Abstract
Therapies targeted at fundamental processes of aging may hold great promise for enhancing the health of a wide population by delaying or preventing a range of age-related diseases and conditions—a concept dubbed the “geroscience hypothesis.” Early, proof-of-concept clinical trials will be a key step in the translation of therapies emerging from model organism and preclinical studies into clinical practice. This article summarizes the outcomes of an international meeting partly funded through the NIH R24 Geroscience Network, whose purpose was to generate concepts and frameworks for early, proof-of-concept clinical trials for therapeutic interventions that target fundamental processes of aging. The goals of proof-of-concept trials include generating preliminary signals of efficacy in an aging-related disease or outcome that will reduce the risk of conducting larger trials, contributing data and biological samples to support larger-scale research by strategic networks, and furthering a dialogue with regulatory agencies on appropriate registration indications. We describe three frameworks for proof-of-concept trials that target age-related chronic diseases, geriatric syndromes, or resilience to stressors. We propose strategic infrastructure and shared resources that could accelerate development of therapies that target fundamental aging processes.
Keywords: Aging, Geroscience Network, Clinical trials
In October 2015, global experts in the field of aging gathered at the “6th Annual Alliance for Healthy Aging Conference” in Newcastle, United Kingdom, which was followed by a retreat focused on how to conceptualize and facilitate early, proof-of-concept clinical trials that target fundamental aging mechanisms. The retreat was funded and organized through the National Institute on Aging (NIA) R24 Geroscience Network, a consortium of 18 aging centers and academic groups across the United States (see Table 1), in partnership with separately supported centers in the European Union (EU). The experts present at the retreat included both basic scientists and clinicians with areas of expertise ranging from biogerontology, molecular biology, geriatrics, physiatry, oncology/stem cell transplantation, and epidemiology as well as members of US and EU research funding and drug regulatory agencies. This article summarizes the outcomes of this retreat, including outlining the goals of proof-of-concept clinical trials involving fundamental biological processes that contribute to aging, developing three consensus frameworks (targeting age-related chronic diseases, geriatric syndromes, and impaired resilience) for such trials, and proposing opportunities for accelerating progress toward translational therapies.
Table 1.
Geroscience Network. Centers on aging and academic groups involved in the Geroscience Network
| Albert Einstein College | University of Alabama at Birmingham |
| Buck Institute | University of Arkansas |
| European Union | University of Connecticut |
| Harvard University | University of Michigan |
| Johns Hopkins University | University of Minnesota |
| Mayo Clinic | University of Texas Health Science Center at San Antonio |
| National Institute on Aging | University of Southern California |
| The Scripps Research Institute | University of Washington |
| Stanford University | Wake Forest University |
Approach
Clinical Trials of Interventions That Target Fundamental Aging Processes: Proof-of-Concept
The “geroscience hypothesis” is that targeting fundamental aging processes might delay, prevent, alleviate, or reverse a wide range of diseases and conditions for which age is the primary non-modifiable risk factor. In recent years, preclinical studies targeting fundamental aging processes have shown promising results in delaying aging-related biological parameters, as well as in demonstrating beneficial effects on measures related to frailty, age-related chronic disease, and overall health (1–7). Some of these approaches involve cellular and molecular mechanisms that may be readily testable in humans through drug repurposing. Examples of this include reducing the burden of senescent cells in human tissues using senolytic drugs (anticancer drugs, high-dose flavonoids, or Bcl-2 family member inhibitors) (5,8,9), inhibiting mammalian target of rapamycin (mTOR) signaling with rapamycin (10), or enhancing AMP-activated protein kinase activity and inhibiting mTOR with 17α-estradiol (11). Advancing these studies “beyond the bench” and into proof-of-concept clinical trials is a key step in the translational continuum to take these interventions toward clinical practice (Figure 1).
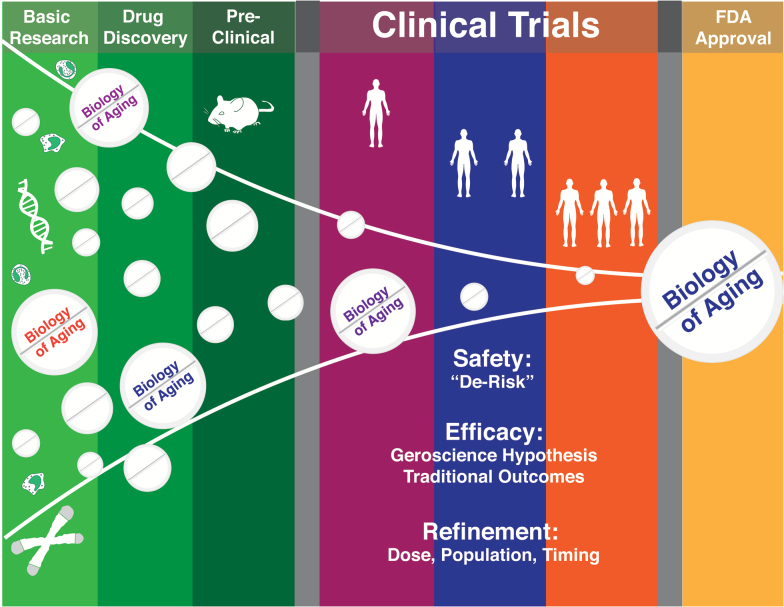
Figure 1.
Pipeline of drug development for interventions that target fundamental aging processes. As in other fields, numerous potential interventions will be winnowed in the preclinical phase, with those showing the most promise for targeting the biology of aging progressing to initial safety studies in humans. Small proof-of-concept clinical trials might then “de-risk” these candidate interventions by providing signals of efficacy and further safety data to better predict success in large clinical trials designed to support Food and Drug Administration approval.
For the purposes of this meeting and paper, proof-of-concept trials are envisioned as small, short, relatively inexpensive studies that provide initial evaluation of safety and dosing, test efficacy toward biological outcomes, and provide supportive data on clinical outcomes to help design and justify larger clinical trials (Figure 2). One goal of a proof-of-concept trial is to “de-risk” an intervention by providing a signal of efficacy in an outcome that is relevant to the ultimate clinical indication as well as data on intervention safety in the target population (12). The key difference between most traditional disease-targeting Phase 2 studies (which target pathways typically identified within the affected tissue) and proof-of-concept studies testing the geroscience hypothesis is that the molecular target in geroscience-oriented studies should be identified from the field of aging, and should not directly act on risk factors specific to a disease process of interest (eg, cholesterol in atherosclerosis studies) (13–15). In order to help test the geroscience hypothesis, some trials might explicitly target a non-traditional primary outcome that is broadly representative of aging, such as a geriatric syndrome (eg, sarcopenia or mild confusion). Other studies with a more traditional primary outcome involving a specific disease might instead incorporate secondary outcomes that help determine if the intervention has broader effects on aging phenotypes. Successive trials would iteratively refine the most effective interventions, doses, timing of interventions, and populations to target in subsequent larger studies.
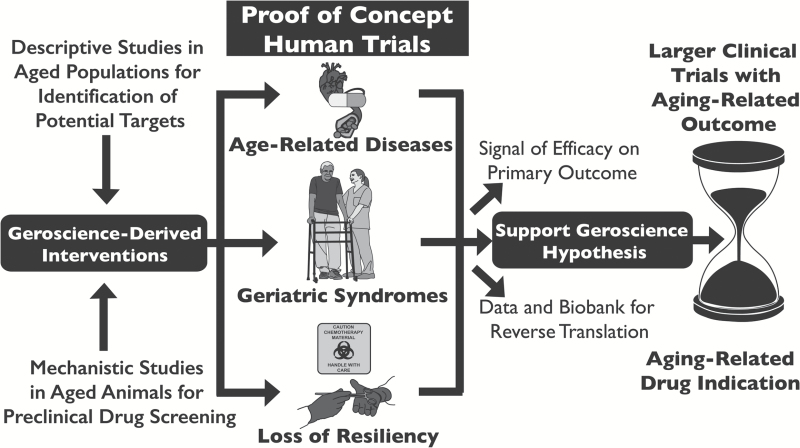
Figure 2.
Frameworks and goals for proof-of-concept clinical trials of interventions that target fundamental aging processes. These interventions are hypothesized to affect multiple age-related diseases and conditions: the “geroscience hypothesis.” Three frameworks are presented for the design of clinical trials to test these interventions for effects on outcomes related to age-related diseases, geriatric syndromes, and resiliency. Proof-of-concept clinical trials would test the geroscience hypothesis, but also provide evidence of efficacy in the outcome being targeted, as well as data and samples for further research. Ultimately, proof-of-concept trials will help justify and in the design of larger clinical trials and support new regulatory indications for interventions that target fundamental aging processes.
Proof-of-concept trials will also help to refine a framework for translational research and advance dialogue with regulatory agencies (16). Standardization of approaches across multiple studies and collection of an array of aging-related molecular and physiological measures would streamline efforts to initiate clinical trials, lend insights into the role of fundamental aging processes across a broad range of diseases, and inspire new basic research directions. Some of the outcomes most representative of the effects of aging on the health of individuals and populations, such as geriatric syndromes, multimorbidity, or dependence in activities of daily living, are not currently recognized as end points for registration by regulatory agencies. Completion of a critical mass of trials testing geroscience-derived compounds will crucially advance discussions with regulatory agencies to expand the definitions of registration end points for therapies that may have broad effects not limited to a single disease process.
Below, we present three related conceptual frameworks for proof-of-concept studies of interventions that target fundamental aging mechanisms, guided by the ultimate goals of reducing disease burden, reducing morbidity/disability, and maintaining or improving daily function and independence in aging populations. The three frameworks involve targeting age-related diseases, geriatric syndromes, and resilience to stressors. Following detailed descriptions of these three frameworks, we present elements common to each, as well as a table highlighting commonalities, differences, strengths, and weaknesses related to each framework (Tables 2 and Supplementary Table S1).
Table 2.
Strengths and Weaknesses of Three Proposed Proof of Concept Clinical Trial Frameworks
| Age-Related Disease | Geriatric Syndromes | Resilience | |
|---|---|---|---|
| Strengths | High clinical impact of multimorbidity | High impact on quality of life, few current therapies | Most disability in older adults associated with acute events |
| Mechanistic studies can leverage known pathophysiology | “Final common pathway” of aging | Can plan intervention | |
| Multifactorial causes well suited to pleiotropic interventions | Potential for short-term, high-incidence, high-impact outcomes | ||
| Weaknesses | Not clear which interventions will target which diseases | Mechanistic studies challenging in multifactorial conditions | Potential heterogeneity in both population and stressor |
| Need to separate specific single-disease effect from underlying effect on aging | Late effect of aging, may be too late to intervene | Possibility for harm with high-impact stressors |
Frameworks for Proof-of-Concept Trials Targeting Fundamental Aging Processes
Trials Targeting Age-Related Diseases
Age-related diseases are defined as conditions in which age is leading risk factor and the dysfunction of an organ system occurs in the absence of a single inciting event. For example, arteriosclerotic vascular disease, osteoarthritis, osteoporosis, type II diabetes mellitus, most cancers, and Alzheimer’s disease and other dementias could all be considered to be “age-related” diseases (17). Proof-of-concept trials would evaluate interventions that affect these diseases ultimately through effects on fundamental aging processes, rather than proximately through a single disease-specific pathophysiology. The geroscience hypothesis predicts that interventions targeting basic aging mechanisms would show efficacy in multiple disease states whose proximate pathophysiology is not directly related. For example, a series of proof-of-concept trials might test if an intervention demonstrates a beneficial molecular effect in separate cohorts of subjects with arteriosclerosis, osteoporosis, and nonvascular dementia. Demonstrating such pleiotropic effects is important to show that geroscience-related interventions targeting fundamental aging processes might be a viable approach to manage or prevent an array of age-related conditions, rather than a single disease.
Population
Age-related diseases are often slowly progressive over years, yet choosing the optimal time to intervene is critical both for the success of proof-of-concept trials and for identification of treatment windows that will provide maximum health benefit to patients. Collaborations between clinicians and researchers are essential to predict key time points for intervention, as the “sweet spot” for maximizing molecular and clinical evidence of benefit is likely to be different for different diseases.
Outcomes
Measurements that provide an index of interactions between a drug and the relevant pathophysiological processes are the ultimate driver of go/no-go decisions when advancing to larger clinical trials. The short duration of proof-of-concept trials suggests that molecular outcomes may provide a stronger signal than more heterogeneous clinical outcomes. When possible, these outcomes should provide evidence that a drug intervention attenuates deleterious molecular changes in the tissue of interest (eg, insulin sensitivity in muscle biopsies from subjects with diabetes). When tissue is not available, biomarkers specifically related to a disease process can be used (eg, circulating markers of bone turnover specific to osteoporosis), and evaluation of phenotypic changes with disease progression can be examined (eg, progression of valve calcification in subjects with aortic valve stenosis using computerized tomography scanning). Finally, outcomes more generally related to aging could be incorporated as practical, using standardized toolkits that can be shared across trials. These aging outcomes ought not to detract from the primary disease-specific proof-of-concept outcome, but would be “value-multipliers” that would provide additional data to help discern between “responders” and “nonresponders” and how geroscience-derived interventions affect fundamental aging mechanisms in different patient populations.
Many of these trials may be designed with the strategy of conducting a short-term trial (eg, weeks to a month) with a prospective plan for longer-term follow-up (eg, months to a year). One year is long for an initial proof-of-concept study, but significant outcomes might become evident within one or a few months. If so, it would be feasible to publish effects detected at early time-points, but also wise to plan for longer-term follow-up in order to (1) increase likelihood of definitively testing the impact of the intervention, rather than concluding the trial at an early point but with an indeterminate outcome, (b) ascertain the durability of any beneficial effects detected soon after the intervention, (c) detect late-appearing side effects, and (d) test if the intervention can be administered over a short time, but have a long-term impact. The latter point would be particularly relevant in the case of interventions that alter tissue composition, such as senolytics, which may prove effective if used intermittently.
Example
Rapamycin for osteoporosis. The geroscience-derived hypothesis is that rapamycin will protect against age-associated bone loss via activation of autophagy (18) in osteocytes (19). In this example, a concept trial would focus on improvement in plasma markers of bone turnover. A reasonable proof-of-concept trial size could be enrollment of 100 patients or less who are 65 years and older with a new diagnosis of age-related osteoporosis and femoral neck T-score ≤2.5 who are also independent in activities of daily living and instrumental activities of daily living. Patients would continue to receive current standard of care treatments for osteoporosis and would be randomized to rapamycin or placebo. The primary outcome measure would be changes in urinary and plasma biomarkers of bone turnover/osteocyte function/osteoclast function at 1, 6, and 12 months of treatment (compared to baseline) and could include alkaline phosphatase, osteocalcin, collagen type 1 cross-linked N-telopeptide, or Fibroblast Growth Factor 23 (FGF23). A secondary outcome could be the change in femoral neck T-score at 1 year compared to enrollment baseline. Clinical outcomes related to osteoporosis (eg, new fractures, falls) within the 12-month treatment period could be tertiary outcomes, although the study would not be powered for these. Importantly, investigators could also obtain a number of standardized measures of physiological aging (eg, grip strength and gait speed) and additional blood samples for future molecular studies.
Trials Targeting Geriatric Syndromes
“Geriatric syndromes” are common conditions in the elderly that have multifactorial causes, usually including several diseases as well as other age-related physiological changes. Examples include frailty, falls, cognitive decline, major mobility disability, delirium, pressure ulcers, and incontinence (20). They can be debilitating, with a major impact on quality of life, independence, and longevity. Geriatric syndromes do not have a single underlying pathophysiology but represent the integration of dysfunction in multiple organ systems. Due to these multifactorial causes, the best evidence-based treatments are multicomponent interventions (21). Critically, this characteristic may make them particularly responsive to interventions that target fundamental processes of aging, which have pleiotropic effects on multiple organ systems. Preclinical studies suggest this may indeed be feasible (22). Senolytic agents delay multiple frailty-associated phenotypes in progeroid mice (8) and Janus kinase (JAK1/2) inhibitors, which attenuate the senescence-associated secretory phenotype, alleviate frailty in older mice (6).
Population
For prevention studies, investigators could select for higher-risk group by enrolling individuals who already meet some clinical criteria for a syndrome (eg, “prefrail”), have elevated biomarker risk profiles, or have multiple chronic diseases that increase risk for developing geriatric syndromes. Alternatively, certain younger populations exhibit accelerated aging and early onset of geriatric syndromes: cancer survivors who underwent chemotherapy or radiotherapy, or individuals with HIV (23–26). For studies aiming to slow progression or reverse geriatric syndromes, individuals with mild to moderate symptoms would provide a robust signal-to-noise ratio for clinical outcomes while not presenting the challenging task of needing to alleviate advanced or severe disease. As with other outcomes related to aging, a goal of initial small trials may be to characterize optimal windows for intervention.
Outcomes
Known molecular pathways contributing to certain syndromes can be used to design intermediate biological and physiological outcomes. For example, frailty and immobility are often closely related to sarcopenia, which has well-characterized physiological markers (eg, grip strength (27)) and emerging molecular markers (eg, senescent cell density or mTOR activity in skeletal muscle (28)). Cognitive tests such as processing speed or executive functioning might be an early physiological marker for cognitive decline (29). Some geriatric syndromes, such as pressure ulcers or incontinence, may not have intermediate outcomes identified so far that are as tractable.
Example
Dasatinib plus quercetin for frailty. The geroscience-derived hypothesis is that reducing senescent cell burden would improve markers of patient frailty. In this example, a concept trial would focus on confirming efficacy of the drug in clearing senescent cells. The study would enroll 10–20 adults 60–80 years old who undergo skin biopsies before and after a single course of dasatinib plus quercetin. The primary outcome would be the degree of senescent cell clearance, measured by senescent markers in biopsy tissue (eg, p16, p21, senescence associated β-galactosidase, telomere associated foci, etc.). A secondary outcome could be evaluation of blood samples for changes in circulating markers of inflammation and cellular senescence following treatment with dasatinib and quercetin. The inclusion of a frailty assessment at enrollment (including standardized tests of grip strength and gait speed) would permit correlations between frailty measures and senescent cell burden, with an optional extension to examine change in frailty measures at 3 months.
Trials targeting resilience
A key aspect of aging is a loss of resilience or a decrease in the capacity to maintain homeostasis and return to baseline function following exposure to stressors, because of decreased physiologic reserve (30). Such stressors can include elective or nonelective surgery, chemotherapy, periods of immobility, and numerous age-associated acute pathophysiologic events (ischemic cardiovascular events, fall-related fractures, infections, etc.). In older adults, 50%–80% of all new disability (defined as new inability to perform activities of daily living) begins suddenly after a hospitalization for injury or illness (31). Preventing even some of this disability could save tens of billions of dollars in medical and long-term care costs every year (32). Aside from the human and financial imperatives, targeting resilience could aid in testing the geroscience hypothesis by providing a more sensitive and specific assay for testing therapeutic interventions compared to measurement of general health under static conditions.
If the stressors can be planned in advance, the investigational agent can be provided beforehand (ie, prehabilitation). Trials of geroscience-derived interventions might be modeled on trials of lifestyle prehabilitation interventions that have proven effective at improving molecular markers and clinical outcomes following surgery (33) or antineoplastic therapies (34,35). Unlike a prehabilitation study design, treatment administration at or immediately following an unplanned event such as stroke, myocardial infarction, or fracture (ie, “opportunistic” patient recruitment) depends on incidence of the unplanned events. Furthermore, time elapsed between the unplanned event and initiation of treatment may be critical. These points make study design and data interpretation challenging—although ultimately the goal must be to apply these interventions to the unplanned stressors that cause most disability in older adults (31).
Population
The population of older adults undergoing significant medical stressors is both readily available for study and unusually amenable to improvements in short-term clinically significant outcomes. Age is an independent risk factor for adverse outcomes from interventions such as cardiac surgical procedures (36), which are commonly pursued in older adults (37). However, this population is confounded by the necessary presence of the disease being treated, and often other comorbid health problems that could act as confounding variables. Thus, an alternative approach for proof-of-concept studies might be to test “artificial” stressors in healthier middle-aged or older adults, such as a skin biopsy to assess wound healing or vaccination to test immune responses. This strategy also provides the advantage of greater scientific control, from study population and initial conditions to selected intervention and outcomes.
Outcomes
The primary outcomes would be specific to the subject population and stressor, and focused on restoration of baseline function following the applied challenge or stressor. Depending on the study design, this could mean, for example, time to healing from a skin biopsy, or proportion of subjects discharged home versus to skilled nursing facilities after surgery. Outcomes could also include more general outcomes, such as grip strength, gait speed, or levels of circulating inflammatory mediators, as well as subjective measures such as patient-reported pain and perceived disability.
Example
Cardiac surgery “prehabilitation” with myostatin inhibitor. The geroscience-derived hypothesis would be that myostatin inhibition begun prior to elective coronary artery bypass surgery will reduce postsurgical muscle atrophy via enhanced satellite cell activation (38). A blocked-randomized design could be employed at a hospital with high surgical volume to enroll ~40 adults aged 70–80 years over several months. Along with usual preoperative care, patients would be randomized to 2 weeks of myostatin inhibitor treatment immediately preoperatively. The primary outcome could be change in quadriceps muscle power and stair-climbing power at 1 week and 1 month postoperatively (39). Secondary outcomes could include biological markers of myostatin activation in skeletal muscle biopsies obtained pre- and postoperatively (eg, Pax7 and MyoD protein levels), as well as clinical measures, such as time to discharge and gait speed at discharge. Tertiary outcomes, which the study would not be powered for, could include proportion discharged home versus to skilled nursing facilities, time to return home, and new disability in activities of daily living at 1 month.
Common Elements of Proof-of-Concept Trials Targeting Fundamental Aging Processes
There are themes that cut across proof-of-concept clinical trials under any of the three frameworks: age-related diseases, geriatric syndromes, and resilience to stressors.
Populations
As noted above, many trials of interventions that target fundamental aging processes would include older subjects, who would make up by far the largest group in which such interventions will ultimately be used. The rationale for selecting specific study populations varies among the frameworks, but a common theme is to balance the risk of expected outcomes against magnitude of expected benefits in order to find an adequate signal-to-noise ratio. In general, subjects who are older or more frail are at higher risk for poor outcomes from usual care—such as new disability and loss of independence after a hospitalization. Enrolling older or frailer subjects could therefore provide higher power to detect improved outcomes in a small trial, and any improvement in outcomes is likely to be highly meaningful to the health of the subjects. However, older and more frail subjects are also more susceptible to clinically significant adverse effects from most treatments, and there may be a point of diminishing benefits from interventions targeting aging. In certain circumstances, there may be a benefit to pursuing proof-of-concept studies in populations with accelerated aging-like states, as described above. Genetic progeroid syndromes are a special group that might be considered, but the established research infrastructure and relative ease of regulatory approval would need to be balanced against the limitations of a small population size and restricted generalizability.
Outcomes
Although all trials include efficacy and safety outcomes that are specific to the particular intervention or indication (disease, syndrome, or stress) under study, it would also be important to study biomarkers that broadly reflect fundamental aging processes. Along a spectrum from more basic to more clinical, such biomarkers might include those of phenomena associated with aging (eg, telomere length, mTOR activity, or senescent cell abundance), physiological measures that are generally impaired in many older people (eg, grip strength or gait speed), and incident diagnosis of a new age-related disease or syndrome during the defined treatment/follow-up period (eg, dementia or metabolic disease). Furthermore, biomarkers that provide an index of drug activity/target activation would be of great utility in early proof-of-concept clinical studies (such as S6 kinase activity in trials of mTOR modulators or senescent cell burden following senolytic treatment), particularly when combined with biomarkers and/or outcomes that provide an index of off-target or unwanted effects of the drug (eg, reductions in muscle protein synthesis with mTOR inhibition). If such efforts were coalesced in the form of a Geroscience Network, this would also allow multiple research groups to evaluate pleiotropic effects of various interventions (eg, possible reductions in senescent cell burden with mTOR inhibition, etc.) across studies in a broad variety of populations.
Interventions
These frameworks can apply broadly to behavioral, dietary, nutraceutical, or pharmacological interventions (40,41). However, the present discussion applies most specifically to either repurposing of existing Food and Drug Administration (FDA)-approved drugs to new indications related to aging, or to new drugs on the pathway to an initial indication. Approved drugs with strong evidence as therapies that target fundamental processes of aging from human and animal studies are reviewed in Newman and colleagues (42) in this issue and include metformin, rapamycin, acarbose, and 17α-estradiol. Other promising compounds with varying degrees of evidence include the senolytic drugs (dasatinib and quercetin in isolation or in combination, navitoclax (9)), mitochondrial-targeted peptides, novel analogues of rapamycin, NAD therapies (nicotinamide riboside and nicotinamide mononucleoside), myostatin, and sirtuin activators. Demonstration of efficacy in preclinical models that accurately recapture clinical scenarios can be challenging, but are essential to increasing the probability of success in proof-of-concept trials [see Huffman and colleagues (43) in this issue].
Pairing an intervention with a study outcome should be informed whenever possible by hypotheses developed from preclinical, translational, or epidemiological data, because targeting a single pathway is not likely to be beneficial in all circumstances. For example, senescent fibroblasts and endothelial cells are essential in the early response to cutaneous insult and subsequent wound healing (44), making perioperative senolytic treatment unadvisable. Similarly, mTOR inhibition suppresses skeletal muscle hypertrophy (45), and so might exacerbate muscle atrophy from immobilization, such as following major surgery.
Interventions will often be tested against standard-of-care, which in many cases will include some form of non-pharmacological (often intensive) intervention, such as cardiac rehabilitation following cardiac surgery. Exercise may be a common comparator and is something of a special case, having evidence of benefit in many diseases and geriatric syndromes but with large variability in subject response (46–49). Along with being compared to the effect of exercise, drug interventions might be evaluated for adding to its effect or providing a greater uniformity of response.
Size, length, and cost
Proof-of-concept studies should be lean and focused and be powered to detect a signal in the primary outcome that would justify further study. Ideally, they might be funded by NIH R01 grants or other relatively small funding mechanisms, and initiated at a single site leveraging shared resources as below and an existing clinical care infrastructure (eg, prehabilitation clinic). They would usually enroll 10 to less than 100 people and run from a month to a year. Multiple, coordinated, smaller studies have a variety of advantages over fewer, larger, monolithic studies for proof-of-concept. Many smaller trials can explore a wider range of concepts, timing of interventions, characteristics of participants most likely to respond, doses, and drug-condition pairs. As early trials suggest areas of focus, conducting multiple, small follow-on trials in parallel will speed iterative refinements. Proof-of-concept can spiral from brief, low-cost trials focusing on biomarker outcomes to larger, longer trials with outcomes ever-further on the clinical spectrum, with each phase providing justification and design information for the next.
Safety
Some proposed interventions are FDA-approved with extensive clinical experience regarding safety and adverse effects (eg, metformin), whereas others will be new chemical entities. The latter will require Phase 1 safety and pharmacokinetic studies, preferably in a population relevant to aging trials, before proceeding to the proof-of-concept trials described here. FDA-approved compounds may also require additional studies to confirm tolerability and pharmacokinetics in the populations targeted by studies testing interventions that target fundamental aging mechanisms, especially if prior data in older people are limited. As with efficacy outcomes, safety outcomes should be designed to capture expected side effects of the specific intervention (eg, nausea for metformin, oral ulcers for rapamycin) as well as nonspecific symptoms and global adverse outcomes, both self-reported and objective where appropriate. Elderly subjects are more susceptible to side effects that would be minor in younger individuals, with serious health problems ensuing as the side effect interacts with underlying organ dysfunction and age-related changes. Study design should therefore pay particular attention to significant secondary adverse outcomes that could be related to more minor side effects, such as weight loss associated with nausea or oral ulcers, falls associated with dizziness, or delirium associated with sedation. Standardization of safety outcomes can help increase the power of multiple closely related proof-of-concept studies of an intervention to detect important safety issues before a decision is made to proceed to larger studies. Ultimately, the decision as to whether any safety risks or adverse effects are tolerable must be made in the context of the risks of the usual course of events and the expected benefit of an intervention, and with input from regulatory bodies and the broader community. For some interventions and trial designs, this decision might be conceptually similar to that for chemotherapy of advanced cancers; for others, more as for primary prevention interventions.
Regulatory issues
Demonstrating safety is a necessary prerequisite to proof-of-concept trials. Institutional Review Board and FDA applications can cross-reference the investigational new drug approval for existing compounds, although safety may need to be separately confirmed in frail, multimorbid, or elderly populations. Proof-of-concept trials will usually not be powered to support registration, or thorough analysis of safety and adverse events. However, they should still be designed with the goal of justifying larger trials targeting an existing registration end point, or supporting the creation of new registration end points that reflect the targeting of fundamental aging processes. The three frameworks might form the foundations of such new end points: composite end points of multiple age-related diseases (multimorbidity), geriatric syndromes with clear clinical definitions, or as adjuvant or preventative therapies for other medical treatments (resilience).
Accelerating Progress
The workshop developed a number of concepts that could help accelerate and sustain progress in translating interventions into and through clinical trials (Figure 3). These suggestions for common resources or strategic infrastructure span the full breadth of the translational pipeline, and are in some cases inspired by similar efforts in fields such as oncology, whereas others leverage existing common infrastructure to assist translational geroscience (14,50,51).
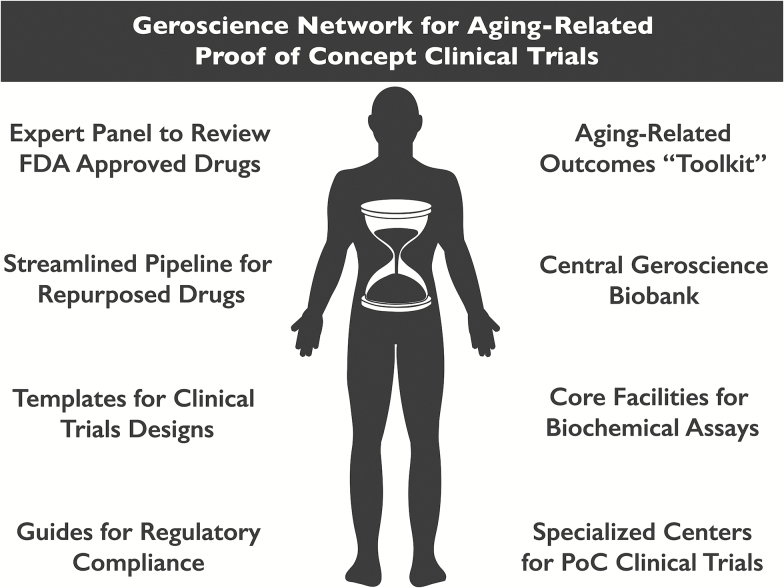
Figure 3.
Proposed development of shared resources and strategic infrastructure to accelerate progress through early clinical trials of interventions targeting fundamental aging processes. Many of these shared resources might be coordinated by new Geroscience Translational Network Centers, which would specialize in particular aspects of preclinical basic research, clinical trial support, and training.
Many of the most promising drugs in the geroscience pipeline are in fact repurposed FDA-approved drugs that have been found to regulate fundamental processes related to aging (eg, metformin, rapamycin, and others). Many other promising drugs may exist, with unrecognized potential. An expert panel might review all FDA-approved drugs for their potential in targeting fundamental processes of aging. Candidate drugs could be rapidly screened in preclinical studies and would be likely to proceed more rapidly to early clinical trials than novel compounds, and the geroscience community could engage existing resources for identifying new indications for repurposed drugs (eg, National Center for Advancing Translational Sciences Drug Repurposing Program).
Beyond “lead compound” identification, however, there are steps that can be taken to accelerate progress in testing geroscience-derived interventions. First, common templates can be developed for clinical trial designs utilizing the frameworks described here, for investigational new drug applications to the FDA, for establishment of data safety monitoring boards, and for applications to Institutional Review Boards. These templates could be developed by trialists within a Geroscience Network, updated regularly to reflect new data and experience gained through early trials, and shared freely with participating investigators and institutions. The NIA’s Clinical Research Study Investigator’s Toolbox (52) might provide a starting point that can be adapted for geroscience trials.
Second, a national network could develop a standardized “toolkit” of aging-related outcomes that can be modularly incorporated into a variety of study designs, as has been developed in other fields (53). The toolkit might include a set of specified biochemical markers with detailed protocols for collection and measurement, and sets of physiological measures or functional outcomes, all of which would be directly comparable across many trials. The toolkit would provide a common data set for assessing how any intervention affects fundamental aging processes, as well as building a database to guide the mechanistic pairing of interventions with different outcomes. A similar standardized toolkit of safety outcomes and adverse events could help ensure that safety concerns are identified early. Network centers could serve as core facilities for efficiently running assays from submitted biological specimens.
Third, biological specimens generated in these trials can be collected into a new national geroscience biobank. Such a biobank could facilitate wide access for the entire scientific community to the diversity of biological specimens that will be generated (54), uniquely enriched in samples from multimorbid, frail, and older individuals. Distribution would be mediated by a scientific review process for vetting and prioritizing their use. The biobank would be a resource invaluable not only for the geroscience community but also for all biomedical researchers studying a disease process that is related to aging. Critically, the value of such a resource would not depend on the success of any or all of the morbidity-specific trials described herein, but instead would allow investigators in multiple fields to understand the interplay between mechanisms thought to contribute to the complex context of organismal aging.
Fourth, the institutions in a Geroscience Network could develop as centers specializing in specific types of clinical trials, facilitating the high-throughput performance of trials that test different interventions, target populations, timing, etc. in a common clinical trial framework, maintaining shared templates for a trial framework, and training investigators from other sites. One center might develop expert basic science and clinical personnel, and physical infrastructure, dedicated to trials targeting frailty, for example, whereas another center might develop equivalent infrastructure for trials involving prehabilitation prior to elective cardiac surgery. Outside investigators would find an open pathway to test their new interventions at centers, and launching trials at noncenter sites would be aided by the standardization and shared resources promoted by centers. Centers would facilitate the rapid and reproducible completion of the iterative trials necessary to bring interventions into effective clinical practice as quickly as possible.
These four concepts are inspired by the remarkable success of cooperative research networks in other biomedical fields, such as the Alliance for Clinical Trials in Oncology and ECOG-ACRIN Cancer Research Group. A further developed Geroscience Translational Network would not duplicate the efforts of such disease-specific cooperatives, but would seek collaborations with them. Existing research infrastructure at Geroscience Network institutions, such as the Clinical and Translational Science Award programs, could be leveraged to reduce startup costs and provide additional shared efficiencies. Importantly, individual investigators outside the Geroscience Network could benefit from engagement at any level with the network resources, making it possible for researchers with diverse interests beyond aging to participate.
Summary
The successful translation of therapies that target fundamental aging processes into routine clinical interventions could transform the practice of medicine and human health (55). A number of candidate drugs (many already FDA-approved for other indications) have shown promise in preclinical studies. This Geroscience Network retreat developed ideas for proof-of-concept clinical trials that could be the next step in translating interventions that target fundamental aging processes into clinical practice. We described three frameworks for proof-of-concept trials, targeted at age-related diseases, geriatric syndromes, and resilience to acute stressors. Some aspects of clinical trial design are common to all three, whereas some require unique consideration in each framework. Importantly, proof-of-concept clinical trials would serve to test and advance the “geroscience hypothesis” that targeting the fundamental biology of aging will affect a range of age-related outcomes. Trial outcomes would be multidimensional and include outcomes related to the mechanism of action of the intervention; specific to the disease, syndrome, or stress under study; related to off-target effects of the intervention; and broadly relevant to the mechanisms and physiology of aging. Finally, several concrete steps could greatly accelerate the progress of clinical trials of interventions that target basic aging processes, including the development of standardized templates for trial design, toolkits for standardized outcome measurements, the establishment of a national geroscience biobank, and the development of specialist trial and training centers in the Geroscience Network.
Supplementary Material
Please visit the article online at http://gerontologist.oxfordjournals.org/ to view supplementary material.
Funding
This work was supported by NIH grant R24AG044396 (J.L.K., S.N.A., N.B.), the Nathan Shock Center of Excellence for the Biology of Aging (P30AG038072, N.B.), the Glenn Center for the Biology of Human Aging (Paul Glenn Foundation Grant, N.B.), the Connor Group, and the Noaber and Ted Nash Foundations (J.L.K.). J.N.J. was supported by T32 AG033534 (P.I.: S. Kritchevsky). J.C.N. was supported by K08 AG048354.
Supplementary Material
Acknowledgments
The authors are grateful for the contributions of the participants in the Geroscience Network retreat in Newcastle, UK, on October 4–5, 2015 and to all of the retreat attendees (Appendix A). We are also grateful to Frederick Baumer, who acted as facilitator for the retreat, Jacqueline Armstrong and Tina Larson-Cronk for organizing it, and the Nathan Shock Center of Excellence for the Biology of Aging (P30AG038072) (N.B.), the Mayo Clinic Robert and Arlene Kogod Center on Aging (J.D.M., S.K.H., and J.L.K.), the Alliance for Healthy Aging, the University of Groningen, Newcastle University, and the Glenn Center for the Biology of Human Aging (Paul Glenn Foundation Grant) (N.B.).
References
- 1. Harrison DE, Strong R, Allison DB, et al. Acarbose, 17-alpha-estradiol, and nordihydroguaiaretic acid extend mouse lifespan preferentially in males. Aging Cell. 2014;13:273–282. doi:10.1111/acel.12170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wilkinson JE, Burmeister L, Brooks SV, et al. Rapamycin slows aging in mice. Aging Cell. 2012;11:675–682. doi:10.1111/j.1474-9726.2012.00832.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Harrison DE, Strong R, Sharp ZD, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi:10.1038/nature08221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Martin-Montalvo A, Mercken EM, Mitchell SJ, et al. Metformin improves health span and lifespan in mice. Nat Commun. 2013;4:2192. doi:10.1038/ncomms3192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Roos CM, Zhang B, Palmer AK, et al. Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice. Aging Cell. [published online ahead of print February 10,2016]. doi:10.1111/acel.12458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Xu M, Tchkonia T, Ding H, et al. JAK inhibition alleviates the cellular senescence-associated secretory phenotype and frailty in old age. Proc Natl Acad Sci USA. 2015;112:E6301–6310. doi:10.1073/pnas.1515386112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xu M, Palmer AK, Ding H, et al. Targeting senescent cells enhances adipogenesis and metabolic function in old age. Elife. 2015;4:e12997. doi:10.7554/eLife.12997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhu Y, Tchkonia T, Pirtskhalava T, et al. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell. 2015;14:644–658. doi:10.1111/acel.12344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhu Y, Tchkonia T, Fuhrmann-Stroissnigg H, et al. Identification of a novel senolytic agent, navitoclax, targeting the Bcl-2 family of anti-apoptotic factors. Aging Cell. 2016;15:428–435. doi:10.1111/acel.12445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bjedov I, Partridge L. A longer and healthier life with TOR down-regulation: genetics and drugs. Biochem Soc Trans. 2011;39:460–465. doi:10.1042/BST0390460 [DOI] [PubMed] [Google Scholar]
- 11. Stout MB, Steyn FJ, Jurczak MJ, et al. 17α-Estradiol alleviates age-related metabolic and inflammatory dysfunction in male mice without inducing feminization. J Gerontol A Biol Sci Med Sci. 2016. doi:10.1093/gerona/glv309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schmidt B. Proof of principle studies. Epilepsy Res. 2006;68:48–52. doi:10.1016/j.eplepsyres.2005.09.019 [DOI] [PubMed] [Google Scholar]
- 13. Kennedy BK, Berger SL, Brunet A, et al. Geroscience: linking aging to chronic disease. Cell. 2014;159:709–713. doi:10.1016/j.cell.2014.10.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Seals DR, Melov S. Translational geroscience: emphasizing function to achieve optimal longevity. Aging. 2014;6:718–730. doi:10.18632/aging.100694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Burch JB, Augustine AD, Frieden LA, et al. Advances in geroscience: impact on healthspan and chronic disease. J Gerontol A Biol Sci Med Sci. 2014;69(suppl 1):S1–S3. doi:10.1093/gerona/glu041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Corr PB, Williams DA. Pathway from idea to regulatory approval: examples for drug development. In: Lo E, Field MJ, eds. Institute of Medicine (US) Committee on Conflict of Interest in Medical Research, Education, and Practice. Washington, DC: National Academies Press; 2009, 375–383. [PubMed] [Google Scholar]
- 17. Hazzard WR, Halter JB. Hazzard’s Geriatric Medicine and Gerontology. 6th ed. New York, NY: McGraw-Hill Medical Publication Division; 2009. [Google Scholar]
- 18. Kim YC, Guan KL. mTOR: a pharmacologic target for autophagy regulation. J Clin Invest. 2015;125:25–32. doi:10.1172/JCI73939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Luo D, Ren H, Li T, Lian K, Lin D. Rapamycin reduces severity of senile osteoporosis by activating osteocyte autophagy. Osteoporos Int. 2016;27:1093–1101. doi:10.1007/s00198-015-3325-5 [DOI] [PubMed] [Google Scholar]
- 20. Inouye SK, Studenski S, Tinetti ME, Kuchel GA. Geriatric syndromes: clinical, research, and policy implications of a core geriatric concept. J Am Geriatr Soc. 2007;55:780–791. doi:10.1111/j.1532-5415.2007.01156.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hshieh TT, Yue J, Oh E, et al. Effectiveness of multicomponent nonpharmacological delirium interventions: a meta-analysis. JAMA Intern Med. 2015;175:512–520. doi:10.1001/jamainternmed.2014.7779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kane AE, Hilmer SN, Boyer D, et al. Impact of longevity interventions on a validated mouse clinical frailty index. J Gerontol A Biol Sci Med Sci. 2016;71:333–339. doi:10.1093/gerona/glu315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brothers TD, Rockwood K. Biologic aging, frailty, and age-related disease in chronic HIV infection. Curr Opin HIV AIDS. 2014;9:412–418. doi:10.1097/COH.0000000000000070 [DOI] [PubMed] [Google Scholar]
- 24. Williams BA, Goodwin JS, Baillargeon J, Ahalt C, Walter LC. Addressing the aging crisis in U.S. criminal justice health care. J Am Geriatr Soc. 2012;60:1150–1156. doi:10.1111/j.1532-5415.2012.03962.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sanoff HK, Deal AM, Krishnamurthy J, et al. Effect of cytotoxic chemotherapy on markers of molecular age in patients with breast cancer. J Natl Cancer Inst. 2014;106:dju057. doi:10.1093/jnci/dju057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cimino T, Steinman MA, Mitchell SL, et al. The course of functional impairment in older homeless adults: disabled on the street. JAMA Intern Med. 2015;175:1237–1239. doi:10.1001/jamainternmed.2015.1562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Roberts HC, Denison HJ, Martin HJ, et al. A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age Ageing. 2011;40:423–429. doi:10.1093/ageing/afr051 [DOI] [PubMed] [Google Scholar]
- 28. Romanick M, Thompson LV, Brown-Borg HM. Murine models of atrophy, cachexia, and sarcopenia in skeletal muscle. Biochim Biophys Acta. 2013;1832:1410–1420. doi:10.1016/j.bbadis.2013.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nilsson J, Thomas AJ, O’Brien JT, Gallagher P. White matter and cognitive decline in aging: a focus on processing speed and variability. J Int Neuropsychol Soc. 2014;20:262–267. doi:10.1017/S1355617713001458 [DOI] [PubMed] [Google Scholar]
- 30. Troncale JA. The aging process. Physiologic changes and pharmacologic implications. Postgrad Med. 1996;99:111–114, 120–122. [PubMed] [Google Scholar]
- 31. Gill TM, Allore HG, Holford TR, Guo Z. Hospitalization, restricted activity, and the development of disability among older persons. JAMA. 2004;292:2115–2124. doi:10.1001/jama.292.17.2115 [DOI] [PubMed] [Google Scholar]
- 32. Guralnik JM, Alecxih L, Branch LG, Wiener JM. Medical and long-term care costs when older persons become more dependent. Am J Public Health. 2002;92:1244–1245. doi:10.2105/ajph.92.8.1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Debes C, Aissou M, Beaussier M. Prehabilitation. Preparing patients for surgery to improve functional recovery and reduce postoperative morbidity. Ann Fr Anesth Reanim. 2014;33:33–40. doi:10.1016/j.annfar.2013.12.012 [DOI] [PubMed] [Google Scholar]
- 34. Demark-Wahnefried W, Clipp EC, Morey MC, et al. Lifestyle intervention development study to improve physical function in older adults with cancer: outcomes from Project LEAD. J Clin Oncol. 2006;24:3465–3473. doi:10.1200/JCO.2006.05.7224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Meynet O, Ricci JE. Caloric restriction and cancer: molecular mechanisms and clinical implications. Trends Mol Med. 2014;20:419–427. doi:10.1016/j.molmed.2014.05.001 [DOI] [PubMed] [Google Scholar]
- 36. Avery GJ, 2nd, Ley SJ, Hill JD, Hershon JJ, Dick SE. Cardiac surgery in the octogenarian: evaluation of risk, cost, and outcome. Ann Thorac Surg. 2001;71:591–596. doi:10.1016/s0003-4975(00)02163-9 [DOI] [PubMed] [Google Scholar]
- 37. Craver JM, Puskas JD, Weintraub WW, et al. 601 octogenarians undergoing cardiac surgery: outcome and comparison with younger age groups. Ann Thorac Surg. 1999;67:1104–1110. doi:10.1016/s0003-4975(99)00154-x [DOI] [PubMed] [Google Scholar]
- 38. Siriett V, Salerno MS, Berry C, et al. Antagonism of myostatin enhances muscle regeneration during sarcopenia. Mol Ther. 2007;15:1463–1470. doi:10.1038/sj.mt.6300182 [DOI] [PubMed] [Google Scholar]
- 39. Kortebein P, Symons TB, Ferrando A, et al. Functional impact of 10 days of bed rest in healthy older adults. J Gerontol A Biol Sci Med Sci. 2008;63:1076–1081. doi:10.1093/gerona/63.10.1076 [DOI] [PubMed] [Google Scholar]
- 40. Longo VD, Antebi A, Bartke A, et al. Interventions to slow aging in humans: are we ready? Aging Cell. 2015;14:497–510. doi:10.1111/acel.12338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Seals DR, Justice JN, LaRocca TJ. Physiological geroscience: targeting function to increase healthspan and achieve optimal longevity. J Physiol. 2016;594:2001–2024. doi:10.1113/jphysiol.2014.282665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Newman JC, Milman S, Hashmi S, et al. Strategies and challenges in clinical trials targeting human aging. J Gerontol A Biol Sci Med Sci. 2016;71: 1424–1434. doi:10.1093/gerona/glw149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Huffman DM, Justice JN, Stout MB, Kirkland JL, Barzilai N, Austad SN. Evaluating healthspan in pre-clinical models of aging and disease: guidelines, challenges and opportunities for geroscience. J Gerontol A Biol Sci Med Sci. 2016;71:1395–1406. doi:10.1093/gerona/glw106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Demaria M, Ohtani N, Youssef SA, et al. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev Cell. 2014;31:722–733. doi:10.1016/j.devcel.2014.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bodine SC, Stitt TN, Gonzalez M, et al. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol. 2001;3:1014–1019. doi:10.1038/ncb1101-1014 [DOI] [PubMed] [Google Scholar]
- 46. Cesari M, Vellas B, Hsu FC, et al. A physical activity intervention to treat the frailty syndrome in older persons-results from the LIFE-P study. J Gerontol A Biol Sci Med Sci. 2015;70:216–222. doi:10.1093/gerona/glu099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Clark AM, Hartling L, Vandermeer B, McAlister FA. Meta-analysis: secondary prevention programs for patients with coronary artery disease. Ann Intern Med. 2005;143:659–672. [DOI] [PubMed] [Google Scholar]
- 48. Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi:10.1056/NEJMoa012512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ngandu T, Lehtisalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385:2255–2263. doi:10.1016/S0140-6736(15)60461-5 [DOI] [PubMed] [Google Scholar]
- 50. Hobin JA, Deschamps AM, Bockman R, et al. Engaging basic scientists in translational research: identifying opportunities, overcoming obstacles. J Transl Med. 2012;10:72. doi:10.1186/1479-5876-10-72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Simon R. Translational research in oncology: key bottlenecks and new paradigms. Expert Rev Mol Med. 2010;12:e32. doi:10.1017/S1462399410001638 [DOI] [PubMed] [Google Scholar]
- 52. Clinical Research Study Investigator’s Toolbox. https://www.nia.nih.gov/research/dgcg/clinical-research-study-investigators-toolbox. Accessed June 30, 2016.
- 53. Gershon RC, Wagster MV, Hendrie HC, Fox NA, Cook KF, Nowinski CJ. NIH toolbox for assessment of neurological and behavioral function. Neurology. 2013;80:S2–S 6. doi:10.1212/WNL.0b013e3182872e5f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Riegman PH, Dinjens WN, Oosterhuis JW. Biobanking for interdisciplinary clinical research. Pathobiology. 2007;74:239–244. doi:10.1159/000104451 [DOI] [PubMed] [Google Scholar]
- 55. Goldman DP, Cutler D, Rowe JW, et al. Substantial health and economic returns from delayed aging may warrant a new focus for medical research. Health Affairs. 2013;32:1698–1705. doi:10.1377/hlthaff.2013.0052 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.