Abstract
Introduction:
Pregnancy is a strong motivator to quit smoking, yet postpartum relapse rates are high. Growing evidence suggests a role of sex hormones in drug abuse behavior and given the precipitous drop in sex hormones at delivery, they may play a role in postpartum relapse. This pilot study evaluates the feasibility and potential role of exogenous progesterone in postpartum smoking relapse.
Methods:
This 12-week double-blind placebo-controlled randomized pilot trial randomized 46 abstinent postpartum women to active progesterone (PRO; 200mg twice a day) versus placebo (PBO) for 4 weeks. Participants were followed for relapse for 12 weeks. Main study outcomes include abstinence (point prevalence), feasibility (compliance per number of clinic visits attended, pill counts and Electronic Data Capture [EDC] completed) and self-reported acceptability. Safety was also measured by depressive symptom scores, adverse events, and breastfeeding.
Results:
Overall retention rate was 87% at week 12. At week 4, abstinence rates were 75% in the PRO group and 68.2% in the PBO group (p = .75). Medication adherence was 68% and clinic visit attendance was 80%, with no differences by randomization. Depressive symptom scores, adverse events, and breastfeeding did not vary by randomization.
Conclusions:
Although the study was not powered to evaluate abstinence rates, we did observe a higher prevalence of abstinence at week 4 in the PRO group. Further, exogenous progesterone was well tolerated and did not adversely affect depressive symptoms or breastfeeding. Thus, the results of this pilot study indicate further investigation into progesterone as a postpartum relapse prevention strategy is warranted.
Implications:
This innovative pilot trial determined the feasibility of delivering exogenous progesterone as a potential prevention of postpartum smoking relapse. We observed high retention and moderate adherence rates, as well as high acceptability among participants. Further, though not statistically significant, more women in the treatment group remained abstinent from smoking during follow-up. This project adds to the growing body of literature on the role of sex hormones in smoking relapse and also provides support for a fully powered clinical trial.
Introduction
Pregnancy is a strong motivator to quit smoking with 23% of women successfully able to quit while pregnant.1 Unfortunately over half relapse within 6 months of delivery and up to 90% relapse after 1 year.2 Postpartum relapse negatively affects not only maternal health, but also infant health through secondhand smoke exposure (SHS). SHS increases health risks ranging from early death (eg, Sudden Infant Death Syndrome) to acute (eg, pneumonia and ear infections) and chronic conditions (eg, asthma) in children.3 To date, effective interventions to prevent postpartum smoking relapse are lacking.
Many postpartum interventions are predominantly psychosocial, such as support groups,4 telephone sessions,5 and web-based interventions.6,7 Others are based on financial incentives8–10 and nicotine replacement therapy.11–13 While some of these interventions have improved cessation during pregnancy, they lack effectiveness for the prevention of postpartum relapse.
There is growing evidence suggesting sex hormones may play an important role in drug abuse behavior. This is strongly supported by preclinical work14,15 but clinical studies are mixed.16–20 In pregnancy, progesterone increases by more than 10 fold and certain estrogen levels increase by up to 1000 fold, then within the first few hours postpartum levels drop to near pre pregnancy levels.21 Sex hormone levels remain low until women begin naturally cycling and sex hormones become more balanced.22 The precipitous drop in progesterone, which is thought to be protective against drug abuse behavior, may play an important role in postpartum relapse. Unfortunately, few studies have explored the potential role of progesterone during this time. Therefore, this pilot double-blind placebo-controlled randomized trial evaluated the safety and feasibility of administering exogenous progesterone (PRO) compared to placebo (PBO) in the postpartum period for smoking relapse prevention. The specific aims were to (1) determine feasibility (compliance and acceptability) with study procedures and the safety of administering progesterone in the postpartum period (adverse events, depressive symptoms and breastfeeding) and (2) collect preliminary information on the role of progesterone (exogenous delivery and serum levels) on postpartum smoking relapse. We hypothesized there would be no significant differences between participants randomized to PRO versus PBO in terms of compliance and acceptability, as well as adverse events, depressive symptoms or breastfeeding. Although this pilot study was not fully powered to detect significant differences in smoking outcome, we also expected to see higher rates of smoking relapse at 4 and 12 weeks postpartum in PBO group compared to the PRO group. Finally, we expected that, regardless of randomization, women who relapse to smoking would have on average a lower level of serum progesterone.
Methods
This study was approved by the University of Minnesota’s Institutional Review Board before any procedures completed.
Participants
Recruitment occurred between June 2013 to February 2015 in Minneapolis/St Paul, Minnesota. Recruitment consisted of presentations at OB/GYN clinics, flyers and advertising on social media. Eligibility included: women 18–35 years old; stable physical/mental health; uncomplicated, single gestational pregnancy with established prenatal care; a signed letter from their OB physician confirming no pregnancy-related complications (ie, gestational diabetes, fetal growth restriction, pregnancy hypertension, history of ≥3 miscarriages, known congenital anomaly); self-report of no smoking for 4 weeks prior to study enrollment (at 32–35 weeks gestation), confirmed with carbon monoxide (CO) < 5ppm23; previous history of five cigarettes per day (CPD) for at least 6 of the past 12 months; motivated to remain abstinent after delivery (≥7 on 10-point Likert-type scale); willing to use nonhormonal birth control during the postpartum follow-up period of the study; English fluency; and ability to provide informed consent. Ineligibility items included: self-reported use of illicit drugs, nicotine replacement therapy, smoking cessation medications, or medications contraindicated to progesterone use (ie, drugs that may inhibit CYP3A4 functioning); contraindications to progesterone treatment (ie, history of thrombophlebitis, deep vein thrombosis, pulmonary embolus, clotting/bleeding disorders, stroke, heart disease, diabetes, liver dysfunction or disease, or an allergy to peanuts (progesterone capsules contain peanut oil).
Procedures
Participants were assessed via phone then invited to a screening visit between 32–35 weeks gestation where informed consent was obtained. Once enrolled, participants attended a baseline visit at 36 weeks gestation and completed several questionnaires, provided a blood sample for hormone measurement (progesterone), had their vital signs assessed (height, weight, blood pressure, heart rate) and were trained on Electronic Data Capture (EDC).
Between 36 weeks gestation and delivery, participants completed EDC daily using either their own or a study-supplied smartphone. Participants were prompted once daily to complete a short assessment at random times between 8 AM and 10 PM. Assessment items included daily Subjective State Scale24,25 for measurement of positive and negative affect, weekly Edinburgh Postnatal Depression Scale26,27 for assessment of depressive symptoms, and in the event of a smoking “slip,” the modified Cigarette Evaluation Questionnaire28 measuring the degree to which subjects experienced the reinforcing effects of smoking (results of the Subjective State Scale and modified Cigarette Evaluation Questionnaire analyses are forthcoming). Weekly phone calls were made to troubleshoot issues, further collect data regarding smoking status and offer brief relapse prevention counseling. Participants also used EDC to alert staff when they went into labor, delivered the baby and completed postpartum daily breastfeeding and vaginal bleeding logs.
At 4 days postpartum (week 0), participants were randomized (1:1) to either oral micronized progesterone (200mg twice daily) or placebo. This visit occurred in their homes, at the research clinic or a location of their choice. At this visit, participants completed questionnaires; provided a blood sample for progesterone and cotinine; did breath CO measurement, had vital signs assessed, were given study medication and instructed to start medication that evening at 8 PM. Participants were instructed to take the medication at 8 AM and 8 PM for the next 4 weeks. Four days postpartum was chosen since breast milk is established by that time.29 Administering progesterone too early can inhibit prolactin and thereby affect breast milk production.30 The readiness to start medication was assessed with a self-report breastfeeding questionnaire.31 If the participant reported delayed onset of lactation the medication start date was postponed. If a participant reported no plans of breastfeeding, the assessment was waived and medication was started on day 4 per protocol.
Over the next 12 weeks, participants were seen at the research clinic four times (weeks 2, 4, 8, and 12). At each visit, participants completed the Edinburgh Postnatal Depression Scale,26 blood samples (progesterone, cotinine) were drawn, breath CO measurement and vital signs were taken, adverse events assessed with a change in general health question including the Columbia-Suicide Severity Rating Scale,32 smoking status (Timeline Followback),33,34 medication compliance (pill count), and a brief (15 minute) relapse prevention counseling session (or cessation counseling if indicated). For the nonclinic week visits (weeks 1, 3, 5, 6, 7, 9, 10, and 11), phone calls were made by study staff to check on EDC progress, troubleshoot EDC issues, collect data regarding smoking status, assess adverse events and offer brief relapse prevention or cessation counseling.
Participants were compensated at each clinic visit in cash plus a $30 gift card to Target or a gift bag for themselves or baby. Gift bags included baby items (weeks 0, 4, and 12) or spa-like gifts for the mother (baseline, weeks 2 and 8). Participants were paid $10 cash for each clinic visit, $1 for each completed EDC, $5 for each weekly phone call with study staff and a cash bonus ($300) upon study completion. Thus, maximum compensation was $782.
Study Medication
Participants were randomized to receive either active (200mg twice a day) or placebo progesterone. The active medication was micronized progesterone capsules manufactured by Teva Pharmaceuticals. It is synthesized from a plant source and chemically identical to progesterone of human ovarian origin. The drug was purchased through Investigational Drug Services at the University of Minnesota, Fairview Medical Center and encapsulated along with an identical placebo. After oral administration, 50%–60% of micronized progesterone is absorbed; it is metabolized by the liver to several steroids. The elimination half-life is 3–4 hours. The study dose (200mg of oral micronized progesterone) provides serum concentrations comparable to the luteal phase of the menstrual cycle (3–25ng/ml).35,36 Common side effects include fatigue, nausea, breast tenderness, spotting, and weight gain.37
Measures
Study data were collected in several ways. First, data from clinic visits (CO, vitals, Timeline Followback, adverse events, pill counts, and study questionnaires) were managed using Research Electronic Data Capture (a secure, web-based application for research studies).38 Next, blood samples at clinic visits were collected using standard venipuncture technique and preparation. Frozen samples were transported to internal labs for the analysis of progesterone and cotinine. Progesterone was analyzed using a solid phase enzyme-linked immunosorbent assay (ELISA) based on the principle of competitive binding. The assay sensitivity is 0.045ng/ml with a standard range of 0–40ng/ml.39 Cotinine was analyzed by liquid chromatography tandem mass spectrometry. The limit of quantitation for these analyses was 0.4ng/ml, and the coefficient of variation 6%.40 Finally, daily data collection at home (EPDS, vaginal bleeding and breastfeeding) was done using EDC.
Baseline Characteristics
The following measures were collected at the Screening Visit: demographics (ie, age, parity, race), previous smoking behavior (CPD, number of previous quit attempts) and environmental exposure to smoking (ie, partner smoking).
Feasibility
Feasibility was defined by compliance and acceptability. Specifically, compliance was measured as the number of visits attended (maximum of five), number of medication pills taken (maximum of 56) and number of EDCs completed (maximum of 84). Acceptability was measured with a nine-item study satisfaction questionnaire where each item was measured via a four-point Likert-type scale (with higher scores indicating higher acceptability). This questionnaire, done at each clinic visit, assessed study protocol, study medication, collateral factors, and EDCs.
Safety
Adverse events were assessed weekly at clinic visits or through phone calls with a single open-ended question about changes in general health. If a participant experienced an adverse event that was “possibly” or “definitely” related to the study medication, this was discussed with the medical personnel and appropriate action was taken (medication decreased/stopped or mental health referral). Participants were encouraged to contact study staff if they experienced any serious medical problems between assessments.
Depression
Depression screening was done with the Edinburgh Postnatal Depression Scale.26 This 10-item instrument is commonly used to screen for postpartum depressive symptoms but is not diagnostic. It has also been validated for prepartum use.27 This item was completed weekly via EDC from 36 weeks gestation through 12 weeks postpartum, and at each clinic visit using Research Electronic Data Capture. In addition, the Columbia-Suicide Severity Rating Scale32 was administered weekly (phone or study staff) since depression was a side effect of progesterone.
Breastfeeding and Vaginal Bleeding
Breastfeeding was initially assessed using the Breastfeeding Assessment Form31 completed by staff at the week 0 visit. Study drug was delayed if breast milk was not yet established. Participants continued to record breastfeeding status daily using EDC. Vaginal bleeding was reported daily by participants via EDC.
Smoking Status
Smoking status or abstinence was confirmed by breath CO measurements (CO < 5ppm indicating abstinence)23 serum cotinine (<4.47ng/ml indicating abstinence)41 analysis and self-report via the Timeline Followback procedure33,34 at each clinic visit. Cotinine levels for weeks 0, 4, and 12 were analyzed. Smokers were considered to have relapsed if: (1) they smoked a single puff or more of a lit cigarette anytime after randomization (defined as no longer achieving continuous abstinence [CA]) (2) if they had a single puff or more of a lit cigarette during 7 days prior to a specific time point (defined as no longer achieving 7-day point prevalence abstinence [PP]) or (3) if they had seven consecutive slips without a 24-hour period between any slip (defined as no longer achieving prolonged abstinence).42 For CA and prolonged abstinence relapse days can range from 0 (smoking on randomization date) to 84 days (not smoking at the end of the study). If participants did have a single puff or more from a lit cigarette, they were instructed to immediately initiate an EDC to capture the event.
Analysis
Descriptive statistics (means and standard deviations for continuous variables and totals/percentages for categorical variables) were used to summarize demographics by randomization group. Feasibility and relapse outcomes were compared between groups. Two group t tests were used to compare continuous variables or outcomes (compliance and acceptability at weeks 4 and 12) between groups. Fisher’s exact tests were used to compare categorical variables or outcomes (PP at weeks 4 and 12) between groups. CA was compared between groups using a log-rank test (participants without a relapse prior to 84 days were censored). A two group t test was used to compare week 0 EPDS scores between groups and a paired t test was used to assess change in EPDS scores from week 0 to week 12. For EPDS scores, a group by time interaction was examined using a linear model with a random subject effect. The associations between baseline demographics and characteristics (listed in Table 1) and outcomes were explored using simple logistic regression (for PP) or simple linear regression (for compliance and acceptability). Associations between outcomes and serum progesterone were assessed with logistic regression (PP) or Cox’s regression (CA). All randomized participants were included in the analysis, however, one participant’s serum cotinine (>4.47ng/ml) indicated current smoking at week 0. Thus, she was excluded as smoking relapse had occurred prior to the delivery of the study intervention, in only the modified intent-to-treat analysis.41 SAS V9.343 was used for the analysis. p Values less than .05 were considered statistically significant. There were no adjustments for multiple comparisons in this pilot study.
Table 1.
Demographics and Baseline Characteristics (N = 46)
| Variable | Total | Progesterone n = 24 | Placebo n = 22 | p a |
|---|---|---|---|---|
| Age, mean (SD) | 26.5 (5.2) | 26.2 (5.5) | 26.7 (5.0) | .7403 |
| Non-Hispanic, n (%) | 42 (91) | 21 (88) | 21 (95) | .6093 |
| Race, n (%) | ||||
| White | 28 (62) | 14 (61) | 14 (64) | 1.0000 |
| Black | 12 (27) | 6 (26) | 6 (27) | |
| Native American | 2 (4) | 1 (4) | 1 (5) | |
| >1 race | 3 (7) | 2 (9) | 1 (5) | |
| 1 missing | ||||
| Education, n (%) | .6702 | |||
| Graduate | 1 (2) | 1 (4) | 0 | |
| College graduate or higher | 7 (15) | 2 (8) | 5 (23) | |
| Some college | 30 (65) | 17 (71) | 13 (59) | |
| High school | 6 (13) | 3 (13) | 3 (14) | |
| <High school | 2 (4) | 1 (4) | 1 (5) | |
| Marital status, n (%) | .3785 | |||
| Never married | 30 (65) | 17 (71) | 13 (59) | |
| Married | 9 (20) | 3 (13) | 6 (27) | |
| Divorced | 6 (13) | 4 (17) | 2 (9) | |
| Remarried | 1 (2) | 0 | 1 (5) | |
| Smoker in household, n (%) | 21 (46) | 10 (42) | 11 (50) | .7675 |
| CPD, mean (SD) | 10.1 (4.5) | 10.1 (4.7) | 10.0 (4.4) | .9776 |
| Age regular smoker, mean (SD) | 17.4 (2.6) | 17.7 (2.8) | 17.0 (2.4) | .3472 |
| Quit attempts, mean (SD) | 4.3 (4.8) | 4.1 (3.1) | 4.6 (6.2) | .7101 |
| Longest quit in days, median (IQR) | 180 (30–300) | 120 (21–365) | 195 (90–270) | .6805 |
| Weight concern scale, mean (SD) | 2.7 (1.2) | 2.5 (1.0) | 2.9 (1.4) | .3888 |
| Week 0 Progesterone ng/ml | 0.82 (0.52) | 0.71 (0.40) | 0.97 (0.66) | .1925 |
| # of Pregnancies | 2.11 (1.30) | 2.25 (1.45) | 1.95 (1.13) | .4487 |
| # of Children | 0.67 (0.90) | 0.88 (1.08) | 0.45 (0.60) | .1060 |
CPD = cigarettes per day; IQR = interquartile range.
aTwo group t test or Fisher’s Exact tests
Results
Baseline Characteristics
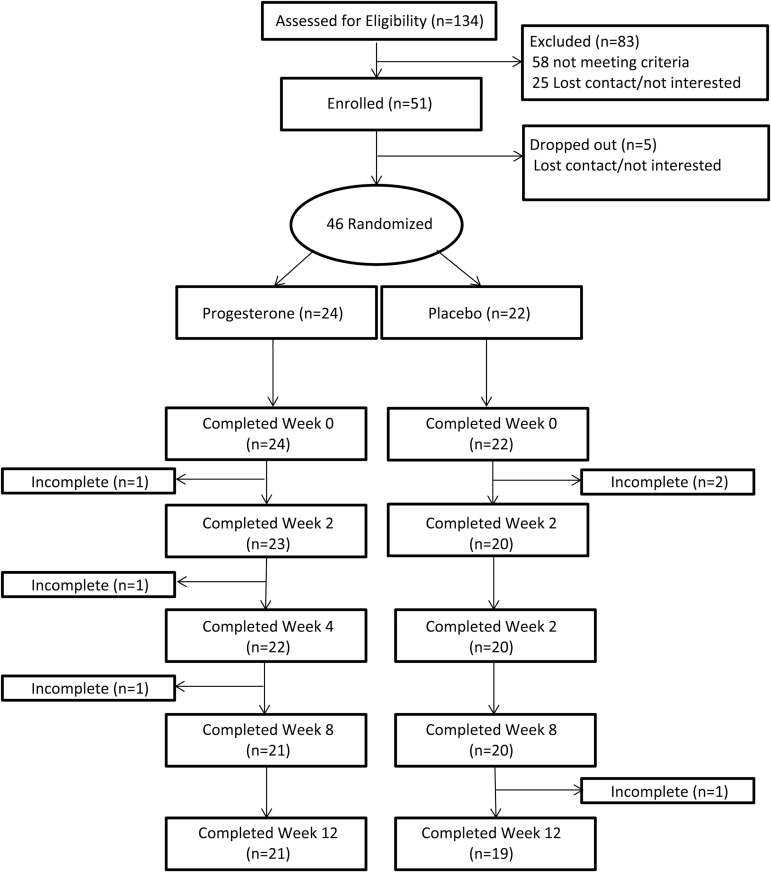
A total of 134 pregnant women were initially assessed for eligibility by phone. Of those, 83 (62%) were not interested or ineligible (smoking <5 CPD prior to quitting, pregnancy complications or unwilling to use nonhormonal birth control for the study duration). Our target N was 40 randomized postpartum women. A total of 51 pregnant women were enrolled between 32–35 weeks gestation. Of the 51 enrolled during pregnancy, five women (10%) were either lost to follow-up or withdrew participation and were labeled “dropouts.” The remaining 46 delivered live babies and were randomized. Participants who were lost to follow-up or withdrew participation after randomization were labeled “incompleters” (Figure 1).
Figure 1.
CONSORT diagram.
Overall, participants (n = 46) were 26.5 (±5.2) years of age, mostly non-Hispanic white (62%), with at least some college education (83%). They smoked a mean of 10.1 (±4.5) CPD prior to quitting, reported a mean of 17.4 (±2.6) years of age when they became a regular smoker, a mean of 4.3 (±4.8) quit attempts throughout their lifetime and a median of 180 days for their longest quit attempt. No differences were observed by randomization (Table 1).
Feasibility
Retention
Study retention after randomization was 87% (40/46) at 12 weeks. This did not differ by randomization (PRO 21/24 and PBO 19/22). Incompleters represented 13% (6/46) and there was no difference by randomization (p = 1.00). Participants cited the following reasons for not completing the protocol: wanting to start hormonal birth control prior to completing the study (n = 3), clinic visits with a new baby were too burdensome (n = 2) and concerns regarding milk production and vaginal bleeding (n = 1).
Compliance
Results indicated number of clinic visits, pill counts and EDC’s completed did not differ by randomization. Adherence rate of medication was 68% (Table 2). The mean (SD) progesterone levels at week 2 for the PRO and PBO groups were 12.72ng/ml (21.01) and 0.21ng/ml (0.33), respectively; p = .0217. However, progesterone levels in 33% (6/18) of participants in PRO were lower than 1.0 at week 2. The majority of participants 72% (33/46) began study medication per protocol on day 4 postpartum. Subsequently, 27% (12/46) were delayed starting medication due to nursing or scheduling issues and one participant refused medication due to breastfeeding complications but remained in the study (1/46).
Table 2.
Feasibility of Study Protocol (N = 46)
| Total | Progesterone n = 24 | Placebo n = 22 | p c | |
|---|---|---|---|---|
| Compliance (mean ± SD) | ||||
| Number of postpartum visits attended (range: 0–5) | 4.5 (1.1) | 4.5 (1.1) | 4.4 (1.2) | .5990 |
| Doses of medication taken (range: 0–56) | 38.2 (20.3) | 38.9 (22.3) | 37.5 (18.3) | .8154 |
| EDCs completed (range: 0–84) | 61.3 (22.7) | 60.9 (21.8) | 61.8 (24.3) | .9002 |
| Acceptability (mean ± SD) | ||||
| Acceptability scorea at week 4, n = 38 | 3.6 (0.3) | 3.6 (0.3) | 3.5 (0.3) | .2205 |
| Acceptability scorea at week 12, n = 39 | 3.5 (0.3) | 3.6 (0.3) | 3.5 (0.4) | .1354 |
| Individual questionsb n (%) | ||||
| 1. How willing would you be to participate in a study like this again? | 37 (80.4) | 20 (83.3) | 17 (77.3) | |
| 2. Overall, how much of a burden was it to participate in this study? | 6 (13.0) | 2 (8.3) | 4 (18.2) | |
| 3. Overall, how much did participating in this study interfere with your usual activities? | 1 (2.2) | 0 | 1 (4.5) | |
| 4. How difficult was it to use the cell phone to complete the study questionnaires? | 1 (2.2) | 1 (4.2) | 1 (4.5) | |
| 5. Overall, how much were you annoyed with the number of times the cell phone beeped during the day? | 1 (2.2) | 0 | 1 (4.5) | |
| 6. How difficult was it to take the study-supplied medication? | 6 (13.0) | 2 (8.3) | 4 (18.2) | |
| 7. How difficult was it to notify the study staff of your child’s birth? | 3 (6.5) | 2 (8.3) | 1 (4.5) | |
| 8. Overall, how much do you think this study has helped you stay smoke- free after your baby was born? | 32 (69.6) | 20 (83.3) | 12 (54.5) | |
| 9. Overall, how difficult was it to work with study staff? | 0 (0) | 0 (0) | 0 (0) | |
EDC = electronic data capture.
aDefined as the average of nine questions (1 and 8 reversed). Scores range between 1–4 with 1 = low acceptability and 4 = high acceptability.
bResponses of “moderately” or “extremely” on week 12 satisfaction survey.
cTwo group t test.
Acceptability
There were no differences in the acceptability scores by randomization (PRO: 3.6±0.3 vs. PBO: 3.5±0.3; p = .22). Higher acceptability was associated with older age at which they became a regular smoker (r = 0.55; p = .0005) and with having more children (r = 0.30; p = .0659).
Safety
Adverse Events
No adverse events were determined “definitely related” to study drug. Medication was not reduced for any participant. Two participants (4%) reported adverse events that were “possibly related.” These included dizziness (n = 1) and depressed mood with anxiety (n = 1). One serious adverse event, not related to study participation, was reported in the PBO group (hospitalization for a postpartum infection). During the 4 weeks of treatment, 22% (10/46) participants reported daily vaginal bleeding: three PRO (with all three breastfeeding) and seven PBO (with only six breastfeeding).
Depression
At week 0, depressive symptoms scores were similar in the two randomization groups (PRO: 5.6±3.8 vs. PBO: 7.3±4.4, respectively; p = .17). Regardless of randomization, depressive symptoms scores significantly decreased by −2.4 (±3.8) from week 0 to week 12 (p = .0023). A group by time interaction was not significant (p = .88). No participants endorsed suicidal ideation during the study.
Breastfeeding
The initiation of study medication was delayed by up to 6 days for 11 participants due to low milk supply or other breastfeeding complications. One additional woman was delayed 13 days for similar reasons. Number of days reported to last breastfeeding were similar (PRO 51±35; PBO 54±35, p = .83).
Progesterone Association With Relapse
Postpartum Smoking Relapse
Based on point prevalence, 71.7% (33/46) of participants remained abstinent at week 4, with 75.0% (18/24) PRO and 68.2% (15/22) PBO (p = .75). At week 12, 47.8% (22/46) of participants were still abstinent: 54.2% (13/24) PRO and 40.9% (9/22) PBO (p = .39; Table 3). Based on CA, the median days to relapse was 81 (PRO: 81 days, PBO: 65 days; p = .95). Of those who relapsed prior to 4 weeks and 12 weeks, the mean days to relapse were 13.6±7.5 days (PRO: 13.4±7.0 days; PBO: 13.8±8.4 days) and 30.0±25.6 days (PRO: 34.2±27.7 days; PBO: 25.2±23.2 days), respectively. Similar results were found using prolonged abstinence whereas 85% had not relapsed prior to week 12 and there were no differences by randomization (p = .067). CPD, age became a regular smoker, smoker in household, number of quit attempts and longest quit attempts were not found to be statistically significantly associated with relapse at weeks 4 and 12.
Table 3.
Summary of Postpartum Smoking Relapse by Point Prevalence
| Outcome | Total | Progesterone n = 24 | Placebo n = 22 | p a |
|---|---|---|---|---|
| PP abstinent at 4 weeks, n (%) | ||||
| ITT | .7462 | |||
| Yes | 33 (71.7) | 18 (75.0) | 15 (68.2) | |
| No | 13 (28.3) | 6 (25.0) | 7 (31.8) | |
| Modified ITTb | 1.0000 | |||
| Yes | 33 (73.3) | 18 (75.0) | 15 (71.4) | |
| No | 12 (26.7) | 6 (25.0) | 6 (28.6) | |
| PP abstinent at 12 weeks, n (%) | ||||
| ITT | .3945 | |||
| Yes | 22 (47.8) | 13 (54.2) | 9 (40.9) | |
| No | 24 (52.2) | 11 (45.8) | 13 (59.1) | |
| Modified ITTb | .5544 | |||
| Yes | 22 (48.9) | 13 (54.2) | 9 (42.9) | |
| No | 23 (51.1) | 11 (45.8) | 12 (57.1) | |
ITT = Intent to treat; PP abstinence = 7-day point prevalence (a single puff or more of a lit cigarette during the 7 days prior to a specific time point is defined as relapse).
aFisher’s exact test.
bOne participant excluded due to smoking at week 0.
Association of Serum Progesterone Levels and Postpartum Smoking Relapse
Serum progesterone level was not associated with PP at week 4 (OR = 1.00, 95% confidence interval [CI] = 0.95–1.04, p = .85) or at week 12 (OR = 1.03, 95% CI = 0.97–1.09, p = .41). Progesterone level was also not associated with time to first slip (hazard ratio = 1.00, 95% CI = 0.98–1.02, p = .89). For the PRO group (with levels greater than 1.0 at week 2 indicating medication compliance) and for the PBO group, the change in progesterone from baseline to week 2 was not associated with relapse as measured by PP.
Discussion
This double-blind randomized controlled pilot study was designed to determine feasibility of administering exogenous progesterone to women in the immediate postpartum period as a relapse prevention strategy. Given the preclinical14,15 and clinical literature16–20 which supports the role of progesterone as attenuating addictive behavior and given the erratic change of gonadal hormones in the postpartum period we hypothesized that administering exogenous progesterone to postpartum women could potentially prevent postpartum relapse. Our observations indicate that there was moderate to high compliance and high acceptability of administering exogenous progesterone to women in the immediate postpartum period. These observations did not differ by randomization. Further, while this study was not powered to assess progesterone effects on quit outcomes, women randomized to the active progesterone group had a higher prevalence of abstinence at 4 weeks postpartum.
Overall, participants were highly compliant with this protocol regardless of randomization. Clinic visit attendance (>80%) was comparable to previous clinical trials including postpartum studies.44–46 Study retention (87%) was also comparable to previous cessation studies for addiction.44,47–50 Number of pills taken (69%) or adherence rate to study medication was moderate compared to previous pharmacological clinical trials.45,46,51 Of note, 33% of the progesterone group at week 2 had low progesterone blood levels suggesting missing pills. Possible reasons include the burden of twice daily dosing or busy with their newborn. This lower adherence rate could contribute to lack of differences in abstinence between randomized groups. This could be improved by novel reminders of dosing or alternate methods of progesterone delivery. Abstinent rates at end of treatment for progesterone versus placebo (75% vs. 68%) and end of study (54% vs. 41%) respectively, were comparable for treatment groups in other pharmaceutical cessation trials for addiction in the general population. For example, in a study comparing varenicline and placebo abstinence rates at 12 weeks were 50% versus 12%, respectively45 and in a comparison of bupropion versus placebo abstinence rates were 29% versus 19%, respectively at 7 weeks.52 A third study compared varenicline plus nicotine replacement therapy (patch) to placebo and observed abstinence rates of 55% versus 41% at 12 weeks.53 One must keep in mind that even though our placebo group had higher abstinence rates this is a limited comparison given the different populations. Our participants were postpartum and other factors could play a role such as new mothers and more concern about passive smoke and influence of breastfeeding. Although the reasoning behind breastfeeding supporting substance abstinence is not completely understood, some hypotheses include more maternal bonding and personal investment in baby’s health, or more time spent with baby equals less time spent smoking.54 Breastfeeding has been highly associated with postpartum cigarette abstinence and duration of breastfeeding is proven to positively correlate with duration of smoking abstinence.55,56 This warrants further study.
Progesterone was well tolerated by participants. Serum levels of progesterone were comparable to previous studies administering similar doses (200mg twice daily) of exogenous progesterone.57 No participants required alteration of dosage. Daily vaginal bleeding was reported in 22% of participants, regardless of randomization, or breastfeeding status and is a common occurrence with progesterone treatment. Drug was not discontinued for any participant because of side effects compared to a large randomized 12-week cessation trial, where study drug was discontinued due to adverse events: for varenicline (8.6%) for bupropion Sustained Release (15.2%) and for placebo (9.0%).45
The pharmaceutical literature indicates that depressive symptoms and lactation problems may be side effects of progesterone administration.37 Given we were administering a safe but high dose of progesterone, there were concerns that participants may experience these adverse effects, but none developed. In the United States, 41% of women report exclusive breastfeeding at 3 months, 19% at 6 months with 79% of women reporting have ever-breastfed.58 Participants who breastfed in this study at 12 weeks were comparable: 42% for PRO and 50% for PBO with 66% reporting progesterone had no effect on breastfeeding and were not delayed in starting study medication. Further, progesterone did not negatively affect breastfeeding once milk was established. Interestingly participants experienced decreased depressive symptoms over the 12 weeks regardless of randomization. Exogenous progesterone effect on postpartum depressive symptoms seemed minimal and consistent with other studies.36,44 However, our study sample was not depressed. In fact, our study sample reported less depressive symptoms than in the general population of women (18.5%)59 and during the postpartum period (7%–13%).60 Therefore, future research should explore how progesterone may alter postpartum smoking behavior in women who are at high risk of developing depressive symptoms as they may receive additional benefit from progesterone treatment.
Finally, participants indicated high levels of acceptability for this study protocol at both week 4 (end of treatment) and at week 12 (end of study) regardless of randomization. Most (70%) felt that this study helped them stay smoke free and the majority (80%) indicated they would be willing to participate in the study again. Few (6/46; 13%) participants reported that it was a burden to participate.
A large proportion of participants in this trial remained abstinent during follow-up; 71.1% at week 4 (75.0% in PRO and 68.2% in PBO) and 47.8% at week 12 (54.2% in PRO and 40.9% in PBO). Though not statistically significantly, the PRO group had a higher prevalence of abstinence. While the current project was a feasibility study and not powered to determine a statistically significant difference, if our observations are valid, a sample size of 231 per group would be needed to detect this difference at 80% power.
Although this project has several strengths (randomized double-blind control trial design, use of EDC), it does have some limitations. First, while consistent with the goals of assessing feasibility and collecting preliminary data, this study included a small homogenous sample. Thus, we are not able to fully assess the effect of progesterone on postpartum smoking relapse nor can we generalize our observations to the general population. Second, low levels of serum progesterone in the progesterone group at week 2 suggests participants were missing pills, limiting the potential effect of progesterone. Further, because there is considerable inter-subject variability in the absorption of oral progesterone,36 non-oral administration may provide a more stable dose from participant to participant and should be considered in future work. Third, history of depression, a strong predictor of postpartum smoking relapse, was not assessed. Future work should assess both a history of depression in the past, as well as postpartum depression, as both limit the likelihood of maintaining abstinence.61–63 Thus, these observations are limited. Future work should consider focusing on women who are at high risk for postpartum relapse due to a history of depression, and/or not breastfeeding.
In summary, postpartum relapse is a major public health problem that may be related to the erratic fluctuation of hormones. Given the encouraging literature, pre-clinical and clinical, regarding progesterone and its dampening effect on addictive behavior, the potential therapeutic value of exogenous progesterone in a larger clinical trial is important. Given the acceptable and feasible results of this study, a fully powered trial to explore the effect of exogenous progesterone on postpartum smoking relapse is warranted.
Funding
This work was supported by the National Institute on Drug Abuse (R21DA034840), National Center for Advancing Translational Sciences (UL1TR000114) and Building Interdisciplinary Research Careers in Women’s Health (K12HD055887). The content is solely the responsibility of the authors and does not necessarily represent the office views of the NIH.
Declaration of Interests
None declared.
Acknowledgments
Laboratory support provided by Behavioral Medicine Laboratories, University of Minnesota Medical School. Brittany Neisen completed in-home visits (week 0). Karin Knutsen assisted in the literature review.
References
- 1. Tong VT, Jones JR, Dietz PM, D’Angelo D, Bombard JM. Trends in smoking before, during, and after pregnancy - Pregnancy Risk Assessment Monitoring System (PRAMS), United States, 31 sites, 2000–2005. Morbidity and mortality weekly report. Surveillance summaries (Washington, D.C. : 2002) 2009. www.cdc.gov/mmwr/preview/mmwrhtml/ss5804a1.htm Accessed November 23, 2015. [PubMed]
- 2. Tong VT, Jones JR, Dietz PM, D’Angelo D, Bombard JM. Trends in smoking before, during, and after pregnancy - Pregnancy Risk Assessment Monitoring System (PRAMS), United States, 31 sites, 2000–2005. MMWR Surveill Summ. 2009;58(4):1–29. www.ncbi.nlm.nih.gov/pubmed/19478726 Accessed December 3, 2015. [PubMed] [Google Scholar]
- 3. US Department of Health and Human Services. The Health Consequences of Smoking- 50 Years of Progress: A Report of the Surgeon General, Executive Summary 2014. www.cdc.gov/tobacco/data_statistics/sgr/50th-anniversary/index.htm. Assessed December 7, 2015.
- 4. Hoedjes M, Berks D, Vogel I, et al. Effect of postpartum lifestyle interventions on weight loss, smoking cessation, and prevention of smoking relapse: a systematic review. Obstet Gynecol Surv. 2010;65(10):631–652. doi:10.1097/OGX.0b013e3182077f64. [DOI] [PubMed] [Google Scholar]
- 5. Jiménez-Muro A, Nerín I, Samper P, et al. A proactive smoking cessation intervention in postpartum women. Midwifery. 2013;29(3):240–245. doi:10.1016/j.midw.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 6. Tong VT, Dietz PM, England LJ. “Smoking cessation for pregnancy and beyond: a virtual clinic,” an innovative web-based training for healthcare professionals. J Womens Health (Larchmt). 2012;21(10):1014–1017. doi:10.1089/jwh.2012.3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Herbec A, Brown J, Tombor I, Michie S, West R. Pilot randomized controlled trial of an internet-based smoking cessation intervention for pregnant smokers (‘MumsQuit’). Drug Alcohol Depend. 2014;140:130–136. doi:10.1016/j.drugalcdep.2014.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lopez AA, Skelly JM, Higgins ST. Financial incentives for smoking cessation among depression-prone pregnant and newly postpartum women: effects on smoking abstinence and depression ratings. Nicotine Tob Res. 2015;17(4):455–462. doi:10.1093/ntr/ntu193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cahill K, Hartmann-Boyce J, Perera R. Incentives for smoking cessation. Cochrane Database Syst Rev. 2015;5:CD004307. doi:10.1002/14651858.CD004307.pub5. [DOI] [PubMed] [Google Scholar]
- 10. Ierfino D, Mantzari E, Hirst J, Jones T, Aveyard P, Marteau TM. Financial incentives for smoking cessation in pregnancy: a single-arm intervention study assessing cessation and gaming. Addiction. 2015;110(4):680–688. doi:10.1111/add.12817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Einarson A, Riordan S. Smoking in pregnancy and lactation: a review of risks and cessation strategies. Eur J Clin Pharmacol. 2009;65(4):325–330. doi:10.1007/s00228-008-0609-0. [DOI] [PubMed] [Google Scholar]
- 12. Hemsing N, Greaves L, O’Leary R, Chan K, Okoli C. Partner support for smoking cessation during pregnancy: a systematic review. Nicotine Tob Res. 2012;14(7):767–776. doi:10.1093/ntr/ntr278. [DOI] [PubMed] [Google Scholar]
- 13. Agboola S, McNeill A, Coleman T, Leonardi Bee J. A systematic review of the effectiveness of smoking relapse prevention interventions for abstinent smokers. Addiction. 2010;105(8):1362–1380. doi:10.1111/j.1360-0443.2010.02996.x. [DOI] [PubMed] [Google Scholar]
- 14. Anker JJ, Carroll ME. The role of progestins in the behavioral effects of cocaine and other drugs of abuse: human and animal research. Neurosci Biobehav Rev. 2010;35(2):315–333. doi:10.1016/j.neubiorev.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lynch WJ, Sofuoglu M. Role of progesterone in nicotine addiction: evidence from initiation to relapse. Exp Clin Psychopharmacol. 2010;18(6):451–461. doi:10.1037/a0021265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Allen SS, Bade T, Center B, Finstad D, Hatsukami D. Menstrual phase effects on smoking relapse. Addiction. 2008;103(5):809–821. doi:10.1111/j.1360-0443.2008.02146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Allen AM, Allen SS, Lunos S, Pomerleau CS. Severity of withdrawal symptomatology in follicular versus luteal quitters: The combined effects of menstrual phase and withdrawal on smoking cessation outcome. Addict Behav. 2010;35(6):549–552. doi:10.1016/j.addbeh.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Franklin TR, Ehrman R, Lynch KG, et al. Menstrual cycle phase at quit date predicts smoking status in an NRT treatment trial: a retrospective analysis. J Womens Health (Larchmt). 2008;17(2):287–292. doi:10.1089/jwh.2007.0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mazure CM, Toll B, McKee SA, Wu R, O’Malley SS. Menstrual cycle phase at quit date and smoking abstinence at 6 weeks in an open label trial of bupropion. Drug Alcohol Depend. 2011;114(1):68–72. doi:10.1016/j.drugalcdep.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wetherill RR, Franklin TR, Allen SS. Ovarian hormones, menstrual cycle phase, and smoking: a review with recommendations for future studies. Curr Addict Reports. 2016;3(1):1–8. doi:10.1007/s40429-016-0093-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Albrecht ED, Pepe GJ. Endocrinology of pregnancy. In: Bazer FW, ed. Endocrinology of Pregnancy. Totowa, NJ: Humana Press; 1998:319–351. doi:10.1007/978-1-4612-1804-3. [Google Scholar]
- 22. Strauss J, Barbieri R. Yen & Jaffe’s Reproductive Endocrinology. 7th ed. Philadelphia, PA: Elsevier Saunders; 2014. [Google Scholar]
- 23. Perkins KA, Karelitz JL, Jao NC. Optimal carbon monoxide criteria to confirm 24-hr smoking abstinence. Nicotine Tob Res. 2013;15(5):978–982. doi:10.1093/ntr/nts205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. al’Absi M, Lovallo WR, McKey BS, Pincomb GA. Borderline hypertensives produce exaggerated adrenocortical responses to mental stress. Psychosom Med. 1994;56(3):245–250. www.ncbi.nlm.nih.gov/pubmed/8084971 Accessed March 25, 2016. [DOI] [PubMed] [Google Scholar]
- 25. al’Absi M, Lovallo WR, McKey B, Sung BH, Whitsett TL, Wilson MF. Hypothalamic-pituitary-adrenocortical responses to psychological stress and caffeine in men at high and low risk for hypertension. Psychosom Med. 1998;60(4):521–527. www.ncbi.nlm.nih.gov/pubmed/9710300 Accessed March 25, 2016. [DOI] [PubMed] [Google Scholar]
- 26. Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150(6):782–786. www.ncbi.nlm.nih.gov/pubmed/3651732 Accessed March 16, 2015. [DOI] [PubMed] [Google Scholar]
- 27. Rubertsson C, Börjesson K, Berglund A, Josefsson A, Sydsjö G. The Swedish validation of Edinburgh Postnatal Depression Scale (EPDS) during pregnancy. Nord J Psychiatry. 2011;65(6):414–418. doi:10.3109/08039488.2011.590606. [DOI] [PubMed] [Google Scholar]
- 28. Cappelleri JC, Bushmakin AG, Baker CL, Merikle E, Olufade AO, Gilbert DG. Confirmatory factor analyses and reliability of the modified cigarette evaluation questionnaire. Addict Behav. 2007;32(5):912–923. doi:10.1016/j.addbeh.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 29. Neville MC, Morton J, Umemura S. Lactogenesis. The transition from pregnancy to lactation. Pediatr Clin North Am. 2001;48(1):35–52. www.ncbi.nlm.nih.gov/pubmed/11236732 Accessed December 7, 2015. [DOI] [PubMed] [Google Scholar]
- 30. Neville MC, Morton J. Physiology and endocrine changes underlying human lactogenesis II. J Nutr. 2001;131(11):3005S–3008. http://jn.nutrition.org/content/131/11/3005S.full Accessed December 7, 2015. [DOI] [PubMed] [Google Scholar]
- 31. Entwistle F. The evidence and rationale for the UNICEF UK Baby Friendly Initiative standards 2013:51 www.unicef.org.uk/BabyFriendly/Resources/Guidance-for-Health-Professionals/Writing-policies-and-guidelines/guide-to-the-baby-friendly-initiative-standards/. Accessed December 7, 2015.
- 32. Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168(12):1266–1277. doi:10.1176/appi.ajp.2011.10111704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Brown RA, Burgess ES, Sales SD, Whiteley JA, Evans DM, Miller IW. Reliability and validity of a smoking timeline follow-back interview. Psychol Addict Behav. 1998;12(2):101–112. doi:10.1037/0893-164X.12.2.101. [Google Scholar]
- 34. Gariti PW, Alterman AI, Ehrman RN, Pettinati HM. Reliability and validity of the aggregate method of determining number of cigarettes smoked per day. Am J Addict. 1998;7(4):283–287. www.ncbi.nlm.nih.gov/pubmed/9809132 Accessed February 12, 2016. [PubMed] [Google Scholar]
- 35. Sofuoglu M, Babb DA, Hatsukami DK. Effects of progesterone treatment on smoked cocaine response in women. Pharmacol Biochem Behav. 2002;72(1–2):431–435. www.ncbi.nlm.nih.gov/pubmed/11900816 Accessed February 12, 2016. [DOI] [PubMed] [Google Scholar]
- 36. McAuley JW, Kroboth FJ, Kroboth PD. Oral administration of micronized progesterone: a review and more experience. Pharmacotherapy. 1996;16(3):453–457. www.ncbi.nlm.nih.gov/pubmed/8726605 Accessed December 29, 2015. [PubMed] [Google Scholar]
- 37. Progesterone. PRD Health website. 2014 www.pdr.net Accessed February 12, 2016.
- 38. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi:10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Elisa DRGP. Progesterone ELISA kit 2007;2007(908):1–10. [Google Scholar]
- 40. Murphy SE, Park S-SL, Thompson EF, et al. Nicotine N-glucuronidation relative to N-oxidation and C-oxidation and UGT2B10 genotype in five ethnic/racial groups. Carcinogenesis. 2014;35(11):2526–2533. doi:10. 1093/carcin/bgu191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Benowitz NL, Bernert JT, Caraballo RS, Holiday DB, Wang J. Optimal serum cotinine levels for distinguishing cigarette smokers and nonsmokers within different racial/ethnic groups in the United States between 1999 and 2004. Am J Epidemiol. 2009;169(2):236–248. doi:10.1093/aje/kwn301. [DOI] [PubMed] [Google Scholar]
- 42. Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: issues and recommendations. Nicotine Tob Res. 2003;5(1):13–25. www.ncbi.nlm.nih.gov/pubmed/12745503 Accessed December 4, 2015. [PubMed] [Google Scholar]
- 43. SAS v9.3. 2010. Cary, NC: SAS Institute Inc.; 2011.
- 44. Yonkers KA, Forray A, Nich C, et al. Progesterone reduces cocaine use in postpartum women with a cocaine use disorder: a randomized,double-blind study. Lancet Psychiatry. 2014;1(5):360–367. doi:10.1016/S2215-0366(14)70333-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Oncken C, Gonzales D, Nides M, et al. Efficacy and safety of the novel selective nicotinic acetylcholine receptor partial agonist, varenicline, for smoking cessation. Arch Intern Med. 2006;166(15):1571–1577. doi:10.1001/archinte.166.15.1571. [DOI] [PubMed] [Google Scholar]
- 46. Jorenby DE. Efficacy of Varenicline, an α4β2 Nicotinic Acetylcholine Receptor Partial Agonist, vs Placebo or Sustained-Release Bupropion for Smoking Cessation<SUBTITLE>A Randomized Controlled Trial</SUBTITLE>. JAMA. 2006;296(1):56. doi:10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- 47. Okuyemi KS, Thomas JL, Hall S, et al. Smoking cessation in homeless populations: a pilot clinical trial. Nicotine Tob Res. 2006;8(5):689–699. doi:10.1080/14622200600789841. [DOI] [PubMed] [Google Scholar]
- 48. Napolitano MA, Lloyd-Richardson EE, Fava JL, Marcus BH. Targeting body image schema for smoking cessation among college females: rationale, program description, and pilot study results. Behav Modif. 2011;35(4):323–346. doi:10.1177/0145445511404840. [DOI] [PubMed] [Google Scholar]
- 49. Bisaga A, Aharonovich E, Garawi F, et al. A randomized placebo-controlled trial of gabapentin for cocaine dependence. Drug Alcohol Depend. 2006;81(3):267–274. doi:10.1016/j.drugalcdep.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 50. López-Pelayo H, Batalla A, Balcells MM, Colom J, Gual A. Assessment of cannabis use disorders: a systematic review of screening and diagnostic instruments. Psychol Med. 2015;45(6):1121–1133. doi:10.1017/S0033291714002463. [DOI] [PubMed] [Google Scholar]
- 51. Leischow SJ, Muramoto ML, Matthews E, Floden LL, Grana RA. Adolescent smoking cessation with bupropion: the role of adherence. Nicotine Tob Res. 2015;18(5):1–4. doi:10.1093/ntr/ntv179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hurt RD, Sachs DP, Glover ED, et al. A comparison of sustained-release bupropion and placebo for smoking cessation. N Engl J Med. 1997;337(17):1195–1202. doi:10.1056/NEJM199710233371703. [DOI] [PubMed] [Google Scholar]
- 53. Koegelenberg CFN, Noor F, Bateman ED, et al. Efficacy of varenicline combined with nicotine replacement therapy vs varenicline alone for smoking cessation: a randomized clinical trial. JAMA. 2014;312(2):155–161. doi:10.1001/jama.2014.7195. [DOI] [PubMed] [Google Scholar]
- 54. Kendzor DE, Businelle MS, Costello TJ, et al. Breast feeding is associated with postpartum smoking abstinence among women who quit smoking due to pregnancy. Nicotine Tob Res. 2010;12(10):983–988. doi:10.1093/ntr/ntq132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. DiSantis KI, Collins BN, McCoy ACS. Associations among breastfeeding, smoking relapse, and prenatal factors in a brief postpartum smoking intervention. Acta Obstet Gynecol Scand. 2010;89(4):582–586. doi:10.3109/00016341003678435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Harmer C, Memon A. Factors associated with smoking relapse in the postpartum period: an analysis of the child health surveillance system data in Southeast England. Nicotine Tob Res. 2013;15(5):904–909. doi:10.1093/ntr/nts221. [DOI] [PubMed] [Google Scholar]
- 57. Sofuoglu M, Mouratidis M, Mooney M. Progesterone improves cognitive performance and attenuates smoking urges in abstinent smokers. Psychoneuroendocrinology. 2011;36(1):123–132. doi:10.1016/j.psyneuen.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Breastfeeding: Data & Statistics website. 2015 www.cdc.gov Accessed February 12, 2016.
- 59. Husky MM, Mazure CM, Paliwal P, McKee SA. Gender differences in the comorbidity of smoking behavior and major depression. Drug Alcohol Depend. 2008;93(1–2):176–179. doi:10.1016/j.drugalcdep.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Thombs BD, Benedetti A, Kloda LA, et al. Diagnostic accuracy of the Edinburgh Postnatal Depression Scale (EPDS) for detecting major depression in pregnant and postnatal women: protocol for a systematic review and individual patient data meta-analyses. BMJ Open. 2015;5(10):e009742. doi:10.1136/bmjopen-2015-009742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hitsman B, Papandonatos GD, McChargue DE, et al. Past major depression and smoking cessation outcome: a systematic review and meta-analysis update. Addiction. 2013;108(2):294–306. doi:10.1111/add.12009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zvolensky MJ, Bakhshaie J, Sheffer C, Perez A, Goodwin RD. Major depressive disorder and smoking relapse among adults in the United States: a 10-year, prospective investigation. Psychiatry Res. 2015;226(1):73–77. doi:10.1016/j.psychres.2014.11.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Allen AM, Prince CB, Dietz PM. Postpartum depressive symptoms and smoking relapse. Am J Prev Med. 2009;36(1):9–12. doi:10.1016/j.amepre.2008.09.020. [DOI] [PubMed] [Google Scholar]