Abstract
Background
Sulfated Polysaccharides (SPs) possess spectrum of pharmacological and therapeutic properties that could attributed to their origins variation, chemical structures and biological activities. Various studies have shown the impact of SPs on proliferation in different cancer cell lines.
Objectives
In this study, we have evaluated the biological effects of λ-carrageenan, a highly SP, extracted from the red seaweed Laurencia papillosa, on MDA-MB-231 cancer cell line.
Materials and Methods
MDA-MB-231 cells have treated with λ-carrageenan, the viability and apoptosis have assessed by the appropriate florescent probes on flow cytometer. The expression levels of mRNA of apoptotic genes have detected by real-time PCR analysis.
Results
Our results have indicated that the signaling pathway of λ-carrageenan inhibited the proliferation of MDA-MB-231 cells by up-regulating the pro-apoptotic genes caspase-8, caspase-9, caspase-3 which have been resulting the increased levels of active caspase-3 protein. Furthermore, This SP had that capacity to disrupt the mitochondrial function by altering the bax/bcl-2 ratio of expression which has considered an important element in apoptosis induction.
Conclusions
The presented results have signposted that λ-carrageenan was a promising bioactive polymer which could be a potential candidate in preventing or treating breast cancer.
Keywords: Anti-Proliferation, λ-Carrageenan, Apoptosis, Breast Cancer, MDA-MB-231
1. Background
Cancer has been responsible for the death of more than 8.2 million people worldwide annually according to the WHO (world health organization). Breast cancer has considered the most prevalent type of cancer among women with 23% of the total cancer cases in 2011 (1). The overall percentage of breast cancer patients, which responded to therapy, remained under 35% (2). Therefore, the attempts for new molecules to treat the breast cancer became increasingly important in particular for patients who have not responded to conventional treatments. Sulfated polysaccharides extracted from red seaweeds have attracted enormous unprecedented attention over the last decays for their multiple chemical structures and biological activities (3). Carrageenan was a small chemical group of linear sulfated polysaccharides with relatively a high molecular weight and composed of 1,3α -1,4β -galactans. It has formed the basic composite of red seaweed cell walls. Carrageenan oligomers have generally classified into three groups: kappa (κ-), iota (ι-) and lambda λ-carrageenan which differed according to their structural characteristics, degree of sulfation and water solubility. The sulfated contents of kappa, iota and lambda-carrageenan were: (15% - 20%), (28% - 30%) and (32% - 39%), respectively (4). λ-carrageenan which had the highest degree of sulfation, had diverse biological activities including anti-coagulant (5), anti-viral (6), anti-oxidant (7), anti-proliferation, anti-angiogenesis and anti-tumor (8, 9). moreover, λ-carrageenan has exhibited an efficient adjuvant effect in preventative and therapeutic vaccine for cancer treatment (10). Recent studies have amended by Jazzara et al proved the efficacy of sulfated carrageenans in the induction of apoptosis on breast cancer MDA-MB-231 cells and glioblastoma T98G cells (11). Moreover, targeting colon cancer (HCT116) cells by coupling an i-carrageenan with magnetic nanoparticle gaudily could induce cellular apoptosis (12).
2. Objectives
In the present study, we have highlighted the pivotal role of λ-carrageenan for inhibiting the growth of breast cancer MDA-MB-231 cells and triggering apoptotic process.
3. Materials and Methods
3.1. Cell culture
MDA MB 231 cells [provided by Professor P. Becuwe from the cancer research unit (EA SIGRETO), Nancy, France] have cultured in RPMI 1640 medium containing 10% fetal bovine serum (FBS), 50 U/mL penicillin/streptomycin and 2 mM L glutamine. The cells have cultured at 37°C in 5% CO2. All materials have used in the cell culture have supplied by Gibco-BRL.
3.2. XTT Assay
Cells have seeded at a density of 5 × 103 in a 96 well plate. After a 24 hours of culture, cells have treated with different concentrations of λ-carrageenan at 0, 6.25, 12.5, 25, 50 μM and incubated for 24, 48, 72 or 96 hours. Cell viability percentage has counted using an XTT assay kit (Roche) following the manufacturer’s instructions. The number of living cells has quantified by measuring absorbance at a wavelength of 450 nm using a microplate reader (Multiskan EX Microplate Readers; Thermo Scientific) and the absorption of the control cells has set to 100%. A graph of cell viability percentage against λ-carrageenan concentration and time treatment has produced from the mean absorbance values, by calculating the percentage growth of the λ-carrageenan treated cells, in comparison with the growth of untreated cells. Treatment with each-carrageenan concentration has performed in triplicate.
3.3. Cell Cycle Distribution
For DNA content analysis, MDA MB 231 cells have seeded at a density of 1 × 105/mL and then cultured for 24 hours. Subsequently, cells have treated with 12.5 and 25 μM λ-carrageenan for 24 hours in complete medium. Cells have harvested by Trypsinization, centrifuged at 200 g for 5 minutes and washed in PBS buffer. After centrifugation, the cell pellets have stained with 125 μg/mL propidium iodide (PI) staining solution (sigma Aldrich) and incubated with 0.1 mg/mL RNaseA in PBS buffer for 15 minutes at room temperature in the dark. PI intensities have measured and analyzed using a FACSCalibur flow cytometer.
3.4. Annexin V Staining
Annexin V staining has performed to examine the apoptosis status. A total of 1 × 105/mL cells have treated with former concentrations of λ-carrageenan for 24 hours. Cells have then harvested and washed with ice-cold PBS buffer and re-suspended with the binding buffer (solution in 0.1 M HEPES, 1.4 M NaCl, 25 mM CaCl2. Diluted to 1X, followed by incubation with Annexin V-FITC (50 µg/mL) (BD PharmingenTM) and PI staining solution (100 µg/mL) (BD PharmingenTM) for 15 minutes at room temperature in the dark, and analyzed using the FACSCalibur flow cytometer.
3.5. Cell Bio-Imaging
Cells (2 × 103) have seeded into a slide chamber (Nalge Nunc International) and cultured for 24 hours, followed by treatment with two concentrations (12.5 and 25 μM) of λ-carrageenan for 24 hours. Formaldehyde fixed cells (4% formaldehyde) have inspected using a 40X objective lens of an Olympus inverted microscope (Olympus CK2, Olympus Corporation). Images have then captured using a microscope branched Olympus DP70 camera (Olympus Corporation).
3.6. DAPI and PI Staining
Cells have first cultured in a slide chamber. After treatment with (12.5 and 25 µM) λ-carrageenan, the cells have incubated in 1 μg/ml DAPI (Sigma Aldrich) (dissolved in methanol) for 5 min in the dark. Slides have mounted and observed using a Nikon ECLIPSE 80i fluorescence microscope (Nikon, Tokyo, Japan). PI staining has obtained by following the same previous steps and incubated in 500 µl PI (4.3 mM/L) (sigma Aldrich) for 5 minutes in the dark. Images have captured using a microscope branched Nikon DS Ri1 camera (Nikon).
3.7. Quantification of the Levels of Active Caspase-3 Protein by Flow Cytometry
MDA-MB-231 cells have seeded at a density of 1 × 105/mL and cultured for 24 hours. Cells were treated with 25 μM λ-carrageenan for 12 or 24 hours. Cells have then harvested after short treatment, (1 minute), with 1X Trypsin solution. Cells have then washed twice with ice-cold PBS buffer, then resuspended in 1 mL of PBS (with FCS 1%, NaN3 0.09%). A one hundred µl of the cell suspension have added into 6 mL sterile tube containing 200 µL of permeabilization/fixation solution [PBS with CaCl2 1 mM, MgSO4 1 mM, HEPES 10 mM, Paraformaldehyde 4%, Saponin 0.1%]. Samples have then incubated at 4°C for 20 minutes in the dark, followed by adding 1 mL of permeabilization/washing buffer (with FCS 2%, NaN2 0.1%, 1 mM, MgSO4 1 mM, HEPES 10 mM Saponin 0.1%); Centrifugation and re-suspended cells in 100 µL prior solution PBS by incubation with 20 μL of fluorescent anti-bodies against the active Caspase-3 for 45 minutes at 4°C in the dark. Finally, Cells have washed in PWB, and re-suspended in 100 μL PBS for analysis.
3.8. Real-Time qPCR Analysis
Cells have seeded at a density of 1 × 105/mL cells and cultured for 24 hours. Cells have treated with 25 μM λ-carrageenan for 12 hours and 24 hours prior to harvesting. Total RNA has extracted using an RNeasy kit (Qiagen). The cDNA has directly prepared from total RNA using the M MLV RT first-strand Synthesis System (Invitrogen Life Technologies) according to the manufacturer’s instructions. Transcript levels of caspase 8, caspase 3, caspase 9, bcl 2, bax genes within the GAPDH reference gene have determined using transcript specific primers (Table 1). A qPCR has performed with a StepOne/Plus real time PCR system (Applied Biosystems) according to the manufacturer’s instructions. Expression of the target genes has normalized to the expression of GAPDH. Data were analyzed by the ΔΔCt method. The relative fold change (R) in genes mRNA quantity in the treated samples has calculated using the equation: R = 2-ΔΔCt.
Table 1. Real-Time qPCR Primer Sequences.
| Gene Name With Accession Number | Primer Sequence (5’ - 3’) |
|---|---|
| Cacpase 8 (NM_001228.4) | |
| F | CATCCAGTCACTTTGCCAGA |
| R | GCATCTGTTTCCCCATGTTT |
| Cacpase 9 (NM_032996.2) | |
| F | TTCCCAGGTTTTGTTTCCTG |
| R | CCTTTCACCGAAACAGCATT |
| Cacpase 3 (NM_004346.3) | |
| F | ACATGGCGTGTCATAAAATACC |
| R | CACAAAGCGACTGGATGAAC |
| Bcl-2 (NM_000633.2) | |
| F | ATCGCCCTGTGGATGACTGAG |
| R | CAGCCAGGAGAAATCAAACAGAGG |
| Bax (NM_138764.4) | |
| F | GGACGAACTGGACAGTAACATGG |
| R | GCAAAGTAGAAAAGGGCGACAAC |
| GAPDH (NM_002046.3) | |
| F | ATGACCCCTTCATTGACC |
| R | GAAGATGGTGATGGGATTTC |
3.9. Statistical Analysis
All data expressed as mean ± SD or mean ± SEM were representative of at least three independent experiments. Data have statistically been evaluated using one-way analysis of variance (ANOVA) test. The values have considered statistically significant when P < 0.05 (signified by*) and P < 0.01 (signified by**).
4. Results
4.1. Evaluation of the Inhibitory Effects of λ-Carrageenan on MDA-MB-231 Cells
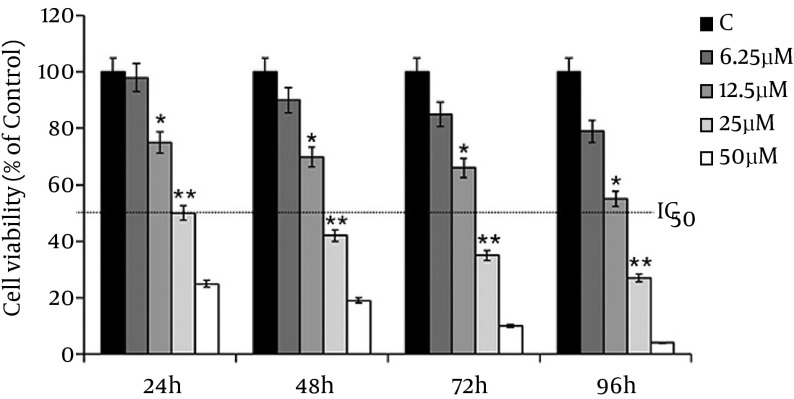
MDA-MB-231 cells have treated with 6.25, 12.5, 25 and 50 µM of λ-carrageenan and then monitored over 24, 48, 72 and 96 hours of treatments. The treated versus untreated cells have shown significant decrease in the viability of exposed cells to the treatment in time /concentration dependent manner. In addition, the results have shown that the treated MDA-MB-231 cells with 25 µM λ-carrageenan for 24 hours has decreased the number of cell to the half without indication of the present dead cells which indicated that the half-maximal inhibitory concentration (IC50) of λ-carrageenan in the treated MDA-231 is 25 µM λ-carrageenan at 24 hours (Figure 1).
Figure 1. Anti-Proliferative Effect of λ-Carrageenan in a Time and Concentration Dependent Manner, Were Studied in MDA-MB-231 Cells in Comparison With Control Cells (C).
Cell viability was measured by the XTT assay. Values represent means ± SEM of three independent experiments.
4.2. Effect of λ-Carrageenan on Cell Cycle Distribution
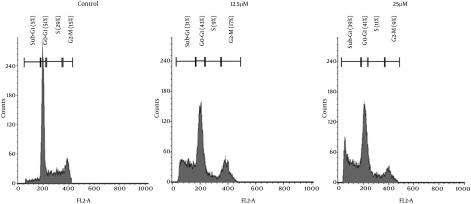
λ-carrageenan-treated MDA-MB-231 has shown cell cycle redistribution as demonstrated in Figure 2. The most affected phase of the cell cycle in the treated cell was the S phase where it has decreased significantly from 29 % in the untreated cells to reach 9 and 11 % of treated cells with 12.5 and 25 µM respectively. The visible and significant changes was the accumulation of the cells in the sub-G1 phase in the treated cells which reflect the increase of DNA fragmentation depending on λ-carrageenan concentration where the more concentration applied the more DNA fragmentation occurred.
Figure 2. λ-Carrageenan Induces an Accumulation in the Sub-G1 Population of MDA-MB-231 Cell Line.
Cells were treated with 12.5 and 25 μM λ-carrageenan or vehicle control, stained with propidium iodide (PI) and analyzed by flow cytometry.
4.3. λ-Carrageenan Induces Morphological and Nuclear Changes in MDA MB 231 Cells
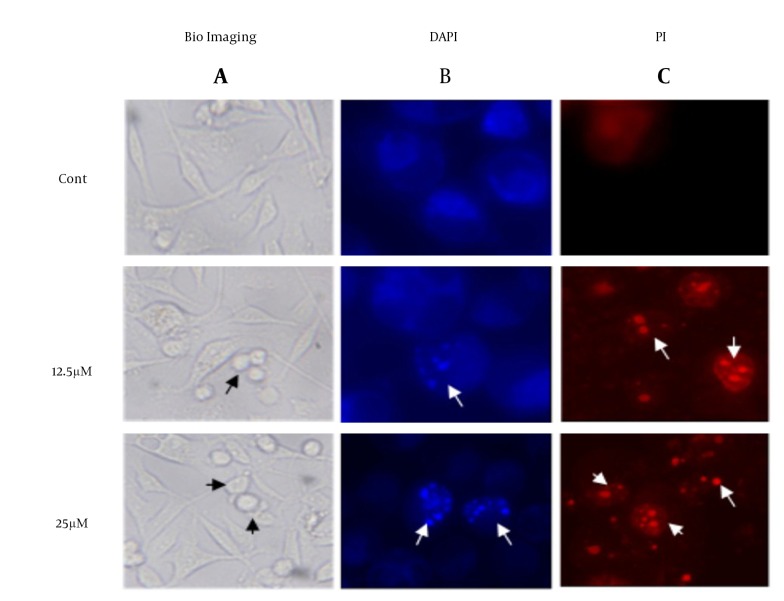
To investigate the morphological changes in the treated MDA-MB-231, cells have investigated under microscope in present of DAPI/PI stain. The images in Figure 3 have shown visible shrinkage in the treated cell with 12.5 and 25 µM of λ-carrageenan in comparison with the untreated one. In addition, the DAPI/PI stain has demonstrated DNA condensation in the treated cells and the condensation has increased in dose dependent manner. The previous observation like DNA fragmentation, cell shrinkage and DNA condensation have indicated that the cell perform a programmed cell death.
Figure 3. Morphological and Nuclear Changes of MDA-MB-231 Cells Treated With 12.5 and 25 μM λ-Carrageenan for 24 Hours.
A, control cells (cont) were treated with culture medium. Cells were observed and photographed under optical microscope lens (100X); Checked cells were then stained with B, DAPI and C, PI to display apoptotic morphological changes which were characterized by DNA condensation and nuclear fragmentation (indicated by arrows) under fluorescence microscope (400X).
4.4. λ-Carrageenan Induced Apoptosis in MDA-MB-231 Cells
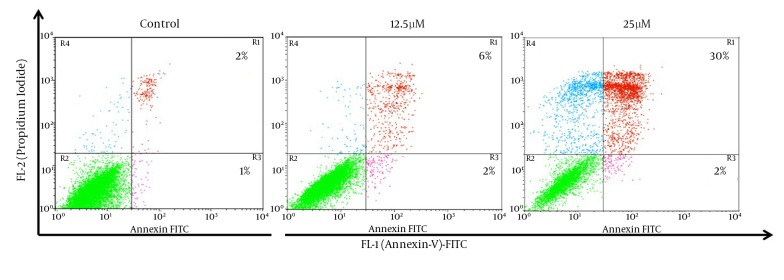
For additional characterization of the MDA-MB-231 cell death that has observed in earlier experiment, we have performed Annexin-V FITC analysis by flow cytometry. The Annexin-V analysis has demonstrated that 8% and 32% of the cells Annexin V+ after treatment with 12.5 and 25 µM of λ-carrageenan for 24 hours respectively, while apoptotic cells have not exceeded 3% in the control (Figure 4). These results have given an extra indication that the treated cells follow a programmed cell death as the Phosphoserine flipped from the inner cell membrane to the surface of the cell membrane.
Figure 4. Apoptosis Induced in MDA-MB-231 Cells After Treatment With 12.5 and 25 μM λ-Carrageenan for 24 hours.
Cells were classified as healthy (green color: Annexin V FITC-/PI-), early apoptotic cells (Pink color: Annexin V FITC+/PI-), late apoptotic cells (red color: Annexin V FITC+/PI+), and harmed cells (blue color: Annexin V FITC-/PI+).
4.5. λ-Carrageenan Alters Apoptotic Genes Activity in Treated MDA MB 231 Cells
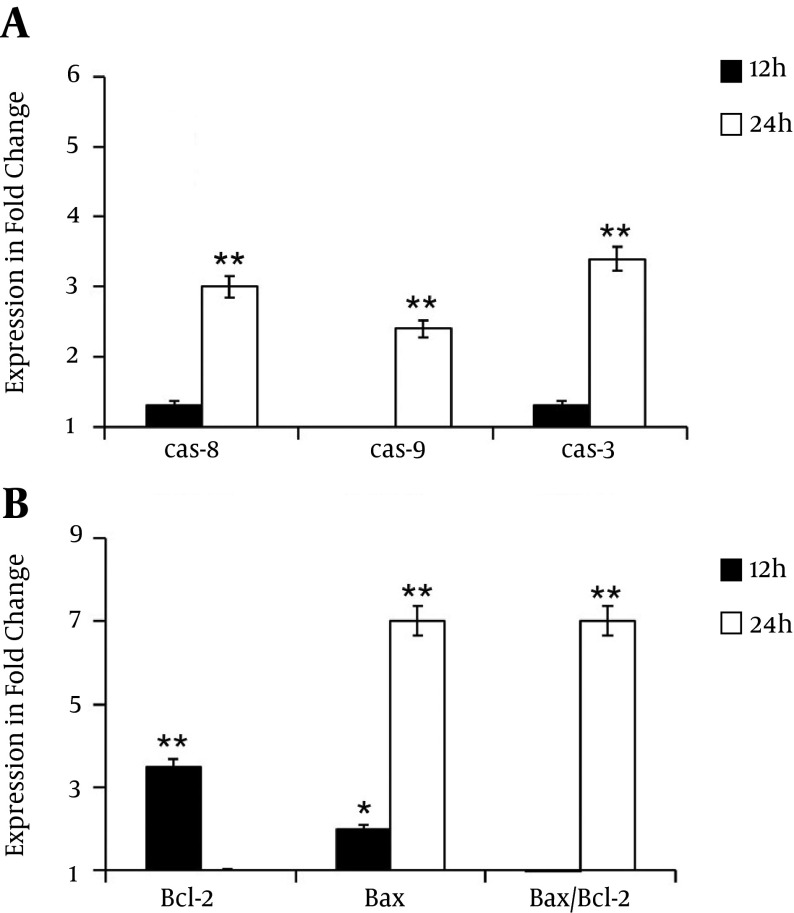
Quantitative RT-PCR has performed to decipher the mechanism of cell death induced by λ-carrageenan. Cells have treated with 25 µM for 12 and 24 hours. Then, the expression of several genes related to apoptotic pathways have analyzed including the key genes caspase 8, caspase 9, caspase 3, bcl 2 and bax. Transcripts levels of caspase 8, caspase 9, caspase 3 have shown a slight increase at 12 hours post-treatment in comparison with untreated cells. Treatments for 24 hours have increased the expression levels of the studied genes to 3, 2.4 and 3.4 fold respectively. Furthermore, the expression ratio of bax/bcl-2 has varied with time. While expression levels of bcl 2 has reduced from 3.5 fold at 12 hours to 1 fold at 24, the transcripts amount of bax has augmented from 2 folds after the first 12 hours to 7 folds after 24 hours. These data have indicated the clear ability of tested oligomer to induce apoptotic process (Figure 5).
Figure 5. Alteration of Apoptotic Genes Expression.
Diagrams are presented the relative expression of A, caspase-8, caspase-9, caspase-3 and B, changes in the bcl-2, bax and bax/bcl-2 expression ratio in MDA-MB-231 cells treated with 25 µM of λ-carrageenan for 12 and 24 hours. The reference gene gapdh was examined as an endogenous control. Values represent means ± SEM of three independent experiments.
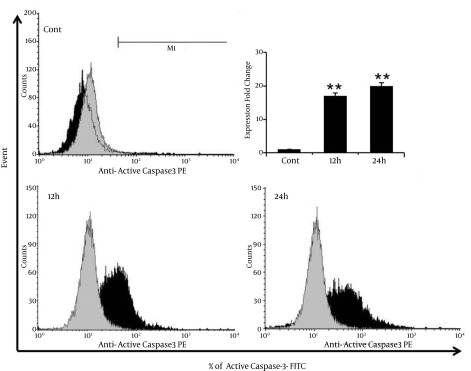
4.6. λ-Carrageenan Induces the Apoptotic Protein Active Caspase-3
The mechanism of apoptosis induction in MDA-MB-231 breast cancer cells have treated with λ-carrageenan has further characterized by assessing the capability of λ-carrageenan to trigger the pro-apoptotic protein expression. Caspases, a family of cysteine proteases have activated by cleavage of their inactive pro-caspase form (13). Results have shown significant increasing of cleaved Caspase-3 from 1% in control cells to 17% and 20%, after treatment with 25 µM λ-carrageenan for 12 and 24 hours respectively (Figure 6). The activation of Caspase-3 has been leading to defective DNA repair and resulted in apoptotic cell death shown repetitively in Figures 3 and 4.
Figure 6. λ-carrageenan Altered the Levels of the Pro-Apoptotic Protein Active Caspase-3 in MDA-MB-231 Cells.
Cells were treated with 25 μM λ-carrageenan for 12 and 24 hours in comparison with control cells, and then immunostained using an anti-active caspase-3 primary antibody/FITC-conjugated secondary antibody combination. Values represent means ± SEM of three independent experiments.
5. Discussion
λ-Carrageenan has been a highly sulfated polysaccharide which was a main component of red seaweed cell walls. The phycocolloid has exerted diverse biological activities in cancer cells such as anti-proliferation and anti-tumor effects (10). In the present study, we have demonstrated that the proliferation of MDA-MB-231 cells has inhibited after treatment with λ-carrageenan in concentration- and time-dependent manners. The anti-proliferative role of λ-carrageenan has previously revealed in mice 4T1 cell lines (10) and human umbilical vein endothelial cell line (HUVEC) (8). In the other hand, degraded κ -carrageenan has shown moderate cytotoxic effects to both intestine Caco-2 and liver HepG2 human cancer cell lines (14). Although, intratumoral injection of λ-carrageenan inhibit tumor growth has induced by melanoma B16-F10 and mammary cancer 4T1 bearing mice. While, λ-carrageenan had low cytotoxicity to the same tumor cells in vitro (10). Moreover, our results of cell cycle assessment have proven that there was a clear increasing in sub-G1 phase (Figure 2), which was the cellular output of nuclear condensation and DNA fragmentation in treated cells with λ-carrageenan. Other study has also demonstrated that the accumulation of cell in sub-G1 initiated apoptosis process (15). Another biological impact of λ-carrageenan has reported by inducing G2/M arrest on intestinal epithelial cells (16). While, lambda-carrageenan oligosaccharides (lambda-CO) has shown the decrease of cells in G0/G1 phase, and increased in S phase in human umbilical vein endothelial cells (HUVECs) (8). These data has promoted the agility of λ-carrageenan to induce diverse biological effects in different cells.
Activation of apoptosis pathways was a key mechanism by which cytotoxic drugs kill tumor cells (17), thus, it has became a major goal in cancer therapy (18). Apoptosis has characterized by cell shrinkage, nuclear DNA fragmentation, and membrane blebbing (19). Our results of annexin-V assay has revealed a significant increase of apoptotic cells number after λ-carrageenan-treatment (Figure 4). Similar results has shown that degraded ι-CGN induced apoptosis in human osteosarcoma cell line HOS cells within 48 of treatment (20). Annexin-V data was in full concordance with the molecular study, where we have found a significant increase in gene expression levels of caspase-8, caspase-9 and caspase-3. Correspondingly, flow cytometry analysis has shown an increasing of active Caspase-3 protein levels after treatment with λ-carrageenan (Figure 5). Active Caspase-3 has played an essential role in the activation of keys molecules which lead to biochemical and morphological changes in cells undergoing apoptosis (21). Otherwise, the antiproliferative effect of degraded k-carrageenan has related to apoptosis together with inactivation of cell proliferating genes as determined by morphological observation and molecular analysis in Caco-2 and HepG2 human cancer cell lines (14).
The cellular commitment to apoptosis induction has also regulated by the imbalanced mitochondrial bax/bcl-2 ratio (22). Our results have also indicated the ability of λ-carrageenan to simultaneously prompt the up-regulation of bax (encoding the pro-apoptotic protein Bax) and the down-regulation of bcl-2 (encoding the anti-apoptotic protein Bcl-2) in time dependent manner (Figure 6). This bioactive role of λ-carrageenan in shifting the bax/bcl-2 ratio of expression has previously studied in HUVEC cells too (8, 9). The imbalance of Bax/Bcl-2 ratio has led to the disruption of mitochondrial inner transmembrane potential (23), and the release of Cytochrome C triggering the formation of cytochrome C/APAF-1/Caspase-9 complex and subsequently enhancing the activation of active Caspase-3 as a positive feedback effect (24, 25).
5.1. Conclusions
λ-carrageenan has shown anti-proliferative activity in the human breast cancer cells MDA-MB-231 via the promotion of caspases-dependent apoptosis, and with the contribution of imbalanced ratio of Bax/Bcl-2 and nuclear fragmentation. This study has exemplified that λ-carrageenan possess both common and distinctive anti-carcinogenic mechanisms, which could further develop into an effective chemotherapeutic agents in the treatment of human breast cancer.
Acknowledgments
This work has supported and funded by the Damascus University. The authors would like to thank the director general of AECS and the Head of Molecular Biology and Biotechnology Department for their support.
Footnotes
Authors’ Contribution:Hossam Murad has designed the study, analyzed the data and written the paper. Ahmed ghannam and Chadi Soukkarieh have contributed to the data entry, literature review and writing-up process. Marie Jazzara has contributed to the study design and analysis. All authors have read and approved the final manuscript
Conflict of Interests:The authors have declared no conflict of interest.
Financial Disclosure:None declared.
Funding/Support:None declared.
References
- 1.DeSantis C, Howlader N, Cronin KA, Jemal A. Breast cancer incidence rates in U.S. women are no longer declining. Cancer Epidemiol Biomarkers Prev. 2011;20(5):733–9. doi: 10.1158/1055-9965.EPI-11-0061. [DOI] [PubMed] [Google Scholar]
- 2.Rajput S, Volk-Draper LD, Ran S. TLR4 is a novel determinant of the response to paclitaxel in breast cancer. Mol Cancer Ther. 2013;12(8):1676–87. doi: 10.1158/1535-7163.MCT-12-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jiao G, Yu G, Zhang J, Ewart HS. Chemical structures and bioactivities of sulfated polysaccharides from marine algae. Mar Drugs. 2011;9(2):196–223. doi: 10.3390/md9020196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liang W, Mao X, Peng X, Tang S. Effects of sulfate group in red seaweed polysaccharides on anticoagulant activity and cytotoxicity. Carbohydr Polym. 2014;101:776–85. doi: 10.1016/j.carbpol.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 5.Shanmugam M, Mody KH. Heparinoid-active sulphated polysaccharides from marine algae as potential blood anticoagulant agents. Curr Sci. 2000;79(12):1672–83. [Google Scholar]
- 6.Carlucci MJ, Scolaro LA, Noseda MD, Cerezo AS, Damonte EB. Protective effect of a natural carrageenan on genital herpes simplex virus infection in mice. Antiviral Res. 2004;64(2):137–41. doi: 10.1016/j.antiviral.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Rocha de Souza MC, Marques CT, Guerra Dore CM, Ferreira da Silva FR, Oliveira Rocha HA, Leite EL. Antioxidant activities of sulfated polysaccharides from brown and red seaweeds. J Appl Phycol. 2007;19(2):153–60. doi: 10.1007/s10811-006-9121-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mi TY, Yan XJ, Chen HM, Lin J, Wang F, Xu WF. [Proliferation inhibition of lambda-carrageenan oligosaccharides on HUVEC and expression of apoptotic relevant genes]. Yao Xue Xue Bao. 2008;43(5):474–9. [PubMed] [Google Scholar]
- 9.Chen HM, Yan XJ, Mai TY, Wang F, Xu WF. λ-Carrageenan oligosaccharides elicit reactive oxygen species production resulting in mitochondrial-dependent apoptosis in human umbilical vein endothelial cells. Int J Mol Med. 2009;24(6):801–6. doi: 10.3892/ijmm_00000295. [DOI] [PubMed] [Google Scholar]
- 10.Luo M, Shao B, Nie W, Wei XW, Li YL, Wang BL, et al. Antitumor and Adjuvant Activity of lambda-carrageenan by Stimulating Immune Response in Cancer Immunotherapy. Sci Rep. 2015;5:11062. doi: 10.1038/srep11062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jazzara M, Ahmed Ghannam A, Chadi Soukarieh C, Hossam Murad H. Anti-proliferative effect of sulfated carrageenan extracted from Laurencia papillosa on T98G glioblastoma cancer cells. 2015 Available from: https://www.researchgate.net/publication/268743704_Anti-proliferative_effect_of_sulfated_carrageenan_extracted_from_Laurencia_papillosa_on_T98G_glioblastoma_cancer_cells.
- 12.Raman M, Devi V, Doble M. Biocompatible iota-carrageenan-gamma-maghemite nanocomposite for biomedical applications - synthesis, characterization and in vitro anticancer efficacy. J Nanobiotechnology. 2015;13:18. doi: 10.1186/s12951-015-0079-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Degterev A, Boyce M, Yuan J. A decade of caspases. Oncogene. 2003;22(53):8543–67. doi: 10.1038/sj.onc.1207107. [DOI] [PubMed] [Google Scholar]
- 14.Ariffin SH, Yeen WW, Abidin IZ, Abdul Wahab RM, Ariffin ZZ, Senafi S. Cytotoxicity effect of degraded and undegraded kappa and iota carrageenan in human intestine and liver cell lines. BMC Complement Altern Med. 2014;14:508. doi: 10.1186/1472-6882-14-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kajstura M, Halicka HD, Pryjma J, Darzynkiewicz Z. Discontinuous fragmentation of nuclear DNA during apoptosis revealed by discrete "sub-G1" peaks on DNA content histograms. Cytometry A. 2007;71(3):125–31. doi: 10.1002/cyto.a.20357. [DOI] [PubMed] [Google Scholar]
- 16.Bhattacharyya S, Borthakur A, Dudeja PK, Tobacman JK. Carrageenan induces cell cycle arrest in human intestinal epithelial cells in vitro. J Nutr. 2008;138(3):469–75. doi: 10.1093/jn/138.3.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Debatin KM. Apoptosis pathways in cancer and cancer therapy. Cancer Immunol Immunother. 2004;53(3):153–9. doi: 10.1007/s00262-003-0474-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Westhoff MA, Karpel-Massler G, Bruhl O, Enzenmuller S, La Ferla-Bruhl K, Siegelin MD, et al. A critical evaluation of PI3K inhibition in Glioblastoma and Neuroblastoma therapy. Mol Cell Ther. 2014;2:32. doi: 10.1186/2052-8426-2-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407(6805):770–6. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 20.Jin Z, Han YX, Han XR. Degraded iota-carrageenan can induce apoptosis in human osteosarcoma cells via the Wnt/beta-catenin signaling pathway. Nutr Cancer. 2013;65(1):126–31. doi: 10.1080/01635581.2013.741753. [DOI] [PubMed] [Google Scholar]
- 21.Choudhury JD, Kumar S, Mayank V, Mehta J, Bardalai D. A review on apoptosis and its different pathway. Int J Biol Pharm Res. 2012;3:848–61. [Google Scholar]
- 22.Salakou S, Kardamakis D, Tsamandas AC, Zolota V, Apostolakis E, Tzelepi V, et al. Increased Bax/Bcl-2 ratio up-regulates caspase-3 and increases apoptosis in the thymus of patients with myasthenia gravis. In Vivo. 2007;21(1):123–32. [PubMed] [Google Scholar]
- 23.Murphy KM, Ranganathan V, Farnsworth ML, Kavallaris M, Lock RB. Bcl-2 inhibits Bax translocation from cytosol to mitochondria during drug-induced apoptosis of human tumor cells. Cell Death Differ. 2000;7(1):102–11. doi: 10.1038/sj.cdd.4400597. [DOI] [PubMed] [Google Scholar]
- 24.Fulda S, Debatin KM. Signaling through death receptors in cancer therapy. Curr Opin Pharmacol. 2004;4(4):327–32. doi: 10.1016/j.coph.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 25.van Loo G, Saelens X, van Gurp M, MacFarlane M, Martin SJ, Vandenabeele P. The role of mitochondrial factors in apoptosis: a Russian roulette with more than one bullet. Cell Death Differ. 2002;9(10):1031–42. doi: 10.1038/sj.cdd.4401088. [DOI] [PubMed] [Google Scholar]