Abstract
Background
The altered levels of some essential trace elements and antioxidant minerals have been observed in diabetic patients.
Objectives
The aim of the present study was to compare the concentrations of essential trace elements, copper (Cu), zinc (Zn), and iron (Fe) in the serum of patients who have type 2 diabetes mellitus (T2DM) with those of their non-diabetic first-degree relatives (FDR) and control subjects. The association between glycated hemoglobin (HbA1c) and levels of metals was also evaluated.
Patients and Methods
We studied 46 subjects with T2DM, 46 FDR, and 50 control subjects matched for age and sex. Serum concentrations of Cu, Zn, and Fe were measured by colorimetric kit. Fasting blood glucose (FBG) and HbA1c were assayed using the standard kit.
Results
An imbalance in the levels of the studied metals was observed in both patients with T2DM and FDR. We found significantly decreased levels of Zn and higher levels of Cu and Fe in the patients with T2DM and FDR when compared with the control subjects (P < 0.05). HbA1c levels were positively correlated with Cu and Fe and inversely correlated with Zn in the patients with T2DM and FDR (P < 0.05).
Conclusions
The patients with T2DM and FDR had altered contents of Cu, Zn, and Fe that might be a predisposing factor to the development of diabetes in future or vice versa the result of diabetes development. Impaired metabolism of these elements may contribute to the augmented risk of developing type 2 diabetes mellitus later in the life of their first-degree relatives.
Keywords: Copper, Zinc, Iron, HbA1c, Type 2 Diabetes Mellitus, Relatives
1. Background
The incidence of diabetes mellitus (DM) as a major global health problem has been increasing throughout the world in recent years. Type 2 diabetes mellitus (T2DM) comprises 90% of people with diabetes (1, 2). Non-diabetic first-degree relatives of diabetic patients (FDR) have obesity, insulin resistance, and increased prevalence of cardiovascular risk and T2DM later in their lives (3, 4).
Trace elements play an important role in various metabolic processes and are crucial for many physiological processes (5). Impaired metabolism of trace elements like copper (Cu) and zinc (Zn) has been reported to result in higher sensitivity to oxidative damage and development of diabetes and diabetic complications (6-11). Both of these metals are involved in glucose homeostasis and their status is modulated in DM (12, 13). Cu, as a pro-oxidant factor, participates in the creation of free radicals and Zn, as a component of many enzymes, plays a significant role in the synthesis, storage, and release of insulin (13-15). Zn homeostasis affects T2DM in different ways and its absence is associated with many chronic illnesses (16, 17).
Altered metabolisms of other micronutrients such as iron (Fe) have been reported in diabetes (18). Fe, as a strong pro-oxidant, catalyzes several cellular reactions that result in the production of reactive oxygen species (ROS) with a subsequent increase in the level of oxidative stress (19). Therefore, high concentrations of free iron could be harmful and its homeostasis is critical. Increasing evidence now suggests a potential influence of Fe metabolism on T2DM (20). The relationship between Fe and insulin is bi-directional as Fe influences insulin activity by getting involved in glucose uptake and consumption (21) and insulin affects Fe uptake and storage by increasing the cell surface transferrin receptors (22). It is documented that Fe affects glucose metabolism and it has been shown that free iron concentrations in the patients with T2DM are higher than those of normal subjects, which could contribute to tissue damage that may potentially elevate the risk of T2DM (23, 24).
HaemoglobinA1c (HbA1c), the major form of glycated haemoglobin, is formed by nonenzymatic reaction of glucose to hemoglobin (25, 26). Increasing levels of HbA1c in diabetic patients has revealed the relationship between HbA1c and blood glucose concentrations (27, 28). There is a hypothesis that glycated proteins binding to transition metals such as Cu and Fe may result in glycocholates creation. Glycocholates play an important role in the etiology of peripheral vascular dysfunction and neuropathies in DM (29, 30).
Awareness of the imbalance of trace elements can be a great advantage for early diagnosis and therapeutic perspectives in diabetic patients and their first-degree relatives.
2. Objectives
According to our literature search, most of the previous studies have compared serum Cu, Zn, and Fe levels in the diabetic patients with healthy control subjects. There is no information about the effect of serum Cu, Zn, and Fe levels on FDR. Thus, the purpose of the current study was to compare the amounts of essential trace elements of the patients with T2DM and FDR with those of control subjects and also evaluate the association between these elements and HbA1c.
3. Patients and Methods
3.1. Subjects
The study was carried out on 46 T2DM patients, 46 FDR, and 50 healthy nondiabetic controls matched for age and sex. The age range was 38 - 80 with the median age of 52 years old. The exclusion criteria for T2DM group were having anemia, cardiovascular disease, hepatic dysfunction, kidney problems, hypothyroidism, hypertension, currently taking nutritional supplements, using estrogen, progesterone, and diuretic drugs, as well as history of smoking and alcohol intake. In addition, healthy controls as well as relatives of diabetic patients with abnormal glucose tolerance, cardiovascular disease, or any other chronic and acute illness, and history of certain drugs use were excluded. None of the participants had taken vitamin and mineral supplements. The study protocol was followed only after obtaining the voluntary consent of the subjects. A full explanation of the research protocol was given to the subjects before their consent was requested. Data were collected from all the participants regarding their age, sex, and other comorbid conditions.
3.2. Blood Sampling and Analysis
From all the groups (n = 142), 5 mL venous blood was taken from the antecubital vein after overnight fasting. The blood was centrifuged at 5200 rpm for 10 minutes. Then, the blood samples were stored in the deep-freezer at -80 °C. At the time of the study, the serum samples were diluted with deionized water. FBG and HbA1c were determined by an enzymatic method with the standard kit (Pars Azmoon Comany, Tehran, Iran). Sensitivity of FBG and HbA1c assays was 5 mg/dL and 3%, respectively. Fe level was evaluated using photometric kit made by Pars Azmoon Company, Tehran, Iran (sensitivity: 5 µg/dL). Cu and Zn levels were measured in all the serum samples using colorimetric kit (Biorex, UK) with the sensitivity of 0.3 and 0.4 µg/dl, respectively. The expected ranges of Cu, Zn, and Fe were 70 - 140, 70 - 114, and 39 - 149 µg/dL, respectively. All the samples were analyzed when internal quality control met the acceptable criteria. Inter- and intra-assay coefficients of variation were 2.8% and 1.3% for Cu, 2.97% and 1.01% for Zn, and 2.48% and 1.02% for Fe, respectively.
3.3. Statistical Analysis
All the values were expressed as mean ± SD and P < 0.05 was considered statistically significant. Statistical significance of the differences between the mean values was analyzed by one way ANOVA followed by Tukey’s HSD post-hoc test using SPSS 16 statistical analysis software. Correlations between different variables were analyzed using Pearson’s correlation coefficients (r). Sample size was calculated using (α = 0.05, β = 0.2, σ = 18, d = 11) formula.
4. Results
Baseline characteristics of the patients with T2DM, FDR of diabetic patients, and control groups as well as the serum concentrations of Cu, Zn, and Fe and Cu/Zn, Cu/Fe, and Fe/Zn ratios are shown in Table 1. FBG and HbA1c of the control groups were less than those of the patients with diabetes (P < 0.05). There was no significant difference in the levels of FBG and HbA1c (P > 0.05) between FDR and control groups; however their amount was less in the control group.
Table 1. The Characteristics of the Study Subjectsa,b.
| T2DM | FDR | Control | P Valuec | P Valued | P Valuee | |
|---|---|---|---|---|---|---|
| N | 46 | 46 | 50 | |||
| Sex, M/F | 26/20 | 22/24 | 25/25 | |||
| Age, y | 54 ± 1.3 | 49.09 ± 1.5 | 51.04 ± 1.7 | |||
| FBG, mg/dL | 197.50 ± 69.8 | 97.20 ± 7.33 | 93.24 ± 8.7 | < 0.001 | < 0.001 | 0.8 |
| HbA1c, % | 7.8 ±1.6 | 5.31 ± 0.42 | 5.22 ± 0.6 | < 0.001 | < 0.001 | 0.8 |
| Cu, µg/dL | 118.15 ± 17.2 | 109 ±16.3 | 99 ± 14.2 | 0.02 | < 0.001 | 0.007 |
| Zn, µg/dL | 93.76 ± 13.45 | 94.59 ± 21.4 | 111.32 ± 14.3 | 0.9 | < 0.001 | < 0.001 |
| Fe, µg/dL | 118.33 ± 27.3 | 98.93 ± 13.9 | 88.96 ± 14.5 | < 0.001 | < 0.001 | 0.03 |
| Cu/Zn | 1.3 ± 0.28 | 1.2 ± 0.26 | 0.89 ± 0.13 | 0.07 | < 0.001 | < 0.001 |
| Cu/Fe | 1.03 ± 0.21 | 1.12 ± 0.23 | 1.14 ± 0.26 | 0.1 | 0.05 | 0.8 |
| Fe/Zn | 1.29 ± 0.37 | 1.07 ± 0.23 | 0.81 ± 0.16 | <0.001 | < 0.001 | < 0.001 |
aValues are expressed as mean ± SD (range).
bN is the number of subjects per group.
cP value between T2DM group and FDR group.
dP value between T2DM group and control group.
eP value between FDR group and control group.
We observed significantly higher levels of Cu and Fe as well as Cu/Zn and Fe/Zn ratios (P < 0.05) and lower levels of Zn and Cu/Fe ratio in the patients with T2DM compared with the control group. We also found significantly lower Zn levels (P < 0.001) and higher values of Cu (P = 0.007), Fe (P = 0.03), Cu/Zn ratio (P < 0.001), and Fe/Zn ratio (P < 0.001) in FDR compared with the control subjects.
There was a significant difference in the levels of FBG, HbA1c, Cu, Fe, as well as Cu/Zn and Fe/Zn ratios (P < 0.05) between T2DM and FDR. However, there was no significant difference in the levels of Zn (P = 0.9) and Cu/Fe ratio (P = 0.1) in T2DM compared with FDR.
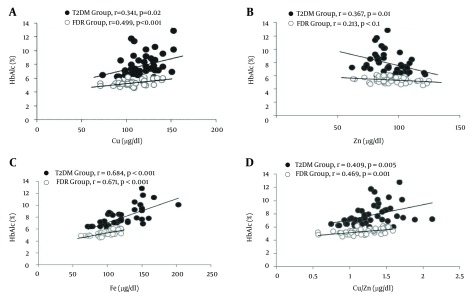
We calculated the associations between the concentrations of serum Cu, Zn, and Fe levels and Cu/Zn, Cu/Fe, and Fe/Zn ratios with HbA1c in T2DM and FDR groups (Figure 1A – 1D). In T2DM group, a positive correlation between serum levels of HbA1c and Cu (r = 0.341,P = 0.02), Fe (r = 0.684, P < 0.001), as well as Cu/Zn (r = 0.409, P = 0.005) and Fe/Zn ratios (r = 0.699,P < 0.001) and a negative correlation between serum levels of HbA1c and Zn (r = −0.367, P = 0.01), and Cu/Fe ratio (r = -0.458, P = 0.001) were shown.
Figure 1. Correlation Between Serum Levels of HbA1c With Cu (a), Zn (b), Fe (c), and Cu/Zn Ratio (d) in T2DM and FDR Groups.
Also, in FDR group, we found positive correlations between HbA1c and Cu (r = 0.499, P < 0.001), Fe (r = 0.671, P < 0.001), Cu/Zn ratio (r = 0.469, P = 0.001), and Fe/Zn ratio (r = 0.610, P < 0.001) and a negative correlation between HbA1c and Zn (r = -0.213, P = 0.1) as well as Cu/Fe ratio (r = -0.152, P = 0.3).
5. Discussion
Several studies have reported that the metabolism of some trace elements is altered in DM and that these alterations might be a contributing factor in the pathogenesis of this disease (6, 12, 31). In the present study, a difference in the level of the studied trace elements was observed in both T2DM and FDR subjects. We observed higher values of Cu, Fe, Fe/Zn ratio, and Cu/Zn ratio and lower Zn levels and Cu/Fe ratio in FDR compared with the control subjects. According to our knowledge, this work was the first study for reporting the analysis of Cu, Zn, and Fe among the FDR of diabetic patients. Ozkaya et al. observed a lower level of selenium in FDR, suggesting selenium deficiency as a risk factor for cardiovascular disease in this group (32). In fact, many essential metals are shown to have interactive connections with DM (33). Alterations in the metabolism of several essential minerals, including Cu, Zn, Mn, Cr, Mg, and Fe have been associated with impaired insulin release, insulin resistance, and glucose intolerance (34).
The crucial role of Cu and Zn in oxidative stress is well-known (9, 35). Cu and Zn are needed for the essential activity of antioxidant enzyme Cu/Zn superoxide dismutase (SOD). Abnormal metabolism of Cu and Zn can affect the function of SOD and result in decreased protection of cells from superoxide radical (29, 30). Changes in the levels of Cu, Zn, and Cu/Zn ratio by influencing the antioxidant defense system might elevate the toxic effect of free radicals. Besides, hyperglycemia and hyperinsulinemia increase the production of free radicals and decrease the efficiency of antioxidant defense systems, which may lead to the complications of diabetes (7, 36).
An increase in Cu concentration has been linked to disorders in the structure of arterial walls, stress, infection, and DM (37). In this study, we observed that serum concentration of Cu was significantly higher in T2DM compared with the control subjects, which was consistent with previous studies on T2DM patients (38-40). Basaki et al. showed that Cu was significantly less in the serum of the patients with diabetes (41). In contrast, Terres-Martos et al. established that serum Cu concentrations were not significantly different in the diabetic patients compared with the controls (22).
It has been frequently reported that Zn deficiency is associated with diabetic complications such as hypertension, retinopathy, thrombosis, reduced insulin secretion, and increased tissue resistance to insulin action in T2DM (6, 8). However, the actual role of Zn deficiency in diabetes is still unclear (42, 43). Consistent with the previous studies on T2DM patients, we observed that the serum concentration of Zn was significantly less in T2DM compared with the control subjects (22). Al-Maroof et al. observed lower serum Zn levels in type 2 diabetics compared with healthy controls. They reported that Zn supplementation for type 2 diabetics had beneficial effects on elevating their serum Zn level (16).
Many studies have suggested a possible relationship between high body Fe stores and metabolic parameters, like serum insulin and glucose (44, 45), hypertension (46), dyslipidemia (47, 48), and obesity (49). In addition, epidemiological studies have reported an association between high Fe stores as a pro-oxidant and increased risk of cardiovascular disease (50), metabolic syndrome (51), gestational diabetes (52, 53), and type 2 diabetes (54-56). In the present study, we observed that serum concentration of Fe was significantly higher in T2DM compared to the control subjects, consistent with previous studies of T2DM patients (57-59). Gohel et al. showed that the serum level of free iron was higher in the patients with poorly controlled type 2 DM (60). In the current study, we excluded the patients with diabetic complications; however, examining Fe levels in the complicated diabetic patients could be evaluated in the future studies.
In this study, we also found that HbA1c levels were positively correlated with Cu as well as with Fe and Cu/Zn ratio and inversely correlated with Zn in T2DM and FDR groups. Consistent with our results, Viktorinova et al. reported that HbA1c levels were positively correlated with Cu as well as Cu/Zn ratio and inversely correlated with Zn in DM patients (12). In addition, Shetty et al. reported that HbA1c levels were positively correlated with free iron in the patients with T2DM (57). In contrast, Dorre et al. showed no significant correlation between serum Zn level and HbA1c (61). The difference between this study and our results might be because of the Hb status of the patients, which was not assessed in our study. Besides, HbA1c level in DM patients may depend on the treatment they receive for diabetes rather than Cu, Zn, and Fe levels. However, it should be noted that lower Zn level might lead to higher blood glucose as a result of low insulin synthesis or action and consequently, higher HbA1c. This issue could be one probable reason for the correlation between HbA1c and Zn, Cu, or Fe, which has negative correlation with Zn in most circumstances.
Besides, some investigators have reported the hypothesis that glycated proteins binding to transition metals such as Cu and Fe may result in glycocholates formation, which plays an important role in the etiology of peripheral vascular dysfunction and peripheral neuropathies in DM (29, 30). However, the association of these elements with HbA1c should be evaluated to better clarify the related mechanisms (12). In addition, for obtaining strong consequences, factors including Hb, BMI, duration of diabetes, lipid hydroperoxides, hypertention and lipoprotein should be also evaluated, which were the limitations in our study.
Taken together, because of significant differences in the levels of the studied elements, despite the lack of differences in FBG and HbA1c levels between FDR and healthy control groups, Cu, Zn, and Fe levels as well as Cu/Zn, Cu/Fe, and Fe/Zn ratios might be useful to estimate the upcoming risk of diabetes in FDR. In addition, improving the levels of these elements by medical interventions might be helpful in decreasing diabetes risk, which should be evaluated in the future studies. Besides, the results of this study should be approved by comprehensive assessment, especially atomic absorption as the standard method, for measuring these elements.
In conclusion, the present results demonstrated an imbalance in the levels of Cu, Zn, and Fe among the patients with T2DM and FDR compared with the control subjects. The difference of Cu and Zn levels may play an important role in the pathogenesis of this disease by the involvement of these elements in the oxidative stress response. On the other hand, increasing Fe as a strong pro-oxidant that catalyzes the formation of hydroxyl radicals and results in the increased levels of oxidative stress might be associated with the risk of diabetes in FDR. We also showed that increased levels of Cu, Fe, and Cu/Zn ratio and decreased levels of Zn were associated with the increased value of HbA1c in diabetic patients and FDR. As an assumption, the imbalance of trace elements in FDR might be the sign of diabetes later in their lives. Besides, it seems necessary to consider possible changes in the metabolism of essential trace elements such as Cu, Zn, and Fe.
Acknowledgments
The authors sincerely appreciate the following organizations and people for their support: laboratory of Imam Khomeini hospital, Urmia, Iran, and its staffs.
Footnotes
Authors’ Contribution:Study concept and design: Fatemeh Kheradmand; analysis and interpretation of data: Somayeh Atari-Hajipirloo, Fatemeh Kheradmand; drafting of the manuscript: Somayeh Atari-Hajipirloo, Fatemeh Kheradmand; critical revision of the manuscript for important intellectual content: Fatemeh Kheradmand, Somayeh Atari-Hajipirloo, Neda Valizadeh, Mohammad-Hassan Khadem-Ansari and Yousef Rasmi; statistical analysis: Somayeh Atari-Hajipirloo.
Financial Disclosure:The Authors declare that they have no conflicts of interest to disclose.
Funding/Support:This study was supported by Urmia University of Medical Sciences, Urmia, Iran.
References
- 1.Groop LC. Insulin resistance: the fundamental trigger of type 2 diabetes. Diabetes Obes Metab. 1999;1 Suppl 1:S1–7. doi: 10.1046/j.1463-1326.1999.0010s1001.x. [DOI] [PubMed] [Google Scholar]
- 2.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15(7):539–53. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 3.Stewart MW, Humphriss DB, Berrish TS, Barriocanal LA, Trajano LR, Alberti KG, et al. Features of syndrome X in first-degree relatives of NIDDM patients. Diabetes Care. 1995;18(7):1020–2. doi: 10.2337/diacare.18.7.1020. [DOI] [PubMed] [Google Scholar]
- 4.Martin BC, Warram JH, Krolewski AS, Bergman RN, Soeldner JS, Kahn CR. Role of glucose and insulin resistance in development of type 2 diabetes mellitus: results of a 25-year follow-up study. Lancet. 1992;340(8825):925–9. doi: 10.1016/0140-6736(92)92814-v. [DOI] [PubMed] [Google Scholar]
- 5.Henkin RI. Trace metals in endocrinology. Med Clin North Am. 1976;60(4):779–97. doi: 10.1016/s0025-7125(16)31861-2. [DOI] [PubMed] [Google Scholar]
- 6.Chausmer AB. Zinc, insulin and diabetes. J Am Coll Nutr. 1998;17(2):109–15. doi: 10.1080/07315724.1998.10718735. [DOI] [PubMed] [Google Scholar]
- 7.Ceriello A. Oxidative stress and glycemic regulation. Metabolism. 2000;49(2 Suppl 1):27–9. doi: 10.1016/s0026-0495(00)80082-7. [DOI] [PubMed] [Google Scholar]
- 8.Soinio M, Marniemi J, Laakso M, Pyorala K, Lehto S, Ronnemaa T. Serum zinc level and coronary heart disease events in patients with type 2 diabetes. Diabetes Care. 2007;30(3):523–8. doi: 10.2337/dc06-1682. [DOI] [PubMed] [Google Scholar]
- 9.Valko M, Morris H, Cronin MT. Metals, toxicity and oxidative stress. Curr Med Chem. 2005;12(10):1161–208. doi: 10.2174/0929867053764635. [DOI] [PubMed] [Google Scholar]
- 10.Prasad AS, Bao B, Beck FW, Kucuk O, Sarkar FH. Antioxidant effect of zinc in humans. Free Radic Biol Med. 2004;37(8):1182–90. doi: 10.1016/j.freeradbiomed.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 11.Opara EC. Oxidative stress, micronutrients, diabetes mellitus and its complications. J R Soc Promot Health. 2002;122(1):28–34. doi: 10.1177/146642400212200112. [DOI] [PubMed] [Google Scholar]
- 12.Viktorinova A, Toserova E, Krizko M, Durackova Z. Altered metabolism of copper, zinc, and magnesium is associated with increased levels of glycated hemoglobin in patients with diabetes mellitus. Metabolism. 2009;58(10):1477–82. doi: 10.1016/j.metabol.2009.04.035. [DOI] [PubMed] [Google Scholar]
- 13.Xu J, Zhou Q, Liu G, Tan Y, Cai L. Analysis of serum and urinal copper and zinc in Chinese northeast population with the prediabetes or diabetes with and without complications. Oxid Med Cell Longev. 2013;2013:635214. doi: 10.1155/2013/635214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kinlaw WB, Levine AS, Morley JE, Silvis SE, McClain CJ. Abnormal zinc metabolism in type II diabetes mellitus. Am J Med. 1983;75(2):273–7. doi: 10.1016/0002-9343(83)91205-6. [DOI] [PubMed] [Google Scholar]
- 15.Fitzharris JW. Abnormal zinc metabolism in type II diabetes mellitus. Am J Med. 1984;76(6):e33273. doi: 10.1016/0002-9343(84)90845-3. A75. [DOI] [PubMed] [Google Scholar]
- 16.Al-Maroof RA, Al-Sharbatti SS. Serum zinc levels in diabetic patients and effect of zinc supplementation on glycemic control of type 2 diabetics. Saudi Med J. 2006;27(3):344–50. [PubMed] [Google Scholar]
- 17.Anderson RA, Roussel AM, Zouari N, Mahjoub S, Matheau JM, Kerkeni A. Potential antioxidant effects of zinc and chromium supplementation in people with type 2 diabetes mellitus. J Am Coll Nutr. 2001;20(3):212–8. doi: 10.1080/07315724.2001.10719034. [DOI] [PubMed] [Google Scholar]
- 18.Kazi TG, Afridi HI, Kazi N, Jamali MK, Arain MB, Jalbani N, et al. Copper, chromium, manganese, iron, nickel, and zinc levels in biological samples of diabetes mellitus patients. Biol Trace Elem Res. 2008;122(1):1–18. doi: 10.1007/s12011-007-8062-y. [DOI] [PubMed] [Google Scholar]
- 19.Zhuang T, Han H, Yang Z. Iron, oxidative stress and gestational diabetes. Nutrients. 2014;6(9):3968–80. doi: 10.3390/nu6093968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fernandez-Real JM, Lopez-Bermejo A, Ricart W. Cross-talk between iron metabolism and diabetes. Diabetes. 2002;51(8):2348–54. doi: 10.2337/diabetes.51.8.2348. [DOI] [PubMed] [Google Scholar]
- 21.Niederau C, Berger M, Stremmel W, Starke A, Strohmeyer G, Ebert R, et al. Hyperinsulinaemia in non-cirrhotic haemochromatosis: impaired hepatic insulin degradation? Diabetologia. 1984;26(6):441–4. doi: 10.1007/BF00262217. [DOI] [PubMed] [Google Scholar]
- 22.Terres-Martos C, Navarro-Alarcon M, Martín-Lagos F, Lopez-Ga De La Serrana H, Perez-Valero V, Lopez-Mart ínez MC. Serum Zinc and Copper Concentrations and Cu/Zn ratios in Patients with Hepatopathies or Diabetes. J Adv Lab Res Biol. 1998;12(1):44–9. doi: 10.1016/s0946-672x(98)80020-5. [DOI] [PubMed] [Google Scholar]
- 23.Ekmekcioglu C, Prohaska C, Pomazal K, Steffan I, Schernthaner G, Marktl W. Concentrations of Seven Trace Elements in Different Hematological Matrices in Patients with Type 2 Diabetes as Compared to Healthy Controls. Biol Trace Elem Res. 2001;79(3):205–19. doi: 10.1385/bter:79:3:205. [DOI] [PubMed] [Google Scholar]
- 24.Fernandez-Real JM, Penarroja G, Castro A, Garcia-Bragado F, Hernandez-Aguado I, Ricart W. Blood letting in high-ferritin type 2 diabetes: effects on insulin sensitivity and beta-cell function. Diabetes. 2002;51(4):1000–4. doi: 10.2337/diabetes.51.4.1000. [DOI] [PubMed] [Google Scholar]
- 25.De Rosa MC, Sanna MT, Messana I, Castagnola M, Galtieri A, Tellone E, et al. Glycated human hemoglobin (HbA1c): functional characteristics and molecular modeling studies. Biophys Chem. 1998;72(3):323–35. doi: 10.1016/s0301-4622(98)00117-3. [DOI] [PubMed] [Google Scholar]
- 26.Bunn HF, Haney DN, Kamin S, Gabbay KH, Gallop PM. The biosynthesis of human hemoglobin A1c. Slow glycosylation of hemoglobin in vivo. J Clin Invest. 1976;57(6):1652–9. doi: 10.1172/JCI108436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rahbar S. An abnormal hemoglobin in red cells of diabetics. Clin Chim Acta. 1968;22(2):296–8. doi: 10.1016/0009-8981(68)90372-0. [DOI] [PubMed] [Google Scholar]
- 28.Shapiro R, McManus M, Garrick L, McDonald MJ, Bunn HF. Nonenzymatic glycosylation of human hemoglobin at multiple sites. Metabolism. 1979;28(4):427–30. doi: 10.1016/0026-0495(79)90050-7. [DOI] [PubMed] [Google Scholar]
- 29.Galhardi CM, Diniz YS, Faine LA, Rodrigues HG, Burneiko RC, Ribas BO, et al. Toxicity of copper intake: lipid profile, oxidative stress and susceptibility to renal dysfunction. Food Chem Toxicol. 2004;42(12):2053–60. doi: 10.1016/j.fct.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 30.Cooper GJ, Chan YK, Dissanayake AM, Leahy FE, Keogh GF, Frampton CM, et al. Demonstration of a hyperglycemia-driven pathogenic abnormality of copper homeostasis in diabetes and its reversibility by selective chelation: quantitative comparisons between the biology of copper and eight other nutritionally essential elements in normal and diabetic individuals. Diabetes. 2005;54(5):1468–76. doi: 10.2337/diabetes.54.5.1468. [DOI] [PubMed] [Google Scholar]
- 31.Aguilar MV, Saavedra P, Arrieta FJ, Mateos CJ, Gonzalez MJ, Meseguer I, et al. Plasma mineral content in type-2 diabetic patients and their association with the metabolic syndrome. Ann Nutr Metab. 2007;51(5):402–6. doi: 10.1159/000108108. [DOI] [PubMed] [Google Scholar]
- 32.Ozkaya M, Sahin M, Cakal E, Gisi K, Bilge F, Kilinc M. Selenium levels in first-degree relatives of diabetic patients. Biol Trace Elem Res. 2009;128(2):144–51. doi: 10.1007/s12011-008-8263-z. [DOI] [PubMed] [Google Scholar]
- 33.Chen MD, Lin PY, Tsou CT, Wang JJ, Lin WH. Selected metals status in patients with noninsulin-dependent diabetes mellitus. Biol Trace Elem Res. 1995;50(2):119–24. doi: 10.1007/BF02789414. [DOI] [PubMed] [Google Scholar]
- 34.Noto R, Alicata R, Sfogliano L, Neri S, Bifarella M. A study of cupremia in a group of elderly diabetics. Acta Diabetol Lat. 1983;20(1):81–5. doi: 10.1007/BF02629133. [DOI] [PubMed] [Google Scholar]
- 35.DiSilvestro RA. Zinc in relation to diabetes and oxidative disease. J Nutr. 2000;130(5S Suppl):1509S–11S. doi: 10.1093/jn/130.5.1509S. [DOI] [PubMed] [Google Scholar]
- 36.Van Campenhout A, Van Campenhout C, Lagrou AR, Abrams P, Moorkens G, Van Gaal L, et al. Impact of diabetes mellitus on the relationships between iron-, inflammatory- and oxidative stress status. Diabetes Metab Res Rev. 2006;22(6):444–54. doi: 10.1002/dmrr.635. [DOI] [PubMed] [Google Scholar]
- 37.Beshgetoor D, Hambidge M. Clinical conditions altering copper metabolism in humans. Am J Clin Nutr. 1998;67(5 Suppl):1017S–21S. doi: 10.1093/ajcn/67.5.1017S. [DOI] [PubMed] [Google Scholar]
- 38.Abou-Seif MA, Youssef AA. Evaluation of some biochemical changes in diabetic patients. Clin Chim Acta. 2004;346(2):161–70. doi: 10.1016/j.cccn.2004.03.030. [DOI] [PubMed] [Google Scholar]
- 39.Zhao C, Wang H, Zhang J, Feng L. [Correlations of trace elements, glucose and body compositions in type 2 diabetics]. Wei Sheng Yan Jiu. 2008;37(5):600–1. 605. [PubMed] [Google Scholar]
- 40.El-Zebda G, Abed AA, El-Ashgar NM. Significance of serum levels of copper and zinc in Type II diabetic, hypertensive, and diabetic hypertensive patients in Gaza City. The Islamic University-Gaza. 2006 [Google Scholar]
- 41.Basaki M, Saeb M, Nazifi S, Shamsaei HA. Zinc, copper, iron, and chromium concentrations in young patients with type 2 diabetes mellitus. Biol Trace Elem Res. 2012;148(2):161–4. doi: 10.1007/s12011-012-9360-6. [DOI] [PubMed] [Google Scholar]
- 42.Ghayour-Mobarhan M, Taylor A, New SA, Lamb DJ, Ferns GA. Determinants of serum copper, zinc and selenium in healthy subjects. Ann Clin Biochem. 2005;42(Pt 5):364–75. doi: 10.1258/0004563054889990. [DOI] [PubMed] [Google Scholar]
- 43.Ruiz C, Alegría A, Barbera R, Farre R, Lagarda MJ. Selenium, Zinc and Copper in Plasma of patients with Type 1 Diabetes Mellitus in Different Metabolic Control States. Trace Elem Med J. 1998;12(2):91–5. doi: 10.1016/s0946-672x(98)80031-x. [DOI] [PubMed] [Google Scholar]
- 44.Ramakrishnan U, Kuklina E, Stein AD. Iron stores and cardiovascular disease risk factors in women of reproductive age in the United States. Am J Clin Nutr. 2002;76(6):1256–60. doi: 10.1093/ajcn/76.6.1256. [DOI] [PubMed] [Google Scholar]
- 45.Sheu WH, Chen YT, Lee WJ, Wang CW, Lin LY. A relationship between serum ferritin and the insulin resistance syndrome is present in non-diabetic women but not in non-diabetic men. Clin Endocrinol (Oxf). 2003;58(3):380–5. doi: 10.1046/j.1365-2265.2003.01729.x. [DOI] [PubMed] [Google Scholar]
- 46.Piperno A, Trombini P, Gelosa M, Mauri V, Pecci V, Vergani A, et al. Increased serum ferritin is common in men with essential hypertension. J Hypertens. 2002;20(8):1513–8. doi: 10.1097/00004872-200208000-00013. [DOI] [PubMed] [Google Scholar]
- 47.Halle M, Konig D, Berg A, Keul J, Baumstark MW. Relationship of serum ferritin concentrations with metabolic cardiovascular risk factors in men without evidence for coronary artery disease. Atherosclerosis. 1997;128(2):235–40. doi: 10.1016/s0021-9150(96)05994-1. [DOI] [PubMed] [Google Scholar]
- 48.Williams MJ, Poulton R, Williams S. Relationship of serum ferritin with cardiovascular risk factors and inflammation in young men and women. Atherosclerosis. 2002;165(1):179–84. doi: 10.1016/s0021-9150(02)00233-2. [DOI] [PubMed] [Google Scholar]
- 49.Gillum RF. Association of serum ferritin and indices of body fat distribution and obesity in Mexican American men--the Third National Health and Nutrition Examination Survey. Int J Obes Relat Metab Disord. 2001;25(5):639–45. doi: 10.1038/sj.ijo.0801561. [DOI] [PubMed] [Google Scholar]
- 50.Alpert PT. New and emerging theories of cardiovascular disease: infection and elevated iron. Biol Res Nurs. 2004;6(1):3–10. doi: 10.1177/1099800404264777. [DOI] [PubMed] [Google Scholar]
- 51.Jehn M, Clark JM, Guallar E. Serum ferritin and risk of the metabolic syndrome in U.S. adults. Diabetes Care. 2004;27(10):2422–8. doi: 10.2337/diacare.27.10.2422. [DOI] [PubMed] [Google Scholar]
- 52.Lao TT, Tam KF. Maternal serum ferritin and gestational impaired glucose tolerance. Diabetes Care. 1997;20(9):1368–9. doi: 10.2337/diacare.20.9.1368. [DOI] [PubMed] [Google Scholar]
- 53.Lao TT, Chan PL, Tam KF. Gestational diabetes mellitus in the last trimester - a feature of maternal iron excess? Diabet Med. 2001;18(3):218–23. doi: 10.1046/j.1464-5491.2001.00453.x. [DOI] [PubMed] [Google Scholar]
- 54.Ford ES, Cogswell ME. Diabetes and serum ferritin concentration among U.S. adults. Diabetes Care. 1999;22(12):1978–83. doi: 10.2337/diacare.22.12.1978. [DOI] [PubMed] [Google Scholar]
- 55.Kaye TB, Guay AT, Simonson DC. Non-insulin-dependent diabetes mellitus and elevated serum ferritin level. J Diabetes Complications. 1993;7(4):246–9. doi: 10.1016/0891-6632(93)90008-5. [DOI] [PubMed] [Google Scholar]
- 56.Thomas MC, MacIsaac RJ, Tsalamandris C, Jerums G. Elevated iron indices in patients with diabetes. Diabet Med. 2004;21(7):798–802. doi: 10.1111/j.1464-5491.2004.01196.x. [DOI] [PubMed] [Google Scholar]
- 57.Shetty JK, Prakash M, Ibrahim MS. Relationship between free iron and glycated hemoglobin in uncontrolled type 2 diabetes patients associated with complications. Indian J Clin Biochem. 2008;23(1):67–70. doi: 10.1007/s12291-008-0016-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ekin S, Mert N, Gunduz H, Meral I. Serum Sialic Acid Levels and Selected Mineral Status in Patients with Type 2 Diabetes Mellitus. Trace Elem Med J. 2003;94(3):193–202. doi: 10.1385/bter:94:3:193. [DOI] [PubMed] [Google Scholar]
- 59.Kundu D, Roy A, Mandal T, Bandyopadhyay U, Ghosh E, Ray D. Relation of iron stores to oxidative stress in type 2 diabetes. Niger J Clin Pract. 2013;16(1):100–3. doi: 10.4103/1119-3077.106776. [DOI] [PubMed] [Google Scholar]
- 60.Gohel M, Sirajwala HB, Chacko A. Serum free iron concentration in patients with type 2 diabetes mellitus with good and poor control and its correlation with glycemic control. Int J of Diab Res. 2013;2(2):33–8. [Google Scholar]
- 61.Dorre F, Rezvanfar M, Ghaseminegad S. Comparison of Serum Zinc Level in Patients with Diabetes Type 1 and 2 and Its' Relation to with HbA1c. Zahedan J Res Med Sci. 2014;16(1):48–50. [Google Scholar]