Abstract
Background Deep roots are a common trait among a wide range of plant species and biomes, and are pivotal to the very existence of ecosystem services such as pedogenesis, groundwater and streamflow regulation, soil carbon sequestration and moisture content in the lower troposphere. Notwithstanding the growing realization of the functional significance of deep roots across disciplines such as soil science, agronomy, hydrology, ecophysiology or climatology, research efforts allocated to the study of deep roots remain incommensurate with those devoted to shallow roots. This is due in part to the fact that, despite technological advances, observing and measuring deep roots remains challenging.
Scope Here, other reasons that explain why there are still so many fundamental unresolved questions related to deep roots are discussed. These include the fact that a number of hypotheses and models that are widely considered as verified and sufficiently robust are only partly supported by data. Evidence has accumulated that deep rooting could be a more widespread and important trait among plants than usually considered based on the share of biomass that it represents. Examples that indicate that plant roots have different structures and play different roles with respect to major biochemical cycles depending on their position within the soil profile are also examined and discussed.
Conclusions Current knowledge gaps are identified and new lines of research for improving our understanding of the processes that drive deep root growth and functioning are proposed. This ultimately leads to a reflection on an alternative paradigm that could be used in the future as a unifying framework to describe and analyse deep rooting. Despite the many hurdles that pave the way to a practical understanding of deep rooting functions, it is anticipated that, in the relatively near future, increased knowledge about the deep rooting traits of a variety of plants and crops will have direct and tangible influence on how we manage natural and cultivated ecosystems.
Keywords: Deep roots, maximum rooting depth, rooting profile, soil carbon, drought tolerance, climate change
INTRODUCTION
Among the myriad of soil biological actors, plant roots are of pivotal importance with regards to many soil processes. Due to roots’ inherent nutritional value as a carbon substrate and the wide range of metabolites that they release to the soil (Rovira, 1965), rhizosphere soil and root surfaces are also the main habitats for many soil organisms. Converging evidence shows that plants deposit considerable amounts of carbon in the soil: fine root production alone has been estimated to represent 22 % of terrestrial net primary production globally (McCormack et al., 2015) and rhizodeposits such as root cap cells, mucilages, soluble exudates and lysates, and decaying tissues (Hutsch et al., 2002; Hawes et al., 2003; Nguyen, 2003) might be the main source of stabilized carbon input to soil (Rasse et al., 2005).
Many of the world’s soils are deeper than 1–1·5 m (Richter and Markewitz, 1995), although this is the depth range that most textbooks generally recommend to consider (FAO, 2006). Many plants grow roots beyond the soil layers, sometimes deep into weathered and/or fractured hard rock and bedrock (Canadell et al., 1996; Schwinning, 2010), thus accessing water and nutrient supplies unavailable to surface roots (Estrada-Medina et al., 2012). Recent research suggests that deep roots could be of pivotal importance to alleviate water stress in many crops (Gewin, 2010). In addition, carbon deposition from deep root growth could be much more substantial than commonly accepted (Harper and Tibbett, 2013). While current estimates of soil organic carbon (SOC) are almost exclusively based on the standard IPCC (Intergovernmental Panel on Climate Change) sampling depth of 0·3 m (Aalde et al., 2006), many of the world’s soils are much deeper than 1 m and there is clear evidence that biological activity extends several metres into the soil and bedrock (Richter and Markewitz, 1995; Maeght et al., 2013). Even though the drivers of deep root growth are still poorly understood, evidence has accumulated that deep rooting could be a more widespread and important trait among plants than usually considered based on the share of overall root biomass that they make up. In particular, several studies suggest that deep roots can forage for water and nutrient supplies totally beyond the reach of surface roots, thereby playing a central role in the cycling of nutrient and water (e.g. Da Silva et al., 2011; Thorup-Kristensen and Rasmussen, 2015; Giambelluca et al., 2016).
Knowledge about deep roots, their functions and their ecological significance, although still relatively limited, has been on the rise during the last decade. A search on the Web of Science™ (http://apps.webofknowledge.com) database as per January 2016 returned 767 references for the expression [deep root* and plant] with citations increasing from <100 to about 2500 per year from 1995 to 2015 (h-index 74); 411 references for the expression [deep root* and tree], with citations increasing from <26 to > 1100 per year from 1995 to 2015 (h-index 55); and 276 references for the expression [‘deep rooting’], with citations increasing from <25 to nearly 900 per year from 1995 to 2015 (h-index 49). By comparison, searching the expressions [‘plant root*’] and [‘fine root*’] yielded >48 000 and 8200 references, respectively. Current research efforts allocated to the study of deep roots remain incommensurate with those devoted to shallow roots, leaving a wide array of fundamental questions regarding deep roots underinvestigated and unresolved, notwithstanding the growing realization that deep roots may be a key functional element of the Critical Zone (Richter and Billings, 2015). The fact that deep roots have yet to be given the attention they deserve is further intensified by the fact that, despite some technological advances, observing and measuring deep roots remains challenging (Maeght et al., 2013).
Here we examine the difficulties inherent in clearly defining deep roots and deep rooting, which leads us to review current uncertainties regarding deep rooting and its determinants. In particular, we briefly present and discuss various pieces of evidence that challenge the widely accepted view that the exponential decrease model is of universal relevance to rooting profiles. Further, we examine controversial evidence regarding the fact that shallow roots are at a competitive advantage compared with deep roots. We then move on to discuss examples of the functional importance of fine and deep roots, in both perennials and annual crops, as well as the role of deep rooting in soil carbon dynamics and ways to improve soil carbon dynamics in agro-ecosystems. Finally, we present and discuss examples that illustrate how deep rooting may be involved in drought tolerance and climate change attenuation and reflect on an alternative paradigm that could be used in the future as a unifying framework to describe and analyse deep rooting.
THE CHALLENGE OF DEFINING DEEP ROOTS
Defining deep roots is challenging. Compiling a large database of published root profiles encompassing virtually all terrestrial biomes, Schenk and Jackson (2002) ascertained that the median depth of root profiles was 0·88 m. Based on this value, Maeght et al. (2013) proposed that ‘deep roots’ be defined as roots growing at soil depths of at least 1 m. Applying such a universal 1 m threshold to all vegetation types in all climates and soil types can of course be seen as highly controversial, as it can be argued among a number of other criticisms that (1) perennials and annuals do not stand on an equal basis regarding their potential to explore large volumes of soils; (2) according to the principle of allometry, plants with smaller/shorter aerial parts may often have a lower potential for deep rooting than larger plants; and (3) environmental conditions certainly affect the deep rooting potential of all plant species, in a way not dissimilar to the so-called bonsai effect observed in aerial parts of plants (Passioura, 2002).
A definition of deep rooting based on some quantitative metrics of root systems, such as the proportion of the cumulative root length or biomass at a given soil depth, would therefore appear more sensible than a universal depth threshold. Yet, the conundrum that ensues from such a definition is that it is totally dependent upon the absolute necessity to sample maximum root depth (MRD) systematically to ensure sufficient accuracy of the underlying metrics. It is known, however, that only a marginal proportion of published root profiles correspond to complete profiles [a proportion estimated to be of the order of 10 % by Schenk and Jackson’s analysis (2002b)], which means that only a marginal proportion of published data contains reliable information about MRD. This major sampling shortcoming was also identified by Yuen et al. (2013) who reported that despite evidence that rooting depths of tropical trees, shrubs and herbaceous plants can reach or exceed depths of 7·0, 5·0 and 2·5 m, respectively (Canadell et al., 1996), very few studies on the rooting depth of tropical vegetation include samples taken below 1 m. It therefore appears that an MRD-related definition of deep roots will remain of limited use as long as we do not have more comprehensive data about the rooting habits of major plant functional groups worldwide.
More promising may be approaches that consider mathematically appreciable variations in the shape of rooting profiles; in particular, the so-called ‘hockey stick’ model (Qiand and Cuffney, 2012) which allows the detection of significant breakpoints in a series of data or robust trend detection estimators such as Sen’s slope estimator (Sen, 1968), might open ways to more objective definitions of deep rooting even in the absence of precise knowledge about MRD. The hockey stick model was thus recently applied to the analysis of 4 m deep root profiles (Cardinael et al., 2015); yet, it must be noted that this model sometimes fails to detect a breakpoint, for example when there are not enough variations in rooting intensity with depth.
Due to their distal position within the root system, it can be said that deep roots inherently encompass a large share of, if not exclusively, fine roots. Yet, there also is a lack of consensus on what a fine root is. Although it is very often considered that fine roots are roots <2 mm in diameter, roots <0·2 mm in diameter can make up to > 50 % of the overall root length (e.g. Pallant et al., 1993; Amato and Pardo, 1994; Pierret et al., 2005). Recently, McCormack et al. (2015) advocated that the traditional fine-root pool, i.e. roots whose diameter is < 2 mm, is in fact a heterogeneous group of roots that are best defined, measured, and modelled separately as absorptive fine roots and transport fine roots. They consequently proposed that an order-based, functional classification would improve our understanding of dynamic root processes in ecosystems dominated by perennial plants. Yet, such an approach is not straightforward to implement and, while its adoption should certainly be promoted, it prompts the need for the development of more automated, image-based procedures, as the suggested order-based classification is time-consuming to implement manually.
To summarize, deep roots encompass a rather poorly defined ensemble of fine roots that stand at soil depths ranging from 0·1 m to several metres below the soil surface, not necessarily but frequently, at least in the case of perennials, penetrating the altered bedrock. While it represents an oversimplification considering the diversity of climates, soils and vegetation types that prevail on the planet, we nevertheless propose that, when considering plant roots in association with soil (including biogeochemical processes) the 1 m threshold still combines the advantages of simplicity, practicality and functional ecological significance.
UNCERTAINTIES REGARDING DEEP ROOTING AND ITS DETERMINANTS
Roots are notably challenging to characterize accurately, particularly under field conditions and, as discussed extensively elsewhere, estimating a single parameter such as root length or root diameter is already subject to controversy (Pierret et al., 2005; Zobel, 2008; Yuen, 2013). As previously mentioned, major uncertainties also surround the determination of rooting depth (or MRD, as it is often referred to) and how this parameter varies depending on biophysical conditions and genetic pre-determinism. On the basis of 56 complete and 463 incomplete root profiles combined with climate and soil texture data, Schenk and Jackson (2005) concluded that deep roots are most likely to occur in seasonally dry, semi-arid to humid tropical regions on coarse- and fine-textured soils and least likely to occur in the coolest and most humid climates on medium textured soil. However, in a previous study, the same authors concluded that at ecosystem level, the absolute rooting depth was positively correlated to mean annual precipitation (Schenk and Jackson, 2002). Concurrently, it appears that (1) shrubs and trees can be deep-rooted, independent of climate as long as this enables them to reach groundwater and (2) rooting depths in tropical vegetation were only weakly correlated with climate but strongly correlated with sampling depths, suggesting that sampling is frequently too shallow to capture the actual MRD (Schenk and Jackson, 2002).
The collection of finely hand-drawn pictures of root systems of hundreds of tree, shrub and grass species that Lore Kutschera and co-workers produced over the course of half a century (Kutschera, 1960; Kutschera et al., 1997) further indicates that assuming a strong relationship between the size of the above-ground part of a given plant and the size of its root system can be misleading. For example, it can been seen in Fig. 1 that the above-ground height/MRD ratio can vary by more than one order of magnitude and that some species with a canopy that does not even extend 1 m above the soil surface can grow roots deeper than 5 m below-ground. Such unique data demonstrate that (1) the aerial part of a plant can literally represent the ‘tip of the iceberg’ with respect to its total extent from the lower end of the root system to the top of the canopy and (2) estimating MRD from excessively shallow sampling jeopardizes the predictive power of allometric relationships between the dimensions of aerial parts and MRD.
Fig. 1.
Drawings of the above- and below-ground extension of the species Pinus sylvestris, Pimpinella saxifrage, Zygophullum xanthoxylo and Convolvulus tragacanthoides. H/D is the ratio of the above-ground plant height divided by the maximum rooting depth (MRD). This figure illustrates the large inter-specific variability in H/D ratio and demonstrates the risk of establishing misleading allometric relationships between these parameters when MRD is not accurately determined (adapted from Kutschera et al., 1997).
More unexpectedly, several reports also demonstrated that smaller plants can also develop deep root systems. For example, Pimpinella saxifraga, a member of the Umbelliferae family, despite being but a modest weed standing hardly beyond 0·5 m in height above the ground, was found to grow roots down to a depth of > 2·5 m (Kutschera et al., 1997); or Nitraria sibirica, a xerophyte found in the Gobi desert, with a canopy <1 m high but growing roots to a depth of > 3 m (Kutschera et al., 1997; Zhou et al., 2015).
Considering published elongation rates of fine roots (e.g. Nodichao et al., 2011; Gonkhamdee et al., 2010a; Germon et al., 2015), ranging from a few millimetres to a few centimetres per day, it is very likely that most plant root systems can reach much greater soil depths, and hence explore much bigger soil volumes, than the ‘classically’ studied 1 m soil profile.
Examples of such deep root systems have long been known and reported in tree species (Stone and Kalisz, 1991). Recently, Christina et al. (2011) reported near-symmetrical above- and below-ground growth rates for Eucalyptus grandis grown in nutrient-poor soils of tropical Brazil, with MRD reaching 9·2 and 15·8 m at ages of 1·5 and 3·5 years, respectively, with an average root front expansion rate of > 12 mm d–1 (with peak values of 18 mm d–1 within the first 10 months after planting). There are also examples of extremely deep rooting in shrubs, such as the mesquite (Prosopis juliflora), a large shrub in the Fabaceae family, native to Mexico, South America and the Caribbean, reaching a maximum height of 10–12 m above ground and reportedly growing roots to a depth of > 53 m (Phillips, 1963).
Finally, and in contrast to widely accepted assumptions, deep rooting also appears to be a common feature of annual crops, for which rooting depth appear to be related to the rate of root penetration [between 0·9 and 2·3 mm °C d–1, depending on the crop (Thorup-Kristensen, 2001)] and the cumulative temperature before anthesis. For example, species such as turnip and radish were observed to reach depths of 2 and 2·5 m, respectively in 3 months, white cabbage grew to a depth of 2 m in 4 months, and winter wheat grew down almost 2 m from a depth of 0·5 to nearly 2·5 m in the 6 month period spanning from March to September (Kristensen and Thorup-Kristensen, 2004a; Thorup-Kristensen et al., 2009); out of 18 catch crop species, Thorup-Kristensen and Rasmussen (2015) found that all but one could grow roots at soil depths between 1·6 and 2·4 m.
To sum up, deep rooting appears to be a common trait among a wide range of species, including annual crop species and under a variety of climates. This could be related to the relatively high root elongation rates observed in most species. Further, there does not seem to be solid ground for assuming a narrow range of proportionality between canopy height and maximum rooting depth across species.
WHAT IS THE HEURISTIC VALUE OF THE EXPONENTIAL DECREASE MODEL?
A point that requires careful (re-)assessment is the widely accepted view that the vast majority of published root profiles show exponential declines of root density with increasing soil depth (e.g. Schenk, 2008b). This model reflects a perception that any researcher working in the field has experienced first-hand while digging and looking for roots; yet as most root profiles are not sampled to MRD the previously discussed conundrum ensues (see ‘The challenge of defining deep roots’). Truncated rooting profiles, i.e. profiles sampled to a soil depth less than the actual MRD, can yield misleading information about the shape of the actual depth distribution of roots; this may in turn result in spurious conclusions regarding root functions and their role in biogeochemical cycles. Such a situation is illustrated in Fig. 2 which shows that insufficiently deep sampling truncates the actual rooting profile and can radically alter its shape: the shallowest sampling would lead to the conclusion that the rooting profile follows a clear exponential decline with depth when the actual profile, sampled down to MRD, has a very different shape (see also Fig. 6A).
Fig. 2.
Illustration of the influence of maximum sampling depth on the shape of the inferred cumulative root profile. The root system drawing (A) depicts a specimen of Raphanus raphanistrum (from Kutschera, 1960). The plot in (B) illustrates the fact that truncated rooting profiles can yield misleading information about the actual depth distribution of roots, which may subsequently result in spurious conclusions regarding root functioning; from grossly undersampled (fine dashed horizontal line and curve in A and B, respectively), to moderately undersampled (solid horizontal line and curve in A and B, respectively) and fully sampled (dashed dotted horizontal line and curve in A and B, respectively).
Fig. 6.
(A) Cumulative dry root biomass fraction in boreholes drilled down to 32 m in a young rubber tree plantation of north-eastern Thailand, established after clearing dipterocarp secondary forest. Given that the rubber trees were very young (<5 years old) at the time of sampling, it is assumed that these rooting profile correspond to the pre-existing forest. The groundwater level at the time of drilling, for each rooting profile is indicated to the right hand side of the plot by horizontal lines whose style and shade of grey matches that of the corresponding rooting profile. (B Scanning electron microscopy image of one of the roots retrieved from a depth of 24 m (Montoroi et al., 2015). Note the well-preserved overall structure of the root and visible cell walls.
In addition, such an exponential decline, if as ubiquitous as generally assumed, does not match the vertical distribution of the major plant nutrients (Jobbagy and Jackson, 2001). Schenk (2008b) interpreted this mismatch as evidence that factors other than nutrient availability determine the average shape of root profiles. Indeed, it has been reported that on average, at the global scale, only 10–30 % of nitrogen, phosphorus and potassium occur in the top 20 cm of soils (Jobbagy and Jackson, 2001). Some research even suggests that long-term leaching in xeric ecosystems could result in sub-soil nitrate accumulations over soil depths of several metres (Walvoord et al., 2003). Nutrient uptake from soil layers well below 1 m has been documented in both annual crops (Kristensen and Thorup-Kristensen, 2004a) and trees (Da Silva et al., 2011) and might be of particular importance in deep, highly weathered tropical soils. For example, Da Silva et al. (2011) demonstrated that the fine roots of eucalypt trees exhibit contrasting potential uptake rates with depth depending on the nutrient; while the highest uptake rates were found in the topsoil and were higher for than for K+ and Ca2+ analogues, the uptake rates per unit of fine root length density for K+ and Ca2+ were the highest at 3 m, suggesting a specialization of upper fine roots in uptake and deep fine roots in potassium and calcium uptake. This example highlights the fact that, although there is not necessarily a strict correlation between rooting densities and root uptake, the presence of deep roots actively involved in resource uptake is a factor likely to alter the universal explanatory value of the exponential decline model. Even if resource extraction is arguably less costly from shallow than deep soil layers, it is indisputable that rooting depths and lateral root spread of several metres have been observed for most plant forms (e.g. Stone and Kalisz, 1991; Kutschera et al., 1997; Schenk and Jackson, 2002; Syahrinudin, 2005; Thorup-Kristensen et al., 2009; Battie-Lacla and Laclau, 2009; Da Silva et al., 2011; Laclau et al., 2013; Maeght et al., 2015). There are now several examples in the literature that correspond to what Mulia and Dupraz (2006) qualified of ‘unusual fine root distributions’ and that provide direct illustrations on how sampling depth influences the shape of rooting profiles (Battie-Laclau and Laclau, 2009; Maeght, 2014; Cardinael et al., 2015; Maeght et al., 2015;).
To summarize, in the absence of more systematic sampling of root profiles down to MRD, it remains impossible to assert with sufficient confidence that the exponential decrease model is the most likely shape of rooting profiles.
ARE SHALLOW ROOTS AT A COMPETITIVE ADVANTAGE OVER DEEP ROOTS?
In an attempt to produce a simple tool for parameterization of global models, Schenk (2008b) proposed that, globally, rooting profiles of plant communities tend to be as shallow as possible and as deep as needed to meet the evapotranspiration (ET) demand. The rationale behind Schenk’s model (2008b) is that many ecological factors tend to favour shallow over deep root placement including (1) lower energy costs for construction, maintenance and resource uptake; (2) lower soil strength near the soil surface; (3) high water availability close to the surface, which is wetted even by small precipitation events; (4) high nutrient availability in the upper soil layers; (5) a lower probability of oxygen deficiency; and (6) because deep roots in dry soil could potentially be a drain for downwards hydraulic redistribution (Schenk, 2008a).
One environmental factor often invoked to support the hypothesis that deep roots are of marginal importance, both functionally and quantitatively, relative to shallow rooting, is that soil strength is lower, therefore more conducive to profuse root growth near the soil surface (Bengough et al., 2011). Yet, while it is correct that soil bulk density, hence soil strength, increases within the first few centimetres of nearly all soil profiles, variations at greater depths are much less systematically predictable. In addition, there are many examples that show that roots can penetrate soil horizons of high mechanical impedance through growth in soil structural features such as cracks or macropores (White and Kirkegaard, 2010; Jin et al., 2013).
Further, although there is a substantial body of evidence that supports the hypothesis that plant organs, whether aerial or sub-terranean, are built according to fundamental traits that favour economically competitive investment strategies of plant assimilates, via a control of the trade-off between resource acquisition and conservation (Osnas et al., 2013; Prieto et al., 2015; Roumet et al., 2016), it remains virtually unknown how this model applies to deep roots. In particular, a recent study reports results that run against the existence of a single root economics spectrum in absorptive roots (Kong et al., 2015), thus suggesting that deep roots, which encompass a large proportion of absorptive roots, might not be grown according to a universal scheme of resource allocation. From a mere geometrical perspective, there is no obvious reason why the construction and maintenance of deep roots would be more costly than that of leaves and stems high above ground level, particularly in perennials that maintain canopies metres to tens of metres away from the collar of their trunks.
Although measurements of fine root turnover have rarely been conducted below the first few centimetres of the soil surface, the fact that fine root turnover has been observed to decrease with soil depth in some studies (Joslin et al., 2006; Germon et al., 2015) could indicate that the high construction costs of deep roots tends to be counter-balanced by lower maintenance costs through longer life spans. On the other hand, Maeght et al. (2015) recently reported increasing fine root turnover with increasing soil depths, at least to a depth of 3 m, which is consistent with the hypothesis that beyond a simple root economics spectrum and the relative weight of endogenous drivers (Abramoff and Finzi, 2015), deep root growth and turnover is probably strongly modulated by a combination of environmental factors (McCormack and Guo, 2014). Under such a scenario, even though deep roots might be more expensive to construct and maintain than shallow roots, these costs are likely to be offset by the advantages that deep roots confer to the plant in terms of resource uptake. It is worth noting that the notion of resource use efficiency, as relevant as it may be to agronomists to achieve food security and environmental quality, might prove deceptive when considering unmanaged systems; in the latter case, access to, for example, marginal amounts of extra water during a critically dry period might prove essential to plant survival, even though it comes at the cost of apparently ‘inefficient’ allocation of photosynthates. At the plant community level, functional redundancy can be described as a feature that ensures the persistence of ecosystem processes following perturbation of species assemblages (Pillar et al., 2013). Likewise, it might be that at the single plant level, deep roots, while arguably a costly and redundant set of absorptive organs at times when shallow resources are readily available, could become an insurance against the potentially fatal consequences of drought under less favourable conditions.
There are documented cases of extreme root system specialization, particularly in nutrient-impoverished environments (Poot and Lambers, 2008) and it seems a reasonable proposition that over geological times, extreme environments have led to the evolution of species of reduced phenotypic plasticity due to the need to adapt to the restrictions imposed by such environments. Root climbers (e.g. Hedera helix – common ivy) that attach to virtually any surface with glandular secretions or by growing into irregularities represent other examples of such cases of extreme root system specialization (Isnard and Silk, 2009). However, the existence of such specific evolutionary cases is not at odds with the overall conceptual framework that consists of proposing that the main driver of root growth, placement and functioning is access to the most readily available resources.
To sum up, although there is solid ground to hypothesize that shallow roots are at competitive advantage over deeper roots, many other factors, such as resilience against climate variability, the relative paucity of certain nutriments in some soils, even in shallow horizons, or competition/facilitation processes between individuals within a plant community are among reasons that probably explain why deep rooting could be a more widespread trait among a variety of plants than commonly acknowledged.
DEEP ROOTING AS A MEANS TO IMPROVE GEOCHEMICAL CYCLING IN AGRO-ECOSYSTEMS
Plant roots are one of the many biological components of the Critical Zone, a concept that encompasses the Earth’s permeable layers extending from the top of the vegetation canopy down to and including the zone of freely circulating fresh groundwater. As part of this concept, there is a growing recognition that the base of the Critical Zone, which also corresponds to ecosystems’ lower boundaries, is deeper and more open, diffuse, heterogeneous and temporally variable than generally thought (Richter and Yaalon, 2012). Plants and associated micro-organisms are known to exert feedback controls over the fate of lithology through the exudation of organic acids, ligands and a range of solubilizing compounds that promote the dissolution of minerals and the release of nutrients in plant-available forms (Hinsinger et al., 2011). The functional importance of deep root systems is probably widely underestimated, even in annual crops. Kristensen and Thorup-Kristensen (2004a, b) and Thorup-Kristensen et al. (2009) reported that crops such as autumn white cabbage (Brassica oleracea L.) and winter wheat (Triticum aestivum L.) can develop root systems to a depth of 2·5 m over a growing period of 3–4 months. Although they had fewer deep than shallow roots, the latter were actively involved in the removal of nutrients, and mineral uptake by deep roots was as substantial as that of shallower roots (Fig. 3). Such observations clearly demonstrate that designing and implementing cropping systems on the sole basis of knowledge about plant functioning within shallow soil layers equates to taking the risk of ignoring some essential eco-physiological mechanisms and thus potentially jeopardizing their efficiency and sustainability. Even though their functions are not yet fully understood, there is a body of evidence suggesting that deep roots could play a role of utmost importance as a ‘safety net’ against surface stress such as drought and soil loss and that, at the landscape scale, they could contribute to prevent water and nutrient losses by deep drainage (Bergeron, 2011; Laclau et al., 2013). For example, Thorup-Kristensen and Rasmussen (2015) found that several cultivated species such as Dyer’s woad or chicory can develop substantial amounts of roots at soil depths >2 m and that such deep rooting significantly improves nitrogen uptake from deep soil layers, thus effectively reducing nutrient losses through deep leaching in agro-ecosystems.
Fig. 3.
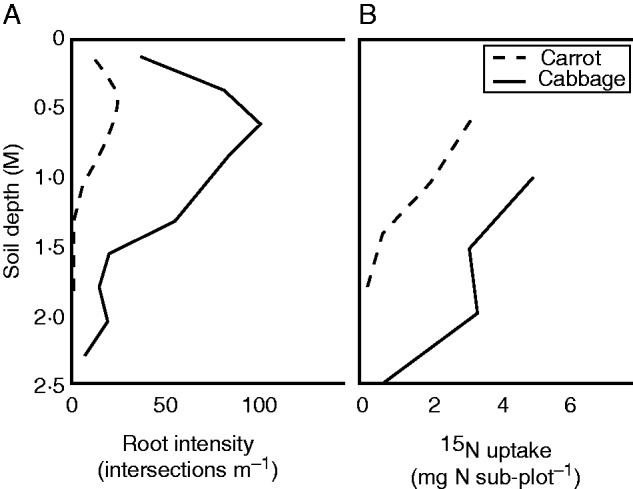
Average deep rooting and nutrient uptake in annual crops (n = 4). (A) Root intensity; (B) plant 15N uptake determined, 6 d after tracer injection, from 15N enrichment in the above-ground biomass of plants grown in sub-plots where 15N was injected at four different soil depth increments (0·6, 1·0, 1·4 and 1·8 m for carrot and 1·0, 1·5, 2·0 and 2·5 m for cabbage) (redrawn from Kristensen and Thorup-Kristensen, 2004a).
Designing and encouraging the adoption of land uses that increase the depth of carbon input by roots should lead to enhanced storage, although this will be modulated by soil mineralogy and microclimate. Developing strategies to enhance carbon storage in soils will also require understanding the controls over plant allocation below-ground and the efficiency of below-ground carbon storage. Among options that could rapidly be scaled up, agroforestry represents an avenue to improve carbon sequestration of agro-ecosystems significantly, due to at least two processes. First, agroforestry is known to promote deeper rooting of trees compared with monoculture (Mulia and Dupraz, 2006; Fig. 4), most probably as a result of a plastic response of trees to the competition with the annual crop. Secondly, in intercropping systems, higher carbon assimilation results in net accumulation of carbon, compared with a monocropping system (Peichl et al., 2006). A recent synthesis (Hamon et al., 2009) of carbon sequestration potential of temperate agroforestry systems concluded that a potential of 2 Mg ha–1 year–1 of sequestered carbon is plausible, which may be more efficient than the additional carbon sequestration that occurs in established forests.
Fig. 4.
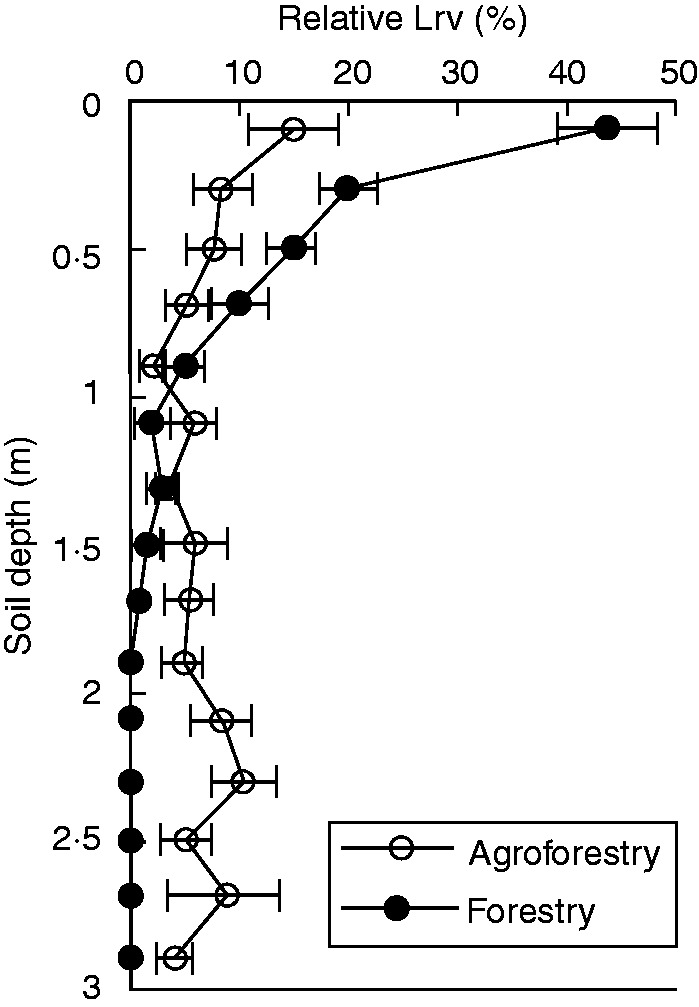
Root distribution profiles of walnut in agroforestry and forestry plots. Horizontal bars extend ± 1 s.e. around mean values for each soil depth. Note enhanced root growth below 1·5 m in the agroforestry plot (redrawn from Mulia and Dupraz, 2006).
In a walnut agroforest of southern France, Germon et al. (2015) reported rarely observed root phenological cycles at soil depths of 1·7–4·7 m. While shallow root growth occurred mainly during the spring and summer, deep root growth occurred at bud break in early spring and throughout the winter, suggesting that root production estimates derived from shallow roots may be underestimated (with fine roots below a depth of 4 m accounting for more than a quarter of the overall root production along the 4·7 m profile). Further, root turnover was between 20 and 50 % higher (depending on root diameter) between the surface and 1·7 m than below 1·7 m (Germon et al., 2015), with only 10 % of fine roots below 4 m dying during a 19 month period.
In a rubber tree plantation of north-eastern Thailand, Maeght et al. (2015) found that fine root turnover increased between soil depths of 0·5–3 m, but decreased again at 4·5 m. This work also showed that shallow fine root emergence occurred shortly after major rainfall events and was in sync with seasonal rainfall variations, i.e. increased with the onset of the rainy season and virtually ceased during the dry season. In contrast, root emergence below 2·5 m increased toward the onset of the dry season and continued to occur during the driest months of the year.
The observed variations in fine root turnover and the fine root emergence reported in the two previous case studies indicate an asynchrony between root growth dynamics close to the surface and at depth in two different tree species and under different climates. Using a dynamic model of plant water uptake (Holdo, 2013), Nippert and Holdo (2015) found that root systems respond to the variability of water availability within the soil volume through morphological and functional plasticity as well as local adjustments in hydraulic conductivity. The two previously discussed examples of differentiated root growth dynamics with soil depth concur with the hypothesis of Nippert and Holdo (2015). While maximum rooting depth sets the lower boundary for resource acquisition by individual plants, thereby enabling some perennials to overcome drought stress or minimize competition, tree root systems also deploy high degrees of morphological and functional plasticity in response to changing soil conditions.
To summarize, even though data about deep root growth dynamics and deep root morphological and functional plasticity remain scarce, recently published evidence suggests that improved knowledge of such processes might lead to novel ways to improve geochemical cycling in agro-ecosystems, including improved water and nutrient use and potentially increased soil carbon deposition and storage.
DEEP ROOTING, DROUGHT TOLERANCE AND HYDRAULIC REDISTRIBUTION
Even though deep rooting may be widespread, its role in water uptake does not seem as straightforward as one would assume upon first assessment; indeed, a recent analysis found that 70 % of 226 forest species from 81 sites worldwide operate with narrow (<1 MPa) hydraulic safety margins against injurious levels of drought stress (Choat et al., 2012). This result indicates that most tree species, especially temperate angiosperms, appear to maximize carbon fixation through a sort of upregulation of stomatal conductance, which in turns results in increased risk of embolism, thus jeopardizing their survival. In contrast, other authors found that during severe drought, the hydraulic conductance of 20 m deep roots increased 2·6-fold from spring values in association with upregulated aquaporin activity, thus avoiding total hydraulic failure (Johnson et al., 2014).
A detailed assessment of ET and water uptake in rubber tree plantations in north-eastern Thailand and central Cambodia revealed sustained high rates of water use throughout the dry season. As surface soil dried up, an increasing proportion of water was extracted by deep roots and, by the end of the dry season, more than half of the water transpired by the trees originated from soil layers below 1·7 m (Giambelluca et al., 2016). In another study of sub-tropical Cerrado forest sites in Brazil, water fluxes of the order of 15–20 mm month–1 during very dry months were found to correspond to deep root uptake (below 25 m) (Christoffersen et al., 2014).
In an attempt to clarify why, in the Amazonian rain forest, the taller trees display higher photosynthetic activity and ET during the dry season than in the wet season, Ivanov et al. (2012) explicitly investigated the concept of ‘root niche separation’ using a mechanistic model that includes tree ‘big leaf’ canopy layers and a 36 m deep soil profile. This concept relies on the assumption that, in a mature forest, overstorey trees develop deep roots which, during the dry season, extract water stored from wet season precipitation, while constantly light-limited understorey develops shallower roots that readily access dry season precipitations. This work demonstrated that while capillary rise was too marginal to explain stress avoidance, there was a deepening of the uptake centroid of taller trees during drought. Such a behaviour combined with the assumption of a shallow rooted understorey, validates the concept of ‘root/water uptake niche separation’ as a possible mechanism of drought avoidance in this environment (Ivanov et al., 2012). Likewise, recent measurements carried out in an agroforest of southern France demonstrated that walnut trees intercropped with durum wheat displayed deeper, more vertically homogeneous fine root profiles, although at much lower root length densities, than trees grown in pure forestry stands. This increased exploration of larger volumes of soil at depth by trees in agroforestry systems was interpreted as a plastic response of trees to reduce competition from crop roots, enabling trees to access deeper water resources beyond the reach of crop roots (Cardinael et al., 2015).
Hydraulic redistribution, or uplift, is the process by which plants can passively transfer water from deep, moist soil layers to shallow, dry soil layers (Caldwell et al., 1998). Although it has attracted recent research interest, a mechanistic understanding of hydraulic redistribution and its implications for ecosystem functioning is still lacking (Prieto et al., 2010). We thus need to assess its importance for crops and forest systems. If we assume that the water quantities involved in this transfer are large, the hydraulic lift could significantly alter the competition between plants with respect to transpiration.
Alternatively, if lifted water quantities are modest, they may nevertheless have a strong environmental impact: water transported from a wet horizon to a drier horizon may have several functions: (a) it could assist in mobilizing nutrients that are beyond reach in dry soil, (b) serve to decrease soil resistance to penetration by roots or (c) allow roots to survive in very dry horizons and be reactive at the return of the rains. Therefore, according to this scenario, the hydraulic lift is used, not to displace large amounts of water, but to use water in order to gain access to limiting resources (nutrients).
It is documented that the leaf water status of some tropical rain forest trees remains rather unchanged during seasonal periods of drought (Stahl et al., 2013a). Such an observation is consistent with the hypothesis that some tropical trees could, at least partly, mitigate the stress corresponding to drought by extracting deep water when shallow soil water becomes scarce. Indeed, based on field measurements involving dual-isotope labelling coupled with modelling, Stahl et al. (2013b) demonstrated that, during a pronounced drought event, more than half of the trees making up a mature rainforest stand relied on soil water extracted below 1 m. However, these authors only found a weak relationship between tree dimensions (height and diameter) and mean depth of water uptake; such a relationship explains only 10–15 % of the observed variability (Stahl et al., 2013a). This result highlights that allometric relationships are likely to be weak predictors of maximum rooting depth and of mean depth of water uptake, despite the fact that they are often used for such purposes (e.g. Gentine et al., 2015).
To sum up, even though published evidence is somewhat contradictory and indicates the likely existence of complex interacting processes, it seems possible that, under a range of biophysical conditions, particularly in tropical and sub-tropical environments, deep rooting plays a central role in drought tolerance. In addition, It might be misleading to appraise the role of deep roots in water uptake considering exclusively the volumes or proportions of water supplied by deep roots vs. that originating from shallow roots, as, even though quantitatively marginal, deep root water uptake might be pivotal to plant survival during critically dry periods. Finally, in rain-fed cropping systems, deep-rooted crops may represent an effective means to capture water otherwise lost to deep drainage (Passioura, 2006) which in turn is demonstrably a strategy to increase productivity in water-limited environments as an extra 10 mm of deep soil water can yield an extra 0·6 t of grain ha−1 (Kirkegaard et al., 2007).
THE ROLE OF DEEP ROOTING IN SOIL CARBON DYNAMICS
The picture that has emerged over the past decade is that there is a lot more deep soil carbon than we once thought, but the underlying processes controlling its turnover are still largely unknown (Schmidt et al., 2011). The potential for soils to soak up atmospheric carbon is primarily affected by the balance between the rate at which fresh photosynthetic material, i.e. roots and exudates, is deposited and the time required by these carbon inputs to get broken down through heterotrophic respiration (Matamala et al., 2003; Strand et al., 2008; Mathieu et al., 2015). Further, as root tissue is more recalcitrant to degradation and mineralization than topsoil litter, root-derived carbon has a long residence time (Rasse et al., 2005). Soil is therefore likely to be the most effective carbon sink in many ecosystems because of the long turnover time of soil organic matter (SOM) compared with most plant tissues, and because of less inter-annual variability or disturbance-driven losses (erosion, mass-wasting, fire), particularly at depth. Nevertheless, investigations on below-ground biogeochemical processes and cycles overwhelmingly focus on the shallowest soil horizons: for example, 90 % of 360 studies on the effects of land use change on SOM sampled the soil to ≤ 30 cm (Richter and Billings, 2015). Such poor characterization of deep soil processes and dynamics may lead to a significant misunderstanding of the evolution and resilience of terrestrial ecosystems: for example, over a century, a change of 0·1 % in SOC in 100 cm of sub-soil, while difficult to detect, would be the same order of magnitude as changes in SOC contents estimated in the upper 20–30 cm (Richter and Billings, 2015). For example, it was found that reforestation in sub-tropical South-eastern USA resulted, after 40 years, in a 60 % increase in SOC in the 0–7·5 cm horizon while SOC remained unchanged between 7·5 and 35 cm and decreased by about 30 % between 35 and 60 cm, with gains in the topsoil being offset by losses in the sub-soil (Mobley et al., 2015).
Fine-root production represents about a quarter of terrestrial net primary production globally (McCormack et al., 2015) and although fresh carbon supply from roots may induce a ‘priming effect’ leading to the breakdown of pre-existing SOM by soil micro-organisms (Fontaine et al., 2007), there are reports that root-derived carbon is more easily retained in soil than carbon inputs from litter fall (Rasse et al., 2005; Schmidt et al., 2011). Hence root biomass is likely to be one of the main components of the terrestrial carbon budget, and storing more carbon in soils, particularly at depth, could be an effective and readily available means to mitigate climate change. Yet existing estimates of root biomass are overwhelmingly derived from allometric relations, the accuracy of which remains largely uncertain (Yuen et al., 2013; see ‘Uncertainties regarding deep rooting and its determinants’ above). Likewise, current estimates of SOC are almost exclusively based on a sampling depth of 0·3 m (Aalde et al., 2006) even though carbon deposition from deep root growth could be much more substantial than commonly accepted: for example, Harper and Tibbett (2013) sampled SOC in deeply weathered regolith in south-western Australia down to 38 m (mean 21 m) and found that 50–75 % of the SOC occurred within the top 5 m of soil profiles, with mean SOC mass densities at least 2–5 times greater than would be reported with standard IPCC sampling depth. In view of such unusual findings, the authors of this study called for (1) a reassessment of the current arbitrary shallow soil sampling depths for assessing carbon stocks; (2) a revision of global SOC estimates; and (3) elucidation of the composition and fate of deep carbon in response to land use and climate change.
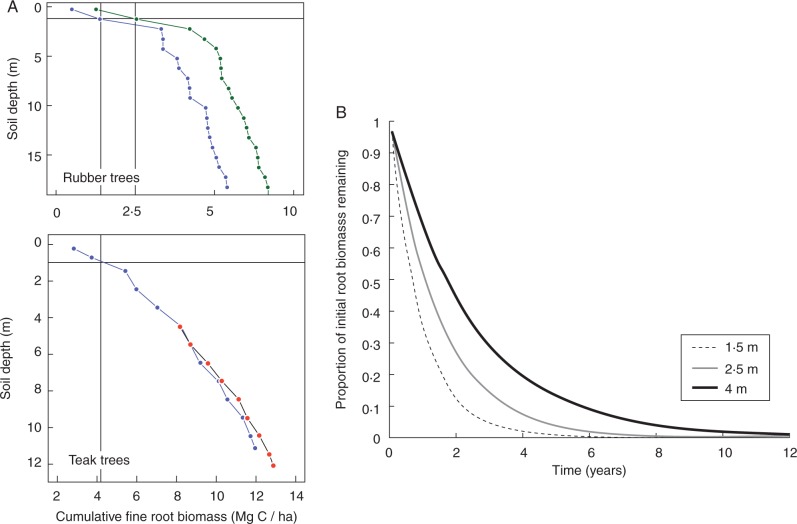
With regards to the dynamics of deep root breakdown, Prieto et al. (2016) recently provided evidence that, across and within plant communities of seven sites world-wide encompassing a land-use gradient ranging from agricultural crops to natural forests, deep roots have a lower decomposability than shallow root. A thorough investigation of the traits of fine roots of 74 species (including graminoids and eudicots) collected in three biomes also suggested that root decomposability is a complex process that depends both on root traits and on interaction with rhizosphere microbial communities (Roumet et al., 2016). Indeed, measurements carried out in rubber and teak tree plantations in north-eastern Thailand and Laos, respectively, showed that, in such tropical environments, even relatively young trees (<20 years) can develop significant amounts of fine roots at soil depths >1 m (Fig. 5A). Further, in the case of rubber trees in north-eastern Thailand, the dynamics of root tissue breakdown tend to be slower at depth than nearer the soil surface (Fig. 5B; Gonkhamdee et al., 2010b), even though this decrease is not monotonous with depth and probably varies depending on, for example, local moisture conditions. Such observations suggest that, in the context of young tropical tree plantations, root carbon deposition below 1 m can be substantial and that the residence time of root-derived carbon at depth may be longer than near the soil surface.
Fig. 5.
(A) Examples of deep rooting profiles of rubber trees in Southern Thailand (top) and teak trees in Laos (bottom). Horizontal and vertical dotted lines indicate a soil depth of 1 m and the amount of fine root C (in Mg ha–1) found in the top first metre of soil, respectively, for each cumulative root profile. Fine root biomass below 1 m is about 3-fold that above 1 m. Note that roots may still be present below the sampled depths. (B) Dynamics of root tissue breakdown at different soil depths in a rubber tree plantation of north-eastern Thailand. Breakdown was the fastest at a depth of 2·5 m, intermediate at 1·5 m and the slowest at 4 m. In relation to rooting profiles shown in (A), which indicate that root carbon deposition below 1 m can be substantial, such differences in root degradation with depth suggest that the residence time of root-derived C at depth may be longer than near the soil surface (adapted from Gonkhamdee et al., 2010b; Maeght, 2014).
In a young rubber tree plantation of north-eastern Thailand established on land converted from dry dipterocarp secondary forest, Montoroi et al. (2015) found unexpectedly high amounts of roots at soil depths ranging from 10 to 32 m (Fig. 6A). As the rubber trees were <5 years old at the time of sampling, the relatively profuse amounts of roots found at soil depths of 10–25 m could only be attributed to the pre-existing forest. A root sample was collected for each 1 m depth increment from the soil surface down to 32 m. Each of these samples was oven-dried for 48 h at 60 °C and subsequently weighed using a precision balance. A sub-sample of 10 individual roots revealed that the carbon content of this deep root material was on average 24·5 ± 10·3 % (s.e.m.), which, while quite variable, corresponds to the presence of substantial amounts of carbon in this material. Scanning electron microscopy images of some root specimens clearly revealed well-preserved cell membranes (Fig. 6B). X-ray energy dispersion (EDX) measurements suggest that cell membranes were coated with a thin layer of 2/1 clay mineral (mainly smectite), probably resulting from the periodic input of minerals by the water, thus protecting root tissues from microbial activity and preserving the original organic material within a sheath of mineral deposits. Such a scenario, if proved correct and sufficiently widespread, could represent a mechanism through which deep root-related carbon deposition is stored in the soil.
Such processes of delayed root carbon breakdown at depth are of particular interest as a potential approach to reduce the recent, human-induced build-up of atmospheric carbon dioxide (CO2), or at least to slow down the turnover (mineralization) of below-ground carbon (BGC). Although this approach forms the basis of global schemes for carbon trading [such as those arising from Reducing Emissions from Deforestation and Forest Degradation (REDD)-type projects], it remains unclear how much carbon can effectively be sequestered in an ecosystem, at what rate and how this can be improved (Jackson et al., 2000; Ziegler et al., 2012; Yuen et al., 2013).
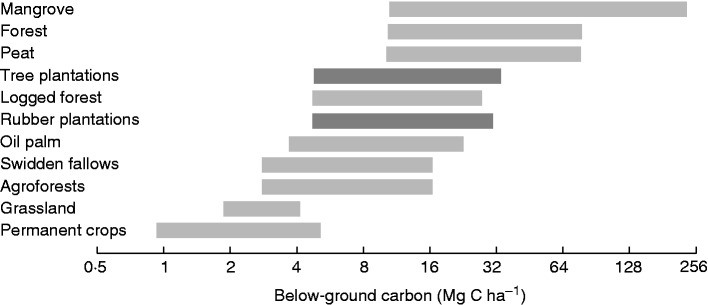
In conclusion, it appears that existing estimates of below-ground biomass are overwhelmingly derived from non-standardized protocols and allometric relations (such as root:shoot ratios) that are not yet sufficiently robust (Yuen et al., 2013; Fig. 7) and that processes that determine the long-term fate of deep root carbon are still very unclear.
Fig. 7.
Below-ground carbon biomass for 11 land-covers in south-east Asia: thick lines correspond to adjusted ranges of values after the removal of outlying values. Ranges corresponding to teak and rubber trees, species for which we provided below-ground carbon profiles in Fig. 5 are indicated in dark grey. Note that (1) the ranges reported here include all root types, not only fine roots as in Fig. 5 and (2) most published rooting profiles are truncated, which results in the wide variability of below-ground carbon estimates (adapted from Yuen et al., 2013).
DEEP ROOTS AND CLIMATE CHANGE ATTENUATION
Accurate knowledge of rooting depth is essential to understand the interactions between plants and the water cycle and their complex feedback effects on the carbon cycle. Although roots have long been recognized as a unique and essential link between the pedosphere, on the one hand, and the biosphere and the atmosphere, on the other hand, they only received greater attention from soil scientists, ecologists and climatologists relatively recently (Clothier and Green, 1997; Feddes et al., 2001). Therefore, it is not surprising to find that climate and hydrological models only include very crude descriptions of root water uptake, due to the lack of robust estimates of the parameters that control this function. Besides possibilities of increasing the residence time of root-derived carbon into soil, especially in deep soil layers, there are other, often more complex interactions between plants, the pedosphere and the atmosphere, that could open up new avenues for climate change mitigation.
For example, several studies report a link between vegetation cover and rainfall patterns at the regional scale (Pitman et al., 2004); according to the so-called ‘biotic pump’ concept, the forest cover drives the ocean-to-land atmospheric moisture transport at the continental scale (Makarieva et al., 2012). On the other hand, others reported that changes in precipitation over continental masses result from complex processes only partly influenced but not controlled by local water sources or vegetation (Angellini et al., 2011). Some of these studies have specifically identified rooting depth of the vegetation as a major undermonitored climate variable that needs to be more accurately documented (Pielke et al., 2007).
There is also a growing interest among climatologists in the possible importance of heat and water exchanges between the atmosphere and deeper soil layers as a means to improve our understanding of the global climate. Indeed, besides the physically driven moisture exchanges between aquifers and overlying soil layers, the roots of many trees have the ability to tap directly into deep water tables, at least during dry periods (Fan and Miguez-Macho, 2010). Krakauer et al. (2013) performed modelling that showed that modifying tree root distributions so that 20 % of their roots stand below 1 m improved access to deeper soil moisture by vegetation and allowed more extensive interactions between the soil and the aquifer layers. This was found to have limited impact on the mean climate but to improve the description of seasonality and inter-annual persistence. Krakauer et al. (2013) indicated that further improvement of the model could be achieved with the inclusion of roots that reach soil depths below 3·5 m (Stone and Kalisz, 1991), whether in permeable deep soil or regolith (Nepstad et al., 1994; Markewitz et al., 2010) or even in fractured bedrock (Roering et al., 2010; Schwinning, 2010).
Other authors have investigated the potential impact of hydraulic lift (also referred to as vertical hydraulic redistribution), i.e. the transfer of soil water from deep and moist soil horizons to drier and shallower parts, on photosynthesis and ET. Incorporating such a mechanism into an atmospheric general circulation model (GCM), Lee et al. (2005) found that in the case of the Amazonian forest, hydraulic lift significantly increased photosynthesis and ET, which in turn affected the seasonal patterns of air temperature, this outlining a direct link between deep plant root functioning and climate. Recent evidence indicates that in annual crops, lower canopy temperatures can be weakly correlated to maximum rooting depth (Wasson et al., 2014), thus further suggesting such a putative causal link between deep rooting and climate.
Another example of complex interactions between plants and climate involves physical and chemical mechanisms by which symbiotic mycorrhizal fungi of forest tree roots potentially enhance carbonate rock weathering. Rhizosphere respiration is known to induce carbonic acid weathering of primary minerals which in turn releases soluble nutrients taken up by deep roots and highly alkaline drainage waters (Richter and Billings, 2015). Such a process of alkalinity export is particularly prevalent in the context of carbonate lithologies, and currently rising atmospheric CO2 might enhance it (Thorley et al., 2014). The deeper part of root systems releases CO2, which, while representing only a very small fraction of soil respiration, participates in a process of biophysical magnification of CO2 partial pressures (Jackson et al., 2009), which induces the release of cations and generates alkalinity in the hydrosphere. Ultimately, a marginal fraction of the system’s total biomass deep in the profile, i.e. deep fine roots and associated microbes, could have an enormous influence, through respiration, on ecosystem structure and function, directly influencing the rates at which soil will form (Richter and Billings, 2015). While on million-year time scales, such a process has no net effect on atmospheric CO2 concentration, on time scales of decades to centuries, it may increase the flux of continental alkalinity to the oceans, thus counteracting ocean acidification (Thorley et al., 2014).
In summary, even though far from fully elucidated, there are several pieces of evidence arising from very diverse research perspectives that indicate that deep rooting could exert both direct and indirect influences on climate change mitigation.
CONCLUDING REMARKS: TOWARDS MORE UNCONVENTIONAL RESEARCH ON DEEP ROOTS AND THEIR FUNCTIONS
Herein, we reviewed diverse research that converges to demonstrate that plant roots consistently explore a considerable soil depth range, and that while roots are certainly much less densely distributed at depth than in the surface layers, deep roots and associated functions are likely to have a significant impact on biogeochemical cycles.
To a large extent, our knowledge of deep fine roots remains extremely limited due to the fact that research on deep roots is hampered by the lack of appropriate methodology to study them accurately. This state of affairs results in a major observational bias, which consists of the vast majority of root research focusing on shallow roots. Indeed, it is not because research questions related to deep roots and deep root functioning lack an adequate empirical or process-based rationale that they are still underinvestigated, but because of the technical and practical challenges they entail. Deep root research also struggles to gain momentum as ‘safer’, more conventional research is more widely supported by donors than more daring, unconventional investigations that involve a larger degree of risk, even though the latter are much more conducive to discovery than the former (Battaglia and Atkinson, 2015).
Further, many of the examples discussed herein also indicate that many broadly accepted views regarding roots and root functioning, particularly at depth, rest on a rather controversial body of evidence. Consequently, we propose a new conceptual model of root growth in which the primary driver is the balance between environmental constraints and opportunities to access resources. While we do not claim that genetic pre-determinism only has a marginal influence on root development, the model that we propose has a genetically pre-set developmental scheme for root growth and root architecture which is most often, and sometimes largely, modulated as a result of the local biophysical conditions experienced by roots and integrated at the scale of the whole plant. Fundamentally, such a model shares common characteristics with that proposed by Schenk (2008b) as it recognizes the importance of resource uptake as a driving factor of root growth and includes the notion that rooting profiles are as deep as is necessary to satisfy the nutritional needs of the plant. It potentially departs from the Schenk model, as it does not assume that the shallowest resources are necessarily the most readily available.
From the practical standpoint, several of the examples that we have examined indicate that deep rooting could help in mitigating climate change by inducing changes in air temperature and moisture, and increasing carbon input to deep soil layers. Further, it is known that deep-rooted trees of the Devonian era and the rise of angiosperms since the Cretaceous have decreased atmospheric CO2 by at least one order of magnitude (Kell, 2011). Such geological evidence indicates that decreases needed to curb current global warning are probably within reach, provided more practical understanding of deep rooting becomes available.
With regards to food production, we have discussed the fact that modern grain crops have been bred with little or no attention to their root traits, particularly rooting depth, despite the fact that many have the potential to extend to at least twice the rooting depth of current cultivars (Kell, 2011). The Green Revolution largely – if not exclusively – relied upon the selection of high-yielding varieties and the addition of external inputs from the soil surface, thus primarily focusing on above-ground and near-surface processes and largely overlooking the impact of below-ground on plant performance (Den Herder et al., 2010). Although assuming that processes relevant to agronomic applications are essentially shallow has not hampered the overall success of the Green Revolution, it nevertheless gave rise to unforeseen problems such as eutrophication of water bodies due to deep nutrient leaching. There is tangible evidence that deeper rooted crops may help in reducing environmental hazard resulting from nutrient leaching (Thorup-Kristensen et al., 2009). In addition, deep roots may be of central importance for accessing water in amounts sufficient to maximize yields, even in fertile soils. Further, decades of increasing global demand for food, timber, fibre and fuel have resulted in a shortage of arable land, which has led to the conversion of land of marginal agronomic value to agriculture, particularly in the least developed countries. Considering the worldwide shortage of cropland, it is evident that deep rooting now represents one of the last frontiers to be explored for the development of a more efficient and sustainable cropping systems.
Understanding the role of deep roots for agronomic purposes holds promises for the design of more environmentally friendly cropping systems. For example, due to its deep root growth and nutrient uptake (down to 2·5 m), winter wheat was found to have better grain yields than spring wheat grown without previous winter catch crop (Thorup-Kristensen et al., 2009). This was due to the fact that deep winter wheat roots retrieved more nitrogen from the lower parts of the profile than shallower rooted spring wheat. Such an example also demonstrates that deep root research is not inherently risky and that, like more conventional areas of root research, it also has potential for short-term return on investment. Deep-rooted crops were also found to capture water otherwise lost to deep drainage, thus increasing water productivity in water-limited environments (Kirkegaard et al., 2007). Such encouraging results clearly outline the need for more research aimed to optimize the exploitation of sub-soil resources by crops as a means to reduce external inputs and nutrient losses to the environment. Despite the many hurdles that pave the way to a practical understanding of deep rooting functions, we anticipate that, in the future, much needed knowledge about the deep rooting traits of a variety of plants and crops will be increasingly influential with respect to how we manage natural and cultivated ecosystems.
ACKNOWLEDGEMENTS
The authors would like to express their deepest gratitude to all the members of the organizing committee of the 9th Symposium of the International Society of Root Research, held in Canberra, Australia from 6 to 9 October 2015, for having invited us to present our research at this event and for having encouraged us to write this viewpoint paper.
LITERATURE CITED
- Aalde H, Gonzalez P, Gytarsky M, et al. 2006. Generic methodologies applicable to multiple land-use categories In: Eggleston S, Buendia L, Miwa K, Ngara T, Tanabe K, eds. IPCC guidelines for national greenhouse gas inventories, vol. 4. Agriculture, forestry and other land use. Kanagawa: IGES. [Google Scholar]
- Abramoff RZ, Finzi AC. 2015. Are above- and below-ground phenology in sync? New Phytologist 205: 1054–1061. [DOI] [PubMed] [Google Scholar]
- Amato M, Pardo A. 1994. Root length and biomass losses during sample preparation with different screen mesh sizes. Plant and Soil 161: 299–303. [Google Scholar]
- Angelini IM, Garstang M, Davis RE, et al. 2011. On the coupling between vegetation and the atmosphere. Theoretical and Applied Climatology 105: 243–261. [Google Scholar]
- Battaglia M, Atkinson MA. 2015. The streetlight effect in type 1 diabetes. Diabetes 64: 1081–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battie-Laclau P, Laclau J-P. 2009. Growth of the whole root system for a plant crop of sugarcane under rainfed and irrigated environments in Brazil. Field Crops Research 114: 351–360. [Google Scholar]
- Bengough AG, McKenzie BM, Hallett PD, Valentine TA. 2011. Root elongation, water stress, and mechanical impedance: a review of limiting stresses and beneficial root tip traits. Journal of Experimental Botany 62: 59–68. [DOI] [PubMed] [Google Scholar]
- Bergeron M, Lacombe S, Bradley RL, et al. 2011. Reduced soil nutrient leaching following the establishment of tree-based intercropping systems in eastern Canada. Agroforestry Systems 83: 321–330. [Google Scholar]
- Caldwell MM, Dawson TE, Richards JH. 1998. Hydraulic lift: consequences of water efflux from the roots of plants. Oecologia 113: 151–161. [DOI] [PubMed] [Google Scholar]
- Canadell J, Jackson R, Ehleringer J, Mooney HA, Sala OE, Schulze ED. 1996. Maximum rooting depth of vegetation types at the global scale. Oecologia 108: 583– 595. [DOI] [PubMed] [Google Scholar]
- Cardinael C, Mao Z, Prieto I, et al. 2015. Competition with winter crops induces deeper rooting of walnut trees in a Mediterranean alley cropping agroforestry system. Plant and Soil 391: 219–235. [Google Scholar]
- Choat B, Jansen S, Brodrib TJ, et al. 2012. Global convergence in the vulnerability of forests to drought. Nature 491: 752–755. [DOI] [PubMed] [Google Scholar]
- Christina M, Laclau J-P, Gonçalves JLM, Jourdan C, Nouvellon Y, Bouillet J-P. 2011. Almost symmetrical vertical growth rates above and below ground in one of the world’s most productive forests. Ecosphere 2: art27. [Google Scholar]
- Christoffersen BO, Restrepo-Coupe N, Arain MA, et al. 2014. Mechanisms of water supply and vegetation demand govern the seasonality and magnitude of evapotranspiration in Amazonia and Cerrado. Agricultural and Forest Meteorology 191: 33–50. [Google Scholar]
- Clothier BE, Green SR. 1997. Roots: the big movers of water and chemicals in soils. Soil Science 162: 534–543. [Google Scholar]
- Den Herder G, Van Isterdael G, Beeckman T, De Smet I. 2010. The roots of a new green revolution. Trends in Plant Science 15: 600–607. [DOI] [PubMed] [Google Scholar]
- Estrada-Medina H, Graham RC, Allen MF, Jiménez-Osornio JJ, Robles-Casolco S. 2012. The importance of limestone bedrock and dissolution karst features on tree root distribution in northern Yucatán, México. Plant and Soil 362: 37–50. [Google Scholar]
- Fan Y, Miguez-Macho G. 2010. Potential groundwater contribution to Amazon evapotranspiration. Hydrology and Earth System Science 14: 2039–2056. [Google Scholar]
- FAO. 2006. Guidelines for soil description, 4th edn Rome: Food and Agriculture Organization of the United Nations. [Google Scholar]
- Feddes RA, Hoff H, Bruen M, et al. 2001. Modelling root water uptake in hydrological and climate models. Bulletin of the American Meteorological Society 82: 2797–2809. [Google Scholar]
- Fontaine S, Barot S, Barré P, Bdioui N, Mary B, Rumpel C. 2007. Stability of organic carbon in deep soil layers controlled by fresh carbon supply. Nature 450: 277–281. [DOI] [PubMed] [Google Scholar]
- Gentine P, Guérin M, Uriarte M, McDowell NG, Pockman WT. 2015. An allometry-based model of the survival strategies of hydraulic failure and carbon starvation. Ecohydrology 9: 529–546. [Google Scholar]
- Germon A, Cardinael R, Prieto I, et al. 2015. Unexpected phenology and lifespan of shallow and deep fine roots of walnut trees grown in a silvoarable Mediterranean agroforestry system. Plant and Soil 401: 409–426. [Google Scholar]
- Gewin V. 2010. An underground revolution. Nature 466: 552–553. [DOI] [PubMed] [Google Scholar]
- Giambelluca TW, Mudd RG, Liu W, et al. 2016. Evapotranspiration of rubber (Hevea brasiliensis) cultivated at two plantation sites in Southeast Asia. Water Resources Research 52: 660–679. [Google Scholar]
- Gonkhamdee S, Pierret A, Maeght J-L, et al. 2010a. Effects of corn (Zea mays L.) on the local and overall root development of young rubber tree (Hevea brasiliensis Muel. Arg). Plant and Soil 334: 335–351. [Google Scholar]
- Gonkhamdee S, Maeght J-L, Do F, Pierret A. 2010b. Growth dynamics of fine Hevea brasiliensis roots along a 4.5-m soil profile. Khon Kaen Agriculture Journal 37: 265–276. [Google Scholar]
- Hamon X, Dupraz C, Liagre F. 2009. L’Agroforesterie, Outil de Séquestration du Carbone en Agriculture. Rapport d’expertise pour le Ministére de l’Agriculture, de l’Alimentation et de la Pêche, Agroof, INRA éditeurs, Montpellier.
- Harper RJ, Tibbett M. 2013. The hidden organic carbon in deep mineral soils. Plant and Soil 368: 641–648. [Google Scholar]
- Hawes MC, Bengough AG, Cassab G, Ponce G. 2003. Root caps and rhizosphere. Journal of Plant Growth Regulation 2: 352–367. [Google Scholar]
- Hinsinger P, Brauman A, Devau N, et al. 2011. Acquisition of phosphorus and other poorly mobile nutrients by roots. Where do plant nutrition models fail? Plant and Soil 348: 29–61. [Google Scholar]
- Holdo RM. 2013. Revisiting the two-layer hypothesis: coexistence of alternative functional rooting strategies in savannas. PLoS One 8: e69625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutsch BW, Augustin J, Merbach W. 2002. Plant rhizodeposition: an important source of carbon turnover in soils. Journal of Plant Nutrition and Soil Science 165: 397–407. [Google Scholar]
- Isnard S, Silk W. 2009. Moving with climbing plants from Charles Darwin’s time into the 21st century. American Journal of Botany 96: 1205–1221. [DOI] [PubMed] [Google Scholar]
- Ivanov VY, Hutyra LR, Wofsy SC, et al. 2012. Root niche separation can explain avoidance of seasonal drought stress and vulnerability of overstory trees to extended drought in a mature Amazonian forest, Water Resources Research 48: W12507. doi:10.1029/2012WR011972. [Google Scholar]
- Jackson RB, Schenk HJ, Jobbagy EG, et al. 2000. Belowground consequences of vegetation change and their treatment in models. Ecological Applications 10: 470–483. [Google Scholar]
- Jackson RB, Cook CW, Pippen JS, Palmer SM. 2009. Increased belowground biomass and soil CO2 fluxes after a decade of carbon dioxide enrichment in a warm temperate forest. Ecology 90: 3352–3360. [DOI] [PubMed] [Google Scholar]
- Jin K, Shen J, Ashton RW, Dodd IC, Parry MAJ, Whalley WR. 2013. How do roots elongate in a structured soil? Journal of Experimental Botany 64: 4761–4777. [DOI] [PubMed] [Google Scholar]
- Jobbagy EG, Jackson RB. 2001. The distribution of soil nutrients with depth: global patterns and the imprint of plants. Biogeochemistry 53: 51–77. [Google Scholar]
- Johnson DM, Sherrard ME, Domec J-C, Jackson RB. 2014. Role of aquaporin activity in regulating deep and shallow root hydraulic conductance during extreme drought. Trees 28: 1323–1331. [Google Scholar]
- Joslin JD, Gaudinski JB, Torn MS, Riley WJ, Hanson PJ. 2006. Fine-root turnover patterns and their relationship to root diameter and soil depth in a 14C-labeled hardwood forest. New Phytologist 172: 523–535. [DOI] [PubMed] [Google Scholar]
- Kell DB. 2011. Breeding crop plants with deep roots: their role in sustainable carbon, nutrient and water sequestration. Annals of Botany 108: 407–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkegaard JA, Lilley JM, Howe GN, Graham JM. 2007. Impact of subsoil water use on wheat yield. Australian Journal of Agricultural Research 58: 303–315. [Google Scholar]
- Kong D, Wang J, Kardol P, et al. 2015. The root economics spectrum: divergence of absorptive root strategies with root diameter. Biogeosciences Discussions 12: 13041–13067. [Google Scholar]
- Krakauer NY, Puma MJ, Cook BI. 2013. Impacts of soil-aquifer heat and water fluxes on simulated global climate. Hydrology and Earth System Sciences Discussions 10: 1185–1212. [Google Scholar]
- Kristensen HL, Thorup-Kristensen K. 2004a. Uptake of15N labeled nitrate by root systems of sweet corn, carrot and white cabbage from 0.2–2.5 meters depth. Plant and Soil 265: 93–100. [Google Scholar]
- Kristensen HL, Thorup-Kristensen K. 2004b. Root growth and nitrate uptake of three different catch crops in deep soil layers. Soil Science Society of America Journal 68: 529–537. [Google Scholar]
- Kutschera L. 1960. Wurzelatlas mitleleuropaïsher Ackerunkräuter und Kulturpflkanzen. Frankfurt am Main, Germany: DLG-Verlags-GmbH. [Google Scholar]
- Kutschera L, Sobotok M, Lichtenegger E, Haas D. 1997. Bewurzelung von Pflanzen in den verschiedenen Lebensräumen. 5 Band der Wurzelatlas-Reihe. Stapfia 49.
- Laclau J-P, da Silva EA, Rodrigues Lambais G, et al. 2013. Dynamics of soil exploration by fine roots down to a depth of 10 m throughout the entire rotation in Eucalyptus grandis plantations. Frontiers in Plant Science 4: 243. doi:10.3389/fpls.2013.00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J-E, Oliveira RS, Dawson TE, Fung I. 2005. Root functioning modifies seasonal climate. In: Proceedings of the National Academy of Sciences, USA 102: 17576–17581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeght J-L. 2014. Effects of climate variability on shallow and deep root growth of mature rubber (Hevea brasiliensis) and teak (Tectona grandis) trees in South East Asian plantations. PhD Thesis, Montpellier II University, Montpellier, France.
- Maeght J-L, Rewald B, Pierret A. 2013. How to study deep roots – and why it matters. Frontiers in Plant Science 4: 299. doi:10.3389/fpls.2013.00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeght J-L, Clement C, Gonkhamdee S, et al. 2015. Empirical evidence of the contribution of fine deep roots to terrestrial carbon stocks. Regional Forum on Climate Change (RFCC) Low Carbon and Climate Resilient Societies: Bridging Science, Practice, and Policy). 1–3 July 2015. Asian Institute of Technology, Thailand.
- Makarieva AM, Gorshkov VG, Li BL. 2012. Revisiting forest impact on atmospheric water vapor transport and precipitation. Theoretical and Applied Climatolology 111: 79–96. [Google Scholar]
- Markewitz D, Devine S, Davidson EA, Brando P, Nepstad DC. 2010. Soil moisture depletion under simulated drought in the Amazon: impacts on deep root uptake. New Phytologist 187: 592–607. [DOI] [PubMed] [Google Scholar]
- Matamala R, Gonzàlez-Meler MA, Jastrow JD, Norby RJ, Schlesinger WH. 2003. Impacts of fine root turnover on forest NPP and soil C sequestration potential. Science 302: 1385–1387. [DOI] [PubMed] [Google Scholar]
- Mathieu JA, Hatte C, Balesdent J, Perent E. 2015. Deep soil carbon dynamics are driven more by soil type than by climate: a worldwide meta-analysis of radiocarbon profiles. Global Change Biology 21: 4278–4292. [DOI] [PubMed] [Google Scholar]
- McCormack ML, Guo D. 2014. Impacts of environmental factors on fine root lifespan. Frontiers in Plant Science 5: 205. doi:10.3389/fpls.2014.00205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack ML, Dickie IA, Eissenstat DM, et al. 2015. Redefining fine roots improves understanding of below-ground contributions to terrestrial biosphere processes. New Phytologist 207: 505–518. [DOI] [PubMed] [Google Scholar]
- Mobley ML, Lajtha K, Kramer M, Bacon AR, Heine PR, Richter DdeB. 2015. Surficial gains and subsoil losses of soil carbon and nitrogen during secondary forest development. Global Change Biology 21: 986–996. [DOI] [PubMed] [Google Scholar]
- Montoroi J-P, Pierret A, Maeght J-L, Chintachao W, Chenyapanich S, Srisuk K. 2015. Deep root biomass and interfluve aquifer. Case of a watershed in Northeast Thailand. International Soils Conference – Sustainable uses of soil in harmony with food security. Cha Am, Thailand, 18–21 August 2015.
- Mulia R, Dupraz C. 2006. Unusual fine root distributions of two deciduous tree species in southern France: what consequences for modelling of tree root dynamics? Plant and Soil 281: 71–85. [Google Scholar]
- Nepstad D, de Carvalho C, Davidson E. 1994. The role of deep roots in the hydrological and carbon cycles of Amazonian forests and pastures. Nature 372: 666–669. [Google Scholar]
- Nguyen C. 2003. Rhizodeposition of organic C by plants: mechanisms and controls. Agronomie 23: 375–396. [Google Scholar]
- Nippert JB, Holdo RM. 2015. Challenging the maximum rooting depth paradigm in grasslands and savannas. Functional Ecology 29: 739–745. [Google Scholar]
- Nodichao L, Chopart J-L, Roupsard O, Vauclin M, Aké S, Jourdan C. 2011. Genotypic variability of oil palm root system distribution in the field. Consequences for water uptake. Plant and Soil 341: 505–520. [Google Scholar]
- Osnas JLD, Lichstein JW, Reich PB, Pacala SW. 2013. Global leaf trait relationships: mass, area, and the leaf economics spectrum. Science 340: 741–744. [DOI] [PubMed] [Google Scholar]
- Pallant E, Holmgren RA, Schuler GE, McCracken KL, Drbal B. 1993. Using a fine-root extraction device to quantify small-diameter corn roots (≤0.025 mm) in field soils. Plant and Soil 153: 273 –279. [Google Scholar]
- Passioura JB. 2002. Soil conditions and plant growth. Plant, Cell and Environment 25: 311–318. [DOI] [PubMed] [Google Scholar]
- Passioura JB. 2006. Increasing crop productivity when water is scarce – from breeding to field management. Agricultural Water Management 80: 176–196. [Google Scholar]
- Peichl M, Thevathasan N, Gordon A, Huss J, Abohassan R. 2006. Carbon sequestration potentials in temperate tree-based intercropping systems, Southern Ontario, Canada. Agroforestry Systems 66: 243–257. [Google Scholar]
- Phillips WS. 1963. Depth of roots in soil. Ecology 44: 424–429. [Google Scholar]
- Pielke RA, Sr, Adegoke J, Beltran-Przekurat A, et al. 2007. An overview of regional land-use and land-cover impacts on rainfall. Tellus 59B: 587–601. [Google Scholar]
- Pierret A, Moran CJ, Doussan C. 2005. Conventional detection methodology is limiting our ability to understand the roles and functions of fine roots. New Phytologist 166: 967–980. [DOI] [PubMed] [Google Scholar]
- Pillar VD, Blanco CC, Muller SC, Sosinski EE, Joner F, Duarte LDS. 2013. Functional redundancy and stability in plant communities. Journal of Vegetation Science 24: 963–974. [Google Scholar]
- Pitman AJ, Narisma GT, Pielke RA, Sr, Holbrook NJ. 2004. Impact of land cover change on the climate of southwest Western Australia. Journal of Geophysical Research 109: D1810. doi:10.1029/2003JD004347. [Google Scholar]
- Poot P, Lambers H. 2008. Shallow-soil endemics: adaptive advantages and constraints of a specialized root-system morphology. New Phytologist 178: 371–381. [DOI] [PubMed] [Google Scholar]
- Prieto I, Zaal Kikvidze Z, Pugnaire FI. 2010. Hydraulic lift: soil processes and transpiration in the Mediterranean leguminous shrub Retama sphaerocarpa (L) Boiss. Plant and Soil 329: 1573–5036. [Google Scholar]
- Prieto I, Roumet C, Cardinael R, et al. 2015. Root community traits along a land use gradient: evidence of a community-level economics spectrum. Journal of Ecology 103: 361–373. [Google Scholar]
- Prieto I, Stokes A, Roumet C. 2016. Root functional parameters predict fine root decomposability at the community level. Journal of Ecology 104: 725–733. [Google Scholar]
- Qian SS, Cuffney TF. 2012. To threshold or not to threshold? That’s the question. Ecological Indicators 15: 1–9. [Google Scholar]
- Rasse DP, Rumpel C, Dignac M-F. 2005. Is soil carbon mostly root carbon? Mechanisms for a specific stabilisation. Plant and Soil 269: 341–356. [Google Scholar]
- Richter DdeB, Billings SA. 2015. ‘One physical system’: Tansley’s ecosystem as Earth’s critical zone. Tansley review. New Phytologist 206: 900–912. [DOI] [PubMed] [Google Scholar]
- Richter DdeB, Yaalon D. 2012. ‘The changing model of soil’ revisited. Soil Science Society of America Journal 76: 766–778. [Google Scholar]
- Richter DD, Markewitz D. 1995. How deep is soil? Bioscience 45: 600–609. [Google Scholar]
- Roering JJ, Marshall J, Booth AM, Mort M, Jin Q. 2010. Evidence for biotic controls on topography and soil production. Earth and Planetary Science Letters 298: 183–190. [Google Scholar]
- Roumet C, Birouste M, Picon-Cochard C, et al. 2016. Root structure–function relationships in 74 species: evidence of a root economics spectrum related to carbon economy. New Phytologist 210: 815–826. [DOI] [PubMed] [Google Scholar]
- Rovira AD. 1965. Interactions between plant roots and soil microorganisms. Annual Review of Microbiology 19: 241–266. [DOI] [PubMed] [Google Scholar]
- Schenk HJ. 2008a. Soil depth, plant rooting strategies and species’ niches. New Phytologist 178: 223–225. [DOI] [PubMed] [Google Scholar]
- Schenk HJ. 2008b. The shallowest possible water extraction profile: a null model for global root distributions. Vadose Zone Journal 7: 1119–1124. [Google Scholar]
- Schenk HJ, Jackson RB. 2002a. The global biogeography of roots. Ecological Monographs 72: 311–328. [Google Scholar]
- Schenk HJ, Jackson RB. 2002b. Rooting depths, lateral root spreads and below-ground/above-ground allometries of plants in water-limited ecosystems. Journal of Ecology 90: 480–494. [Google Scholar]
- Schenk HJ, Jackson RB. 2005. Mapping the global distribution of deep roots in relation to climate and soil characteristics. Geoderma 126: 129–140 [Google Scholar]
- Schmidt MWI, Torn MS, Abiven S, et al. 2011. Persistence of soil organic matter as an ecosystem property. Nature 478: 49–56. [DOI] [PubMed] [Google Scholar]
- Schwinning S. 2010. The ecohydrology of roots in rocks. Ecohydrology 245: 238–245. [Google Scholar]
- Sen PK. 1968. Estimates of the regression coefficient based on Kendall’s tau. Journal of the American Statistical Association 63: 1379–1389. [Google Scholar]
- da Silva EV, Bouillet J-P, de Moraes Gonçalves JL, et al. 2011. Functional specialization of Eucalyptus fine roots: contrasting potential uptake rates for nitrogen, potassium and calcium tracers at varying soil depths. Functional Ecology 25: 996–1006. [Google Scholar]
- Stahl C, Burban B, Wagner F, Goret J-Y, Bompy F, Bonal D. 2013a. Influence of seasonal variations in soil water availability on gas exchange of tropical canopy trees. Biotropia 45: 155–164 [Google Scholar]
- Stahl C, Hérault B, Rossi V, Burban B, Bréchet C, Bonal D. 2013b. Depth of soil water uptake by tropical rainforest trees during dry periods: does tree dimension matter? Oecologia 173: 1191–1201. [DOI] [PubMed] [Google Scholar]
- Stone EL, Kalisz PJ. 1991. On the maximum extent of tree roots. Forest Ecology and Management 46: 59–102. [Google Scholar]
- Strand AE, Pritchard SG, McCormack ML, Davis MA, Oren R. 2008. Irreconcilable differences: fine-root life spans and soil carbon persistence. Science 319: 456–458. [DOI] [PubMed] [Google Scholar]
- Syahrinudin. 2005. The potential of oil palm and forest plantations for carbon sequestration on degraded land in Indonesia Ecology and Development Series No. 28. Göttingen: Cuvillier Verlag.
- Thorley RMS, Taylor LL, Banwart SA, Leake JR, Beerling DJ. 2014. The role of forest trees and their mycorrhizal fungi in carbonate rock weathering and its significance for global carbon cycling. Plant, Cell and Environment 38: 1947–1961. [DOI] [PubMed] [Google Scholar]
- Thorup-Kristensen K. 2001. Are differences in root growth of nitrogen catch crops important for their ability to reduce soil nitrate-N content, and how can this be measured? Plant and Soil 230: 185–195. [Google Scholar]
- Thorup-Kristensen K, Rasmussen CR. 2015. Identifying new deep-rooted plant species suitable as undersown nitrogen catch crops. Journal of Soil and Water Conservation 70: 399–409. [Google Scholar]
- Thorup-Kristensen K, Salmerón Cortasa M, Loges R. 2009. Winter wheat roots grow twice as deep as spring wheat roots, is this important for N uptake and N leaching losses? Plant and Soil 322: 101–114. [Google Scholar]
- Walvoord MA, Phillips FM, Stonestrom DA, et al. 2003. A reservoir of nitrate beneath desert soils. Science 5647: 1021–1024. [DOI] [PubMed] [Google Scholar]
- Wasson AP, Rebetzke GJ, Kirkegaard JA, Christopher J, Richards RA, Watt M. 2014. Soil coring at multiple field environments can directly quantify variation in deep root traits to select wheat genotypes for breeding. Journal. of Experimental Botany 65: 6231–6249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White RG, Kirkegaard JA. 2010. The distribution and abundance of wheat roots in a dense, structured subsoil – implications for water uptake. Plant, Cell and Environment 33: 133–148. [DOI] [PubMed] [Google Scholar]
- Yuen JQ, Ziegler AD, Webb EL, Ryan CM. 2013. Uncertainty in below-ground carbon biomass for major land covers in Southeast Asia. Forest Ecology and Management 310: 915–926. [Google Scholar]
- Zhou H, Zhao W, Zheng X, Li S. 2015. Root distribution of Nitraria sibirica with seasonally varying water sources in a desert habitat. Journal of Plant Resources and Environment 128: 613–622. [DOI] [PubMed] [Google Scholar]
- Ziegler AD, Phelps J, Qi Yuen J, et al. 2012. Carbon outcomes of major land-cover transitions in SE Asia: great uncertainties and REDD+ policy implications. Global Change Biology 18: 3087–3099. [DOI] [PubMed] [Google Scholar]
- Zobel RW. 2008. Hardware and software efficacy in assessment of fine root diameter distributions. Computers and Electronics in Agriculture 60: 178–189. [Google Scholar]