Abstract
The neurobiology of anorexia nervosa remains incompletely understood. Here we utilized PET imaging with the radiotracer [11C]raclopride to measure striatal dopamine type 2 (D2) receptor availability in patients with anorexia nervosa. 25 women with anorexia nervosa who were receiving treatment in an inpatient program participated, as well as 25 control subjects. Patients were scanned up to two times with the PET tracer [11C]raclopride: once while underweight, and once upon weight restoration. Control subjects underwent one PET scan. In the primary analyses, there were no significant differences between underweight patients (n=21) and control subjects (n=25) in striatal D2 receptor binding potential. Analysis of subregions (sensorimotor striatum, associative striatum, limbic striatum) did not reveal differences between groups. In patients completing both scans (n=15), there were no detectable changes in striatal D2 receptor binding potential after weight restoration. In this sample, there were no differences in striatal D2 receptor binding potential between patients with anorexia nervosa and control subjects. Weight restoration was not associated with a change in striatal D2 receptor binding. These findings suggest that disturbances in reward processing in this disorder are not attributable to abnormal D2 receptor characteristics, and that other reward-related neural targets may be of greater relevance.
Keywords: eating disorders, Positron Emission Tomography, striatum, dopamine D2 receptor, [11C]raclopride, neuroimaging
1. Introduction
The neurobiology of anorexia nervosa (AN) is incompletely understood. Current theories regarding the neurobiology of this illness are wide-ranging, and include hypotheses relevant to the possible placement of AN within the “internalizing” spectrum of disorders, including mood disorders, anxiety disorders, and obsessive compulsive disorder (OCD) (Forbush et al., 2010), as well as hypotheses which suggest that brain reward systems may be altered in AN, thus altering motivation and responses towards food reward stimuli [reviewed in (Kaye et al., 2013)]. As the striatal dopamine (DA) system bears potential relevance to both conceptualizations of this illness, the current study sought to assess brain reward circuitry in AN through evaluation of striatal dopamine D2 receptor levels (specifically, D2 receptor availability for tracer binding) using Positron Emission Tomography (PET) neuroimaging with the radiotracer [11C]raclopride.
The striatal D2 receptor is one of five dopamine (DA) receptor subtypes in the brain, with high concentration in the striatum. Within the striatum, the D2 receptor acts as a presynaptic autoreceptor, regulating the release, re-uptake, and synthesis of DA; postsynaptically, it is also present on medium spiny neurons regulating outflow tracts from the striatum (Beaulieu and Gainetdinov, 2011; Ford, 2014). The striatal D2 receptor’s role in reward processing has been considered in previous clinical studies. For example, studies of the striatal D2 receptor in healthy human subjects have been performed using PET neuroreceptor neuroimaging with tracers such as [11C]raclopride, which binds the D2 family of receptors (including D3 and D4 receptors), indicating that striatal D2 receptor availability (as measured by “binding potential”) may influence preferences for rewards (Volkow et al., 1999; Volkow et al., 2002). Whether such findings might relate to clinical expression of the extreme avoidance of food reward seen in AN is as yet unknown. Additionally, the striatal DA system, and specifically the D2 receptor, is thought to influence cognitive flexibility, a neurobiological dimension thought to be impaired in disorders including OCD (Klanker et al., 2013) and AN (Tchanturia et al., 2014; Treasure and Schmidt, 2013). Therefore, the striatal D2 receptor may bear relevance to the behaviors of AN from a variety of neurobiological perspectives. Studies of the striatal D2 receptor have also been conducted across a large range of mood, anxiety, and substance use disorders – disorders which are all of potential relevance to AN. While findings across mood and anxiety disorders have been somewhat discrepant (reviewed in the discussion), a large number of studies utilizing PET imaging techniques with [11C]raclopride have been conducted in substance use disorders, demonstrating decreased striatal D2 receptor availability in these conditions [reviewed in (Trifilieff and Martinez, 2014; Volkow et al., 2009)]. Similar findings of diminished striatal D2 receptors have been seen in a smaller number of studies of obesity (de Weijer et al., 2011; Volkow et al., 2008; Wang et al., 2001), and a trend towards diminished striatal D2 receptors was observed in a previous study in bulimia nervosa (Broft et al., 2012).
To our knowledge, only two published studies to date have evaluated striatal D2 receptor availability in patients with AN compared to control subjects. Both of these studies evaluated patients recovered from AN: an earlier study reported increased D2 receptor availability relative to control subjects in the anteroventral striatum, an area of the striatum particularly associated with reward salience (Frank et al., 2005), though a subsequent study reported no difference between recovered patients and control subjects in any striatal subregions (Bailer et al., 2013).
To our knowledge, no study of striatal D2 receptor availability in AN has been conducted in an acutely underweight (non-recovered) population, and no study has been conducted in a longitudinal fashion, testing subjects before and after nutritional restoration. Here we utilized PET imaging with the radiotracer [11C]raclopride to measure striatal D2 receptor availability in patients with AN. In this study, we chose to examine a population of patients acutely underweight, who were in the early stages of undergoing inpatient treatment for AN. We also studied patients with AN at a second timepoint – i.e. once they had achieved weight restoration. This design allowed us to study acute illness, while also partially accounting for the confounder of acute malnutrition, in that (1) underweight patients had been acutely stabilized nutritionally, through the beginnings of participation in an inpatient program, and (2) a direct, within-subject comparison between D2 findings in an underweight vs. weight-restored (non-malnourished) state was possible. On the basis of the previous published study in weight-restored patients with a history of AN, we hypothesized that we would find (1) increased striatal D2 receptor availability in the striatum in acutely-underweight patients with AN, compared to control subjects and (2) a decrease in striatal D2 receptor availability after weight restoration (reflecting normalization of receptors over the course of treatment).
2. Methods
The study was conducted at the Eating Disorders Research Unit of the New York State Psychiatric Institute/Columbia University Medical Center, as well as at Weill Cornell Medical College. The study was reviewed and approved by the New York State Psychiatric Institute/Columbia University IRB as well as the Weill Cornell Medical College IRB, and was registered with clinicaltrials.gov.
2.1. Calculation of sample size
Based on the effect sizes from PET studies of differences in D2 receptor availability reported in individuals with substance abuse vs. controls (Martinez et al., 2004), and in PET studies of obese vs. normal weight individuals (Wang et al., 2001), we initially calculated that a sample size of 15 patients with AN and 15 control participants would provide sufficient (80%) power to detect a moderate (e.g. 15%) difference In D2 receptor availability with α = 0.05 (two-tailed). [Based on (Martinez et al., 2004), and comparing a main effect of diagnosis on two independent means (control subjects’ striatal D2 receptor binding potential 4.13 +/− 0.49; patients with cocaine use disorder: 3.58 +/− 0.40): Cohen’s d=1.23, which suggests a minimum sample size of 12 patients/12 control subjects. Here, an initial sample size of 15 patients with AN and 15 control subjects was calculated to provide 90% power to detect a similar relative difference in striatal D2 binding potential, or 80% power to detect a 12% difference (Faul F, 2009). A second scanner site was later introduced, due to the temporary closure of the primary site’s PET center. At the time, we had recruited and scanned 11 patients with AN and 17 control subjects; we subsequently adjusted our planned sample size to recruit a similar number of subjects at each site, ultimately yielding a sample size of 50 subjects. In the cases of subjects with AN who completed both the baseline (underweight) and weight-restored scans, both scans were completed at the same scan site; therefore, scanner type was not confounded with baseline vs. follow-up imaging.
2.2. Recruitment and screening
Women seeking inpatient treatment for AN were recruited via self-referral and referral from clinicians; control participants responded to notices and advertising in local media. Subjects with both subtypes of AN (AN, restricting subtype, “AN-R”; and AN, binge-purge subtype, “AN-BP”) were recruited. 17/25 of the control subjects presented here had been previously incorporated for comparison purposes in a concurrent PET imaging study evaluating the striatal D2 receptor in patients with bulimia nervosa (Broft et al., 2012). During the subject’s initial phone call to the clinic, potential participants were told about the study by a research coordinator, and information was collected from the subject after verbal consent was obtained. Following this telephone assessment, those participants who continued to be interested and eligible underwent a longer assessment, including (1) full psychiatric and medical assessment, including physical exam, (2) a complete blood count, basic metabolic panel, liver function tests, thyroid stimulating hormone, and serum pregnancy test, (3) urine toxicology, (4) electrocardiogram, (5) Structured Clinical Interview for DSM-IV (First, 1995), and the Eating Disorder Examination-12 (Fairburn, 1993).
2.3. Inclusion/exclusion
Participants were excluded if they met DSM-IV-TR criteria for current or past Axis I disorders, other than anorexia nervosa, for the patient group. Patients with AN were not excluded by the presence of mild or moderate depressive and anxiety symptoms; patients with severe anxiety and depressive symptoms requiring specialized treatment, such as medications, were not eligible. Patients who reported a diagnosis of current ADHD during the telephone assessment or in-person interview were excluded from the study. In addition, participants were excluded for the presence of (1) past histories of abuse or dependency on alcohol or other drugs (assessed by phone interview, in-person MD clinical interview, and urine drug screen on the screening day), (2) active suicidal ideation, (3) use of psychoactive medications in the 4 weeks prior to the study, other than nicotine patch and occasional sleep medications, (4) ongoing medical or neurological illness, (5) pregnancy, (6) exposure to radiation in the workplace, or nuclear medicine procedures during the previous year, and (7) presence of metallic implants that could be adversely affected by MRI procedures. Patients were eligible for inclusion in the second, post-weight restoration PET scan if they had achieved a minimum of 90% of ideal body weight according to Metropolitan Life Insurance actuarial data typically used in the treatment program for this assessment, at >2 consecutive weight assessments.
Participants who continued to be eligible and interested provided written informed consent. Patients were offered treatment for AN as part of their participation in the study; control participants were given financial compensation. All patients with anorexia nervosa received treatment at the same inpatient center (New York State Psychiatric Institute, New York, NY).
2.4. Scanning protocol
PET scanning was conducted at two sites: at Columbia University Medical Center (first phase of study, ECAT EXACT HR+ camera), and at the Weill Cornell Medical College (GE Discovery LS). For control subjects, and for patients with AN who were not amenorrheic at the time of the scan, we attempted, when possible, to perform PET studies during the early follicular phase of the menstrual cycle, due to previous work suggesting variation in brain DA-related measures including D2 receptor binding over the menstrual cycle (Czoty et al., 2009). As follicular phase scanning was sometimes not possible, we also attempted to match patients and control participants for menstrual cycle status. Menstrual cycle status was determined through a combination of self-monitoring menstrual status (subjects kept a log from the time of recruitment to the time of their scans) as well as reproductive hormone measures via blood draw on the day of their scans. Ultimately, hormonal status was necessarily somewhat different between the patient and control groups (see Results section), as 16 out of 21 underweight patients with AN were not menstruating due to their eating disorder and resultant medical status at the time of the underweight scan.
The radiotracer [11C]raclopride (maximum dose 15 mCi/scan), was synthesized on-site immediately prior to scanning. This radiotracer has been used extensively in several psychiatric populations (Mawlawi et al., 2001). Due to concerns regarding risk/benefit of PET scan participation in patients with higher levels of medical compromise (e.g. concern for risk of syncope and falls during transport and procedures in patients who are the most malnourished), inpatients with AN were scanned at a weight not less than 75% of ideal body weight (BMI~16.5). In some cases, this meant that patients’ initial scan was conducted after several weeks of treatment. In some cases (e.g. for clinical or logistical reasons), a small number of patients with AN were not able to participate until reaching 90%, or close to 90%; these subjects participated in the weight-restored scan only to maximize data collection, and a more limited number of analyses were possible with these data. Both patients and control participants were given a standardized meal of an English muffin, 1 pat of butter, and 8 fl oz apple juice, for whatever meal (breakfast or lunch) immediately preceded scanning procedures. When possible, patients with AN were invited to participate in one scan with [11C]raclopride while acutely underweight, and a second scan, upon weight restoration. Some patients were invited to participate in the weight-restored scan only, in cases where patients were admitted to the program well above 75% of ideal body weight, or in other circumstances where participation during the underweight phase of their treatment had not been possible. An MRI was acquired for co-registration of PET data.
2.5. Analyses
PET data were co-registered to each subject’s MRI according to methods previously used by the Division of Functional Brain Mapping, Columbia University Medical Center (Mawlawi et al., 2001) for anatomical localization of regions of interest. Five ROI were identified on the MRI, including the ventral striatum, the caudate rostral to the anterior commissure (dorsal caudate), the caudate caudal to the anterior commissure (posterior caudate), the putamen rostral to the anterior commissure (anterior putamen), and the putamen caudal to the anterior commissure (posterior putamen). Activities from left and right regions were averaged. The activity of the striatum as a whole was derived as the spatially-weighted average of the ROIs. The cerebellum was used as the reference region. After regions were drawn on the MRI, their boundaries were applied to the coregistered PET and average activity in each ROI at each time point was used to generate time activity curves for kinetic analysis. Functional ROI were also determined as volume-weighted averages of the manually drawn ROI: the sensorimotor striatum (defined as the posterior putamen), the associative striatum (caudate plus anterior putamen), and the limbic striatum (ventral striatum), consistent with the functionality of the cortico-striatal-thalamo-cortical loops contained within these subregions (e.g. sensorimotor striatum’s role in locomotion; limbic striatum’s role in drive and motivation; see (Martinez et al., 2003) for a more detailed description of the rationale for this organization). In order to limit the number of anatomical regions the statistical model, these regions were used in the primary analysis. As [11C]raclopride does not demonstrate significant binding to extrastriatal D2 receptors, no analysis was completed outside of the hypothesized ROIs.
The simplified reference tissue model (SRTM) (Lammertsma and Hume, 1996) was used for derivation of the binding potential (BPND) (Innis et al., 2007) implemented in MATLAB (The Math Works, Inc., South Natick, Massachusetts), using the cerebellum as the reference region. BPND is defined as the ratio of specifically bound to nondisplaceable radioligand at equilibrium and can also be described as:
where Bmax is the concentration of D2/3 receptors, Kd is the equilibrium dissociation constant of the radiotracer for the receptor, and fnd is the free fraction in the nonspecific distribution volume of the brain (Innis et al., 2007; Slifstein and Laruelle, 2001). [11C]raclopride has a similar affinity for D2 and D3 receptors (Sokoloff et al., 1990), and the signal from these receptors cannot be distinguished.
2.6. Statistical modeling
Two primary analyses were conducted. Effects of diagnosis and region on primary outcome measures (striatal BPND) were statistically tested using a linear mixed models analysis (SPSS 21 for Mac) of the relationship between D2 receptor BPND and diagnosis. As fixed effects, we entered region (as repeated measure), diagnosis (underweight patient vs. healthy control), and age. Scan site was entered as a random effect. For the second main analysis, an analysis of the effect of condition on striatal BPND in only the 15 patients with AN who completed testing at both timepoints [ΔBPND, the relative change across conditions] was completed, via paired t-test. Associations between D2 receptor BPND and a limited number of clinical measures (age, BMI, Beck Depression Inventory, Beck Anxiety Inventory, Harm Avoidance Subscale of the Temperment and Character Inventory, and Barratt Impulsiveness Subscale), were tested in the underweight patients with AN, and separately in the weight-restored patients with AN, via Pearson correlation, and using partial correlation methods when controlling for age. All weight-restored patients (including those only having a weight-restored scan) were included in these correlation analyses.
3. Results
3.1. Clinical characteristics
25 patients with AN participated in either one or two (underweight and/or weight-restored) scans. 21 patients with AN completed scanning while acutely underweight, and 19 patients with AN completed scanning upon weight restoration. 15 patients with AN completed scans at both time points. 25 control subjects completed a PET [11C]raclopride scan, for comparison to patient data.
Clinical characteristics of the sample are summarized in Table 1. Age [known to be inversely associated with striatal D2 receptor availability (Kim et al., 2011)], BMI, and scan site were considered as factors for incorporation into subsequent statistical modeling. Consistent with previous findings (Kim et al., 2011), age was observed to be associated with decreased striatal D2 receptor BPND (see Figure 1) in underweight patients with AN (r=−0.655, p=0.001) and in weight-restored patients with AN (r=−0.663, p=0.002); the relationship between age and striatal BPND in control subjects was not statistically significant (r=−0.303; p=0.140). Despite the fact that the groups were well-matched for age, age was included in the subsequent statistical model in the event of any potential differences in the relationship between age and D2 between the patient and the control group. We also tested models with and without age as a covariate, which did not influence the overall result.
Table 1.
Characteristics of patients with Anorexia Nervosa (AN) and Controls (CTR)a
| AN | CTR | Statistic | |
|---|---|---|---|
| Number enrolled | 25 (12 AN-R; 13 AN-BP) |
25 | |
| # patients completing underweight scan | 21 | ||
| # patients completing weight-restored scan | 19 | ||
| # patients completing both scans (underweight and weight-restored) | 15 | ||
| Mean Age (years) | 25.2 +/− 7.3 (underweight) 27.5 +/− 8.6 (wt-restored) (range: 18.0–45.8) |
25.6 +/− 5.0 25.6 +/− 5.0 (range: 19.1–40.1) |
p=0.80 p=0.37 |
| Mean Age (years) – patients (n=15) completing both scans | 26.36 +/− 8.20 (underweight) 26.48 +/− 8.19 (wt-restored) (range: 18.0–45.8) |
||
| Mean BMI (kg/m2) – underweight patientsb | 17.2 +/− 0.6 (n=21) |
21.3 +/− 1.4 (n=25) |
p=0.003 |
| Mean BMI (kg/m2) – weight-restored patients | 20.2 +/− 0.9 (n=19) |
21.3 +/− 1.4 (n=25) |
p=0.006 |
| Ethnicity | 22 Caucasian 3 Non-Caucasian |
16 Caucasian 9 Non-Caucasian |
|
| Duration of illness (years) | 11.1 +/− 8.2 | ||
| Scan site (Columbia vs. Cornell) | 11 Columbia 14 Cornell |
17 Columbia 8 Cornell |
|
| For underweight patients: | |||
| EDE-Global | 3.9 +/− 0.9 | ||
| Mean BDI score | 33.1 +/− 11.1 | 0.7 +/− 1.5 | p<0.001 |
| Mean BAI score | 26.8 +/− 12.0 | 0.5 +/− 1.6 | p<0.001 |
| For weight-restored patients: | |||
| EDE-Global | 2.5 +/− 1.3 | ||
| Mean BDI score | 16.1 +/− 10.3 | 0.7 +/− 1.5 | p<0.001 |
| Mean BAI score | 15.3 +/− 9.5 | 0.5 +/− 1.6 | p<0.001 |
Abbreviations: AN = Anorexia Nervosa; CTR = Healthy Control participants; AN-R = Anorexia Nervosa, Restricting Subtype; AN-BP = Anorexia Nervosa, Binge-Purge Subtype; BMI = Body Mass Index (kilograms per square meter); EDE = Eating Disorder Examination; BDI = Beck Depression Inventory; BAI = Beck Anxiety Inventory; PET = positron emission tomography.
Figure 1.
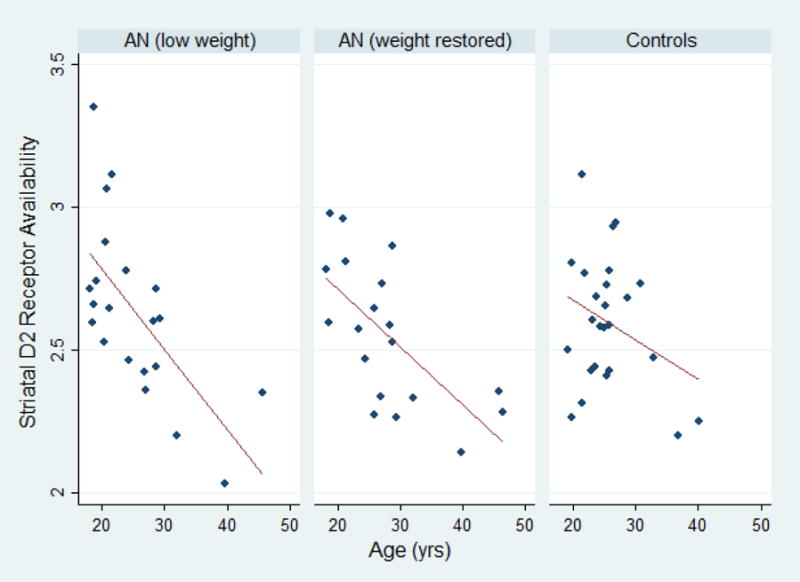
Distribution of striatal D2 receptor availability (“BPND”, unitless) vs. age. The left panel represents all measures in underweight patients with AN. The center panel represents all measures in weight-restored patients with AN. The right panel represents all measures in control subjects.
Mean BMI of underweight patients with AN was 17.2 +− 0.6 (range 16.7–18.8 kg/m2; one subject had BMI>18.5 kg/m2, due to fluid shifts shortly after admission). Mean BMI of healthy control subjects was 21.3 +/− 1.4 (range 18.3–23.6 kg/m2). As preliminary analyses demonstrated no differences in striatal D2 receptor availability between underweight and weight-restored patients, and no relationship between BMI and striatal BPND, we did not include BMI in the statistical modeling of the between-group comparison, in order to limit the number of parameters in the model.
With respect to other categorical variables, subjects with AN and HC subjects did not significantly differ with respect to ethnicity (Fisher’s exact test, p=0.10) or smoking status (Fisher’s exact test, p=0.667). With respect to reproductive hormone status: in underweight patients, 16 out of 21 patients with AN were amenorrheic on the day of their scan, 3 were in the follicular phase, one was in the ovulatory phase, and 1 was in the luteal phase. In weight-restored patients, 10 remained amenorrheic, 4 were in the follicular phase, and 4 were in the luteal phase of their cycle on the day of their scan. In control subjects, 18 of 25 were classified as being in the follicular phase of their menstrual cycle, felt to be the most appropriate control condition for patients with amenorrhea. (Specifically, 9 of 25 control subjects were on oral contraceptive pills (OCP) and classified to be in the follicular phase by hormone measures on the day of the scan; 9 were in the follicular phase but not on OCP; 4 were in the luteal phase; 3 were unknown.) Excluding those that were unknown, there was no effect of menstrual status (follicular, vs luteal, vs OCP) on striatal D2 receptor BPND in the control group (F(2,21)=2.253, p=0.132).
3.2. Scanning procedures
The mean injected dose of [11C]raclopride did not vary between patients and controls. This was the case both when comparing scans of underweight patients to control subjects [underweight patients: 11.30 mCi +/− 2.95 mCi; control subjects: 11.60 mCi +/− 2.22 mCi; p=0.70], as well as when comparing scans of underweight patients to weight-restored patients [underweight patients: 11.30 mCi +/− 2.95 mCi; weight-restored patients: 11.06 mCi +/− 2.96 mCi; p=0.80]. The mean injected mass of raclopride also did not differ when comparing underweight patients to control subjects [underweight patients: 3.48 μg +/− 1.32 μg; control subjects: 3.87 μg +/− 1.81 μg; p=0.40], and it did not differ when comparing underweight patients to weight-restored patients [underweight patients: 3.48 μg +/− 1.32 μg; weight-restored patients: 3.48 μg +/− 1.59 μg; p=0.99].
Additionally, because we were comparing binding in underweight patients and control subjects, we assessed ROI volumes, to evaluate for the need for partial volume effect (PVE) correction, since group differences in region size (e.g. brain atrophy in the patient group) could potentially lead to an underestimation of binding per unit brain volume. PVE correction might correct systematic errors due to one group having regional volume differences, but at the cost of amplified high spatial frequency noise, which reduces power and may introduce its own bias. We found that the ratio of group mean volumes in AN to group mean volumes in control subjects was 95% in whole striatum, 94% in AST, 93% in posterior putamen and 102% in VST, and that none of these reached statistical significance for group differences. Given that the group mean difference in BPND was less than 2% in all regions, and that effect sizes were small (≤ 0.3) in all regions, it is highly unlikely that the differences would become significant or that their magnitude would become biologically meaningful following PVE correction.
With respect to possible differences in striatal BPND measures between the two scan sites (Columbia vs. Cornell): for underweight patients, striatal BPND (Columbia) = 2.75+/− 0.28; striatal BPND (Cornell) = 2.50 +/− 0.29; p=0.06; for control subjects, striatal BPND (Columbia) = 2.64 +/− 0.24; striatal BPND (Cornell) = 2.55 +/− 0.19; p=0.32. As previously noted, scan site was included as a random effect in the subsequent statistical modeling.
3.3. Striatal D2 receptor BPND, underweight patients vs. control subjects
With respect to striatal D2 receptor BPND, in underweight patients vs. control subjects, there was no effect of diagnosis (patient vs control) on D2 receptor BPND [F(1,42.47)=0.45; p=0.50]. As expected, there was an effect of region on BPND [F(2,88.00)=620.96; p<0.001]. There was also no region by diagnosis effect [F(2,88.00)=1.56; p=0.22]. Table 2 summarizes results.
Table 2.
Regional BPND in underweight patients with Anorexia Nervosa (AN) vs. Control Subjects (CTR)a
| Region | AN, underweight (SE) n=21 [95% CI] |
CTR (SE) n=25 [95% CI] |
Statistic (underweight AN vs CTR) |
Cohen’s d |
|---|---|---|---|---|
| Striatum, whole | 2.60 (0.077) [2.15–3.05] |
2.55 (0.076) [2.04–3.06] |
[F(1,42.47)=0.45; p=0.50] | 0.14 |
| Sensorimotor striatum | 3.06 (0.122) [2.17–3.94] |
3.05 (0.121) [2.08–4.02] |
[F(1,42.32)=0.003; p=0.95] | 0.02 |
| Associative striatum | 2.53 (0.060) [2.29–2.77] |
2.47 (0.057) [2.18–2.76] |
[F(1,42.79)=0.80; p=0.38] | 0.22 |
| Limbic striatum | 2.21 (0.055) [2.00–2.42] |
2.14 (0.052) [1.89–2.40] |
[F(1,42.81)=1.18; p=0.28] | 0.27 |
Regional BPND (unitless measure) with standard error are presented, for 21 underweight patients with AN, compared to 25 healthy control subjects without history of an eating disorder. For the primary analysis (differences in D2 BPND in the striatum as a whole, between underweight patients with AN and control subjects), outcome measure estimates and statistics were derived from a mixed models analysis incorporating all 3 regions, with fitted parameters of intercept, diagnosis, and age. For derivation of the subregion outcome measures, parallel F and p statistics, and 95% confidence intervals (CI’s), a similar mixed models analysis was completed for each individual region. (Estimates of the subregion BPNDs were also generated from the full mixed model analysis, and were very similar [not shown here].)
3.4. Striatal D2 receptor BPND, pre/post weight-restoration
In the within-subject analysis of only those patients completing both a baseline and post-weight-restoration scan (n=15; 8 patients with AN-R, 7 patients with AN-BP), there were no change in striatal D2 receptor BPND over the course of weight restoration (see Table 3). Average length of time between underweight and weight-restored scans was 44 +/− 10 days.
Table 3.
Regional BPND in patients with Anorexia Nervosa (AN), pre/post weight restoration (patients completing both scans)
| Region | AN, underweight (n=15)a | AN, weight-restored (n=15)a,e | ΔBPND, averageb (pre vs post weight-restored) |
Statc | Cohen’s dd |
|---|---|---|---|---|---|
| Striatum, whole | 2.59 +/− 0.33 | 2.59 +/− 0.26 | 0.4% +/− 7.2% | p=0.99 | 0.06 |
| Sensorimotor striatum | 3.01 +/− 0.39 | 3.05 +/− 0.29 | 1.7% +/−8.0% | p=0.62 | 0.22 |
| Associative striatum | 2.49 +/− 0.32 | 2.47 +/− 0.27 | −0.3% +/− 7.3% | p=0.70 | 0.05 |
| Limbic striatum | 2.18+/− 0.31 | 2.22 +/− 0.28 | 2.4% +/− 8.3% | p=0.41 | 0.29 |
Data represent regional BPND averages +/− standard deviation (SD)’s in the subset of patients (n=15; 8AN-R, 7 AN-BP) completing both the underweight and weight-restored scans (i.e. a subgroup of the subjects represented in Table 2).
ΔBPND, average represents the average change in binding potential between the pre- and post-weight-restored scans.
P values represent paired t-tests between the pre- vs post-weight restoration scans, within this subgroup of 15 patients.
Cohen’s d (underweight patients vs. weight-restored patients, within-subject comparison) is calculated based on average +/− SD ΔBPND.
3.5. Associations with clinical measures
There were no associations between striatal D2 receptor BPND and BMI at the time of the scan, in either the underweight (r=−0.326, p=0.161) or weight-restored (r=0.130, p=0.608) states. Similarly, there were no associations between striatal D2 receptor BPND and admission BMI. Duration of illness was associated with striatal D2 receptor binding potential in underweight patients with AN (r=−0.661, p=0.001), but this association was not significant when age was factored into the analysis (r=−0.108, p=0.650). A similar pattern was seen when examining the relationship between duration of illness and striatal BPND in weight-restored subjects (r=−0.178; p=0.480 after controlling for age). Examination of striatal D2 receptor BPND in underweight patients with AN revealed a trend towards an association between D2 receptor BPND and global EDE scores (r=0.454, p=0.067); this association was not present in examination of the weight-restored patients (r=0.312; p=0.278).
With respect to co-morbid mood and anxiety symptoms: in underweight patients, striatal D2 receptor BPND was not associated with total scores on the Beck Depression Inventory (r=−0.224, p=0.357), Beck Anxiety Inventory (r=0.003, p=0.991), or Barrett Impulsiveness Scale (r=−0.262, p=0.264), nor was striatal D2 receptor BPND associated with the Harm Avoidance Subscale of the Temperament and Character Inventory (r=−0.059, p=0.804). Similar results were seen on adjusting for age, and when analyzing associations between striatal D2 BPND and these clinical measures in the weight-restored patients with AN.
3.6. Subgroup analysis (AN, restricting subtype vs AN, binge/purge subtype)
Subgroup testing did not reveal a significant difference in D2 receptor BPND between AN-R and AN-BP (underweight patients with AN-R: striatal BPND=2.68 +/− 0.30; underweight patients with AN-BP: striatal BPND=2.58 +/− 0.32; p=0.48).
4. Discussion
The results of the current study do not support the hypothesis of altered striatal D2 receptor availability in AN. We failed to see any significant difference when comparing the underweight group to the control group. We also failed to see any change in D2 receptor availability over the course of inpatient treatment and weight restoration. The current study was able to examine a dopamine receptor thought to be important in other disorders involving compulsive behaviors, including substance use disorders (Trifilieff and Martinez, 2014) and obsessive-compulsive disorder [reviewed in (Nikolaus et al., 2010)]. The lack of a finding here may indicate that other parts of the mesolimbic dopamine system and/or other parts of reward circuitry are more relevant to AN.
The current results differ from that of an earlier study of striatal D2 receptor availability in AN (Frank et al., 2005), that reported elevated D2 receptor availability in the anteroventral striatum, anatomically equivalent here to the limbic striatum. Several differences between the current study and that of Frank et al may contribute to the difference in findings. Only 10 patients were examined in that study, and all had been recovered from AN for at least 1 year. It is possible that elevated anteroventral D2 receptors is a feature of those patients who successfully recover, and that the difference between this study and the Frank et al study pertains to stage of recovery.
However, the lack of a difference in striatal D2 receptor availability in patients with AN in the current study does not exclude the possibility of other DA-related abnormalities. No data are available regarding striatal DA levels, and animal studies suggest that weight loss is associated with decreases in such levels (Pothos et al., 1995). In addition, other human data (e.g., CSF DA and metabolites; plasma DA metabolite studies; genetic studies) suggest the possibility of dopaminergic abnormalities [reviewed in (Kontis and Theochari, 2012)].
Nonetheless, this study has implications for current models of the neurobiology of AN. A variety of studies continue to examine reward processing and cite the potential role of striatal DA in this illness. The rationale for this is sound. First, the mesolimbic DA system plays an important role in the learning of reward value, and one hypothesis suggests that differences in the neural substrates related to this type of learning may contribute to some of the core symptoms of AN. A modest number of recent fMRI neuroimaging studies [e.g. (Frank et al., 2012)] have suggested that mesolimbic circuitry in AN functions differently during reward learning and responding, relative to its functioning in control subjects. However, the present study suggests that such abnormalities are not based on the reduced availability of D2 receptors in the striatum. Secondly, clinicians and patients frequently compare AN to an “addiction” (i.e., a substance use disorder). A reduction in striatal D2 receptor availability is a well-replicated finding across substance use disorders, suggesting that, at least at this level, there are important differences between AN and “addictions”.
There are strengths and limitations of the current study. Strengths of the study include that this is the first study to evaluate the striatal D2 receptor in patients with AN when underweight and immediately after weight gain, as opposed to subjects in long-term remission. The longitudinal design, allowing for assessment of the outcome measure at two different timepoints, is an additional strength. The sample size is relatively large compared to other PET neuroimaging studies of eating disorders. Subjects with AN were hospitalized, eating 100% of daily prescribed calories; while this meant that there was some variation in daily caloric intake, physiological status on the day of scan was relatively stable and consistent across patients.
Limitations include the fact that we were not able to evaluate subjects, due to concerns for maximizing safety of procedures, until they attained a weight of 75% of ideal body weight. In several cases, patients received care for several weeks prior to achieving this weight; thus, they had already undergone significant treatment prior to study. This may have masked differences between the underweight and weight-restored conditions. Similarly, the average length of time between underweight and weight-restored scans (e.g. 6 weeks), and the average BMI change of 3.0 kg/m2, may have limited the ability to detect change. Also, patients with AN were undergoing nutritional rehabilitation at the time of the scans, and may have been in an increased metabolic state relative to control subjects at the time of scanning, which could have affected findings, though the lack of within-subject change in striatal D2 binding over the study suggests that D2 binding was stable to within-subject metabolic changes. Participants were also not always scanned at the same time of day, which may have introduced variability into the imaging data.
Another limitation is that we were unable to exclude patients with mild to moderate symptoms of depression and anxiety, given the frequent comorbidity of AN with depressive and anxiety disorders, and because these symptoms are often either caused or exacerbated by the underweight state; these co-morbid symptoms may have served as a confounder of D2 receptor measures. However, of note, PET/SPECT studies of striatal DA measures in unipolar depression have been discrepant and, in many cases, have not strongly demonstrated striatal DA alterations between patients with major depressive illness and control participants (Hirvonen et al., 2008; Parsey et al., 2001). Similarly, studies of striatal D2 receptor availability across anxiety disorders have shown inconclusive findings in OCD (Perani et al., 2008; Schneier et al., 2008); as well as in social anxiety disorder (Schneier et al., 2009; Schneier et al., 2000; Schneier et al., 2008); despite this, the possibility that co-morbid anxiety problems may confound examination of striatal DA in this population remains a limitation.
Finally, since the radiotracer used here ([11C]raclopride) is sensitive to competition from endogenous dopamine, D2 receptor measures may have been confounded by endogenous dopamine levels, which may differ between patients and controls; the possibility that ‘true’ D2 receptor levels were masked in this way cannot be excluded. [11C]raclopride is also known to be sensitive to D3 receptor binding, and the possible contribution of this receptor subgroup to findings cannot be excluded. [11C]raclopride is also not able to assess other brain regions where DA abnormalities might exist (e.g. cortical regions).
In conclusion, this study failed to detect a significant difference in striatal D2 receptor availability in patients with AN vs. control subjects. The study also failed to detect a significant change in D2 receptor availability in AN over the course of inpatient treatment and restoration to a minimally normal weight. While previous studies have implicated the mesolimbic DA system as relevant to food reward, disturbances in food intake and in psychological functioning in AN cannot be attributed to significant abnormalities in striatal D2 receptor availability.
Highlights.
This study measured striatal D2 receptor availability in anorexia nervosa using PET.
Patients were scanned while underweight, and after nutritional restoration.
No differences in D2 availability between patients and controls subjects were seen.
Striatal D2 receptor availability was not associated with clinical measures.
Acknowledgments
The authors acknowledge those who contributed to this study, including Mary Bongiovi MD/PhD, Ingrid Carretero, Lilya Deshchenko, Michael Devlin MD, Jenna Kaufman, Dileep Kumar PhD, Laurel Mayer, MD, Dan Richter MD, Lilja Solnes MD, and Joanna Steinglass MD. Financial Support: This publication was supported by NIMH grant R01MH079397.
Dr. Walsh has received research support from AstraZeneca. Dr. Attia has received research support from Eli Lilly. Dr. Slifstein has consulted for GlaxoSmithKline and Amgen and has received research support from IntraCellular Therapies, Pierre-Fabre, and Forest Labs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
Dr. Broft served as the Columbia site PI. Dr. Slifstein oversaw all image processing/analysis and collaborated on statistical analysis. Dr. Osborne was the Cornell site PI. Drs. Kothari and Vallabhajosula were radiochemists for the study, and provided and analyzed radioligand data. Mr. Morim oversaw PET suite operations at Cornell. Ms. Shingleton and Ms. Kenney were clinical research coordinators who collected and analyzed clinical characteristics data. Dr. Martinez provided expert input on study design and analysis. Drs. Attia and Walsh are the director of the Columbia Eating Disorders Unit, the recruitment site for the study, and provided expert guidance through all aspects of study design, implementation, and analysis.
Conflict of Interest
The other authors report no conflict of interest.
References
- Bailer UF, Frank GK, Price JC, Meltzer CC, Becker C, Mathis CA, Wagner A, Barbarich-Marsteller NC, Bloss CS, Putnam K, Schork NJ, Gamst A, Kaye WH. Interaction between serotonin transporter and dopamine D2/D3 receptor radioligand measures is associated with harm avoidant symptoms in anorexia and bulimia nervosa. Psychiatry Research. 2013;211(2):160–168. doi: 10.1016/j.pscychresns.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacological Reviews. 2011;63(1):182–217. doi: 10.1124/pr.110.002642. [DOI] [PubMed] [Google Scholar]
- Broft A, Shingleton R, Kaufman J, Liu F, Kumar D, Slifstein M, Abi-Dargham A, Schebendach J, Van Heertum R, Attia E, Martinez D, Walsh BT. Striatal dopamine in bulimia nervosa: a PET imaging study. International Journal of Eating Disorders. 2012;45(5):648–656. doi: 10.1002/eat.20984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoty PW, Riddick NV, Gage HD, Sandridge M, Nader SH, Garg S, Bounds M, Garg PK, Nader MA. Effect of menstrual cycle phase on dopamine D2 receptor availability in female cynomolgus monkeys. Neuropsychopharmacology. 2009;34(3):548–554. doi: 10.1038/npp.2008.3. [DOI] [PubMed] [Google Scholar]
- de Weijer BA, van de Giessen E, van Amelsvoort TA, Boot E, Braak B, Janssen IM, van de Laar A, Fliers E, Serlie MJ, Booij J. Lower striatal dopamine D2/3 receptor availability in obese compared with non-obese subjects. EJNMMI Res. 2011;1(1):37. doi: 10.1186/2191-219X-1-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairburn C. The Eating Disorder Examination. 12th. Guilford; New York: 1993. [Google Scholar]
- Faul F, E E, Lang AG, Buchner A. GPower 3.1.9.2 2009 [Google Scholar]
- First M, Gibbon M, Williams JBW. Structured Clinical Interview for DSMIV Axis I Disorders, Patient Edition (SCIDP), version 2. New York State Psychiatric Institute, Biometrics Research; New York: 1995. [Google Scholar]
- Forbush KT, South SC, Krueger RF, Iacono WG, Clark LA, Keel PK, Legrand LN, Watson D. Locating eating pathology within an empirical diagnostic taxonomy: evidence from a community-based sample. Journal of Abnormal Psychology. 2010;119(2):282–292. doi: 10.1037/a0019189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford CP. The role of D2-autoreceptors in regulating dopamine neuron activity and transmission. Neuroscience. 2014 doi: 10.1016/j.neuroscience.2014.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank GK, Bailer UF, Henry SE, Drevets W, Meltzer CC, Price JC, Mathis CA, Wagner A, Hoge J, Ziolko S, Barbarich-Marsteller N, Weissfeld L, Kaye WH. Increased dopamine D2/D3 receptor binding after recovery from anorexia nervosa measured by positron emission tomography and [11c]raclopride. Biological Psychiatry. 2005;58(11):908–912. doi: 10.1016/j.biopsych.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Frank GK, Reynolds JR, Shott ME, Jappe L, Yang TT, Tregellas JR, O’Reilly RC. Anorexia nervosa and obesity are associated with opposite brain reward response. Neuropsychopharmacology. 2012;37(9):2031–2046. doi: 10.1038/npp.2012.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirvonen J, Karlsson H, Kajander J, Markkula J, Rasi-Hakala H, Nagren K, Salminen JK, Hietala J. Striatal dopamine D2 receptors in medication-naive patients with major depressive disorder as assessed with [11C]raclopride PET. Psychopharmacology. 2008;197(4):581–590. doi: 10.1007/s00213-008-1088-9. [DOI] [PubMed] [Google Scholar]
- Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, Holden J, Houle S, Huang SC, Ichise M, Iida H, Ito H, Kimura Y, Koeppe RA, Knudsen GM, Knuuti J, Lammertsma AA, Laruelle M, Logan J, Maguire RP, Mintun MA, Morris ED, Parsey R, Price JC, Slifstein M, Sossi V, Suhara T, Votaw JR, Wong DF, Carson RE. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. Journal of Cerebral Blood Flow and Metabolism. 2007;27(9):1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- Kaye WH, Wierenga CE, Bailer UF, Simmons AN, Wagner A, Bischoff-Grethe A. Does a shared neurobiology for foods and drugs of abuse contribute to extremes of food ingestion in anorexia and bulimia nervosa? Biological Psychiatry. 2013;73(9):836–842. doi: 10.1016/j.biopsych.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Son YD, Kim HK, Lee SY, Cho SE, Kim YB, Cho ZH. Effects of age on dopamine D2 receptor availability in striatal subdivisions: a high-resolution positron emission tomography study. European Neuropsychopharmacology. 2011;21(12):885–891. doi: 10.1016/j.euroneuro.2011.03.009. [DOI] [PubMed] [Google Scholar]
- Klanker M, Feenstra M, Denys D. Dopaminergic control of cognitive flexibility in humans and animals. Frontiers in Neuroscience. 2013;7:201. doi: 10.3389/fnins.2013.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontis D, Theochari E. Dopamine in anorexia nervosa: a systematic review. Behavioural Pharmacology. 2012;23(5–6):496–515. doi: 10.1097/FBP.0b013e328357e115. [DOI] [PubMed] [Google Scholar]
- Lammertsma AA, Hume SP. Simplified reference tissue model for PET receptor studies. Neuroimage. 1996;4(3 Pt 1):153–158. doi: 10.1006/nimg.1996.0066. [DOI] [PubMed] [Google Scholar]
- Martinez D, Broft A, Foltin RW, Slifstein M, Hwang DR, Huang Y, Perez A, Frankle WG, Cooper T, Kleber HD, Fischman MW, Laruelle M. Cocaine dependence and d2 receptor availability in the functional subdivisions of the striatum: relationship with cocaine-seeking behavior. Neuropsychopharmacology. 2004;29(6):1190–1202. doi: 10.1038/sj.npp.1300420. [DOI] [PubMed] [Google Scholar]
- Martinez D, Slifstein M, Broft A, Mawlawi O, Hwang DR, Huang Y, Cooper T, Kegeles L, Zarahn E, Abi-Dargham A, Haber SN, Laruelle M. Imaging human mesolimbic dopamine transmission with positron emission tomography. Part II: amphetamine-induced dopamine release in the functional subdivisions of the striatum. Journal of Cerebral Blood Flow and Metabolism. 2003;23(3):285–300. doi: 10.1097/01.WCB.0000048520.34839.1A. [DOI] [PubMed] [Google Scholar]
- Mawlawi O, Martinez D, Slifstein M, Broft A, Chatterjee R, Hwang DR, Huang Y, Simpson N, Ngo K, Van Heertum R, Laruelle M. Imaging human mesolimbic dopamine transmission with positron emission tomography: I. Accuracy and precision of D(2) receptor parameter measurements in ventral striatum. Journal of Cerebral Blood Flow and Metabolism. 2001;21(9):1034–1057. doi: 10.1097/00004647-200109000-00002. [DOI] [PubMed] [Google Scholar]
- Nikolaus S, Antke C, Beu M, Muller HW. Cortical GABA, striatal dopamine and midbrain serotonin as the key players in compulsive and anxiety disorders–results from in vivo imaging studies. Reviews in the Neurosciences. 2010;21(2):119–139. doi: 10.1515/revneuro.2010.21.2.119. [DOI] [PubMed] [Google Scholar]
- Parsey RV, Oquendo MA, Zea-Ponce Y, Rodenhiser J, Kegeles LS, Pratap M, Cooper TB, Van Heertum R, Mann JJ, Laruelle M. Dopamine D(2) receptor availability and amphetamine-induced dopamine release in unipolar depression. Biological Psychiatry. 2001;50(5):313–322. doi: 10.1016/s0006-3223(01)01089-7. [DOI] [PubMed] [Google Scholar]
- Perani D, Garibotto V, Gorini A, Moresco RM, Henin M, Panzacchi A, Matarrese M, Carpinelli A, Bellodi L, Fazio F. In vivo PET study of 5HT(2A) serotonin and D(2) dopamine dysfunction in drug-naive obsessive-compulsive disorder. Neuroimage. 2008;42(1):306–314. doi: 10.1016/j.neuroimage.2008.04.233. [DOI] [PubMed] [Google Scholar]
- Pothos EN, Creese I, Hoebel BG. Restricted eating with weight loss selectively decreases extracellular dopamine in the nucleus accumbens and alters dopamine response to amphetamine, morphine, and food intake. Journal of Neuroscience. 1995;15(10):6640–6650. doi: 10.1523/JNEUROSCI.15-10-06640.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneier FR, Abi-Dargham A, Martinez D, Slifstein M, Hwang DR, Liebowitz MR, Laruelle M. Dopamine transporters, D2 receptors, and dopamine release in generalized social anxiety disorder. Depression and Anxiety. 2009;26(5):411–418. doi: 10.1002/da.20543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneier FR, Liebowitz MR, Abi-Dargham A, Zea-Ponce Y, Lin SH, Laruelle M. Low dopamine D(2) receptor binding potential in social phobia. American Journal of Psychiatry. 2000;157(3):457–459. doi: 10.1176/appi.ajp.157.3.457. [DOI] [PubMed] [Google Scholar]
- Schneier FR, Martinez D, Abi-Dargham A, Zea-Ponce Y, Simpson HB, Liebowitz MR, Laruelle M. Striatal dopamine D(2) receptor availability in OCD with and without comorbid social anxiety disorder: preliminary findings. Depression and Anxiety. 2008;25(1):1–7. doi: 10.1002/da.20268. [DOI] [PubMed] [Google Scholar]
- Slifstein M, Laruelle M. Models and methods for derivation of in vivo neuroreceptor parameters with PET and SPECT reversible radiotracers. Nuclear Medicine and Biology. 2001;28(5):595–608. doi: 10.1016/s0969-8051(01)00214-1. [DOI] [PubMed] [Google Scholar]
- Sokoloff P, Giros B, Martres MP, Bouthenet ML, Schwartz JC. Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature. 1990;347(6289):146–151. doi: 10.1038/347146a0. [DOI] [PubMed] [Google Scholar]
- Tchanturia K, Lounes N, Holttum S. Cognitive remediation in anorexia nervosa and related conditions: a systematic review. Eur Eat Disord Rev. 2014;22(6):454–462. doi: 10.1002/erv.2326. [DOI] [PubMed] [Google Scholar]
- Treasure J, Schmidt U. The cognitive-interpersonal maintenance model of anorexia nervosa revisited: a summary of the evidence for cognitive, socio-emotional and interpersonal predisposing and perpetuating factors. J Eat Disord. 2013;1:13. doi: 10.1186/2050-2974-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifilieff P, Martinez D. Imaging addiction: D2 receptors and dopamine signaling in the striatum as biomarkers for impulsivity. Neuropharmacology. 2014;76(Pt B):498–509. doi: 10.1016/j.neuropharm.2013.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Baler R, Telang F. Imaging dopamine’s role in drug abuse and addiction. Neuropharmacology. 2009;56(Suppl 1):3–8. doi: 10.1016/j.neuropharm.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Gifford A, Hitzemann R, Ding YS, Pappas N. Prediction of reinforcing responses to psychostimulants in humans by brain dopamine D2 receptor levels. American Journal of Psychiatry. 1999;156(9):1440–1443. doi: 10.1176/ajp.156.9.1440. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Thanos PP, Logan J, Gatley SJ, Gifford A, Ding YS, Wong C, Pappas N. Brain DA D2 receptors predict reinforcing effects of stimulants in humans: replication study. Synapse. 2002;46(2):79–82. doi: 10.1002/syn.10137. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Thanos PK, Logan J, Alexoff D, Ding YS, Wong C, Ma Y, Pradhan K. Low dopamine striatal D2 receptors are associated with prefrontal metabolism in obese subjects: possible contributing factors. Neuroimage. 2008;42(4):1537–1543. doi: 10.1016/j.neuroimage.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W, Netusil N, Fowler JS. Brain dopamine and obesity. Lancet. 2001;357(9253):354–357. doi: 10.1016/s0140-6736(00)03643-6. [DOI] [PubMed] [Google Scholar]


