Abstract
BACKGROUND
There are no proven strategies to prevent atrial fibrillation (AF) in patients with type 2 diabetes (T2DM). We compared standard blood pressure (BP) lowering vs. intensive BP lowering in reducing incidence of AF or P-wave indices (PWI—ECG markers of left atrial abnormality that are considered intermediate phenotypes of AF) in patients with T2DM.
METHODS
We analyzed data from the ACCORD BP trial—a randomized controlled nonblinded trial (2001–2009) which randomized patients with T2DM and systolic BP (SBP) 130−180mm Hg on ≤3 antihypertensive medications aged 40−79 years with cardiovascular disease (CVD) or aged 55−79 years with subclinical CVD or ≥2 CVD risk factors to standard BP lowering (SBP <140mm Hg) vs. intensive BP lowering (SBP <120mm Hg). The primary outcome was a composite of incident AF and PWI.
RESULTS
Data from 3,087 participants (mean age, 62.2 years; women, 48.2%; non-White, 39.2%) were analyzed. During a mean follow-up of 4.4 years, the primary outcome occurred in 1,063 participants (incidence rate, 84.5 per 1,000 person-years in the standard-therapy group vs. 73.9 per 1,000 person-years in the intensive-therapy group). The adjusted hazard ratios (95% confidence intervals) of intensive-therapy group for the primary outcome and for incident PWI alone were 0.87 (0.77−0.98), P = 0.02 and 0.87 (0.76−0.98), P = 0.02, respectively. The effect of intensive therapy on the incidence of AF alone did not reach statistical significance.
CONCLUSIONS
In patients with T2DM, intensive BP lowering reduces the incidence of the composite outcome of AF and PWI, suggesting a potential benefit from stringent BP control in patients with T2DM.
clinical trials registration
Trial Number NCT00000620
Keywords: atrial fibrillation, blood pressure, diabetes mellitus, hypertension, left atrial abnormality, P-wave indices, randomized controlled trial
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia in the general population. AF afflicts more than 2 million Americans, and this figure is projected to increase to 5–12 million by 2050.1,2 Type 2 diabetes (T2DM) is one of the most common chronic diseases worldwide. In the United States, 23 million adults have T2DM, a figure that is increasing by approximately 1 million each year.3 As in the general population, AF in patients with T2DM is associated with an increased risk of all-cause mortality, cardiovascular death, stroke, or heart failure.4 Hence, preventing AF in patients with T2DM is an important clinical and public health imperative.
Of the many established risk factors for AF, elevated blood pressure (BP) is the most important contributor to the burden of AF.5 Observational data in women suggest that high-normal systolic blood pressure (SBP) (130–139mm Hg) or diastolic blood pressure (DBP) (85–89mm Hg) confer an increased risk of AF compared with SBP <120mm Hg or DBP <65mm Hg.6 However, there are no data from randomized controlled trials to indicate that intensive BP lowering to SBP of <120mm Hg reduces AF incidence compared with standard BP lowering to SBP of <140mm Hg.
Recent evidence suggests that P-wave indices (PWI)—ECG markers of left atrial abnormality7 that are considered intermediate phenotypes of AF8–14—are also associated with ischemic stroke and vascular brain injury, independent of AF.15,16 We hypothesized that compared with standard BP lowering, intensive BP lowering will reduce the incidence of AF or PWI in patients with T2DM. We tested our hypothesis in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial—a randomized, multicenter trial involving middle-aged and older participants with T2DM who are at high risk for cardiovascular disease (CVD) events because of existing CVD or additional risk factors.
METHODS
Study population and design
ACCORD was a randomized trial conducted at 77 clinical sites organized into 7 networks in the United States and Canada (http://www.accordtrial.org/ClinicalTrials.gov Identifier: NCT00000620).17 The trial enrolled 10,251 high-risk participants with T2DM. All participants were randomly assigned to either intensive or standard glycemic control (the ACCORD glycemia trial).18 In addition, 5,518 of the ACCORD participants were also randomly assigned (in a 2-by-2 factorial design) to either simvastatin plus fenofibrate or simvastatin plus placebo (the ACCORD lipid trial),19 and the remaining 4,733 participants were also randomly assigned (in a 2-by-2 factorial design) to either intensive or standard BP control (the ACCORD BP trial).20 This report is based on the ACCORD BP trial. The study was conducted in accordance with the Declaration of Helsinki, and it was approved by the relevant institutional ethics committees.
Inclusion criteria for ACCORD have been previously published.17 In brief, participants were eligible if they had T2DM and a glycated hemoglobin level ≥7.5% and were aged ≥40 years with CVD or ≥55 years with anatomical evidence of a substantial amount of atherosclerosis, albuminuria, left ventricular hypertrophy, or ≥2 additional risk factors for CVD (dyslipidemia, hypertension, smoking, or obesity). In addition, participants with a SBP between 130 and 180mm Hg who were taking ≤3 antihypertensive medications and who had the equivalent of a 24-hour protein excretion rate of less than 1.0g were eligible for the BP trial.
The ACCORD BP trial was a nonblinded trial in which participants were randomly assigned to intensive therapy that targeted SBP of <120mm Hg or standard therapy that targeted SBP of <140mm Hg. The ACCORD BP trial was a study of a treatment strategy to achieve specific SBP goals, rather than an evaluation of any specific drug regimen. Therefore, all available antihypertensive medications were used to lower BP. The approach to the management of BP has been described elsewhere.20
For the primary composite outcome of incident AF or PWI, participants in the ACCORD BP trial with prevalent AF or PWI (n = 1,234), missing or uninterpretable ECG (n = 137), or without any follow-up (n = 275) were excluded, leaving 3,087 participants for analysis.
Outcomes
The primary outcome was a composite outcome of incident AF or PWI; only the first event was counted for the primary outcome. Secondary outcomes were components of the primary outcome: incident AF and PWI.
Incident AF cases were ascertained from 12-lead ECGs that were obtained at the biennial follow-up visits (i.e., every 2 years) and close-out visit. In all ACCORD sites, ECGs were digitally acquired using a GE MAC 1200 electrocardiograph (GE, Milwaukee, WI) at 10mm/mV calibration and a speed of 25mm/s. ECG reading was performed centrally at the Epidemiological Cardiology Research Center, Wake Forest School of Medicine, Winston-Salem, NC. All ECGs were initially inspected visually for technical errors and inadequate quality before being automatically processed using GE 12-SL Marquette Version 2001 (GE).
Electrocardiographic PWI traditionally used as criteria for ECG-based left atrial abnormality are considered intermediate phenotypes for AF.8–14 In this study, incident PWI was ascertained from 12-lead ECGs based on Novacode ECG criteria: P-wave duration in lead II ≥120ms, P′ (negative) amplitude in V1 ≥100 µV, or P-terminal force in V1 ≥−4,000 µV·ms.21 P-wave duration and amplitudes were automatically measured using GE 12-SL Marquette Version 2001 (GE). These automatically calculated measurements were used to mathematically derive the PWI of interest.
Covariates
Race or ethnicity was self-reported. Left ventricular hypertrophy was defined by ECG voltage criteria or echocardiography (if an echocardiogram report was available from the participant). History of CVD included prior myocardial infarction, stroke, arterial revascularization, angina with ischemic changes on ECG at rest, changes on a graded exercise test, and positive cardiac imaging test results.
Statistical analyses
We report means with SDs for continuous variables and counts with percentages for categorical variables to describe baseline characteristics. Person-years at risk were calculated from the date of randomization until the date of the first occurrence of an outcome or the last available visit if a participant remained event-free.
Incidence rates are expressed as the number of events per 1,000 person-years, taking into account censoring of follow-up data. Kaplan–Meier estimates were used to calculate the cumulative incidence of an event over time for both BP treatment groups and were compared using log-rank tests. To estimate the association of BP treatment group with primary (composite of AF and PWI) and secondary outcomes (AF and PWI separately), we used Cox proportional hazards models to calculate hazard ratios (HRs) and 95% confidence intervals (CIs) with the standard BP lowering as the referent group. Model 1 was unadjusted, Model 2 was adjusted for glucose-lowering assignment, and Model 3 was additionally adjusted for the presence of CVD. For secondary analysis of the primary outcome, we additionally adjusted for other baseline covariates in Model 4. In addition, sex- and race-stratified analyses were conducted in relation to the primary outcome. To assess the impact of glycemia control on the relationship between BP control and the primary outcome, we performed an additional analysis stratified by glycemia trial assignment.
Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC). All P values reported were 2-sided, and statistical significance threshold was chosen as 5%.
RESULTS
Study participants
Of 3,087 participants who were included in the analysis, 1,536 were assigned to standard BP control and 1,551 were assigned to intensive BP control. Table 1 shows the distribution of baseline characteristics that are associated with an increased risk of AF; they were similar between the 2 groups. The mean (SD) age of the participants was 62.2 (6.6) years, 48.2% were women, 39.2% were non-White, and 30.9% had CVD at baseline. The mean (SD) duration of follow-up for the primary outcome was 4.4 (1.6) years.
Table 1.
Baseline characteristics of study participants in the Action to Control Cardiovascular Risk in Diabetes blood pressure trial
| Characteristics | Total (n = 3,087) | Standard blood pressure lowering (n = 1,536) | Intensive blood pressure lowering (n = 1,551) | P a |
|---|---|---|---|---|
| Age (years) (SD) | 62.2 (6.6) | 62.1 (6.7) | 62.2 (6.6) | 0.90 |
| Female sex | 1,487 (48.2) | 736 (47.9) | 751 (48.4) | 0.78 |
| Race/ethnicity | 0.86 | |||
| White | 1,876 (60.8) | 926 (60.3) | 950 (61.3) | |
| Latino | 201 (6.5) | 101 (6.6) | 100 (6.4) | |
| Black | 650 (21.1) | 320 (20.8) | 330 (21.3) | |
| Asian | 185 (6.0) | 97 (6.3) | 88 (5.7) | |
| Other | 175 (5.7) | 92 (6.0) | 83 (5.4) | |
| Smoking status | 0.96 | |||
| Never | 1,402 (45.5) | 695 (45.3) | 707 (45.6) | |
| Past | 1,310 (42.5) | 656 (42.7) | 654 (42.2) | |
| Current | 372 (12.1) | 184 (12.0) | 188 (12.1) | |
| Heart rate (bpm) (SD) | 72.6 (11.3) | 72.7 (11.1) | 72.6 (11.6) | 0.96 |
| Body mass index (kg/m2) (SD) | 32.1 (5.5) | 31.9 (5.4) | 32.2 (5.6) | 0.22 |
| Left ventricular hypertrophy | 131 (4.2) | 68 (4.4) | 63 (4.1) | 0.61 |
| Systolic blood pressure (mm Hg) (SD) | 138.8 (15.7) | 138.6 (15.2) | 138.9 (16.2) | 0.69 |
| Diastolic blood pressure (mm Hg) (SD) | 75.7 (10.2) | 75.7 (10.1) | 75.8 (10.3) | 0.94 |
| History of cardiovascular disease | 955 (30.9) | 452 (29.4) | 503 (32.4) | 0.07 |
| Use of ACE inhibitors | 1,616 (52.3) | 789 (51.4) | 827 (53.3) | 0.28 |
| Use of angiotensin receptor blockers | 525 (17.0) | 270 (17.6) | 255 (16.4) | 0.40 |
| Assigned to intensive glucose lowering | 1,553 (50.3) | 789 (51.4) | 764 (49.3) | 0.24 |
Data are presented as number (%) of participants unless otherwise stated.
Abbreviations: ACE, angiotensin converting enzyme; bpm, beats per minute.
a P comparing participants’ characteristics in the standard vs. intensive blood pressure-lowering arms.
Blood pressure
As reported in the ACCORD BP trial,20 after the first year of therapy, the average SBP was 133.5mm Hg (95% CI, 133.1−133.8) in the standard-therapy group and 119.3mm Hg (95% CI, 118.9−119.7) in the intensive-therapy group, resulting in an average between-group difference of 14.2mm Hg (95% CI, 13.7−14.7). The corresponding mean DBPs were 70.5 (95% CI, 70.2−70.8) and 64.4 (95% CI, 64.1−64.7), for an average difference of 6.1mm Hg (95% CI, 5.7−6.5). These levels of BP control in the 2 groups were maintained throughout the study.
Outcomes
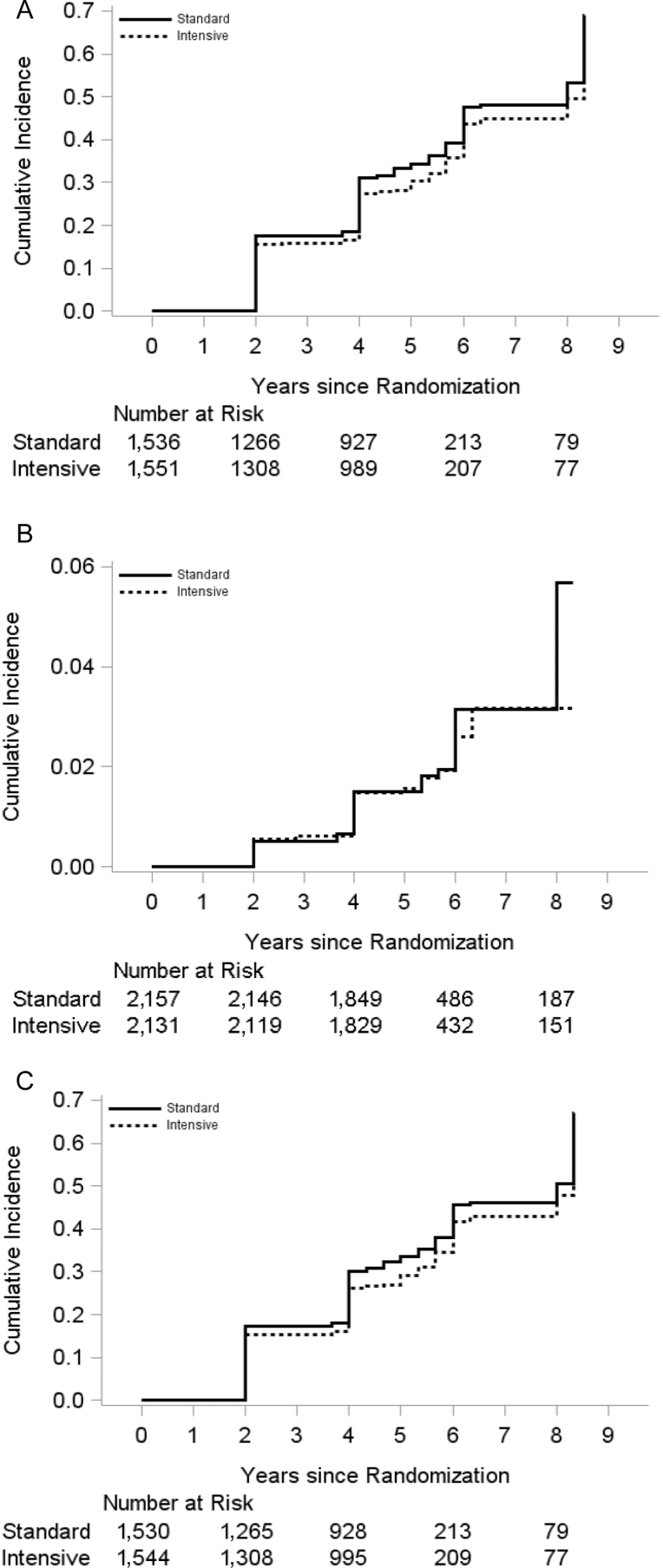
During a mean follow-up of 4.4 years, the primary composite outcome of incident AF or PWI occurred in 1,063 participants. The incidence rate was 84.5 per 1,000 person-years in the standard-therapy group as compared with 73.9 per 1,000 person-years in the intensive-therapy group. Figure 1A shows the cumulative incidence of the primary outcome by BP treatment group. The unadjusted HR (95% CI) of intensive-therapy group for the primary outcome was 0.88 (0.78−0.99), P = 0.04. After adjustment for glycemia trial assignment and baseline CVD, there remained a significant between-group difference (HR with intensive therapy, 0.87 (95% CI, 0.77−0.98), P = 0.02) (Table 2). Our results remained unchanged after additional adjustment for other baseline covariates (Supplementary Table 1).
Figure 1.
(A) Cumulative incidence of the primary outcome by blood pressure treatment group. (B) Cumulative incidence of incident atrial fibrillation by blood pressure treatment group. (C) Cumulative incidence of incident P-wave indices by blood pressure treatment group.
Table 2.
Risks of the primary outcome, atrial fibrillation, and P-wave indices in intensive vs. standard blood pressure-lowering treatment arms in the Action to Control Cardiovascular Risk in Diabetes blood pressure trial
| Outcome | Standard blood pressure- lowering arm | Intensive blood pressure- lowering arm | Model 1: unadjusted | Model 2: adjusted for glycemia trial assignment | Model 3: adjusted for glycemia trial assignment and baseline CVD | |||
|---|---|---|---|---|---|---|---|---|
| Events (n)/event rate per 1,000 person-years | Events (n)/event rate per 1,000 person-years | Hazard ratio (95% CI) | P | Hazard ratio (95% CI) | P | Hazard ratio (95% CI) | P | |
| Primary outcome: atrial fibrillation or P-wave indices | 562/84.5 | 501/73.9 | 0.88 (0.78−0.99) | 0.04 | 0.88 (0.78−0.99) | 0.04 | 0.87 (0.77−0.98) | 0.02 |
| Atrial fibrillation | 45/4.2 | 37/3.5 | 0.86 (0.56−1.33) | 0.49 | 0.86 (0.55−1.32) | 0.48 | 0.85 (0.55−1.32) | 0.48 |
| P-wave indices | 538/81.5 | 478/70.7 | 0.87 (0.77−0.99) | 0.03 | 0.87 (0.77−0.99) | 0.03 | 0.87 (0.76−0.98) | 0.02 |
Abbreviations: CI, confidence interval; CVD, cardiovascular disease.
Incident AF occurred in 45 and 37 participants in the standard BP-lowering and intensive BP-lowering groups, respectively. Figure 1B shows the cumulative incidence of incident AF by BP treatment group. After adjustment for glycemia trial assignment and baseline CVD, the HR (95% CI) of intensive BP lowering for incident AF was 0.85 (0.55−1.32), P = 0.48 (Table 2). There were 538 and 478 incident PWI cases in the standard BP-lowering and intensive BP-lowering groups, respectively. The cumulative incidence of incident PWI by BP treatment group is shown in Figure 1C. In the model adjusted for glycemia trial assignment and baseline CVD, the HR (95% CI) of intensive BP lowering for incident PWI was 0.87 (0.76−0.98), P = 0.02.
Sex- and race-stratified analyses were conducted in relation to the primary outcome. We found that compared with standard therapy, intensive therapy resulted in a nonsignificant lower incidence of the primary outcome in both men and women, and Whites and non-Whites (Table 3). There was no interaction by sex (P for sex interaction = 0.98) or by race (P for race interaction = 0.59). In addition, in stratified analysis by glycemia trial assignment, we found that compared with standard BP-lowering therapy, intensive BP-lowering therapy resulted in a nonsignificant lower incidence of the primary outcome in participants randomized to standard and intensive glycemia control (P for interaction = 0.90) (Table 3).
Table 3.
Risks of the primary outcome in intensive vs. standard blood pressure-lowering treatment arms stratified by sex, race, and glycemia trial assignment in the Action to Control Cardiovascular Risk in Diabetes blood pressure trial
| Stratifying variable | Standard blood pressure- lowering arm | Intensive blood pressure- lowering arm | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|---|---|
| Events (n)/event rate per 1,000 person-years | Events (n)/event rate per 1,000 person-years | Hazard ratio (95% CI) | P | Hazard ratio (95% CI) | P | Hazard ratio (95% CI) | P | |
| Sex | ||||||||
| Men | 302/87.7 | 270/77.4 | 0.88 (0.74−1.03) | 0.11 | 0.87 (0.74−1.03) | 0.11 | 0.86 (0.73−1.01) | 0.07 |
| Women | 260/81.1 | 231/70.1 | 0.88 (0.74−1.05) | 0.15 | 0.88 (0.74−1.05) | 0.15 | 0.88 (0.73−1.05) | 0.14 |
| Race | ||||||||
| White | 320/79.7 | 300/71.7 | 0.90 (0.77−1.06) | 0.21 | 0.91 (0.77−1.06) | 0.21 | 0.90 (0.77−1.06) | 0.20 |
| Non-White | 242/92.0 | 201/77.4 | 0.85 (0.70−1.02) | 0.08 | 0.85 (0.70−1.02) | 0.08 | 0.84 (0.69−1.01) | 0.06 |
| Glycemia trial assignment | ||||||||
| Standard glycemia control | 277/86.2 | 258/74.9 | 0.88 (0.74−1.04) | 0.13 | 0.87* (0.74−1.04) | 0.12* | NA | NA |
| Intensive glycemia control | 285/83.0 | 243/72.8 | 0.88 (0.74−1.04) | 0.14 | 0.86* (0.73−1.03) | 0.10* | NA | NA |
Model 1: unadjusted. Model 2: adjusted for glycemia trial assignment. Model 2 for glycemia trial assignment subgroups is adjusted for baseline CVD*. Model 3: adjusted for glycemia trial assignment and baseline CVD.
Abbreviations: CI, confidence interval; CVD, cardiovascular disease; NA, not applicable.
DISCUSSION
In this post hoc analysis of a randomized controlled trial comprising patients with T2DM, we found that compared with standard BP lowering (targeted SBP of <140mm Hg), intensive BP lowering (targeted SBP of <120mm Hg) reduced the rate of incident AF or PWI by 13%. This benefit was consistent in men and women, Whites and non-Whites, those randomized to standard and intensive glycemia control, and was driven by a reduction in incident PWI. This observation suggests a potential benefit from intensive BP lowering in reducing risk of AF or development of left atrial abnormality in patients with T2DM.
There is robust epidemiological evidence that hypertension is strongly associated with incident AF.22–24 Of the many established risk factors for AF, elevated BP is the most important contributor to the burden of AF. It explains more than one fifth of all AF cases and nearly one quarter if borderline BP is also considered.5 More recently, evidence has emerged that BP values below the current threshold for the diagnosis of hypertension are significantly associated with incident AF.6 The Women’s Health Study (WHS) reported that women with high-normal SBP (130–139mm Hg) or DBP (85–89mm Hg) at baseline had a 28% and 53% increased risk of AF compared with women with SBP <120mm Hg or DBP <65mm Hg, respectively.6 These observations suggest that tight BP control may be helpful in the prevention of AF.
Although there is epidemiological evidence to indicate that tight BP control may prevent AF, no randomized controlled trial has demonstrated a reduction in AF incidence from intensive BP lowering compared with standard BP lowering in patients with T2DM. To the best of our knowledge, the present report is the first to present evidence to suggest that compared with standard BP lowering, intensive BP lowering in patients with T2DM reduces the incidence of AF or PWI. In this study, we defined PWI using Novacode ECG criteria: P-wave duration in lead II ≥120ms, P′ (negative) amplitude in V1 ≥100 µV, or P-terminal force in V1 ≥−4,000 µV·ms.21 These ECG variables have shown good and consistent predictive accuracy for future AF.10–13 Hence, it is reasonable to consider PWI as a surrogate for incident AF. That the point estimates for incident AF (HR = 0.85) and PWI (HR = 0.87) are very similar (the only difference being a wider CI for incident AF which reflects the lower number of events) also underscores the validity of PWI as a surrogate for incident AF. More importantly, evidence is emerging to suggest that PWI are entities that are worthy of further investigation as clinical outcomes, in and of themselves. PWI are ECG markers of left atrial abnormality7 and have been shown to be associated with higher risk of stroke10,15 and vascular brain injury,16 even in the absence of AF. In addition, PWI have been shown to be associated with heart failure hospitalizations,25 all-cause death,26 and sudden cardiac death27—outcomes that are also associated with AF.
Our study has relevant clinical and public health implications. In the ACCORD BP trial, intensive BP lowering did not significantly reduce the primary cardiovascular outcome (composite of nonfatal myocardial infarction, nonfatal stroke, or death from cardiovascular causes) or the rate of death from any cause.20 However, intensive therapy did reduce the rate of 2 prespecified secondary outcomes—total stroke and nonfatal stroke. The number needed to undergo intensive BP management to prevent one stroke over the course of 5 years was 89.20 In our study, the absolute risk reduction of the primary outcome over 5 years was 5.5%. Thus, the number needed to undergo intensive BP treatment to prevent one AF or PWI over the course of 5 years was 18. In a post hoc analysis from the ADVANCE trial, AF in patients with T2DM was associated with a 61% greater risk of all-cause mortality and comparable higher risks of cardiovascular death, stroke, or heart failure.4 Given the benefit in stroke reduction, the AF-associated morbidities in patients with T2DM, and the low number needed to treat to prevent the composite endpoint of AF or PWI, it might be reasonable to consider intensive BP lowering in selected patients with T2DM. This consideration is particularly timely given the recent JNC 8 (Eighth Joint National Committee) recommendation which relaxed the threshold for initiation of BP-lowering treatment from 130 to 140mm Hg for all individuals with T2DM.28 A recent meta-analysis reported that BP-lowering therapy in diabetic patients with initial SBP level of 140mm Hg or treatment to a SBP level below 130mm Hg was associated with lower rates of stroke, retinopathy, and progression of albuminuria.29 We extend this finding by demonstrating that BP treatment to a target SBP lower than the current JNC recommendation may also be associated with another important clinical outcome—AF or PWI. Although the JNC 8 recommendation for a more relaxed threshold should apply to most patients with T2DM, the findings of our paper and the recent meta-analysis29 underscore that more research is needed to identify patients with T2DM who will benefit from intensive BP lowering. Specifically, more research is warranted to evaluate whether patients with T2DM and abnormal PWI will benefit from intensive BP lowering to prevent AF.
The principal strengths of this study include the random assignment of patients to study groups, a high rate of follow-up of a large number of patients according to a common protocol, and achievement and maintenance of an average between-group difference in SBP of 14mm Hg throughout the study. Several limitations should be noted. First, the trial had an open-label design; however, this design was not likely to have affected BP measurement or ascertainment of outcomes. Second, the incidence rates of AF in individuals with T2DM were low in the ACCORD BP trial (4.2 and 3.5 per 1,000 person-years in the standard-therapy and intensive-therapy groups, respectively) compared with previous observational studies: 9.0 per 1,000 person-years in the ARIC study30 and 9.1 per 1,000 person-years in an HMO registry.31 The lower incidence is due to under-ascertainment of incident AF by relying solely on biennial study ECGs and not on outpatient medical records, hospitalization discharge records, or longer ambulatory ECG monitoring for ascertainment of AF.
CONCLUSIONS
In patients with T2DM at high risk for cardiovascular events, targeting a SBP <120mm Hg, as compared with <140mm Hg, reduced the risk of AF or development of left atrial abnormality. This finding suggests that stringent BP control in patients with T2DM may prevent AF or development of left atrial abnormality and motivates additional research to identify patients with T2DM who will benefit from stringent BP control.
SUPPLEMENTARY MATERIAL
Supplementary materials are available at American Journal of Hypertension (http://ajh.oxfordjournals.org).
DISCLOSURE
The authors declared no conflict of interest.
Supplementary Material
ACKNOWLEDGMENTS
Dr Chen receives support from National Institute on Aging (R21AG042660-01A1). The ACCORD BP trial was supported by contracts from the National Heart, Lung, and Blood Institute (N01-HC-95178, N01-HC-95179, N01-HC-95180, N01-HC-95181, N01-HC-95182, N01-HC-95183, N01-HC-95184, and IAA#Y1-HC-9035 and IAA#Y1-HC-1010).
The funding agencies had no role in the design or conduct of the study, in the collection, management, analysis, or interpretation of the data, or in the preparation, review, or approval of the manuscript. The content is solely the responsibility of the authors and does not necessarily reflect the official views of the National Heart, Lung, and Blood Institute, Veterans Administration, or the Department of Health and Human Services.
REFERENCES
- 1. Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001; 285:2370–2375. [DOI] [PubMed] [Google Scholar]
- 2. Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, Seward JB, Tsang TS. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation 2006; 114:119–125. [DOI] [PubMed] [Google Scholar]
- 3. American Diabetes Association. Economic costs of diabetes in the U.S. in 2007. Diabetes Care 2008; 31:596–615. [DOI] [PubMed] [Google Scholar]
- 4. Du X, Ninomiya T, de Galan B, Abadir E, Chalmers J, Pillai A, Woodward M, Cooper M, Harrap S, Hamet P, Poulter N, Lip GY, Patel A; ADVANCE Collaborative Group. Risks of cardiovascular events and effects of routine blood pressure lowering among patients with type 2 diabetes and atrial fibrillation: results of the ADVANCE study. Eur Heart J 2009; 30:1128–1135. [DOI] [PubMed] [Google Scholar]
- 5. Huxley RR, Lopez FL, Folsom AR, Agarwal SK, Loehr LR, Soliman EZ, Maclehose R, Konety S, Alonso A. Absolute and attributable risks of atrial fibrillation in relation to optimal and borderline risk factors: the Atherosclerosis Risk in Communities (ARIC) study. Circulation 2011; 123:1501–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Conen D, Tedrow UB, Koplan BA, Glynn RJ, Buring JE, Albert CM. Influence of systolic and diastolic blood pressure on the risk of incident atrial fibrillation in women. Circulation 2009; 119:2146–2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hancock EW, Deal BJ, Mirvis DM, Okin P, Kligfield P, Gettes LS, Bailey JJ, Childers R, Gorgels A, Josephson M, Kors JA, Macfarlane P, Mason JW, Pahlm O, Rautaharju PM, Surawicz B, van Herpen G, Wagner GS, Wellens H; American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; American College of Cardiology Foundation; Heart Rhythm Society. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part V: electrocardiogram changes associated with cardiac chamber hypertrophy: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol 2009; 53:992–1002. [DOI] [PubMed] [Google Scholar]
- 8. Dilaveris PE, Gialafos EJ, Sideris SK, Theopistou AM, Andrikopoulos GK, Kyriakidis M, Gialafos JE, Toutouzas PK. Simple electrocardiographic markers for the prediction of paroxysmal idiopathic atrial fibrillation. Am Heart J 1998; 135:733–738. [DOI] [PubMed] [Google Scholar]
- 9. Andrikopoulos GK, Dilaveris PE, Richter DJ, Gialafos EJ, Synetos AG, Gialafos JE. Increased variance of P wave duration on the electrocardiogram distinguishes patients with idiopathic paroxysmal atrial fibrillation. Pacing Clin Electrophysiol 2000; 23:1127–1132. [DOI] [PubMed] [Google Scholar]
- 10. Soliman EZ, Prineas RJ, Case LD, Zhang ZM, Goff DC., Jr Ethnic distribution of ECG predictors of atrial fibrillation and its impact on understanding the ethnic distribution of ischemic stroke in the Atherosclerosis Risk in Communities (ARIC) study. Stroke 2009; 40:1204–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Magnani JW, Johnson VM, Sullivan LM, Gorodeski EZ, Schnabel RB, Lubitz SA, Levy D, Ellinor PT, Benjamin EJ. P wave duration and risk of longitudinal atrial fibrillation in persons ≥ 60 years old (from the Framingham Heart Study). Am J Cardiol 2011; 107:917–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Magnani JW, Williamson MA, Ellinor PT, Monahan KM, Benjamin EJ. P wave indices: current status and future directions in epidemiology, clinical, and research applications. Circ Arrhythm Electrophysiol 2009; 2:72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Magnani JW, Zhu L, Lopez F, Pencina MJ, Agarwal SK, Soliman EZ, Benjamin EJ, Alonso A. P-wave indices and atrial fibrillation: cross-cohort assessments from the Framingham Heart Study (FHS) and Atherosclerosis Risk in Communities (ARIC) study. Am Heart J 2015; 169:53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Magnani JW, Lopez FL, Soliman EZ, Maclehose RF, Crow RS, Alonso A. P wave indices, obesity, and the metabolic syndrome: the atherosclerosis risk in communities study. Obesity (Silver Spring) 2012; 20:666–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kamel H, Soliman EZ, Heckbert SR, Kronmal RA, Longstreth WT, Jr, Nazarian S, Okin PM. P-wave morphology and the risk of incident ischemic stroke in the Multi-Ethnic Study of Atherosclerosis. Stroke 2014; 45:2786–2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kamel H, Bartz TM, Longstreth WT, Jr, Okin PM, Thacker EL, Patton KK, Stein PK, Gottesman RF, Heckbert SR, Kronmal RA, Elkind MS, Soliman EZ. Association between left atrial abnormality on ECG and vascular brain injury on MRI in the Cardiovascular Health Study. Stroke 2015; 46:711–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Buse JB, Bigger JT, Byington RP, Cooper LS, Cushman WC, Friedewald WT, Genuth S, Gerstein HC, Ginsberg HN, Goff DC, Jr, Grimm RH, Jr, Margolis KL, Probstfield JL, Simons-Morton DG, Sullivan MD. Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial: design and methods. Am J Cardiol 2007; 99:21i–33i. [DOI] [PubMed] [Google Scholar]
- 18.Gerstein HC, Miller ME, Byington RP, Goff DC, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH, Probstfield JL, Simons-Morton DG, Friedewald WT. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358: 2545–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ginsberg HN, Elam MB, Lovato LC, Crouse JR III, Leiter LA, Linz P, Friedewald WT, Buse JB, Gerstein HC, Probstfield J, Grimm RH, Ismail-Beigi F, Bigger JT, Goff DC Jr, Cushman WC, Simons-Morton DG, Byington RP. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med 2010; 362:1563–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cushman WC, Evans GW, Byington RP, Goff DC Jr, Grimm RH Jr, Cutler JA, Simons-Morton DG, Basile JN, Corson MA, Probstfield JL, Katz L, Peterson KA, Friedewald WT, Buse JB, Bigger JT, Gerstein HC, Ismail-Beigi F. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362:1575–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rautaharju PM, Park LP, Chaitman BR, Rautaharju F, Zhang ZM. The Novacode criteria for classification of ECG abnormalities and their clinically significant progression and regression. J Electrocardiol 1998; 31:157–187. [PubMed] [Google Scholar]
- 22. Benjamin EJ, Levy D, Vaziri SM, D’Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA 1994; 271:840–844. [PubMed] [Google Scholar]
- 23. Psaty BM, Manolio TA, Kuller LH, Kronmal RA, Cushman M, Fried LP, White R, Furberg CD, Rautaharju PM. Incidence of and risk factors for atrial fibrillation in older adults. Circulation 1997; 96:2455–2461. [DOI] [PubMed] [Google Scholar]
- 24. Krahn AD, Manfreda J, Tate RB, Mathewson FA, Cuddy TE. The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba Follow-Up Study. Am J Med 1995; 98:476–484. [DOI] [PubMed] [Google Scholar]
- 25. Liu G, Tamura A, Torigoe K, Kawano Y, Shinozaki K, Kotoku M, Kadota J. Abnormal P-wave terminal force in lead V1 is associated with cardiac death or hospitalization for heart failure in prior myocardial infarction. Heart Vessels 2013; 28:690–695. [DOI] [PubMed] [Google Scholar]
- 26. Tereshchenko LG, Shah AJ, Li Y, Soliman EZ. Electrocardiographic deep terminal negativity of the P wave in V1 and risk of mortality: the National Health and Nutrition Examination Survey III. J Cardiovasc Electrophysiol 2014; 25:1242–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tereshchenko LG, Henrikson CA, Sotoodehnia N, Arking DE, Agarwal SK, Siscovick DS, Post WS, Solomon SD, Coresh J, Josephson ME, Soliman EZ. Electrocardiographic deep terminal negativity of the P wave in V(1) and risk of sudden cardiac death: the Atherosclerosis Risk in Communities (ARIC) study. J Am Heart Assoc 2014; 3:e001387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, Smith SC, Jr, Svetkey LP, Taler SJ, Townsend RR, Wright JT, Jr, Narva AS, Ortiz E. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311:507–520. [DOI] [PubMed] [Google Scholar]
- 29. Emdin CA, Rahimi K, Neal B, Callender T, Perkovic V, Patel A. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA 2015; 313:603–615. [DOI] [PubMed] [Google Scholar]
- 30. Huxley RR, Alonso A, Lopez FL, Filion KB, Agarwal SK, Loehr LR, Soliman EZ, Pankow JS, Selvin E. Type 2 diabetes, glucose homeostasis and incident atrial fibrillation: the Atherosclerosis Risk in Communities study. Heart 2012; 98:133–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nichols GA, Reinier K, Chugh SS. Independent contribution of diabetes to increased prevalence and incidence of atrial fibrillation. Diabetes Care 2009; 32:1851–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.