Abstract
Background:
Research suggests that the central nervous system (CNS) and mobility are closely linked. CNS-mediated mobility impairment may represent a potentially new and prevalent syndrome within the older adult populations. Interventions targeting this group may have the potential to improve mobility and cognition and prevent disability.
Methods:
In 2012, the Gerontological Society of America (GSA) and the National Institute on Aging (NIA) sponsored a 3-year conference workshop series, “Aging, the CNS, and Mobility.” The goal of this third and final conference was to (i) report on the state of the science of interventions targeting CNS-mediated mobility impairment among community-dwelling older adults and (ii) partnering with the NIA, explore the future of research and intervention design focused on a potentially novel aging syndrome.
Results:
Evidence was presented in five main intervention areas: (i) pharmacology and diet; (ii) exercise; (iii) electrical stimulation; (iv) sensory stimulation/deprivation; and (v) a combined category of multimodal interventions. Workshop participants identified important gaps in knowledge and key recommendations for future interventions related to recruitment and sample selection, intervention design, and methods to measure effectiveness.
Conclusions:
In order to develop effective preventive interventions for this prevalent syndrome, multidisciplinary teams are essential particularly because of the complex nature of the syndrome. Additionally, integrating innovative methods into the design of interventions may help researchers better measure complex mechanisms, and finally, the value of understanding the link between the CNS and mobility should be conveyed to researchers across disciplines in order to incorporate cognitive and mobility measurements into study protocols.
Keywords: CNS, Cognition, Disability
The relationship between the central nervous system (CNS) and mobility has been studied extensively in animal models; these rigorously controlled experiments indicate that physical movement in an environment is intimately connected to the CNS at the level of molecules, neurons, signaling pathways, and behavior (1). Evidence from human studies in patient populations with neurological disorders also confirms that the CNS is an important contributor to mobility and gait function (2). In community-dwelling older adult populations, free of neurologic disorders, clinically diagnosable gait abnormalities, including slowed gait speed and altered pace and rhythm, in addition to worse dual-task walking performance, are also prevalent (3). Dual-task walking paradigms are defined as those designed to test a mobility function while imposing a cognitive demand. Researchers in multidisciplinary fields ranging from epidemiology and neurology to rehabilitation are beginning to study older adults with gait abnormalities because they are at high risk of a range of adverse health outcomes including mortality, disability, and falls (4–6). Evidence suggests that gait abnormalities are also risk factors for cognitive decline, mild cognitive impairment, and dementia (7–9); conversely, impaired cognition is also a risk factor for preclinical gait abnormalities, mobility limitations, and falls (10,11). Taken together, these findings suggest that the CNS and mobility are closely linked and at least partially dependent on each other, and abnormalities or impairments in both may share common underlying pathophysiology (10). Clearly understanding these associations and overlaps in mechanistic pathways can lead to targeted interventions that have great potential in the treatment of mobility and cognitive impairment as well as the prevention of disability.
In 2012, the Gerontological Society of America (GSA) and the National Institute on Aging (NIA) sponsored a 3-year conference workshop series, “Aging, the CNS, and Mobility,” that was focused on defining, exploring, and understanding potentially novel signs and symptoms among a large community-dwelling older adult population with mobility impairment who are free of overt neurologic disorders and major disabilities. This large and at-risk group with age-related mobility decline represents an important target for novel interventions specific to underlying CNS-mobility impairments and pathology that may halt the disablement process from impairment to disability (12).
The first CNS and mobility workshop, held in San Diego, California in November 2012, reviewed the evidence and associated risk factors from basic and clinical to epidemiologic studies examining the relationship between CNS and mobility (13). Cognitive domain-specific associations with gait were identified, with the strongest correlations observed for information processing and executive function; changes or disruptions in gait were identified as strong predictors of cognitive decline and dementia; and emphasis was placed on understanding shared brain structure and function that may explain declines in both cognitive and motor functions, including general CNS abnormalities associated with aging (eg, brain atrophy, white matter hyperintensities, and small vessel disease) and shared neural networks including the prefrontal cortex. Workshop 1 additionally identified knowledge gaps, including the lack of a clear understanding of the pathophysiology and neural mechanisms that can be targeted by interventions, and emphasized the importance of multidisciplinary research efforts that include a wide range of mobility, cognitive, and brain health measures to test pharmaceutical and nonpharmaceutical preventive and treatment interventions (13).
Workshop 2 was held in New Orleans, LA, in November 2013. It focused on exploring and describing the neural mechanisms underpinning age-related and pathologic mobility impairment and disability with the goal of identifying pathways to serve as targets for interventions (14). The workshop identified four major age-associated mechanisms of CNS-mediated mobility impairment (CNS-MMI): (i) neurovasculature and associated risk factors including hypertension and cerebrovascular pathology, including white matter hyperintensities, cerebral small vessel disease, and impaired cerebral vasoreactivity; (ii) genetic and metabolic mechanisms and associated risk factors and disorders including hereditary cerebral arteriopathy (eg, cerebral autosomal dominant arteriopathy with subcortical infarcts and leucoencephalopathy [CADASIL]) caused by mutations of the Notch 3 gene on Chromosome 19; dysregulation of the renin–angiotensin system and alterations in the production of neurotophins (eg, brain-derived neurotrophic factor); (iii) inflammatory pathways and bioenergetics also relevant to multiple sclerosis and Parkinson’s disease (PD); and (iv) neuromotor control and activation pathways and other systems, including the autonomic nervous system and sensory systems. Workshop 2 additionally emphasized the importance of promoting research on age-associated mobility decline through integrating multidisciplinary perspectives and shared research paradigms, as well as the development of innovative approaches, including systems level investigation of neural networks, to understand CNS networks (14).
The third and final workshop in the “Aging, the CNS, and Mobility” series was held prior to the GSA conference in November 2014 in Washington, DC. The goals of this workshop were to (i) report on the state of the science of interventions targeting CNS-MMI among community-dwelling older adults and (ii) partnering with the NIA, explore the future of research and intervention design focused on a novel aging phenotype or syndrome. This article provides a summary of the third workshop.
Approach
Workshop 3 built on Workshops 1 and 2, which summarized risk factors and mechanisms supporting the associations between the CNS and mobility. This workshop critically evaluated interventions that target modifiable risk factors associated with mobility and cognitive impairment, by bringing together a multidisciplinary panel of intervention experts in the fields of epidemiology, geriatrics, gerontology, neurology, neuropsychology, ophthalmology, otolaryngology, physical therapy, and rehabilitation. The workshop that included a series of lectures and discussions, group break-out sessions, round table meetings, and a junior investigator poster session focused on the latest findings from research on CNS, cognition, and mobility. In addition, NIA scientific officers provided guidance and recommendations to support the design of the next generation of interventions. Please see Supplementary Appendix 1 for a list of workshop attendees.
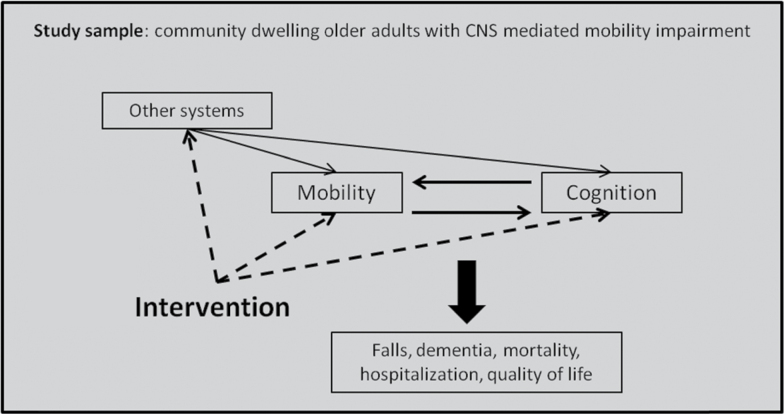
As indicated in Figure 1, interventions for populations with CNS-MMI can target mobility, cognition, other systems related to both mobility and cognition, or multiple targets (ie, multifactorial or multimodal interventions). These interventions often focus on specific mechanisms of age-associated impairment; effectiveness is often measured using cognitive, mobility, or distal health outcomes, including dementia diagnosis, falls, and mortality. Workshop 3 explored five main intervention areas: (i) pharmacology and diet; (ii) exercise; (iii) electrical stimulation; (iv) sensory stimulation/deprivation; and (v) a combined category of multimodal interventions. Below, we review the design, proof of concept, mechanistic targets, and evidence for each intervention area covered in the workshop. We additionally summarize the results of workshop discussions focused on recruiting, designing, and testing emerging and promising next-generation interventions for individuals CNS-MMI.
Figure 1.
Targets for interventions in older adults with CNS-mediated mobility impairment.
Evidence Presented
Pharmacology and Diet
Pharmaceutical therapies for dementia, including acetylcholinesterase inhibitors (eg, donepezil, galantamine, and rivastigmine) and N-methyl-d-aspartate receptor antagonists (eg, memantine), are used most often to stabilize cognitive and functional symptoms of Alzheimer’s disease (AD) without treating the underlying pathology. Considering that gait disorders are prevalent in all forms of dementia with variations in type and severity, recent research has explored whether these therapeutic dementia drugs have an impact on gait performance. These drugs may have an impact on gait through cognitively mediated mechanisms, such as executive function and attention, or through motor function, potentially impacting locomotor centers or neurovasculature. One open label study and randomized clinical trial (RCT) of donepezil among individuals with mild AD found improved gait velocity in the treatment group (15); positive RCT results were found in both short (1 month) and longer (6 months) tests and were dose dependent. Another small pilot RCT found that donepezil reduced falls among patients with PD with advanced postural instability (16). Rivastigmine was also found to improve gait stability and reduce falls in patients with PD (17), and Galantamine was also found to improve stride time under dual-task conditions in patients with AD (18). Although one systematic review and meta-analysis found inconclusive results in the effects of these drugs on gait in individuals with AD (19), an updated review suggested that acetylcholinesterase inhibitors may improve stride time parameters under single and dual-task conditions, and memantine may improve stride time parameters under single-task conditions (20). These effects may be driven by improved neural networks through the supplementation of cholinergic loss (acetylcholinesterase inhibitors) and prevention of the loss of glutamatergic neurons (memantine) (19,21).
Diet, including nutrition and vitamins, has been shown to be related to changes in mobility, including physical function, gait, and mobility. Vitamin D and vitamin B12/folate (homocysteine) in particular have been shown to be associated with reduced falls and improved physical performance among older adults (22,23). Amino acid or dietary supplements, acylcarnitine, and a Mediterranean diet have also shown some benefits to mobility (24–26). The majority of evidence for the effects of diet is observational; however, one ongoing RCT, the Gait, Memory, Dietary and Vitamin D trial (GAME-D2), is exploring the benefits of vitamin D and calcium on gait parameters, posture, and executive function performance in women aged 65 and older.
Exercise
Neurobiologic evidence in human and animal models indicates that physical activity and aerobic exercise may have positive effects on mobility and the structural and functional components of the CNS. A number of recent interventions have shown that exercise and improvements in fitness impact mobility among sedentary adults aged 70–89 years (27) and cognition in a range of older adult study samples (1,28). However, a large-scale, 24-month RCT of a moderate-intensity physical activity program compared to a health education program in sedentary older adults did not result in improved global or domain-specific cognitive functions (29). In order to improve mobility, interventions can target motor and physical function pathways or cognitive and related neural pathways. Trial results indicate that exercise training, including aerobic and resistance training, may improve mobility, including reduced fall risk and improved gait, through improvements in executive function in women aged 65–75 years with intact cognitive functioning (30,31) and reduction in white matter lesions and other neuropathology among a similar sample of women with evidence of white matter lesions at baseline (32).
Disruptions to neural circuitry, particularly in the prefrontal, parietal, putamen, cerebellum, and connecting tracts associated with aging may result in gait and mobility impairments (33). For older adults exhibiting these impairments, motor learning exercise interventions may restore motor skills, leading to smooth, efficient, and automatic movements when walking (34,35). These interventions are goal-oriented, practiced to accuracy, and reward-based (36), and have shown effectiveness in improving the energy cost of walking, gait quality, walking confidence, and physical function in older adults with a range of mobility limitations (37–39). More recent interventions focused on mobility outcomes have combined aerobic exercise with motor learning exercise in adults aged 65 and older who could ambulate independently (40).
Electric Stimulation
Noninvasive, electric stimulation, including transcranial direct current stimulation can be used to safely modulate cortical activity in brain regions and networks involved in standing and walking (41). Transcranial direct current stimulation interventions using sham controls have shown effectiveness in improving ability on dual-task paradigms during standing and walking in healthy older adults (41) and have shown benefits in preliminary experiments with healthy older adults (42). Transcranial direct current stimulation has also been shown to improve cognition across a number of domains related to risk for dementia including executive function (43) and working memory (44) in older adults with a range of cognitive functioning. Evidence suggests that this intervention may target both cognitive and motor mediated mechanisms.
Sensory Stimulation/Deprivation
Sensory deprivation and impairments, including vision and hearing loss, are associated with decreased mobility, decreased ability to complete activities of daily living, increased falls and fractures, and cognitive decline (45). Hearing loss may be associated with decreased mobility and physical function, including slowed gait speed, through cognitive mechanisms, including increased cognitive load and executive function impairments (46,47), shared pathology with balance (impairment of the cochlear and vestibular sense organs) (48), and/or through microvascular disease or inflammation mechanisms (49). Vision loss may be associated with increased falls and gait impairment, including poor postural balance and stability, due to the importance of visual input to the CNS (50) for maintaining posture (51) and for regulating mobility performance (52). One ongoing trial, the Falls in Glaucoma Study (FIGS), is exploring the relationship between glaucoma and mobility impairment (see http://www.fallsinglaucoma.org/). Rehabilitative interventions for improving hearing, including use of a hearing aid, may impact both cognitive and mobility-related health outcomes by improving executive function and decreasing cognitive load (53,54). Currently, there is limited evidence for effectiveness; however, planning grant for a trial of hearing loss treatment among older adults with a range of hearing impairments in the Atherosclerosis Risk in Communities cohort is underway (54). Interventions for improving vision may impact sensory systems associated with gait and mobility. Vision correction independently—in adults aged 65–79 years at high risk for falls (55,56)—and in combination with exercise in adults aged 70 and older living at home (57) has been shown in trials to reduce falls and improve gait.
In addition to vestibular and visual components, standing upright and maintaining balance is related to somatosensation or the process of conveying information regarding the interaction of the body’s surface with the environment. Foot sole somatosensation is associated with activation in the primary and secondary somatosensory cortex and the paracentral gyrus (58). Age-related impairments in somatosensation are associated with abnormal gait (59) and balance (60) and may result in falls and injury (61). Interventions to improve somatosensation by applying subsensory, random mechanical vibrations (white noise) to the foot sole have been shown to improve balance (62) and gait in adults aged 71–91 years experiencing recurrent falls (63) and healthy adults aged 65–90 years (64).
Multimodal Activity
Multimodal or multifactorial interventions combine multiple intervention modalities to improve mobility, cognition, and/or other health outcomes for older adults with gait abnormalities. Virtual reality treadmill gait training, which promotes motor learning while introducing graded motor and cognitive challenges, is one multifactorial treatment approach that has shown success in patients with stroke and PD (65). Participants with PD showed significant post-test improvements in gait speed, dual-task learning, and executive function; effects on dual-task waking were greater in treadmill training with virtual reality compared to treadmill training without virtual reality (65) 2011. V-TIME, a recent RCT including older adults with a history of falls without neuropathology (66), used functional magnetic resonance imaging and functional near infrared spectroscopy (67) to explore intervention effects on frontal activation during dual-tasking tests, gait variability, and falls; 302 participants have completed training and the results are forthcoming.
Another multimodal intervention that combines physical activity and cognitive challenge is an interactive video dance paradigm based on the Dance Dance Revolution system (68). A recent RCT recruited postmenopausal, sedentary, overweight women and explored the effect of video dancing on physical and cognitive functions, including functional magnetic resonance imaging assessments of psychomotor function (digit symbol substitution test) and task-switching (69). Results are forthcoming.
An intervention that extends both physical and cognitive components to a real-world setting is the Experience Corps (EC) intervention. The Baltimore EC RCT explored the mobility and cognitive benefits of a high-intensity volunteer service program embedded within the Baltimore City public elementary school system (70). Participants were healthy adults aged 60 and older at elevated sociodemographic risk for physical and cognitive functional declines. Using accelerometers and brain magnetic resonance imaging measures to evaluate mechanisms of benefit, the trial found modest increases in lifestyle activities (71) postintervention and in objectively measured physical activity among women only (72), and increased hippocampal volume and better executive function, primarily in men (73). A forthcoming paper is exploring intervention-specific mobility effects, including gait speed. EC and other multimodal interventions have the potential to simultaneously target mobility and cognitive impairment through everyday activity.
Results of the Workshop
Considering that clinically diagnosable mobility impairments are prevalent among older adults (13), and that a large body of evidence indicates that those impairments are intimately connected to the CNS (2), interventions that target underlying CNS-mobility impairment mechanisms and pathology have great potential to prevent and treat both mobility disability and cognitive impairment. Discussion points from the conference led to the identification of important gaps in knowledge and key recommendations related to the recruitment and sample selection, intervention design, and methods to measure effectiveness. These are summarized in Table 1.
Table 1.
Gaps in Knowledge and Recommendations for Future Interventions Targeting CNS-mediated Mobility Impairment: Summary of Workshop Round Table Discussions
| Recommendations | |
|---|---|
| Recruitment and sample selection | Develop consensus inclusion and exclusion criteria for the novel phenotype |
| Determine the gait measures required to define criteria | |
| Identify community-based and clinical recruitment centers (eg, ADRCs) | |
| Recruit from diverse and understudied populations | |
| Intervention design | Consider adaptive and individually tailored interventions |
| Focus on multimodal interventions that can target multiple pathways simultaneously | |
| Strive for enjoyable and challenging interventions to increase adherence and maintenance | |
| Consider variable impact by subgroups (eg, sex, age, disability) | |
| Carefully select control participants | |
| Include cross-disciplinary teams in intervention development | |
| Methods to measure effectiveness | Focus on multiple and dependent pathways |
| Incorporate qualitative and quantitative markers of brain health | |
| Develop a detailed baseline phenotype of study participants including multiple cognitive and mobility measures | |
| Consider noninvasive, wearable technology to assess outcomes continuously in daily life |
Note: ADRC = Alzheimer’s Disease Research Center; CNS = central nervous system.
Recruitment and Sample Selection
Of primary importance in recruitment of a study sample for targeted interventions is precisely defining the phenotype and identifying a novel syndrome associated with CNS-mobility impairment. Currently, various research groups have proposed different criteria, one of which builds on current criteria for mild cognitive impairment, including absence of dementia, cognitive complaints, preserved activities of daily living, and slowed gait speed (74). Other criteria have emphasized the importance of excluding individuals with other well-defined neurologic disorders, including PD, and stroke. Similar to the development of diagnostic guidelines for dementia and mild cognitive impairment due to AD, the development of consensus criteria to define a novel syndrome of CNS-MMI is required prior to the testing and evaluation of interventions specific to this group. Additionally, defining the alteration(s) in motor function to be included as criteria is critical; although many studies have used gait speed because of its ease and low cost of implementation and interpretation, other gait measures, including gait variability and velocity, as well as dual-task paradigms, may be more appropriate to define the target population (21,75,76). Recent evidence suggests that groups defined by multiple gait variables may show differences in cognitive profiles and risk factors (76).
Additional recommendations related to recruitment of target populations included the importance of identifying appropriate centers and clinics for recruitment. Although Alzheimer’s Disease Research Centers (ADRCs) have long served as rich recruitment sites for well-characterized volunteers to study cognitive impairment, they also serve individuals who come in with motor dysfunctions as their primary impairment, as well as individuals who may be included based on their cognitive profile, but also have gait and mobility impairments. Finally, workshop attendees emphasized the importance of recruiting from a diverse and specifically underserved target population particularly because those populations may have a higher prevalence of CNS-MMI and may benefit most from targeted interventions (77).
Intervention Design
As indicated above, a number of interventions have recruited populations with or at risk for CNS-MMI and have shown effectiveness in targeting mechanistic pathways of potential benefit. Workshop attendees were asked in small groups to consider these interventions and brainstorm on a series of characteristics that may define the most effective interventions.
Groups emphasized the importance of interventions that are adaptive or individually tailored to participants. These interventions are sequential, where intervention type, intensity, frequency, etc. can be modified at critical decision points, and tailored to participants. For example, participants with a specific gait and cognitive phenotype and specific interests may be matched with a customized intervention that can adapt based on participants’ progress and needs (eg, (66)). Interventions are also increasingly targeting multiple pathways simultaneously through multimodal programs embedded within daily generative activities that are meaningful to individuals (70). In order to increase adherence and maintenance postintervention, intervention design could consider what makes the cost of walking worthwhile for individuals. Similar to virtual reality for gait training that introduces tailored and graded motor and cognitive challenges (66), interventions could strive to be enjoyable and challenging. Intervention design may benefit from the appeal of goals and stages used by online gaming (78). Intervention design could also consider variable impact by subgroups, including sex, age, and disability because intervention effectiveness, interest, and adherence may vary by these and other factors related to increased risk for impairment and disability. Additionally, care must be taken to select appropriate control subjects particularly when exploring the effectiveness of interventions in older adults with cognitive and mobility limitations. Finally, workshop attendees emphasized the importance of cross-disciplinary teams, including individuals from basic sciences, geriatrics, neurology, neuropsychology, exercise physiology, engineering, and rehabilitation, in the design of effective interventions.
Methods to Measure Effectiveness
Because mechanisms of CNS-MMI are diverse, complex, and associated with multiple systems and pathways including neurovasculature, inflammation, genes and metabolism, and neuromotor control and networks (14), it will be important to directly measure and better understand the intersection of these multiple and often dependent pathways, rather than focus in a linear fashion on an individual pathway. For example, cognitive measures could incorporate noninvasive qualitative and quantitative markers of brain health including structural and functional magnetic resonance imaging and functional near infrared spectroscopy measures, as well as biomarkers that allow researchers to understand intervention effects at the cellular and molecular level (eg, neuromuscular junctions).
Prior to intervention onset, researchers could create a detailed baseline phenotype of study participants that incorporates multiple cognitive and mobility measures including executive function, speed, fall history, and speeded gait measures that include variability (75). Assessing one’s mobility while under cognitive demand (ie, dual-task paradigms) is also essential for characterizing baseline CNS-mediated mobility impairment to test effectiveness of interventions. Dual-task paradigms are particularly important because of the intrinsic link between gait and cognition. Finally, interventions can now capitalize on and incorporate noninvasive wearable technology, including smartphones (eg, (79), accelerometers, and wearable insoles, to assess complex cognitive and mobility functions more precisely. This will allow researchers to better understand and assess mobility and other measures continuously and within community settings (eg, (80)).
Naming the Syndrome
Critical to developing interventions as well as effective recruitment and measurement methods, is developing an appropriate term for this prevalent syndrome. Workshop attendees brainstormed a number of potential names including CNS-MMI, motoric cognitive risk syndrome (3), central dysmobility of aging, mobility impairment of aging, and bradypedia. This process highlighted the differential emphases placed on components of this syndrome. With continued research, we believe the field will move toward a consensus name and criteria.
Conclusions and Future Directions
The 3-year conference workshop series, “Aging, the CNS, and Mobility,” reviewed evidence, causal mechanisms, and novel interventions for a potentially new and prevalent syndrome within the older adult population characterized by CNS-mediated mobility impairment. Considering the complex nature of this impairment, a substantive methodological leap and paradigm shift is required in order to develop effective preventive interventions. First, a multidisciplinary and collaborative approach that includes basic, clinical, and translational scientists is essential particularly because of the multiple mechanistic pathways that link the CNS to mobility impairment, decline, and disability. Second, innovative and cutting edge methods that are just emerging in cognitive aging and geriatrics (eg, wearable technology and gait mats) or mobility-related fields (eg, magnetic resonance imaging) could be effectively applied within the design of interventions. Finally, the value of understanding the associations between the CNS and mobility can be conveyed clearly to researchers across disciplines so that new and ongoing interventions and longitudinal studies can incorporate both cognitive and mobility measurements into study protocols.
The conference series provided a framework for future work focused on better understanding and treating this complex and potentially novel syndrome affecting a large proportion of older adults. Although we are in the nascent stages of defining this syndrome and understanding the underlying biologic mechanisms that may connect the CNS to age-related mobility decline, interventions reviewed above have shown some effectiveness in improving both mobility and cognition by targeting multiple systems in both diseased and healthy older adults. These interventions have provided valuable insights into the design and evaluation of potentially effective interventions among those with age-related CNS-MMI. Moving forward, we seek to build on these conference reviews to develop small, hands-on workshops designed to use available data from ongoing studies to critically evaluate and disseminate novel and potentially more precise metrics of (i) CNS function and (ii) mobility in everyday life, where the complex integration of CNS systems is most tested and most needed.
Considering that individuals with mobility impairment are at high risk for dementia, disability, reduced quality of life, falls, and hospitalizations, population-based strategies that can even modestly shift one or more disease courses may have tremendous potential to reduce burden at the level of the individual, family, and society.
Supplementary Material
Please visit the article online at http://gerontologist.oxfordjournals.org/ to view supplementary material.
Funding
This work was supported by a cooperative conference grant (National Institute on Aging, U13-AG-041613-01) and a postdoctoral training grant (National Institute on Aging, 5T32AG000247).
Supplementary Material
Acknowledgment
We thank Judie Lieu for her role in helping to coordinate with the GSA CNS workshops.
References
- 1. Voss MW, Vivar C, Kramer AF, van Praag H. Bridging animal and human models of exercise-induced brain plasticity. Trends Cogn Sci. 2013;17:525–544. doi:10.1016/j.tics.2013.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Snijders AH, van de Warrenburg BP, Giladi N, Bloem BR. Neurological gait disorders in elderly people: clinical approach and classification. Lancet Neurol. 2007;6:63–74. doi:10.1016/S1474-4422(06)70678-0 [DOI] [PubMed] [Google Scholar]
- 3. Verghese J, Annweiler C, Ayers E, et al. Motoric cognitive risk syndrome: multicountry prevalence and dementia risk. Neurology. 2014;83:718–726. doi:10.1212/WNL.0000000000000717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305:50–58. doi:10.1001/jama.2010.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561. doi:10.1056/NEJM199503023320902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hausdorff JM, Rios DA, Edelberg HK. Gait variability and fall risk in community-living older adults: a 1-year prospective study. Arch Phys Med Rehabil. 2001;82:1050–1056. doi:10.1053/apmr.2001.24893 [DOI] [PubMed] [Google Scholar]
- 7. Verghese J, Lipton RB, Hall CB, Kuslansky G, Katz MJ, Buschke H. Abnormality of gait as a predictor of non-Alzheimer’s dementia. N Engl J Med. 2002;347:1761–1768. doi: 10.1056/NEJMoa020441 [DOI] [PubMed] [Google Scholar]
- 8. Mielke MM, Roberts RO, Savica R, et al. Assessing the temporal relationship between cognition and gait: slow gait predicts cognitive decline in the Mayo Clinic Study of Aging. J Gerontol A Biol Sci Med Sci. 2013;68:929–937. doi:10.1093/gerona/gls256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aggarwal NT, Wilson RS, Beck TL, Bienias JL, Bennett DA. Motor dysfunction in mild cognitive impairment and the risk of incident Alzheimer disease. Arch Neurol. 2006;63:1763–1769. doi:10.1001/archneur.63.12.1763 [DOI] [PubMed] [Google Scholar]
- 10. Hausdorff JM, Buchman AS. What links gait speed and MCI with dementia? A fresh look at the association between motor and cognitive function. J Gerontol A Biol Sci Med Sci. 2013;68:409–411. doi:10.1093/gerona/glt002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sheridan PL, Hausdorff JM. The role of higher-level cognitive function in gait: executive dysfunction contributes to fall risk in Alzheimer’s disease. Dement Geriatr Cogn Disord. 2007;24:125–137. doi:10.1159/000105126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Verbrugge LM, Jette AM. The disablement process. Soc Sci Med. 1994;38:1–14. [DOI] [PubMed] [Google Scholar]
- 13. Rosso AL, Studenski SA, Chen WG, et al. Aging, the central nervous system, and mobility. J Gerontol A Biol Sci Med Sci. 2013;68:1379–1386. doi:10.1093/gerona/glt089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sorond FA, Cruz-Almeida Y, Clark DJ, et al. Aging, the central nervous system, and mobility in older adults: neural mechanisms of mobility impairment. J Gerontol A Biol Sci Med Sci. 2015;70:1526–1532. doi:10.1093/gerona/glv130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Montero-Odasso M, Muir-Hunter SW, Oteng-Amoako A, et al. Donepezil improves gait performance in older adults with mild Alzheimer’s disease: a phase II clinical trial. J Alzheimers Dis. 2015;43:193–199. doi:10.3233/JAD-140759 [DOI] [PubMed] [Google Scholar]
- 16. Chung KA, Lobb BM, Nutt JG, Horak FB. Effects of a central cholinesterase inhibitor on reducing falls in Parkinson disease. Neurology. 2010;75:1263–1269. doi:10.1212/WNL.0b013e3181f6128c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Henderson EJ, Lord SR, Brodie MA, et al. Rivastigmine for gait stability in patients with Parkinson’s disease (ReSPonD): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol. 2016;15:249–258. doi:10.1016/S1474-4422(15)00389-0 [DOI] [PubMed] [Google Scholar]
- 18. Assal F, Allali G, Kressig RW, Herrmann FR, Beauchet O. Galantamine improves gait performance in patients with Alzheimer’s disease. J Am Geriatr Soc. 2008;56:946–947. doi:10.1111/j.1532-5415.2008.01657.x [DOI] [PubMed] [Google Scholar]
- 19. Beauchet O, Launay CP, Allali G, Annweiler C. Changes in gait variability with anti-dementia drugs: a systematic review and meta-analysis. CNS Drugs. 2014;28:513–518. doi:10.1007/s40263-014-0170-6 [DOI] [PubMed] [Google Scholar]
- 20. Beauchet O, Launay CP, Montero-Odasso M, Annweiler C, Allali G. Anti-dementia drugs-related changes in gait performance while single and dual tasking in patients with Alzheimer disease: a meta-analysis. Curr Alzheimer Res. 2015;12:761–771. [DOI] [PubMed] [Google Scholar]
- 21. Montero-Odasso M, Verghese J, Beauchet O, Hausdorff JM. Gait and cognition: a complementary approach to understanding brain function and the risk of falling. J Am Geriatr Soc. 2012;60:2127–2136. doi:10.1111/j.1532-5415.2012.04209.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Annweiler C, Schott AM, Berrut G, Fantino B, Beauchet O. Vitamin D-related changes in physical performance: a systematic review. J Nutr Health Aging. 2009;13:893–898. [DOI] [PubMed] [Google Scholar]
- 23. Nelson JM. Vitamin B12 deficiency in the elderly. A major contributor to falls. Adv Nurse Pract. 2001;9:39–41. [PubMed] [Google Scholar]
- 24. Kim H, Suzuki T, Saito K, Kojima N, Hosoi E, Yoshida H. Long-term effects of exercise and amino acid supplementation on muscle mass, physical function and falls in community-dwelling elderly Japanese sarcopenic women: a 4-year follow-up study. Geriatr Gerontol Int. 2016;16:175–181. doi:10.1111/ggi.12448 [DOI] [PubMed] [Google Scholar]
- 25. Lum H, Sloane R, Huffman KM, et al. Plasma acylcarnitines are associated with physical performance in elderly men. J Gerontol A Biol Sci Med Sci. 2011;66:548–553. doi:10.1093/gerona/glr006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Milaneschi Y, Bandinelli S, Corsi AM, et al. Mediterranean diet and mobility decline in older persons. Exp Gerontol. 2011;46:303–308. doi:10.1016/j.exger.2010.11.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pahor M, Guralnik JM, Ambrosius WT, et al. ; LIFE study investigators Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA. 2014;311:2387–2396. doi: 10.1001/jama.2014.5616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: exercise effects on brain and cognition. Nat Rev Neurosci. 2008;9:58–65. doi:10.1038/nrn2298 [DOI] [PubMed] [Google Scholar]
- 29. Sink KM, Espeland MA, Castro CM, et al. ; LIFE Study Investigators Effect of a 24-month physical activity intervention vs health education on cognitive outcomes in sedentary older adults: the LIFE Randomized Trial. JAMA. 2015;314:781–790. doi:10.1001/jama.2015.9617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Best JR, Nagamatsu LS, Liu-Ambrose T. Improvements to executive function during exercise training predict maintenance of physical activity over the following year. Front Hum Neurosci. 2014;8:353. doi:10.3389/fnhum.2014.00353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu-Ambrose T, Nagamatsu LS, Graf P, Beattie BL, Ashe MC, Handy TC. Resistance training and executive functions: a 12-month randomized controlled trial. Arch Intern Med. 2010;170:170–178. doi:10.1001/archinternmed.2009.494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bolandzadeh N, Tam R, Handy TC, et al. Resistance training and white matter lesion progression in older women: exploratory analysis of a 12-month randomized controlled trial. J Am Geriatr Soc. 2015;63:2052–2060. doi:10.1111/jgs.13644 [DOI] [PubMed] [Google Scholar]
- 33. Holtzer R, Epstein N, Mahoney JR, Izzetoglu M, Blumen HM. Neuroimaging of mobility in aging: a targeted review. J Gerontol A Biol Sci Med Sci. 2014;69:1375–1388. doi:10.1093/gerona/glu052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Seidler RD, Bernard JA, Burutolu TB, et al. Motor control and aging: links to age-related brain structural, functional, and biochemical effects. Neurosci Biobehav Rev. 2010;34:721–733. doi:10.1016/j.neubiorev.2009.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Milton J, Solodkin A, Hlustík P, Small SL. The mind of expert motor performance is cool and focused. Neuroimage. 2007;35:804–813. doi:10.1016/j.neuroimage.2007.01.003 [DOI] [PubMed] [Google Scholar]
- 36. Abe M, Schambra H, Wassermann EM, Luckenbaugh D, Schweighofer N, Cohen LG. Reward improves long-term retention of a motor memory through induction of offline memory gains. Curr Biol. 2011;21:557–562. doi:10.1016/j.cub.2011.02.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Brach JS, Vanswearingen JM. Interventions to improve walking in older adults. Curr Transl Geriatr Exp Gerontol Rep. 2013;2:230–238. doi:10.1007/s13670-013-0059-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. VanSwearingen JM, Perera S, Brach JS, Cham R, Rosano C, Studenski SA. A randomized trial of two forms of therapeutic activity to improve walking: effect on the energy cost of walking. J Gerontol A Biol Sci Med Sci. 2009;64:1190–1198. doi:10.1093/gerona/glp098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. VanSwearingen JM, Perera S, Brach JS, Wert D, Studenski SA. Impact of exercise to improve gait efficiency on activity and participation in older adults with mobility limitations: a randomized controlled trial. Phys Ther. 2011;91:1740–1751. doi:10.2522/ptj.20100391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Brach JS, Francois SJ, Van Swearingen JM, Gilmore S, Perera S, Studenski SA. Translation of a motor learning walking rehabilitation program into a group-based exercise program for community-dwelling older adults. PM R. 2015. pii:S1934-1482(15)01064-3. doi:10.1016/j.pmrj.2015.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhou D, Zhou J, Chen H, Manor B, Lin J, Zhang J. Effects of transcranial direct current stimulation (tDCS) on multiscale complexity of dual-task postural control in older adults. Exp Brain Res. 2015;233:2401–2409. doi:10.1007/s00221-015-4310-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Manor B, Zhou J, Jor’dan A, Zhang J, Fang J, Pascual-Leone A. Reduction of dual-task costs by noninvasive modulation of prefrontal activity in healthy elders. J Cogn Neurosci. 2016;28:275–281. doi:10.1162/jocn_a_00897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nitsche MA, Cohen LG, Wassermann EM, et al. Transcranial direct current stimulation: State of the art 2008. Brain Stimul. 2008;1:206–223. doi:10.1016/j.brs.2008.06.004 [DOI] [PubMed] [Google Scholar]
- 44. Brasil-Neto JP. Learning, memory, and transcranial direct current stimulation. Front Psychiatry. 2012;3:80. doi:10.3389/fpsyt.2012.00080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dessai M, Patt LA, Lentzner H, Robinson KN. Trends in vision and hearing among older Americans. In: N.C.F.H. Statistics, ed. Aging Trends. Hyattsville, MD: National Center for Health Statistics; 2001, No. 2. [DOI] [PubMed] [Google Scholar]
- 46. Wingfield A, Peelle JE. The effects of hearing loss on neural processing and plasticity. Front Syst Neurosci. 2015;9:35. doi:10.3389/fnsys.2015.00035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lin FR. Hearing loss and cognition among older adults in the United States. J Gerontol A Biol Sci Med Sci. 2011;66:1131–1136. doi:10.1093/gerona/glr115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Agrawal Y, Carey JP, Della Santina CC, Schubert MC, Minor LB. Disorders of balance and vestibular function in US adults: data from the National Health and Nutrition Examination Survey, 2001–2004. Arch Intern Med. 2009;169:938–944. doi:10.1001/archinternmed.2009.66 [DOI] [PubMed] [Google Scholar]
- 49. Nash SD, Cruickshanks KJ, Zhan W, et al. Long-term assessment of systemic inflammation and the cumulative incidence of age-related hearing impairment in the epidemiology of hearing loss study. J Gerontol A Biol Sci Med Sci. 2014;69:207–214. doi:10.1093/gerona/glt075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lord SR, Smith ST, Menant JC. Vision and falls in older people: risk factors and intervention strategies. Clin Geriatr Med. 2010;26:569–581. doi:10.1016/j.cger.2010.06.002 [DOI] [PubMed] [Google Scholar]
- 51. Ray CT, Horvat M, Croce R, Mason RC, Wolf SL. The impact of vision loss on postural stability and balance strategies in individuals with profound vision loss. Gait Posture. 2008;28:58–61. doi:10.1016/j.gaitpost.2007.09.010 [DOI] [PubMed] [Google Scholar]
- 52. Turano KA, Broman AT, Bandeen-Roche K, Munoz B, Rubin GS, West S; SEE Project Team Association of visual field loss and mobility performance in older adults: Salisbury Eye Evaluation Study. Optom Vis Sci. 2004;81:298–307. [DOI] [PubMed] [Google Scholar]
- 53. Chen DS, Genther DJ, Betz J, Lin FR. Association between hearing impairment and self-reported difficulty in physical functioning. J Am Geriatr Soc. 2014;62:850–856. doi:10.1111/jgs.12800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Deal JA, Sharrett AR, Albert MS, et al. Hearing impairment and cognitive decline: a pilot study conducted within the atherosclerosis risk in communities neurocognitive study. Am J Epidemiol. 2015;181:680–690. doi:10.1093/aje/kwu333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Harwood RH, Foss AJ, Osborn F, Gregson RM, Zaman A, Masud T. Falls and health status in elderly women following first eye cataract surgery: a randomised controlled trial. Br J Ophthalmol. 2005;89:53–59. doi:10.1136/bjo.2004.049478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Haran MJ, Lord SR, Cameron ID, et al. Preventing falls in older multifocal glasses wearers by providing single-lens distance glasses: the protocol for the VISIBLE randomised controlled trial. BMC Geriatr. 2009;9:10. doi:10.1186/1471-2318-9-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Day L, Fildes B, Gordon I, Fitzharris M, Flamer H, Lord S. Randomised factorial trial of falls prevention among older people living in their own homes. BMJ. 2002;325:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hao Y, Manor B, Liu J, et al. Novel MRI-compatible tactile stimulator for cortical mapping of foot sole pressure stimuli with fMRI. Magn Reson Med. 2013;69:1194–1199. doi:10.1002/mrm.24330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Deshpande N, Ferrucci L, Metter J, et al. Association of lower limb cutaneous sensitivity with gait speed in the elderly: the health ABC study. Am J Phys Med Rehabil. 2008;87:921–928. doi:10.1097/PHM.0b013e31818a5556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kars HJ, Hijmans JM, Geertzen JH, Zijlstra W. The effect of reduced somatosensation on standing balance: a systematic review. J Diabetes Sci Technol. 2009;3:931–943. doi:10.1177/193229680900300441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Richardson JK, Hurvitz EA. Peripheral neuropathy: a true risk factor for falls. J Gerontol A Biol Sci Med Sci. 1995;50:M211–M215. doi:10.1093/gerona/50A.4.M211 [DOI] [PubMed] [Google Scholar]
- 62. Priplata AA, Niemi JB, Harry JD, Lipsitz LA, Collins JJ. Vibrating insoles and balance control in elderly people. Lancet. 2003;362:1123–1124. doi:10.1016/S0140-6736(03)14470-4 [DOI] [PubMed] [Google Scholar]
- 63. Galica AM, Kang HG, Priplata AA, et al. Subsensory vibrations to the feet reduce gait variability in elderly fallers. Gait Posture. 2009;30:383–387. doi:10.1016/j.gaitpost.2009.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lipsitz LA, Lough M, Niemi J, Travison T, Howlett H, Manor B. A shoe insole delivering subsensory vibratory noise improves balance and gait in healthy elderly people. Arch Phys Med Rehabil. 2015;96:432–439. doi:10.1016/j.apmr.2014.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Mirelman A, Maidan I, Herman T, Deutsch JE, Giladi N, Hausdorff JM. Virtual reality for gait training: can it induce motor learning to enhance complex walking and reduce fall risk in patients with Parkinson’s disease? J Gerontol A Biol Sci Med Sci. 2011;66:234–240. doi:10.1093/gerona/glq201 [DOI] [PubMed] [Google Scholar]
- 66. Mirelman A, Rochester L, Reelick M, et al. V-TIME: a treadmill training program augmented by virtual reality to decrease fall risk in older adults: study design of a randomized controlled trial. BMC Neurol. 2013;13:15. doi:10.1186/1471-2377-13-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Mirelman A, Maidan I, Bernad-Elazari H, et al. Increased frontal brain activation during walking while dual tasking: an fNIRS study in healthy young adults. J Neuroeng Rehabil. 2014;11:85. doi:10.1186/1743-0003-11-85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Smith ST, Sherrington C, Studenski S, Schoene D, Lord SR. A novel Dance Dance Revolution (DDR) system for in-home training of stepping ability: basic parameters of system use by older adults. Br J Sports Med. 2011;45:441–445. doi:10.1136/bjsm.2009.066845 [DOI] [PubMed] [Google Scholar]
- 69. Jovancevic J, Rosano C, Perera S, Erickson KI, Studenski S. A protocol for a randomized clinical trial of interactive video dance: potential for effects on cognitive function. BMC Geriatr. 2012;12:23. doi:10.1186/1471-2318-12-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Fried LP, Carlson MC, McGill S, et al. Experience Corps: a dual trial to promote the health of older adults and children’s academic success. Contemp Clin Trials. 2013;36:1–13. doi:10.1016/j.cct.2013.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Parisi JM, Kuo J, Rebok GW, et al. Increases in lifestyle activities as a result of experience Corps® participation. J Urban Health. 2015;92:55–66. doi:10.1007/s11524-014-9918-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Varma VR, Tan EJ, Harris G, et al. Effect of community volunteering on physical activity: a randomized controlled trial. Am J Prev Med. 2016; 50(1):106–110. doi:10.1016/j.amepre.2015.06.015 [DOI] [PMC free article] [PubMed]
- 73. Carlson MC, Kuo JH, Chuang YF, et al. Impact of the Baltimore Experience Corps Trial on cortical and hippocampal volumes. Alzheimers Dement. 2015;11:1340–1348. doi:10.1016/j.jalz.2014.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Verghese J, Wang C, Lipton RB, Holtzer R. Motoric cognitive risk syndrome and the risk of dementia. J Gerontol A Biol Sci Med Sci. 2013;68:412–418. doi:10.1093/gerona/gls191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Hausdorff JM. Gait variability: methods, modeling and meaning. J Neuroeng Rehabil. 2005;2:19. doi:10.1186/1743-0003-2-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Allali G, Ayers EI, Verghese J. Motoric cognitive risk syndrome subtypes and cognitive profiles. J Gerontol A Biol Sci Med Sci. 2016;71:378–384. doi:10.1093/gerona/glv092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Schoeni RF, Martin LG, Andreski PM, Freedman VA. Persistent and growing socioeconomic disparities in disability among the elderly: 1982–2002. Am J Public Health. 2005;95:2065–2070. doi:10.2105/AJPH.2004.048744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Griffiths MD, Davies MN, Chappell D. Breaking the stereotype: the case of online gaming. Cyberpsychol Behav. 2003;6:81–91. doi:10.1089/109493103321167992 [DOI] [PubMed] [Google Scholar]
- 79. Sharma V, Mankodiya K, De La Torre F, et al. SPARK: personalized Parkinson disease interventions through synergy between a smartphone and a smartwatch. In: Marcus A, ed. Design, User Experience, and Usability: User Experience Design for Everyday Life Applications and Services. Bern, Switzerland: Springer; 2014:103–114. [Google Scholar]
- 80. Weiss A, Herman T, Giladi N, Hausdorff JM. Association between community ambulation walking patterns and cognitive function in patients with Parkinson’s disease: further insights into motor- cognitive links. Parkinsons Dis. 2015;2015:547065. doi:10.1155/2015/ 547065 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.