Abstract
Background There has been renewed global interest in both genetic and management strategies to improve root system function in order to improve agricultural productivity and minimize environmental damage. Improving root system capture of water and nutrients is an obvious strategy, yet few studies consider the important interactions between the genetic improvements proposed, and crop management at a system scale that will influence likely success.
Scope To exemplify these interactions, the contrasting cereal-based farming systems of Denmark and Australia were used, where the improved uptake of water and nitrogen from deeper soil layers has been proposed to improve productivity and environmental outcomes in both systems. The analysis showed that water and nitrogen availability, especially in deeper layers (>1 m), was significantly affected by the preceding crops and management, and likely to interact strongly with deeper rooting as a specific trait of interest.
Conclusions In the semi-arid Australian environment, grain yield impacts from storage and uptake of water from depth (>1 m) could be influenced to a stronger degree by preceding crop choice (0·42 t ha–1), pre-crop fallow management (0·65 t ha–1) and sowing date (0·63 t ha–1) than by current genetic differences in rooting depth (0·36 t ha–1). Matching of deep-rooted genotypes to management provided the greatest improvements related to deep water capture. In the wetter environment of Denmark, reduced leaching of N was the focus. Here the amount of N moving below the root zone was also influenced by previous crop choice or cover crop management (effects up to 85 kg N ha–1) and wheat crop sowing date (up to 45 kg ha–1), effects which over-ride the effects of differences in rooting depth among genotypes. These examples highlight the need to understand the farming system context and important G × E × M interactions in studies on proposed genetic improvements to root systems for improved productivity or environmental outcomes.
Keywords: Australia, Denmark, root system
INTRODUCTION
In the decades ahead, there is a need for increased agricultural production. This cannot be achieved by increasing the agricultural area, and agriculture is facing climate change and resource limitations, and is challenged to increase production without adverse environmental effects. These challenges have led to a renewed interest in understanding and improving crop root systems (Lynch, 2007; Gregory et al., 2013). Improved root systems can help crops use soil resources more effectively, and thereby be a pathway to improved productivity and environmental outcomes. Studies have been made of both genetic (Munns et al., 2010; Richard et al., 2015) and management strategies (Ford et al., 2006; Rasmussen and Thorup-Kristensen, 2016) to improve root system function. In order to make such efforts successful, it is necessary to identify the root traits which ensure improved capture of soil resources by roots. However, it is equally important to identify the interactions between the crops and their root systems with the soil and environmental conditions. We need to do this to understand when root system performance is a limiting factor, and how to improve root performance, by genetics or management to actually improve crop use of soil resources.
A key objective of this review is to analyse how different aspects of root system growth and function can limit agricultural crop performance, and how this is influenced by the environmental conditions and crop management within specific farming systems. It is a general assumption that improved root systems can improve crop performance (Lynch, 2007; Gregory et al., 2013), but, for this to happen, there must be soil resources available [water, nitrogen (N) or other nutrients] which are not used effectively by crops, demonstrably due to limitations in root growth or function. Both criteria [(1) unused resources in the soil and (2) limitations in root growth or function] must be analysed critically to justify expensive root-based crop improvement research.
In many situations, crops can use practically all of the available water and N resources from the soil during the time available, e.g. plant-available water in dryland farming systems on shallow soils (<1 m) (Lilley and Kirkegaard, 2016), or mineral N when N fertilization is not higher than crop demand (Heumann et al., 2013; Rasmussen et al., 2015). Under other circumstances where soil resources are available in excess, uptake can be limited by above-ground crop demand for the resources, rather than by root system performance, e.g. due to high precipitation, irrigation and heavy fertilization, or sub-optimal crop growth for other reasons. Under these circumstances, modified root growth may not improve productivity.
Crucially it may not be total resource acquisition, but its timing that limits productivity. Increasing total seasonal resource capture may not be valuable if there are specific periods of high resource demand at critical crop growth stages where root performance is unable to satisfy crop demand. Limited early resource capture may reduce leaf area, tiller production and yield, even when water and nutrient uptake is not limiting at later stages (Raun et al., 2011; Bredemeier et al., 2013). Conversely, in resource-limited environments, high early resource uptake may lead to early depletion of limited resources during vegetative growth, leading to a resource deficiency at later critical stages such as flowering or grain filling (Passioura, 2006).
A goal of ‘more roots’ represents a simplification of improved root growth and function. If the resources in question are water or N in the sub-soil, higher root density or mass in the topsoil are less important than an appropriate root depth distribution to provide more timely and complete utilization of the available resources at depth (Barraclough et al., 1989; Manschadi et al., 2006; McKenzie et al., 2009; Maeght et al., 2013). Increasing the total rooted volume through deeper roots may bring the crop root system into contact with soil resources which would otherwise be out of reach (Thorup-Kristensen, 2006a; Thorup-Kristensen et al., 2009; Maeght et al., 2013). Depending on the conditions, and which nutrients are limiting, many other root traits may also be important, such as differences in root diameter, root hair formation, root longevity, root growth responses to local resource availability (Gorska et al., 2010) or nutrient uptake efficiency by various mechanisms. In short, we do not necessarily need more roots, but need root systems which are better adapted to take up the growth-limiting water and nutrients from the soil.
Finally, and in contrast to the above-ground plant parts which must be optimized towards the acquisition of light energy and CO2 only, root systems must be optimized to acquire water and at least 14 different mineral elements, many of which are present in different chemical forms [e.g. N as ammonium, nitrate or dissolved organic nitrogen (DON)] and depths. These requirements may sometimes be contradictory – most obviously those for acquiring the major nutrients phosphorus (P) and N. Efficient P uptake will often require high root density in shallow soil layers and extensive root hair formation (Lynch, 2011), whereas efficient N (and water) utilization will be better achieved with less investment in roots in the uppermost soil layers, and more root growth to deeper soil layers (Barraclough et al., 1989; Lynch, 2011; Wasson et al., 2012).
In this review, we focus on water and N. Water is the most yield-limiting soil resource on a global scale, and N is the major yield-limiting nutrient. The uncontrolled loss of water and N from farming systems also leads to major environmental problems. Water and N are somewhat similar in that they are both mobile in the soil. They will easily move to deeper soil layers and can also move towards the roots more easily than other soil resources, reducing the need for high root densities for effective capture. However, water and N also differ in significant ways, e.g. in the sense that the amount of water taken up by plants is approx. 10 000 times higher than the amount of N, so that physical resistances to water movement become important (Doussan et al., 2006; Garrigues et al., 2006; Watt et al., 2008). Further, the mobility of water in the soil depends strongly on its availability. When the soil is moist, water can move freely towards the roots, but as the soil dries out the mobility of the water declines, making high root length density more important for water use as the soil dries.
Due to the important effects of soil, climate and timing, and the often conflicting requirements (trade-offs) for roots to capture different soil resources, a thorough understanding of the farming system is critical when considering how to improve crop root systems. Often the improved resource capture sought by modifying roots may be more simply achieved, by other means, and it will interact strongly with other potential management changes (Lilley and Kirkegaard, 2011; Dresboll and Thorup-Kristensen, 2014). An obvious example is the earlier sowing of crops, which sets the trajectory of both above-ground crop demand and root system depth into an entirely different context with respect to the availability of resources (Lilley and Kirkegaard, 2007, 2011; Rasmussen and Thorup-Kristensen, 2016). Early sowing is but one of the many ‘in-crop’ management interventions that can be considered, together with, for example, row spacing, tillage system, fertilizer application and weed management. The effects of these management interventions will overlap and interact with the management of the previous crops (crop rotation) and management during the pre-crop transition period, including weed control, crop residue and cover crop management (Kirkegaard and Hunt, 2010). It is the improvement of root system performance within this broader crop system context that is the focus of this review, using case studies from the contrasting wheat-farming systems of Denmark and Australia.
Background to Danish and Australian wheat-farming systems
The Australian and Danish wheat cropping systems provide specific contrasting case studies to exemplify the important system-level influences on root-based limitations to productivity, for opportunities to improve it and the important interactions within the farming system.
The Danish systems are broadly representative of intensive, high-yielding European systems where long-season winter wheat is grown. In Denmark, wheat is grown on light textured soils from sandy to sandy loam soils, commonly sown in late September and ready for harvest in early August of the following year (Figs 1 and 2). Spring wheat is only grown to a small extent, spring barley being by far the most widespread spring-sown cereal crop. Spring sowing is typically in early April followed by harvest in early August. Yearly precipitation of 600–950 mm is generally higher than evapotranspiration (Fig. 3A), generating high winter wheat yield potential (mean yields approx. 7·5 t ha–1), but accompanied by a high risk of nutrient leaching and run-off into groundwater. Farming practices in Denmark and elsewhere in the EU (Larsson et al., 2005; Wendland et al., 2009) have been highly regulated to reduce N losses and, in Denmark, the overall N use in wheat has declined by 40 % with no change in national yields during the last 20 years (Kyllingsbaek and Hansen, 2007; Kronvang et al., 2008; Dalgaard et al., 2014).
Fig. 1.
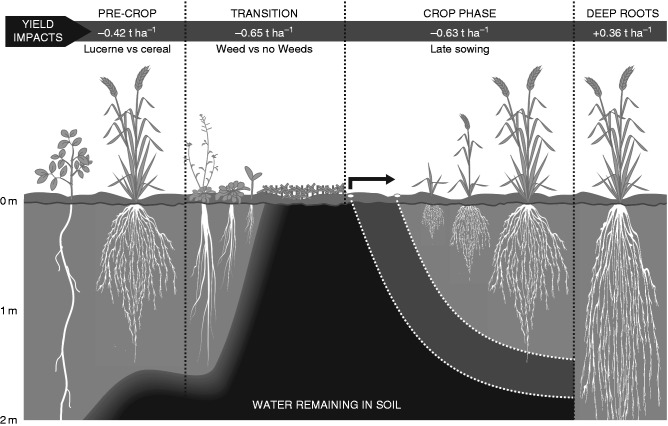
Typical 2 year crop sequences in the wheat-based cropping systems of Denmark (upper) and Australia (lower) showing the pre-crop, transition and current crop phases aligned by season in both systems. Major factors influencing the availability and uptake of water and nitrogen from the sub-soil by the roots of the current wheat crop during each phase are listed in the central panel.
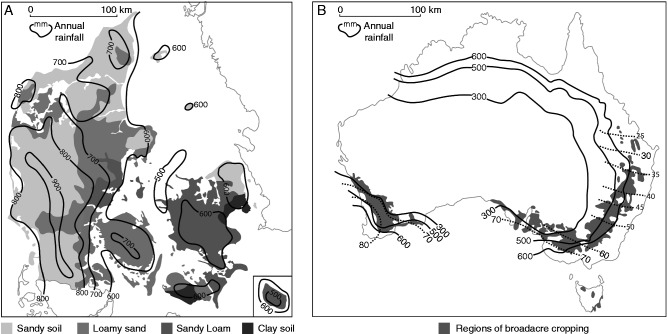
Fig. 2.
Danish (A) and Australian (B) wheat-growing regions with annual rainfall isohyets shown. The shading for the Danish map shows the distribution of sandier soils which are more prone to leaching, and the dotted lines in the Australian graph show the percentage of rain falling during the growing season.
Fig. 3.
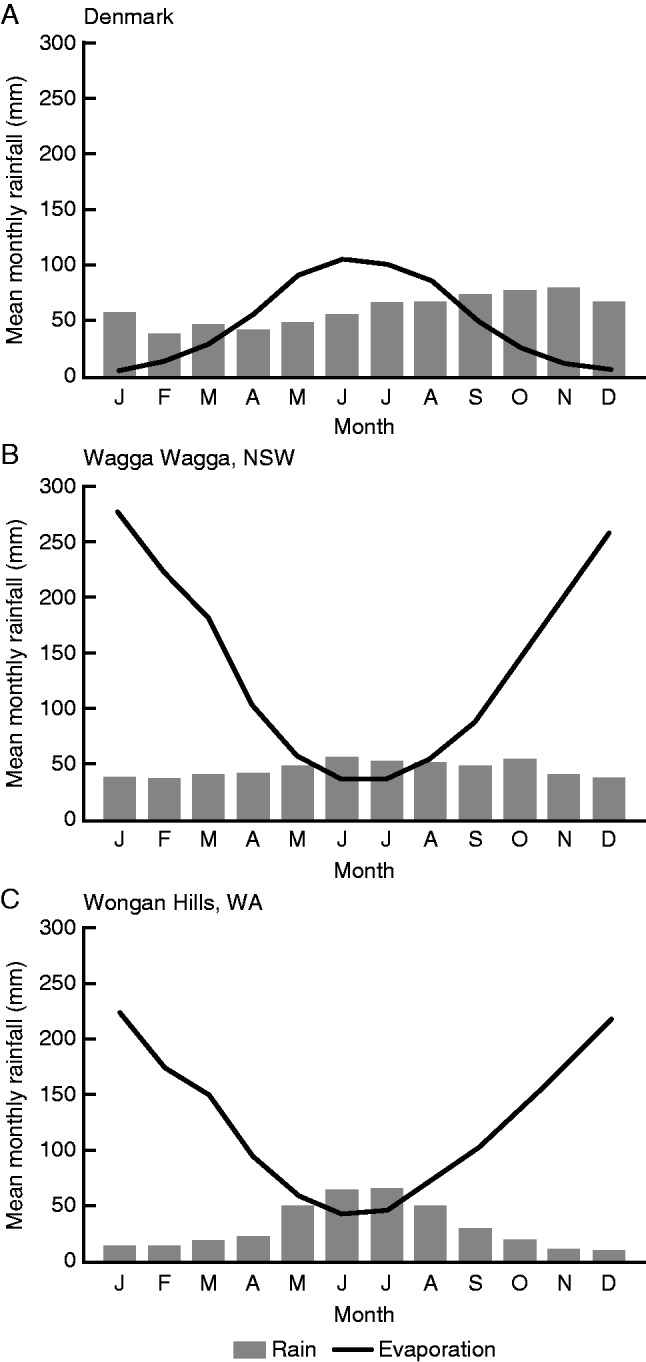
Monthly rainfall and evaporation for (A) Denmark, (B) an equi-seasonal rainfall location (Wagga Wagga) in south-eastern Australia, and (C) a Mediterranean rainfall location (Wongan Hills) in Western Australia. The significant winter surplus of rainfall is clear for the Danish environment compared with Australia, and the much larger deficit outside winter in Australia is also clear.
In contrast, broad-acre wheat-farming systems in southern Australia occur in a semi-arid 350–550 mm rainfall zone on gradational clay, clay loam or duplex soils (sand over clay) where seasonal water deficits are common, and autumn-sown spring wheat currently achieves an average yield of around 2 t ha–1 in the 6–7 month growing season (Kirkegaard et al., 2011; Figs 1 and 2). These systems are typical of other semi-arid dryland farming systems such as those in the North American Great Plains, and areas of China, India and the Middle East. The focus is on maximizing the capture, storage and efficient use of limited rainfall, and matching N inputs to the water-limited yield potential. Leaching risks are low, but over-use of N is risky from a financial perspective because N fertilizer constitutes a major variable cost. Overuse can also lead to excessive vegetative biomass and exhaustion of soil water prior to grain filling, reducing both yield and quality (pinched grains) through ‘haying-off’ (van Herwaarden et al., 1998).
Although different in detail and circumstance, both European and Australian wheat-based farming systems are focused on matching the N supply of the crop to the yield potential, satisfying specific grain quality targets and reducing both the financial and environmental risks associated with inefficient resource use. Further details of the broader wheat-farming system in both environments are provided below, with a specific focus on aspects relevant to improved capture and uptake of sub-soil water and N by wheat root systems.
Danish wheat-farming systems.
In Denmark, wheat is mostly grown on pig-producing farms in sequence with spring barley, winter barley and winter oilseed rape, and the main source of fertilizer is pig slurry. On specialized crop production farms, there are often more speciality crops in the rotations such as grass crops for seed production, sugar beet or potatoes. Specialized dairy farms may grow smaller areas of wheat in rotations with clover grass ley crops and maize for silage.
Wheat is grown on one-third of the total agricultural land, and other cereals [barley (Hordeum vulgare), rye (Secale cereale) and oats (Avena sativa)] make up another third, so that wheat is often grown after wheat or another cereal. Where possible, farmers try to grow wheat after beneficial pre-crops, especially after oilseed rape (as shown in Fig. 1). Intensive wheat production is especially common on the most fertile sandy loam soils of eastern Denmark but less common on the sandier, less fertile soils in the west (Fig. 2). Precipitation is distributed throughout the year, with a maximum intensity in the autumn (Fig. 3A). Thus the autumn period is when much of the nitrate leaching can occur, and the period when cover crops are targeted to reduce the risk of N leaching. It is also the period when the winter wheat is established, but autumn growth is limited and the 5–20 kg N ha–1 taken up before winter (Thorup-Kristensen et al., 2009; Rasmussen and Thorup-Kristensen, 2016; Rasmussen et al., 2015) has little effect on the N leaching risk.
In wheat cropping systems, Danish environmental regulations include limits on the amount of N fertilizer farmers are allowed to apply (80–90 % of economic optimum), on the timing of the fertilizer application (usually from early spring) and on the use of cover crops (N catch crops) (Dalgaard et al., 2014; Kronvang et al., 2015). The autumn application of N fertilizers is banned for most crops, including wheat, though small amounts can be added to oilseed (Brassica napus) rape with its much stronger growth and N uptake in the autumn (Dresboll et al., 2015b). The total N content in the slurry is calculated as 75 % effective, an efficacy which is difficult to achieve in practice. Together with fertilizer restrictions, wheat is fertilized with well under its optimal N level, and with less N fertilizer than in neighbouring European countries. A result of these policies is evident in the low protein content of Danish wheat (8–9 %). Nitrogen fertilizer is typically applied in one application in the early spring and, where fertilized with a combination of slurry and inorganic fertilizer, the fertilizer is usually applied as a later split application but always before the onset of stem elongation.
The environmental regulations oblige farmers to grow cover crops on 10–14 % of their area in the autumn. It is common to sow cover crops immediately after winter wheat (Fig. 1), or to broadcast into the wheat crop a few weeks before harvest. The short period between two winter crops has also generated interest in short-term cover crops using an approx. 6 week period between cereal harvest and sowing of the next wheat crop, which again requires the cover crops to be broadcast into the wheat, to establish beneath the wheat before harvest.
The downward movement of water and N means that these resources are available to crops in deeper layers. Winter wheat can reach rooting depths of 2 m on the sandy loam soils, around twice the rooting depth of spring cereals under the same conditions (Thorup-Kristensen, 2006a; Thorup-Kristensen et al., 2009; Rasmussen et al., 2015). Rooting depth appears to be more limited on the sandy soils, and the amount of N and water in deeper soil layers is lower, reducing the value of deeper roots on sandy soils.
The focus for improved rooting systems in Danish wheat is to target deeper roots to intercept and reduce the leaching of N into groundwater, and to capture deep resources of water and N during the grain-filling stage to satisfy the high demand of high-yielding (>10 t ha–1) Danish wheat.
Australian dryland wheat systems.
In contrast to Danish conditions, Australian wheat cropping occurs on extensive dryland mixed livestock–cropping enterprises under semi-arid conditions (Kirkegaard et al., 2011) (Fig. 2). The soils in the vast majority of the zone are either naturally deficient or depleted in the major crop nutrients (P and N) and average annual rainfall is generally low (<550 mm) and extremely variable by world standards. As a consequence, extensive agricultural production involves significant attention to the management of business risk, due to the probabilistic nature of the outcomes of most important management decisions.
The Australian cropping zone can be divided into two broad zones on the basis of climate; the northern sub-tropical cropping zone and the southern temperate cropping zone, which we focus on here. The southern temperate zone comprises areas in the states of Western Australia, South Australia, Victoria, Tasmania and southern New South Wales. Rainfall patterns range from equi-seasonal (New South Wales) (Fig. 3B) to strongly winter dominant (Western Australia) (Fig. 3C), and are generally inadequate during the hot summers to enable summer cropping. Hence, annual spring crops are sown in autumn, grown through winter–spring and are harvested in late spring or early summer. Short (4–6 month) plant-free summer fallow occurs between crops (Fig. 1). A key goal during such ‘short fallows’ is to store water and N for the following crop (Kirkegaard et al., 2011). Sowing time is generally dictated by suitable rainfall events after April, and the flowering of crops is timed to occur in spring, after the damaging winter frosts, but before the onset of terminal heat and drought.
Sequences of crops are generally grown in rotation with phases (2–5 years) of annual or perennial legume-based pastures (Puckridge and French, 1983). Around 80 % of the area cropped each year is sown to wheat and other cereals, but oilseed rape/canola (B. napus) and winter grain legumes such as field pea (Pisum sativum) and narrow-leaved lupin (Lupinus angustifolius) are also common. Long fallows (plant-free for 12–18 months) were traditionally used in some semi-arid areas to conserve water for the following crops, but these are now rare due to improved no-till cropping systems. There are also areas using phases of perennial rather than annual pastures, particularly of lucerne (Medicago sativa) (Angus et al., 2001; Latta et al., 2001).
In general, the focus in Australian wheat-farming systems is to capture, store and efficiently use as much rainfall as possible to support crop growth, while managing crop inputs (including N) to achieve the water-limited yield potential and grain quality requirements (Kirkegaard et al., 2014). Nitrogen fertilizer application can be risky because excessive N in seasons with limited spring rainfall can cause crops to ‘hay-off’ with lower yield, lower quality and no return on the N investment. On average, N application rates are low (approx. 40 kg ha–1 N), and significant amounts of crop N are derived from mineralized legume residues, although reliance on fertilizer has increased as pasture area has declined (Angus, 2001). Farmers must make decisions about expected yields in mid-winter so that N can be applied while there is sufficient water for crop uptake. The risk of N leaching is very low, but the value of water stored at depth for crop yield is high.
The focus for improved rooting systems in Australian wheat is deeper, more effective roots to capture water during the grain-filling stage when rainfall is low and variable, and deep stored water contributes significantly to yield.
FARMING SYSTEM PHASES: THE CONTEXT FOR IMPROVED ROOT PERFORMANCE
Figure 1 compares the general wheat cropping systems in Denmark and Australia aligned by season so that the various pre-crop, transition and in-crop phases of the farming systems in both countries across a 2 year sequence can be compared. In both countries, pre-crop options include cereals, oilseeds and grain legumes, and, while the mechanisms of influence are common, the magnitude is influenced by the different climatic conditions. The transition period differs markedly, with cover crops mandated in Denmark to reduce nitrate leaching loss, while in Australia the summer fallow period is focused on protecting and recharging dry and N-depleted soils. Both systems share modern in-crop agronomic management options, with each tailored to the yield potential of the environment. In the following sections, we consider briefly how management decisions made within each of these phases can ultimately influence the availability and capture of the water and N resources by the current wheat crop.
Pre-crops and their management
The sequence of crops or pastures preceding a wheat crop can affect the availability and use of deep resources by current wheat crops both indirectly, through effects on plant health, weed burdens and soil conditions, and directly, by affecting the availability of deep resources. These effects have been extensively studied and reviewed worldwide (Kirkegaard et al., 2008; Persson et al., 2008; Angus et al., 2015) and on average wheat grown after broad-leaf break crops yield between 0·6 and 0·8 Mg ha–1 more grain than wheat grown after wheat. Here we consider briefly how pre-crops and their management can interact with cereal root systems in subsequent seasons
Plant health.
Improved root health and the related improvements in shoot growth can influence the capacity for deep root growth and proliferation, the shoot demand for water and N, and the effective utilization of deep sub-soil water. Despite this, there are relatively few published studies directly relating soil-borne disease to root performance. Work in Australia by Whish et al. (2014) on the root lesion nematode (Pratylenchus thornei) showed that planting susceptible wheat varieties into high populations reduced water uptake rates, reduced the root extraction velocity and effectively changed the lower limit of soil drying by the crop. This impeded the early canopy development and reduced water and nutrient uptake, and yield was reduced by 34 %. Root-rotting fungal diseases such as Take-all (Gaeumannomyces graminis) can also infect roots early, and hyphal proliferation within the main xylem vessels reduces the uptake of water and nutrients when demand for grain filling is high. Improved water and nutrient uptake from depth following Brassica break crops has been demonstrated under both Australian (Angus et al., 1991; Kirkegaard et al., 1994) (Fig. 4) and European (Sieling and Christen, 2015) conditions.
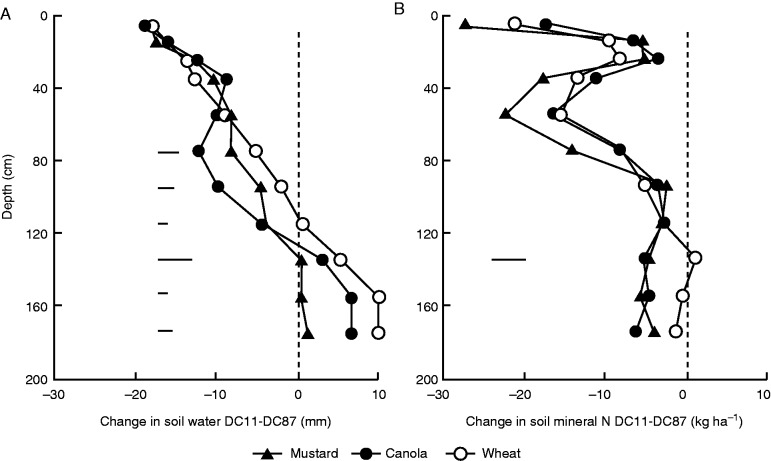
Fig. 4.
The effect of previous crops (wheat , canola or mustard) on the change in (A) soil water content and (B) soil mineral nitrogen from establishment to maturity in wheat in southern NSW Australia. The horizontal bars show LSD at depths where significant differences (P < 0·05) were found. The healthier crop and root system in wheat after break crops increased the uptake of water and N from the sub-soil (redrawn from Kirkegaard et al., 1994).
In a 14 year study of crop rotation, Sieling and Christen (2015) found that the yield increase in wheat after an oilseed rape break crop was high in dry years at approx. 1·5 Mg ha–1, but almost absent in years with above-average precipitation during the main growing season, indicating that the yield increase is caused mainly by a healthier root system allowing the crop to take up more soil water under dry conditions. Also soil- or stubble-borne foliar diseases can cause rotational effects on root efficiency, as they can reduce root growth (Ford et al., 2006) and reduce overall plant growth and demand for uptake of sub-soil resources. Thus the legacies of higher root or shoot disease inoculum after different pre-crops and rotations can clearly influence the performance of current wheat crops, including new varieties with improved root characteristics, unless they have specific resistance to the diseases. For many soil-borne diseases of cereals (e.g. G. grammins or Rhizoctonia solani), there is no known genetic resistance, and effective use of pre-crops remains important to ensure optimum root performance in subsequent wheat crops.
Residual water and nutrient profiles
Species and cultivar differences
Crop and pasture species differ significantly in rooting patterns and soil resource use, creating differences in residual deep water and N profiles (Thorup-Kristensen, 2006a; Thorup-Kristensen et al., 2009, 2012; Kirkegaard and Ryan, 2014; Angus et al., 2015; Fig. 5A, B). Seasonal conditions also influence the patterns of demand and uptake, and the movement of water and N within the soil profile during the season (Burns, 1984; Lilley and Kirkegaard, 2007, 2011; Pedersen et al., 2009). Despite the variability generated by crop choice and seasonal conditions, some general observations can be made. Most agricultural crops can use the available water and N resources in the topsoil (1·0 m) so residual levels at harvest are low in the upper soil layers but may increase with depth. The duration of crop growth has a significant impact on the depth and extent of resource use, both within and between species (Thorup-Kristensen, 2006b; Zhou et al., 2008). For example, a later-maturing lupin crop dried the soil to a greater extent than earlier-maturing mustard in Australia (Kirkegaard and Ryan, 2014), and longer duration winter wheat has deeper roots and greater sub-soil water and N depletion than spring wheat (Thorup-Kristensen et al., 2009). In general, legumes will deplete soil N less than non-legumes, and the subsequent mineralization of N-rich residues will add to soil N residues after harvest. Deeper-rooted Brassicas generally use more N from depth than cereals, but also often leave higher residual N at the surface due to the mineralization of fallen leaf material and other remaining plant residues (Ryan et al., 2006; Dresboll et al., 2015b). Exceptions may occur where yield was limited by other factors (disease, drought, nutrient deficiencies, partial winter kill).
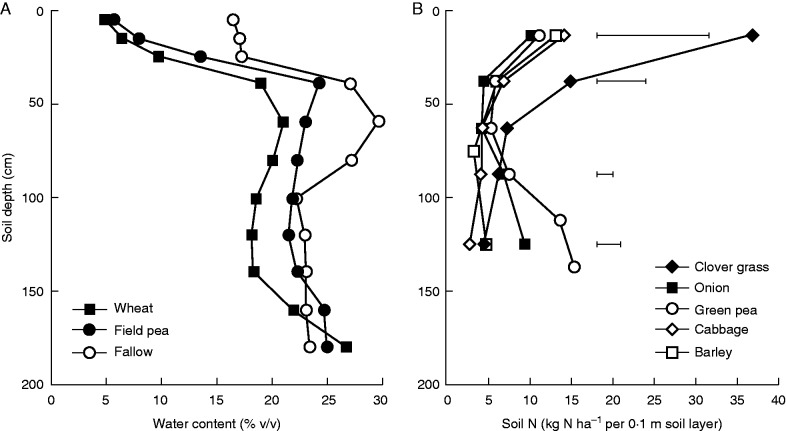
Fig. 5.
Differences in (A) residual water following a range of crops (wheat, field pea and fallow) in southern Australia, and (B) residual mineral nitrogen following diverse crops in Denmark. [A, data reproduced with permission from Angus et al. (2015); B from an 8 year rotation study (K. Thorup-Kristensen, unpubl.)].
Perennial crops/pastures such as lucerne (M. sativa) grown in sequence with annual crops can have extreme impacts on residual soil resources, especially in drier environments. Lucerne can dry out the soil profile to the wilting point to significant depths (2–3 m) which requires significant subsequent rainfall to re-fill (Angus et al., 2001; Sauer et al., 2002). Nitrogen mineralization in the sub-soil is slow, so the legacy of low residual soil water and N can influence subsequent annual crops substantially, as shown by Thorup-Kristensen (2006a) for N, and the termination time of a crop can be critical to avoid crop failure due to low sub-soil water content (Angus et al., 2000).
Management of pre-crops
Several aspects of pre-crop management can influence the residual profiles of water and nutrients. Improved management of legume crops to increase biomass and N fixation will increase the levels of residual N to improve subsequent cereals (Seymour et al., 2012). The management of fertilizer N in the pre-crop can also have a significant influence on the residual N following the pre-crop (Beaudoin et al., 2005; Zhou et al., 2008), and in some cases this can over-ride those of crop type, as shown by Thorup-Kristensen and Sørensen (1999). Under Australian conditions, rotational N effects are likely to be common and significant as excessive N will be preserved in dry seasons and remain in the soil to influence growth significantly (Kirkegaard and Ryan, 2014). In North-Western Europe, the winter season loss of soil N depends on soil type and yearly precipitation surplus, and therefore varies among regions and years (Burns, 1984). In Denmark, N leaching losses occur much faster than in Australia, and pre-crop effects on N availability are less likely to be preserved from one season to the next (Pedersen et al., 2009). The application of high N levels to, for example, vegetable pre-crops can still have a significant influence on the soil-available N for the next crop, though the remaining N will be moved deeper into the sub-soil by the surplus precipitation on the often light textured soils (Thorup-Kristensen, 2006a).
Earlier maturity or termination of the pre-crop will allow more time for soil N and water resources to build up in the soil. In Australia, the clearest example is the need for early termination of perennial legume pastures prior to cropping to increase the availability of soil water and N for subsequent wheat (Angus et al., 2000). Lilley and Kirkegaard (2011) compared the impact of annual vs. perennial pre-crops across a range of seasons using simulation modelling. The study showed that following the lucerne pre-crop, the sub-soil did not re-fill in 76 % of seasons compared with 13 % after annual crops. The availability of deep resources for the next crop was driven by the depth of rewetting. In recent years, driven by the need to control herbicide-resistant grass weeds, early termination of legume cover crops as hay or manures in Australia has been shown to preserve water and N in the sub-soil, significantly improving the growth of subsequent cereals (Hunt et al., 2013).
In Denmark, there is generally less flexibility in harvest time, and the warm and wet conditions following harvest generate high N mineralization rates and increased leaching risk. In contrast to the Australian situation, it may therefore be an advantage to delay pre-crop termination. Such effects have mainly been studied with cover crops (Lahti and Kuikman, 2003), but the same principles apply to main crops where crop termination time is open to management decisions.
Soil structure and soil biology.
The choice of pre-crop can influence soil structural and biological processes, but it is often difficult to isolate and quantify the persistence and impact of these on the growth of subsequent crops. For example, surface soil structure is improved following canola compared with cereals in both Australia (Chan and Heenan, 1996) and Germany (Schönhammer and Fishbeck, 1987), but the effects may be transitory, especially under conventional cultivation. Changes in sub-soil structure may be more persistent, but the review by Cresswell and Kirkegaard (1995) concluded that these effects from annual break crops were either small, not evident or could not be adequately distinguished from other effects of break crops, though Asseng et al. (1998) found evidence of deeper wheat root growth after lupin. Perennial legumes such as lucerne (M. sativa) were able to improve the macro-porosity of the sub-soil layers (McCallum et al., 2004). Persistent influences of this type appear to have limited effects through improved rooting of subsequent crops.
Fate of resources following pre-crops
In a recent review, Angus et al. (2015) concluded that the effects of different pre-crops rarely persisted into a second year under higher yielding wetter regions of northern Europe, but could persist up to 4 years under drier Australian conditions. Under higher rainfall European conditions, the soil profile almost inevitably re-fills during the winter season. The N left in the soil at harvest is at risk of leaching loss during winter, and the retention of N from one season until the next is highly variable (Burns, 1984; Pedersen et al., 2009). Effects persisting into a second year are limited, though examples have been documented (Thorup-Kristensen, 2006a). As a consequence, there has been significant attention paid to managing N residues during the transition period to avoid leaching loss, rather than management to preserve residual soil water and N for the following crop. In the Australian cropping systems, less N fertilizer is applied to crops, and lower spring and summer rainfall means that relatively low levels of residual water and N are normal at harvest. Here the focus is much more on capturing summer rainfall to re-fill the soil profile, and preserving residual water and N for the following crop by protecting the soil surface and controlling summer weeds. Strategies to manage this transition phase are the topic of the following section.
MANAGING THE TRANSITION PHASE
The residual resources left in the soil by the previous crop as described above are subject to various change processes which can include loss or accumulation of available water and N, and a change in their position within the profile. With sufficient surplus precipitation, water and N will move deeper into the soil, making deep rooting especially important for recovery of resources left over from previous crops or accumulated during the transition period itself (Thorup-Kristensen, 2006a; Thorup-Kristensen et al., 2009; Lilley and Kirkegaard, 2011; Gabriel et al., 2012; Fig. 5). The demand for deep roots to recover accumulated deep resources differs from the demands on the root system for using newly added surface resources from rain, irrigation, N mineralization or fertilization in the topsoil.
The processes of resource accumulation, downwards movement and loss are influenced by management of the transition phase. We consider three different management strategies in the transition phase: (1) managing plant growth during the transition (weeds, volunteers or sown cover crops); (2) managing crop residues (removal, incorporation or surface retention); and (3) soil tillage. In practical terms, these are strongly interconnected, as incorporation of residues and cover crops involves soil tillage. We focus here on how these processes can influence accumulation of deep soil resources and influence their uptake by subsequent crop roots.
Managing plants during transition
Plants growing during the transition phase remove water and N from the soil, but the effect of this is complex and depends on the alternative fate of this water or N (Thorup-Kristensen et al., 2003). This is most easily understood with water, as any plant use of water leads to its loss from the system.
Under Danish conditions, high precipitation during the transition phase usually refills the soil and generates losses through leaching or drainage (Fig. 2). Plant water use during the transition phase reduces water loss but rarely the amount present in the soil at the start of the next growing season. In contrast, under Australian conditions, the soil profile is typically not fully recharged during the transition phase (Kirkegaard and Lilley, 2007), and the water used by plants reduces that available for the next crop. Here water uptake by weeds or cover crops occurs in full ‘pre-emptive competition’ with the next crop, and can reduce yield
With N it is more complicated, as the N taken up by weeds or cover crops is not removed from the system, but taken from an available pool of inorganic soil N and bound into organic N compounds in the plants. The N is still in the system, but is unavailable to other plants until it has been mineralized. If it is N which would otherwise have been lost (i.e. by leaching), there is no impact, but if it would have stayed in the soil available for the next crop, the uptake also occurs in pre-emptive competition with the next crop (Thorup-Kristensen, 1993). Usually some re-mineralization of the N will occur, often in the range of 10–50 % of the N taken up (Thorup-Kristensen et al., 2003), which counteracts the pre-emptive competition effect (Fig. 6). When pre-emptive competition is low, i.e. the leaching of N is intense, the N effect of weeds or cover crops may become positive, as they capture, store and re-mineralize N that would otherwise be lost to the system.
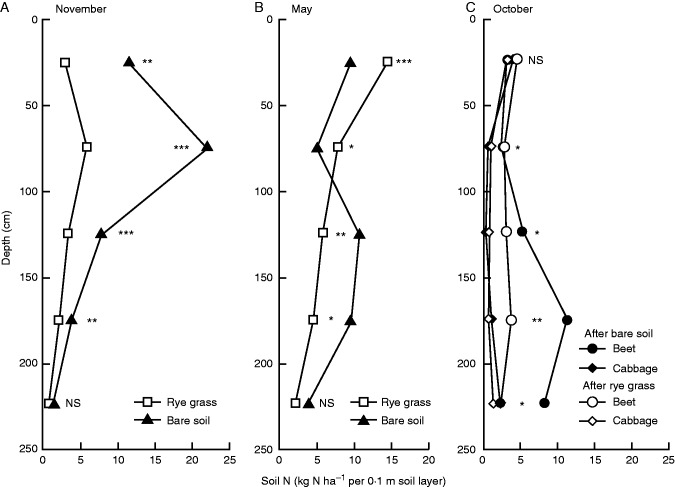
Fig. 6.
Effects of a cover crop on soil N content to 2·5 m depth in a crop sequence study that included subsequent crops with different rooting depth (Thorup-Kristensen, 2006). In the first year, the soil was either left fallow during the autumn or a cover crop was grown. Soil N was measured in the late autumn at the time of cover crop termination (A), and again in the next spring in May (B). In the second year, main crops with different root depth were grown, and soil N was measured again after the harvest of red beet and cabbage (C). The rooting depth of red beet was approx. 1·5 m while the roots of cabbage grew to at least 2·5 m.
Where pre-emptive competition is strong, avoiding weed or cover crop growth during the transition period makes sense, e.g. by strict weed control during summer fallow in Australia. If such competition is low, cover crops can be grown to improve N availability for the next crop, protect against erosion, add organic matter to the soil or provide other advantages. Thorup-Kristensen and Dresboll (2010) found that cover crops increased N supply for the succeeding crop in two out of three years where precipitation was high, but in the third drier year, cover crops strongly reduced N availability for the next crop. Effects of year to year variation in precipitation on N retention on different soil types are shown in the model simulation study by Pedersen et al. (2009).
In intermediate situations, pre-emptive competition is dependent on the rate of loss of resources which would otherwise happen before the next crop can use it. Uptake of the resources during the early transition phase or from larger soil depths will cause less pre-emptive competition than uptake happening later or from upper soil layers (Thorup-Kristensen et al., 2003). This allows farmers to optimize the effects. Termination time is an important management tool, and choosing cover crops with fast, deep rooting and a short growing season will be optimal in such situations (Thorup-Kristensen and Dresboll, 2010; Alonso-Ayuso et al., 2014). Termination of weed or cover crop growth may be made with mechanical treatments, herbicides (Sanderson et al., 1999) or sometimes using natural winter kill off. Pre-emptive competition is often stronger before deep-rooted crops than before shallow-rooted crops (Fig. 6; Thorup-Kristensen, 2006a), as much more of the resources are leached below the rooting zone of a shallow-rooted crop than below a deep-rooted crop (Thorup-Kristensen, 2006a, b). Cover crops should therefore preferentially be grown before shallow-rooted crops in the rotation.
The depth distribution of water and N is also influenced by management during the transition phase. If water is used during the transition phase, it will not reach as deep into the soil (Gabriel et al., 2012), an effect even stronger for N due to uptake and N mineralization from residues in the uppermost soil layers. It has been repeatedly shown that after cover crops, sub-soil N availability is reduced while topsoil N availability is increased (Thorup-Kristensen, 2006a; Thorup-Kristensen et al., 2009). Rotations including cover crops generally contain less sub-soil N (Beaudoin et al., 2005), and Thorup-Kristensen et al. (2012) found that systematic use of cover crops reduced sub-soil N content (1–2·5 m depth) by 70 % across the rotation. Thus cover crops or weeds can reduce the need for deep rooting in the succeeding crop and may actually directly reduce the root development of crops in deeper soil layers (Kirkegaard and Lilley, 2007; Kristensen and Thorup-Kristensen, 2007).
Under Australian conditions, the focus during the transition phase is generally to maximize water storage during the summer fallow period to re-fill the soil profile. In southern Australia, the summer period between winter crops is rarely sown to crops due to insufficient rainfall and high temperatures. Until recently, careful management of the transition period in southern Australia was not a focus as it was assumed that growing season rainfall was sufficient to grow the crops. Sheep were usually grazed on weedy summer fallows, but recent studies have shown significant benefits for water and N accumulation, and yield in subsequent crops, by strict weed control in the summer fallow period (Hunt and Kirkegaard, 2011; Kirkegaard et al., 2014). When such strategies are successful and increase the soil water storage, water will move deeper into the soil, making deeper rooting of the next crop more important and valuable (Lilley and Kirkegaard, 2011; McMaster et al., 2015; Fig. 7).
Fig. 7.
The effect of the level of weed control during the summer fallow transition phase in southern Australia on the profiles of (A) water and (B) mineral N at the sowing of the subsequent crop. Weeds reduced both water and N content to significant depth in the soil profile (McMaster et al., 2015).
Summer weeds can also host root, crown and stubble-borne diseases, acting as a ‘green bridge’ to maintain or increase disease inoculum or pest populations during the transition phase. This has the same effects as poor pre-crop choices by increasing root diseases, with all the same consequences for deep resource use as described in the previous section.
While the transition phase before winter wheat under Danish conditions is in principle very short (approx. 1 month, Fig. 1), the reality is somewhat different. The winter wheat grows very little during autumn and winter (September to March) and has little effect on soil water and N dynamics compared with a fallow field. Soil water accumulates and N moves as occurs during the transition phase (Barraclough and Leigh, 1984; Addiscott and Darby, 1991; Thorup-Kristensen et al., 2009; Rasmussen et al., 2015). The main difference compared with the spring cereals is that winter wheat develops its root system in the autumn and it does not die back. It continues to develop a much deeper root system and utilizes much more of the deep N leached during autumn and winter than a spring cereal (Thorup-Kristensen et al., 2009; Rasmussen and Thorup-Kristensen, 2016).
The chance to grow cover crops before winter wheat is quite limited by available time under Danish conditions, while before spring wheat or other spring-sown cereals, efficient cover crops can be grown (Larsson et al., 2005; Thorup-Kristensen et al., 2009; Fig. 1). Before winter wheat, short-term cover crops may be grown in the 4–6 week period (Thomsen and Hansen, 2014), but their effect is likely to be low.
Tillage and residue management during the transition
Crop residues can be incorporated into the soil, left on the soil surface or removed from the field (Myrbeck et al., 2012), and this will influence both the infiltration and evaporation of soil water. Soil water movement will also affect N leaching through the profile. Soil N will be influenced indirectly through soil moisture levels influencing mineralization and by the removal of crop residues which might either have released N by mineralization or caused net N immobilization (Hansen et al., 2015). In Australia, crop residues are mostly left intact on the surface under no-till systems to protect the soil and improve water capture and storage, and weeds are controlled with herbicides to maximize water and N accumulation.
In the Danish context, tillage and residue management will mostly influence soil N dynamics because the soil water profiles will generally re-fill. Most crop residues such as cereal straw have low N content and a high C/N ratio, so the amount of N left with the residues is typically low, and their initial effect will be to immobilize N (Hansen et al., 2015). Under leaching conditions, this may reduce the amount of N lost or leached deeper into the soil. In some crops, such as ley crops, potatoes and vegetables, the N content in the crop residue is high and mineralized N is at high risk of subsequent leaching depending on timing, soil type and precipitation regime (Pedersen et al., 2009). In such situations, both cover crops and growing deep-rooted crops in the following year are options to reduce leaching and recover some of the N leached deep in the soil (Dresboll and Thorup-Kristensen, 2014). Delaying or omitting incorporation is a strategy that has been implemented in environmental regulation in both Denmark and Sweden (Larsson et al., 2005; Kronvang et al., 2008) but the effect of this on soil N dynamics as compared with early straw incorporation are not well understood (Munoz-Romero et al., 2010; Myrbeck et al., 2012; Hansen et al., 2015).
Australian farmers generally spray weeds during the transition phase, as there is little risk of leaching, and N use by weeds therefore occurs in direct pre-emptive competition with subsequent crops (Kirkegaard et al., 2014).
IN-CROP PHASE
The previous sections have detailed how the management of preceding crops and the transition phase can significantly influence the water and N resources available to subsequent crops at the time of sowing, and its depth distribution in the soil. In this section we consider how management of the crop itself interacts with previous management and can be manipulated to influence the access and uptake of soil water and N by the root systems.
The availability of water and N resources in the sub-soil
In dry environments such as Australia, depth of re-wetting at sowing can be a key factor determining maximum rooting depth of wheat (Kirkegaard and Lilley, 2007). In addition, many Australian soils are constrained by various chemical or physical conditions such as acidity, salinity or gravel layers that may limit maximum root depth to < 1 m (McDonald et al., 2012). The frequency with which deep water is likely to be available for crops, and hence the opportunity to benefit from deeper roots, varies markedly across sites and from season to season (Lilley and Kirkegaard, 2007, 2011, 2016). The subsequent in-season winter rainfall is typically below the potential evapotranspiration, so that soil profiles may often not re-fill prior to spring. As a consequence, water stored in the transition period is critical for crop growth, with an average of approx. 50 mm representing a 33 % contribution to yield (3–72 %) depending on soil type and season (Hunt and Kirkegaard, 2011). Wheat yields are in the range of 2–4 t ha–1, and stored sub-soil water is then worth on average 1 t ha–1.
In contrast, in Denmark, the growing season rainfall will exceed evapotranspiration significantly until early spring, typically until March, and re-wetting the soil is not a problem. The re-wetting thus occurs in what is the early growing season of winter wheat, and the transition phase before spring cereals. When the main growing period starts in the spring, Danish soil profiles are usually at field capacity, and the amount of available water in deeper soil layers varies from 4–7 vol% on sandy soils to 15–20 vol% on sandy loam soils (Jacobsen, 1989). On tile-drained sandy loam soils, sub-soil water content may be well above field capacity at the start of the growing season, potentially offering an even larger supply of soil water to crops. On a very sandy soil, increasing the effective rooting depth by 10 cm may add 5 mm of extra available water (worth <2 d of crop transpiration), whereas on sandy loam soils it may add >15 mm of available water (5 d of crop transpiration). As the sandy loam soils are generally considered more conductive to root growth, the potential to access extra water by increasing rooting depth on such soils is high. Summer rainfall is significant in Denmark, but quite variable and well below crop demand. Thus, in most of Denmark, wheat crops rely on water stored in the soil for significant periods, and deep water can be highly valuable for the crop growth and yield. Compared with Australia, a larger fraction of the crop water use is generated by rain falling during crop growth, and average wheat yields are in the range of 6–11 Mg ha–1.
In both countries, the soil N content is typically highest at the surface and declines with depth when crops are sown. As discussed above, the level of sub-soil N is affected by many factors, but fertilizers and crop residues are repeatedly added to the surface soil where soil organic matter levels are highest and where most N mineralization thus occurs. In deeper layers, N is primarily added through leaching from above, and typically the water moving downwards will not carry very high N levels in arable rotations. Exceptions occur, if late season mineralization from crop residues or soil organic matter is high, e.g. when following ley crops (Hansen et al., 2007), horticultural crops (Rahn et al., 2003) or even legumes in the rotation (Thorup-Kristensen et al., 2012).
Rasmussen et al. (2015) found that soil N levels in spring were generally low, in the range of 1–5 kg N ha–1 per 10 cm soil layer, while others have found slightly higher values; for example, Beaudoin et al. (2005) found levels between 5 and 10 kg N ha–1. In both Denmark and Australia, the level of N available to wheat within deeper layers has been found to vary between 1 and 15 kg N ha–1 per 10 cm soil (Thorup-Kristensen et al., 2009; Kirkegaard et al., 2015). In the high soil N examples, between 100 and 200 kg of N or more per hectare is found in the sub-soil below 0·50 m depth. Utilizing this N increases crop yield or quality and reduces N leaching. However, even with the low levels of 1–5 kg N ha–1 per 10 cm mentioned above, the total content between 0·5 and 2 m depth will be in the range of 15–75 kg ha–1, which is significant compared with the amounts available in the topsoil.
Under Australian conditions, N levels at sowing are generally only high after legume pre-crops and pastures, and wet transition periods which facilitate N mineralization in the absence of crop uptake (Kirkegaard and Ryan, 2014; Angus et al., 2015). Losses of N through leaching are low in highly productive cropping systems, even in circumstances where water drains below the root zone (Smith et al., 2000). However, a few studies have shown that N can accumulate in a ‘bulge’ at 40–80 cm in the soil profile on clay loam soils and can leach below 1·5 m on coarse-textured soils in higher rainfall areas (Lilley et al., 2004). Typically mineral N levels below 1 m are equivalent to 1–5 kg ha–1 per 10 cm soil (Angus et al., 1998, 2006; Kirkegaard and Ryan, 2014) and there is little risk of further leaching as N applications are generally low and delayed until crop stem elongation, when roots have reached considerable depth. Continuous cereal crop sequences lead to low levels of sub-soil N, as pasture residues are exhausted, and late, surface-applied N fertilizer is not subject to loss.
Accessing deep resources effectively
The usual pattern of wheat rooting depth and density has been studied extensively. During the early stage of seedling development, the plant may be allocating approx. 50 % of assimilate to root growth, which reduces to approx. 5 % after anthesis, when the developing grains become the dominant sink (Gregory and Atwell, 1991; Ford et al., 2006). Studies in field soils around the world have shown that after the initial establishment period, a near-linear pattern of root depth penetration persists up to just after anthesis when root depth penetration ceases (Gregory et al., 1978; Kirkegaard and Lilley, 2007; Thorup-Kristensen et al., 2009). As the roots enter a new layer, root length density increases in a sigmoidal pattern within that layer as the root front extends to deeper layers. The seminal roots, especially the root axes emerging from the seed, are thought to be the most important for accessing deep soil resources (Watt et al., 2008). Nodal or ‘secondary’ roots emerging from tillers or leaf nodes on the main stem emerge later, and they are generally thought to be restricted to the upper soil layers (1·0 m) because of the delay in their emergence and growth. The deepest wheat roots generally consist of main seminal axes and first- and second-order branches (Watt et al., 2008).
In considering the access to deep water and N by roots, it is helpful to consider the soil–root interactions in three conceptual layers (Fig. 8): zone 1, an upper layer with high root density for most of the season where virtually all the available water and N can be taken up; zone 3, a deep soil layer with no roots but with available resources that are not exploited by the crop; and zone 2, an intermediate layer where root density is low, declines with depth, and in which the roots of annual crops are present only during the late part of the crop growth period. The late arrival of roots in zone 2 and their limited time of activity lead to only partial use of water and N resources, even in the face of shoot demand. Growing deeper roots extends the lower boundaries between the three layers deeper into the soil (Fig. 8), while increasing the density or efficiency of the deep roots helps by increasing the use of resources from zone 2, as illustrated for water in the study of Lilley and Kirkegaard (2011). In some cases, the boundaries of these conceptual layers may differ for water and N. For example, some results indicate that the upper layer where all the available resources may be used by the crop is deeper when considering N than when considering water.
Fig. 8.
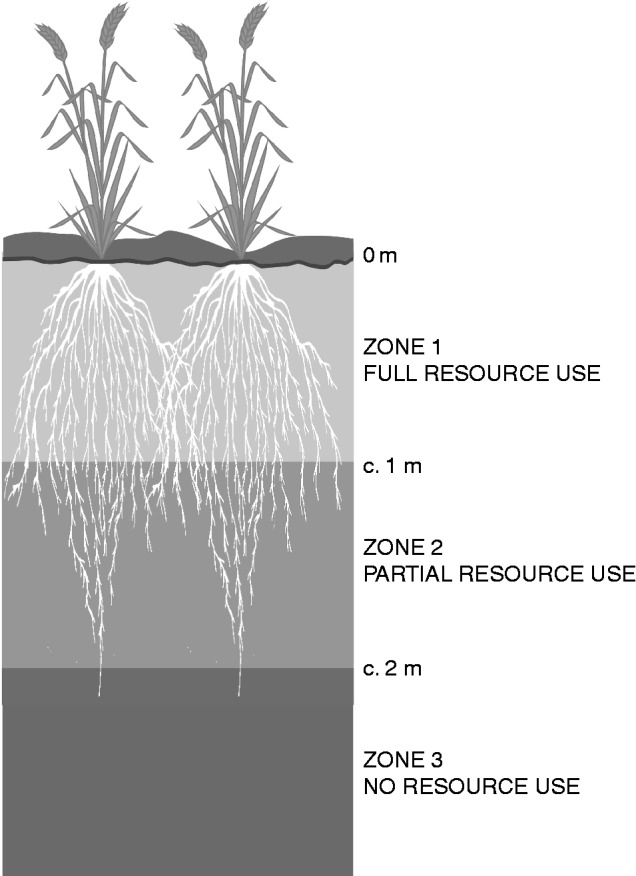
Schematic diagram illustrating zones of root exploitation by wheat crops. Zone 1 is the upper part of the root system where the root system is able to use all available water and N. The roots develop in this zone during early growth, and develop a high final root density there. Zone 2 is the zone where roots can use some but not all of the available water and N. The crop develops roots in zone 2, but they appear later and develop a lower final density. Zone 3 is the soil below the root zone, and there is no uptake of water and N.
Thus currently, deeper rooting varieties benefit mostly from access to new resources late in the season in zone 2 when demand is high. Under leaching conditions, earlier uptake may be important too, as uptake from zones 1 and 2 may restrict the subsequent leaching loss to N to zone 3 below the root zone. Differences in soil N uptake due to differences in rooting depth are obvious in the cover crop study of Thorup-Kristensen (2001) where the top 0·5 m soil layer was clearly zone 1, roots were not limiting N uptake, while the 0·5–1·5 m depth was zone 2, showing a strong effect of root growth on N uptake from this layer.
In zone 2, where we aim to get more roots, the uptake of resources is incomplete due to limited time, low root length density and uneven root distribution. Under such conditions, root uptake rates (unit uptake per metre of root per second) become a further parameter for improvement, in contrast to the situation in zone 1 where roots are active for a longer period, and resources can be effectively used even if uptake rates are lower.
The resource uptake rate of the crop roots will depend on total crop demand for the resource. If the crop is already well supplied with N, or there is still a high N availability in the topsoil, the uptake will be determined by crop growth rate, as uptake will not exceed shoot demand (Gastal and Lemaire, 2002). In this case, the uptake activity per unit root length will be reduced (Lemaire et al., 2007), leading to lower total N uptake than the basic potential of the root system. In zone 2, this will result in reduced uptake of the resources available there (Pedersen et al., 2010). As a result, adding extra N to the topsoil can often increase the amount of N left in the sub-soil to a greater extent than that left in the topsoil [see the example in the Danish case study below and in Thorup-Kristensen (1993)]. Any factors reducing yield potential (diseases, poor establishment, etc.) can limit crop sink strength at late growth stages and reduce the demand and utilization of resources from zone 2. By optimizing the availability of water and N simultaneously, it may be possible to increase the use of deep resources, e.g. by adding N to maintain green leaf area to increase deep water use.
Factors influencing deep root growth
Genotype selection for improved water and N use.
Currently there is a lot of interest in the opportunity to select crop genotypes with deeper and more effective root systems to improve water and N use in both water-limited environments (Palta et al., 2011; Wasson et al., 2012) and higher rainfall environments (Semenov et al., 2009; Lynch, 2013; Carvalho et al., 2014; Ober et al., 2014). In Australia, Kirkegaard and Lilley (2007) reviewed data on rooting depth in wheat and found little evidence for genotypic variation in commercial varieties used up to that time. They argued that the root depth penetration rate was a more useful selection parameter than rooting depth, as it integrated the impacts of effects such as soil conditions, and corrects for the fact that later maturation in itself leads to deeper rooting. The important confounding factors were the depth of soil wetting, length of the vegetative phase and soil type, and the depth penetration rate ranged from 1·0 to 1·2 cm d–1. The maximum rates recorded on Australian soils were remarkably similar to the rates found in Denmark by Thorup-Kristensen et al. (2009) for winter and spring wheat. In most cases, the root system characteristics thought useful for water extraction are also thought useful for N extraction (Lynch, 2013), as N is also easily mobile in the soil, through both diffusion and mass flow.
Prospects for genetic improvements to the root penetration rate of wheat are under investigation and have been discussed in recent papers (Palta et al., 2011; Wasson et al., 2012), and some evidence for varietal differences has been suggested (Wasson et al., 2012, 2014; Ytting et al., 2014; Rasmussen et al., 2015), though these are yet to be adequately separated from influences of flowering time and length of vegetative stage. A study from Western Australia showed a significant interaction between soil conditions and genotypes for deep rooting, indicating that it may be difficult to breed genotypes with a general ability to form deeper root systems (Acuna and Wade, 2012). The traits of interest include increased depth penetration rate, less surface branching and increased deep branching, root angle and cost of root tissue formation (Lynch, 2013), and these traits may be under separate genetic control. Currently these factors are being studied in many places around the world, various attempts to develop high-throughput screening facilities are being developed, and genetic markers for traits of interest are identified. Most of these studies are being carried out on very young plants, often grown in artificial media to simplify phenotyping (Hund et al., 2009; Richard et al., 2015). While this allows efficient phenotyping, it leaves an uncertainty about how this relates to root growth until maturity of crop stands growing in field soil. A few attempts have been made to link lab results to field performance (Wasson et al., 2012), as there is a general need to strengthen the links between lab-based basic studies and their application for solving real-life problems (Dresboll et al., 2015a).
While studies have shown that deeper rooting genotypes improve sub-soil water use, this has not been clearly shown for N. In the results of Rasmussen et al. (2015), the winter wheat genotype ‘Hereford’ grew deeper roots than other genotypes, and it was related to more efficient use of sub-soil N. To test this relationship more thoroughly, we need more knowledge of specific cultivar differences in rooting depth, to conduct experiments where N fertilization is kept at moderate levels and where sub-soil N levels are high enough to allow good detection of differences in its uptake. A previous study on silage maize in Germany showed correlations between rooting depth and sub-soil N levels at harvest time (Wiesler and Horst, 1994) but, again in this study, it was not easy to disentangle the effects of root growth from differences in phenology.
The impact of soil N concentration on root growth independent of overall crop N supply is of interest as nutrient ‘patches’ are known to influence root branching. If low sub-soil N availability reduces root growth and branching there, it may affect the ability of the root system to use water and other nutrients from the deeper layers. Most studies have been conducted using very high concentrations and young plants in artificial systems. Cover crop experiments in the field, where cover crops leave the sub-soil with low N content, have demonstrated that roots of crop plants can proliferate when they encounter high levels of residual N remaining in the deep soil layers (Kristensen and Thorup-Kristensen, 2007; Weligama et al., 2010a), but also that rooting depth may be reduced if they encounter deep layers with low N concentrations. Assuming a crop is adequately supplied with N to satisfy healthy root and shoot growth, species or varietal differences in response to deep soil N concentrations are of interest (NK Ytting, unpubl. res.). The evidence that species or genotypes differ in such a response is limited, but is important especially if a minimum or threshold external N availability is required for branching and if there are varietal differences in this threshold.
Management interactions
Deep root growth and effective functioning of the deepest roots can be significantly manipulated with various in-crop management strategies (Fig. 1).
Sowing time.
Early sowing of genotypes with appropriate phenological response to specific environments will maximize the time for downward root growth during the vegetative period and it is one of the most effective management factors influencing deep root growth and function. As the downward root growth period extends from sowing to anthesis, longer vegetative periods generally increase rooting depth on deep soils (Barraclough and Leigh, 1984; Vincent and Gregory, 1989; Kirkegaard and Lilley, 2007; Rasmussen et al., 2015). Indeed, Kirkegaard and Hunt (2010) emphasized the importance of early sowing in Australia to capture the benefits of water and N resources stored by improved pre-crop and fallow management.
In most cases, in Denmark and Australia, the uptake of N by wheat in autumn is low, limiting the significance of differences in rooting depth during this early growth period. However, the deeper rooting achieved by earlier sowing continues into the main growth season, allowing more deep water and N uptake which can significantly improve yield in both environments and reduce leaching potential in the Danish environment. In the study by Rasmussen and Thorup-Kristensen (2016), it was found that increases in root depth achieved by 2–4 weeks earlier sowing in the autumn were maintained even until harvest where early sowing led to approx. 0·3 and 0·6 m deeper final rooting depth.
In Denmark and other European systems, earlier sowing also improves yield potential as well as reducing the risk of N leaching (Rasmussen and Thorup-Kristensen, 2016), and has large effects compared with the potential effects of breeding improved genotypes (Dresboll and Thorup-Kristensen, 2014). It is critical to note that in both systems other agronomic adjustments will often be required to avoid some of the associated risks of early sowing including increased weed growth, excessive above-ground biomass production and the increased risk of root and foliar disease. Recent availability of resistant varieties, seed and foliar fungicides and a range of pre- and post-emergent herbicides help make some of these issues manageable (Ishikawa et al., 2012; Kirkegaard et al., 2014).
Plant density and spatial arrangement.
The spatial arrangement of crops which includes inter-row spacing, intra-row spacing and the spatial arrangement can also influence the pattern of root growth in the soil, although the above-ground interactions are an important consideration for cereals in this regard (Olsen et al., 2012; Qin et al., 2013). Higher plant density gives rise to a larger number of early initiated seminal roots having more time to grow deep (Dai et al., 2014). This was also seen in Australian spring wheat systems; the seminal roots are often the deepest, and a recent study showed that higher seed density did increase the density of roots at depth (J. Kirkegaard, pers. comm.). Finally, deeper rooting may arise from the earlier onset of ‘kin’ interactions (Mercer and Eppley, 2014) that can influence the tendency of roots from the same genotype to grow away from each other and force roots to grow downwards in a dense stand. The value of deeper rooting due to higher plant density should be weighed against extra seed cost and potential disadvantages in the above-ground plant stand.
Crop nutrition.
Plant nutrient management can affect crop use of soil water and nutrients both if the supply become too high and if nutrient deficits occur. Crops that are deficient in one or more essential nutrients and have restricted overall growth may obviously have limits to root growth. We do not consider deficiencies here but focus on how the management of crop nutrition for generally well-managed crops influences the pattern of growth and function of roots into deeper soil layers.
When nutrient availability approaches the uptake capacity of the crop, it will reduce the ability of the crop to take up all that is available, and more will be left in the soil (Beaudoin et al., 2005; Rasmussen et al., 2015), not because of limitations to the root system, but because the uptake capacity of the shoot is not high enough (Gastal and Lemaire, 2002; Lemaire et al., 2008). This situation will not only affect the uptake of the excess fertilizer added, but will also reduce uptake of the available soil N pool, including N uptake from deep soil layers (Thorup-Kristensen and Sørensen, 1999; Pedersen et al., 2010).
A range of methods exist to help farmers adjust their N fertilization to the amount of N already available in the soil, based on measuring plant N content (Lukina et al., 2001; Le Bail et al., 2005; Sripada et al., 2006), soil N content (Liu et al., 2003; Chen et al., 2006) or predicting the availability based on pre-crops and recent weather, using model simulation or choice diagrams as in the English N advice in the Fertiliser Manual RB209 (Department of Environment, Food and Rural Affairs, 2011). Using such methods to try to avoid excessive N supply will help the crops use sub-soil N resources, and should be employed whenever relevant. Results so far show little effect of N fertilizer levels on rooting depth for various crops (Barraclough et al., 1989; Thorup-Kristensen and Van den Boogaard, 1998), though total root growth can be significantly affected. It was found by Rasmussen et al. (2015) that root density but not rooting depth of winter wheat was reduced at low N supply.
One important limitation is that while sub-soil N content may vary strongly, N fertilization of crops is not reduced accordingly. Sub-soil N can vary more strongly than deep soil water availability (Sauer et al., 2002; Thorup-Kristensen et al., 2012), but it is also more difficult to predict, as more processes are involved in the N dynamics. Direct measurement is expensive as it requires deep soil sampling and, though we can to some extent predict it with model simulation, the predictions of deep soil N are not well validated by measured data. Understanding and predicting deep soil N, and using it in fertilization and rotation planning could offer significant improvements in cropping system N use and in reduction in N leaching loss in the future.
A special but important case here is lack of water, which will reduce the ability of crops to take up N. Uptake will be limited due to reduced crop growth and thereby N uptake potential, and also by soil layers being or becoming too dry for efficient N uptake by crop roots (Kirkegaard and Lilley, 2007; Dijkstra and Cheng, 2008).
Leaf and root disease management.
Healthy, well-managed crops grow larger root systems, have higher demand for water and nutrients and can therefore remove more resources from the soil (Fig. 3). The demand-driven uptake of resources by healthy crops was discussed earlier in relation to crop rotation (pre-crop section), and in-crop management using disease-resistant varieties, and fungicides will also have a significant impact on crop health and resource use (Whish et al., 2014). Leaf diseases appearing later during crop growth can reduce the demand for water and nutrients later in the season (Ford et al., 2006).
Dynamic nature of water and N
The effects of genotype and management on root growth and sub-soil resource use discussed above play out in a dynamic environment. Water is coming and going with rainfall events, and N is both mineralizing at the top of the profile and moving down with excess water through the profile. The synchrony between the root growth and branching through the profile and the movement of water and N in the profile is critical. In Australian dryland systems, this leads to a distinction in the types of roots systems suggested for wheat in Mediterranean climates where rainfall is in season, and those where stored soil water makes up a large proportion of the water availability to the crop (Palta et al., 2011; Kirkegaard et al., 2014). The latter implies a more static water and N environment in which crops must ‘meter-out’ water and N supply to ensure enough is available for critical periods and the risk of loss is low, whereas the former represents a situation where the capture of resources before they are lost below the root zone may be more important. The high-yielding wheat crops grown under Danish conditions represent an intermediate situation. To achieve the high yields, it is necessary that the crops can use stored water in the soil as well as water added by rainfall during the main crop growth period.
Value of deep rooting and resources for yield formation
When considering the possibilities for recovering deep water and N resources, we need to realize that their value may differ from the value of similar resources assimilated closer to the soil surface. Uptake from the deepest layers occurs late in the season, i.e. for wheat, it will occur during the grain-filling phase.
The value of deep resources, and therefore of improved rooting depth, interacts significantly with seasonal conditions and with crop management. For example in Australia, the value of deeper rooting in the 1·3–1·7 m layer for wheat was estimated to be on average 33 kg grain ha–1 mm–1 of extra water accessed, but this ranged from 0 to 104 kg ha–1 mm–1 across seasons. The variation arose because of interactions between crop yield potential, weather and use of sub-soil water in each of the years (Kirkegaard and Lilley, 2007; Lilley and Kirkegaard, 2007, 2011). Thus, counter-intuitively, deep resources were often of more value in good seasons and on good soils. In those studies, no account was taken of the additional N that may be available. In high-yielding environments in Australia, and especially in Denmark, the additional N along with deeper water may contribute to maintenance of green leaf area, to larger grain size or to higher grain protein.
Other evidence for the value of deep nutrients comes from studies on deep placement of chemical and biological fertilizers into sub-soils which have been demonstrated to have a high impact on crop yield in high rainfall environments in Australia. The impacts were greater than those of similar nutrients applied on the surface at the same time (Weligama et al., 2010b; Gill et al., 2012). Here deep placement of organic amendments had benefits that persisted over several seasons, similar to other examples where sodic sub-soils were ameliorated with gypsum (Western Australia). Such effects may be very durable, a fact which is exploited in archaeology. In dry seasons, improved crop growth can be observed where people have been digging for buildings or other purposes thousands of years ago (http://sciencenordic.com/denmark%E2%80%99s-past-viewed-above), as the addition of organic matter even to moderate depths still clearly improves the water availability for plants growing at such spots today.
Under high rainfall conditions, uptake from deep layers may be masked by continual replenishment by percolation from upper layers, but where uptake has been observed it also contributes to higher yield and protein (King et al., 2003; Semenov et al., 2009). Similar benefits have been demonstrated in maize (Hammer et al., 2009), though much of this has been modelled and small improvements in N or water availability that may occur each day to maintain high photosynthesis may not be picked up in daily time-step crop growth models.
CASE STUDIES OF SYSTEM CONTEXT
Few studies have captured the dynamics of water and N utilization through a sequence of crops to consider how different pre- and in-crop management strategies can interact to influence the uptake of deep soil water and N resources by crops. We present two case studies, one involving N management in Danish systems and the second focused on efficient water use in Australian systems. The Australian case study focuses on wheat and its interaction with soil water. The Danish study focuses on the fate of soil N residues over three crop seasons in a leaching environment. Though the study involves vegetable crops rather than wheat, it clearly illustrates the principles related to surplus N, its movement and availability in deeper soil layers and interaction with crop management and root growth through a crop sequence. It serves to highlight key points about the dynamics of N and its fate in a rotation under Danish soil and weather conditions.
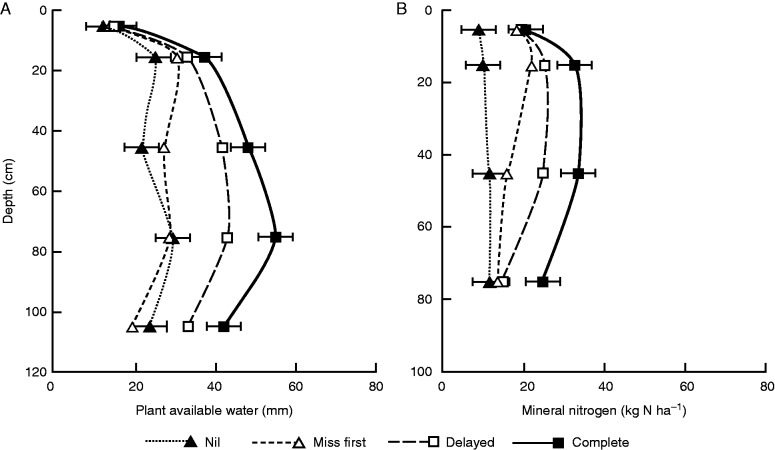
Value of deep roots for improved water uptake in Australian wheat-growing systems: relative effects and interactions of modified root genotypes and management
A series of experiments (Kirkegaard et al., 2007) and related simulation papers (Lilley and Kirkegaard, 2007, 2011, 2016) have investigated the value of deep roots for wheat crops in southern Australia, including the relative and interactive effects of different sites, soils and management scenarios. A summary of this work provides useful insights into the importance of the farming systems context when assessing the value of deep roots (Fig. 9). Kirkegaard et al. (2007) showed experimentally that deep water was used very efficiently by crops to increase grain yield by approx. 60 kg ha–1 mm–1. The subsequent simulation analyses of Lilley and Kirkegaard (2007, 2011) reported mean potential yield benefits of 0·3 – 0·4 t ha–1 from faster growing and more efficient roots across five deep-soil sites in Australia, although there was considerable seasonal and site variation (range in benefits –0·1 to 1·4 t ha–1). The studies also showed that the mean yield impacts of management (pre-crop, transition phase and in-crop) often exceeded those of root modification by influencing the depth of profile wetting and duration of root descent. For example, the individual effects of preceding management (e.g. 0·42 t ha–1 less after lucerne compared with cereal), weed control in the transition phase (0·65 t ha–1 less if weeds were not controlled) and earlier sowing (0·63 t ha–1 less for later sowing) all exceeded the potential 0·3 – 0·4 t ha–1 benefit of modified roots (see Fig. 9). Furthermore, significant interactions occurred. For example, the predicted benefit of genetically improved wheat roots shown in Fig. 9 of 0·36 t ha–1 in wheat following wheat dropped to 0·10 t ha–1 following lucerne, as the soil re-wet below 1 m less frequently. For later-sown crops, the benefit dropped from 0·36 to 0·28 as a result of the reduced depth of rooting and lower yield potential. In a separate study by Lilley and Kirkegaard (2007), uncontrolled summer weeds reduced the predicted benefits of deeper roots from 0·5 to 0·3 t ha–1, again due to incomplete wetting of the soil profile. In a more recent study (Lilley and Kirkegaard, 2016), these legacy effects of the modified roots themselves were investigated in continuous crop simulations to account for the fact that water used by the improved roots in one season would also leave the soil drier and may not be available in the following season when the profile does not re-fill. Indeed the predicted yield benefits of more extensive roots were reduced from 0·3–0·4 t ha–1 to 0 – 0·3 t ha–1, and were more variable compared with the usual annual re-setting due to drier soils (9–31 mm drier at sowing). In the same study on shallow soils (approx. 1 m) there was no mean yield benefit of more extensive roots, and yield benefits of > 0·2 t ha–1 were rare (2–9 % of years) compared with deeper soils (1·6 to 2·5 m) where yield benefits occurred in 10–50 % of years.
Fig. 9.
Schematic diagram showing the impact of different management options during the pre-crop (lucerne vs. cereal), transition (weed control vs. no control) and current crop (late vs. early sowing) phases on the water availability and yield of the subsequent wheat crop in Australia. The magnitude of these management effects (mean) are larger than the mean benefit from a modified deeper wheat root system when grown after cereal, with weed control and sown early (0·36 t ha–1). The data are derived from the experimental and simulation studies of Kirkegaard et al. (2007) and Lilley and Kirkegaard (2007, 2011) for wheat growing on red loam soils in southern NSW, Australia. The darker the colour in the diagram, the more residual water in the soil.
Thus the value of deeper roots for improved water access and yield in Australian wheat crops was highly dependent on the farming systems context, including soil type, the type of pre-crop, fallow management and sowing date. All can have a significant and interactive effect on the availability of soil water for crop growth, rooting depth and the value of modified root traits.
Danish 3 year study of the fate of fertilizer N applied to vegetable crops
A study was conducted to follow the fate of fertilizer N added to crops and its availability to succeeding crops over 3 years.
In the first year of the study (2003), cauliflower was grown, a crop with a high fertilizer N demand and a low N harvest index so that significant amounts of N were left in the soil at harvest. Fertilizer was applied at rates from 0 to 390 kg N ha–1 to establish differences in soil N profiles that were followed through the next two seasons. The cauliflower also had two different harvest times in order to study the significance of harvest time (i.e. the effective length of transition phase) on the fate of the N left in the system.
In the first year, soil N was measured to 2·5 m depth to quantify the profiles of soil N left in the soil immediately after the two harvest dates in early August and in late October (Fig. 10A). The measurements were repeated over the winter season (December and March), and again in May 2004 (Fig. 10B), just prior to the establishment of the subsequent crop. More N was left in the soil after cauliflower with high N fertilization, but only in the top 1 m of the soil (Fig. 10A). The following spring, the effect of cauliflower N application was still evident, mainly below 1 m depth after the early cauliflower harvest; however, after the late cauliflower harvest, differences were evident in the upper soil layers as well (Fig. 10B).
Fig. 10.
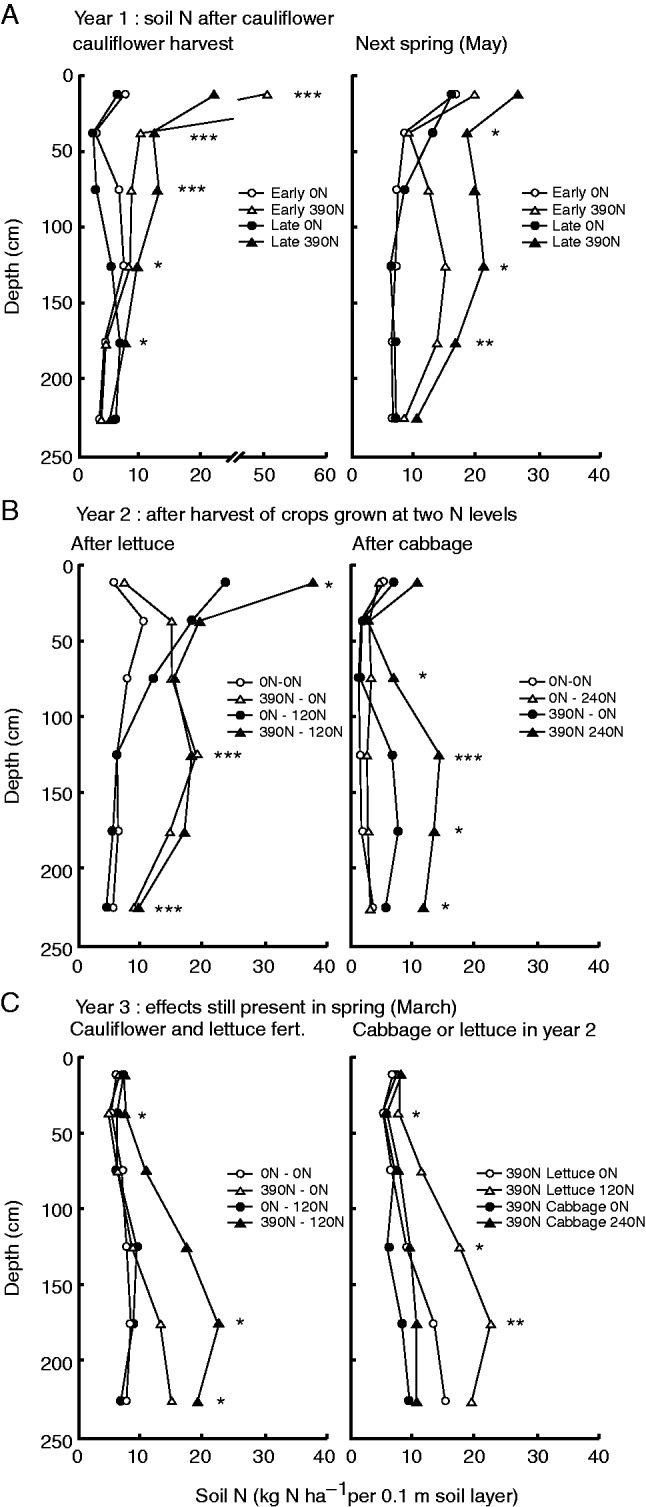
The six figures show selected data from a study made during 2003–2005 in Denmark in order to follow the carry-over of N across seasons in a cropping system. The study included variation in harvest time and duration of the transition phase, in crop N fertilization rates and in crop species with different rooting depths. The experiment was part of a crop N modelling project, and has been used in the validation of the EU-Rotate_N model (Rahn et al., 2010). Detailed explanation of the data shown in the figure is given in the description of the Danish case study on N in the text.
In year 2 (2004), we grew crops with different root growth patterns with either no fertilizer N, or at a normal fertilizer N rate to study the effects of these management changes on the assimilation of the residual N pool. Deep-rooted cabbage (>2 m depth; Thorup-Kristensen, 2006a) and shallow-rooted lettuce (approx. 0·5 m depth; Thorup-Kristensen, 2006b) were chosen for comparison. The soil N data measured at harvest after shallow-rooted lettuce (Fig. 10C) and deep-rooted cabbage (Fig. 10D) harvest include combinations of lettuce and cabbage grown after late-harvested cauliflower fertilized with 0 or 390 kg N ha–1. The lettuce itself was fertilized with 0 or 120 kg N ha–1 and cabbage with 0 or 240 kg N ha–1.
Growing lettuce in 2004 had little effect on soil N (Fig. 10C), although unfertilized lettuce reduced soil N in the upper 0·5 m. After cabbage, soil N was reduced through the whole profile, with total reductions of between 130 and 180 kg N ha–1 measured (Fig. 10D). The effect of cabbage fertilization on soil N interacted strongly with the previous fertilization. Fertilizing cabbage led to an increase in soil N of 19 vs. 136 kg ha–1 where preceding cauliflower had received 0 or 390 kg N ha–1, respectively. This corresponded to 8 % vs. 57 % of the applied 240 kg N ha–1, respectively. The effect was seen mainly in soil layers below 0·5 m, demonstrating that it was not the fertilizer N which was left unused, but fertilization caused the crop to use less of the sub-soil N.
In year 3 (December 2004 and March 2005), measurements were repeated in selected plots. Soil N in March 2005 is shown, where lettuce was grown in 2004 (Fig. 10E) and where either lettuce or cabbage were grown with high fertilizer application (Fig. 10F). Soil inorganic N levels in March 2005 were generally high throughout the soil profile, and in the sub-soil they remained higher when 390 kg N ha–1 had been applied to the cauliflower crop in 2003 (Fig. 10E), and this effect was especially strong where 120 kg N ha–1 had also been applied to the lettuce crop in 2004. Growing cabbage in 2004 led to lower soil inorganic N levels in 2005 (Fig. 10F), but the effect of fertilizer applied to cabbage in 2004 persisted in the sub-soil.
This example of N dynamics in deep soil layers throughout a 3 year vegetable crop sequence exemplifies the fate of inorganic N left in the soil, and its interactions with various management practices, root growth of the next crop, and N utilization and leaching risks from deep soil layers. Here measurements on the same plots across three seasons demonstrate the impacts seen in wheat systems studied for a shorter time in other experiments in relation to previous crops and cover crops (Thorup-Kristensen et al., 2009), sowing dates (Rasmussen and Thorup-Kristensen, 2016) and N management (Rasmussen et al., 2015), and serve to exemplify the impacts of these management and crop choice decisions.
CONCLUSIONS
Water and N are arguably the most important resources taken up by agricultural crops, and rooting depth is accepted as one of the most important root traits to improve their use. There are many ways to make crop roots grow deeper, and currently there is much focus on genetics and breeding, and this seems promising.
However, our review of Danish and Australian cropping systems has demonstrated that both the availability of water and N within the soil profile, and the potential rooting depth are strongly influenced by crop management factors. Indeed management of the fields in the years and months before the crop, as well as of the crop itself, can have larger impacts on rooting depth and water and N uptake than those achieved by breeding.
More importantly, we have demonstrated that the best outcomes in terms of effective root function were provided by combinations of management and genetics designed with an understanding of the farming systems context. Capturing the synergies offered by these G × E × M interactions will be necessary to exploit fully the promise of novel root traits designed to improve productivity and environmental efficiency.
LITERATURE CITED
- Acuna T, Wade L. 2012. Genotype × environment interactions for root depth of wheat. Field Crops Research 137: 117–125. [Google Scholar]
- Addiscott TM, Darby RJ. 1991. Relating the nitrogen fertilizer needs of winter wheat crops to the soil mineral nitrogen. Influence of the downward movement of nitrate during winter and spring. Journal of Agricultural Science 117: 241–249. [Google Scholar]
- Alonso-Ayuso M, Gabriel JL, Quemada M. 2014. The kill date as a management tool for cover cropping success. PLoS One 9 :e109587. doi:10.1371/journal.pone.0109587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angus JF. 2001. Nitrogen supply and demand in Australian agriculture. Australian Journal of Experimental Agriculture 41: 277–288. [Google Scholar]
- Angus JF, Van Herwaarden AF, Howe GN. 1991. Productivity and break crop effects of winter-growing oilseeds. Australian Journal of Experimental Agriculture 31: 669–677. [Google Scholar]
- Angus JF, van Herwaarden AF, Fischer RA, Howe GN, Heenan DP. 1998. The source of mineral nitrogen for cereals in south-eastern Australia. Australian Journal of Agricultural Research 49: 511–522. [Google Scholar]
- Angus JF, Gault RR, Good AJ, Hart AB, Jones TD, Peoples MB. 2000. Lucerne removal before a cropping phase. Australian Journal of Agricultural Research 51: 877–890. [Google Scholar]
- Angus JF, Gault RR, Peoples MB, Stapper M, van Herwaarden AF. 2001. Soil water extraction by dryland crops, annual pastures, and lucerne in south-eastern Australia. Australian Journal of Agricultural Research 52: 183–192. [Google Scholar]
- Angus JF, Bolger TP, Kirkegaard JA, Peoples MB. 2006. Nitrogen mineralisation in relation to previous crops and pastures. Australian Journal of Soil Research 44: 355–365. [Google Scholar]
- Angus JF, Kirkegaard J, Hunt J, Ryan M, Ohlander L, Peoples M. 2015. Break crops and rotations for wheat. Crop and Pasture Science 66: 523–552. [Google Scholar]
- Asseng S, Fillery IRP, Gregory PJ. 1998. Wheat response to alternative crops on a duplex soil. Australian Journal of Experimental Agriculture 38: 481–488. [Google Scholar]
- Barraclough PB, Kuhlmann H, Weir AH. 1989. The effects of prolonged drought and nitrogen-fertilizer on root and shoot growth and water-uptake by winter-wheat. Journal of Agronomy and Crop Science-Zeitschrift fur Acker und Pflanzenbau 163: 352–360. [Google Scholar]
- Barraclough PB, Leigh RA. 1984. The growth and activity of winter wheat roots in the field: the effect of sowing date and soil type on root growth of high-yielding crops. Journal of Agricultural Sciece 103: 59–74. [Google Scholar]
- Beaudoin N, Saad JK, Van Laethem C, Machet JM, Maucorps J, Mary B. 2005. Nitrate leaching in intensive agriculture in Northern France: effect of farming practices, soils and crop rotations. Agriculture Ecosystems and Environment 111: 292–310. [Google Scholar]
- Bredemeier C, Variani C, Almeida D, Rosa AT. 2013. Wheat yield potential estimation using active optical sensor for site-specific nitrogen fertilization. Ciencia Rural 43: 1147–1154. [Google Scholar]
- Burns IG. 1984. Estimation of annual variations in leaching of residual nitrate under deep-rooted crops during winter In: The nitrogen requirements of cereals, MAFF/ADAS Reference book 385. London: Her Majesty’s Stationary Office, 95–101. [Google Scholar]
- Carvalho P, Azam-Ali S, Foulkes MJ. 2014. Quantifying relationships between rooting traits and water uptake under drought in Mediterranean barley and durum wheat. Journal of Integrative Plant Biology 56: 455–469. [DOI] [PubMed] [Google Scholar]
- Chan KY, Heenan DP. 1996. The influence of crop rotation on soil structure and soil physical properties under conventional tillage. Soil and Tillage Research 37: 113–125. [Google Scholar]
- Chen X, Zhang F, Romheld V, et al. 2006. Synchronizing N supply from soil and fertilizer and N demand of winter wheat by an improved N-min method. Nutrient Cycling in Agroecosystems 74: 91–98. [Google Scholar]
- Cresswell HP, Kirkegaard JA. 1995. Subsoil amelioration by plant-roots – the process and the evidence. Australian Journal of Soil Research 33: 221–239. [Google Scholar]
- Dai XL, Xiao LL, Jia DY, et al. 2014. Increased plant density of winter wheat can enhance nitrogen-uptake from deep soil. Plant and Soil 384: 141–152. [Google Scholar]
- Dalgaard T, Hansen B, Hasler B, et al. 2014. Policies for agricultural nitrogen management – trends, challenges and prospects for improved efficiency in Denmark. Environmental Research Letters 9. [Google Scholar]
- Department of Environment, Food and Rural Affairs. 2011. Fertiliser Manual (RB209), 8th edn, with 2011 errata. [Google Scholar]
- Dijkstra FA, Cheng WX. 2008. Increased soil moisture content increases plant N uptake and the abundance of N-15 in plant biomass. Plant and Soil 302: 263–271. [Google Scholar]
- Doussan C, Pierret A, Garrigues E, Pages L. 2006. Water uptake by plant roots: II – Modelling of water transfer in the soil root-system with explicit account of flow within the root system – Comparison with experiments. Plant and Soil 283: 99–117. [Google Scholar]
- Dresboll DB, Christensen B, Thorup-Kristensen K. 2015a. Approaches to translational plant science. Advances in Agronomy 131: 305–335. [Google Scholar]
- Dresboll DB, Rasmussen IS, Thorup-Kristensen K. 2015b. The significance of litter loss and root growth on N efficiency in normal and semi-dwarf winter oilseed rape genotypes. Field Crops Research 186: 166–178. [Google Scholar]
- Dresboll DB, Thorup-Kristensen K. 2014. Will breeding for nitrogen use efficient crops lead to nitrogen use efficient cropping systems?: a simulation study of G × E × M interactions. Euphytica 199: 97–117. [Google Scholar]
- Ford KE, Gregory PJ, Gooding MJ, Pepler S. 2006. Genotype and fungicide effects on late-season root growth of winter wheat. Plant and Soil 284: 33–44. [Google Scholar]
- Gabriel JL, Munoz-Carpena R, Quemada M. 2012. The role of cover crops in irrigated systems: water balance, nitrate leaching and soil mineral nitrogen accumulation. Agriculture Ecosystems and Environment 155: 50–61. [Google Scholar]
- Garrigues E, Doussan C, Pierret A. 2006. Water uptake by plant roots: I – Formation and propagation of a water extraction front in mature root systems as evidenced by 2D light transmission imaging. Plant and Soil 283: 83–98. [Google Scholar]
- Gastal F, Lemaire G. 2002. N uptake and distribution in crops: an agronomical and ecophysiological perspective. Journal of Experimental Botany 53: 789–799. [DOI] [PubMed] [Google Scholar]
- Gill JS, Clark GJ, Sale PW, Peries RR, Tang C. 2012. Deep placement of organic amendments in dense sodic subsoil increases summer fallow efficiency and the use of deep soil water by crops. Plant and Soil 359: 57–69. [Google Scholar]
- Gorska A, Lazor JW, Zwieniecka AK, Benway C, Zwieniecki MA. 2010. The capacity for nitrate regulation of root hydraulic properties correlates with species’ nitrate uptake rates. Plant and Soil 337: 447–455. [Google Scholar]
- Gregory PJ, Atwell BJ. 1991. The fate of carbon in pulse-labeled crops of barley and wheat. Plant and Soil 136: 205–213. [Google Scholar]
- Gregory PJ, McGowan M, Biscoe PV, Hunter B. 1978. Water relations of winter wheat 1. Growth of the root system. Journal of Agricultural Science 91: 91–102. [Google Scholar]
- Gregory PJ, Atkinson CJ, Bengough AG, et al. 2013. Contributions of roots and rootstocks to sustainable, intensified crop production. Journal of Experimental Botany 64: 1209–1222. [DOI] [PubMed] [Google Scholar]
- Hammer GL, Dong ZS, McLean G, et al. 2009. Can changes in canopy and/or root system architecture explain historical maize yield trends in the US corn belt? Crop Science 49: 299–312. [Google Scholar]
- Hansen EM, Eriksen J, Vinther FP. 2007. Catch crop strategy and nitrate leaching following grazed grass–clover. Soil Use and Management 23: 348–358. [Google Scholar]
- Hansen EM, Munkholm LJ, Olesen JE, Melander B. 2015. Nitrate leaching, yields and carbon sequestration after noninversion tillage, catch crops, and straw retention. Journal of Environmental Quality 44: 868–881. [DOI] [PubMed] [Google Scholar]
- van Herwaarden AF, Farquhar GD, Angus JF, Richards RA, Howe GN. 1998. ‘Haying-off’, the negative grain yield response of dryland wheat to nitrogen fertiliser. I. Biomass, grain yield, and water use. Australian Journal of Agricultural Research 49: 1067–1082. [Google Scholar]
- Heumann S, Fier A, Hassdenteufel M, et al. 2013. Minimizing nitrate leaching while maintaining crop yields: insights by simulating net N mineralization. Nutrient Cycling in Agroecosystems 95: 395–408. [Google Scholar]
- Hund A, Trachsel S, Stamp P. 2009. Growth of axile and lateral roots of maize: I. Development of a phenotying platform. Plant and Soil 325: 335–349. [Google Scholar]
- Hunt J, Kirkegaard J. 2011. Re-evaluating the contribution of summer fallow rain to wheat yield in southern Australia. Crop and Pasture Science 62: 915–929. [Google Scholar]
- Hunt JR, Browne C, McBeath TM, Verburg K, Craig S, Whitbread AM. 2013. Summer fallow weed control and residue management impacts on winter crop yield through soil water and N accumulation in a winter-dominant, low rainfall region of southern Australia. Crop and Pasture Science 64: 922–934. [Google Scholar]
- Ishikawa S, Hare MC, Kettlewell PS. 2012. Effects of strobilurin fungicide programmes and fertilizer nitrogen rates on winter wheat: leaf area, dry matter yield and nitrogen yield. Journal of Agricultural Science 150: 427–441. [Google Scholar]
- Jacobsen OH. 1989. Umættet hydraulisk ledningsevne i nogle danske jorde, metode og jordtypekarakterisering (Unsaturated hydraulic conductivity for some Danish soils, Methods and characterization of soils). Tidsskrift for Planteavls Specialserie 2030. [Google Scholar]
- King J, Gay A, Sylvester-Bradley R, et al. 2003. Modelling cereal root systems for water and nitrogen capture: towards an economic optimum. Annals of Botany 91: 383–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkegaard JA, Hunt J. 2010. Increasing productivity by matching farming system management and genotype in water-limited environments. Journal of Experimental Botany 61: 4129–4143. [DOI] [PubMed] [Google Scholar]
- Kirkegaard JA, Lilley JM. 2007. Root penetration rate – a benchmark to identify soil and plant limitations to rooting depth in wheat. Australian Journal of Experimental Agriculture 47: 590–602. [Google Scholar]
- Kirkegaard JA, Ryan MH. 2014. Magnitude and mechanisms of persistent crop sequence effects on wheat. Field Crops Research 164: 154–165. [Google Scholar]
- Kirkegaard JA, Gardner PA, Angus JF, Koetz E. 1994. Effect of Brassica break crops on the growth and yield of wheat. Australian Journal of Agricultural Research 45: 529–545. [Google Scholar]
- Kirkegaard JA, Hocking PJ, Angus JF, Howe GN, Gardner PA. 1997. Comparison of canola, Indian mustard and Linola in two contrasting environments. 2. Break-crop and nitrogen effects on subsequent wheat crops. Field Crops Research 52: 179–191. [Google Scholar]
- Kirkegaard JA, Lilley JM, Howe GN, Graham JM. 2007. Impact of subsoil water use on wheat yield. Australian Journal of Agricultural Research 58: 303–315. [Google Scholar]
- Kirkegaard JA, Christen O, Krupinsky J, Layzell D. 2008. Break crop benefits in temperate wheat production. Field Crops Research 107: 185–195. [Google Scholar]
- Kirkegaard JA, Peoples MB, Angus J.A, Unkovich M. 2011. Diversity and evolution of rain-fed farming systems in southern Australia In: Tow P, Cooper I, Partridge I, Birch C, eds. Rainfed farming systems. Dordrecht, The Netherlands: Springer, 715–754. [Google Scholar]
- Kirkegaard JA, Hunt JR, McBeath TM, et al. 2014. Improving water productivity in the Australian Grains industry – a nationally coordinated approach. Crop and Pasture Science 65: 583–601. [Google Scholar]
- Kirkegaard JA, Lilley J, Hunt J, et al. 2015. Effect of defoliation by grazing or shoot removal on the root growth of field-grown wheat (Triticum aestivum L.). Crop and Pasture Science 66: 249–259. [Google Scholar]
- Kristensen HL, Thorup-Kristensen K. 2007. Effects of vertical distribution of soil inorganic nitrogen on root growth and subsequent nitrogen uptake by field vegetable crops. Soil Use and Management 23: 338–347. [Google Scholar]
- Kronvang B, Andersen HE, Borgesen C, et al. 2008. Effects of policy measures implemented in Denmark on nitrogen pollution of the aquatic environment. Environmental Science and Policy 11: 144–152. [Google Scholar]
- Kronvang B, Windolf J, Larsen SE, Bogestrand J. 2015. Background concentrations and loadings of nitrogen in Danish surface waters. Acta Agriculturae Scandinavica Section B: Soil and Plant Science 65: 155–163. [Google Scholar]
- Kyllingsbaek A, Hansen JF. 2007. Development in nutrient balances in Danish agriculture 1980–2004. Nutrient Cycling in Agroecosystems 79: 267–280. [Google Scholar]
- Lahti T, Kuikman PJ. 2003. The effect of delaying autumn incorporation of green manure crop on N mineralization and spring wheat (Triticum aestivum L.) performance. Nutrient Cycling in Agroecosystems 65: 265–280. [Google Scholar]
- Larsson MH, Kyllmar K, Jonasson L, Johnsson H. 2005. Estimating reduction of nitrogen leaching from arable land and the related costs. Ambio 34: 538–543. [PubMed] [Google Scholar]
- Latta RA, Blacklow LJ, Cocks PS. 2001. Comparative soil water, pasture production, and crop yields in phase farming systems with lucerne and annual pasture in Western Australia. Australian Journal of Agricultural Research 52: 295–303. [Google Scholar]
- Le Bail M, Jeuffroy MH, Bouchard C, Barbottin A. 2005. Is it possible to forecast the grain quality and yield of different varieties of winter wheat from Minolta SPAD meter measurements? European Journal of Agronomy 23: 379–391. [Google Scholar]
- Lemaire G, Oosterom E, Sheehy J, Jeuffroy MH, Massignam A, Rossato L. 2007. Is crop N demand more closely related to dry matter accumulation or leaf area expansion during vegetative growth? Field Crops Research 100: 91–106. [Google Scholar]
- Lemaire G, van Oosterom E, Jeuffroy MH, Gastal F, Massignam A. 2008. Crop species present different qualitative types of response to N deficiency during their vegetative growth. Field Crops Research 105: 253–265. [Google Scholar]
- Lilley JM, Kirkegaard JA. 2007. Seasonal variation in the value of subsoil water to wheat: simulation studies in southern New South Wales. Australian Journal of Agricultural Research 58: 1115–1128. [Google Scholar]
- Lilley JM, Kirkegaard JA. 2011. Benefits of increased soil exploration by wheat roots. Field Crops Research 122: 118–130. [Google Scholar]
- Lilley JM, Kirkegaard JA. 2016. Farming systems context drives the value of deep roots in Australia. Journal Experimental Botany 67: 3665–3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilley JM, Probert M., Kirkegaard JA. 2004. Simulation of deep drainage under a 13 year crop sequence in southern NSW. In: Proceedings of the 4th International Crop Science Congress, 26 September–1 October 2004.
- Liu XJ, Ju XT, Zhang FS, Chen XP. 2003. Nitrogen recommendation for winter wheat using N-min test and rapid plant tests in North China Plain. Communications in Soil Science and Plant Analysis 34: 2539–2551. [Google Scholar]
- Lukina EV, Freeman KW, Wynn KJ, et al. 2001. Nitrogen fertilization optimization algorithm based on in-season estimates of yield and plant nitrogen uptake. Journal of Plant Nutrition 24: 885–898. [Google Scholar]
- Lynch JP. 2007. Roots of the second green revolution. Australian Journal of Botany 55: 493–512. [Google Scholar]
- Lynch JP. 2011. Root phenes for enhanced soil exploration and phosphorus acquisition: tools for future crops. Plant Physiology 156: 1041–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JP. 2013. Steep, cheap and deep: an ideotype to optimize water and N acquisition by maize root systems. Annals of Botany 112: 347–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeght JL, Rewald B, Pierret A. 2013. How to study deep roots – and why it matters. Frontiers in Plant Science 4: 299. doi:10.3389/fpls.2013.00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manschadi AM, Christopher J, deVoil P, Hammer GL. 2006. The role of root architectural traits in adaptation of wheat to water-limited environments. Functional Plant Biology 33: 823–837. [DOI] [PubMed] [Google Scholar]
- McCallum MH, Kirkegaard JA, Green TW, et al. 2004. Improved subsoil macroporosity following perennial pastures. Australian Journal of Experimental Agriculture 44: 299–307. [Google Scholar]
- McDonald GK, Taylor JD, Verbyla A, Kuchel H. 2012. Assessing the importance of subsoil constraints to yield of wheat and its implications for yield improvement. Crop and Pasture Science 63: 1043–1065. [Google Scholar]
- McKenzie B, Bengough A, Hallett P, Thomas W, Forster B, McNicol J. 2009. Deep rooting and drought screening of cereal crops: a novel field-based method and its application. Field Crops Research 112: 165–171. [Google Scholar]
- McMaster C, Graham N, Kirkegaard JA, Hunt J, Menz I. 2015. ‘Buying a spring’ – the water and nitrogen cost of poor winter fallow weed control In: Building productive, diverse and sustainable landscapes. In: Proceedings of the 17th ASA Conference, 20–24 September 2015, Hobart, Australia. [Google Scholar]
- Mercer CA, Eppley SM. 2014. Kin and sex recognition in a dioecious grass. Plant Ecology 215: 845–852. [Google Scholar]
- Munns R, James RA, Sirault XRR, Furbank RT, Jones HG. 2010. New phenotyping methods for screening wheat and barley for beneficial responses to water deficit. Journal of Experimental Botany 61: 3499–3507. [DOI] [PubMed] [Google Scholar]
- Munoz-Romero V, Benitez-Vega J, Lopez-Bellido RJ, Fontan JM, Lopez-Bellido L. 2010. Effect of tillage system on the root growth of spring wheat. Plant and Soil 326: 97–107. [Google Scholar]
- Myrbeck A, Stenberg M, Arvidsson J, Rydberg T. 2012. Effects of autumn tillage of clay soil on mineral N content, spring cereal yield and soil structure over time. European Journal of Agronomy 37: 96–104. [Google Scholar]
- Ober ES, Werner P, Flatman E, Angus WJ, Jack P, Smith-Reeve L, Tapsell C. 2014. Genotypic differences in deep water extraction associated with drought tolerance in wheat. Functional Plant Biology 41: 1078–1086. [DOI] [PubMed] [Google Scholar]
- Olsen JM, Griepentrog HW, Nielsen J, Weiner J. 2012. How important are crop spatial pattern and density for weed suppression by spring wheat? Weed Science 60: 501–509. [Google Scholar]
- Palta JA, Chen X, Milroy SP, Rebetzke GJ, Dreccer MF, Watt M. 2011. Large root systems: are they useful in adapting wheat to dry environments? Functional Plant Biology 38: 347–354. [DOI] [PubMed] [Google Scholar]
- Passioura J. 2006. Increasing crop productivity when water is scarce – from breeding to field management. Agricultural Water Management 80: 176–196. [Google Scholar]
- Pedersen A, Thorup-Kristensen K, Jensen LS. 2009. Simulating nitrate retention in soils and the effect of catch crop use and rooting pattern under the climatic conditions of Northern Europe. Soil Use and Management 25: 243–254. [Google Scholar]
- Pedersen A, Zhang KF, Thorup-Kristensen K, Jensen LS. 2010. Modelling diverse root density dynamics and deep nitrogen uptake – a simple approach. Plant and Soil 326: 493–510. [Google Scholar]
- Persson T, Bergkvist G, Kaetterer T. 2008. Long-term effects of crop rotations with and without perennial leys on soil carbon stocks and grain yields of winter wheat. Nutrient Cycling in Agroecosystems 81: 193–202. [Google Scholar]
- Qin XL, Weiner J, Qi L, Xiong YC, Li FM. 2013. Allometric analysis of the effects of density on reproductive allocation and Harvest Index in 6 varieties of wheat (Triticum). Field Crops Research 144: 162–166. [Google Scholar]
- Rahn CR, Bending GD, Turner MK, Lillywhite RD. 2003. Management of N mineralization from crop residues of high N content using amendment materials of varying quality. Soil Use and Management 19: 193–200. [Google Scholar]
- Rahn CR, Zhang K, Lillywhite R, et al. 2010. EU-Rotate_N – a decision support system to predict environmental and economic consequences of the management of nitrogen fertiliser in crop rotations. European Journal of Horticultural Science 75: 20–32. [Google Scholar]
- Rasmussen IS, Thorup-Kristensen K. 2016. Does earlier sowing improve root growth and N uptake by winter wheat? Field Crops Research, doi:10.1016/j.fcr.2016.05.009, in press.
- Rasmussen IS, Dresboll DB, Thorup-Kristensen K. 2015. Winter wheat cultivars and nitrogen (N) fertilization – effects on root growth, N uptake efficiency and N use efficiency. European Journal of Agronomy 68: 38–49. [Google Scholar]
- Raun WR, Solie JB, Stone ML. 2011. Independence of yield potential and crop nitrogen response. Precision Agriculture 12: 508–518. [Google Scholar]
- Richard CAI, Hickey LT, Fletcher S, Jennings R, Chenu K, Christopher JT. 2015. High-throughput phenotyping of seminal root traits in wheat. Plant Methods 11: 13. doi:10.1186/s13007-015-0055-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan MH, Kirkegaard JA, Angus JF. 2006. Brassica crops stimulate soil mineral N accumulation. Australian Journal of Soil Research 44: 367–377. [Google Scholar]
- Sanderson JB, MacLeod JA, Kimpinski J. 1999. Glyphosate application and timing of tillage of red clover affects potato response to N, soil N profile, and root and soil nematodes. Canadian Journal of Soil Science 79: 65–72. [Google Scholar]
- Sauer S, Haussmann W, Harrach T. 2002. Effective rooting depth, percolation water, and nitrate leaching in deeply developed loess soils of a water-shortage area. Journal of Plant Nutrition and Soil Science-Zeitschrift fur Pflanzenernahrung und Bodenkunde 165: 269–273. [Google Scholar]
- Schönhammer A, Fishbeck G. 1987. Investigations on cereal crop rotations and monocultures. III Changes in soil properties. Bayerisches Landwirtschaftliches Jahrbuch 64: 681–694. [Google Scholar]
- Semenov MA, Martre P, Jamieson PD. 2009. Quantifying effects of simple wheat traits on yield in water-limited environments using a modelling approach. Agricultural and Forest Meteorology 149: 1095–1104. [Google Scholar]
- Seymour M, Kirkegaard JA, Peoples MB, White PF, French RJ. 2012. Break-crop benefits to wheat in Western Australia – insights from over three decades of research. Crop and Pasture Science 63: 1–16. [Google Scholar]
- Sieling K, Christen O. 2015. Crop rotation effects on yield of oilseed rape, wheat and barley and residual effects on the subsequent wheat. Archives of Agronomy and Soil Science 61: 1531–1549. [Google Scholar]
- Smith CJ, Dunin FX, Poss R, Angus JF. 2000. Nitrogen budget of wheat growing on a Riverine clay soil. Australian Journal of Agricultural Research 51: 867–876. [Google Scholar]
- Sripada RP, Heiniger RW, White JG, Meijer AD. 2006. Aerial color infrared photography for determining early in-season nitrogen requirements in corn. Agronomy Journal 98: 968–977. [Google Scholar]
- Thomsen IK, Hansen EM. 2014. Cover crop growth and impact on N leaching as affected by pre- and postharvest sowing and time of incorporation. Soil Use and Management 30: 48–57. [Google Scholar]
- Thorup-Kristensen K. 1993. The effect of nitrogen catch crops on the nitrogen nutrition of a succeeding crop. I. effects through mineralization and pre-emptive competition. Acta Agriculturae Scandinavica Section B: Soil and Plant Science 43: 74–81. [Google Scholar]
- Thorup-Kristensen K. 2001. Are differences in root growth of nitrogen catch crops important for their ability to reduce soil nitrate-N content, and how can this be measured? Plant and Soil 230: 185–195. [Google Scholar]
- Thorup-Kristensen K. 2006a. Effect of deep and shallow root systems on the dynamics of soil inorganic N during three year crop rotations. Plant and Soil 288: 233–248. [Google Scholar]
- Thorup-Kristensen K. 2006b. Root growth and nitrogen uptake of carrot, early cabbage, onion and lettuce following a range of green manures. Soil Use and Management 22: 29–38. [Google Scholar]
- Thorup-Kristensen K, Dresboll DB. 2010. Incorporation time of nitrogen catch crops influences the N effect for the succeeding crop. Soil Use and Management 26: 27–35. [Google Scholar]
- Thorup-Kristensen K, Sørensen JN. 1999. Soil nitrogen depletion by vegetable crops with variable root growth. Acta Agriculturae Scandinavica Section B: Soil and Plant Science 49: 92–97. [Google Scholar]
- Thorup-Kristensen K, Van den Boogaard R. 1998. Temporal and spatial root development of cauliflower (Brassica oleracea L. var. botrytis L.). Plant and Soil 201: 37–47. [Google Scholar]
- Thorup-Kristensen K, Magid J, Jensen LS. 2003. Catch crops and green manures as biological tools in nitrogen management in temperate zones. Advances in Agronomy 79: 227–301. [Google Scholar]
- Thorup-Kristensen K, Cortasa MS, Loges R. 2009. Winter wheat roots grow twice as deep as spring wheat roots, is this important for N uptake and N leaching losses? Plant and Soil 322: 101–114. [Google Scholar]
- Thorup-Kristensen K, Dresboll DB, Kristensen HL. 2012. Crop yield, root growth, and nutrient dynamics in a conventional and three organic cropping systems with different levels of external inputs and N re-cycling through fertility building crops. European Journal of Agronomy 37: 66–82. [Google Scholar]
- Vincent CD, Gregory PJ. 1989. Effects of temperature on the development and growth of winter wheat roots. II. Field studies of temperature, nitrogen and irradiance. Plant and Soil 119: 99–110. [Google Scholar]
- Wasson A, Richards R, Chatrath R, et al. 2012. Traits and selection strategies to improve root systems and water uptake in water-limited wheat crops. Journal of Experimental Botany 63: 3485–3498. [DOI] [PubMed] [Google Scholar]
- Wasson A, Rebetzke G, Kirkegaard J, Christopher J, Richards R, Watt M. 2014. Soil coring at multiple field environments can directly quantify variation in deep root traits to select wheat genotypes for breeding. Journal of Experimental Botany 65: 6231–6249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt M, Magee LJ, McCully ME. 2008. Types, structure and potential for axial water flow in the deepest roots of field-grown cereals. New Phytologist 178: 135–146. [DOI] [PubMed] [Google Scholar]
- Weligama C, Sale PWG, Conyers MK, Liu DL, Tang C. 2010a. Nitrate leaching stimulates subsurface root growth of wheat and increases rhizosphere alkalisation in a highly acidic soil. Plant and Soil 328: 119–132. [Google Scholar]
- Weligama C, Tang C, Sale PWG, Conyers MK, Liu DL. 2010b. Application of nitrogen in NO (3) (–) form increases rhizosphere alkalisation in the subsurface soil layers in an acid soil. Plant and Soil 333: 403–416. [Google Scholar]
- Wendland F, Behrendt H, Goemann H, et al. 2009. Determination of nitrogen reduction levels necessary to reach groundwater quality targets in large river basins: the Weser basin case study, Germany. Nutrient Cycling in Agroecosystems 85: 63–78. [Google Scholar]
- Whish JPM, Thompson JP, Clewett TG, Lawrence JL, Wood J. 2014. Pratylenchus thornei populations reduce water uptake in intolerant. wheat cultivars. Field Crops Research 161: 1–10. [Google Scholar]
- Wiesler F, Horst WJ. 1994. Root growth and nitrate utilization of maize cultivars under field conditions. Plant and Soil 163: 267–277. [Google Scholar]
- Ytting N, Andersen S, Thorup-Kristensen K. 2014. Using tube rhizotrons to measure variation in depth penetration rate among modern North-European winter wheat (Triticum aestivum L.) cultivars. Euphytica 199: 233–245. [Google Scholar]
- Zhou SL, Wu YC, Wang ZM, Lu LQ, Wang RZ. 2008. The nitrate leached below maize root zone is available for deep-rooted wheat in winter wheat-summer maize rotation in the North China Plain. Environmental Pollution 152: 723–730. [DOI] [PubMed] [Google Scholar]