Abstract
Background and Aims Plants modulate defence signalling networks in response to various biotic stresses via inter-organ communications. The root-mediated transmission of systemic acquired resistance (SAR) against soil-borne and air-borne plant pathogens from SAR-induced plants to neighbouring plants subjected to local chemical and pathogen treatments was evaluated.
Methods The first two plants out of ten Nicotiana benthamiana seedlings were pre-treated with the SAR-triggering chemical benzothiadiazole (BTH). All ten seedlings were then challenged with two pathogenic bacteria, i.e. the root (bacterial wilt) pathogen Ralstonia solanacearum and the leaf (wildfire) pathogen Pseudomonas syringae pv. tabaci, at 7 d after SAR induction.
Key Results Disease severity was noticeably lower in BTH-pre-treated plants than in the control. Surprisingly, two plants located next to BTH-treated plants exhibited reduced disease symptoms indicating that SAR signal transmission occurred through the root system. Determinant(s) secreted from the root system were search for and it was found that salicylic acid (SA) is a major molecule involved in SAR transmission through the root. Analysis of the expression of the defence-related genes N. benthamiana pathogenesis-related gene 1a (NbPR1a) and NbPR2 confirmed that BTH treatment elicited SAR via root–root transmission between plants. Plants with knock-down of the multiple resistance component SGT1 and SA biosynthesis-related gene ICS1 by Tobacco rattle virus-mediated virus-induced gene silencing exhibited a lack of root-mediated SAR transmission. The biological relevance of this finding was validated by challenge with the SAR-inducing avirulent pathogen P. syringae pv. syringae instead of BTH, which produced similar results.
Conclusions Our findings demonstrated that SAR is transmissible through the root system from SAR-triggered plants to neighbouring plants.
Keywords: Systemic acquired resistance, root-to-root transmission, signal transduction, benzothiadiazole, salicylic acid, Nicotiana benthamiana
INTRODUCTION
Plants have developed various resistance mechanisms to help them adapt to pathogen and insect attack (Green and Ryan, 1972; Agrawal, 1998; Jones and Dangl, 2006). The plant resistance system comprises constitutive defences, which are always present, and induced defences, which are subsequently expressed in plant parts distant from the primary site of attack. Systemic protection against subsequent invasion is referred to as ‘systemic acquired resistance’ (SAR) (Sticher et al., 1997; Hammerschmidt, 2009). SAR generally leads to the development of broad-spectrum and long-lasting responses against pathogens (Görlach et al., 1996; Hammerschmidt, 2009). SAR was initially reported to be induced by incompatible plant pathogens such as fungi, viruses and bacteria (Ross, 1961; Hammerschmidt, 2009). SAR is elicited by biological compounds, including chitins, ergosterols, glucans, lipopolysaccharides, proteins, peptides, salicylic acid (SA) and sphingolipids (Lyon, 2007). In addition to biological compounds, chemicals such as 2,6-dichloroisonicotinic acid (INA) and benzo[1,2,3]thiadiazole-7-carbothioic acid S-methyl ester (BTH) can also trigger SAR. BTH, the first commercialized agrochemical to trigger SAR (under the trade names Bion and Actigard in Europe and the USA, respectively), is effective against a broad spectrum of diseases and insect pests, including oomycetes in wheat and tobacco (Nicotiana tabacum), and even insects in tomato (Solanum lycopersicum) (Tally et al., 1999). BTH increases endogenous SA levels and activates SA-dependent signalling pathways in plants (Gaffney et al., 1993; Anand et al., 2008; Hammerschmidt, 2009). Reducing SA accumulation in plants through transformation with the SA-degrading gene NahG from the soil bacterium Pseudomonas putida significantly compromises their ability to combat pathogens (Delaney et al., 1994, Molina et al., 1998). SAR marker genes, including pathogenesis-related (PR) genes, have been identified and characterized; their induction is highly correlated with the onset of induced resistance in systemic tissues (Thomma et al., 2001). Chemical application of SA induces strong expression of defence-related genes, such as the defence genes, PR-1, PR-2 and PR-5, which are often used as marker genes in Arabidopsis (Thomma et al., 2001).
In addition to endogenous signal transmission, SAR can be transmitted to neighbouring plants. The well-known neighbouring SAR transmission system is an indirect form of defence involving transmission of volatile organic compounds (VOCs) and a common root–root network via fungal mycelia (Heil and Karban, 2010; Song et al., 2010). First, following wounding by a herbivore, certain plant tissues produce VOCs such as methyl jasmonate (MeJA), which plays an important role as an alarm signal for undamaged neighbours, resulting in increased levels of toxin and repellent production or the attraction of natural enemies of the herbivore (Paré and Tumlinson, 1999; Kessler et al., 2006; Heil, 2014). Secondly, mycorrhizal networks can transfer carbon, nitrogen and phosphorus from one plant to its neighbours (He et al., 2003; Simard et al., 2012). By transferring defence-related signalling compounds, root–root interactions also help boost defensive enzyme activities and defence-related gene expression in neighbouring plants (Farmer and Ryan, 1990; Song et al., 2010). However, root-mediated transmission of SAR signal has not been fully elucidated to date, while interest in root–root interactions has increased over the past several decades.
Most plant roots exude small molecular weight compounds into the rhizosphere, such as amino acids, organic acids, sugars and phenolics, as well as high molecular weight compounds such as polysaccharides and proteins (Walker et al., 2003; Bais et al., 2006). Our knowledge of root–root interactions, however, is quite limited, as it is still difficult to identify the signalling molecules responsible for such interactions. Root–root interactions via root exudate secretion can have both negative and positive effects. Negative effects include the allelopathic mechanism present in most plant species, i.e. plants interfere with the growth of neighbouring plants by exuding phytotoxins around the rhizosphere (Bertin et al., 2003; Bais et al., 2006). The positive effects of these interactions include the increased herbivore resistance induced in neighbouring plants through root exudates, although there are only a few examples of this phenomenon (Bais et al., 2004, 2006; Badri and Vivanco, 2009). For instance, Elytrigia repens produces carboline for defence against aphids for both its own defence response and the defence responses of neighbouring plants, such as Hordeum vulgare (Glinwood et al., 2003). In addition to having direct effects on herbivore behaviour, some root exudates induce defence responses in neighbouring plants that indirectly reduce herbivore populations by attracting predators and parasites of the offending herbivore. For example, Vicia faba plants under attack release root exudates that induce green leaf volatile production in undamaged V. faba plants, which in turn attract aphid parasitoids (Chamberlain et al., 2001). Similarly, Phaseolus lunatus (lima bean) plants under attack by spider mites produce root exudates that induce VOC production in undamaged P. lunatus plants, attracting predatory mites (Guerrieri et al., 2002).
In this study, we investigated whether SAR is transmissible through root–root transmission from a plant exhibiting SAR to its neighbouring plants. The bacterial wilt caused by Ralstonia solanacearum in roots and wildfire caused by Pseudomonas syringae pv. tabaci (Pta) on leaves were significantly attenuated in neighbouring plants of SAR-induced plants. Further study determined that SA is a major molecule involved in this SAR transmission. Tobacco rattle virus (TRV)-based virus-induced gene silencing (VIGS)-mediated knock-down of isochorismate synthase 1 (ICS1) and suppressor of G2 allele of skp1 (SGT1) in N. benthamiana plants compromised the protective effect of SAR transmission against two pathogens. The biological relevance of this phenomenon was also validated by challenging the plants with biologically SAR-inducing avirulent pathogen such as P. syringae pv. syringae (Psy) instead of BTH, which produced similar results. Our results clearly demonstrate that SAR is transmissible through root–root interactions between plants exhibiting SAR and neighbouring plants through secretion of SA from roots, thereby priming the SAR response.
MATERIALS AND METHODS
Plant preparation and treatments
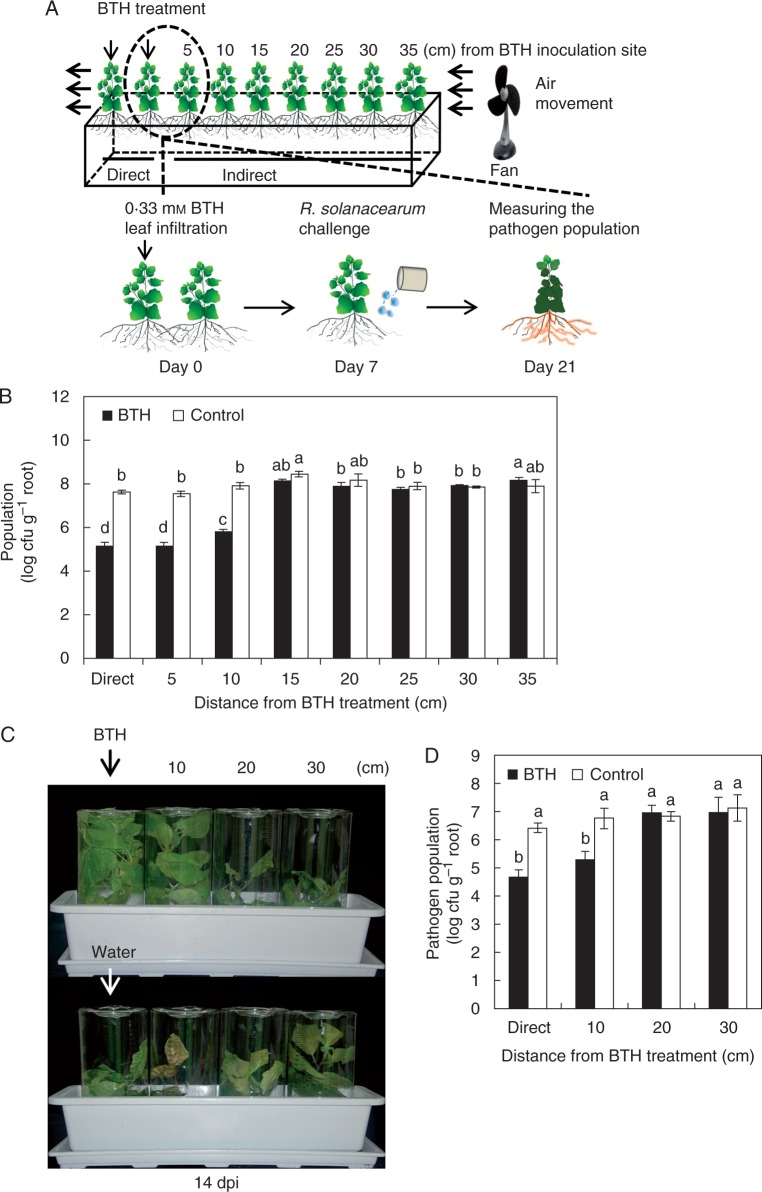
Seeds of Nicotiana benthamiana were surface sterilized with 6 % sodium hypochlorite, washed four times with sterilized distilled water and sown on autoclaved soil-less potting medium (Punong, Co. Ltd, Gyeongju, South Korea) that contained zeolite, perlite, colour dust and lime (pH range from 4·5 to 7·5). Nutrients included in the soil-less potting medium were nitrate-nitrogen, ammonium-nitrogen, phosphorus, potassium, calcium, magnesium, boron, copper, iron, manganese, zinc, molybdenum, sodium and aluminium. Nicotiana benthamiana plants were cultivated in a growth chamber at 25 °C under a 16 h/8 h light/dark photocycle. Each pot (60 × 15 × 15 cm) contained ten N. benthamiana plants. Five leaves from each of two plants (3 week old) were separately pressure infiltrated with 5 ml of 0·33 mM BTH (“Direct” treatment) or 5 ml of sterilized distilled water (control) using a needleless syringe. To eliminate SAR transmission via volatiles, we have used an electric fan (Model no. WDF-100C; BKW Inc., Anyang, South Korea) to keep volatiles from BTH-treated plants from moving to neighbouring plants (Fig. 1A). To block leaf-to-leaf contact between plants, the plants were grown in plastic cylinders (Fig. 1C). Each pot (45 × 15 × 15 cm) contained four N. benthamiana plants at 10 cm distance. Five leaves from the first plant out of four N. benthamiana were treated with BTH as described above.
Fig. 1.
Root-mediated transmission of systemic acquired resistance (SAR) against a soil-borne pathogen R. solanacearum. (A) Schematic representation of the experimental design described in the Materials and Methods. (B) The bacterial cell numbers were measured at 14 d after challenge with 108 cfu mL–1 R. solanacearum in pre-treated plants. (C) Root-mediated transmission of SAR upon blocking direct contact of foliar tissues between N. benthamiana plants. Representative photograph taken at 14 days post-inoculation (dpi) with R. solanacearum. (D) The bacterial cell numbers were was measured at 12 d after pathogen inoculation. Bars represent the mean ± s.e. m. (sample size, n = 5 replications per treatment). Different letters indicate significant differences between treatments (P = 0·05 according to least significant difference). The experiment was repeated three times with similar results.
Pathogen challenge
Ralstonia solanacearum, Pta and Psy were grown on solid Casamino acid-Peptone-Glucose (CPG) and King’s B medium respectively containing 100 μg mL–1 rifampicin for selection at 30 °C for 2 d, scraped off the plates and re-suspended in 10 mm MgCl2 (Roberts et al., 1988; Song et al., 2015). A 50 mL aliquot of bacterial suspension of R. solanacearum at OD600 = 1 was freshly prepared and drenched into the root system of every N. benthamiana seedling (Chandrasekaran et al., 2016). A 10 mL suspension of Pta (OD600 = 1) was sprayed on the leaves of N. benthamiana plants at 1 week after leaf infiltration with the chemical BTH (Figs 1 and 3). The severity of R. solanacearum symptoms was scored from 0 to 5 as follows: 0, no leaves wilted; 1, 1–20 % of leaves wilted; 2, 21–40 % of leaves wilted; 3, 41–60 % of leaves wilted; 4, 61–80 % of leaves wilted; and 5, 81–100 % of leaves wilted (Roberts et al., 1988). The severity of Pta symptoms was scored from 0 to 5 as follows: 0, no symptoms; 1, yellowish colour; 2, chlorosis only; 3, partial necrosis and chlorosis; 4, necrosis of the inoculated area and expanded chlorosis; and 5, complete necrosis of the inoculated area. The total number of R. solanacearum in the rhizosphere was counted at 0 and 14 d after drench application. Each whole root was collected without soil particles, placed in a flask containing 200 mL of sterilized distilled water and incubated with shaking for 30 min at 30 °C. The liquid from the flask was then plated on CPG agar containing 100 μg mL–1 rifampicin. The total number of Pta in leaves was counted at 0 and 7 d after spray application. Leaf discs (1 cm diameter) had been ground in 10 mm MgCl2, and serial dilutions of bacterial solution were spread onto selection medium (King’s B agar medium containing 100 μg mL–1 rifampicin), and incubated for 2 d in a 30 ºC chamber. For evaluation of biological relevance by the elicitation of SAR by Psy, 7 d after challenge with the avirulent pathogen Psy (OD600 = 0·01 in 10 mm MgCl2) inducing SAR on the first two out of ten plants similar to the previous experiment with BTH, bacterial suspensions of 108 colony-forming units (cfu) mL–1 Pta were sprayed onto the all leaves of two plants. This experiment had five replicates and one plant per replicate. The experiment was repeated three times with similar results.
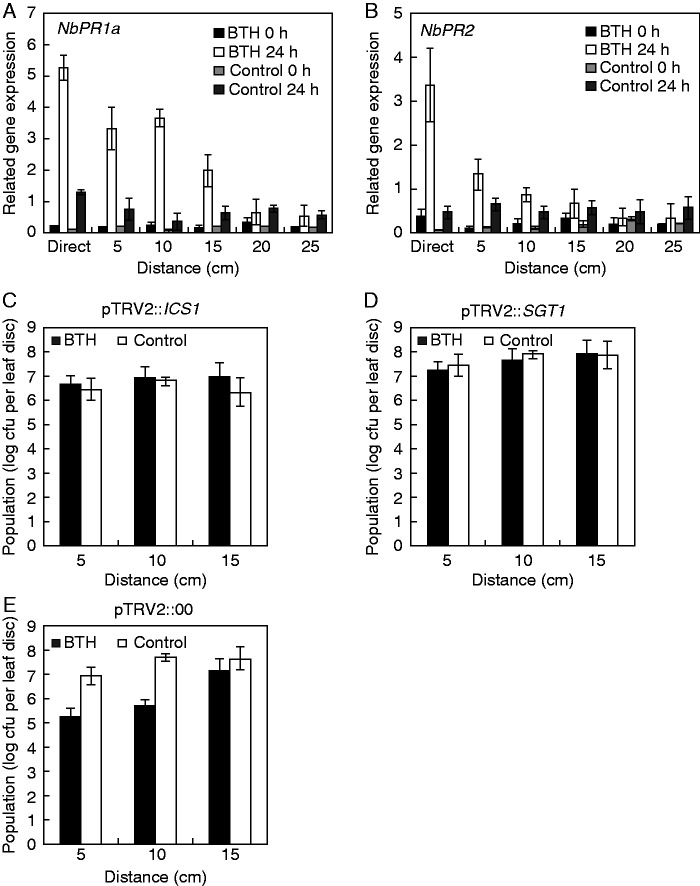
Fig. 3.
Transcriptional expression of defence genes and virus-induced gene silencing to validate salicylic acid (SA)-dependent signalling. The expression levels of SA-dependent resistance genes NbPR1 (A) and NbPR2 (B) were assessed by qRT–PCR. Bars represent the mean value ± s.e.m. (n = 4). The housekeeping gene NbActin was used as a control. The experiment was repeated twice with similar results. Effect of virus-induced gene silencing (VIGS) of NbICS1 (C) and NbSGT1 (D) on root-mediated transmission of systemic acquired resistance. Two-week-old N. benthamiana seedlings were infiltrated with Tobacco rattle virus 2-vector (TRV2) vector control (E) or TRV2 harbouring NbICS1 (C) and NbSGT1 (D), and monitored for 2 weeks. The silenced N. benthamiana plants were treated with BTH or water (control) for 7 d and inoculated with 108 cfu mL–1 R. solanacearum in plants pre-treated with BTH or water and their neighbouring plants. The experiment was repeated three times with similar results.
Root exudate collection
In vitro, N. benthamiana seeds were surface sterilized and germinated as described above. Four-day-old seedlings were transferred to plates (60 × 15 mm, SPL) containing 26 mL of 0·5× Murashige and Skoog liquid medium (Song et al., 2015). Plates were placed in a plastic container (Phytohealth, 103 × 78·6 mm, SPL) (Fig. 2A). Three leaves of each 3-week-old plant were pressure-infiltrated with 0·2 mL of 0·33 mm BTH (BTH) or 0·2 mL of sterilized water (control) using a needleless syringe. Root exudates were collected at 7 d after treatment, when the plants were 28 d old. For each replicate (containing two plants), 20 mL of root exudate was collected from the plates. No medium contamination was observed during the experiment. In the rhizosphere, soil was collected at 7 d after treatment as described above. Then 5 g of soil was extracted with 30 mL of methanol containing an internal standard and evaporated. An isotope-labelled standard (salicylic acid-D4) was used as an internal standard. The residues were dissolved with 70 % methanol, vortexed for 15 min and centrifuged at 15 000 rpm for 10 min at 4 °C. The final supernatants were transferred to a liquid crystallography (LC) vial and injected into the LC/mass spectrometry (MS) system. This experiment had 12 replicates and one plant per replicate. The experiment was repeated three times with similar results.
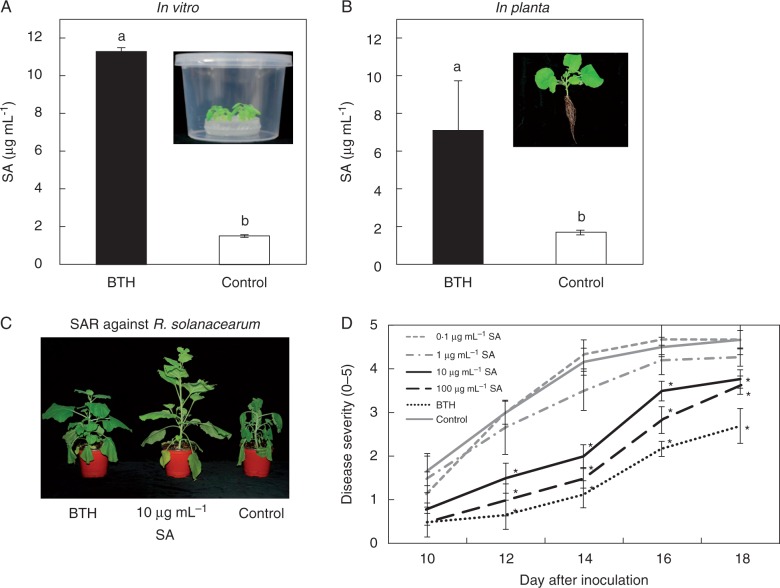
Fig. 2.
Salicylic acid (SA) as a determinant from root exudates of systemic acquired resistance (SAR)-induced plants. (A inset) Schematic representation of the experimental set-up. (A) Three-week-old N. benthamiana plants grown in 0·1× MS liquid medium and (B) in the soil were treated with BTH or water (control). (B inset) The representative photo of N. benthamiana seedling in the soil. Endogenous total SA production was measured in root exudates from BTH-infiltrated plants. (C) Representative photograph of symptoms taken at 14 d post-inoculation with R. solanacearum. (D) Disease severity (0–5) measured at 10–18 d after challenge with 108 cfu mL–1 R. solanacearum in plants pre-treated with different concentrations of SA. Bars represent the mean ± s.e.m (sample size, n = 12 replications per treatment). Different letters indicate significant differences between treatments (P = 0·05 according to least significant difference). The experiment was repeated three times with similar results.
Quantification of endogenous plant defence hormone levels in the root exudate
Five replicate samples per treatment were analysed to quantify SA concentrations in response to R. solanacearum in root exudates of BTH- and water-treated plants in in vitro and in planta conditions. Ultra-performance liquid chromatography (UPLC) analysis was performed using an ACQUITY®UPLC system (Waters Corp., Milford, MA, USA) coupled to a QTOF instrument (XEVO G2XS; Waters Corp.). The chromatographic separation was carried out on an ACQUITY®UPLC BEH C18 column (100 × 2·1 mm, id, 1·7 μm) connected to an ACQUITY®UPLC BEH C18 VanGuard™ pre-column (5 × 2·1 mm, id, 1·7 μm). The mobile phases consisted of solvent A (0·1 % formic acid) and solvent B (acetonitrile). The gradient elution mode was programmed as follow: 20–25 % B for 0·0–5·0 min and 25–35 % B for 5·0–10·0 min. The column was then washed with 95 % B for 3 min and equilibrated with 20 % B for 2 min. All samples were kept at 10 °C during the analysis. The flow rate and injection volume were 0·4 mL min–1 and 2 μL, respectively. MS analysis was conducted in the negative ion mode with electrospray ionization (ESI). The MS conditions were optimized as follows; capillary voltage, 3 kV; cone voltage, 40 V; source temperature, 130 °C; desolvation temperature, 400 °C; cone gas flow, 50 L h–1; desolvation gas flow, 900 L h–1.
Virus-induced gene silencing (VIGS)
For VIGS, 2-week-old tobacco seedlings were infiltrated with TRV-based VIGS vectors (pTRV2) containing N. benthamiana homologues of the plant defence-related and plant defence hormone signalling genes NbSGT1 and NbICS1 or with the control vector pTRV2::00, as described previously (Anand et al., 2008; Senthil-Kumar and Mysore, 2014; Kim et al., 2016). The Agrobacterium tumefaciens strain GV2260 containing TRV-VIGS vectors was used for VIGS experiments. Agrobacterium tumefaciens were grown at 28 °C on Luria–Bertani (LB) broth with appropriate antibiotics. Agrobacterium inoculation buffer (10 mm MgCl2, 10 mm MES pH 5·6, 150 μm acetosyringone) was adjusted to a final OD600 of 1·0 (for both TRV1 and TRV2) and shaken for 4 – 6 h at room temperature before infiltration. For leaf infiltration, each Agrobacterium strain containing TRV1 and TRV2 vectors was mixed in a 1:1 ratio and infiltrated into the leaves of 3-week-old plants with a needleless syringe. The infiltrated plants were monitored for approx. 14 d. This experiment had nine replicates and one plant per replicate. The experiment was repeated three times with similar results.
Extraction of plant RNA, cDNA synthesis and real-time quantitative PCR
Following inoculation with pathogen, the plants were returned to the growth chamber, and root tissue was harvested 0 and 24 h after inoculation with R. solanacearum and used for total RNA isolation. Total RNA was isolated using an RNeasy® plus mini kit according to the manufacturer’s protocol (Qiagen, USA). First-strand cDNA was synthesized using 2 μg of RNA, oligo(dT) primer, dNTP and Moloney murine leukaemia virus reverse transcriptase (M-MLV RT; Enzynomics, Daejeon, South Korea). Quantitative reverse transcription–PCR (qRT–PCR) was carried out using a Chromo4 real-time PCR system (Bio-Rad, CA, USA). The reaction mixture contained 2× Brilliant SYBR Green qRT-PCR Supermix (Bio-Rad), cDNA and 0·5 μm of each gene-specific primer. The expression of candidate priming genes was analysed using the following primers: 5′-AATATCCCACTCTTGCCG-3′ (NbPR1a-F), 5′-CCTGGAGGATCATAGTTG-3′ (NbPR1a-R), 5′-ACCATCAGACCAAGATGT-3′ (NbPR2-F) and 5′-TGGCTAAGAGTGGAAGGT-3′ (NbPR2-R). Relative RNA levels were calibrated and normalized relative to the level of NbACT mRNA (GenBank accession no. U60489). The sequences were amplified using the following thermocycler parameters: 10 min at 95 °C, followed by 44 cycles of 30 s at 95 °C, 30 s at 60 °C and 42 s at 72 °C.
Statistical analysis
Analysis of variance (ANOVA) for experimental data sets was performed using JMP software version 5·0 (SAS Institute Inc., Cary, NC, USA; www.Sas.com). Significant effects of treatment were determined based on the magnitude of the F-value (P = 0·05). When a significant F-test was obtained, separation of means was accomplished by Fisher’s protected LSD at P = 0·05.
RESULTS
Induction of systemic resistance by induced systemic resistance in neighbouring plants against an above-ground pathogen
To test the hypothesis that SAR in BTH-elicited plants could induce SAR in neighbouring plants via root transmission, we assessed the total R. solanacearum bacterial population at 0 and 14 days post-inoculation (dpi). The initial R. solanacearum population comprised 104 cfu mL−1 no difference in population levels between treatments was observed on day 0 (data not shown). The R. solanacearum population sizes in plants 5 and 10 cm from direct SAR-induced plants at 14 dpi were lower than those observed with water-treated plants (Fig. 1B). Similarly, the population density in plants 15 cm from direct SAR-induced plants was not reduced relative to the water-treated control (Fig. 1B). Nicotiana benthamiana seedlings grown inside the plastic cylinder to eliminate the possibility of leaf-to-leaf contact (Fig. 1C) showed a similar pattern to that obtained in the experiment without the plastic cylinder (Fig. 1B, D), indicating that the signal transmission through leaf contact is unlikely to occur.
SA is a major component of SAR root-to-root
To determine the substances in root exudates that are transmitted in the root–root interaction, we developed an in vitro root exudate collection system (Song et al., 2015). We attempted to search for the plant determinant corresponding to the root–root transmission signal. In our previous similar study, the leaf infestation of whitefly elicited SA secretion in the root system (Song et al., 2015). Due to similar induction of SA-dependent signalling between whitefly and BTH treatment, we examined SA secretion from the root after leaf infiltration with BTH and successfully detected SA in the root exudate (Song et al., 2015; Fig. 2A, B). To verify the accumulation of total SA in root exudates from SAR-induced plants, we measured total SA levels in root exudate samples (Fig. 2A). SA levels in root exudates were approx. 7·6-fold higher in SA-induced plants than in control plants (Fig. 2A). We also measured total SA levels in the rhizosphere; SA levels in the rhizosphere were approx. 3·8-fold higher in SA-induced plants than in control plants. The average SA amount was 4·1 μg mL–1 less than that in the in vitro experiment. To validate the effectiveness of SA of which concentration was detected from root exudate (Fig. 2A, B), we performed drench application of 10 μg mL–1 and 100 μg mL–1 SA reduced disease severity at 10 to 18 dpi (every 2 d), respectively, compared with the controls (Fig. 2C, D). Drench application of the positive control 0·33 mm BTH reduced disease symptom development in N. benthamiana plants infected with R. solanacearum (Fig. 2C, D).
Defence gene expression in neighbouring plant roots of SAR-induced plants
To examine whether SAR-related defence genes in neighbouring plants were upregulated by SAR-induced plants, we measured transcript levels of SA signalling-related genes such as PR1a and PR2. Specifically, we investigated the expression of the defence-related N. benthamiana genes PR1a (NbPR1a) and NbPR2 in neighbouring plant roots after 0 and 24 h of pathogen challenge using qRT–PCR. NbPR1a transcript levels increased 20-, 14- and 12-fold from 0 to 24 hours post-inoculation (hpi) in plants 5, 10 and 15 cm from SAR-induced plants, respectively, while 3·5-, 3·2- and 2·8-fold increases were detected in control neighbouring plants, respectively (Fig. 3A). NbPR2 transcript levels increased 10-fold from 0 to 24 hpi in plants 5 cm from SAR-induced plants, while a 4·5-fold increase was detected in control neighbouring plants (Fig. 3B). Unlike NbPR1a expression, NbPR2 expression did not differ between plants 10 and 15 cm from SAR-induced plants and the control (Fig. 3B). In addition, in plants 20 and 25 cm from SAR-induced plants, neither NbPR1a nor NbPR2 expression was altered compared with the control (Fig. 3A, B). Silencing the SA biosynthetic genes ICS1 and SGT1 compromised the protective effect of SAR-induced plants on neighbouring plants against R. solanacearum. To identify plant SA biosynthetic genes involved in root-mediated transmission, we used VIGS with TRV 2-vector to silence N. benthamiana NbSGT1 and NbICS1. We then infiltrated these plants with 0·33 mm BTH. After 7 d, neighbouring plants of the SAR-induced plants were inoculated with R. solanacearum as described above. For the empty vector (pTRV2::00) controls, the R. solanacearum population was significantly lower in plants 5 and 10 cm from SAR-induced plants vs. neighbouring plants of the water-treated control (Fig. 3E). In contrast, when NbICS1 and NbSGT1 were silenced, the R. solanacearum population sizes in neighbouring plants of SAR-induced plants were similar to those of the water-treated controls (Fig. 3C, D).
Root-mediated transmission of SAR against above-ground pathogens
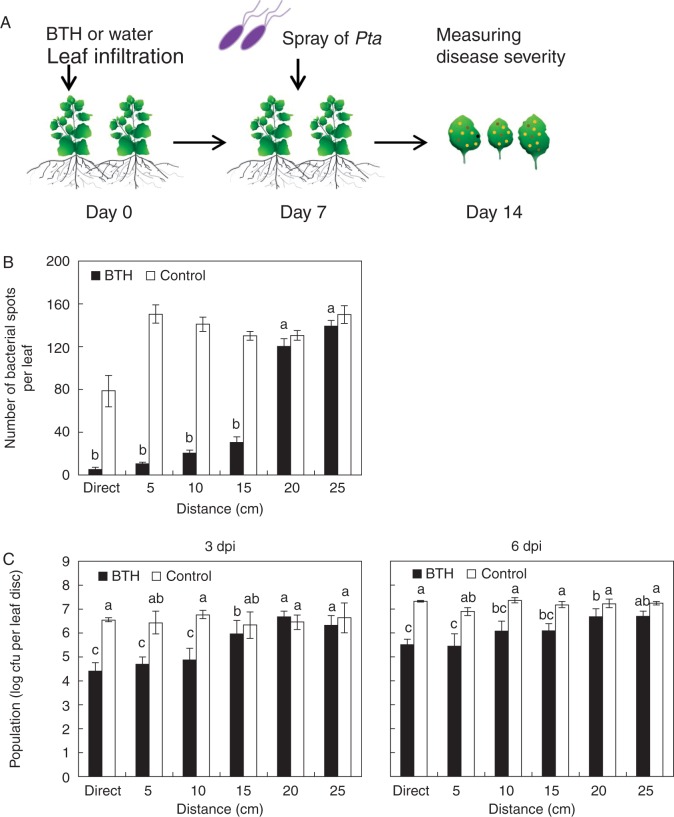
We then evaluated whether SAR-induced plants can induce resistance against above-ground pathogens in neighbouring plants using Pta (wildfire) as the pathogen. One week after initial treatments as described above, we inoculated N. benthamiana leaves with suspensions of Pta (OD600 = 1) (Fig. 4A) and assessed symptom development at 7 dpi. The number of bacterial spots was significantly reduced in plants 5, 10 and 15 cm from SAR-induced plants, with reductions of 14·5-, 6·8- and 4·3-fold compared with the control, respectively (Fig. 4B).
Fig. 4.
Root-mediated signal transmission of systemic acquired resistance against the foliar pathogen P. syringae pv. tabaci (Pta) (A) Schematic representation of the experimental design. (B) Number of bacterial spots measured at 7 d after challenge with 108 cfu mL–1 Pta in plants pre-treated with 0·33 mM BTH. (C) The bacterial cell count was measured 3 and 6 d after pathogen inoculation. Bars represent the mean ± s.e.m. (sample size, n = 5 replications per treatment). Different letters indicate significant differences between treatments (P = 0·05 according to least significant difference). The experiment was repeated three times with similar results.
At 3 dpi, the bacterial population sizes in plants 5, 10 and 15 cm from SAR-induced plants was log 4·7, 4·9 and 5 cfu per leaf disc, respectively, while those of the control were log 6·4, 6·8 and 6·3 cfu per leaf disc (Fig. 4C). At 6 dpi, the bacterial population sizes in plants 5, 10 and 15 cm from SAR-induced plants were reduced compared with the control. The differences in bacterial population sizes between neighbouring plants of BTH- and water-treated plants might be associated with the differences observed in symptom development (Fig. 4B, C).
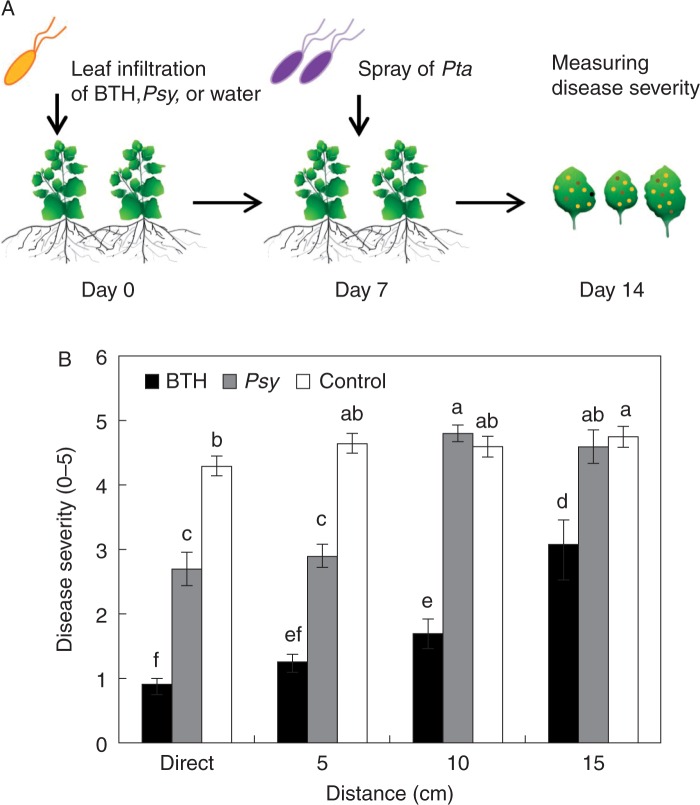
Root-mediated transmission of SAR biologically
To evaluate whether SAR-induced plants biologically (induced by an avirulent pathogen) can induce resistance against above-ground pathogens in neighbouring plants, we used an avirulent bacterial pathogen Psy to elicit SAR. Specifically, we infiltrated 3-week-old N. benthamiana plants with Psy, which was used as a biological elicitor of SAR (Fig. 5A). The disease severity index was 2·9 in plants 5 cm from Psy-treated, SAR-induced plants, 1·3 in plants 5 cm from BTH-treated, SAR-induced plants, and 4·6 in the untreated controls (Fig. 5B). However, plants 10 cm from Psy-treated, SAR-induced plants did not exhibit root-mediated transmission of SAR (Fig. 5B).
Fig. 5.
Root-mediated transmission of the avirulent pathogen P. syringae pv. syringae (Psy)-elicited systemic acquired resistance (SAR). (A) Schematic representing the experimental design. (B) Disease severity (0 – 5) measured at 7 d after challenge with 108 cfu mL–1 P. syringae pv. tabaci (Pta) in plants pre-treated with Psy (an avirulent pathogen) at various distances from the SAR-induced plant (cm). Bars represent the mean ± s.e.m. (sample size, n = 5 replications per treatment). Different letters indicate significant differences between treatments (P = 0·05 according to least significant difference). The experiment was repeated three times with similar results.
DISCUSSION
Here, we provide the first evidence of transmission of chemically and biologically elicited SAR from one plant to its neighbouring plants via root–root signal transmission. Further study demonstrated that SA’s role as a root-derived signal and determinant for root–root communication was confirmed in vitro and in the soil. Our results are in agreement with previous findings that plants perceive and adaptively respond to their environment based on subtle biotic signals (Farmer and Ryan, 1990; Baldwin et al., 2006; Yi et al., 2009).
Similarly, mycelial networks appear to transmit signalling cues to neighbouring plants through root–root interactions (Giovannetti et al., 2004; Song et al., 2010; Falik et al., 2012). Previous studies demonstrated that plant communication via common mycelial networks leads to increased disease resistance in uninfected tomato plants (Song et al., 2010; Barto et al., 2012) and that allelochemicals released by marigold (Tagetes tenuifolia Millsp.) are transported through arbuscular mycorrhizal (AM) fungal networks to inhibit the growth of neighbouring plants (Barto et al., 2011; Gorzelak et al., 2015). In addition to transmission of biological- and chemical-derived cues through root systems, abiotic stresses including osmotic stress and drought induced stomatal closure in both stressed Pisum sativum plants and their unstressed neighbours (Falik et al., 2011, 2012). Most studies of plant-to-plant interactions via common mycelial networks did not eliminate airborne-mediated SAR transmission between plants (Simard et al., 2012). For instance, lima bean (Phaseolus lunatus) and tobacco (Nicotiana tabacum) plants release modified VOCs upon SAR induction (Shulaev et al., 1997; Yi et al., 2009). Among the modified VOCs, nonanal and methyl salicylic acid were present in the headspace of BTH-treated and Tobacco mosaic virus-challenged plants, respectively, resulting in the reduced appearance of symptoms in the exposed neighbouring plants (Shulaev et al., 1997; Yi et al., 2009).
To support SAR root–root signal transmission, two possible signal transmissions need to be excluded: (1) mycelial network-based signal transduction; and (2) VOC-mediated induced resistance. First, the soil and seeds were sterilized before conducting all experiments to eliminate the mycelial network-based signal transduction (Mahmood et al., 2014). The possibility of any mycelial connection between plant roots was examined at the end of the experiment and it was confirmed that there was no fungal contamination (data not shown). Intriguingly, plants growing within 10 cm of BTH-treated plants displayed a lower population density of the root pathogen R. solanacearum (Fig. 1) suggesting that root pathogen colonization is negatively affected by specific root exudate. Alternatively, it can also affect both parasite and saprophyte leading to general effect on the rhizosphere. Secondly, to eliminate the effects of VOCs, we prevented volatiles in BTH-treated plants from moving to untreated plants by a constant flow system (Fig. 1A). Using a digital anemometer, we also confirmed that the air flow reached the last plant at as much as 0·1 – 0·3 m s–1 (data not shown). In addition, to block any leaf-to-leaf contact between plants, the isolated plants were grown in plastic cylinders, and root-mediated transmission of the SAR signal was examined and confirmed (Fig. 1C, D; Mousavi et al., 2013; Appel and Cocroft, 2014).
We still do not fully understand why the resistance to R. solanacearum was only exhibited in plants 10 cm away from the BTH-treated plant (Fig. 1). From measuring SA contents in the soil (Fig. 2B) and the disease assay with different concentrations of SA (Fig. 2D), we speculate that 10 μg mL–1 SA application was sufficient for induction of resistance against R. solanacearum (Figs 1B and 2D). The expression level of the SA signalling marker gene NbPR1a compared with the water control was coupled with SAR against Pta, and NbPR2 expression with that against R. solanacearum because SAR was maintained 10 cm away from BTH-treated plants against R. solanacearum and 15 cm away against Pta (Figs 1, 3 and 4). In this system, even within 24 h, the SAR signal was transmitted at least 10 cm away that is critical to protect the plant itself against each pathogen, indicating that constant production of an SAR signal may not have occurred or initial secretion is enough to elicit SAR in the adjacent plant (Datta and Muthukrishnan, 1999). To validate the hypothesis, the SA threshold to elicit the SAR from the root exudate in our system can be determined. Similarly, the stomata of P. sativum plants tend to close to prevent water loss under drought conditions (Falik et al., 2012). Such closure was detected in unstressed plants within 15 min of contact between these plants and stressed P. sativum plants (Falik et al., 2012). When the roots were lined up in order, the stomata in all plants closed within 1 h. Moreover, at 14 d after drought induction, only drought-treated plants (i.e. stressed P. sativum plants) and the neighbouring plant, which received the strongest signals, suffered weight loss compared with the control group (Falik et al., 2011, 2012).
To identify the signalling molecules transmitted from root to root, we cultivated plants in vitro and collected root exudates using a modified method that we previously designed (Fig. 2A; Song et al., 2015). Due to our previous experience with identification of signal molecules from root exudate, we hypothesized that BTH treatment induces the plant to secrete large amounts of SA from roots (Badri and Vivanco, 2009; Strehmel et al., 2014; Song et al., 2015). The measurement of SA levels in root exudates demonstrated that SA levels in BTH-treated plant root exudates were 7·6-fold higher than those in the control group (Fig. 2B). Further experiments supported the involvement of SA in silencing NbSGT1 and NbICS in N. benthamiana, thereby compromising SAR against R. solanacearum (Fig. 3C, D). These findings strongly suggest that SA is a signalling compound transmitted from root to root that is involved in SAR against leaf and root pathogens. SA at 10 μg mL–1 does not have a direct antagonistic effect on R. solanacearum or other diverse pathogens (data not shown), indicating that it represents SA only involved in SAR (Rivas-San Vicente and Plasencia, 2011)
We also examined whether root–root SAR transmission occurs in response to Psy (a biological SAR elicitor in the natural environment) (Fig. 5A; Casarrubias-Castillo et al., 2014). Like BTH-treated plants, Psy-challenged plants also transmitted SAR to neighbouring plants (Fig. 5). However, unlike BTH-treated plants, such transmission only occurred to plants located 5 cm away from the SAR-induced plants (vs. 10 cm for BTH-treated plants). It is possible that resistance genes are less strongly induced by the avirulent pathogen during the process of SAR transmission (Cameron et al., 1994; Kohler et al., 2002).
In conclusion, we first demonstrated that SAR is transmissible through the root system from SAR-triggered plants to neighbouring plants against R. solanacearum and P. syringae pv. tabaci. SA as a major signal molecule involved in SAR transmission in root exudates was validated by silencing of SA biosynthesis gene and transcriptional expression of SA-dependent PR genes. Our results suggest new evidence that dynamic signal transduction occurred through root systems in addition to the mycelial network and VOC-mediated plant resistance elicitation against plant pathogens.
ACKNOWLEDGEMENTS
We thank Dr. Dong Won Bae for measurement of SA contents. This research was supported by grants from the Industrial Source Technology Development Program of the Ministry of Knowledge Economy (10044909) of Korea, the cooperative Research Program for Agricutlture and Technology Development (Agenda Project No. PJ011707), and the KRIBB initiative program, South Korea.
LITERATURE CITED
- Agrawal AA. 1998. Induced responses to herbivory and increased plant performance. Science 279: 1201–1202. [DOI] [PubMed] [Google Scholar]
- Anand A, Uppalapati SR, Ryu C-M, et al. 2008. Salicylic acid and systemic acquired resistance play a role in attenuating crown gall disease caused by Agrobacterium tumefaciens. Plant Physiology 146: 703–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appel H, Cocroft R. 2014. Plants respond to leaf vibrations caused by insect herbivore chewing. Oecologia 175: 1257–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badri DV, Vivanco JM. 2009. Regulation and function of root exudates. Plant, Cell & Environment 32: 666–681. [DOI] [PubMed] [Google Scholar]
- Bais HP, Park S-W, Weir TL, Callaway RM, Vivanco JM. 2004. How plants communicate using the underground information superhighway. Trends in Plant Science 9: 26–32. [DOI] [PubMed] [Google Scholar]
- Bais HP, Weir TL, Perry LG, Gilroy S, Vivanco JM. 2006. The role of root exudates in rhizosphere interactions with plants and other organisms. Annual Review of Plant Biology 57: 233–266. [DOI] [PubMed] [Google Scholar]
- Baldwin IT, Halitschke R, Paschold A, Von Dahl CC, Preston CA. 2006. Volatile signaling in plant–plant interactions: ‘talking trees’ in the genomics era. Science 311: 812–815. [DOI] [PubMed] [Google Scholar]
- Barto EK, Hilker M, Müller F, Mohney BK, Weidenhamer JD, Rillig MC. 2011. The fungal fast lane: common mycorrhizal networks extend bioactive zones of allelochemicals in soils. PLoS One 6: e27195. doi:10.1371/journal.pone.0027195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barto EK, Weidenhamer JD, Cipollini D, Rillig MC. 2012. Fungal superhighways: do common mycorrhizal networks enhance below ground communication? Trends in Plant Science 17: 633–637. [DOI] [PubMed] [Google Scholar]
- Bertin C, Yang X, Weston LA. 2003. The role of root exudates and allelochemicals in the rhizosphere. Plant and Soil 256: 67–83. [Google Scholar]
- Cameron RK, Dixon RA, Lamb CJ. 1994. Biologically induced systemic acquired resistance in Arabidopsis thaliana. The Plant Journal 5: 715–725. [Google Scholar]
- Casarrubias-Castillo K, Martínez-Gallardo NA, Délano-Frier JP. 2014. Treatment of Amaranthus cruentus with chemical and biological inducers of resistance has contrasting effects on fitness and protection against compatible Gram positive and Gram negative bacterial pathogens. Journal of Plant Physiology 171: 927–939. [DOI] [PubMed] [Google Scholar]
- Chamberlain K, Guerrieri E, Pennacchio F, et al. 2001. Can aphid-induced plant signals be transmitted aerially and through the rhizosphere? Biochemical Systematics and Ecology 29: 1063–1074. [Google Scholar]
- Chandrasekaran M, Subramanian D, Yoon E, Kwon T, Chun SC. 2016. Meta-analysis reveals that the genus Pseudomonas can be a better choice of biological control agent against bacterial wilt disease caused by Ralstonia solanacearum. Plant Pathology Jounal 32: 216–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta SK, Muthukrishnan S. 1999. Pathogenesis-related proteins in plants. Boca Raton, FL: CRC Press. [Google Scholar]
- Delaney TP, Uknes S, Vernooij B, Friedrich L. 1994. A central role of salicylic acid in plant disease resistance. Science 266: 1247–1250. [DOI] [PubMed] [Google Scholar]
- Falik O, Mordoch Y, Ben-Natan D, Vanunu M, Goldstein O, Novoplansky A. 2012. Plant responsiveness to root–root communication of stress cues. Annals of Botany 110: 271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falik O, Mordoch Y, Quansah L, Fait A, Novoplansky A. 2011. Rumor has it…: relay communication of stress cues in plants. PLoS One 6: e23625. doi:10.1371/journal.pone.0023625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer EE, Ryan CA. 1990. Interplant communication: airborne methyl jasmonate induces synthesis of proteinase inhibitors in plant leaves. Proceedings of the National Academy of Sciences, USA 87: 7713–7716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffney T, Friedrich L, Vernooij B, et al. 1993. Requirement of salicylic acid for the induction of systemic acquired resistance. Science, 261: 754–754. [DOI] [PubMed] [Google Scholar]
- Giovannetti M, Sbrana C, Avio L, Strani P. 2004. Patterns of below-ground plant interconnections established by means of arbuscular mycorrhizal networks. New Phytologist 164: 175–181. [DOI] [PubMed] [Google Scholar]
- Glinwood R, Pettersson J, Ahmed E, Ninkovic V, Birkett M, Pickett J. 2003. Change in acceptability of barley plants to aphids after exposure to allelochemicals from couch-grass (Elytrigia repens). Journal of Chemical Ecology 29: 261–274. [DOI] [PubMed] [Google Scholar]
- Görlach J, Volrath S, Knauf-Beiter G, et al. 1996. Benzothiadiazole, a novel class of inducers of systemic acquired resistance, activates gene expression and disease resistance in wheat. The Plant Cell 8: 629–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorzelak MA, Asay AK, Pickles BJ, Simard SW. 2015. Inter-plant communication through mycorrhizal networks mediates complex adaptive behaviour in plant communities. AoB PLANTS 7: plv050. doi:10.1093/aobpla/plv050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green T, Ryan CA. 1972. Wound-induced proteinase inhibitor in plant leaves: a possible defense mechanism against insects. Science 175: 776–777. [DOI] [PubMed] [Google Scholar]
- Guerrieri E, Poppy G, Powell W, Rao R, Pennacchio F. 2002. Plant-to-plant communication mediating in-flight orientation of Aphidius ervi. Journal of Chemical Ecology 28: 1703–1715. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt R. 2009. Systemic acquired resistance. Advances in Botanical Research 51: 173–222. [Google Scholar]
- He X-H, Critchley C, Bledsoe C. 2003. Nitrogen transfer within and between plants through common mycorrhizal networks (CMNs). Critical Reviews in Plant Sciences 22: 531–567. [Google Scholar]
- Heil M. 2014. Herbivore-induced plant volatiles: targets, perception and unanswered questions. New Phytologist 204: 297–306. [Google Scholar]
- Heil M, Karban R. 2010. Explaining evolution of plant communication by airborne signals. Trends in Ecology and Evolution, 25: 137–144. [DOI] [PubMed] [Google Scholar]
- Jones JD, Dangl JL. 2006. The plant immune system. Nature 444: 323–329. [DOI] [PubMed] [Google Scholar]
- Kessler A, Halitschke R, Diezel C, Baldwin IT. 2006. Priming of plant defense responses in nature by airborne signaling between Artemisia tridentata and Nicotiana attenuata. Oecologia 148: 280–292. [DOI] [PubMed] [Google Scholar]
- Kim KH, Lim S, Kang YJ, et al. 2016. Optimization of a virus-induced gene silencing system with Soybean yellow common mosaic virus for gene function studies in soybeans. Plant Pathology Journal 32: 112–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler A, Schwindling S, Conrath U. 2002. Benzothiadiazole-induced priming for potentiated responses to pathogen infection, wounding, and infiltration of water into leaves requires the NPR1/NIM1 gene in Arabidopsis. Plant Physiology 128: 1046–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon G. 2007. Agents that can elicit induced resistance In: Walters DR, Newton AC, Lyon GD, eds Induced resistance for plant defence. A sustainable approach to crop protection. Oxford: Blackwell Publishing Ltd, 9–29. [Google Scholar]
- Mahmood T, Mehnaz S, Fleischmann F, Ali R, Hashmi Z, Iqbal Z. 2014. Soil sterilization effects on root growth and formation of rhizosheaths in wheat seedlings. Pedobiologia 57: 123–130. [Google Scholar]
- Molina A, Hunt MD, Ryals JA. 1998. Impaired fungicide activity in plants blocked in disease resistance signal transduction. The Plant Cell 10: 1903–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousavi SA, Chauvin A, Pascaud F, Kellenberger S, Farmer EE. 2013. GLUTAMATE RECEPTOR-LIKE genes mediate leaf-to-leaf wound signalling. Nature 500: 422–426. [DOI] [PubMed] [Google Scholar]
- Paré PW, Tumlinson JH. 1999. Plant volatiles as a defense against insect herbivores. Plant Physiology 121: 325–332. [PMC free article] [PubMed] [Google Scholar]
- Rivas-San Vicente M, Plasencia J. 2011. Salicylic acid beyond defence: its role in plant growth and development. Journal of Experimental Botany 62: 3321–3338. [DOI] [PubMed] [Google Scholar]
- Roberts D, Denny T, Schell M. 1988. Cloning of the egl gene of Pseudomonas solanacearum and analysis of its role in phytopathogenicity. Journal of Bacteriology 170: 1445–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross AF. 1961. Systemic acquired resistance induced by localized virus infections in plants. Virology 14: 340–358. [DOI] [PubMed] [Google Scholar]
- Senthil-Kumar M, Mysore KS. 2014. Tobacco rattle virus-based virus-induced gene silencing in Nicotiana benthamiana. Nature Protocols 9: 1549–1562. [DOI] [PubMed] [Google Scholar]
- Shulaev V, Silverman P, Raskin I. 1997. Airborne signalling by methyl salicylate in plant pathogen resistance. Nature 385: 718–721. [Google Scholar]
- Simard SW, Beiler KJ, Bingham MA, Deslippe JR, Philip LJ, Teste FP. 2012. Mycorrhizal networks: mechanisms, ecology and modelling. Fungal Biology Reviews 26: 39–60. [Google Scholar]
- Song GC, Lee S, Hong J, et al. 2015. Aboveground insect infestation attenuates belowground Agrobacterium-mediated genetic transformation. New Phytologist 207: 148–158. [DOI] [PubMed] [Google Scholar]
- Song YY, Zeng RS, Xu JF, Li J, Shen X, Yihdego WG. 2010. Interplant communication of tomato plants through underground common mycorrhizal networks. PLoS One 5: e13324. doi:10.1371/journal.pone.0013324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sticher L, Mauch-Mani B, Métraux JP. 1997. Systemic acquired resistance. Annual Review of Phytopathology 35: 235–270. [DOI] [PubMed] [Google Scholar]
- Strehmel N, Böttcher C, Schmidt S, Scheel D. 2014. Profiling of secondary metabolites in root exudates of Arabidopsis thaliana. Phytochemistry 108: 35–46. [DOI] [PubMed] [Google Scholar]
- Tally A, Oostendorp M, Lawton K, et al. 1999. Commercial development of elicitors of induced resistance to pathogens In: Agrawal AA, Tuzun S, Bent E, eds. Induced plant defenses against pathogens and herbivores: biochemistry, ecology, and agriculture. St Paul, MN: APS Press, 357–369. [Google Scholar]
- Thomma BP, Penninckx IA, Cammue BP, Broekaert WF. 2001. The complexity of disease signaling in Arabidopsis. Current Opinion in Immunology 13: 63–68. [DOI] [PubMed] [Google Scholar]
- Walker TS, Bais HP, Grotewold E, Vivanco JM. 2003. Root exudation and rhizosphere biology. Plant Physiology 132: 44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi H-S, Heil M, Adame-Álvarez RM, Ballhorn DJ, Ryu C-M. 2009. Airborne induction and priming of plant defenses against a bacterial pathogen. Plant Physiology 151: 2152–2161. [DOI] [PMC free article] [PubMed] [Google Scholar]