means of silicon tubing. Experiments started 4 h after the start of the photoperiod by excising the shoot. In some experiments, this was preceded by 20 min of ABA treatment. After 1 h, the capillary containing osmotically driven bleeding sap was disconnected from the root system and weighed; the root system was also weighed to determine its fresh weight (f. wt). Bleeding sap from each capillary was diluted five times to provide sufficient sample for measurement of osmotic potential using a freezing point depression osmometer (Osmomat 030, Gonotech, Berlin, Germany). In preliminary experiments, proportionality of the effect of dilution on the obtained values was checked. Root hydraulic conductivity, LpRoot, was calculated according to equation: LpRoot=J/((Ψs − Ψx) × f. wt) where J is the bleeding sap flow rate and (Ψs − Ψx) the difference in osmotic pressure between xylem sap and root medium: a root solute reflection coefficient of 1·0 was used (Knipfer and Fricke, 2010). To inhibit AQP activity, hydroxyl radicals (*OH) were produced through the Fenton reaction (Fe2++H2O2= Fe3++OH−+*OH) by mixing equal volumes of 6 mm H2O2 and 6 mm FeSO4 (Ye and Steudle, 2006). Roots of barley plants were placed in the solution. Preliminary experiments showed that inhibition of transpiration by the Fenton reagent was reversible, as transpiration returned to pretreatment levels within 30 min after substitution of the culture medium for the one without Fenton reagent.
Cell pressure probe analyses
Turgor, halftime of water exchange (T1/2), cell elastic modulus (ε) and LpCell were determined through the cell pressure probe technique as described previously (e.g. Fricke and Peters, 2002; Knipfer et al., 2011; Suku et al., 2014). Cell osmotic pressure, which is required for calculation of LpCell, was determined through picolitre osmometry of sap extracted from cells (Fricke and Peters, 2002), and the dimension of cells (volume, surface area) was determined through free-hand cross-sections assuming that cells were shaped like cylinders (data not shown). The cells which were analysed in the root hair region were cortex cells, and were located in the two cortical cell layers beneath the epidermis.
Generation of antibodies against AQPs, protein expression in oocytes and Western analysis
Polyclonal antibodies for HvPIP2s were raised in rabbits against synthetic oligopeptides (Medical & Biological Laboratories Co., Tokyo, Japan) corresponding to the amino acid sequences in the N-region of HvPIP2;1 (Katsuhara et al., 2002), HvPIP2;2 (Horie et al., 2011) and HvPIP2;5 (EVMETGGGGDFAAKD, in the present study).
The specificity of HvPIP2;5 antibodies was tested through expression of HvPIP2;5 isoform in oocytes of the toad Xenopus laevis and subsequent analysis of membrane protein fraction through Western analyses. Expression of HvPIP2;5 in Xenopus oocytes was performed according to Katsuhara et al. (2002). Briefly, the coding region of HvPIP2;5 cDNAs was sub-cloned into pXβG-ev1, and corresponding cRNA was synthesized and injected into oocytes. Total membranes of oocytes expressing HvPIP2;5 protein were extracted according to Leduc-Nadeau et al. (2007). All membrane protein corresponding to one oocyte was used as a sample and subjected to solubilization, SDS-PAGE and Western blotting as described previously (Katsuhara et al., 2002).
Immunoassay of ABA
ABA was immunoassayed as previously described (Vysotskaya et al., 2007) in the roots of control plants of ‘Steptoe’ and Az34 and those exposed to 10–5 m ABA in solution. Aqueous residues of ethanol extracts were diluted with distilled water, acidified with HCl to pH 2·5 and partitioned twice with peroxide-free diethyl ether (ratio of organic to aqueous phases was 1 : 3). Subsequently, hormones were transferred from the organic phase into 1 % sodium hydrocarbonate (pH 7–8, ratio of organic to aqueous phases was 3 : 1), acidified with HCl to pH 2·5, re-extracted with diethyl ether, methylated with diazomethane and immunoassayed using antibodies to ABA (Veselov et al., 1992). ABA recovery calculated in model experiments was about 80 %. Reducing the amount of extractant, based on the calculated distribution of ABA in organic solvents, increased the selectivity of hormone recovery and the reliability of immunoassay. The reliability of the immunoassay for ABA was enabled by both specificity of antibodies and purification of hormones according to a modified scheme of solvent partitioning (Veselov et al., 1992).
Immunolocalization of ABA and AQPs
Immunolocalization was carried out on root sections prepared from the root hair zone (3–5 mm from the root tip, Fig. 1). Specific rabbit antisera against ABA and HvPIP AQPs were used for immunolocalization of these antigens. Sections of roots were harvested from control plants and from those exposed to an ABA solution for about 1 h. To prevent ABA washing out from tissues during fixation and dehydration, root tip segments 3–5 mm in length were fixed in 4 % 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (Sigma, St Louis, MO, USA) for 4 h (under vacuum during the first 30 min of fixation) and then with 4 % formaldehyde for a night as described earlier (Vysotskaya et al., 2007). In this process, ABA carboxylic groups were linked with protein amino groups. Following fixation in formaldehyde, root segments were dehydrated in ethanol solutions of increasing grades (up to 96 %). Segments were then embedded in methacrylate resin (JB-4, Electron Microscopy Sciences, Hatfield, PA, USA) as recommended by the manufacturer. Histological sections (1·5 μm) were cut with a rotary microtome (HM 325, MICROM Laborgeräte, Berlin, Germany) and placed on slides.
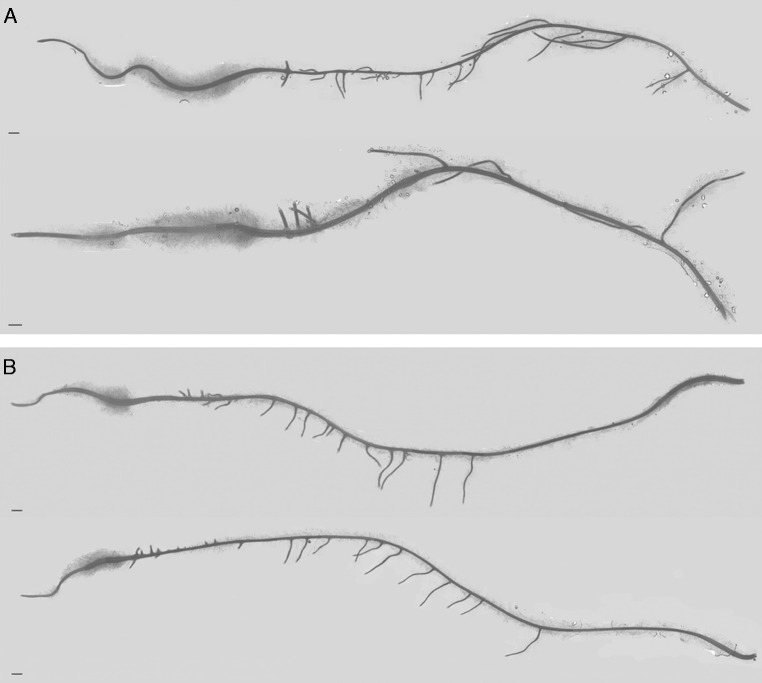
Fig. 1.
Image of the roots of ‘Steptoe’ (A) and Az34 (B) plants. Scale bar = 1 mm.
Sections were treated for 30 min with 0·1 m Na-phosphate buffer (pH 7·3) containing 0·2 % gelatin and 0·05 % Tween 20 (PGT), washed with distilled water and incubated for 2 h in a moist chamber at room temperature with immune rabbit anti-ABA or anti-HvPIP2 sera (20 μL) diluted with PGT at a ratio of 1 : 50 or 1 : 200 (when anti-ABA serum was applied to the sections of ‘Steptoe’ roots in some cases). To check the specificity of immunostaining, some sections were treated with non-immune serum at similar dilution. To visualize antibodies bound to either ABA or AQPs, sections were treated for 1 h in a moist chamber with the second goat antibodies raised against rabbit immunoglobulin labelled with colloidal gold (1 : 40 in PGT; Aurion, Hatfield, PA, USA). After three washes with phosphate buffer (PB), samples were post-fixed in 2 % glutaraldehyde in PB for 5 min. The sections were then washed with distilled water, and incubated with silver enhancer (Aurion) for 30 min. Excess silver was removed with distilled water and sections were examined under a light microscope (Carl Zeiss, Jena, Germany) equipped with an AxioCam MRc5 digital camera (Carl Zeiss).
The intensity of immunostaining of plasmalemma AQPs was estimated from 8-bit grayscale images using ImageJ software (1·48, National Institutes of Health, Bethesda, MD, USA). Circles of fixed dimensions were marked along cell membranes of the epidermis and cortical cell layer beneath epidermis at regular intervals around the entire perimeter of the root section and mean pixel intensities were measured within the regions of interest (ROI). Staining values, obtained by determining the pixel intensity for every circle, were averaged for each root section (about 160 circles per image of one root section). The intensity of root section staining for ABA was measured by using the ‘Freehand Selections’ tool of the same software by selecting the entire area of root sections and measuring mean pixel intensities within the ROI. Images were taken from nine independent sections per genotype or ABA treatment. The intensity of staining was expressed in arbitrary units, with maximal staining taken as 100 % and minimal as 0.
Statistics
Data were expressed as means ± s.e., which were calculated in all treatments using MS Excel. Significant differences between means were analysed by a t-test and two-way analysis of variance (ANOVA) with genotype and ABA treatment as main factors, and a least significance difference (LSD) test to discriminate means.
RESULTS
Root hydraulic conductivity (LpRoot) was about two times lower in Az34 than ‘Steptoe’ (Table 1). Inhibiting AQP activity by producing reactive hydroxyl radicals during the Fenton reaction decreased hydraulic conductivity of ABA-treated and untreated plants of both genotypes (Table 1).
Table 1.
Root hydraulic conductance (mg h–1 g–1 root f. wt MPa–1) and ABA concentration (pmol g–1 root f. wt) of barley plants treated with 10–5 m ABA, Fenton reagent and control
| Characteristic | Genotype, treatment | –ABA | +ABA |
|---|---|---|---|
| Hydraulic conductance | ‘Steptoe’, –Fenton | 290 ± 35c | 610 ± 55d |
| Az34, –Fenton | 136 ± 21b | 390 ± 40cd | |
| ‘Steptoe’, +Fenton | 170 ± 23b | 184 ± 30b | |
| Az34, +Fenton | 68 ± 8a | 89 ± 14a | |
| ABA concentration | ‘Steptoe’ | 23 ± 3b | 117 ± 19c |
| Az34 | 8±1a | 38 ± 11b |
Significantly different means for each variable are labelled with different letters (n = 5, LSD test).
Root fresh weight did not differ significantly (P > 0·1, t-test) between the two genotypes (63±4 and 54±6 mg in ‘Steptoe’ and Az34, respectively), and thus the difference in LpRoot between the two genotypes could not be attributed to the difference in root mass. Root hairs appeared closer to the root tip (1·1±0·1 mm from root tip) in Az34 compared with ‘Steptoe’ (2·3±0·2 mm from root tip). Moreover, the length of the root hair zone was shorter in Az34 than in ‘Steptoe’ (Fig. 1) (13±1 and 32±3 mm in Az34 and ‘Steptoe’, respectively, the difference being significant at P = 0·001).
Bulk ABA concentration in Az34 roots was only one-third that in ‘Steptoe’. ABA treatment increased root ABA concentrations by five-fold in both genotypes (Table 1). ABA treatment increased LpRoot of both genotypes similarly by two-fold or more (no significant genotype × ABA interaction: P = 0·13, two way ANOVA). ABA treatment of Az34 raised endogenous root ABA concentrations and LpRoot. Application of exogenous ABA to the root medium of Az34 plants increased the LpCell of root cortex cells almost three-fold (P < 0·001) (Table 2). This was due to a much decreased T1/2 (P < 0·001) while changes in ε were minor and not significant. Exogenous ABA had no effect on the turgor of cortical cells.
Table 2.
Water relations parameters of root cortical cells of the ABA-deficient barley mutant Az34 in the absence (–ABA) and presence (+ABA) of exogenous ABA in the root medium (10 μm ABA)
| Variable | –ABA | +ABA | P |
|---|---|---|---|
| Cell turgor (MPa) | 0·48 ± 0·02 | 0·49 ± 0·01 | 0·673 |
| Cell elastic modulus (MPa) | 1·62 ± 0·19 | 1·34 ± 0·11 | 0·212 |
| Cell half-time of water exchange, T1/2 (s) | 9·48 ± 0·89 | 5·11 ± 0·63 | <0·001*** |
| Cell hydraulic conductivity, Lp (m s–1 MPa–1) | 1·90 ± 0·27 × 10–7 | 4·54 ± 0·60 × 10–7 | <0·001*** |
Plants were analysed between 20 min and 2 h following the addition of ABA to the root medium. Cells were located within the root hair zone. Results are means ± s.e. of n = 23 cell analyses, which were obtained from the analysis of four roots each.
P < 0·001 (Student’s t-test).
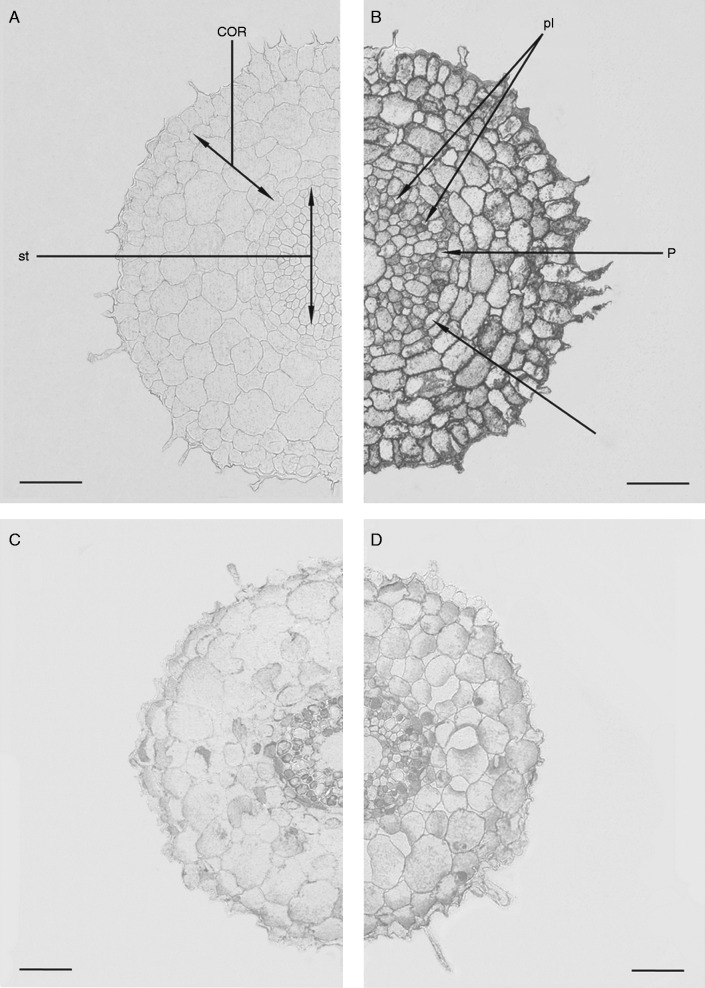
Root sections treated with non-immune serum were weakly stained (Fig. 2A). ABA immunolocalization showed strong labelling of cells in all root tissues of ‘Steptoe’ (Fig. 2B). Staining for ABA was much lower in root cells of Az34, which was most noticeable in the weakly labelled cortex (Fig. 2C). Application of ABA to Az34 plants increased immunostaining of cortical cells, especially those which were located closer to the root periphery (intensity of root immunolabelling for ABA was statistically higher in ABA-treated plants at P < 0·05) (Fig. 2D, Table 3). The strong labelling of ABA in sections of ‘Steptoe’ roots prior to application of ABA impaired the detection of differences in labelling between ABA-treated and untreated plants (data not shown). Dilution of anti-ABA serum decreased immunostaining of ‘Steptoe’ roots, but enabled detection of increased staining of the ABA-treated ‘Steptoe’ roots (Table 3). Even after dilution of the serum, the sections were more strongly stained for ABA in the case of ‘Steptoe’ compared with Az34 (despite the use of more concentrated serum in the case of the mutant).
Fig. 2.
Immunolocalization of ABA in root sections (3–5 mm from the root tip) of ‘Steptoe’ (A and B) and Az34 (C and D) treated (D) and untreated (A, B, C) with 10–5 m ABA. Similar dilutions of anti-ABA serum were applied to the sections of either ‘Steptoe’ or Az34. (A) Section of ‘Steptoe’ roots treated with normal non-immune serum. COR, cortex; P, pericycle; pl, phloem; st, stele; e, endodermis. Scale bars = 100 µm.
Table 3.
Intensity of staining for ABA and PIP2 aquaporins (means ± s.e., arbitrary units, maximal staining taken as 100 %, minimal as 0 %) of control and ABA-treated Az34 roots
| Staining for: | ‘Steptoe’ |
Az34 |
||
|---|---|---|---|---|
| Control | ABA-treated | Control | ABA-treated | |
| ABA | 41 ± 7b | 65 ± 9c | 21 ± 7a | 79 ± 5c |
| PIP2;1 | 23 ± 5a | 75 ± 4b | 26 ± 9a | 68 ± 13b |
| PIP2;2 | 21 ± 4a | 85 ± 7b | 12 ± 7a | 87 ± 12b |
| PIP2;5 | 73 ± 8a | 56 ± 5a | 61 ± 12a | 45 ± 19a |
Anti-ABA serum was diluted four-fold when applied to the sections of ‘Steptoe’ roots as compared with the procedure of ABA immunolocalization in Az34. Significantly different means for each variable within a row are labelled with different letters (n = 5, LSD test).
Western blotting showed specificity of antibodies raised against a synthetic oligopeptide corresponding to the amino acid sequences in the N-region of HvPIP2;5 (Fig. 3). These antibodies recognized the band in membrane proteins of oocytes expressing HvPIP2;5 and did not recognize other PIP2 proteins. The specificity of antibodies used to detect HvPIP2;1 and HvPIP2;2 has been shown previously (Horie et al., 2011).
Fig. 3.

Western blot analysis of membrane proteins of oocytes expressing cRNA of the coding region of HvPIP2;5 using antibodies against synthetic oligopeptides corresponding to the amino acid sequences in the N-region of HvPIP2;5.
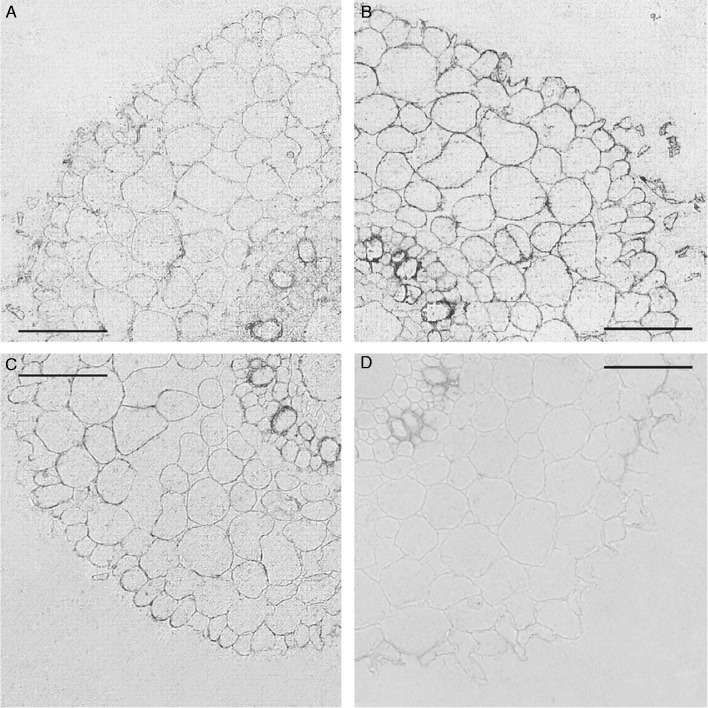
Staining of sections, which was indicative of the presence of candidate PIP2 AQPs, was barely visible on sections treated with non-immune serum [Fig. 4D (‘Steptoe’) and Fig. 5D (Az34)]. This changed when antibodies against HvPIP2 AQPs were used for immunostaining. Cell boundaries, which included the plasma membrane, were clearly visualized due to immunolabelling of plasma membrane AQPs (Figs 4A–C and 5A–C). Labelling of boundaries of, or next to, metaxylem cells was most intense. Cytoplasm was also immunostained for AQPs, although less than cell boundaries. Immunostaining was rather low for HvPIP2;5 and stronger for HvPIP2;1 and HvPIP2;2.
Fig. 4.
Immunolocalization of AQPs in roots (3–5 mm from the root tip) of ‘Steptoe’ plants using antibodies against HvPIP2;1 (A), HvPIP2;2 (B) and HvPIP2;5 (C). (D) Sections treated with non-immune serum. Scale bars = 50 µm.
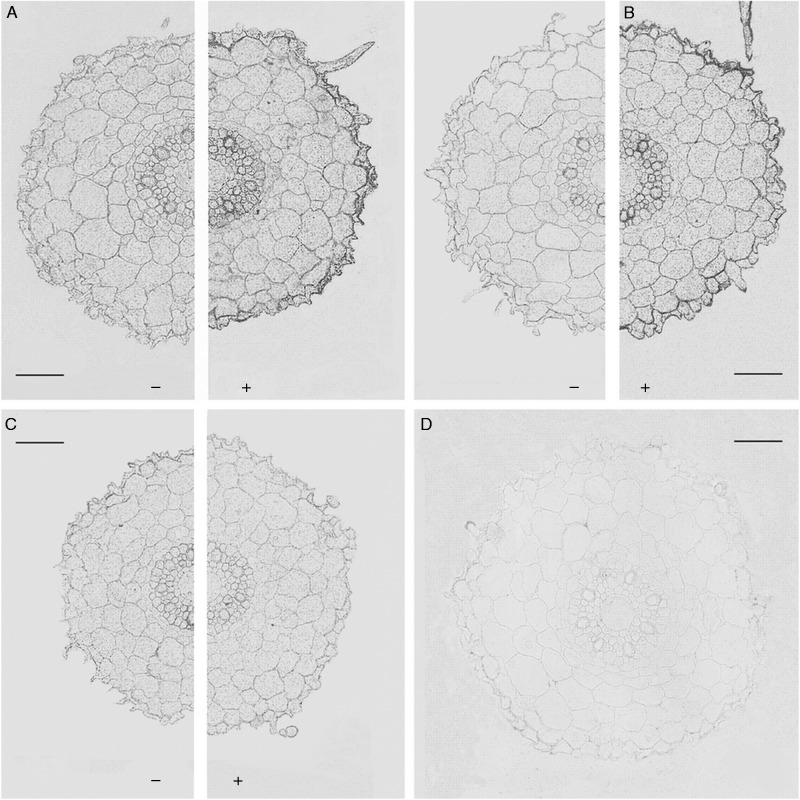
Fig. 5.
Immunolocalization of AQPs in roots (3–5 mm from the root tip) of Az34 plants using antibodies against HvPIP2;1 (A), HvPIP2;2 (B) and HvPIP2;5 (C) AQPs treated (+) and untreated (–) with 10–5 m ABA. (D) Sections treated with non-immune serum. Scale bars = 50 µm.
Root sections of Az34 and ‘Steptoe’ did not differ visibly in the immunostaining for any of the HvPIP2 AQPs (cf. Fig. 4 and the left part of Fig. 5A–C). This was supported by a quantitative analysis of immunostaining intensity using ImageJ software (Table 3). ABA-treatment increased immunostaining of roots cells of Az34 and ‘Steptoe’ for HvPIP2;1 and HvPIP2;2 antibodies (Table 3, means for immunostaining intensity along cell membranes of root periphery different at P < 0·05), whereas the level of staining did not change for the HvPIP2;5 antiserum. The increase in immunostaining was most pronounced at the root periphery (Fig. 5).
DISCUSSION
Comparing Az34 with ‘Steptoe’
Previous measurements of bulk root ABA concentrations showed that ABA levels in Az34 plants were 70 % lower than in their wild-type parent ‘Steptoe’ (Kudoyarova et al., 2014). The present study extends these analyses to the tissue level and shows that all major tissues which are located along the radial path of water movement across roots have lower ABA levels in Az34 compared with ‘Steptoe’.
Initially, the longer-term, and possibly developmental, effect of differences in root ABA levels on root water uptake properties were compared between genotypes. The decreased root hydraulic conductivity of Az34 (compared with ‘Steptoe’) was accompanied by a decreased ABA concentration consistent with the role of ABA in regulating water uptake across roots (Parent et al., 2009). However, the abundance of HvPIP2;1 and HvPIP2;2 was similar in roots of both genotypes and did not match the genotypic difference in LpRoot. The latter could mean either that longer-term lowered levels of ABA reduce activity of AQPs at the post-translational level, or that the reduction in LpRoot does not involve changes in AQP activity.
Root hydraulic conductivity depends not only on AQP activity, but also on other root attributes such as root hair development. The length of the root hair zone, which can be extremely important to overall water uptake by the plant (Segal et al., 2008), was shorter in Az34 than in ‘Steptoe’ and this may have also contributed to a reduced ability of Az34 roots to take up and conduct water. This observation agrees with earlier reports that accumulation of ABA under moderate drought enhances root hair development (Xu et al., 2013). Further experiments are needed to study the mechanism(s) responsible for the decreased LpRoot in Az34 compared with ‘Steptoe’.
Effects of exogenous ABA on root (cell) hydraulics in Az34
By comparing root hydraulics of Az34 and ‘Steptoe’ in the presence and absence of exogenously added ABA, short-term effect of differences in root ABA levels on root water uptake properties were revealed. Thus, the response of the two genotypes to exogenous ABA was similar. These experiments excluded the possibility that ABA affects root hydraulics through developmental changes and provided a more convenient model for relating tissue ABA levels to AQP abundance and hydraulics at a cellular level. Comparison of the distribution of immunostaining between cells shows some similarity in the increased labelling of ABA and AQPs in response to application of ABA. ABA staining increased especially in the root periphery, at the epidermis and cortical cell layers located beneath, and in these tissues staining of HvPIP2;1 and HvPIP2;2 increased too. In addition, the stimulation of LpRoot by exogenous ABA (Table 1) was accompanied by enhanced LpCell (Table 2). As LpCell reflects membrane transport properties, these results indicate that exogenous ABA stimulates LpRoot by stimulating the water transport properties of root cells, or at least those root cortex cells which were analysed in the present study. Furthermore, because membrane water transport properties involve AQP function, and because exogenous ABA increased immunostaining of HvPIP2;1 and HvPIP2;2 in cortex cells, we conclude that exogenous ABA increased LpRoot through an increase in the abundance, and activity, of at least two HvPIP2 isoforms (HvPIP2;1, HvPIP2;2), but not that of HvPIP2;5 in root cortex cells. Parent et al. (2009) showed up-regulation of expression of all ZmPIP AQP isoforms by ABA in maize plants. The present data show that ABA also increases the protein level of some (HvPIP2;1, HvPIP2;2) yet not all PIP AQPs (HvPIP2;5).
ABA can induce expression of AQP genes (Maurel et al., 2008). We do not know whether, in shorter term experiments like ours (about 1 h), any changes in AQP expression are likely to influence corresponding protein levels. Rather, effects of ABA at the post-transcriptional level of AQPs are more likely to occur. As ABA can regulate the activity of AQPs through their phosphorylation (Chaumont and Tyerman, 2014, and references therein), rapid ABA-induced changes in hydraulic conductivity could be explained through this mechanism. In addition, ABA has been suggested to alter the conformation and gating and, through this, water permeability of AQPs (Wan et al., 2004).
The present data show that application of exogenous ABA to the ABA-deficient barley mutant Az34 and its parental cultivar ‘Steptoe’ increases root and root cell hydraulic conductivity parallel to an increased abundance of particular PIP2 AQP isoforms in root epidermal and cortex cells closer to the root periphery. The difference in root hydraulic conductivity between Az34 and its ABA-sufficient parent, ‘Steptoe’, may also involve developmental effects of ABA on the timing and formation of root hairs during root development.
ACKNOWLEDGEMENTS
This work was partially supported by the Russian Ministry of Education and Science [N 01201456413] and Russian Foundation for Basic Research [N 14-04-97077].
LITERATURE CITED
- Akhiyarova G, Fricke W, Veselov D, Kudoyarova G, Veselov S. 2006. ABA accumulation and distribution in the leaf tissues shows its role in regulation of stomatal conductance under short-term salinity. Tsitologiya 48: 918–923. [PubMed] [Google Scholar]
- Aroca R, Vernieri P, Irigoyen JJ, Sanchez-Diaz M, Tognoni F, Pardossi A. 2003. Involvement of abscisic acid in leaf and root of maize (Zea mays L.) in avoiding chilling-induced water stress. Plant Science 165: 671–679. [Google Scholar]
- Carvajal M, Cooke DT, Clarkson DT. 1996. Responses of wheat plants to nutrition deprivation may involve the regulation of water-channel function. Planta 199: 372–381. [Google Scholar]
- Chaumont F, Tyerman SD. 2014. Aquaporins: highly regulated channels controlling plant water relations. Plant Physiology 164: 1600–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd IC. 2005. Root-to-shoot signalling: assessing the roles of ‘up’ in the up and down world of long-distance signalling in plants. Plant and Soil 274: 257–275. [Google Scholar]
- Dodd IC. 2013. Abscisic acid and stomatal closure: a hydraulic conductance conundrum? New Phytologist 197: 6–8. [DOI] [PubMed] [Google Scholar]
- Fricke W, Peters WS. 2002. The biophysics of leaf growth in salt-stressed barley. A study at the cell level. Plant Physiology 129: 374–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricke W, Akhiyarova G, Veselov D, Kudoyarova G. 2004. Rapid and tissue-specific changes in ABA and in growth rate in response to salinity in barley leaves. Journal of Experimental Botany 55: 1115–1123. [DOI] [PubMed] [Google Scholar]
- Horie T, Kaneko T, Sugimoto G, et al. 2011. Mechanisms of water transport mediated by PIP aquaporins and their regulation via phosphorylation events under salinity stress in barley roots. Plant Cell Physiology 52: 663–675. [DOI] [PubMed] [Google Scholar]
- Hose E, Steudle E, Hartung W. 2000. Abscisic acid and hydraulic conductance of maize roots: a study using cell- and root-pressure probes. Planta 211: 874–882. [DOI] [PubMed] [Google Scholar]
- Katsuhara M, Akiyama Y, Koshio K, Shibasaka M, Kasamo K. 2002. Functional analysis of water channels in barley roots. Plant Cell Physiology 43: 885–893. [DOI] [PubMed] [Google Scholar]
- Knipfer T, Fricke W. 2010. Root pressure and a solute reflection coefficient close to unity exclude a purely apoplastic pathway of radial water transport in barley (Hordeum vulgare). New Phytologist 187: 159–170. [DOI] [PubMed] [Google Scholar]
- Knipfer T, Besse M, Verdeil J-L, Fricke W. 2011. Aquaporin-facilitated water uptake in barley (Hordeum vulgare L.) roots. Journal of Experimental Botany 62: 4115–4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudoyarova G, Veselova S, Hartung W, Farhutdinov R, Veselov D, Sharipova G. 2011. Involvement of root ABA and hydraulic conductance in the control of water relations in wheat plants exposed to increased evaporative demand. Planta 233: 87–94. [DOI] [PubMed] [Google Scholar]
- Kudoyarova G, Veselov D, Sharipova G, Akhiyarova G, Dodd IC, Veselov S. 2014. Water relations and growth of original barley plants and its ABA deficient mutants at increased air temperature. Russian Journal of Plant Physiology 61: 188–193. [Google Scholar]
- Leduc-Nadeau A, Lahjouji K, Bissonnette P, Lapointe JY, Bichet DG. 2007. Elaboration of a novel technique for purification of plasma membranes from Xenopus laevis oocytes. American Journal of Physiology – Cell Physiology 292: 1132–1136. [DOI] [PubMed] [Google Scholar]
- Ludewig M, Dorffling K, Seifert H. 1988. Abscisic acid and water transport in sunflowers. Planta 175: 325–333. [DOI] [PubMed] [Google Scholar]
- Mahdieh M, Mostajeran A. 2009. Abscisic acid regulates root hydraulic conductance via aquaporin expression modulation in Nicotiana tabacum. Journal of Plant Physiology. 166: 1993–2003. [DOI] [PubMed] [Google Scholar]
- Martin-Vertedor AI, Dodd IC. 2011. Root-to-shoot signalling when soil moisture is heterogeneous: increasing the proportion of root biomass in drying soil inhibits leaf growth and increases leaf abscisic acid concentration. Plant, Cell and Environment 34: 1164–1175. [DOI] [PubMed] [Google Scholar]
- Maurel C, Verdoucq L, Luu DT, Santoni V. 2008. Plant aquaporins: membrane channels with multiple integrated functions. Annual Review of Plant Physiology 59: 595–624. [DOI] [PubMed] [Google Scholar]
- Mulholland BJ, Black CR, Taylor IB, Roberts JA. 1996. Effect of soil compaction on barley (Hordeum vulgare) growth. II. Are increased xylem sap ABA concentrations involved in maintaining leaf expansion in compacted soils? Journal of Experimental Botany 47: 551–556. [Google Scholar]
- Pantin F, Monnet F, Jannaud D, et al. 2013. The dual effect of abscisic acid on stomata. New Phytologist 197: 65–72. [DOI] [PubMed] [Google Scholar]
- Parent B, Hachez C, Redondo E, Simonneau T, Chaumont F, Tardieu F. 2009. Drought and abscisic acid effects on aquaporin content translate into changes in hydraulic conductance and leaf growth rate: a trans-scale approach. Plant Physiology 149: 2000–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal E, Kushnir T, Mualem Y, Shani U. 2008. Water uptake and hydraulics of the root hair rhizosphere. Vadose Zone Journal 7: 1027–1034. [Google Scholar]
- Steudle E, Jeschke WD. 1983. Water transport in barley roots. Planta 158: 237–248. [DOI] [PubMed] [Google Scholar]
- Steudle E, Peterson CA. 1998. How does water get through roots? Journal of Experimental Botany 49: 775–788. [Google Scholar]
- Suku S, Knipfer T, Fricke W. 2014. Do root hydraulic properties change during the early vegetative stage of plant development in barley (Hordeum vulgare)? Annals of Botany 113: 385–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker-Simmons M, Kudrna DA, Warner RL. 1989. Reduced accumulation of ABA during water stress in a molybdenum cofactor mutant of barley.. Plant Physiology 90: 728–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veselov SU, Kudoyarova GR, Egutkin NL, Gyuli-Zade VG, Mustafina AR, Kof EK. 1992. Modified solvent partitioning scheme providing increased specificity and rapidity of immunoassay for indole 3-acetic acid. Physiologia Plantarum 86: 93–96. [Google Scholar]
- Vysotskaya LB, Veselov SYu, Veselov DS, et al. 2007. Immunohistological localization and quantification of IAA in studies of root growth regulation. Russian Journal of Plant Physiology 54: 827–832. [Google Scholar]
- Wan X, Steudle E, Hartung W. 2004. Gating of water channels (aquaporins) in cortical cells of young corn roots by mechanical stimuli (pressure pulses): effects of ABA and of HgCl2. Journal of Experimental Botany 55: 411–422. [DOI] [PubMed] [Google Scholar]
- Xu W, Jia L, Shi W, et al. 2013. Abscisic acid accumulation modulates auxin transport in the root tip to enhance proton secretion for maintaining root growth under moderate water stress. New Phytologist 197: 139–150. [DOI] [PubMed] [Google Scholar]
- Ye Q, Steudle E. 2006. Oxidative gating of water channels (aquaporins) in corn roots. Plant, Cell & Environment 29: 459–470. [DOI] [PubMed] [Google Scholar]
- Zhang J, Zhang X., Liang J. 1995. Exudation rate and hydraulic conductivity of maize roots are enhanced by soil drying and abscisic acid treatment. New Phytologist 131: 329–336. [Google Scholar]