Abstract
Background:
Habitual (non-exercise) physical activity (PA) declines with age, and aging-related increases in inflammation and fatigue may be important contributors to variability in PA.
Methods:
This study examined the association of objectively-measured PA (accelerometry over 7 days) with inflammation (plasma interleukin-6 and C-reactive protein) and with self-reported fatigue (SF-36 Vitality) at baseline and 18 months after a diet-induced weight loss, exercise, or diet-induced weight loss plus exercise intervention in 167 overweight/obese, middle-aged, and older adults.
Results:
At baseline, individuals with higher plasma interleukin-6, as well as those who reported feeling less energetic (more fatigued), took less steps per day and had lower PA energy expenditure and minutes of light and moderate–vigorous PA (p < .05 for all). At the 18-month follow-up, inflammation was lower in both weight loss groups, fatigue was reduced in all three groups with larger decreases in the combined group, and mean levels of habitual PA were not changed in any group. In longitudinal analyses with all groups combined, we found that participants reporting larger increases in vitality (eg, declines in fatigue) had greater increases in PA (p < .05 for all). Also, changes in steps/d and physical activity energy expenditure were indirectly associated with changes in interleukin-6 (β [SEM] for steps/d = −565 [253]; β [SEM] for physical activity energy expenditure = −22.4 [10.17]; p < .05).
Conclusions:
Levels of habitual PA are lower in middle-aged and older adults with higher levels of chronic inflammation and greater self-reported fatigue. In addition, participants who experienced greater declines in inflammation during the interventions had greater declines in fatigue and larger increases in PA.
Keywords: Accelerometry, C-reactive protein, Interleukin-6, SF-36 Vitality subscale
Physical activity (PA) is comprised of both “purposeful” exercise, as well as non-exercise movement comprised primarily of mobility-related activities occurring throughout the day. This habitual (non-exercise) activity declines across the lifespan (1), an observation that is biologically inherent to aging, as it is evident even among highly fit older exercisers (2) and is observed across species (3). Habitual PA is inversely related to sedentary behavior (sitting or lying down), and more sedentary behavior is associated with worse physical function and loss of mobility in older adults, even independent of structured exercise (4–6).
Despite the fundamental ubiquitous nature of reduced habitual PA with age, very little is known regarding the biological, environmental, and/or psychosocial factors that contribute to one’s propensity to move and to inter-individual variation in movement. An important factor underlying less habitual activity may be fatigue, or the subjective lack of physical and/or mental energy (7). Fatigue also increase with age (8,9), and some studies report an association between fatigue and low levels of PA and greater sedentary behavior (10–17). However, the biological link between fatigue and sedentary behavior is not known. Acute inflammation from infection results in fatigue (ie, “sickness behavior”) and chronic inflammation underlies fatigue symptoms common to specific diseases (18–21). Aging is characterized by an increasingly elevated pro-inflammatory state, but one that is far below that observed during acute infections or disease states (22–24). This persistent, low-grade inflammation is likely a contributing factor to loss of muscle function and onset of disability with age (25,26); however, its role in fatigue and reduced PA is less studied (14,15,27,28).
The purpose of this study was to determine the cross-sectional and longitudinal relationships of objectively-measured habitual PA to biomarkers of inflammation and self-reported fatigue in middle-aged and older adults. We examined these relationships using baseline and follow-up data collected from participants before and 18 months after either: diet-induced weight loss alone, exercise alone, or diet-induced weight loss plus exercise. We hypothesized that individuals with higher inflammation would report feeling more fatigued and have lower levels of PA at baseline; and that individual changes in habitual PA over the 18 months of intervention would be associated with individual changes in inflammation and fatigue, independent of treatment arm.
Methods
Study Design and Participants
This analysis used data from a random sub-sample (n = 167) of 454 total participants enrolled in the Intensive Diet and Exercise for Arthritis study (IDEA; conducted between July 2006 and April 2011), an 18-month, randomized, controlled trial of the effects of three interventions: Diet-induced weight loss only (Diet, N = 49); Exercise only (Exercise, N = 60); and Exercise in combination with diet-induced weight loss (Diet+Exercise, N = 58). The participants in the sub-sample were those randomly assigned at baseline to additional/ancillary assessments outside the primary outcomes, including accelerometer measurement of PA. Complete details of the trial design, methodology, and main results are published (29).
Briefly, ambulatory, middle-aged and older (≥55 years), overweight or obese (body mass index [BMI] = 27–41kg/m2) men and women were enrolled if they met all inclusion/exclusion criteria, including: (a) Kellgren Lawrence grade II-III (mild to moderate) radiographic tibiofemoral osteoarthritis of one or both knees; (b) not performing more than 30min/wk of exercise in past 6 months; (c) no cognitive impairment or depression; (d) no knee surgery in prior 6 months; and (e) no clinically relevant comorbid disease that would pose a safety threat or impair ability to participate. The Wake Forest University Health Sciences Institutional Review Board approved the study and all participants gave written informed consent to participate.
Interventions
Details of the interventions are published (29). The diet-induced weight loss intervention consisted of group and individual nutrition education and behavioral sessions, as well as an individualized dietary prescription plan providing an energy-intake deficit of 800–1,000 kcals/d to reach an average weight loss goal of 10% of baseline weight. The prescribed diet provided an energy intake deficit of 800 to 1,000 kcal/d as predicted by estimated weight-maintenance energy expenditure (estimated resting metabolic rate × 1.2 activity factor) with at least 1,100 kcal/d for women and 1,200 kcal/d for men. There was one individual session and three group sessions per month for the first 6 months. From months 7–18, participants attended biweekly group sessions, with an individual appointment every 2 months. The Exercise-only group was not counseled to restrict caloric intake during the study.
The exercise intervention took place 3 days per week. Each session consisted of a 5-minute dynamic warm-up, an aerobic phase (over ground walking) for 15 minutes, followed by light strength training (20 minutes) and a second aerobic phase of over ground walking for 15 minutes, and then a cool-down with stretching (10 minutes). During the first 6 months, each session was supervised and center-based. Starting at month 7, participants could continue to exercise in the research center for all 3d/wk (chosen by 67% of participants), or could opt for a home-based program (11%), or a combination of center- and home-based (22%). Exercise prescriptions were individualized based on a participant’s ability. Briefly, the walking intensity prescription progressed from 50% to 60% of heart rate reserve initially to 75%–85% of HRR; the strength training workload was also progressive with participants increasing the weight lifted every fifth to sixth session during the first 6 months, and then maintained that weight for 12 months.
Measurements
Demographic characteristics were obtained through self-report questionnaires, and included age, sex, race, and years of education. Body height and weight were assessed in the clinic without shoes or heavy clothing on a calibrated scale using standard techniques. Self-reported pain and function were assessed using the Western Ontario McMasters Universities Osteoarthritis Index (WOMAC). Perception of fatigue was assessed using the Vitality domain on the original Medical Outcomes Study 36-item short-form (SF-36) measure (30). The Vitality sub-scale consists of four items assessing vitality/fatigue levels in the past month on a 6-point scale from 1 (“all of the time”) to 6 (“none of the time”). The questions are: (a) Did you feel tired?; (b) Did you feel worn out?; (c) Did you feel full of pep?; and (d) Did you have a lot of energy? Scores are linearly transformed to a scale of 0 to 100, with higher scores indicating higher vitality (ie, less fatigue). A summary vitality/fatigue score was calculated by averaging the four scores.
Physical activity
The Kenz Lifecorder EX accelerometer (Suzuken Co., Ltd) was used to objectively quantify daily PA levels. The device was attached to a belt or waistband of the participant’s clothing during all waking hours (excluding time for swimming or bathing) for seven consecutive days and the display was locked to avoid providing feedback. The device provides valid and reliable measures of steps/d, physical activity energy expenditure (PAEE, in kcal/d), and minutes of light, moderate, and vigorous PA in free-living conditions (31). A maximum pulse over 4 seconds was taken as the acceleration value and activities were categorized based on intensity levels (1 or minimal intensity to 9 or maximal intensity). When the sensor detected three acceleration pulses or more for four consecutive seconds, the activity was recognized as PA. If an acceleration pulse was not immediately followed by another acceleration pulse then it was not counted as a 0 but as a 0.5. This assumed isolated spurts of acceleration were changes in posture and not PA. PA was categorized as light (<3 Metabolic Equivalents), moderate (3–6 Metabolic Equivalents), or vigorous (>6 Metabolic Equivalents) intensity (32). Participants maintained accelerometer diaries to note the time of day the device was worn. The accelerometer data were uploaded to a computer for analysis and outcome variables included: total steps/d, PAEE, minutes of light physical activity (Light PA), and minutes of moderate or vigorous physical activity (Mod–Vig PA).
Inflammatory biomarkers
Blood samples were collected in the early morning after a 12-hour fast. All follow-up samples were collected at least 24 hours after an exercise session and blood sampling was postponed (1–2 weeks after recovery of all symptoms) in the event of an acute respiratory, urinary tract, or other infection. Plasma interleukin-6 (IL-6) assays were run using Quantikine enzyme-linked immunosorbent kits from R&D systems (Minneapolis, MN) and high-sensitivity CRP was assessed using an automated immunoanalyzer (IMMULITE; Diagnostics Products Corporation, Los Angeles, CA) (31).
Statistical Analysis
Baseline characteristics are presented overall using descriptive measures. Inflammatory biomarkers were assessed on the log-scale due to right skewness. Pearson partial correlations were estimated between baseline PA measures and both fatigue and inflammation at baseline, adjusted for age, gender, race, and BMI. Treatment effects on PA, inflammation, and fatigue at 6 and 18 months was assessed using mixed linear models adjusted for visit, visit-by-treatment interaction, gender, baseline BMI, and baseline values of the outcome; visit-specific means and p-values were estimated using contrast statements, assuming an AR (1) covariance. Associations between 6- and 18-month changes in PA and 6- and 18-month changes in fatigue and inflammation were analyzed using Pearson partial correlations, adjusted for baseline age, sex, race, and baseline BMI. We also analyzed these associations averaging across time points using mixed linear models to account for repeated measures. One model of the association between change in PA and change in vitality/fatigue, and another model between change in PA and change in inflammatory biomarker, were adjusted for randomization group, visit, group-by-visit interaction, sex, baseline BMI, and baseline value of the PA outcome.
All tests were performed assuming a 2-sided alternative hypothesis at a .05 level of significance. Due to the exploratory nature of the substudy, no adjustment for multiplicity was performed. All analyses were conducted using SAS v9.4.
Results
Participant Baseline Characteristics
Demographic and other characteristics of the study sample at baseline are shown in Table 1. Briefly, participants were predominantly women, college-educated, and white. All were either overweight or obese and the average number of comorbidities was 1.8±1.1. The CRP and IL-6 levels in this overweight/obese sample of middle-aged/older adults are considered to be elevated compared to the general population (33); however, there was large variability in these inflammatory markers. The SF-36 Vitality (fatigue) scores, as well as the PA data, also indicate large inter-individual variability among the study sample.
Table 1.
Baseline Demographic and Health Characteristics of the Study Sample
| Mean ± SD or # (%) | |
|---|---|
| Age (y) | 66.2±6.4 |
| Female, n (%) | 116 (70%) |
| White, n (%) | 135 (81%) |
| Weight (kg) | 92.9±14.5 |
| Education | |
| Less than High School | 49 (29%) |
| College or Grad school | 118 (71%) |
| Body mass index (kg/m2) | 33.5±3.7 |
| Percentage body fat (%) | 39.6±7.0 |
| Self-reported comorbidities, n (%) | |
| Hypertension | 105 (64%) |
| Cardiovascular disease | 13 (8%) |
| Diabetes | 24 (15%) |
| Osteoporosis | 18 (11%) |
| Any Cancer | 35 (22%) |
| WOMAC pain score | 5.9±2.8 |
| WOMAC function score | 22.3±10.3 |
| Inflammation | |
| CRP (mg/L) | 8.2±10.1 |
| IL-6 (pg/mL) | 3.1±2.0 |
| SF-36 Vitality questions | |
| Feeling tired | 59.3±23.0 |
| Feeling worn-out | 69.0±22.7 |
| Feeling energetic | 44.3±25.0 |
| Feeling peppy | 46.5±24.0 |
| SF-36 Vitality summary score | 54.8±19.5 |
| Accelerometer-measured physical activity (PA) | |
| Steps/d | 6,143±2,558 |
| PA energy expenditure (kcal/d) | 234±125 |
| Light PA (min/d) | 130±39 |
| Mod–Vig PA (min/d) | 10.5±9.3 |
Note: N = 167; SF-36 scale (each question scaled as 0 = fatigued to 100 = energetic; higher scores indicate feeling more energetic/less fatigued);
CRP = C-reactive protein; IL-6 = interleukin-6; Mod–Vig PA = moderate or vigorous physical activity; WOMAC = Western Ontario and McMaster Universities Osteoarthritis Index.
Cross-sectional Associations Among Inflammation, Fatigue, and PA at Baseline
To determine whether individuals with higher chronic inflammation and greater fatigue have lower levels of habitual PA, we calculated Pearson correlation coefficients (adjusting for age, sex, race, and BMI) among all variables at baseline. There was a significant inverse relationship between IL-6 concentrations and PA, such that those with higher plasma IL-6 were less active across each measure of PA (steps/d: r = −.20; PAEE: r = −.21; light PA: r = −.16; Mod–Vig PA: r = −.19; all p < .05). CRP concentrations only correlated with PAEE (r = −.16, p < .05). In addition, there were small, but significant, positive correlations between PA and the SF-36 Vitality score, such that those who reported feeling more energetic (less fatigued) were more active (steps/d: r = .19, p < .05; PAEE: r = .16, p < .05; light PA: r = .18, p < .05; Mod–Vig PA: r = .14, p = .07). Self-reported vitality/fatigue levels did not correlate with CRP or IL-6 concentrations.
Treatment Effects on Inflammation, Fatigue, and PA
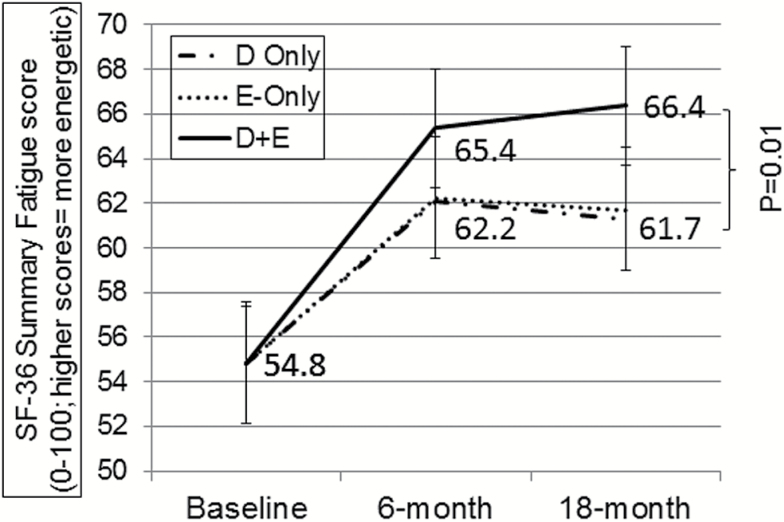
By design, there was a significant loss of body mass in both Diet and Diet+Exercise compared to Exercise, but no difference in weight lost between weight loss groups (Diet = −10.7±7.3kg, 14.1%; Diet+Exercise = −11.0±9.5kg, 14.3%; Exercise = 0.1±3.6kg, 0.2%; p < .0001). Table 2 shows the 6-month and 18-month treatment effects on the inflammation, vitality/fatigue, and PA variables, adjusted for baseline BMI, sex, and baseline value of each measure. Treatment effects on inflammation showed that both Diet and Diet+Exercise had lower CRP and IL-6 than Exercise at both 6- and 18-months; this is consistent with what was reported in the entire cohort previously (29,31). The treatment effects on self-reported vitality/fatigue are shown in Table 2 and Figure 1. Overall, there were significant increases across all three groups in their SF-36 Vitality score (eg, decrease in fatigue). By the 18-month time point, the combined group of Diet+Exercise reported feeling more energetic (less fatigued) than either Exercise or Diet (p < .05). The number of minutes of Mod–Vig PA was greater in the Exercise groups (Exercise and Diet+Exercise); however, there were no group differences in any of the other PA measures at 6 or 18 months.
Table 2.
Inflammation, SF-36 Vitality Score, and Physical Activity by Treatment Group
| Diet Only | Exercise Only | Diet+Exercise | Overall | ||
|---|---|---|---|---|---|
| Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | p-value | ||
| Log CRP (mg/L) | Baseline | 1.48 (1.14; 1.83) | 1.72 (1.46; 1.97) | 1.39 (1.08; 1.69) | |
| 6-month | 1.10 (0.94; 1.26) | 1.51 (1.36; 1.67) | 1.15 (1.00; 1.30) | <.001 | |
| 18-month | 0.90 (0.74; 1.06) | 1.37 (1.21; 1.53) | 0.88 (0.73; 1.04) | <.0001 | |
| Log IL-6 (pg/mL) | Baseline | 0.82 (0.62; 1.01) | 0.92 (0.77; 1.06) | 1.09 (0.93; 1.26) | |
| 6-month | 0.75 (0.65; 0.85) | 0.91 (0.82; 1.01) | 0.79 (0.70; 0.88) | .04 | |
| 18-month | 0.72 (0.62; 0.82) | 0.96 (0.86; 1.06) | 0.72 (0.63; 0.82) | <.001 | |
| SF-36 Vitality score | Baseline | 57.0 (51.4; 62.7) | 53.6 (48.5; 58.7) | 54.1 (49.0; 59.1) | |
| 6-month | 62.1 (59.3; 64.9) | 62.2 (59.5; 65.0) | 65.4 (62.7; 68.0) | .14 | |
| 18-month | 61.3 (58.6; 64.0) | 61.7 (59.0; 64.4) | 66.4 (63.7; 69.1) | .01 | |
| Steps/d | Baseline | 5,886 (5,198; 6,573) | 6,548 (5,914; 7,182) | 5,942 (5,211; 6,673) | |
| 6-month | 6,258 (5,462; 7,053) | 5,521 (4,755; 6,287) | 6,207 (5,475; 6,939) | .29 | |
| 18-month | 5,252 (4,176; 6,329) | 4,686 (3,745; 5,627) | 5,963 (4,775; 7,151) | .23 | |
| PAEE (kcal/d) | Baseline | 219 (187; 251) | 252 (220; 285) | 228 (193; 264) | |
| 6-month | 223 (189; 258) | 213 (179; 246) | 223 (191; 255) | .86 | |
| 18-month | 182 (136; 228) | 164 (123; 204) | 206 (156; 257) | .40 | |
| Light PA (min/d) | Baseline | 135 (123; 147) | 132 (123; 142) | 124 (115; 134) | |
| 6-month | 133 (118; 148) | 119 (104; 134) | 126 (112; 140) | .39 | |
| 18-month | 109 (87; 131) | 107 (88; 126) | 131 (107; 155) | .23 | |
| Mod–Vig PA (min/d) | Baseline | 7.9 (5.8; 10.0) | 12.1 (9.7; 14.6) | 11.1 (8.4; 13.8) | |
| 6-month | 9.8 (7.2; 12.4) | 8.8 (6.2; 11.3) | 12.4 (10.0; 14.7) | .09 | |
| 18-month | 7.9 (4.1; 11.7) | 6.3 (3.1; 9.6) | 15.7 (11.6; 19.9) | <.01 | |
Note: No differences between groups at baseline: Diet (Baseline N = 49, 18-month N = 41); Exercise (Baseline N = 60, 18-month N = 41); Diet+Exercise (Baseline N = 58, 18-month N = 43). SF-36 Vitality scale (higher scores indicate feeling more energetic/less fatigued);
CI = confidence interval; CRP = C-reactive protein; IL-6 = interleukin-6; Mod–Vig PA = moderate or vigorous physical activity; PAEE = physical activity energy expenditure.
Figure 1.
Treatment effects on perceived energy/fatigue from SF-36 Vitality questions. 6- and 18-month means are adjusted for baseline body mass index (BMI), sex, and baseline Vitality score. Significant (p < .05) increases within all three groups; Significant (p = .01) 18-month difference between Diet+Exercise and Exercise-Only or Diet-Only.
Longitudinal Associations Among Inflammation, Fatigue, and PA
Pearson correlation coefficients (adjusted for baseline age, sex, race, and BMI) among changes in inflammation, fatigue and PA are shown in Table 3. Changes in all of the PA variables correlated positively with changes in the SF-36 Vitality score, especially at the 6-month time point, whereby individuals with a larger increase in vitality (ie, greater reduction in fatigue) exhibited greater increases in PA. There were negative correlations between changes in both CRP and IL-6 and changes in the SF-36 Vitality score, such that greater increases in vitality (ie, greater reductions in fatigue) correlated with larger declines in inflammation. Changes in PA were not related to changes in CRP at either time point; however, changes in steps per day and light PA correlated negatively with changes in IL-6 at 18-months.
Table 3.
Pearson Correlations Among 6- and 18-Month Changes in the SF-36 Vitality Score, Inflammation, and Physical Activity
| N | Steps/d | PAEE | Light PA | Mod–Vig PA | Log CRP | Log IL-6 | ||
|---|---|---|---|---|---|---|---|---|
| SF-36 Vitality score | 6-monthΔ | 87 | 0.41 (p < .01) | 0.40 (p < .01) | 0.24 (p < .05) | 0.39 (p < .01) | −0.28 (p = .01) | −0.29 (p = .01) |
| 18-monthΔ | 39 | 0.34 (p < .05) | 0.30 (p = .08) | 0.24 (p = .17) | 0.37 (p < .05) | −0.14 (p = .43) | −0.26 (p = .13) | |
| Log CRP | 6-monthΔ | 88 | −0.06 | −0.01 | −0.04 | −0.05 | — | — |
| 18-monthΔ | 39 | −0.04 | 0.03 | −0.03 | −0.14 | — | — | |
| Log IL-6 | 6-monthΔ | 88 | −0.16 | −0.16 | −0.05 | −0.12 | — | — |
| 18-monthΔ | 39 | −0.32 (p = .07) | −0.27 (p = .12) | −0.36 (p < .05) | −0.15 | — | — | |
Note: Change scores calculated as Post-value at 6- or 18-months minus Pre-value. SF-36 Vitality score (greater negative change indicates more fatigued). Δ = 6-month or 18-month changes from baseline;
CRP = C-reactive protein; IL-6 = interleukin-6; Mod–Vig PA = moderate or vigorous physical activity; PAEE = physical activity energy expenditure.
We also determined whether longitudinal changes in PA were associated with changes in vitality/fatigue or inflammation using mixed model linear regression. Modeling the associations between changes in vitality/fatigue and the PA variables showed there were positive associations among changes in each PA variable and changes in the SF-36 Vitality score (β [SEM] for steps/d = 38.31 [9.74]; PAEE = 1.61 [0.40]; light PA = 0.44 [0.19]; Mod–Vig PA = 0.13 [0.03]; p < .05 for all). Modeling associations between change in inflammation and the PA variables showed changes in PA were not associated with changes in CRP, but changes in steps/d and PAEE were indirectly associated with changes in IL-6 levels (steps/d = −565 [253]; PAEE = −22.4 [10.17]; p < .05).
Discussion
This paper took advantage of existing data from a lifestyle intervention trial to examine the cross-sectional and longitudinal relationships of objectively-measured habitual PA with biomarkers of chronic inflammation and levels of perceived fatigue, two factors likely to contribute to one’s propensity to move throughout the day. In cross-sectional analyses with baseline data, we found relatively weak, but statistically significant, correlations between inflammation and PA, and between fatigue and activity. Participants with higher inflammation (especially IL-6) had fewer steps per day and fewer minutes of light, moderate and vigorous activity, independent of age, sex, race, and baseline BMI. These results are consistent with several prior observational studies reporting an inverse relationship between inflammation and PA (34–36). However, the premise of most of these studies was that PA is the predictor variable, and conclusions drawn suggest engaging in more activity, especially that of a higher-intensity, lowers inflammation (37,38). Our premise is that the converse is also true, that inflammation contributes to lowering of activity, possibly through increasing fatigue, as we (in this study), and others (10–17), find that those who report more fatigue are less active. Though in our data from overweight/obese older adults with prevalent knee osteoarthritis, there was no cross-sectional association between inflammation and fatigue using the Vitality domain of the SF-36, a few other studies do show a link between inflammation and fatigue in healthy (15,27,28) and diseased (18–21) populations.
The link between habitual PA and inflammation may be a “vicious cycle” whereby elevated chronic inflammation leads to fatigue and less activity throughout the day (eg, more time spent lying or sitting or in sedentary behavior), and in turn, inactivity leads to higher inflammation, making it difficult to decipher exact causal links between them. This idea is supported by recent work showing individuals who spend more time sitting have an elevated inflammatory profile, independent of time spent in moderate or vigorous PA (39–41). Disrupting this cycle with experimental studies that decrease inflammation are needed to definitively confirm the idea that not only acute, but also chronic, inflammation is a risk factor for sedentary behavior and reduced habitual activity.
Although not the primary purpose of our study, we also reported mean effects of the interventions on inflammation, fatigue, and habitual PA. These analyses confirmed the already known effects of weight loss for lowering inflammation (35,42) as both CRP and IL-6 levels were much lower in the diet groups after treatment, compared to exercise only. However, despite greater weight loss and less inflammation in both diet groups, the combined diet and exercise group reported less fatigue at 18-months compared to either exercise or diet alone. The positive effects of the diet and exercise combination on the SF-36 Vitality domain is in line with the overall conclusion of a recent systematic review (of studies in younger and middle-aged individuals) which found vitality was the most responsive sub-scale of the SF-36 to small decreases in body weight in response to lifestyle interventions (43). Notably, there were no group differences in habitual PA, although the combined treatment group engaged in approximately 8–9 more minutes of Mod–Vig activity per day at the 18-month follow-up compared to the other treatment arms.
We took advantage of the 18-month interventions to examine temporal relationships between PA and inflammation and fatigue. In correlation analyses, using data collapsed across treatment groups, participants with larger individual declines in both CRP and IL-6 had greater improvements in their SF-36 Vitality score (indicative of less fatigue), independent of treatment group and demographic characteristics. Importantly, those with larger declines in IL-6 and greater decreases in fatigue were those that exhibited a greater increase in number of steps/d and PAEE. Thus, it appears that the cycle between inflammation and propensity to move throughout the day may have been “disrupted” in those who experienced a larger decrease in inflammation. Of course, since, to our knowledge, these are the first data to show a longitudinal association of individual changes in habitual PA with changes in inflammation, additional research is needed to confirm that treating or lowering inflammation per se will result in less fatigue and greater habitual movement among older adults.
The results of this study need to be interpreted in light of the limitations to our study design, subject sample, and assessments. First, this was a retrospective analysis of existing data from a study that was designed for a separate purpose. Therefore, the findings are limited to middle-aged and older adults who were overweight or obese and had knee osteoarthritis, but were otherwise fairly healthy and functional. Also, there was no non-intervention control group that did not undergo either a diet or exercise intervention. Thus, we are not able to definitively determine the effects of either the dietary weight loss or exercise intervention on study outcomes; but can only determine the effects of the combination of diet plus exercise compared to either treatment alone. The measures of inflammation were limited to two biomarkers. The fatigue measure available in this study, the SF-36 Vitality sub-score, is a commonly used measure, but is not specific for older adults. Also, it does not incorporate the construct of fatigability, or fatigue in the context of a standardized task, that accounts for the concept of self-pacing in order to lower exertion or activity to reduce perceived fatigue. Finally, there are other factors that we were unable to measure, including diet, comorbid disease, medication use, etc., which can affect PA, inflammation and fatigue and may have confounded the associations we observed.
Taken together, the results of this study advance our understanding of the biological and psychosocial correlates of individual variation in habitual PA among middle-aged and older adults with obesity. Additional work is needed to determine whether increases in chronic inflammation with aging and chronic disease directly contribute to aging-related increases in fatigue, which in turn lead to less propensity to move and greater time spent in sedentary behavior with aging. Additional research is also needed to determine whether intervening to lower inflammation will result in less fatigue and sedentary behavior which would have major relevance for maintaining health and function in older adults.
Funding
This work was supported by an independent research grant from the National Institutes of Health (R01 AR052528), as well as the Wake Forest University Claude D. Pepper Center Older Americas Independence Center (P30-AG21332) and Wake Forest University General Clinical Research Center (M01-RR07122); and General Nutrition Centers, Inc.
Conflict of Interest
None to declare.
References
- 1. Westerterp KR. Daily physical activity and ageing. Curr Opin Clin Nutr Metab Care. 2000;3:485–488. [DOI] [PubMed] [Google Scholar]
- 2. Madden KM, Ashe MC, Chase JM. Activity profile and energy expenditure among active older adults, British Columbia, 2011-2012. Prev Chronic Dis. 2015;12:E112. doi:10.5888/pcd12.150100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ingram DK. Age-related decline in physical activity: generalization to nonhumans. Med Sci Sports Exerc. 2000;32:1623–1629. [DOI] [PubMed] [Google Scholar]
- 4. Chmelo E, Nicklas B, Davis C, Miller GD, Legault C, Messier S. Physical activity and physical function in older adults with knee osteoarthritis. J Phys Act Health. 2013;10:777–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gennuso KP, Gangnon RE, Matthews CE, Thraen-Borowski KM, Colbert LH. Sedentary behavior, physical activity, and markers of health in older adults. Med Sci Sports Exerc. 2013;45:1493–1500. doi:10.1249/MSS.0b013e318288a1e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Santos DA, Silva AM, Baptista F, et al. Sedentary behavior and physical activity are independently related to functional fitness in older adults. Exp Gerontol. 2012;47:908–912. doi:10.1016/j.exger.2012.07.011 [DOI] [PubMed] [Google Scholar]
- 7. Avlund K. Fatigue in older adults: an early indicator of the aging process? Aging Clin Exp Res. 2010;22:100–115. [DOI] [PubMed] [Google Scholar]
- 8. Cheng H, Gurland BJ, Maurer MS. Self-reported lack of energy (anergia) among elders in a multiethnic community. J Gerontol A Biol Sci Med Sci. 2008;63:707–714. [DOI] [PubMed] [Google Scholar]
- 9. Junghaenel DU, Christodoulou C, Lai JS, Stone AA. Demographic correlates of fatigue in the US general population: results from the patient-reported outcomes measurement information system (PROMIS) initiative. J Psychosom Res. 2011;71:117–123. doi:10.1016/j.jpsychores.2011.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. O’Connor PJ, Puetz TW. Chronic physical activity and feelings of energy and fatigue. Med Sci Sports Exerc. 2005;37:299–305. [DOI] [PubMed] [Google Scholar]
- 11. Puetz TW. Physical activity and feelings of energy and fatigue: epidemiological evidence. Sports Med. 2006;36:767–780. [DOI] [PubMed] [Google Scholar]
- 12. Moreh E, Jacobs JM, Stessman J. Fatigue, function, and mortality in older adults. J Gerontol A Biol Sci Med Sci. 2010;65:887–895. doi:10.1093/gerona/glq064 [DOI] [PubMed] [Google Scholar]
- 13. Resnick HE, Carter EA, Aloia M, Phillips B. Cross-sectional relationship of reported fatigue to obesity, diet, and physical activity: results from the third national health and nutrition examination survey. J Clin Sleep Med. 2006;2:163–169. [PubMed] [Google Scholar]
- 14. Silva JP, Pereira DS, Coelho FM, Lustosa LP, Dias JM, Pereira LS. Clinical, functional and inflammatory factors associated with muscle fatigue and self-perceived fatigue in elderly community-dwelling women. Rev Bras Fisioter. 2011;15:241–248. [PubMed] [Google Scholar]
- 15. Valentine RJ, Woods JA, McAuley E, Dantzer R, Evans EM. The associations of adiposity, physical activity and inflammation with fatigue in older adults. Brain Behav Immun. 2011;25:1482–1490. doi:10.1016/j.bbi.2011.06.002 [DOI] [PubMed] [Google Scholar]
- 16. Egerton T, Chastin SF, Stensvold D, Helbostad JL. Fatigue may contribute to reduced physical activity among older people: an observational study. J Gerontol A Biol Sci Med Sci. 2016;71:670–676. doi:10.1093/gerona/glv150 [DOI] [PubMed] [Google Scholar]
- 17. Ward-Ritacco CL, Adrian AL, O’Connor PJ, et al. Feelings of energy are associated with physical activity and sleep quality, but not adiposity, in middle-aged postmenopausal women. Menopause. 2015;22:304–311. doi:10.1097/GME.0000000000000315 [DOI] [PubMed] [Google Scholar]
- 18. Dantzer R, Heijnen CJ, Kavelaars A, Laye S, Capuron L. The neuroimmune basis of fatigue. Trends Neurosci. 2014;37:39–46. doi:10.1016/j.tins.2013.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bower JE, Lamkin DM. Inflammation and cancer-related fatigue: mechanisms, contributing factors, and treatment implications. Brain Behav Immun. 2013;30(Suppl):S48–S57. doi:10.1016/j.bbi.2012.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Johansson P, Riegel B, Svensson E, et al. Sickness behavior in community-dwelling elderly: associations with impaired cardiac function and inflammation. Biol Res Nurs. 2014;16:105–113. doi:10.1177/1099800412466170 [DOI] [PubMed] [Google Scholar]
- 21. Lasselin J, Layé S, Dexpert S, et al. Fatigue symptoms relate to systemic inflammation in patients with type 2 diabetes. Brain Behav Immun. 2012;26:1211–1219. doi:10.1016/j.bbi.2012.03.003 [DOI] [PubMed] [Google Scholar]
- 22. Nash SD, Cruickshanks KJ, Klein R, et al. Long-term variability of inflammatory markers and associated factors in a population-based cohort. J Am Geriatr Soc. 2013;61:1269–1276. doi:10.1111/jgs.12382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ferrucci L, Corsi A, Lauretani F, et al. The origins of age-related proinflammatory state. Blood. 2005;105:2294–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Franceschi C, Capri M, Monti D, et al. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev. 2007;128:92–105. [DOI] [PubMed] [Google Scholar]
- 25. Singh T, Newman AB. Inflammatory markers in population studies of aging. Ageing Res Rev. 2011;10:319–329. doi:10.1016/j.arr.2010.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Penninx BW, Kritchevsky SB, Newman AB, et al. Inflammatory markers and incident mobility limitation in the elderly. J Am Geriatr Soc. 2004;52:1105–1113. [DOI] [PubMed] [Google Scholar]
- 27. Cho HJ, Kivimäki M, Bower JE, Irwin MR. Association of C-reactive protein and interleukin-6 with new-onset fatigue in the Whitehall II prospective cohort study. Psychol Med. 2013;43:1773–1783. doi:10.1017/S0033291712002437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bautmans I, Gorus E, Njemini R, Mets T. Handgrip performance in relation to self-perceived fatigue, physical functioning and circulating IL-6 in elderly persons without inflammation. BMC Geriatr. 2007;7:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Messier SP, Mihalko SL, Legault C, et al. Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: the IDEA randomized clinical trial. JAMA. 2013;310:1263–1273. doi:10.1001/jama.2013.277669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 31. Van RH, Giavedoni S, Raste Y, et al. Validity of activity monitors in health and chronic disease: a systematic review. Int J Behav Nutr Phys Act. 2012;9:84. doi:10.1186/1479-5868-9-84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kumahara H, Schutz Y, Ayabe M, et al. The use of uniaxial accelerometry for the assessment of physical-activity-related energy expenditure: a validation study against whole-body indirect calorimetry. Br J Nutr. 2004;91:235–243. [DOI] [PubMed] [Google Scholar]
- 33. Woloshin S, Schwartz LM. Distribution of C-reactive protein values in the United States. N Engl J Med. 2005;352:1611–1613. [DOI] [PubMed] [Google Scholar]
- 34. Abramson JL, Vaccarino V. Relationship between physical activity and inflammation among apparently healthy middle-aged and older US adults. Arch Intern Med. 2002;162:1286–1292. [DOI] [PubMed] [Google Scholar]
- 35. Nicklas BJ, You T, Pahor M. Behavioural treatments for chronic systemic inflammation: effects of dietary weight loss and exercise training. CMAJ. 2005;172:1199–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Adams SA, Wirth MD, Khan S, et al. The association of C-reactive protein and physical activity among a church-based population of African Americans. Prev Med. 2015;77:137–140. doi:10.1016/j.ypmed.2015.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Plaisance EP, Grandjean PW. Physical activity and high-sensitivity C-reactive protein. Sports Med. 2006;36:443–458. [DOI] [PubMed] [Google Scholar]
- 38. Hawkins M, Belalcazar LM, Schelbert KB, Richardson C, Ballantyne CM, Kriska A. The effect of various intensities of physical activity and chronic inflammation in men and women by diabetes status in a national sample. Diabetes Res Clin Pract. 2012;97:e6–e8. doi:10.1016/j.diabres.2012.03.019 [DOI] [PubMed] [Google Scholar]
- 39. Henson J, Yates T, Biddle SJ, et al. Associations of objectively measured sedentary behaviour and physical activity with markers of cardiometabolic health. Diabetologia. 2013;56:1012–1020. doi:10.1007/s00125-013-2845-9 [DOI] [PubMed] [Google Scholar]
- 40. Gennuso KP, Gangnon RE, Matthews CE, Thraen-Borowski KM, Colbert LH. Sedentary behavior, physical activity, and markers of health in older adults. Med Sci Sports Exerc. 2013;45:1493–1500. doi:10.1249/MSS.0b013e318288a1e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Leon-Latre M, Moreno-Franco B, Andres-Esteban EM, et al. Sedentary lifestyle and its relation to cardiovascular risk factors, insulin resistance and inflammatory profile. Rev Esp Cardiol (Engl Ed). 2014;67:449–455. doi:10.1016/j.rec.2013.10.015 [DOI] [PubMed] [Google Scholar]
- 42. Beavers KM, Beavers DP, Newman JJ, et al. Effects of total and regional fat loss on plasma CRP and IL-6 in overweight and obese, older adults with knee osteoarthritis. Osteoarthritis Cartilage. 2015;23:249–256. doi:10.1016/j.joca.2014.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kroes M, Osei-Assibey G, Baker-Searle R, Huang J. Impact of weight change on quality of life in adults with overweight/obesity in the United States: a systematic review. Curr Med Res Opin. 2016;32:485–508. doi:10.1185/03007995.2015.1128403 [DOI] [PubMed] [Google Scholar]