Abstract
Introduction:
Previous studies suggest female smoking increases time-to-pregnancy (TTP), a couple-dependent reproductive outcome, while associations with male smoking are more ambiguous. Furthermore, despite small increases in smokeless tobacco use in the United States, no prior study has evaluated TTP among smokeless tobacco users.
Methods:
Using population-based sampling in 16 counties in Michigan and Texas, 501 couples discontinuing contraception to become pregnant were followed until positive pregnancy test or 12 months of trying. Participants were interviewed on lifetime and current cigarette, cigar, and chew/snuff (smokeless) use and provided blood samples for quantification of heavy metals and cotinine. Fecundability odds ratios (FORs) and 95% confidence intervals (95% CIs) were estimated, adjusted for demographics/lifestyle. FORs less than 1 reflect longer TTP.
Results:
Eleven percentage of females and 15% of males smoked cigarettes. Among men, 14% smoked cigars, 9% used snuff, and 2% used chew. Compared with never tobacco users, male (FOR: 0.41, 95% CI: 0.24, 0.68) and female (FOR: 0.53, 95% CI: 0.33, 0.85) smoking were individually associated with longer TTP; males’ smoking remained significant (FOR: 0.46, 95% CI: 0.27, 0.79) when modeling partners together. Cadmium levels were higher in smokers than smokeless tobacco and never users; adjusting for cadmium attenuated the cigarette–TTP association, particularly among women. TTP was shorter among smokeless tobacco users relative to smokers (FOR: 2.86, 95% CI: 1.47, 5.57).
Conclusions:
Compared with never users, smokeless tobacco did not alter TTP in our cohort; however, TTP was shorter compared with smokers. We observed longer TTP in male and female smokers; cadmium may partially contribute.
Implications:
Both partners’ preconception smoking contributed to longer TTP, highlighting the importance of both partners’ lifestyles in healthy reproduction and underscores the need for couple-based preconception guidance. The male’s contribution is a new finding. Higher cadmium levels may partially contribute to longer TTP in smokers, particularly among females. Though we do not observe longer TTP among a small sample of smokeless tobacco users compared with never tobacco users, we observe shorter TTP compared with smokers. Further work is needed to more thoroughly delineate the relationship between smokeless tobacco use and TTP and possible mechanisms of tobacco use’s effects on reproduction.
Introduction
Several types of tobacco products are available in the United States, including cigarettes, cigars, pipe and waterpipe tobacco, chewing tobacco, snuff, dip, and electronic cigarettes. The most recent available data from the United States show cigarettes are still the tobacco product most often consumed ever (43%) and currently (17%)1 among adults, though smokeless tobacco, including chew/snuff/dip, is the second most commonly used tobacco ever (14%) and currently (3%).2 While small decreases in cigarette smoking have been noted among working adults in the past decade, small increases in smokeless tobacco use have been observed, particularly among adults aged 18–44 years.3 In other parts of the world, smokeless tobacco use is relatively more common and may be more common than smoking, particularly among women.4,5
Given the morbidity and mortality associated with cigarette smoking, particularly lung cancer and respiratory diseases, some researchers have touted smokeless tobacco as a harm reduction tool for smokers who cannot cease nicotine use completely.6 If smokers, particularly those of reproductive age hopeful for future children, are to be properly counseled about the risks and benefits of smokeless tobacco for harm reduction, then the reproductive toxicity of smokeless tobacco needs to be disclosed. Unfortunately, there is a dearth of epidemiological research on the reproductive harms of smokeless tobacco particularly relative to cigarette use for which smokeless tobacco would be substituted.
Reproductive toxicity can be assessed by several endpoints measured in males, females, and couples. Couple-mediated endpoints provide the greatest insight into reproductive capacity as reproductive outcomes (eg, pregnancy, live birth) can be observed.7 Time-to-pregnancy (TTP), defined as the number of menstrual cycles or months of unprotected intercourse required to achieve pregnancy, is a sensitive couple-mediated endpoint for assessing reproductive toxicity and has been used to evaluate both persistent8 and short-lived chemicals.9,10 As TTP is couple-dependent, potentially toxic exposures should be measured in both male and female partners to potentially avoid erroneous conclusions based upon a single partner; ideally, biological samples would be obtained from both partners to measure toxicant concentrations (eg, blood metal concentrations) associated with more readily measured behaviors (eg, self-reported tobacco product use).8
Despite the need for couple-mediated endpoints in assessing reproductive toxicity, the existing literature on reproductive (non-obstetric) toxicity of smokeless tobacco comprises exclusively studies of semen quality, a male-specific endpoint. Most studies were conducted among men attending infertility clinics in India where the composition of chewing tobacco products is varied and largely different from the composition of chewing tobacco in Western countries. With that caveat, azoospermia (no sperm) is more common in tobacco chewers than nonusers11 and more common in heavier than lighter users.12 Compared with nonusers of tobacco, chewing tobacco users in India13,14 and snuff users in Sweden15 have lower sperm count, concentration, motility, and normal morphology among men seeking infertility treatment. In contrast, among a voluntary sample of 242 male Swedish military conscripts, snuff use was not associated with semen parameters or reproductive hormones.16
We are unaware of any studies on the relationship between smokeless tobacco use and TTP. Previous preconception cohort studies have evaluated the relationship between preconception cigarette use and TTP. Maternal preconception smoking is associated with longer TTP in most17–22 but not all23 studies. The relationship between paternal preconception smoking and TTP is more ambiguous, with one study reporting a nonsignificantly longer TTP,19 a second reporting a significantly shorter TTP,23 and two reporting no association with TTP20,22 among male smokers compared with nonsmokers. All of these studies used self-reported behavior; none were able to compare chemical concentrations in self-reported exposed and unexposed individuals to elucidate possible biological mechanisms.
Given the knowledge gap regarding reproductive outcomes associated with smokeless tobacco use and a need to further investigate the relationship between paternal preconception smoking and TTP, we assessed the relationship between couples’ preconception tobacco use (smokeless and combustible) and prospectively observed TTP. We also evaluated blood heavy metal and serum cotinine concentrations among various tobacco product users and nonusers to determine if specific chemicals may contribute to changes in TTP.
Methods
Study Population
The purpose of the Longitudinal Investigation of Fertility and the Environment (LIFE) Study was to examine the relationships between lifestyle, including environmental chemicals, and human fecundity. Using population-based sampling, 501 couples who were discontinuing contraception for purposes of becoming pregnant in 16 counties in Michigan and Texas, 2005–2009, enrolled in the LIFE Study. These geographic areas were chosen to ensure a range of environmental exposures among study participants. Participants were selected from marketing databases (Michigan) or state wildlife and fish registry (Texas). Further details on the study design are provided elsewhere.24 Following enrollment, couples were followed daily until a positive home pregnancy test or 12 months of trying. To be inclusive of the wide range of couples attempting pregnancy, only couples in which at least one partner had been told they would require medical assistance to become pregnant were excluded. Inclusion criteria were both partners spoke English or Spanish, were discontinuing contraception to attempt pregnancy or were off contraception not more than 2 months, male was aged 18 years and older, female was aged 18–40 years, had menstrual cycle length of 21–42 days, and had not received injectable contraception in the past year.
Exposure Ascertainment
At enrollment, each partner was administered a questionnaire by trained study personnel on their lifetime and current use of several tobacco products including cigarettes, cigars, snuff, and chewing tobacco. Specifically, for cigarettes, participants were asked if they had smoked more than 100 cigarettes (five packs) during their lifetime. If yes, they were asked if they had smoked in the past 12 months, and if so, whether they smoked now. For other tobacco products, participants were asked if they had smoked a pipe, smoked cigars, used sniff, or used chewing tobacco at least 20 times. If yes, for each product participants were asked if they currently used the product. For the main analysis, we are interested in those participants who never used any tobacco product (never users) and those who were currently using specific tobacco products regardless of past use of other tobacco products. As very few women reported using a tobacco product other than cigarettes at enrollment, women are categorized as never users or current cigarette users (regardless of intensity). Any women not meeting criteria for these categories were assigned as “other” and their estimates are not reported due to their exposure heterogeneity though the women were retained in models. For men, categories of current tobacco use (regardless of intensity) include never users, exclusively using cigarettes, exclusively using cigars, exclusively using smokeless tobacco (chew/snuff), or using both cigarettes and smokeless tobacco at enrollment. Any men not meeting criteria for these categories were assigned “other” and their estimates are not reported due to their exposure heterogeneity though the men were retained in models.
Outcome Ascertainment
TTP was prospectively observed and measured in menstrual cycles. At enrollment, women were provided with and instructed in the use of the digital ClearBlue Easy fertility monitor, which measures urinary estrone-3-glucuronide and luteinizing hormone to provide visual prompts of high and peak fertility days to facilitate intercourse timed to impending ovulation. On the first day of each cycle (first day of menses), women pressed the “m” button on the monitor. Women also recorded intensity of any vaginal bleeding in a daily diary. Together, the data from the fertility monitor and the daily diaries were used to define menstrual cycles used for determining TTP.24 Women were provided with highly sensitive (25 IU/L) home pregnancy tests25 that provide digital readouts of “pregnant” and “not pregnant” to ensure accuracy reading results26 and were instructed to begin testing on the day of expected menses. A single positive pregnancy test denoted an human chorionic gonadotropin pregnancy.
Covariate Ascertainment
During the enrollment interview, each partner reported their age, race/ethnicity, educational attainment, income, and alcohol and caffeine use. Height and weight were measured by study staff using a standardized protocol for calculation of body mass index. Missing data for demographic and lifestyle variables (n = 28) were singly imputed using the median or mode value for participants with the same tobacco exposure. At enrollment, each partner provided a blood specimen for quantification of blood metals (cadmium, lead) and serum cotinine. Participants missing values for metal or cotinine concentrations (n = 28) had values multiply imputed.
Statistical Analysis
Descriptive analyses of participants’ characteristics by tobacco use were evaluated using chi-square test for categorical and Kruskal–Wallis test for continuous variables. Geometric means and 95% confidence intervals (95% CIs) of chemicals and metals were computed and compared across tobacco use types using nonparametric tests.
Cox proportional odds models for discrete survival data27 accounting for left truncation (to account for time off contraception before enrollment) and right censoring (to account for loss to follow-up or end of study) were used to estimate fecundability odds ratios (FORs), the relative odds of achieving pregnancy in a cycle conditional on not becoming pregnant in the previous cycle, where FOR less than 1 indicates a longer TTP.28 Robust standard errors were used to estimate 95% CIs. Associations in which 95% CI exclude 1.00 are considered statistically significant.
For both partners, cigarette users were compared with never users for each partner modeled separately and together. We first ran models with each partner’s cigarette use modeled separately from the other partner’s cigarette use (separate models) and then ran models with both partners’ cigarette use modeled together, adjusting for the other partner’s cigarette use (together models). For males only, models were also run for each tobacco type (cigarettes, cigars, smokeless, cigarettes and smokeless) compared with never users. All models were adjusted for confounders selected a priori and included demographic and lifestyle factors listed above. In models run for each partner’s tobacco use, only confounding factors of the relevant partner (male/female) were included while in models run with both partners’ tobacco use, both partners’ confounding factors were included. Due to concerns about collinearity with respect to partners’ ages, the mean age of the partners and the difference of their ages were included in models where both partners were modeled together.
Some heavy metals are commonly found in tobacco products as metals are absorbed by the tobacco plant from the soil due to application of agricultural pesticides or from polluted air or water.29,30 Cadmium and lead, two heavy metals, are also associated with longer TTP in the LIFE Study.31,32 Thus, we hypothesized that these metals may be partially responsible for any observed association between tobacco use and TTP (potential mediators). Metals with significantly different blood level concentrations across tobacco types used were included as covariates in additional multivariable models to determine if any observed relationship between tobacco use and TTP was attenuated.
We also wanted to evaluate serum cotinine, a nicotine metabolite, as a measure of nicotine exposure, rather than self-reported tobacco use, to potentially better reflect the relationship between tobacco use and TTP. Thus, we used various cotinine cutoffs33,34 as exposure variables in sensitivity models. Effects from models using multiply imputed values for metals or cotinine were estimated using Rubin’s combining rules in SAS (PROC MIANALYZE).
Results
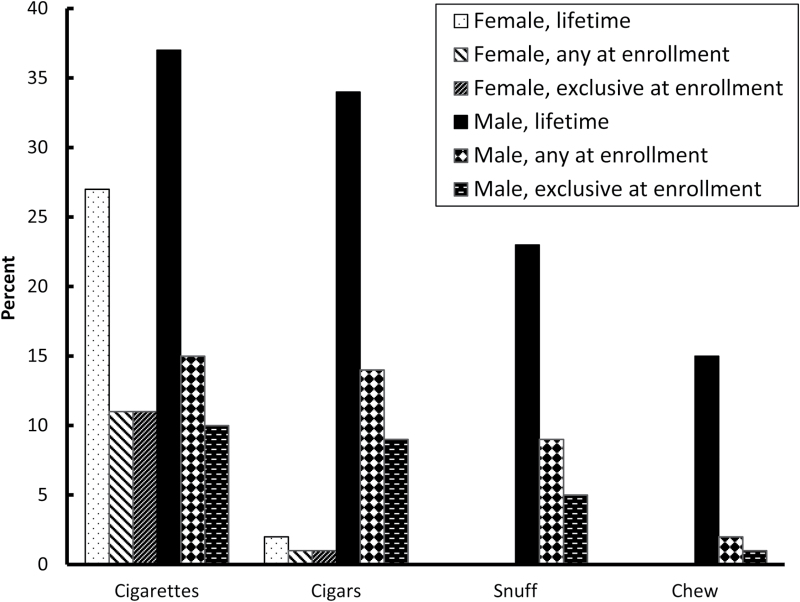
Lifetime tobacco use is common among couples attempting to conceive. Specifically, among male partners, self-reported lifetime use of tobacco products was 37% for cigarettes, 34% for cigars, 23% for snuff, and 15% for chewing tobacco (Figure 1). Among female partners, 27% reported lifetime cigarette and 2% reported lifetime cigar use.
Figure 1.
Prevalence of tobacco use among couples in the Longitudinal Investigation of Fertility and the Environment Study (n = 501).
Tobacco use by both partners persisted into the beginning of the pregnancy attempt with 15% of male and 11% of female partners reporting any current cigarette use (Figure 1). Ten percentage and 11% of male and female partners, respectively, exclusively used cigarettes. Among men who smoked, 44% had a female partner who also smoked (Supplementary Table 1).
Substantial numbers of male partners used other combustible and/or smokeless tobacco products at the start of the pregnancy attempt (Figure 1). Among male partners, any and exclusive cigar use was 14% and 9%, respectively, any and exclusive snuff use was 9% and 5%, respectively, and any and exclusive chewing tobacco use was 2% and 1%, respectively. Two percent of males used cigarettes along with snuff or chewing tobacco.
Significant differences by tobacco use at enrollment were noted in male partners for education, income, alcohol use, and caffeine use (Table 1) and in female partners for income, alcohol use, caffeine use, age, and body mass index (Table 2).
Table 1.
Characteristics Associated With Exclusive Enrollment Use and Never Use of Tobacco Products Among Males (n = 501)
| Never used (n = 208) | Cigarettes (n = 48) | Cigars (n = 46) | Snuff/chew (n = 28) | Cigarettes + snuff/ chew (n = 12) | Other pattern (n = 159) | |
|---|---|---|---|---|---|---|
| n (%) | ||||||
| Race/ethnicity | ||||||
| Non-Hispanic white | 166 (80) | 31 (65) | 34 (74) | 25 (96) | 11 (92) | 127 (80) |
| Non-Hispanic black | 12 (6) | 4 (8) | 3 (7) | 0 (0) | 0 (0) | 4 (3) |
| Hispanic | 15 (7) | 9 (19) | 5 (11) | 0 (0) | 0 (0) | 16 (10) |
| Other | 14 (7) | 4 (8) | 4 (9) | 1 (4) | 1 (8) | 12 (8) |
| Education* | ||||||
| High school or below | 6 (3) | 15 (31) | 2 (4) | 4 (15) | 5 (42) | 12 (8) |
| Some college or above | 199 (97) | 33 (69) | 44 (96) | 22 (85) | 7 (58) | 147 (92) |
| Annual income* | ||||||
| <$50 000 | 29 (14) | 18 (38) | 2 (4) | 3 (12) | 2 (17) | 23 (14) |
| $50–100 000 | 96 (47) | 20 (43) | 25 (54) | 13 (50) | 7 (58) | 76 (48) |
| >$100 000 | 80 (39) | 9 (19) | 19 (41) | 10 (38) | 3 (25) | 56 (35) |
| Alcohol use past year* | ||||||
| Noa | 48 (23) | 4 (8) | 1 (2) | 3 (11) | 0 (0) | 17 (11) |
| Yes, less than weekly | 67 (32) | 17 (35) | 8 (17) | 7 (25) | 4 (33) | 48 (30) |
| Yes, weekly or more | 93 (45) | 27 (56) | 37 (80) | 18 (64) | 8 (67) | 94 (59) |
| Caffeine use, daily* | ||||||
| <4 cups | 174 (84) | 28 (58) | 35 (76) | 19 (68) | 3 (25) | 107 (67) |
| ≥4 cups | 34 (16) | 20 (42) | 11 (24) | 9 (32) | 9 (75) | 52 (33) |
| Mean (SD) | ||||||
| Age at enrollment | 31.1 (4.5) | 32.6 (6.5) | 31.8 (5.6) | 32.6 (4.8) | 32.9 (2.9) | 32.2 (4.7) |
| BMI at enrollment | 29.2 (5.6) | 31.1 (5.6) | 30.6 (5.2) | 29.6 (4.1) | 29.4 (4.9) | 30.1 (5.9) |
BMI = body mass index.
aConsumed less than 12 alcoholic drinks in the year prior to enrollment.
*P value less than .05 (chi-square test for categorical and Kruskal–Wallis test for continuous variables).
Table 2.
Characteristics Associated With Exclusive Enrollment Use and Never Use of Tobacco Products Among Females (n = 501)
| Never used (n = 357) | Cigarettes (n = 55) | Other pattern (n = 89) | |
|---|---|---|---|
| n (%) | |||
| Race/ethnicity | |||
| Non-Hispanic white | 271 (76) | 43 (78) | 79 (89) |
| Non-Hispanic black | 20 (6) | 4 (7) | 0 (0) |
| Hispanic | 41 (12) | 5 (9) | 4 (5) |
| Other | 23 (6) | 3 (5) | 5 (6) |
| Education | |||
| High school or below | 21 (6) | 5 (9) | 1 (1) |
| Some college or above | 334 (94) | 50 (91) | 86 (97) |
| Annual income* | |||
| <$50 000 | 56 (16) | 20 (36) | 13 (15) |
| $50–100 000 | 162 (46) | 23 (42) | 49 (55) |
| >$100 000 | 132 (38) | 12 (22) | 24 (27) |
| Alcohol use past yeara,* | |||
| No | 102 (29) | 10 (18) | 15 (17) |
| Yes, less than weekly | 156 (44) | 22 (40) | 40 (45) |
| Yes, weekly or more | 97 (27) | 23 (42) | 34 (38) |
| Caffeine use, daily* | |||
| <4 cups | 328 (92) | 35 (65) | 80 (90) |
| ≥4 cups | 28 (8) | 19 (35) | 8 (9) |
| Mean (SD) | |||
| Age at enrollment* | 29.7 (4.2) | 30.7 (3.9) | 30.8 (3.9) |
| BMI at enrollment* | 27.0 (7.1) | 30.1 (8.1) | 28.3 (7.4) |
BMI = body mass index.
aConsumed less than 12 alcoholic drinks in the year prior to enrollment.
*P value less than .05 (chi-square test for categorical and Kruskal–Wallis test for continuous variables).
Among male partners, serum cotinine concentrations were significantly higher in cigarette smokers and smokeless tobacco users than among never users, though not significantly different between cigarette and smokeless tobacco users (Supplementary Table 2a). While both cigarette and smokeless tobacco users had higher lead concentrations than never users, the difference between male tobacco users was not different. Cigarette users had significantly higher concentrations of blood cadmium than both never users and smokeless tobacco users. Among female partners, cigarette smokers had higher cotinine, lead, and cadmium levels than never users (Supplementary Table 2b).
Among tobacco types used by male partners, in models adjusted for demographics and lifestyle factors, only cigarette use was associated with significantly longer TTP compared with never users among male partners (adjusted FOR [aFOR]: 0.41, 95% CI: 0.24, 0.68; Table 3). After adjustment for cadmium, the result was slightly attenuated (aFOR: 0.44, 95% CI: 0.24, 0.79; Table 4). For female partners, use of cigarettes was also associated with significantly longer TTP after adjustment for demographic and lifestyle factors (aFOR: 0.53, 95% CI: 0.33, 0.85; Table 4) compared with never tobacco users. This association was attenuated after adjusting for blood cadmium levels and was no longer significant (aFOR: 0.75, 95% CI: 0.42, 1.32; Table 4).
Table 3.
FORs for Enrollment Determined Use Compared With Never Use of Tobacco Products Among Male and Female Partners (n = 501)a
| FOR | 95% CI | aFORb | 95% CI | aFORc | 95% CI | |
|---|---|---|---|---|---|---|
| Male partners | ||||||
| Cigarettes only | 0.35 | 0.21, 0.56 | 0.41 | 0.24, 0.68 | 0.44 | 0.24, 0.79 |
| Cigars only | 0.75 | 0.49, 1.13 | 0.70 | 0.45, 1.08 | 0.70 | 0.45, 1.09 |
| Snuff and/or chew only | 1.09 | 0.67, 1.79 | 1.17 | 0.70, 1.95 | 1.17 | 0.70, 1.95 |
| Cigarettes and snuff/chew | 0.73 | 0.32, 1.67 | 0.76 | 0.32, 1.82 | 0.79 | 0.32, 1.96 |
| Female partners | ||||||
| Cigarettes only | 0.44 | 0.28, 0.70 | 0.53 | 0.33, 0.85 | 0.75 | 0.42, 1.32 |
BMI = body mass index; CI = confidence interval; aFOR = adjusted fecundability odds ratio.
aModels are stratified by partner sex.
bAdjusted for race/ethnicity, education, income, age, alcohol use, caffeine use, BMI in each partner.
cAdjusted for race/ethnicity, education, income, age, alcohol use, caffeine use, BMI, blood cadmium in each partner.
Table 4.
FORs for Exclusive Use of Cigarettes at Enrollment Compared With Never Tobacco Use in Both Partners Modeled Separately and Together (n = 501)
| Females | Males | |||
|---|---|---|---|---|
| FOR | 95% CI | FOR | 95% CI | |
| Separatelya | ||||
| Unadjusted | 0.44 | 0.28, 0.70 | 0.35 | 0.21, 0.56 |
| Adjustedb | 0.53 | 0.33, 0.85 | 0.41 | 0.24, 0.68 |
| Adjustedc | 0.75 | 0.42, 1.32 | 0.44 | 0.24, 0.79 |
| Togetherd | ||||
| Unadjusted | 0.56 | 0.34, 0.91 | 0.42 | 0.25, 0.70 |
| Adjustede | 0.63 | 0.37, 1.05 | 0.46 | 0.27, 0.79 |
| Adjustedf | 0.81 | 0.44, 1.48 | 0.50 | 0.27, 0.91 |
BMI = body mass index; CI = confidence interval; aFOR = adjusted fecundability odds ratio.
aEach partner is modeled alone (eg, sex stratified).
bAdjusted for race/ethnicity, education, income, age, alcohol use, caffeine use, BMI in each partner.
cAdjusted for race/ethnicity, education, income, age, alcohol use, caffeine use, BMI, blood cadmium in each partner.
dBoth partners are modeled together (eg, accounting for the other partner’s tobacco use and sociodemographic characteristics).
eAdjusted for both partners’ race/ethnicity, education, income, alcohol use, caffeine use, BMI, couple’s mean age, difference in partners’ ages.
fAdjusted for both partners’ race/ethnicity, education, income, alcohol, caffeine, BMI, blood cadmium, couple’s mean age, difference in partners’ ages.
When both partners’ tobacco use were conditionally modeled together, male smoking compared with never use was associated with a significantly longer TTP (aFOR: 0.46, 95% CI: 0.27, 0.79) and female smoking was associated with a nonsignificantly longer TTP (aFOR: 0.63, 95% CI: 0.37, 1.05; Table 4). Results were attenuated after adjustment for cadmium for both male (aFOR: 0.50, 95% CI: 0.27, 0.91) and female (aFOR: 0.81, 95% CI: 0.44, 1.48) partners.
When we examined serum cotinine as an exposure variable instead of self-reported tobacco use, the association between female cotinine levels and TTP was stronger than for male cotinine levels whether partners’ levels were modeled separately or together (Supplementary Table 3, a–c). Only female cotinine greater than 9 ng/mL modeled separately remained significant after adjusting for demographic and lifestyle factors, and both male and female associations were further attenuated after additionally adjusting for blood cadmium levels. In a post hoc analysis, we also examined the association of cotinine levels and TTP among male cigarette and smokeless tobacco users, separately. We found that cotinine was not associated with TTP among cigarette users; however, among smokeless tobacco users, a cutoff above 10ng/mL was associated with longer TTP (aFOR: 0.09, 95% CI: 0.01, 0.95) though cutoff above 200ng/mL was not (aFOR: 0.54, 95% CI: 0.14, 2.08). A shorter TTP was observed for smokeless tobacco users compared with cigarette users (aFOR: 2.86, 95% CI: 1.47, 5.57). When restricting to those men with cotinine levels greater than 10ng/mL, a shorter TTP in smokeless tobacco compared with cigarette users remained (aFOR: 3.66, 95% CI: 1.60, 8.40).
Discussion
Among couples attempting pregnancy in the LIFE Study, 15% of men and 11% of women smoked cigarettes and 11% of men used smokeless tobacco alone or in combination with cigarettes. Smoking prevalence in the LIFE Study was less than reported among recently delivered mothers35 and reproductive aged men,3 but smokeless tobacco use is greater.3 To our knowledge, this is the first study to investigate smokeless tobacco use and TTP. In our sample, we did not observe longer TTP in couples where the male partner reported using smokeless tobacco at the beginning of the pregnancy attempt compared with never tobacco users. However, we observed shorter TTP among smokeless tobacco users compared with smokers.
Among couples where one or both partners smoke, we observed longer TTP in couples in which the female partner smoked when partners were modeled separately, consistent with previous work. A new finding is the longer observed TTP in couples in which the male partner smoked. When modeled together and controlling for the other partner’s tobacco use and demographic and lifestyle factors, male smoking is associated with a longer TTP.
When modeling serum cotinine as the exposure instead of self-reported tobacco use, the association with longer TTP appears stronger for female partners than for male partners. This likely reflects the source of cotinine exposure in both partners. For women, the main source of nicotine is from smoking cigarettes whereas the source of nicotine exposure is more varied among men.
Nicotine is unlikely to be the culprit behind delayed pregnancy in cigarette smokers as serum cotinine levels in male smokeless tobacco users, who did not have longer TTP, were as high as or higher than male cigarette smokers, who did have longer TTP, in this study. When investigating the relationship between cotinine levels and TTP among male cigarette and smokeless tobacco users, no relationship is observed among cigarette users. Longer TTP is observed for cotinine levels greater than 10ng/mL for smokeless tobacco users, though this relationship does not persist at the highest cotinine levels (>200ng/mL). Furthermore, among cigarette and smokeless tobacco users with cotinine levels greater than 10ng/mL, smokeless tobacco users had shorter TTP than cigarette users. Blood cadmium concentrations are significantly different between male smokeless tobacco and cigarette users; cadmium levels are also significantly higher in both female and male smokers than in never users. Adjusting for blood cadmium attenuates the relationship between smoking and TTP for females and males. This suggests that cadmium may be responsible for some of the observed association between cigarette smoking and longer TTP with greater attenuation of the association observed in females.
Studies on cadmium levels among infertility seeking populations show adverse effects on achieving pregnancy. Higher concentrations of cadmium in semen were associated with fewer pregnancies after infertility treatment,36 and higher blood cadmium concentrations in women were associated with decreases in clinical and biochemical pregnancies.37 Among women receiving infertility treatment for endometriosis and tubal infertility, higher cadmium concentrations in follicular fluid were associated with decreased pregnancy rates.38
Cadmium may act in several ways to impair reproductive capacity in females and males. Cadmium contributes to oxidative stress in the body by inducing the formation of reactive oxygen species,39 and higher cadmium levels have been associated with higher levels of reactive oxygen species in follicular fluid in women undergoing infertility treatment.38 Cadmium may also disrupt the endocrine system. In premenopausal women, higher blood cadmium levels were associated with decreased follicle-stimulating hormone.40 Higher urinary cadmium concentrations among men were associated with higher levels of total and free testosterone and estradiol before adjusting for smoking.41 In addition to potential effects of cadmium on the reproductive system, cigarette smoke contains more than 5000 other chemicals, including several which have been characterized as having adverse effects on reproduction,42 and these need to be explored further in relation to couples’ capacity to conceive.
While chewing tobacco use reportedly reduces semen quality among heavy users in India, it is unclear whether smokeless tobacco use is harmful in men planning a pregnancy in Western countries, where constituents of smokeless tobacco are different. One small study of Swedish men seeking infertility treatment found reduced semen quality in snuff users,15 though another study of Swedish military conscripts did not show an association between snuff use and semen parameters.16 We did not observe an effect of male smokeless tobacco use on TTP compared with never users of tobacco; however, we did observe male smokeless tobacco users had a shorter TTP compared with cigarette users.
Chief among the study’s limitations was its small numbers of smokeless tobacco users. Only 28 men used smokeless tobacco exclusively at enrollment, so we may be underpowered to detect a difference and cannot conclude that smokeless tobacco has no effect on TTP. Furthermore, women in our sample did not use smokeless tobacco so we could not evaluate the contribution of female smokeless tobacco use on TTP. Another limitation was that we did not have data on electronic cigarette use as this study was conducted before electronic cigarettes came into widespread use in the United States. Twenty percent (n = 99) of the study population was lost to follow-up. Because men and women lost to follow-up were more likely to be current smokers than couples completing the study, our findings may underestimate the association between cigarette use and TTP. We did use all available data (eg, all time that was observed) in our Cox models including data provided from couples lost to follow-up; however, since we observed cigarette smokers have longer TTP, our findings may have been stronger if we had observed all trying time for those lost to follow-up.
Alongside these limitations were several strengths. To our knowledge, this is the first study to evaluate smokeless tobacco use in relation to the couple-dependent reproductive outcome, TTP. By interviewing partners on their use of several types of tobacco products during their lifetime and at the start of the pregnancy attempt, we were able to evaluate the relationships between several types of tobacco products at different times and TTP. By interviewing couples at the start of their pregnancy attempt, we also minimized recall bias associated with reporting exposures after the outcome is known. Preconception recruitment of couples allowed us to prospectively observe trying time for pregnancy without relying upon retrospective report of trying time, which has been shown to be accurate in short43 but not long44 term recall thereby minimizing measurement error of the outcome. We were also able to investigate possible biological mechanisms underlying the observed associations using measured cotinine and heavy metal concentrations from biological samples.
Overall, the contribution of the male partner’s smoking habit to longer TTP highlights the importance of both partners’ lifestyles in healthy reproduction. The Centers for Disease Control and Prevention’s preconception guidance is now targeted to both partners and urges men and women to quit smoking.45,46 In light of the call for smokeless tobacco use as a harm reduction tool for smokers, increasing smokeless tobacco use among young adults in the United States, and the relatively greater use of smokeless tobacco in other countries, further investigations into the potential reproductive toxicity of smokeless tobacco are warranted. Scandinavian populations may be well suited for future work on this topic as smokeless tobacco use is common,15 even among females,47 and surveillance systems for reproduction (eg, semen analysis among military conscripts and national birth and health registers) are already in place. Additionally, elucidating the biological mechanisms by which tobacco products may interfere with couples’ biological capacity to conceive will provide more information of the potential harms of tobacco use, both combustible and noncombustible, in couples trying to conceive.
Supplementary Material
Supplementary Tables 1–3 can be found online at http://www.ntr.oxfordjournals.org
Funding
This work was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) (contracts N01-HD-3-3355, N01-HD-3-3356, and N01-HD-3-3358, HHSN27500001).
Declaration of Interests
None declared.
Supplementary Material
Acknowledgments
This work was presented in part at the 2015 Society for Epidemiologic Research Annual Meeting, Denver, CO, and the 2015 National Conference on Health Statistics, North Bethesda, MD.
References
- 1. Jamal A, Homa DM, O’Connor E, et al. Current cigarette smoking among adults–United States, 2005–2014. MMWR Morb Mortal Wkly Rep. 2015;64(44):1233–1240. www.cdc.gov/mmwr/preview/mmwrhtml/mm6444a2.htm Accessed January 14, 2016. [DOI] [PubMed] [Google Scholar]
- 2. Agaku IT, King BA, Husten CG, et al. Tobacco product use among adults—United States, 2012–2013. MMWR Morb Mortal Wkly Rep. 2014;63(25):542–547. www.ncbi.nlm.nih.gov/pubmed/24964880 Accessed September 29, 2015. [PMC free article] [PubMed] [Google Scholar]
- 3. Mazurek JM, Syamlal G, King BA, Castellan RM. Smokeless tobacco use among working adults—United States, 2005 and 2010. MMWR Morb Mortal Wkly Rep. 2014;63(22):477–482. www.ncbi.nlm.nih.gov/pubmed/24898164 Accessed September 29, 2015. [PMC free article] [PubMed] [Google Scholar]
- 4. Sreeramareddy CT, Pradhan PMS, Mir IA, Sin S. Smoking and smokeless tobacco use in nine South and Southeast Asian countries: prevalence estimates and social determinants from Demographic and Health surveys. Popul Health Metr. 2014;12:22. doi:10.1186/s12963-014-0022-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sreeramareddy CT, Pradhan PMS, Sin S. Prevalence, distribution and social determinants of tobacco use in 30 sub-Saharan African countries. BMC Med. 2014;12(1):243. doi:10.1186/s12916-014-0243-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kozlowski LT. Effect of smokeless tobacco product marketing and use on population harm from tobacco use: policy perspective for tobacco-risk reduction. Am J Prev Med. 2007;33(6):S379–S386. doi:10.1016/j.amepre.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 7. US Environmental Protection Agency. Guidelines for Reproductive Toxicity Risk Assessment 1996. www.epa.gov/sites/production/files/2014-11/documents/guidelines_repro_toxicity.pdf Accessed January 14, 2016. [PubMed]
- 8. Louis GMB. Persistent environmental pollutants and couple fecundity: an overview. Reproduction. 2014;147(4):R97–R104. doi:10.1289/ehp.1205301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Louis GMB, Chen Z, Kim S, Sapra KJ, Bae J, Kannan K. Urinary concentrations of benzophenone-type ultraviolet light filters and semen quality. Fertil Steril. 2015;104(4):989–996. doi:10.1016/j.fertnstert.2015.07.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Louis GMB, Sundaram R, Sweeney AM, Schisterman EF, Maisog J, Kannan K. Urinary bisphenol a, phthalates, and couple fecundity: the Longitudinal Investigation of Fertility and the Environment (LIFE) Study. Fertil Steril. 2014;101(5):1359–1366. doi:10.1016/j.fertnstert.2014.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dikshit RK, Buch JG, Mansuri SM. Effect of tobacco consumption on semen quality of a population of hypofertile males. Fertil Steril. 1987;48(2):334–336. www.ncbi.nlm.nih.gov/pubmed/3609347 Accessed September 29, 2015. [DOI] [PubMed] [Google Scholar]
- 12. Said TM, Ranga G, Agarwal A. Relationship between semen quality and tobacco chewing in men undergoing infertility evaluation. Fertil Steril. 2005;84(3):649–653. doi:10.1016/j.fertnstert.2005.03.052. [DOI] [PubMed] [Google Scholar]
- 13. Banerjee A, Pakrashi A, Chatterjee S, Ghosh S, Dutta SK. Semen characteristics of tobacco users in India. Arch Androl. 1993;30(1):35–40. doi:10.3109/01485019308988366. [DOI] [PubMed] [Google Scholar]
- 14. Sunanda P, Panda B, Dash C, Ray PK, Padhy RN, Routray P. Prevalence of abnormal spermatozoa in tobacco chewing sub-fertile males. J Hum Reprod Sci. 2014;7(2):136. doi:10.4103/0974-1208.138873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Parn T, Grau Ruiz R, Kunovac Kallak T, et al. Physical activity, fatness, educational level and snuff consumption as determinants of semen quality: findings of the ActiART study. Reprod Biomed Online. 2015;31(1):108–119. doi:10.1016/j.rbmo.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 16. Richthoff J, Elzanaty S, Rylander L, Hagmar L, Giwercman A. Association between tobacco exposure and reproductive parameters in adolescent males. Int J Androl. 2008;31(1):31–39. http://onlinelibrary.wiley.com/doi/10.1111/j.1365-2605.2007.00752.x/epdf Accessed September 29, 2015. [DOI] [PubMed] [Google Scholar]
- 17. Buck Louis GM, Dmochowski J, Lynch C, Kostyniak P, McGuinness BM, Vena JE. Polychlorinated biphenyl serum concentrations, lifestyle and time-to-pregnancy. Hum Reprod. 2009;24(2):451–458. doi:10.1093/humrep/den373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hakim RB, Gray RH, Zacur H. Alcohol and caffeine consumption and decreased fertility. Fertil Steril. 1998;70(4):632–637. doi:10.1016/S0015-0282(98)00257-X. [DOI] [PubMed] [Google Scholar]
- 19. Jensen TK, Henriksen TB, Hjollund NH, et al. Adult and prenatal exposures to tobacco smoke as risk indicators of fertility among 430 Danish couples. Am J Epidemiol. 1998;148(10):992–997. doi:10.1093/oxfordjournals.aje.a009576. [DOI] [PubMed] [Google Scholar]
- 20. Radin RG, Hatch EE, Rothman KJ, et al. Active and passive smoking and fecundability in Danish pregnancy planners. Fertil Steril. 2014;102(1): 183–191. doi:10.1016/j.fertnstert.2014.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Weinberg CR, Wilcox AJ, Baird DD. Reduced fecundability in women with prenatal exposure to cigarette smoking. Am J Epidemiol. 1989;129(5):1072–1078. www.ncbi.nlm.nih.gov/pubmed/2705427 Accessed September 29, 2015. [DOI] [PubMed] [Google Scholar]
- 22. De Mouzon J, Spira A, Schwartz D. A prospective study of the relation between smoking and fertility. Int J Epidemiol. 1988;17(2):378–384. doi:10.1093/ije/17.2.378. [DOI] [PubMed] [Google Scholar]
- 23. Florack EI, Zielhuis GA, Rolland R. Cigarette smoking, alcohol consumption, and caffeine intake and fecundability. Prev Med. 1994;23(2): 175–180. doi:10.1006/pmed.1994.1024. [DOI] [PubMed] [Google Scholar]
- 24. Buck Louis GM, Schisterman EF, Sweeney AM, et al. Designing prospective cohort studies for assessing reproductive and developmental toxicity during sensitive windows of human reproduction and development—the LIFE Study. Paediatr Perinat Epidemiol. 2011;25(5):413–424. doi:10.1111/j.1365-3016.2011.01205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cole LA. The utility of six over-the-counter (home) pregnancy tests. Clin Chem Lab Med. 2011;49(8):1317–1322. doi:10.1515/CCLM.2011.211. [DOI] [PubMed] [Google Scholar]
- 26. Johnson SR, Miro F, Barrett S, Ellis JE. Levels of urinary human chorionic gonadotrophin (hCG) following conception and variability of menstrual cycle length in a cohort of women attempting to conceive. Curr Med Res Opin. 2009;25(3):741–748. doi:10.1185/03007990902743935. [DOI] [PubMed] [Google Scholar]
- 27. Cox DR. Regression models and life tables (with discussion). J Royal Stat Soc. 1972;20:187–220. [Google Scholar]
- 28. Weinberg CR, Wilcox AJ. Methodologic issues in reproductive epidemiology. In: Rothman KJ, Greenland S, Lash TL, eds. Modern Epidemiology. Philadelphia, PA: Lippincott Williams & Wilkins; 2008:620–640. [Google Scholar]
- 29. Panta YM, Qian S, Cross CL, Cizdziel JV. Mercury content of whole cigarettes, cigars and chewing tobacco packets using pyrolysis atomic absorption spectrometry with gold amalgamation. J Anal Appl Pyrolysis. 2008;83(1):7–11. doi:10.1016/j.jaap.2008.05.006. [Google Scholar]
- 30. Gichner T, Patková Z, Száková J, Demnerová K. Toxicity and DNA damage in tobacco and potato plants growing on soil polluted with heavy metals. Ecotoxicol Environ Saf. 2006;65(3):420–426. doi:10.1016/j.ecoenv.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 31. Buck Louis GM, Sundaram R, Schisterman EF, et al. Heavy metals and couple fecundity, the LIFE Study. Chemosphere. 2012;87(11):1201–1207. doi:10.1016/j.chemosphere.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Buck Louis GM, Sundaram R, Schisterman EF, et al. Persistent environmental pollutants and couple fecundity: the LIFE Study. Environ Health Perspect. 2013;121(2):231–236. doi:10.1289/ehp.1205301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Benowitz NL, Bernert JT, Caraballo RS, Holiday DB, Wang J. Optimal serum cotinine levels for distinguishing cigarette smokers and nonsmokers within different racial/ethnic groups in the United States between 1999 and 2004. Am J Epidemiol. 2009;169(2):236–248. doi:10.1093/aje/kwn301. [DOI] [PubMed] [Google Scholar]
- 34. Wall MA, Johnson J, Jacob P, Benowitz NL. Cotinine in the serum, saliva, and urine of nonsmokers, passive smokers, and active smokers. Am J Public Health. 1988;78(6):699–701. doi:10.2105/AJPH.78.6.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tong VT, Dietz PM, Morrow B, et al. Trends in smoking before, during, and after pregnancy—pregnancy risk assessment monitoring system, United States, 40 sites, 2000–2010. MMWR Surveill Summ. 2013;62(6):1–19. www.cdc.gov/mmwr/preview/mmwrhtml/ss6206a1.htm?utm_source=rss&utm_medium=rss&utm_campaign=trends-in-smoking-before-during-and-after-pregnancy-pregnancy-risk-assessment-monitoring-system-united-states-40-sites-20002010 Accessed September 29, 2015. [PubMed] [Google Scholar]
- 36. Wu H-M, Lin-Tan D-T, Wang M-L, et al. Cadmium level in seminal plasma may affect the pregnancy rate for patients undergoing infertility evaluation and treatment. Reprod Toxicol. 2008;25(4):481–484. doi:10.1016/j.reprotox.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 37. Bloom MS, Fujimoto VY, Steuerwald AJ, Cheng G, Browne RW, Parsons PJ. Background exposure to toxic metals in women adversely influences pregnancy during in vitro fertilization (IVF). Reprod Toxicol. 2012;34(3):471–481. doi:10.1016/j.reprotox.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 38. Singh AK, Chattopadhyay R, Chakravarty B, Chaudhury K. Markers of oxidative stress in follicular fluid of women with endometriosis and tubal infertility undergoing IVF. Reprod Toxicol. 2013;42:116–124. doi:10.1016/j.reprotox.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 39. Nair AR, Degheselle O, Smeets K, Van Kerkhove E, Cuypers A. Cadmium-induced pathologies: where is the oxidative balance lost (or not)? Int J Mol Sci. 2013;14(3):6116–6143. doi:10.3390/ijms14036116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pollack AZ, Schisterman EF, Goldman LR, et al. Cadmium, lead, and mercury in relation to reproductive hormones and anovulation in premenopausal women. Environ Health Perspect. 2011;119(8):1156–1161. doi:10.1289/ehp.1003284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Menke A, Guallar E, Shiels MS, et al. The association of urinary cadmium with sex steroid hormone concentrations in a general population sample of US adult men. BMC Public Health. 2008;8:72. doi:10.1186/1471-2458-8-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Talhout R, Schulz T, Florek E, Van Benthem J, Wester P, Opperhuizen A. Hazardous compounds in tobacco smoke. Int J Environ Res Public Health. 2011;8(2):613–628. doi:10.3390/ijerph8020613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zielhuis GA, Hulscher ME, Florack EI. Validity and reliability of a questionnaire on fecundability. Int J Epidemiol. 1992;21(6):1151–1156. http://ije.oxfordjournals.org/content/21/6/1151.long Accessed January 14, 2016. [DOI] [PubMed] [Google Scholar]
- 44. Cooney MA, Louis GMB, Sundaram R, McGuiness BM, Lynch CD. Validity of self-reported time to pregnancy. Epidemiology. 2009;20(1):56. doi:10.1097/EDE.0b013e31818ef47e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Centers for Disease Control and Prevention. Preconception Health and Health Care: Information for Men 2015. www.cdc.gov/preconception/men.html Accessed September 16, 2015.
- 46. Centers for Disease Control and Prevention. Preconception Health and Health Care: Women 2015. http://www.cdc.gov/preconception/women.html Accessed September 16, 2015.
- 47. Lundqvist A, Johansson I, Wennberg A, et al. Reported dietary intake in early pregnant compared to non-pregnant women—a cross-sectional study. BMC Pregnancy Childbirth. 2014;14(1):373. doi:10.1186/s12884-014-0373-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.