Abstract
Objectives. To assess trends in continuing and new prescriptions for sedative-hypnotic medications, including benzodiazepines (BZDs) and non-BZD receptor agonists (nBZRAs).
Methods. Data came from the National Ambulatory Medical Care Survey and comprised 287 288 randomly sampled patient visits. Physicians reported medications prescribed and whether they were “continuing” or “new” prescriptions. We assessed trends in continuing BZD, new BZD, continuing nBZRA, and new nBZRA prescriptions from 2005 to 2012.
Results. Proportions of visits with continuing prescriptions increased from 3.4% in 2005 to 4.7% in 2012 (P < .01) for BZDs, and from 1.0% to 1.7% (P < .01) for nBZRAs. We noted no changes in new prescriptions. We observed the same patterns across patient age and physician specialties, except psychiatry. Despite no growth over time, the prevalence of visits involving continuing and new BZD and nBZRA prescriptions was much higher in psychiatry than in primary care and other specialties.
Conclusions. Increased sedative-hypnotic prescribing in recent years may be attributable to long-term growth in continuing prescriptions, rather than new prescriptions.
Public Health Implications. Findings call for renewed efforts to limit continuing prescribing of sedative-hypnotics to reduce their use in the population.
Benzodiazepines (BZDs) and non-BZD receptor agonists (nBZRAs) are part of the broad group of sedative-hypnotic medications commonly prescribed for the treatment of insomnia and anxiety disorders. Their popularity has grown over the past decade1–4 despite ongoing debate regarding the appropriate use of these drugs.5,6
Research studies highlight the association of BZD and nBZRA use with adverse health outcomes,7–9 particularly in older adults, including falls7 and increased disability.9 Consequently, the US Food and Drug Administration (FDA) has approved the majority of BZDs and nBZRAs10,11 for short-term use, typically no longer than 2 to 3 weeks. Although the more extended use of some nBZRAs has received FDA approval in more recent years12,13—eszopiclone was approved for use of up to 6 months in 200412 and the indications for a controlled release version of zolpidem were extended from 3 weeks to 6 months in 200713—regulatory and medical groups continue to discourage physicians from prescribing BZDs and nBZRAs on a long-term basis.14–16 This is partly based on research suggesting that long-term use of these agents is potentially associated with lasting cognitive impairment8,17 and worsening psychiatric symptoms,18 among other adverse outcomes.19
The concerns regarding the potential adverse effects of these medications have led a number of physician groups and medical organizations to develop and disseminate guidelines to discourage their use, especially in older adults, and to encourage alternative treatments.14,20 Notably, the American College of Physicians recently recommended Cognitive Behavioral Therapy for Insomnia as the first-line treatment of chronic insomnia and that the use of sedative-hypnotics should be added only if this therapy alone was not effective.21 Nevertheless, the use of BZDs and nBZRAs has grown in recent years,1–4 and there is some evidence that the long-term prescribing of BZDs is common,3 especially for older adults.3
Although previous studies have examined trends in the prescription and use of BZDs and nBZRAs, no studies have examined trends in new versus continuing prescriptions of sedative-hypnotic medications. It is not known whether the growth in the prescribing of these agents is attributable to larger numbers of continuing prescriptions or of new prescriptions. A recent study by Bachhuber et al. found that the median quantity of BZDs prescribed each year increased from 1996 to 2013, suggesting that patients were receiving either higher doses or a greater number of prescriptions each year.1 A study by Olfson et al. using data from the LifeLink LRx Longitudinal Prescription database (IMS Health, Danbury, CT) found that long-term prescribing of BZDs was particularly common in nonpsychiatric settings (e.g., primary care) and also increased with patient age.3 However, the Olfson et al. study only used data for 2008 and did not include prescriptions to those older than 80 years, a group that may be at greatest risk for adverse health outcomes from long-term sedative-hypnotic use. Further studies are needed to assess trends over time, and for individuals older than 80 years. Furthermore, the prescribing of nBZRAs is already common and fast growing.4 Little is known about trends in repeat and new prescription of these medications over time.
We assessed trends in continuing and new prescriptions for sedative-hypnotic medications between 2005 and 2012, and assessed differences in these trends by patient age and physician specialty using data from the National Ambulatory Medical Care Survey (NAMCS). In a previous report using NAMCS data, we found significant increases in the prescribing of BZDs and nBZRAs between 1993 and 2010.4 However, in that analysis, we did not examine continuing prescriptions separately from new prescriptions. On the basis of our earlier findings, we hypothesized that both continuing and new prescriptions for BZDs and nBZRAs increased between 2005 and 2012. On the basis of the findings of Olfson et al.,3 we also hypothesized that these trends in the continuing prescription groups for BZDs and nBZRAs would be more pronounced in older than younger patients, and in visits to primary care physicians and other nonpsychiatrist physicians than in visits to psychiatrists.
METHODS
We used 2005–2012 data from the NAMCS. The NAMCS is an annual cross-sectional survey that examines health care delivery in a nationally representative sample of ambulatory health care settings in the United States and is conducted by the National Center for Health Statistics (NCHS).22 The NAMCS samples physicians from all specialties and asks them to report on a random selection of patient visits in a random week; therefore, the unit of observation in this data set is the patient visit. Information regarding services and lab tests ordered, reimbursements, diagnoses, and medications prescribed were reported to the NAMCS for each visit on the basis of medical records. We included 287 288 patient visits from the 2005–2012 NAMCS in these analyses (range = 25 665–76 330 per year).
Measures
Medications.
The NAMCS records all prescriptions and over-the-counter drugs that were “ordered, supplied, administered or continued” during the patient visit. Between 2005 and 2010, the NAMCS recorded a maximum of 8 medications; starting in 2011, it increased the number to 10. We examined only the first 8 medications listed for all years to make the available data comparable across years. We included the following as BZDs: alprazolam, clonazepam, clorazepate, chlordiazepoxide, diazepam, estazolam, flurazepam, lorazepam, oxazepam, temazepam, and triazolam; we included zolpidem, zaleplon, and eszopiclone as nBZRAs.
Beginning in the 2005 NAMCS, for each medication prescribed, a notation was also made indicating whether the medication was a “continuing” or “new” medication. For each medication group (i.e., for BZDs and nBZRAs separately), we created 2 variables indicating whether the visit resulted in a new or continuing prescription. Information on continuing versus new medication was missing for 2.4% of BZD and nBZRA prescriptions (2.2% for other specialties, 2.1% for primary care, and 3.1% for psychiatry); we did not include these prescriptions in the analyses.
Patient age.
Physicians were asked to report the age of the patient at the time of the visit. To be consistent in part with the categorization of Olfson et al.,3 we identified each visit as including patients younger than 18 years, 18 to 35 years, 36 to 50 years, 51 to 64 years, 65 to 80 years, and 81 years and older.
Physician specialty.
Physician specialty was recorded by the NAMCS as listed in the American Medical Association Master File. Physicians were asked to confirm this specialty at study enrollment. For this study, we categorized physicians as practicing in primary care (including general or family practice and internal medicine), psychiatry, or other specialties (pediatricians, general surgeons, urologists, etc.).
Analyses
We conducted analyses in 2 stages. First, we assessed overall trends in the proportion of visits across the 8-year period that resulted in continuing and new prescriptions for BZDs and nBZRAs, respectively, using logistic regression models; survey year was the main predictor variable in these models. On the basis of recommendations from the NCHS,23 we combined years into 2-year intervals to improve the stability of our estimates and numbered them from 1 to 4 (1 = 2005–2006, 2 = 2007–2008, etc.). Additionally, to facilitate interpretation of the resulting odds ratios from the regression analyses, we transformed this time variable by subtracting 1 and dividing by 3 (the number of combined years between 2005–2012 minus 1), resulting in values between 0 and 1. This enabled us to interpret the odds ratio as the change in odds of prescribing across the entire study period. Second, we repeated these analyses, stratifying them into separate models according to patient age as well as the specialty of the physician (i.e., primary care, other specialty, and psychiatry).
We conducted analyses in Stata SE version 13 (StataCorp LP, College Station, TX) and used Taylor linearized estimation to account for the complex sampling design and weights to make results representative of office-based physician visits in the United States. Use of these survey design elements has been described elsewhere.22
RESULTS
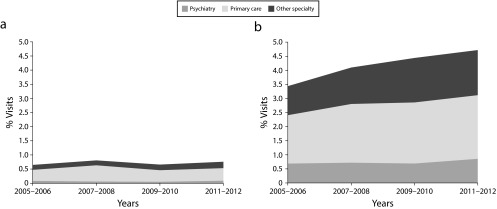
From 2005 to 2012, the proportion of visits resulting in a BZD prescription increased from 4.1% in the 2005–2006 period to 5.4% in the 2011–2012 period (odds ratio [OR] = 1.33; 95% confidence interval [CI] = 1.16, 1.52). The proportion of visits for continuing prescriptions of BZDs increased from 3.4% to 4.7% (OR = 1.37; 95% CI = 1.19, 1.58), whereas there was no significant change in the proportion of visits with new BZD prescriptions (OR = 1.07; 95% CI = 0.86, 1.34; Table 1). Among age groups, we found an increasing trend of continuing prescription of BZDs for all groups, although this was not significant for visits by those younger than 18 years and those aged 65 to 80 years. Across physician specialties, we found increases in continuing BZD prescribing for visits to physicians in primary care and other specialties, but no change among psychiatrists. Growth of continued BZD prescribing as a whole was primarily driven by prescribing increases in nonpsychiatry specialties (Figure 1). We observed no significant trends in stratified analyses for new BZD prescriptions except for a significant decline in prescribing for visits with patients aged 36 to 50 years. Despite no changes over the study period, the prevalence of BZD prescriptions among psychiatrists was the highest among the specialties (24.7% for continuing and 2.4% for new prescriptions over the 2005–2012 period).
TABLE 1—
Trends in Prescribing Continuing and New Prescriptions for Benzodiazepines: National Ambulatory Medical Care Survey, United States, 2005–2012
| Yearsa |
|||||
| Characteristic of Visit | 2005–2006, % | 2007–2008, % | 2009–2010, % | 2011–2012, % | Trend (2005–2012), ORb (95% CI) |
| Continuing prescriptions | |||||
| All visits | 3.44 | 4.10 | 4.44 | 4.72 | 1.37 (1.19, 1.58) |
| Age, y | |||||
| < 18 | 0.20 | 0.16 | 0.17 | 0.28 | 1.46 (0.71, 3.01) |
| 18–35 | 2.21 | 2.52 | 3.37 | 3.51 | 1.67 (1.31, 2.13) |
| 36–50 | 4.12 | 5.97 | 6.29 | 6.53 | 1.53 (1.30, 1.81) |
| 51–64 | 5.29 | 5.76 | 6.20 | 6.64 | 1.27 (1.06, 1.53) |
| 65–80 | 4.72 | 5.13 | 5.05 | 5.76 | 1.20 (0.98, 1.48) |
| ≥ 81 | 4.56 | 5.46 | 6.14 | 6.56 | 1.45 (1.16, 1.82) |
| Physician specialty | |||||
| Other | 1.76 | 2.20 | 2.61 | 2.59 | 1.47 (1.23, 1.76) |
| Primary care | 4.48 | 5.43 | 5.95 | 6.50 | 1.46 (1.21, 1.76) |
| Psychiatry | 24.10 | 25.25 | 24.13 | 25.32 | 1.04 (0.79, 1.38) |
| New prescriptions | |||||
| All visits | 0.65 | 0.81 | 0.66 | 0.76 | 1.07 (0.86, 1.34) |
| Age, y | |||||
| < 18 | 0.08 | 0.05 | 0.06 | 0.06 | 0.79 (0.26, 2.46) |
| 18–35 | 0.88 | 0.97 | 0.95 | 1.37 | 1.51 (0.96, 2.38) |
| 36–50 | 1.03 | 1.58 | 0.88 | 0.89 | 0.74 (0.55, 1.00) |
| 51–64 | 0.84 | 0.94 | 0.88 | 1.06 | 1.22 (0.88, 1.70) |
| 65–80 | 0.58 | 0.64 | 0.67 | 0.60 | 1.04 (0.70, 1.56) |
| ≥ 81 | 0.42 | 0.63 | 0.38 | 0.67 | 1.33 (0.62, 2.88) |
| Physician specialty | |||||
| Other | 0.30 | 0.30 | 0.33 | 0.37 | 1.24 (0.86, 1.78) |
| Primary care | 1.03 | 1.50 | 1.13 | 1.29 | 1.10 (0.86, 1.42) |
| Psychiatry | 2.97 | 2.19 | 1.91 | 2.64 | 0.88 (0.54, 1.42) |
Note. CI = confidence interval; OR = odds ratio. Benzodiazepines include the following: alprazolam, clonazepam, clorazepate, chlordiazepoxide, diazepam, estazolam, flurazepam, lorazepam, oxazepam, temazepam, and triazolam.
Statistics correspond to the percentage of physicians in a specific year that had prescribed the respective medication within strata.
Odds ratios come from logistic regression models and correspond to the difference in odds of being a prescriber of the respective medication across the entire study period (i.e., 2005–2012).
FIGURE 1—
Visits Involving Benzodiazepine Prescriptions That Were (a) New and (b) Continuing: National Ambulatory Medical Care Survey, United States, 2005–2012
Note. Shades indicate the proportion of visits in study years that were attributable to each specialty group.
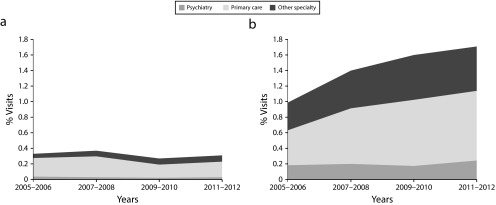
Over the study period, the proportion of visits resulting in an nBZRA prescription increased from 1.3% in the 2005–2006 period to 2.0% in the 2011–2012 period (OR = 1.45; 95% CI = 1.24, 1.71). Whereas the proportion of visits with continuing nBZRA prescriptions increased from 1.0% to 1.7% over this time (OR = 1.65; 95% CI = 1.38, 1.98), there was no significant change in the proportion of visits with a new nBZRA prescription (OR = 0.86; 95% CI = 0.64, 1.17; Table 2). In stratified analyses, we found increases in continuing nBZRA prescribing for all age groups older than 36 years, but not in the younger age groups. As with BZDs, we found increases in continuing nBZRAs for visits to physicians in primary care and other specialties, but no change in visits to psychiatrists. The growth of continued nBZRA prescribing as a whole was attributable to prescribing increases in nonpsychiatry specialties (Figure 2). We observed no significant trends in stratified analyses for new nBZRA prescriptions. Although we saw no changes over the study period, the prevalence of continuing and new nBZRA prescriptions for psychiatrists was highest among the specialties (6.7% for continuing and 1.0% for new prescriptions).
TABLE 2—
Trends in Prescribing Continuing and New Prescriptions for Nonbenzodiazepine Receptor Agonists: National Ambulatory Medical Care Survey, United States, 2005–2012
| Yearsa |
|||||
| Characteristic of Visit | 2005–2006, % | 2007–2008, % | 2009–2010, % | 2011–2012, % | Trend (2005–2012), ORb (95% CI) |
| Continuing prescriptions | |||||
| All visits | 1.00 | 1.40 | 1.60 | 1.71 | 1.65 (1.38, 1.98) |
| Age, y | |||||
| < 18 | 0.07 | 0.03 | 0.02 | 0.03 | 0.27 (0.05, 1.56) |
| 18–35 | 0.61 | 0.69 | 0.76 | 0.86 | 1.41 (0.88, 2.26) |
| 36–50 | 1.00 | 2.08 | 1.76 | 2.13 | 1.74 (1.36, 2.22) |
| 51–64 | 1.96 | 2.22 | 2.87 | 2.93 | 1.56 (1.23, 1.97) |
| 65–80 | 1.22 | 1.67 | 2.16 | 2.24 | 1.83 (1.37, 2.43) |
| ≥ 81 | 1.05 | 1.70 | 1.64 | 1.96 | 1.67 (1.08, 2.57) |
| Physician specialty | |||||
| Other | 0.61 | 0.83 | 0.95 | 0.92 | 1.47 (1.10, 1.96) |
| Primary care | 1.18 | 1.86 | 2.33 | 2.57 | 2.08 (1.65, 2.63) |
| Psychiatry | 6.46 | 7.04 | 6.08 | 7.22 | 1.07 (0.74, 1.55) |
| New prescriptions | |||||
| All visits | 0.33 | 0.37 | 0.27 | 0.31 | 0.86 (0.64, 1.17) |
| Age, y | |||||
| < 18 | 0.01 | 0.00 | 0.02 | 0.00 | 0.78 (0.09, 6.46) |
| 18–35 | 0.34 | 0.36 | 0.31 | 0.30 | 0.85 (0.42, 1.74) |
| 36–50 | 0.69 | 0.57 | 0.32 | 0.61 | 0.75 (0.40, 1.40) |
| 51–64 | 0.38 | 0.57 | 0.48 | 0.47 | 1.11 (0.63, 1.95) |
| 65–80 | 0.23 | 0.38 | 0.24 | 0.24 | 0.88 (0.52, 1.48) |
| ≥ 81 | 0.27 | 0.24 | 0.16 | 0.09 | 0.37 (0.12, 1.12) |
| Physician specialty | |||||
| Other | 0.09 | 0.12 | 0.13 | 0.13 | 1.37 (0.74, 2.55) |
| Primary care | 0.62 | 0.70 | 0.46 | 0.57 | 0.82 (0.58, 1.18) |
| Psychiatry | 1.30 | 1.03 | 0.79 | 0.87 | 0.63 (0.33, 1.23) |
Note. CI = confidence interval; OR = odds ratio. Nonbenzodiazepine receptor agonists include zolpidem, zaleplon, and eszopiclone.
Statistics correspond to the percentage of physicians in a specific year that had prescribed the respective medication within strata.
Odds ratios come from logistic regression models and correspond to the difference in odds of being a prescriber of the respective medication across the entire study period (i.e., 2005–2012).
FIGURE 2—
Visits Involving Nonbenzodiazepine Receptor Agonist Prescriptions That Were (a) New and (b) Continuing: National Ambulatory Medical Care Survey, United States, 2005–2012
Note. Shades indicate the proportion of visits in study years that were attributable to each specialty group.
Although the trend in continuing prescription was larger than for new prescriptions for both BZDs and nBZRAs, the ratio of continued over new prescriptions grew more drastically for nBZRAs than for BZDs. For BZDs, the continuing–new ratio changed from 5.3 in the 2005–2006 period to 6.2 in the 2011–2012 period, an almost 17% increase; for nBZRAs, the ratio changed more than 80%, from 3.0 to 5.5.
As the prevalence of BZD and nBZRA prescribing was highest among psychiatrists, we further investigated whether the increasing trend in visits involving continuing BZD and nBZRA prescriptions was in part attributable to an increase in the proportion of visits to psychiatrists among these visit types. Specifically, we examined time trends in the odds of visits to psychiatrists among all visits that involved continuing prescriptions for BZDs and nBZRAs. For this, we conducted logistic regression analyses with physician specialty (psychiatry vs nonpsychiatry) as the outcome and time as the predictor. The results of these analyses indicated that between 2005 and 2012, there was a moderately declining albeit not significant change in the odds of visits to psychiatrists. Among visits involving continuing BZD prescriptions, 20.0% were to psychiatrists in the 2005–2006 period compared with 18.2% in the 2011–2012 period (OR = 0.87; 95% CI = 0.60, 1.27; P = .48). Similarly, 18.5% of visits involving continuing nBZRA prescriptions were to psychiatrists in the 2005–2006 period compared with 14.3% in the 2011–2012 period (OR = 0.72; 95% CI = 0.42, 1.24; P = .23). Thus, the increasing trend in continuing prescriptions of BZDs and nBZRAs cannot be attributed to a larger proportion of psychiatry visits.
DISCUSSION
To our knowledge, this is the first study to assess national prescribing trends in continuing and new prescriptions for sedative-hypnotic medications. We found that the prevalence of visits at which a BZD or an nBZRA was prescribed increased in the 2005–2012 period, consistent with findings from our study of an earlier period in the NAMCS4 and those of other investigators.1–3 Furthermore, in the present study we observed that the increasing trend was limited to continuing prescriptions. Whereas the prevalence of visits in which these medications were started (i.e., new prescriptions) did not increase over the 8-year period of the study, visits involving continuing prescriptions for BZDs increased by 32% and those involving continued nBZRA prescriptions increased by 54%. We also found that between 2005 and 2012, there were significant increases in the percentage of visits with continued BZD prescriptions across patient age and physician specialty strata, with the exception of psychiatry. Our findings indicate that the cross-sectional results reported by Olfson et al. showing a relatively high prevalence of long-term BZD use in nonpsychiatry specialties in 20083 may be attributable to a broader increasing trend that preceded 2008 and continued until 2012.
Although the findings regarding temporal trends were clear, the reasons for the growing trends in continuing prescription of these medications remain elusive. Changes in public and prescriber attitudes toward these medications and their safety profiles may have contributed to this trend. During the study period, there were marketing efforts to promote the safety of opioid pain medications, and these efforts may have had “spillover” effects, creating a sense of safety for the prescribing of benzodiazepines. Additionally, at the beginning of our study period, eszopiclone and the controlled release version of zolpidem were approved with indications for up to 6 months, which may have led to acceptance of continuing prescribing of nBZRAs and perhaps BZDs. However, these potential influences were countered by the growing recognition of the potential harms associated with these medications9,17,19,24 and the growing use of selective serotonin reuptake inhibitors, which are indicated for the treatment of anxiety disorders. More research should examine the impact of these various factors on the observed trends in BZD and nBZRA prescriptions, and assess their impact on continuing prescribing in the years following 2012.
We found that continued prescribing of BZDs at visits of the very old (i.e., 81 years and older) was as prevalent, if not more so, as continued prescribing to younger groups. Continued nBZRA prescribing was also more common in this older age group. These findings extend those of Olfson et al,3 who examined ages up to 80 years. We further found that the proportion of visits involving continued prescribing of BZDs as well as nBZRAs significantly grew in the 81-years-and-older age group between 2005 and 2012. This finding is concerning in light of the inclusion of BZDs and, more recently, of nBZRAs on the Beers Criteria, which identify medications to be avoided in older age groups.14 With the growing population of older adults in the United States, it will be important that long-term prescribing among older adults is monitored and managed.
The growth of continued prescriptions was seen almost exclusively in visits to practices with nonpsychiatry specialties (i.e., primary care and other specialties). Although the prevalence of BZDs and nBZRAs in visits to psychiatrists was quite high, especially for continued prescribing, these providers did not contribute to the growing trends in prescribing these medications over time. This finding is consistent with other research indicating that psychotropic prescribing in general has become more common in primary care practices in recent years.25 Efforts have sought to limit the prescribing of sedative-hypnotics in practices by a variety of means,15 but in view of the growing prescription trends, continued implementation and evaluation of these efforts is clearly needed.
Minimizing long-term prescribing will require a multicomponent effort that includes patients, providers, and payers. The opioid abuse epidemic in recent years has brought about many efforts to stop long-term opioid prescribing, including public education programs26 and the development of state guidelines.27 These efforts have been somewhat successful at preventing long-term prescribing of these medications. Lessons learned from the opioid control efforts may benefit future efforts aimed at curbing long-term prescribing of BZDs and nBZRAs. The New York State triplicate prescribing program implemented in 1989 is a successful example of such policy initiatives. The program resulted in an immediate 50% decline in the prescribing of BZDs to patients previously prescribed BZDs.24
At the same time, it will also be important to improve access to alternative treatment options, including safer medications as well as behavioral treatments. There is some evidence to suggest that efforts to decrease use of certain medications may unintentionally result in substitutions with less-safe alternatives. For example, the exclusion of BZD reimbursement following the implementation of Medicare Part D in 2006 coincided with an increase in the prescribing of other non-BZD psychotropic medications (e.g., antidepressants and anxiolytics).28 Regarding sleep disorders, despite the demonstrated efficacy of nonpharmacological treatment options (e.g., sleep hygiene education and cognitive behavioral therapy for insomnia), there are few providers trained in their administration.29 Future efforts should consider offering training and incentives to encourage the delivery and dissemination of these promising treatments.
Limitations
Our study has a number of limitations. First, data were based on medical records and some were recorded by nonphysician staff, making them open to clerical errors. Second, trends in the prescription of a medication does not directly translate into trends in the use of the medication. Patients may not fill the prescription or not use the medication consistently. Third, some of the “continued” medications may have been prescribed to be used “as needed,” in which case the medication may not have been used continuously. Fourth, a physician might have marked a prescription as new when a previous prescription for the same (or similar) medication had been provided by another physician. If that is the case, the prevalence of continuing sedative-hypnotic prescriptions in this study may have been an underestimate. Fifth, we could not assess the appropriateness of the continued prescriptions, because the NAMCS did not inquire about the indication for prescribed medications. Although the NAMCS did collect data on diagnoses at the visit, only a small proportion of sedative-hypnotic visits were associated with diagnoses that could be the target of these prescriptions (e.g., anxiety disorders, insomnia).4 Finally, the specific patient instructions for the prescription were not recorded; for example, information such as how long the medication was prescribed for, whether the medication was to be used as needed or on a regular basis, and the dose was not available.
Public Health Implications
Acknowledging the limitations noted in the previous section, this study provides evidence for an increasing prevalence of visits involving continued prescriptions for BZDs and nBZRAs in the United States. Future research needs to examine the association of this practice pattern with long-term use of these medications and explore society-, physician-, and patient-level factors driving these trends. Our findings also highlight the need for renewed efforts to monitor the long-term prescribing of sedative-hypnotics to vulnerable patients—especially older adults. Part of these efforts could include disseminating information with guidance on safe prescribing practices for these medications through continuing education credits, classes or seminars, and information packets, or by reminders or alerts in electronic medical records warning about prescribing to particularly vulnerable groups. Provider organizations with expertise in sleep and anxiety disorders might also advise and provide resources for physicians in nonpsychiatry specialties on alternative treatment options available. More importantly, efforts need to be made to increase the availability and appropriate use of safer alternative treatments, including medications (e.g., selective serotonin reuptake inhibitors) and cognitive behavioral therapies for anxiety disorders and insomnia.30–33 Such efforts could potentially modify physicians’ prescribing styles and ultimately decrease the public health burden associated with long-term use of sedative-hypnotic medications.
ACKNOWLEDGMENTS
C. N. Kaufmann received funding from University of California San Diego’s T32 Research Training Program in Geriatric Mental Health (T32MH019934). A. P. Spira is supported in part by National Institute on Aging grants AG050507, AG050745, and AG049872.
We thank Daniel F. Kripke, MD, for his helpful feedback on the manuscript.
HUMAN PARTICIPANT PROTECTION
This study used de-identified publically available data and was thus exempt from institutional review board review.
REFERENCES
- 1.Bachhuber MA, Hennessy S, Cunningham CO, Starrels JL. Increasing benzodiazepine prescriptions and overdose mortality in the United States, 1996–2013. Am J Public Health. 2016;106(4):686–688. doi: 10.2105/AJPH.2016.303061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ford ES, Wheaton AG, Cunningham TJ, Giles WH, Chapman DP, Croft JB. Trends in outpatient visits for insomnia, sleep apnea, and prescriptions for sleep medications among US adults: findings from the National Ambulatory Medical Care Survey 1999–2010. Sleep. 2014;37(8):1283–1293. doi: 10.5665/sleep.3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olfson M, King M, Schoenbaum M. Benzodiazepine use in the United States. JAMA Psychiatry. 2015;72(2):136–142. doi: 10.1001/jamapsychiatry.2014.1763. [DOI] [PubMed] [Google Scholar]
- 4.Kaufmann CN, Spira AP, Alexander GC, Rutkow L, Mojtabai R. Trends in prescribing of sedative-hypnotic medications in the USA: 1993–2010. Pharmacoepidemiol Drug Saf. 2016;25(6):637–645. doi: 10.1002/pds.3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moore N, Pariente A, Bégaud B. Why are benzodiazepines not yet controlled substances? JAMA Psychiatry. 2015;72(2):110–111. doi: 10.1001/jamapsychiatry.2014.2190. [DOI] [PubMed] [Google Scholar]
- 6.Fava GA, Balon R, Rickels K. Benzodiazepines in anxiety disorders. JAMA Psychiatry. 2015;72(7):733–734. doi: 10.1001/jamapsychiatry.2015.0182. [DOI] [PubMed] [Google Scholar]
- 7.Herings RM, Stricker BH, de Boer A, Bakker A, Sturmans F. Benzodiazepines and the risk of falling leading to femur fractures. Dosage more important than elimination half-life. Arch Intern Med. 1995;155(16):1801–1807. [PubMed] [Google Scholar]
- 8.Billioti de Gage S, Moride Y, Ducruet T et al. Benzodiazepine use and risk of Alzheimer’s disease: case–control study. BMJ. 2014;349:g5205. doi: 10.1136/bmj.g5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gray SL, LaCroix AZ, Hanlon JT et al. Benzodiazepine use and physical disability in community-dwelling older adults. J Am Geriatr Soc. 2006;54(2):224–230. doi: 10.1111/j.1532-5415.2005.00571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Food and Drug Administration. Ambien approval letter. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/nda/pre96/019908_S000_AP&AE_LTRS&FPL.pdf. Accessed June 18, 2014.
- 11.Food and Drug Administration. Sonata approval letter. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/appletter/1999/20859ltr.pdf. Accessed June 17, 2014.
- 12.Food and Drug Administration. Lunesta prescribing information. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/021476s005s008lbl.pdf. Accessed March 2, 2016.
- 13.Food and Drug Administration. Letter confirming supplemental new drug application: Ambien CR. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/appletter/2007/021774s003,s004,s005,s007,s008ltr.pdf. Published December 20, 2007. Accessed March 2, 2016.
- 14.American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2015;63(11):2227–2246. doi: 10.1111/jgs.13702. [DOI] [PubMed] [Google Scholar]
- 15.Smith AJ, Tett SE. Improving the use of benzodiazepines—is it possible? A non-systematic review of interventions tried in the last 20 years. BMC Health Serv Res. 2010;10(1):321. doi: 10.1186/1472-6963-10-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.American Psychiatric Association. Practice guideline for the treatment of patients with panic disorder. Available at: http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/panicdisorder.pdf. Accessed August 13, 2014.
- 17.Gallacher J, Elwood P, Pickering J, Bayer A, Fish M, Ben-Shlomo Y. Benzodiazepine use and risk of dementia: evidence from the Caerphilly Prospective Study (CaPS) J Epidemiol Community Health. 2012;66(10):869–873. doi: 10.1136/jech-2011-200314. [DOI] [PubMed] [Google Scholar]
- 18.Guina J, Rossetter SR, DeRhodes BJ, Nahhas RW, Welton RS. Benzodiazepines for PTSD: a systematic review and meta-analysis. J Psychiatr Pract. 2015;21(4):281–303. doi: 10.1097/PRA.0000000000000091. [DOI] [PubMed] [Google Scholar]
- 19.Kripke DF. Chronic hypnotic use: deadly risks, doubtful benefit. Sleep Med Rev. 2000;4(1):5–20. doi: 10.1053/smrv.1999.0076. [DOI] [PubMed] [Google Scholar]
- 20.Gallagher P, Ryan C, Byrne S, Kennedy J, O’Mahony D. STOPP (Screening Tool of Older Person’s Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment). Consensus validation. Int J Clin Pharmacol Ther. 2008;46(2):72–83. doi: 10.5414/cpp46072. [DOI] [PubMed] [Google Scholar]
- 21.Qaseem A, Kansagara D, Forciea MA, Cooke M, Denberg TD Clinical Guidelines Committee of the American College of Physicians. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165(2):125–133. doi: 10.7326/M15-2175. [DOI] [PubMed] [Google Scholar]
- 22.Hing E, Hall MJ, Xu J. National Hospital Ambulatory Medical Care Survey: 2006 outpatient department summary. Natl Health Stat Report. 2008;(4):1–31. [PubMed] [Google Scholar]
- 23.Hsiao C-J. Understanding and using NAMCS and NHAMCS data. Available at: https://www.cdc.gov/nchs/ppt/nchs2010/03_hsiao.pdf. Accessed August 4, 2016.
- 24.Wagner AK, Ross-Degnan D, Gurwitz JH et al. Effect of New York State regulatory action on benzodiazepine prescribing and hip fracture rates. Ann Intern Med. 2007;146(2):96–103. doi: 10.7326/0003-4819-146-2-200701160-00004. [DOI] [PubMed] [Google Scholar]
- 25.Olfson M, Kroenke K, Wang S, Blanco C. Trends in office-based mental health care provided by psychiatrists and primary care physicians. J Clin Psychiatry. 2014;75(3):247–253. doi: 10.4088/JCP.13m08834. [DOI] [PubMed] [Google Scholar]
- 26.Spoth R, Trudeau L, Shin C et al. Longitudinal effects of universal preventive intervention on prescription drug misuse: three randomized controlled trials with late adolescents and young adults. Am J Public Health. 2013;103(4):665–672. doi: 10.2105/AJPH.2012.301209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Franklin G, Sabel J, Jones CM et al. A comprehensive approach to address the prescription opioid epidemic in Washington State: milestones and lessons learned. Am J Public Health. 2015;105(3):463–469. doi: 10.2105/AJPH.2014.302367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ong MK, Zhang L, Xu H, Azocar F, Ettner SL. Medicare Part D benzodiazepine exclusion and use of psychotropic medication by patients with new anxiety disorders. Psychiatr Serv. 2012;63(7):637–642. doi: 10.1176/appi.ps.201100331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vitiello MV, McCurry SM, Rybarczyk BD. The future of cognitive behavioral therapy for insomnia: what important research remains to be done? J Clin Psychol. 2013;69(10):1013–1021. doi: 10.1002/jclp.21948. [DOI] [PubMed] [Google Scholar]
- 30.Reinhold JA, Mandos LA, Rickels K, Lohoff FW. Pharmacological treatment of generalized anxiety disorder. Expert Opin Pharmacother. 2011;12(16):2457–2467. doi: 10.1517/14656566.2011.618496. [DOI] [PubMed] [Google Scholar]
- 31.Buysse DJ, Germain A, Moul DE et al. Efficacy of brief behavioral treatment for chronic insomnia in older adults. Arch Intern Med. 2011;171(10):887–895. doi: 10.1001/archinternmed.2010.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jungquist CR, O’Brien C, Matteson-Rusby S et al. The efficacy of cognitive-behavioral therapy for insomnia in patients with chronic pain. Sleep Med. 2010;11(3):302–309. doi: 10.1016/j.sleep.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olatunji BO, Cisler JM, Deacon BJ. Efficacy of cognitive behavioral therapy for anxiety disorders: a review of meta-analytic findings. Psychiatr Clin North Am. 2010;33(3):557–577. doi: 10.1016/j.psc.2010.04.002. [DOI] [PubMed] [Google Scholar]