Abstract
Background and Aims It is not clear how plants adjust the rate of root water uptake to that of shoot water loss. The aim of this study on rice was to test the idea that root aquaporins (AQPs) and xylem tension play a role in this adjustment.
Methods Three-week-old rice (Oryza sativa L.) plants, which were grown hydroponically, had their entire shoot system removed, and root hydraulic conductivity (exudation analyses) and gene expression (quantitative real-time PCR) of root plasma membrane intrinsic aquaporin proteins (PIPs) was followed within 60 min after shoot excision.
Key Results All three PIP1 genes (OsPIP1;1, OsPIP1;2 and OsPIP1;3) and three of the six PIP2 genes tested (OsPIP2;1, OsPIP2;4 and OsPIP2;5) showed a rapid (5 min) and lasting (60 min) decrease in gene expression. Expression decreased by up to 85 % within 60 min. The other three PIP2 genes tested (OsPIP2;2, OsPIP2;3 and OsPIP2;6) showed a varied response, with expression decreasing either only initially (5 min) or after 60 min, or not changing at all. In a follow-up experiment, plants had their shoot system removed and the detached root system immediately connected to a vacuum pump through which some tension (80 kPa) was applied. This application of tension prevented any significant decrease in PIP expression.
Conclusions Shoot removal leads to a rapid decrease in expression of all PIP1s and some PIP2s in roots of rice. Xylem tension plays some role in this process.
Keywords: Aquaporin, rice (Oryza sativa L.), root hydraulic conductivity, transpiration, xylem tension
Introduction
Plants must adjust the supply of water through the root system to the demand for water by the shoot to sustain transpirational water loss and also carbon fixation. Plants must also ensure that excess water (and mineral nutrients) is not taken up through the root system at times when delivery to the shoot is impaired. How this fine-tuning between root water supply and shoot water demand is achieved is not known, but root aquaporins (AQPs) provide an ideal target for such a regulation. Root AQPs have been shown repeatedly to be involved in the regulation of root hydraulic properties (for reviews, see Aroca et al., 2012; Chaumont and Tyerman, 2014; Li et al., 2014).
It is thought that AQPs exert their controlling function on root water uptake through regulating the transmembrane component of the radial cell to cell transport route of water across the root cylinder (Frensch and Steudle, 1989; Zhu and Steudle, 1991; Steudle and Peterson, 1998). The extent to which AQPs control root water uptake and root hydraulic conductivity (Lp, water uptake per unit time, root surface area and biophysical driving force; e.g. Suku et al., 2014) depends on the relative contribution of the apoplast and the cell to cell path to radial water movement. This contribution varies with species, time of day and environmental conditions (e.g. Frensch and Steudle, 1989; Zhu and Steudle, 1991; Steudle and Peterson, 1998; Steudle, 2000; Bramley et al., 2009; Knipfer and Fricke, 2010, 2011; Aroca et al., 2012). There is increasing evidence which points to a role for AQPs in also regulating the root hydraulic response to rather sudden changes in shoot transpirational water loss or to ‘catastrophic’ events such as injury and removal of the shoot (Levin et al., 2009; Almeida-Rodriguez et al., 2011; Sakurai-Ishikawa et al., 2011; Vandeleur et al., 2014). Sakurai-Ishikawa et al. (2011) reported for rice that the gene expression level of OsPIP2;4 and OsPIP2;5 increased in roots when plants were transferred from high (90 %) to low relative humidity (45 %). The increase in AQP expression occurred parallel to an increase in plant transpiration rate and root water uptake. A similar observation of rather rapid (10–30 min) changes in root AQP expression has been reported for maize and grapevine in response to (partial) shoot removal, which also reduced shoot transpirational water loss significantly (Vandeleur et al., 2014).
The aim of the present study on rice (Oryza sativa L.) was to follow up these previous observations and to address in particular two questions. First, we wanted to test whether expression of PIP (plasma membrane intrinsic aquaporin protein) AQPs could change significantly in response to shoot removal in even less time than previously reported (Vandeleur et al., 2014). Secondly, we wanted to test whether xylem tension plays a role in facilitating any rapid changes in root AQP expression following shoot removal, as proposed previously (Vandeleur et al., 2014).
Previous studies (Sakurai et al., 2005, 2008; Guo et al., 2006; Sakurai-Ishikawa et al., 2011) and our own preliminary experiments (not shown) on the Bala rice cultivar analysed showed that all three PIP1 genes and all PIP2 genes except OsPIP2;7 and OsPIP2;8 are expressed in roots at significant, or at least reproducibly detectable levels. Therefore, the latter two rice PIP genes were not included in the present analyses. In one set of ‘shoot removal’ experiments, the root system of plants was harvested for quantitative real-time PCR (qPCR) analyses immediately (‘t-0’), or 5 min (t-5), 30 min (t-30) and 60 min (t-60) following complete removal of the shoot. In another set of ‘vacuum’ experiments, the root system of plants, following shoot excision, was immediately connected through silicon tubing to a vacuum pump, and a vacuum was applied for 30 min, after which time the root was harvested and AQP expression analysed. This experiment was aimed at simulating xylem tension. It was expected that if the loss of xylem tension was the signal which caused the reduction in AQP expression in roots following shoot removal, application of a vacuum to detached root systems should lead to a smaller, or to no reduction in AQP expression. To test the effect of shoot removal on root hydraulic properties, root Lp was also determined. This was achieved through analysing the exudation rate of detached root systems within the first hour following shoot removal. In addition, root Lp was analysed for detached root systems either by using a pressure chamber (positive xylem hydrostatic pressure) or by application of a vacuum (sub-atmospheric xylem pressure) to induce water flow.
Materials and Methods
Plant material
Rice (Oryza sativa ‘Bala’) seeds were germinated and plants were grown on aerated Yoshida’s nutrient solution [macronutrients in mm: NH4NO3 1·4, NaH2PO4 0·32, K2SO4 0·52, CaCl2 1·0, MgSO4 1·6; micronutrients in μm: MnCl2 9·5, (NH4)6·Mo7O24 0·075, H3BO3 18·8, ZnSO4 0·15, CuSO4 0·15, FeCl3 35·6, citric acid 70; pH adjusted with 1 m NaOH], in a growth chamber (Microclima 1000VHOE, CEC Technology Ltd, Glasgow, UK) with the settings at 12 h light/12 h dark, and a temperature of 30 °C/25 °C. The relative humidity was 75 %. Photosynthetic active radiation at the leaf level was between 350 and 450 μmol m–2 s–1. Seeds were germinated in the dark on pH 5·0 Yoshida’s nutrient solution for 3 d and exposed to light for a further 3 d while remaining on Yoshida’s nutrient solution. Four seedlings each were then transferred to 1 L glass beakers, whose sides were wrapped in aluminium foil to keep out light, and which contained 900 mL of pH 5·5 Yoshida’s nutrient solution. Analyses were carried out on 3-week- (21 d) old plants, and all experiments were conducted 3–4 h into the photoperiod.
Shoot removal experiment: root system exudation analyses
Exudation experiments were carried out under ambient (18–20 °C) laboratory conditions. Root systems were excised with a double-sided razor blade at the shoot base and inserted into a short piece of silicon tubing which was connected to a narrow glass capillary (inner diameter: 0·58 mm). The root systems were transferred into a plastic tray filled with nutrient solution and the exudate flow in the capillary was recorded at 5 min intervals up to 1 h (see Knipfer and Fricke, 2011; Suku et al., 2014). Following the completion of the exudation experiment, the entire root system was scanned with a Canoscan 9900F scanner (Canon, Japan) and an aliquot of the nutrient solution in the tray and the exudate sap in the glass capillary was collected for subsequent analyses of osmotic pressure. The surface area of scanned images of root systems was determined using imageJ software (http://imagej.nih.gov/ij/). Surface area was calculated by treating the roots as a cylinder [surface = π × length × diameter; for details, see Suku et al. (2014) and Fricke et al. (2014)]. The osmotic pressure of the root medium (πmedium) and exudate (πxylem) was analysed using a Wescor osmometer (VAPRO 5600, Wescor, Inc., Logan, UT, USA). It was assumed that an osmolality of 40·75 mosmol kg–1 corresponded to an osmotic pressure of 0·1 MPa. Root hydraulic conductivity (Lp, m s–1 MPa–1) was calculated according to Lp = Q/(Δψ × RSA), where Q is the exudation rate (m3 s–1), RSA is the root surface area (m2), and Δψ is the driving force (MPa) for root water uptake during exudation, which calculates as the difference in water potential between the root medium (–πmedium) and xylem (–πxylem; assuming a root reflection coefficient for solutes of 1·0).
Shoot removal and vacuum experiment: root system gene expression analyses
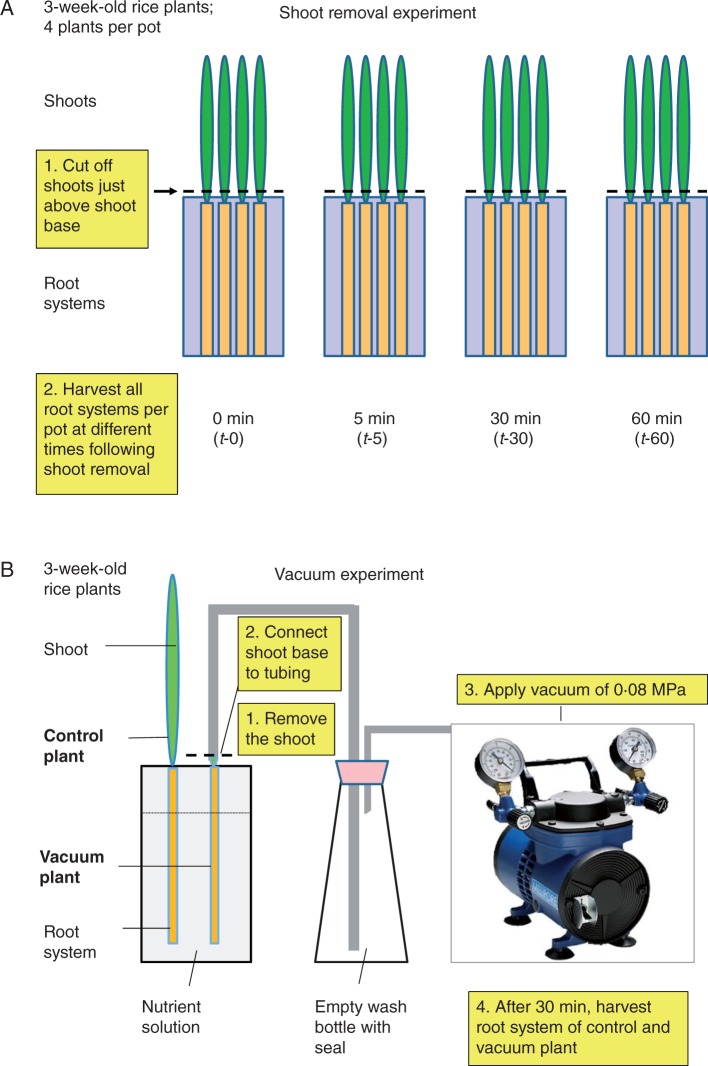
The set-up of the shoot removal experiment is outlined in Fig. 1A. Throughout the experiment, plants and detached root systems were kept in the growth chamber, with the root system in nutrient solution. Plants had been grown in glass beakers, with four plants growing in each beaker. To avoid untangling of roots, which would have delayed the harvest of individual root systems following shoot removal, all four plants in a beaker had their shoot removed and were harvested at the same time. That meant that every harvest of plants consisted of the combined root system of four plants (representing one biological, or ‘experimental’, replicate). A shoot removal experiment was started by having four beakers, with four plants each, aligned in the growth chamber. The shoot of all plants in each beaker was removed with a razor blade just above the base of shoot which extruded from the foam pieces which held the plant in position in the lids of the pots. The pot which had the shoot of plants removed last was taken as the t-0 control, with root systems being harvested immediately following shoot excision; the remaining pots were harvested after 5 min (t-5), 30 min (t-30) and 60 min (t-60) following the removal of the shoot. The detached root systems were transferred into liquid nitrogen for harvest and stored at –80 °C until being used for the extraction of RNA. This experiment was carried out 3–4 times independently (n = 3–4).
Fig. 1.
Scheme detailing the approach of the (A) shoot removal and (B) vacuum application experiment in rice (Oryza sativa L.).
To test the role of xylem tension in facilitating any changes in AQP expression in detached root systems, a set of ‘vacuum’ experiments was carried out (see Fig. 1B). The set-up of experiment was as detailed above, except that individual plants were harvested as a vacuum could only be applied to one root system at a time (only one vacuum pump was available). The shoot system of a plant was cut off 2 cm above the base of the shoot. With the root system in nutrient solution, the remaining shoot base was immediately connected to a vacuum pump using silicon tubing (Helix Medical, Kaiserslautern, Germany) and the junction between the shoot base and tubing was sealed with super glue. A vacuum tension of 80 kPa was applied to the root to simulate some transpirational tension. The root system was harvested after 30 min of applying tension. Following this harvest of ‘vacuum-treated’ plants, a set of ‘control’ plants had their shoot removed and harvested immediately, without any application of a vacuum. Throughout the shoot removal and vacuum experiments, the root medium was aerated. Shoot removal and vacuum experiments were carried out four times independently (n = 4).
Expression of candidate AQP genes using qPCR
Stored samples from the shoot removal and vacuum experiments were ground in liquid nitrogen. The entire root system of either four collectively harvested plants (shoot removal experiments) or individual plants (vacuum experiments) was ground into a fine powder, and an aliquot of this powder was used for extraction of RNA. RNA was extracted, genomic DNA digested and cDNA synthesized as described previously (Besse et al., 2011) using the RNeasy Plant Mini Kit (Qiagen, Tokyo, Japan), DNase I Amplification Grade (Invitrogen, San Diego, CA, USA) and SuperScript II Reverse Transcriptase (Invitrogen). Expression of candidate PIP genes was quantified at the mRNA level by qPCR using a qPCR thermocycler (VIIA 7, Applied Biosystems, USA) (for details, see Boscari et al., 2009; Besse et al., 2011; Knipfer et al., 2011). Nine rice PIP AQP genes (OsPIP1;1, OsPIP1;2, OsPIP1;3, OsPIP2;1, OsPIP2;2, OsPIP2;3, OsPIP2;4, OsPIP2;5 and OsPIP2;6) and three housekeeping genes (UBQ, ubiquitin; EF-2, elongation factor 2; and GAPDH, glyceraldehyde phosphate dehydrogenase) were analysed. Expression was quantified using the 2–ΔCt method (Pfaffl, 2001; Besse et al., 2011); sequences of primers are listed in Supplementary Data Table S1.
Shoot vacuum experiment: root system hydraulic analyses
The aim was to obtain the Lp of detached root systems which were in a vacuum set-up as used for expression analyses. For this purpose, Lp was analysed in two ways, through: (1) application of a vacuum and (2) positive root pressure (pressure chamber). The latter approach was included to compare the ‘vacuum Lp’ with a root system Lp where root xylem was under significant positive pressure. (1) One set of detached root systems had water flow induced through application of a vacuum, using the same set-up as for experiments involving expression analyses, except that the root system (in a container with nutrient solution) was placed onto an analytical balance (Mettler) which allowed root water uptake to be determined gravimetrically. A tension of 80 kPa was applied and water flow was determined at 5 min intervals over a period of 25–30 min. The average of the flow rate was used together with values of root surface area for the respective root system and driving force to calculate a ‘vacuum Lp’. (2) Another set of detached root systems was used for determination of a ‘pressure chamber Lp’, by inserting each root system (in nutrient medium) in a pressure chamber (‘plant moisture vessel’ SKPM 1400, Skye, UK) and applying a pressure of 80 kPa for 25–30 min. Exudate was collected through pre-weighed cotton (compare Ehlert et al., 2009), which was contained in microcentrifuge tubes and placed above the extruding (from the pressure chamber) end of the root system. Tubes with cotton were replaced at 5 min intervals, weighed, and the increase in weight was used to calculate the average rate of root water uptake per 5 min interval. This rate was used together with a value of root surface area of the respective root system to calculate a pressure chamber Lp. The above experiments were repeated on 7–8 root systems (n = 7–8).
Statistical analyses
One-way analysis of variance (ANOVA) followed by Tukey analyses was carried out using the General Linear Model function in Minitab 16·0 (Minitab Inc.). A P-value of < 0·05 was considered significant.
Results
Expression of OsPIP genes following shoot removal
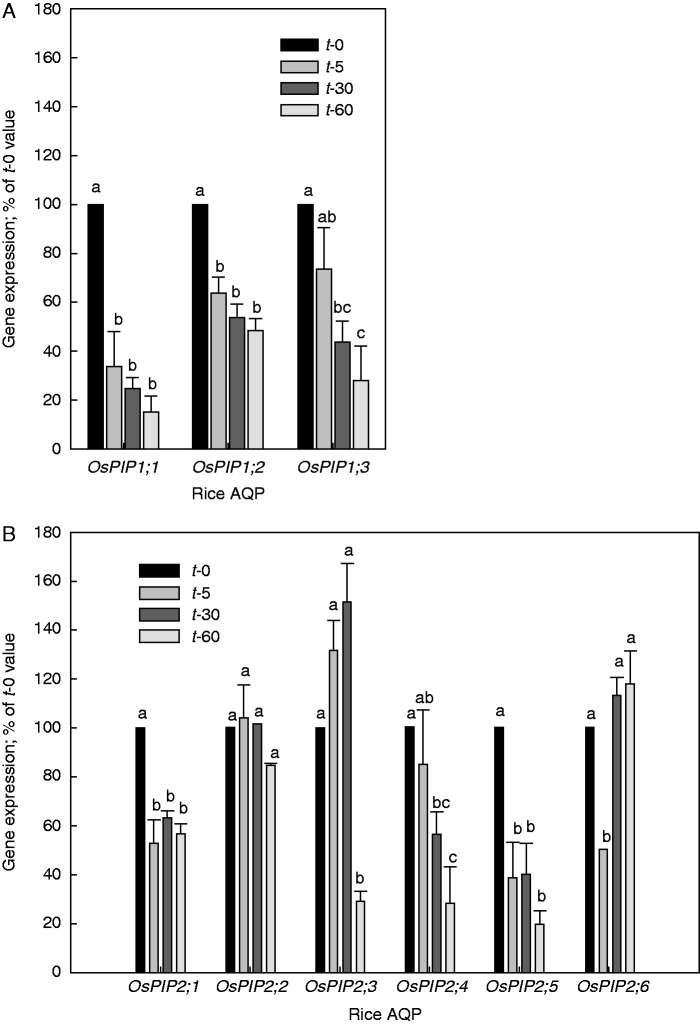
All three rice OsPIP1 genes and three of the rice PIP2 genes tested (OsPIP2;1, OsPIP2;4 and OsPIP2;5) showed a rapid (5 min) decrease in expression in response to shoot removal, becoming larger with time (30–60 min) (Fig. 2A, B). The decrease in expression was significant already at 5 min following shoot excision for OsPIP1;1, OsPIP1;2, OsPIP2;1 and OsPIP2;5, and amounted to between 36 % (OsPIP1;2) and 66 % (OsPIP1;2) of the t-0 value. At 60 min following shoot excision, expression of OsPIP1;1, OsPIP1;2, OsPIP1;3, OsPIP2;1, OsPIP2;4 and OsPIP2;5 had decreased by 43 % (OsPIP2;1) to 85 % (OsPIP1;2). In contrast, the expression of the remaining three PIP2 genes tested (OsPIP2;2, OsPIP2;3 and OsPIP2;6) showed a mixed response to shoot removal. Expression of OsPIP2;2 hardly changed over the course of the experiment, whereas expression of OsPIP2;6 decreased initially (5 min value) by 50 %, only to increase back close to the t-0 value at 30 and 60 min following shoot removal. Expression of OsPIP2;3 increased by up to 50 % in the first 30 min following shoot removal, though this increase was not significant, and decreased significantly to 29 % of the t-0 value after 60 min following shoot removal. The expression of the three reference genes was not affected by shoot removal. The average Ct value of the three references genes, for all experiments, ranged from 21·5 to 21·9 between the four time points (t-0, t-5, t-30 and t-60 min; not shown).
Fig. 2.
(A) Expression of PIP1 and (B) PIP2 aquaporins (AQPs) in rice roots following shoot removal. Values are expressed as a percentage of the 100 % value at the start of the experiment (t-0). The root system was harvested either immediately (t-0) or 5 min (t-5), 30 min (t-30) or 60 min (t-60) following removal of the shoot. The expression of PIP AQP genes was related to that of references genes, and the resulting value at t-0 (harvested immediately) was set to 100 %. The expression of PIP genes at the other time points was expressed as a percentage of this 100 % value. Results are the averages and s.e. (error bar) of 3–4 independent experiments. Values for a particular AQP which do not share a lower case letter differ significantly from each other (P < 0·05, one-way ANOVA followed by Tukey analyses).
Expression of OsPIP genes following application of a vacuum to detached root systems
When the root system was subjected to 80 kPa of tension for 30 min following shoot excision, the expression of all PIP genes tested hardly changed and was either just above or below the control value (whose root system was harvested immediately following shoot removal) (Fig. 3). The expression of reference genes was not affected by the application of a vacuum (not shown).
Fig. 3.
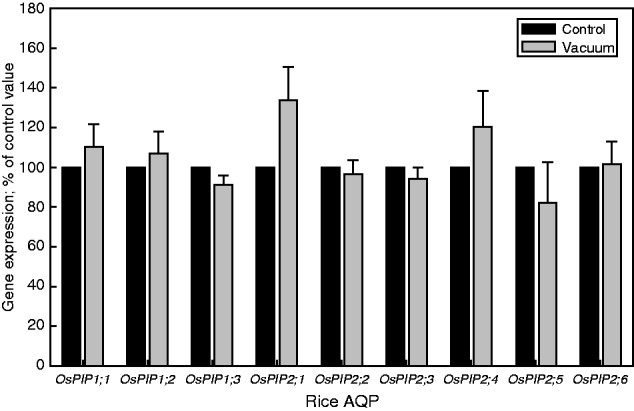
Expression of PIP aquaporins (AQPs) in rice roots at 30 min following shoot removal and application of 80 kPa tension (‘vacuum’) to the detached root system. Following shoot removal of one plant, the excised root system of that plant was connected to a vacuum pump and 80 kPa of vacuum was applied. After 30 min of vacuum application, this ‘vacuum’ plant was harvested. At the same time, the shoot of a ‘control’ plant was excised and the root system harvested immediately. The expression of PIP AQP genes was related to that of reference genes (UBQ, EF-2 and GAPDH), and the resulting value of the control was set to 100 %. The expression of PIP genes in root systems which had a vacuum applied for 30 min was expressed as a percentage of this 100 % value. Results are the averages and s.e. (error bar) of four independent experiments. Expression values did not differ significantly between control and vacuum plants for a particular AQP (P < 0·05, one-way ANOVA followed by Tukey analyses).
Root hydraulic conductivity (Lp)
Excised root systems were analysed in root exudation experiments to determine root Lp at different times following shoot excision. To this aim, root systems were excised and connected to a glass capillary through some silicon tubing. The rate of exudate formation in the glass capillary was followed at 5 min intervals over a period of 1 h. It always required some time to fix the root system to the tubing/capillary and for exudate to appear. This meant that the earliest time point for which the exudation rate could be determined was at 5 min following shoot excision. The osmotic pressure of root (xylem) exudate had to be known to calculate the driving force for water uptake during root exudation, which in turn was required to calculate root Lp. The amount of exudate which formed during a 5 min interval was too small to be analysed for osmotic pressure, and frequent sampling of liquid from the capillary also led to a rapid formation of leak at the root–capillary junction. Therefore, we decided to carry out an additional set of experiments in which root exudate was sampled at 20 and 60 min of exudation and compared the osmotic pressure between these two sampling points. The underlying rationale was that if the osmotic pressure at 20 and 60 min did not differ from each other, it would be reasonable to assume (though not being proof per se) that exudate osmotic pressure did not change between 20 and 60 min. In addition, it was not possible to determine exudate osmotic pressure at 5–15 min of exudation, due to an insufficient amount of exudate. Therefore, it was also assumed that the osmotic pressure of the exudate was similar to that at 20–60 min. When the osmotic pressure of the exudate was determined at 20 and 60 min following shoot removal, osmotic pressure averaged 6·16 × 10–2 ± 0·89 × 10–2 MPa at 20 min compared with 5·83 × 10–2 ± 0·81 × 10–2 MPa (means ± s.d., n = 4) at 60 min (not shown). The two values differed by only 5 % from each other, and the difference was not significant (P = 0·6, t-test). Therefore, it was considered appropriate to use in all other experiments exudate collected after 60 min following shoot excision for the determination of exudate osmotic pressure for the period 20–60 min, and also 5–20 min, for calculation of exudation root hydraulic conductivity (Lp).
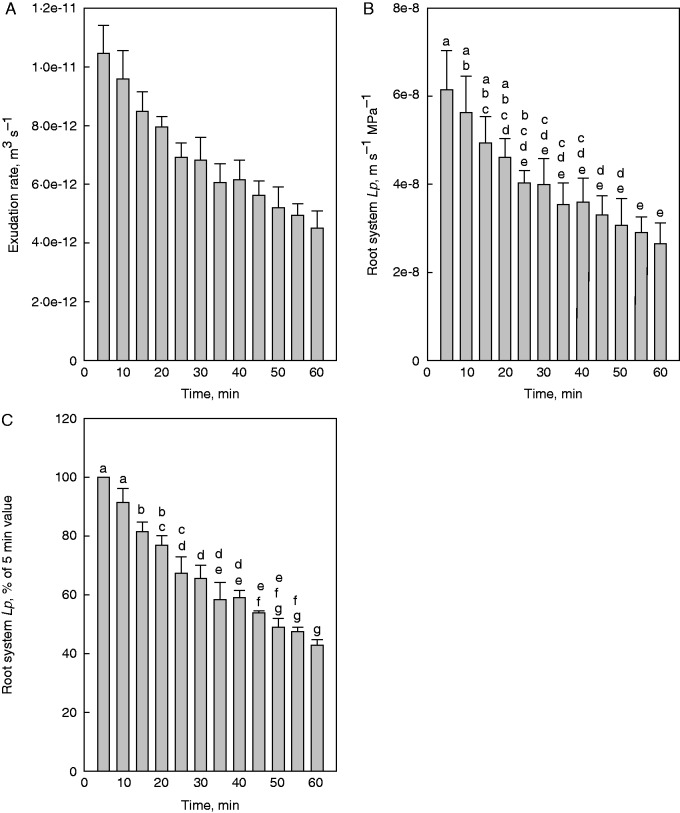
The rate of exudation averaged 1·04 × 10–11 m3 s–1 per root system (n = 5) at 5 min, and decreased continuously during exudation experiments (and following shoot removal) (Fig. 4A). The same applied to exudation root Lp, which averaged 6·14 × 10–8 ± 0·87 × 10–8 m s–1 MPa–1 (means ± s.e., n = 5) at 5 min (Fig. 4B). When the root Lp value at 5 min was set to 100 %, Lp decreased to 65·4 ± 4·6 % and to 42·9 ±1·8 % (mean ± s.e., n = 5) of this value at 30 and 60 min, respectively, during exudation (and following shoot removal; Fig. 4C).
Fig. 4.
Changes in root exudation rate and hydraulic conductivity (Lp) following shoot removal in rice. The shoot of a plant was removed just above the shoot base and the excised root system was connected to a glass capillary. The rate of exudate formation in the glass capillary was followed with time. To calculate Lp, the surface area of the root system was determined at the end of an experiment, and exudate and root medium were analysed for osmotic pressure to calculate the driving force for root water uptake. (A) Absolute values of root exudation rate and (B) Lp are shown, together with the (C) percentage value of Lp; to calculate the latter, the exudation root Lp of each root system at 5 min following shoot removal was set to 100 %. For technical reasons, it was not possible to calculate root Lp for t-0. Results are the means and s.e. of five plants. Values which do not share a lower case letter differ significantly from each other (P < 0·05, one-way ANOVA followed by Tukey analyses).
Discussion
Rice root PIP AQP expression in response to shoot removal
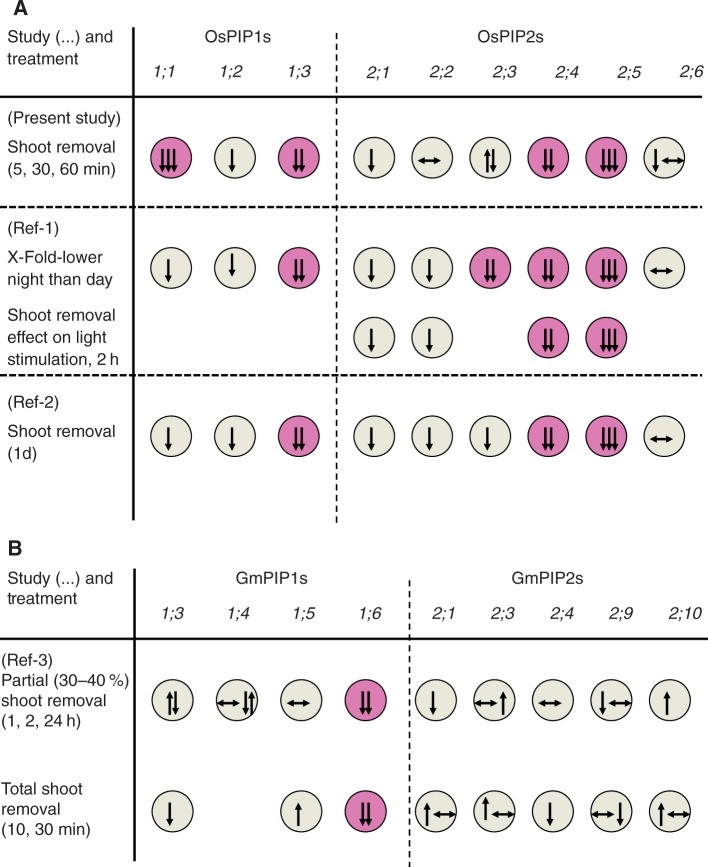
Most but not all of the investigated rice root OsPIP genes decreased in expression following shoot removal. The decrease in expression was substantial and of similar magnitude to the parallel decrease in root Lp as determined through exudation experiments, where osmotic forces drive water flow along the cell to cell path including PIP AQPs (Steudle, 2000) Together, these data suggest that the decrease in root Lp in rice plants following removal of the entire shoot and transpiring surface involved regulation of plasma membrane-localized AQPs, at least at the level of gene transcript abundance. Similarly, Ahamed et al. (2012) observed for rice that the expression of most PIP AQPs in roots decreased in response to shoot removal, though analyses were carried out 1 d following shoot removal. Sakurai-Ishikawa et al. (2011) investigated the role of transpiration in diurnal changes in root AQP expression and Lp in rice. The authors observed that shoot removal prevented the increase in AQP expression and root Lp following the transition from a dark to a light period. Both Ahamed et al. (2012) and Sakurai-Ishikawa et al. (2011) emphasized the role which OsPIP2;5 plays in the Lp response of rice roots. The authors observed that protein levels of OsPIP2;5 behaved similarly to levels of mRNA expression and explained the role of OsPIP2;5 in regulation of root Lp through its tissue localization within the proximal end of the endodermis and the cell surface next to the xylem. In the present study, OsPIP2;5 showed a large decrease in expression following shoot removal, at all time points analysed, and parallel to a decrease in root Lp. These data suggest that OsPIP2;5 not only governs the longer term (hours to days) response of root Lp in rice to changes in transpirational water flow but is also involved in the shorter term response (minutes to hours). Figure 5 summarizes the findings of Ahamed et al. (2012), Sakurai-Ishikawa et al. (2011) and the present study on rice, and also includes the only comparable study on another plant species, soybean (Glycine max. L) by Vandeleur et al. (2014). It can be seen that compared with rice, soybean PIPs show a much more varied response to shoot removal, and only one PIP gene (GmPIP1;6) displays a consistent and significant downregulation in gene expression. In rice, OsPIP1;3 and OsPIP2;4 are the two PIP genes, besides OsPIP2;5, which show the largest, and significant decrease in expression in response to treatments which reduce shoot transpirational water loss. Similarly to OsPIP2;5, OsPIP1s (including OsPIP1;3) and GmPIP1;6 are localized in most root tissues and in particular in the endodermis; GmPIP1;6 is also localized in stelar tissue (Sakurai et al., 2008; Vandeleur et al., 2014). Together these data point to PIP AQPs, which are localized in the endodermis or stele, as the prime facilitators for mediating the decrease in root Lp in response to shoot removal and changes in shoot transpirational demand. To what extent the general downregulation in PIP expression in response to shoot removal is a species-specific feature of rice needs to be tested further.
Fig. 5.
Schematic summary of changes in expression of (A) rice and (B) soybean PIP aquaporins in response to shoot removal or other treatments which affect shoot transpirational water loss. Data for rice were taken from the present study, or from the study by Ahamed et al. (2012; ‘Ref-1’) and Sakurai-Ishikawa et al. (2011; ‘Ref-2’); data on soybean were taken from Vandeleur et al. (2014; ‘Ref-3’). The change in AQP expression in response to either (partial) shoot removal or change in shoot transpiration (Ref-1, comparing night-time with daytime expression) is indicated through the direction of the arrow: down/up/level corresponds to decrease/increase/no change in expression, respectively. The magnitude of expression change is indicated by the number of arrows: one arrow, small change; three arrows, largest change. Those PIPs which showed the largest or most consistent decrease in expression across studies are highlighted in pink. As some of the studies involved time-courses, changes in the expression response with time are indicated through a sequence of arrows (arrow furthest left, first time point; arrow furthest right, last time point).
The signal affecting root AQP expression and Lp
To test the hypothesis that shoot transpiration regulates root AQP expression in rice through transpirational tension, the expression of PIP AQPs was investigated in root systems which had their shoot removed yet xylem tension simulated through the application of a 0·08 MPa vacuum (sub-atmospheric pressure). In root systems treated in that way, none of the PIPs showed a major or significant reduction in gene expression. Even though OsPIP2;5 showed slightly lower expression (Fig. 3) in vacuum-treated root systems compared with root systems at the time of shoot removal (time zero), this small reduction could be due to the circumstance that the vacuum applied (0·08 MPa) was smaller than the xylem tension in intact transpiring plants. The latter was as negative as –0·40 MPa, based on leaf water potential determinations using a pressure chamber (see also Table 1). The present data provide the most direct evidence so far for the idea that xylem tension is involved in the rapid transcriptional response of root AQPs to sudden changes in the plant transpiration rate (Vandeleur et al., 2014) and that a wounding effect does not play any major role in such a response.
Table 1.
Water flow rate, hydraulic conductivity (Lp) and xylem pressure of rice root systems under various experimental conditions used to determine Lp
| Experimental condition | Flow rate (m3 s–1) | Xylem pressure (MPa)* | Lp (m s–1 MPa–1) |
|---|---|---|---|
| Exudation | 1·0 × 10–11 | 0·007† | 6·2 × 10–8 |
| Transpiration | 4·4 × 10–10 | –0·4‡ | 2·4 × 10–7 |
| Vacuum | 2·1 × 10–10 | –0·08 | 5·8 × 10–7 |
| Pressure chamber | 4·6 × 10–11 | 0·08 | 1·2 × 10–7 |
Results are averages of 5–8 plant analyses.
*All values are relative to normal atmospheric pressure, which is set to zero.
†Calculated based on Haagen–Poiseuille’s law (Nobel, 1991), using a metaxylem radius of 15 µm, three metaxylem vessels per root, a minimum of five roots per root system and a total length of root system of approx. 20 cm (Meng and Fricke, unpublished; see also Sakurai et al., 2008).
‡Value derived from leaf water potential measurements; represents a most negative estimate of xylem pressure.
Sakurai-Ishikawa et al. (2011) concluded for rice that diurnal changes in root AQP expression and Lp are not so much due to a circadian clock but rather to diurnal changes in the transpiration rate, with rates being high (day) at times when root AQP expression and Lp are high. Similarly, Ahamed et al. (2012) observed for rice that the increase in root AQP expression and Lp in response to several days of low root temperature treatment could be prevented through removal of the shoot; in other words, plant transpiration was required to achieve elevated levels of root AQP expression. Vandeleur et al. (2014) analysed the role of plant transpiration and xylem tension in the transcriptional response of root AQPs to partial shoot topping in more detail in soybean and grapevine (Vitis vinifera L.). The authors concluded that phloem-borne signals were not required for the AQP expression response in roots. Rather, xylem-mediated hydraulic signals might have been involved in the response, as turgor of root cortical cells decreased significantly, by about 0·05 MPa, within 30 min following shoot topping.
In the present study, the application of a vacuum to decapitated root systems generated some xylem tension. This xylem tension will have had two major and direct effects on roots compared with roots which were decapitated and did not have any vacuum applied to them. First, the applied vacuum set all cells within the stele of roots under an apoplastic tension; secondly, the applied vacuum lowered the water potential of root xylem and, through this, increased the radial water flow rate across the root cylinder. The increased radial water flow rate will also have increased the water flow rate through each root AQP channel, as a significant proportion of water taken up by rice roots moves along the cell to cell path (Miyamoto et al., 2001). Either or both the increased apoplastic tension or water flow rate through AQPs could have been the signal which prevented a decrease in AQP expression in roots which had a vacuum applied to them – and in decapitated root systems which did not a have vacuum applied, the decreased water flow rate or decreased apoplastic tension would have caused AQP expression to decrease. Based on the present data, we cannot distinguish between these two possible primary signals. Xylem (apoplast) tension as the signal would be consistent with the present observation that OsPIP2;5, OsPIP1;1 and OsPIP1;3 were the most affected in gene expression by shoot removal, as these PIP genes are found at the endodermis (Sakurai-Ishikawa et al., 2011). At the same time, the results for the three reference genes and in particular also OsPIP2;6 (see Fig. 5) show that there exist genes in rice roots which do not respond, or respond only a little (OsPIP2;6) in their expression to shoot removal and associated changes in apoplast tension.
One could argue that changes in apoplast tension are a rather diffuse signal compared with the more specific signal of water flow rate through an AQP channel. This would favour the latter as the signal which regulates AQP expression in roots and could explain a PIP isoform-specific response as observed in particular for soybean (compare Fig. 5). Wan et al. (2004) proposed a gating mechanism for AQPs which involves the water flow rate through AQPs. This gating mechanism will affect cellular water status and, through this, could provide the link between xylem tension, cellular water flow rate and nuclear AQP transcription response. Table 1 provides a comparison of water flow rates, Lp and xylem pressure of rice root systems under various experimental conditions used to determine Lp. The Lp of root systems was determined either through (1) exudation analyses (compare Fig. 4); (2) using values of plant transpiration (to derive root water uptake rate) and leaf water potential (to derive xylem water potential and the driving force for root radial water uptake; Suku et al., 2014); (3) application of a vacuum to detached root systems; or (4) application of positive pressure to detached root systems using a pressure chamber (see the Materials and Methods). On the one hand, the approach causing the smallest rate of water flow (exudation analyses) yielded the smallest Lp; on the other hand, the approach associated with the largest flow rate, and also the largest xylem tension (plant transpiration), did not result in the largest Lp; the latter was obtained through application of a vacuum to detached root systems and may, in part, be due to water moving axially along the cortex and by-passing any endodermal hydraulic barrier (as the vacuum applied to a cut root system can cause water flow across the entire root cross-sectional surface, unlike xylem tension in an intact plant which acts just on the stele; see also Knipfer and Fricke, 2011). We do not know whether such an axial cortical by-pass also operates under conditions where the root is exposed to above-atmospheric pressures in the root environment, such as in the pressure chamber approach in Table 1. If so, the much lower Lp obtained through the pressure chamber compared with the vacuum application approach, while applying pressure changes of the same magnitude (+0·08 MPa and –0·08 MPa), could reflect the circumstance that a vacuum, but not positive pressure, prevented AQP expression and Lp from decreasing in the detached root systems.
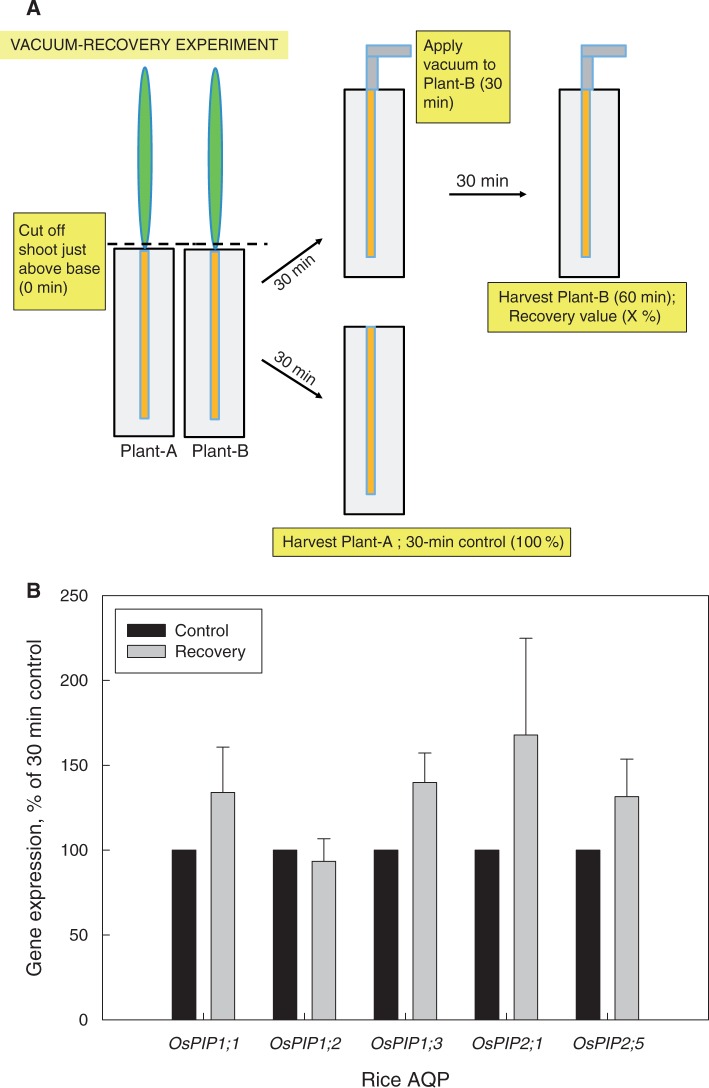
Finally, based on the above, one could also ask whether a vacuum applied to detached root systems not only prevents a decrease in AQP expression following shoot removal, but also plays a part in the recovery of any decreased AQP expression. To test this, we analysed rice plants in pairs. Both plants had their shoot removed at time zero and were then left like this for 30 min. After this time, one root system was harvested (‘control’), and the other root system had a vacuum applied to it and was left for another 30 min before being harvested. The gene expression level of candidate PIPs (which had shown large and consistent decreases in expression in Fig. 2) in this ‘recovery’ root system was related to the expression in the 30 min control root system. Results of four pairs of plants are shown in Fig. 6. Four of the five PIPs tested showed a partial recovery in gene expression in response to vacuum application, though the recovery was statistically not significant due to considerable plant to plant variation in response. Nonetheless, the data point to a vacuum, and xylem tension, also being involved in the recovery of root PIP AQP expression.
Fig. 6.
(A) Scheme detailing the vacuum recovery experiment and (B) associated gene expression data of candidate rice PIPs. A set of two plants (here: plant-A and plant-B) had their shoot removed at time zero, with the root system still in nutrient solution. The root systems of both plants were left like this for 30 min. After this time, one root system (here: plant-A) was harvested and used as the ‘30 min control’. The other root system (here: plant-B) was connected to a vacuum pump; a vacuum was applied for 30 min, after which time this ‘(vacuum-)recovery’ root system was harvested. The candidate PIPs were chosen based on their large and significant decrease in expression within the first 30 min following shoot removal (compare Fig. 2). A total of four sets of plants (n = 4) were analysed by qPCR as detailed in the text for the other experiments (compare Figs 2 and 3). For each set of plants, the expression value of the 30 min control was set to 100 % and the value of the recovery root system was expressed as a percentage of this 100 % value. Results are the averages and s.e. (error bars). Values of the 30 min control and recovery root system did not differ significantly from each (paired t-test, not shown).
In conclusion, the present study shows that shoot removal leads to a rapid (5 min) and lasting (60 min) reduction in expression of most PIPs in roots of rice and that xylem tension plays some role in this process. Water flow rate through AQPs, as an alternative signal regulating root AQP expression, could also explain diurnal changes in root AQP expression (e.g. Henzler et al., 1999; Lopez et al., 2003; Hachez et al., 2008; Vandeleur et al., 2009; Sakurai-Ishikawa et al., 2011). The question needs to be addressed of at which time scale protein levels of rice root AQPs change in response to shoot removal. Do protein levels change at all within the first 30 min following shoot removal, or do xylem tension and water flow rate affect cellular trafficking and gating of root AQPs (e.g. Hachez et al., 2014; Chaumon and Tyerman, 2014) parallel to the fast transcriptional response?
Supplementary Data
Supplementary data are available online at www.aob.oxfordjournals.org and consist of Table S1: details of primers which were used for the qPCR expression analysis of rice aquaporin and reference genes.
Acknowledgements
The authors would like to thank the Chinese Scholarship Council and University College Dublin for jointly funding a CSC PhD studentship (to D.M.). Thanks also to the School of Biology and Environmental Sciences for providing some additional funds and to Wan for a helpful discussion about the design of vacuum experiments. Particular thanks also to Prof. Adam Price (Aberdeen University, UK) for providing Bala seeds and some invaluable advice.
Literature Cited
- Ahamed A, Murai-Hatano M, Ishikawa-Sakurai J, Hayashi H, Kawamura Y, Uemura M. 2012. Cold stress-induced acclimation in rice is mediated by root-specific aquaporins. Plant and Cell Physiology 53: 1445–1456. [DOI] [PubMed] [Google Scholar]
- Almeida-Rodriguez AM, Hacke UG, Laur J. 2011. Influence of evaporative demand on aquaporin expression and root hydraulics of hybrid poplar. Plant, Cell & Environment 34: 1318–1331. [DOI] [PubMed] [Google Scholar]
- Aroca R, Porcel R, Ruiz-Lozano JM. 2012. Regulation of root water uptake under abiotic stress conditions. Journal of Experimental Botany 63: 43–57. [DOI] [PubMed] [Google Scholar]
- Besse M, Knipfer T, Miller AJ, Verdeil JL, Jahn TP, Fricke W. 2011. Developmental pattern of aquaporin expression in barley (Hordeum vulgare L.) leaves. Journal of Experimental Botany 62: 4127–4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boscari A, Clement M, Volkov V, et al. 2009. Potassium channels in barley: cloning, functional characterisation and expression analyses in relation to leaf growth and development. Plant, Cell & Environment 32: 1761–1777. [DOI] [PubMed] [Google Scholar]
- Bramley H, Turner NC, Turner DW, Tyerman SD. 2009. Roles of morphology, anatomy, and aquaporins in determining contrasting hydraulic behavior of roots. Plant Physiology 150: 348–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaumont F, Tyerman SD. 2014. Aquaporins: highly regulated channels controlling plant water relations. Plant Physiology 164: 1600–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlert C, Maurel C, Tardieu F, Simonneau T. 2009. Aquaporin-mediated reduction in maize root hydraulic conductivity impacts cell turgor and leaf elongation even without changing transpiration. Plant Physiology 150: 1093–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frensch J, Steudle E. 1989. Axial and radial hydraulic resistance to roots of maize (Zea mays L.). Plant Physiology 91: 719–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricke W, Bijanzadeh E, Emam Y, Knipfer T. 2014. Root hydraulics in salt-stressed wheat. Functional Plant Biology 41: 366–378. [DOI] [PubMed] [Google Scholar]
- Guo L, Wang ZY, Lin H, et al. 2006. Expression and functional analysis of the rice plasma-membrane intrinsic protein gene family. Cell Research 16: 277–286. [DOI] [PubMed] [Google Scholar]
- Hachez C, Heinen RB, Draye X, Chaumont F. 2008. The expression pattern of plasma membrane aquaporins in maize leaf highlights their role in hydraulic regulation. Plant Molecular Biology 68: 337–353. [DOI] [PubMed] [Google Scholar]
- Hachez C, Laloux T, Reinhardt H, et al. 2014. Arabidopsis SNAREs SYP61 and SYP121 coordinate the trafficking of plasma membrane aquaporin PIP2;7 to modulate the cell membrane water permeability The Plant Cell 26: 3132–3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henzler T, Waterhouse RN, Smyth AJ, et al. 1999. Diurnal variations in hydraulic conductivity and root pressure can be correlated with the expression of putative aquaporins in the roots of Lotus japonicus. Planta 210: 50–60. [DOI] [PubMed] [Google Scholar]
- Knipfer T, Fricke W. 2010. Root pressure and a solute reflection coefficient close to unity exclude a purely apoplastic pathway of radial water transport in barley (Hordeum vulgare L.). New Phytologist 187: 159–170. [DOI] [PubMed] [Google Scholar]
- Knipfer T, Fricke W. 2011. Water uptake by seminal and adventitious roots in relation to whole-plant water flow in barley (Hordeum vulgare L.). Journal of Experimental Botany 62: 717–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipfer T, Besse M, Verdeil JL, Fricke W. 2011. Aquaporin-facilitated water uptake in barley (Hordeum vulgare L.) roots. Journal of Experimental Botany 62: 4115–4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M, Resnick N, Rosianskey Y, et al. 2009. Transcriptional profiling of Arabidopsis thaliana plants’ response to low relative humidity suggests a shoot–root communication. Plant Science 177: 450–459. [Google Scholar]
- Li G, Santoni V, Maurel C. 2014. Plant aquaporins: roles in plant physiology. Biochimica et Biophysica Acta 1840: 1574–1582. [DOI] [PubMed] [Google Scholar]
- Lopez F, Bousser A, Sissoëff I, et al. 2003. Diurnal regulation of water transport and aquaporin gene expression in maize roots: contribution of PIP2 proteins. Plant and Cell Physiology 44: 1384–1395. [DOI] [PubMed] [Google Scholar]
- Miyamoto N, Steudle E, Hirasawa T, Lafitte R. 2001. Hydraulic conductivity of rice roots. Journal of Experimental Botany 52: 1835–1846. [DOI] [PubMed] [Google Scholar]
- Nobel PS. 1991. Physicochemical and environmental plant physiology. San Diego: Academic Press. [Google Scholar]
- Pfaffl MW. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Research 29: e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai-Ishikawa J, Murai-Hatano M, Hayashi H, et al. 2011. Transpiration from shoots triggers diurnal changes in root aquaporin expression. Plant, Cell & Environment 34: 1150–1163. [DOI] [PubMed] [Google Scholar]
- Sakurai J, Ahamed A, Murai M, Maeshima M, Uemura M. 2008. Tissue and cell-specific localization of rice aquaporins and their water transport activities. Plant and Cell Physiology 49: 30–39. [DOI] [PubMed] [Google Scholar]
- Sakurai J, Ishikawa F, Yamaguchi T, Uemura M, Maeshima M. 2005. Identification of 33 rice aquaporin genes and analysis of their expression and function. Plant and Cell Physiology 46: 1568–1577. [DOI] [PubMed] [Google Scholar]
- Steudle E. 2000. Water uptake by plant roots: an integration of views. Plant and Soil 226: 45–56. [Google Scholar]
- Steudle E, Peterson CA. 1998. How does water get through roots? Journal of Experimental Botany 49: 775–788. [Google Scholar]
- Suku S, Knipfer T, Fricke W. 2014. Do root hydraulic properties change during the early vegetative stage of plant development in barley (Hordeum vulgare)? Annals of Botany 113: 385–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandeleur RK, Mayo G, Shelden M.C, Gilliham M, Kaiser BN, Tyerman SD. 2009. The role of plasma membrane intrinsic protein aquaporins in water transport through roots: diurnal and drought stress responses reveal different strategies between isohydric and anisohydric cultivars of grapevine. Plant Physiology 149: 445–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandeleur RK, Sullivan W, Athman A, et al. 2014. Rapid shoot-to-root signalling regulates root hydraulic conductance via aquaporins. Plant, Cell & Environment 37: 520–538. [DOI] [PubMed] [Google Scholar]
- Wan XC, Steudle E, Hartung W. 2004. Gating of water channels (aquaporins) in cortical cells of young corn roots by mechanical stimuli (pressure pulses): effects of ABA and of HgCl2. Journal of Experimental Botany 55: 411–422. [DOI] [PubMed] [Google Scholar]
- Zhu GL, Steudle E. 1991. Water transport across maize roots: simultaneous measurement of flows at the cell and root level by double pressure probe technique. Plant Physiology 95: 305–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.