Abstract
Elevated LDL concentration in mid-life increases the risk of developing Alzheimer’s disease (AD) in later life. Increased oxidative modification (oxLDL) and nitration is observed during dementia and hypercholesterolemia.
We investigated the hypothesis that statin intervention in mid-life mitigates the inflammatory effects of oxLDL on the microvasculature. Human microvascular endothelial cells (HMVEC) were maintained on transwells to mimic the microvasculature and exposed to patient and control LDL. Blood was obtained from statin-naïve, normo- and hyperlipidaemic subjects, AD with vascular dementia (AD-plus) and AD subjects (n=10/group) at baseline. Only hyperlipidaemic subjects with normal cognitive function received 40mg simvastatin intervention/day for three months. Blood was re-analysed from normo- and hyper-lipidaemic subjects after three months.
LDL isolated from statin-naïve hyperlipidaemic, AD and AD-plus subjects was more oxidised (agarose gel electrophoretic mobility, protein carbonyl content and 8-isoprostane F2α) compared to control subjects. Statin intervention decreased protein carbonyls (2.5±0.4 Vs 3.95±0.2nmol/mg; P<0.001) and 8-isoprostane F2α (30.4±4.0 pg/ml Vs 43.5±8.42 pg/ml; P<0.05). HMVEC treatment with LDL-lipids from hyperlipidaemic, AD and AD-plus subjects impaired endothelial tight junction expression and decreased total glutathione levels (AD; 18.61±1.3, AD-plus; 16.5±0.7nmol/mg protein) compared to untreated cells (23.8±1.2 vs nmol/mg protein). Basolateral IL-6 secretion was increased by LDL-lipids from hyperlipidaemic (78.4±1.9 pg/ml), AD (63.2±5.9 pg/ml) and AD-plus (80.8±0.9 pg/ml) groups compared to healthy subject lipids (18.6±3.6 pg/ml). LDL-Lipids isolated after statin intervention did not affect endothelial function.
In summary, LDL-lipids from hypercholesterolaemic, AD and AD-plus patients are inflammatory to HMVEC. In vivo intervention with statins reduces the damaging effects of LDL-lipids on HMVEC.
Keywords: blood brain barrier, lipids, statins, Alzheimer’s, endothelium
Introduction
In the adult brain, primary cholesterol synthesis occurs in astrocytes and to a lesser extent in neurons [1]. Cholesterol is transported within the brain by local lipoproteins. Brain cholesterol metabolism is discrete from metabolism in peripheral tissues and the central nervous system (CNS), and plasma cholesterol/lipoprotein compartments are strictly segregated by the blood brain barrier (BBB).
The BBB is created by the tight junctions between the endothelial cells of brain microvascular tissue assisted by the astrocyte foot processes surrounding the capillary endothelial cells [2, 3]. Transfer across the BBB to the CNS is achieved via ATP-dependent transporters, facilitated diffusion, transmembrane diffusion, and leakage through extracellular pathways.
There is no evidence that normal lipoprotein cholesterol (C) (e.g. high density lipoprotein-C; HDL-C and low density lipoprotein-C; LDL-C) originating in the plasma compartment routinely crosses the BBB to be transported into the CNS [4]. In support of this, knockout of the LDL receptor gene in mice and rabbits or disruption of peripheral SR-BI or ABCA1 in mice changes neither the cholesterol synthesis rate nor the cholesterol concentration in the brain [5, 6]. Taken together, these data are in agreement with the view that plasma cholesterol is not transported to the brain because plasma lipoproteins do not cross the BBB. Nevertheless, prospective studies have shown a reduced risk for AD in users of cholesterol-lowering statin drugs [7]. Many previous studies demonstrated the beneficial effects of statins on endothelial dysfunction and chronic inflammation [8, 9]. In addition, an association between previous statin use and reduced neurofibrillary tangle burden post-mortem has also been reported [10, 11]. In the TgCRND8 mouse model of AD, a reduction in β amyloid accumulation was been observed in the brain after treatment pravastatin, a non-hydrophilic, non-blood brain barrier (BBB)-permeant statin [12], supporting the hypothesis that peripheral cholesterol metabolism is important to brain pathology.
Hypercholesterolaemia is an important contributor to oxidative stress [13], a condition of an imbalance between oxygen free radical production and removal by antioxidant enzymes and micronutrients. If radical production exceeds that which can be controlled by antioxidants, irreversible damage to proteins, lipids and DNA occurs. Evidence for oxidation and nitration of proteins to form carbonyl groups and 3-nitrotyrosine is frequent in plasma from both hypercholesterolaemic and dementia patients [14, 15]. Similarly, free radical-oxidised lipids (e.g. isoprostanes, lipid hydroperoxides) are more prevalent in plasma from AD and hypercholesterolaemic subjects compared to controls [16]. Together, these studies implicate systemic lipid/protein oxidation as common a common feature of AD and hypercholesterolaemia. Whilst the beneficial effects of statins against cardiovascular disease in the latter group have been attributed to reduction in cholesterol, recent studies suggest that benefit is due to oxidised LDL removal [17]. Indeed, clinical trials showing protective effects of statins against AD do not show that a reduction in plasma cholesterol correlates with reduced risk for AD, indicating that an alternative mechanism for the beneficial effects of statins may be present [18]. It is not known whether removal of oxidised LDL accounts for the protective effects of statin-usage in mid-life against the later AD development.
Therefore, we have examined the hypothesis that systemic oxidised LDL affects the function of microvascular endothelial cells to secrete inflammatory mediators into the brain compartment predisposing to AD and that statin intervention renders LDL less inflammatory. We have undertaken qualitative and quantitative analysis of lipid oxidation products in LDL isolated from mid-life subjects with elevated plasma cholesterol, investigated whether statin intervention in hypercholesterolaemic subjects can prevent modifications to LDL in vivo and whether any protection of LDL oxidation restores normal microvascular endothelial cell function.
Materials and Methods
Plasma sample preparation
Twenty mid-life male adults (40-60 years old, mean age 46.9 years) were recruited from general medical practices in the Birmingham area with (total cholesterol >6.5mM measured) and without hypercholesterolaemia. All hypercholesterolaemic subjects were unresponsive to lifestyle change, were statin-naïve and were not taking any disease modifying anti-inflammatory medication or nutritional supplements. All subjects were healthy by careful clinical history and examination, and scored at least 27 on the mini-mental state examination. The research has been carried out in accordance with the Declaration of Helsinki (2008) of the World Medical Association and ethical approval was obtained from the Birmingham and Black Country Local Research Ethics Committee (REC 09/H1202/87). Participants provided informed written consent.
After an overnight fast, 5mL whole blood was drawn from the antecubital vein of each participant and collected into ethylenediaminetetraacetic acid (EDTA) coated tubes (Greiner Bio-One Ltd, UK) between 8:00 and 10:30 am as a baseline measure. All ten statin-naïve, hypercholesterolaemic subjects were started on simvastatin intervention (40mg/day) as a revision to their routine management after lifestyle changes had failed to reduce blood cholesterol, whereas normolipidaemic subjects maintained habitual diets and lifestyles without intervention. According to NICE guidelines (guidance.nice.org.uk/cg181) patients were recruited after 3 months to a second blood sampling. All ten hypercholesterolaemic patients completed the intervention for the study duration of 3 months. AD subjects were recruited from the Unit of Cognitive Frailty, Neurology Outpatient Clinic, Cologne, Germany, after diagnosis of AD using NINCDS–ADRDA criteria either in the presence of vascular comorbidities and risk factors (high cholesterol, hypertension, stroke, type 2 diabetes mellitus, myocardial infarction and heart arrhythmia; AD-Plus group) or without cardiovascular comorbidities and risk factors (AD group) [19]. Three patients in the AD group were diagnosed with hypertension alone. After an overnight fast, 5mL whole blood was drawn from the antecubital vein of each participant and collected into ethylenediaminetetraacetic acid (EDTA). Informed consent was obtained from the patients or their care givers according to severity of disease and the study was approved by the local ethics committee. Blood plasma was prepared by centrifugation (200 g, 30 min) and stored immediately at -80°C prior to analysis and extract LDL.
Isolation, modification, characterisation of LDL and LDL-lipids
All blood samples were obtained using LDL was isolated, modified and characterised as previously [20]. LDL protein concentration was analysed by Bicinchoninic acid assay (Sigma Aldrich, UK). In addition, LDL lipid content was analysed by Amplex® Red cholesterol assay kit (Life Technologies, UK) and phospholipid assay. The degree of lipid oxidation of LDL and oxLDL was determined as 8-isoprostane F2α levels (15.5±1 pg/mg of LDL and 26±2.5 pg/mg of oxLDL) by EIA method according to manufacturer’s instructions (Cayman Chemicals). LDL-lipids (LDL-L) or oxLDL-lipids (oxLDL-L) were extracted from LDL using the Folch method by addition of 160 µl of ice-cold methanol (containing 50 µg/ml BHT) followed by the addition of 320 µl of ice-cold chloroform and incubation for 20 min on ice with occasional vortex mixing. High-purity water (150 µl) was added and the sample kept on ice for an additional 10 min with occasional mixing. The sample was centrifuged for 5 min at 200 g and the upper (aqueous) phase was removed and re extracted by addition of 250 µl of ice-cold chloroform: methanol (2:1,v/v) as above. The upper phase was discarded and both organic phases were combined, dried under nitrogen gas, and kept at -80°C until further use. Extracted lipids for cell culture experiments were conjugated to fatty acid-free bovine serum albumin (BSA) in serum-free RPMI 1640 [25].
Phospholipid assay
Total phospholipid content in HMVEC cell lysates were analysed as described previously with some modifications [21]. A phospholipid standard curve (0.005- 0.05 mg/ml egg yolk lecithin) was prepared by diluting 0.1 mg/ml stock solution in chloroform in glass vials. 1ml of 0.1N ammonium ferrothiocyanate (2.7 g ferric chloride hexahydrate ( FeC13.6H20) and 3.04 g ammonium thiocyanate (NH4SCN) in deionized distilled water and made up to 100ml) was added to 1ml of lipid standards or sample and vortexed for 1 min. The layers were allowed to separate for 5min before removing chloroform layer into a new glass tube. Samples and standards were read in spectrophotometer at 488nm using a quartz cuvette.
Agarose gel electrophoresis
Lipoprotein was assessed by 1% agarose gel electrophoresis in barbital buffer as described previously [22]. Briefly, bromophenol blue in glycerol (50%; 4 µl) was added to LDL samples (20 µg) before electrophoresis in barbital buffer (sodium barbital 10.6 g/L, pH 8.6) for 2 h at 55 V. Gels were stained with Coomassie brilliant blue to visualise proteins for around 45 min. The gel was destained (40% methanol, 10% acetic acid, 50% water), for 30 min, prior to exchange for fresh destain and then left overnight. Rf values were calculated for each sample as the distance the sample moved from origin (mm) divided by the distance travelled by the dye front (mm).
Microvascular endothelial model
Human microvascular endothelial cells (HMVEC, Invitrogen, Life technologies, UK) were seeded (3×105/ml) onto 24-well polycarbonate inserts and cultured for 7days. LDL-L or oxLDL-L (0.4-8 µg) or control/patient LDL-L (4µg) was added onto endothelial barrier for 24 hours before measuring the change in barrier permeability after treatment with 200µl of 1mg/ml FITC-dextran apically for 24 hours. The basolateral and apical media was collected, the apical surface washed twice, the washes collected and combined with the apical media collected. Fluorescence of the media was read at the following wavelengths; excitation 488nm and emission wavelength 520nm using a Spectra Max Gemini XS fluorimeter (Molecular Devices). The percentage fluorescence in the basolateral compartment was calculated as a measure of cell permeability.
ELISA
Following the cell treatments with lipids, cell culture media was collected and cells were pelleted by centrifugation (200g, 10mins), cell free media containing secreted cytokines was stored at -20°C until analysis for interleukin (IL)-6 and tumour necrosis factor (TNF)α by ELISA (Peprotech, UK).
Cell viability assay
Cell viability was measured using the Cell Titer-Blue® viability assay. After incubation with 100µl of Cell Titer-Blue®, supernatants from Transwell® inserts were removed to a 96 well plate and fluorescence measured at excitation (Ex) 560nm emission (Em) 590nm using a Spectra Max Gemini XS fluorimeter (Molecular Devices).
Zona occludens-1 (ZO-1) staining
HMVEC were seeded onto transwell inserts and after 7 days cells were fixed with 100% methanol (pre-cooled) prior to permeabilisation with 0.1% (v/v) Triton X-100. Cells were then blocked with 100µl 1% (v/v) normal goat serum before incubating with mouse monoclonal anti-ZO-1 diluted 1:250 in 1% bovine serum albumin (BSA) overnight at 4°C. Cells were then stained with goat anti-mouse fluorescein isothiocyanate (FITC)-conjugated secondary antibody diluted 1:100 in 1% BSA and mounted with hard set mounting medium containing 4',6-diamidino-2-phenylindole (DAPI) for 48 hours at 4°C in the dark. Images were visualized using a Zeiss LSM 510 META confocal laser-scanning microscope (Leica Microsystems, Milton Keynes, UK) with the x63 oil objective, the green filter cube (495nm) and the blue filter cube (365nm) for visualisation. Images were analysed using the LAS AD Lite software (Leica Microsystems, Milton Keynes, UK).
Glutathione (GSH) assay
HMVEC GSH levels were measured by GSH- Glo assay (Promega).
Lipid peroxidation analysis
Plasma 8-isoprostane F2α was measured by ELISA (Cayman Chemicals). This kit provided Inter-assay variation (%CV) of 16.6 and intra assay variation (%CV) of 16.2. A lower limit of detection was 20pg/ml.
Statistical analysis
Statistical significance was tested by using ANOVA with Tukey’s post-test, student’s T test or Wilcoxon’s matched paired T test using Prism 6 (Graphpad). Unless specified all data are presented as the mean ± SEM of at least three independent experiments, each performed in triplicate.
Results
LDL and oxLDL-lipids increase microvascular endothelial permeability in vitro
Previously we have shown that LDL and its lipids are more oxidised in dementia, therefore we examined the effects of LDL and LDL-lipids that had been isolated from patients with hypercholesterolemia or dementia and their respective controls directly on HMVEC.
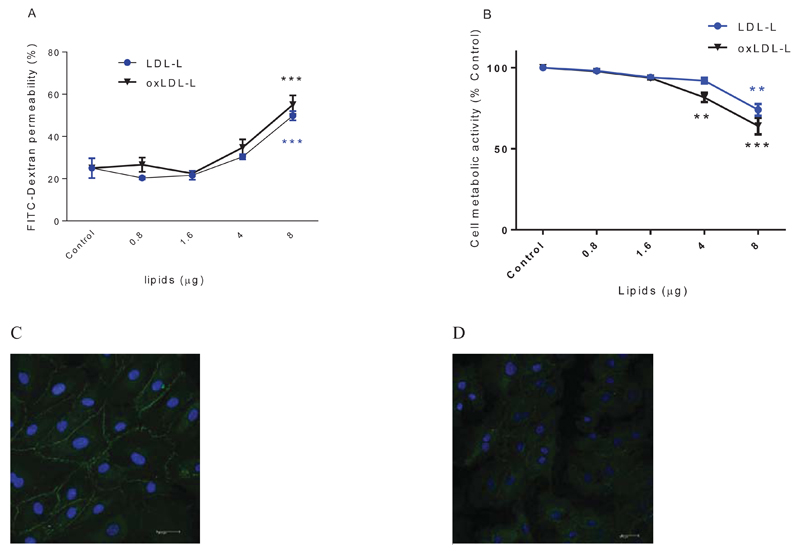
HMVEC were cultured on transwells until a barrier was formed with ZO-1 expression localised to membrane junctions at day 7. Monolayers were subsequently exposed to control LDL from healthy donors with and without oxidation for 24 hours. LDL and oxLDL treatments (≤ 4µg) did not affect endothelial function as measured by permeability to FITC dextran or cell viability (supplementary figure 1). To further explore whether the loss of endothelial function after 8µg of oxLDL is due to protein or lipid fractions, LDL was sub-fractionated. LDL-lipids and oxLDL-lipids increased barrier permeability significantly in a concentration dependent manner (Figure 1A), while LDL-protein had no significant effect (supplementary figure 2). Lipids fractionated from oxLDL and LDL caused a significant inhibition of metabolic activity after addition of >4µg and 8µg respectively (Figure 1B). Despite evidence of cell stress, measured as metabolic inhibition, there was no significant release of lactate dehydrogenase (LDH) when treated with 4µg LDL- or oxLDL-lipids (supplementary figure 3). Addition of LDL or lipids isolated from 4 µg LDL led to a three-fold increase in cellular cholesterol concentration (supplementary figure 4).
Figure 1. The effect of LDL, oxLDL-lipids on endothelial barrier permeability.
HMVEC cells (1×105cells) were seeded in transwell inserts for 2 weeks before lipid treatments. HMVEC were treated with increasing concentrations of LDL-lipids and oxLDL-lipids for 24 hours. Barrier tightness was measured by FITC dextran permeability (A). Cell metabolic activity was measured by CellTiter-Blue® assay (B). Tight junction protein ZO-1 is stained in green, nuclei stained in blue DAPI stain in HMVEC cells before (C) and after (D) treating with LDL-lipids (from 4µg LDL). ** P<0.01, *** P<0.001, n=3 independent experiments.
To determine whether the disruption of tight junction-associated protein was linked to loss of barrier integrity, we immuno-stained HMVEC cells for ZO-1 before and after LDL-lipid treatments. After treatment with a non-toxic but barrier-disturbing concentration of LDL-lipids (4µg), ZO-1 staining was diffuse (Figure 1D) and ZO-1 was not visible on the margins of the cytoplasmic membrane surface; nevertheless, membranes appeared intact. Staining after LDL-lipid addition was unlike the ZO-1 staining pattern that was observed for untreated, control HMVEC.
LDL- and oxLDL-lipids increase NF-kB-dependent basolateral secretion of TNF-α and IL-6
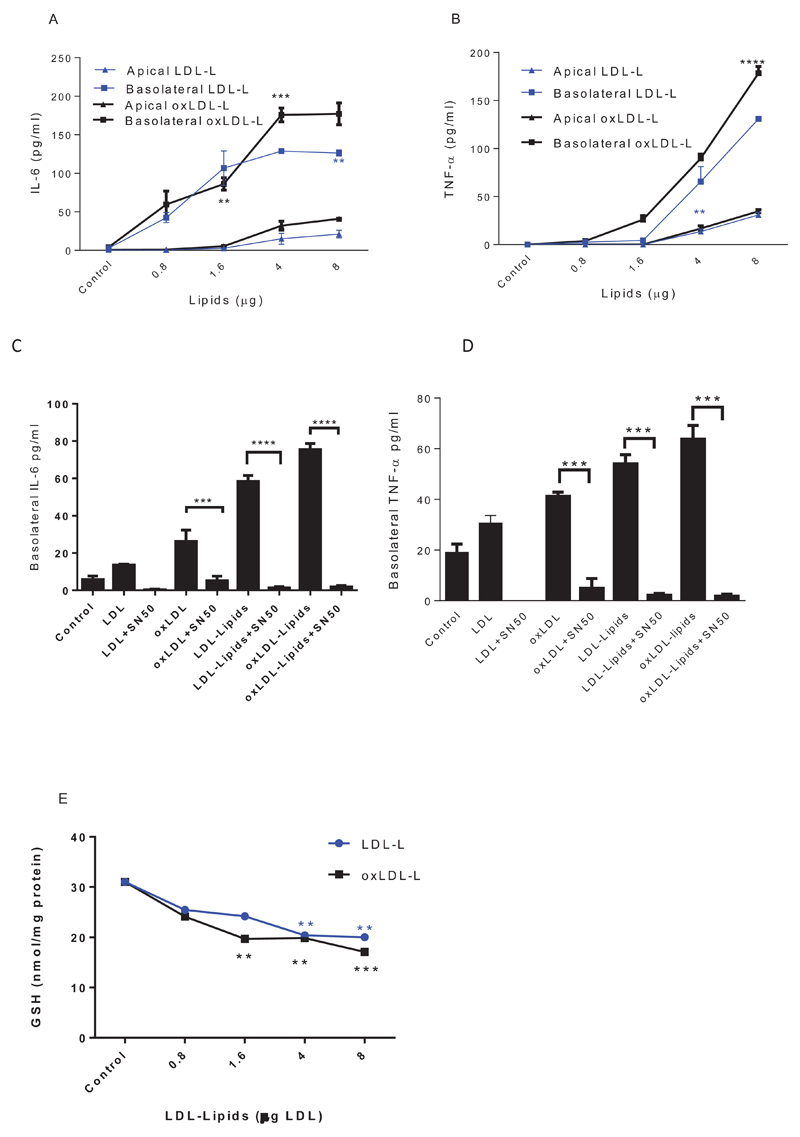
To explore whether LDL, oxLDL and sub fractions of LDL can trigger endothelial inflammatory responses, HMVEC were cultured on transwells until a tight barrier was formed, then exposed apically to LDL- and oxLDL-L. Directional secretion of pro-inflammatory mediators was analysed in both apical and basolateral compartments. oxLDL-L lipids significantly increased both apical and basolateral secretion of TNF-α (Figure 2A) and IL-6 (Figure 2B) compared to LDL-L treated cells.
Figure 2. The effect of LDL-L and oxLDL-L on endothelial barrier inflammation.
HMVEC cells (1×105 cells) were seeded in transwell inserts for 2 weeks before lipid treatments. HMVEC were treated with increasing concentrations of LDL-lipids for 24 hours (A&B) or LDL (4µg; C&D). Cells were co-incubated with 20µM SN-50 before measuring IL-6 (A&C) and TNF-α (B&D) in both apical and basolateral media by ELISA. The level of cellular GSH (E) was determined by the GSH-glo assay. Data are expressed as mean ± SEM, *P<0.05, ** P<0.01, ***P<0.001, ****P<0.0001, n=3 independent experiments.
To determine whether the increase in cytokine secretion was due to activation of NFkB, the inhibitory peptide SN50 was included when HMVEC were exposed to lipid fractions of LDL and oxLDL. SN50 significantly inhibited IL-6 (Figure 2C) and TNF-α (Figure 2D) production, without interfering with cell viability (supplementary figure 5) confirming the importance of NFkB activation in the effects of LDL and oxLDL-lipids on HMVEC.
To understand whether the increase in cytokine secretion that is elicited by LDL-L and oxLDL-L in an NF-kB-dependent manner is associated with an altered redox state, we examined intracellular GSH in HMVEC after over 2 hours of lipid treatments. LDL-L (oxidised and control) significantly depleted cellular GSH concentration (Figure 2E) and this mirrored the patterns of cytokine secretion.
Effect of hypercholesterolemia and statin intervention on LDL oxidation in midlife
Statin intervention in mid-life is reported to reduce incidence of dementia in later life, therefore we have undertaken a qualitative and quantitative analysis of lipid oxidation products in LDL from hypercholesterolaemic patients compared to age-matched controls whose cholesterol levels are within the normal range; their baseline characteristics are reported in Table 1.
Table 1. Demographics of healthy control and hypercholesterolaemic patient populations.
The healthy control subjects continued a normal lifestyle and hypercholesterolaemic patients receievd simvastatin (40mg/d) for 3 months.
| Baseline | 3 months follow up | |||||
|---|---|---|---|---|---|---|
| Control (n=10) | Hypercholesterolaemic (n=10) | p | Control (n=10) | Hyper-cholesterolaemic (n=10) | p | |
| Weight (Kg) | 62 ± 2.47 | 63.8 ± 2.69 | >0.05 | 61±2.3 | 64±2.7 | >0.05 |
| BMI Kg/m2 | 24.88 ± 0.74 | 26.35 ± 1.1 | >0.05 | 24.7±0.68 | 26.3±1.2 | >0.05 |
| Cholesterol (mM) | 4.08 ± 0.18 | 6.72 ± 0.78 | <0.001 | 3.8±0.13 | 4.63±0.31 | >0.05 |
| Age (years) | 46.4 ± 1.7 | 47.4 ± 1.7 | >0.05 | 46.4 ± 1.7 | 47.4 ± 1.7 | >0.05 |
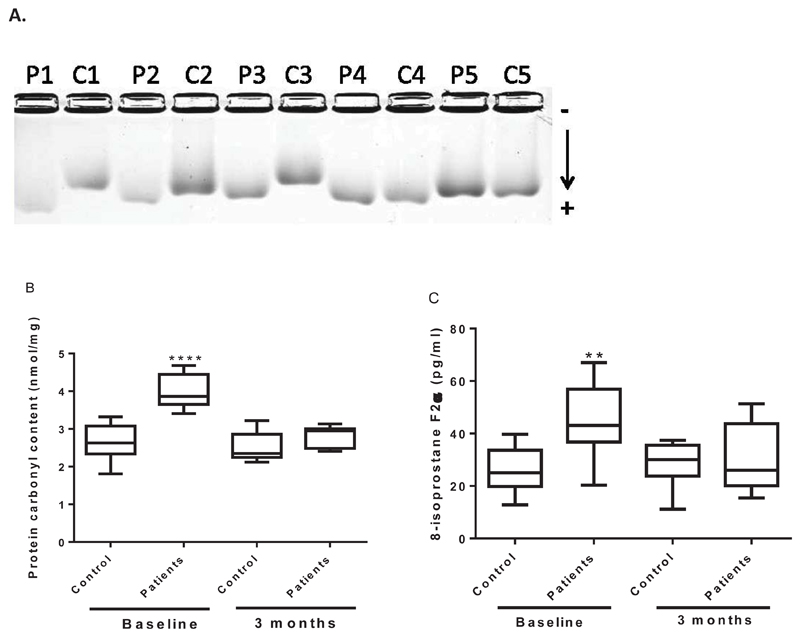
LDL isolated from statin-naïve hypercholesterolaemic subjects showed a trend for higher electrophoretic mobility at baseline by agarose gel electrophoresis (Rf; 0.53±0.06) compared to control subjects (Rf; 0.46±0.05), Figure 3A. To investigate levels of circulating protein and lipid oxidation in the patients and controls, plasma was analysed for protein carbonyls and 8-isoprostane F2α (Figure 3B and C). Statin-naïve hyperlipidaemic subjects had higher plasma carbonyls (3.95±0.2 nmol/mg; P<0.001) and 8-isoprostane F2α (43.5±8.42 pg/ml; P<0.01) compared to control subjects (2.65±0.15 nmol/mg and 24.2±5.37 pg/ml respectively). Statin treatment for 3 months significantly decreased blood cholesterol levels (from 6.72 ± 0.78mM to 4.63 ±0.31 mM; P<0.001), plasma carbonyl (2.5±0.4; P<0.001) and 8-isoprostane F2α levels (30.4±4.0 nmol/mg; P<0.05).
Figure 3. LDL from statin-naïve hypercholesterolaemic patients is more oxidised than LDL from healthy controls and statin treatments reduced LDL oxidation.
Following density gradient ultracentrifugation, LDL electronegativity was examined by agarose gel electrophoresis (A). Protein carbonyls (B) and 8-isoprostane F2α (c) were determined in plasmas from 10 controls and 10 patients analysed in triplicate by ELISAs. **P<0.01 and *** P<0.001.
Effect of LDL from hypercholesterolemic patients pre- and post-statin intervention on endothelial function
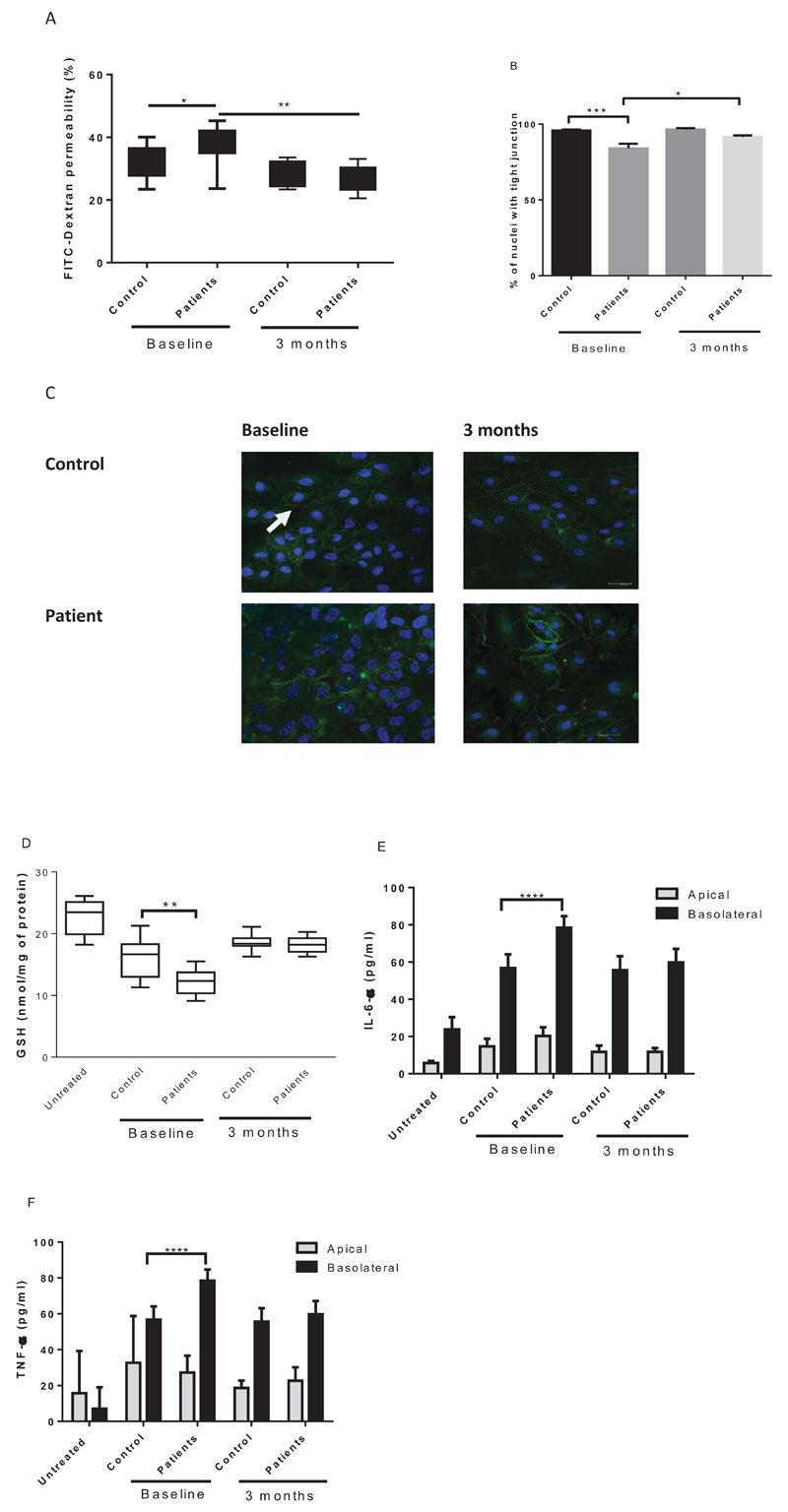
Compared to HMVEC treatment with the LDL-lipids (4µg of LDL) from normolipidaemic subjects, LDL-lipids from hyperlipidaemic subjects increased endothelial permeability as observed by FITC dextran permeability (Figure 4A; P<0.05) and immunohistochemistry for tight junctions was decreased (Figure 4B and C; P<0.001). At the baseline, the percentage of endothelial cells with tight junctions was significantly decreased upon patient LDL-lipids treatment. After 3 months of statin intervention, LDL-lipids did not change the percentage of cells expressing junctional ZO-1 compared to control LDL-lipids. At the baseline, LDL-lipids from patients decreased GSH (15.94±1.0 nmol/mg v 12.2±0.6 nmol/mg; P<0.01 untreated cells 22.75±1.2 nmol/mg of protein; N=10/group), Figure 4D. After 3 months treatment with statins, there was no difference between the effects of LDL isolated from either patients or controls on HMVEC function.
Figure 4. Inflammatory effects of LDL-lipids are removed by statin intervention in hypercholesterolaemic patients.
LDL-lipids (4µg) from normolipidemic (n=10) and statin-naïve hypercholesterolaemic subject plasmas (n=10) pre- and post-statin intervention were investigated for effects on HMVEC barrier function in duplicate (A); distribution of tight junction protein ZO-1 in duplicate (B and C); intracellular GSH concentration by GSH glo in duplicate (D); secreted IL-6 by ELISA in duplicate (E); and TNF-α by ELISA in duplicate (F). *P<0.05, ** P<0.01, ***P<0.001.
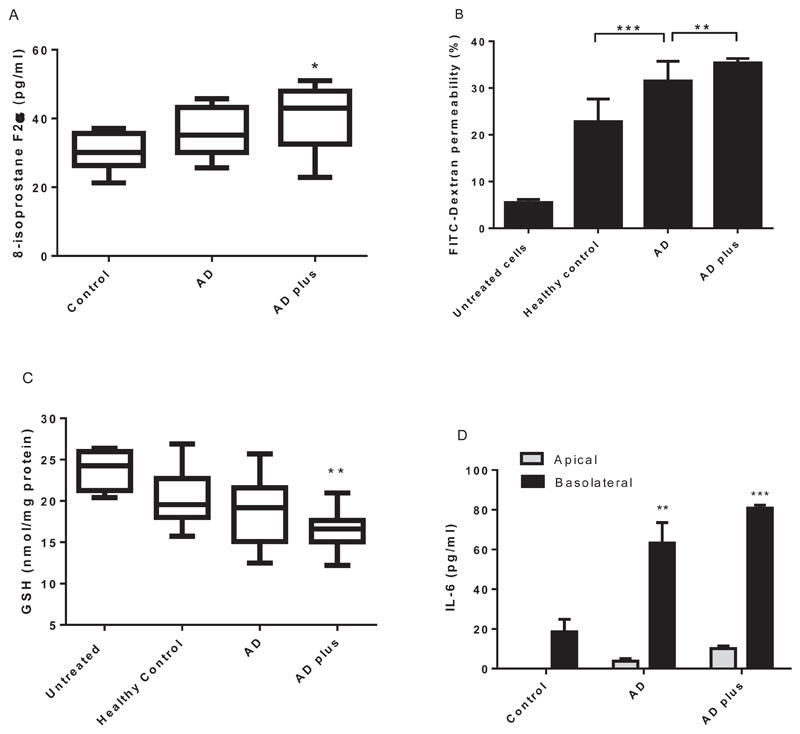
LDL-lipids from patients with Alzheimer’s disease act as inflammatory triggers in microvascular endothelial cells
To explore whether LDL-lipids from patients with dementia are more oxidised than controls and to investigate their inflammatory effects, the endothelial model was treated with 4µg of LDL-lipids from each patient individually from the three groups, AD, AD-Plus and control subjects. Although the total cholesterol and LDL cholesterol levels between subject groups were not different (Table 2), AD-Plus patients had higher plasma 8-isoprostane F2α levels (Figure 5A; 39.93±3 pg/ml) compared to the healthy control group (30.08±1.8 pg/ml). There was an increase of endothelial permeability with AD and AD-Plus LDL-lipid treatments and intracellular GSH levels were decreased (23.8±1.2 vs 16.5±0.7 nmol/mg protein). AD-Plus LDL-lipids were also more inflammatory as measured by basolateral secretion of IL-6 (80.8±1 Vs 18.6±3.6pg/ml).
Table 2. Demographics of mid-life adult volunteers.
| Control (n=10) | AD (n=10) | AD-Plus (n=10) | p | |
|---|---|---|---|---|
| Age (years) | 73 ± 2.5 | 81 ± 1.4 | 80 ± 1.7 | >0.05 |
| BMI Kg/m2 | 23.7 ± 0.34 | 24.1 ± 0.69 | 25.8 ± 0.36 | >0.05 |
| Total Cholesterol (mmol/L) | 5.67 ± 0.17 | 5.24 ± 0.32 | 4.9 ± 0.2 | >0.05 |
| LDL Cholesterol (mg/dL) | 121.5 ± 7.6 | 113.2 ± 11.1 | 108.3 ± 10.0 | >0.05 |
Figure 5. LDL-lipids are more oxidised and inflammatory in AD (n=10) and AD-Plus patients (n=10) compared to controls (n=10).
Plasma 8-isoprostane F2α was determined for each plasma in triplicate (A). LDL-lipids (4µg) from patients with AD and AD-Plus were investigated for effects on microvascular endothelial barrier function (B) and intracellular GSH concentration by GSH glo assay (C) in duplicate for each plasma. Secreted IL-6 (D) levels were measured by IL-6 ELISA in duplicate for each plasma. NS: not significant *P<0.05, **P<0.01 and *** P<0.001.
Discussion
In this study we show that the activation of microvascular endothelial cells with LDL-lipids increased the secretion of inflammatory mediators TNF-α, IL-6 and decreased the membrane localisation of the tight junction protein, ZO-1. LDL-lipids isolated from patients with hypercholesterolaemia were more oxidised and inflammatory towards endothelial cells than those from healthy age-matched subjects. Similarly, LDL-lipids from AD-Plus patients were found to contain higher level of lipid peroxidation and promoted more inflammatory IL-6 secretion from endothelial cells compared to controls. After, simvastatin intervention for three months in hypercholesterolaemia patients, LDL-lipid oxidation was decreased and the inflammatory effects of isolated LDL on endothelial cells were reduced. These findings support the hypothesis that elevated cholesterol in mid-life increases the concentrations of oxidised LDL molecules which in turn can elicit a directional inflammatory response and results in tissue inflammation. It has been described previously that inflammatory cytokines secreted by endothelial cells can exert an autocrine disruption of endothelial tight junction integrity [23]. During neurodegenerative conditions the barrier property of brain microvascular endothelial cells is thought to be damaged by disruption of junctional proteins resulting in barrier leakage [24]. Moreover, cerebral endothelium disruption and white matter lesions typical of dementia are observed more frequently in diabetes and hypercholesterolaemic patients compared to healthy controls [25].
The BBB consists of microvascular endothelial cells that are adjoined by specific protein tight junctions (e.g. occludins, ZO-1, ZO-2, ZO-3, claudins and cingulins) with their basement membrane, underlying brain pericytes and astroglial foot processes [25]. In healthy brains these layers display specific and tightly regulated transport mechanisms between the blood and ventricular cerebrospinal fluid [25]. Although normal ageing increases BBB permeability [25], oxLDL promotes endothelial activation including increased monocyte adhesion to the vascular wall and induction of NFκB. There is controversy over whether ageing alone associates with increased BBB permeability; Pelegri et al [26] have reported an early increase in permeability in the senescence-accelerated mouse model SAMP-8 at 12 months which is not present in control animals and is suggested to underlie deficits in learning and memory. SAMP-8 mice are also characterised by early loss of antioxidant enzymes i.e. of altered control of the scavenging of oxidative species [27].
In a model of localised excitotoxicity-induced neurodegeneration, BBB breakdown in itself was not sufficient to elicit cell death; a subsequent peroxynitrite-mediated event was required [28]. Peroxynitrite is lipophilic and can cross membranes via diffusion in the absence of a transporter. While the BBB requires the presence of carrier/proteins and transporters for rapid molecular transport of nutrients e.g. fatty acids, direct uptake of lipid hydroperoxides proceeds at an eight times faster rate than diffusion-controlled uptake of parent lipids [29]. Thus, clear pathways exist for transport of reactive species and modified lipids from the periphery to the CNS where they are neurotoxic to hippocampal neurones.
To better understand whether oxidised LDL was disruptive to an endothelial barrier, in vitro minimally modified LDL was prepared [19] from a pool of healthy subjects. This approach was taken to represent range of oxidation present in LDL. CuSO4 based method to prepare minimally oxidised LDL is well characterised in literature showing similar levels of oxidation to those seen in patients with vascular disease [24]. In line with literature, oxLDL displayed significantly higher inflammatory activity towards endothelial cells than freshly prepared LDL from control subjects [30]. Prior to ascribing physiological significance to these findings, oxLDL lipid effects on the BBB should be tested in vivo.
After the LDL was sub-fractionated, it was the lipid but not the protein components that mediated cell stress. We did not measure any quantitative difference in the uptake of lipids whether they were delivered by LDL or bound to albumin after 24 hours. This does not preclude a possible difference in rate of delivery. OxLDL-lipids elicited the greatest loss of GSH which has been reported by others to lead to an increase in NFkB activation in endothelial cells [31]. In turn, NFκB can regulate multiple proinflammatory target genes (21). NFkB activation and downstream expression of cytokines is controlled at least in part by the intracellular redox state. A shift towards a more oxidised environment increases phosphatase oxidation and inactivation, thus propagating inhibitor kappa kinase cascade activity and IKK dissociation from NFkB (23).
Oxidised LDL are known to cause barrier disruption in mice which has been attributed to a decrease in the expression of actin-depolymerizing factor (ADF) [32]. Others showed that overexpression of ADF attenuated ox-LDL-induced disruption of endothelial barrier marked by restoration of transendothelial electrical resistance, permeability of Evans Blue and expression of tight junction-associated proteins including ZO-1 and occludin, and blocked ox-LDL-induced oxidative stress (22). Differential expression of inflammatory and actin remodelling proteins by endothelial cells has also been shown after exposure to serum from patients with chronic kidney disease [33] suggesting a common association between endothelial cell response to plasma components.
Consistent with previous reports, we observed that LDL is more oxidised in hypercholesterolaemia patients [17]. Of note, plasma protein carbonyl and isoprostane concentrations described previously in AD-Plus patients [14, 20] were not different to those reported here for hypercholesterolaemic patients. Importantly, after patients were treated with statins their LDL oxidation measured as both protein and lipid oxidation was no different from healthy control subjects. However, we acknowledge the fact that this study involved small cohort size (n=20) and this limits the statistical power.
The present data show for the first time that lipids from LDL of untreated hypercholesterolaemic patients trigger the loss of microvascular barrier integrity. Moreover, the increased lipid toxicity in LDL from hypercholesterolaemic patients compared to control subject LDL towards microvascular endothelial cells was prevented after intervention with simvastatin for 3 months. In a similar manner, when LDL was examined from patients with AD and AD-Plus, isoprostane concentrations were higher as previously described [14]. Moreover, their lipids were more proinflammatory to endothelial cells promoting a significant increase in basolateral IL-6 directional secretion associated with loss of GSH. When considered alongside the importance of BBB integrity for brain health, these observations suggest an explanation for the observed reduction in dementia incidence for those receiving statins, independently of a reduction in plasma cholesterol and which may be attributed to statin effects on lipid oxidation [34].
These data support the hypothesis that statins exert a protective effect against in microvascular damage by modulating LDL by a mechanism that may not only be dependent on reducing cholesterol concentration and may be due to their capacity to prevent LDL oxidation. This may account for the protective effects of statin-usage in mid-life against the later dementia development and offers a rationale for managing patients with elevated oxLDL and mild or subjective cognitive impairment using statins.
In conclusion, this study demonstrated that LDL-lipids from AD and AD-Plus patients are more damaging to endothelial barrier properties and increase release of inflammatory cytokines. These data support the hypothesis that in vivo intervention with statins modifies LDL lipid oxidation and exert a protective effect against in microvascular damage in a manner that is independent of cholesterol concentration. This may account for the protective effects of statin-usage in mid-life against the later development of AD.
Supplementary Material
Clinical Perspectives.
We have explored why patients who have been prescribed lipid-lowering drugs in mid-life are at lower risk for dementia. We identified a novel anti-inflammatory effect for statins in patients with hypercholesterolaemia, by preventing LDL-lipid oxidation.
The inflammatory and oxidising effects of LDL fractions towards microvascular endothelial cells (measured as directional cytokine secretion and cellular glutathione) were greatest for oxLDL-lipids and then LDL-lipids with oxLDL-protein being the least inflammatory. Moreover, in common with adults with hypercholesterolemia, LDL from AD-Plus and AD patients was more oxidised, elicited more endothelial glutathione depletion, more IL-6 secretion and impaired endothelial tight junctions.
Thus, the mechanisms triggering LDL oxidation and the ability of oxidised LDL to endothelial dysfunction could be targeted by statins to reduce the risk for vascular involvement and dementia development.
Summary statement.
We have established a novel role of statins for mid-life adults with high blood cholesterol in depleting inflammation and oxidation of fats in the “bad” cholesterol particle, LDL. Oxidised LDL-fats were also higher in the blood of patients with dementia and caused inflammatory damage to cells that line blood vessels.
Acknowledgments
The authors acknowledge technical support of Ms Charlotte Bland, Aston Research Centre for Healthy Ageing, on the confocal scanning multiphoton microscope.
Funding
This work was supported by The Dunhill Medical Trust [grant number R92/1108].
Footnotes
Author contribution
HKID collected samples from hypercholesterolaemic subjects, undertook experiments, data analysis and manuscript preparation; CB undertook cell culture experiments, viability and cytokine analyses. GL supervised patient recruitment, data analysis and manuscript preparation; MCP collected samples from AD and AD-Plus patient groups, data analysis and manuscript preparation; HRG designed the study, analysed data and drafted the manuscript. All authors read and approved the final manuscript.
References
- 1.Vance JE, Hayashi H, Karten B. Cholesterol homeostasis in neurons and glial cells. Seminars in cell & developmental biology. 2005;16:193–212. doi: 10.1016/j.semcdb.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 2.Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 3.Abbott NJ, Patabendige AAK, Dolman DEM, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiology of Disease. 37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 4.Turley SD, Burns DK, Rosenfeld CR, Dietschy JM. Brain does not utilize low density lipoprotein-cholesterol during fetal and neonatal development in the sheep. J Lipid Res. 1996;37:1953–61. [PubMed] [Google Scholar]
- 5.Dietschy JM, Kita T, Suckling KE, Goldstein JL, Brown MS. Cholesterol synthesis in vivo and in vitro in the WHHL rabbit, an animal with defective low density lipoprotein receptors. J Lipid Res. 1983;24:469–80. [PubMed] [Google Scholar]
- 6.Yu L, von Bergmann K, Lutjohann D, Hobbs HH, Cohen JC. Selective sterol accumulation in ABCG5/ABCG8-deficient mice. J Lipid Res. 2004;45:301–7. doi: 10.1194/jlr.M300377-JLR200. [DOI] [PubMed] [Google Scholar]
- 7.Cramer C, Haan MN, Galea S, Langa KM, Kalbfleisch JD. Use of statins and incidence of dementia and cognitive impairment without dementia in a cohort study. Neurology. 2008;71:344–50. doi: 10.1212/01.wnl.0000319647.15752.7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Satoh Mamoru, Takahashi Yuji, Tabuchi Tsuyoshi, Minami Yoshitaka, Tamada Makiko, Takahashi Kan, Itoh Tomonori, Morino Yoshihiro, Nakamura M. Cellular and molecular mechanisms of statins: an update on pleiotropic effects. Clin Sci. 2015;129:93–105. doi: 10.1042/CS20150027. [DOI] [PubMed] [Google Scholar]
- 9.Lahera V, Goicoechea M, de Vinuesa SG, Miana M, de las Heras N, Cachofeiro V, L J. Endothelial dysfunction, oxidative stress and inflammation in atherosclerosis: beneficial effects of statins. Curr Med Chem. 2007;14:243–8. doi: 10.2174/092986707779313381. [DOI] [PubMed] [Google Scholar]
- 10.Li G, Larson EB, Sonnen JA, Shofer JB, Petrie EC, Schantz A, Peskind ER, Raskind MA, Breitner JC, Montine TJ. Statin therapy is associated with reduced neuropathologic changes of Alzheimer disease. Neurology. 2007;69:878–85. doi: 10.1212/01.wnl.0000277657.95487.1c. [DOI] [PubMed] [Google Scholar]
- 11.Li G, Shofer JB, Rhew IC, Kukull WA, Peskind ER, McCormick W, Bowen JD, Schellenberg GD, Crane PK, Breitner JC, Larson EB. Age-varying association between statin use and incident Alzheimer's disease. Journal of the American Geriatrics Society. 2010;58:1311–7. doi: 10.1111/j.1532-5415.2010.02906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chauhan NB, Siegel GJ, Feinstein DL. Effects of lovastatin and pravastatin on amyloid processing and inflammatory response in TgCRND8 brain. Neurochemical research. 2004;29:1897–911. doi: 10.1023/b:nere.0000042217.90204.8d. [DOI] [PubMed] [Google Scholar]
- 13.Hjuler Nielsen MIH, Vedel S, Raungaard B, Beck-Nielsen H, Handberg A. Elevated Atherosclerosis-Related Gene Expression, Monocyte Activation and Microparticle-Release Are Related to Increased Lipoprotein-Associated Oxidative Stress in Familial Hypercholesterolemia. PLoS ONE. 2015;10 doi: 10.1371/journal.pone.0121516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Polidori MC, Mattioli P, Aldred S, Cecchetti R, Stahl W, Griffiths H, Senin U, Sies H, Mecocci P. Plasma antioxidant status, immunoglobulin g oxidation and lipid peroxidation in demented patients: relevance to Alzheimer disease and vascular dementia. Dementia and geriatric cognitive disorders. 2004;18:265–70. doi: 10.1159/000080027. [DOI] [PubMed] [Google Scholar]
- 15.Griffiths HR, Aldred S, Dale C, Nakano E, Kitas GD, Grant MG, Nugent D, Taiwo FA, Li L, Powers HJ. Homocysteine from endothelial cells promotes LDL nitration and scavenger receptor uptake. Free radical biology & medicine. 2006;40:488–500. doi: 10.1016/j.freeradbiomed.2005.08.039. [DOI] [PubMed] [Google Scholar]
- 16.Pratico D, Clark CM, Liun F, Rokach J, Lee VY, Trojanowski JQ. Increase of brain oxidative stress in mild cognitive impairment: a possible predictor of Alzheimer disease. Archives of neurology. 2002;59:972–6. doi: 10.1001/archneur.59.6.972. [DOI] [PubMed] [Google Scholar]
- 17.Ishigaki Y, Katagiri H, Gao J, Yamada T, Imai J, Uno K, Hasegawa Y, Kaneko K, Ogihara T, Ishihara H, Sato Y, et al. Impact of plasma oxidized low-density lipoprotein removal on atherosclerosis. Circulation. 2008;118:75–83. doi: 10.1161/CIRCULATIONAHA.107.745174. [DOI] [PubMed] [Google Scholar]
- 18.Jick H, Zornberg GL, Jick SS, Seshadri S, Drachman DA. Statins and the risk of dementia. Lancet. 2000;356:1627–31. doi: 10.1016/s0140-6736(00)03155-x. [DOI] [PubMed] [Google Scholar]
- 19.Li L, Willets RS, Polidori MC, Stahl W, Nelles G, Sies H, Griffiths HR. Oxidative LDL modification is increased in vascular dementia and is inversely associated with cognitive performance. Free Radical Research. 2010;44:241–248. doi: 10.3109/10715760903440153. [DOI] [PubMed] [Google Scholar]
- 20.Dias IHK, Mistry J, Fell S, Reis A, Spickett CM, Polidori MC, Lip GYH, Griffiths HR. Oxidized LDL lipids increase β-amyloid production by SH-SY5Y cells through glutathione depletion and lipid raft formation. Free Radical Biology and Medicine. 2014;75:48–59. doi: 10.1016/j.freeradbiomed.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.S JC. Colorimetric Determination of Phospholipids with Ammonium Ferrothiocyanate. Analytical Biochemistry. 1980;104:10–14. doi: 10.1016/0003-2697(80)90269-9. [DOI] [PubMed] [Google Scholar]
- 22.Griffiths HR, Aldred S, Dale C, Nakano E, Kitas GD, Grant MG, Nugent D, Taiwo FA, Li L, Powers HJ. Homocysteine from endothelial cells promotes LDL nitration and scavenger receptor uptake. Free Radical Biology and Medicine. 2006;40:488–500. doi: 10.1016/j.freeradbiomed.2005.08.039. [DOI] [PubMed] [Google Scholar]
- 23.Lutgendorf MA, Ippolito DL, Mesngon MT, Tinnemore D, Dehart MJ, Dolinsky BM, Napolitano PG. Effect of Dexamethasone Administered With Magnesium Sulfate on Inflammation-Mediated Degradation of the Blood–Brain Barrier Using an In Vitro Model. Reproductive Sciences. 2014;21:483–491. doi: 10.1177/1933719113503410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ehara S, Ueda M, Naruko T, Haze K, Itoh A, Otsuka M, Komatsu R, Matsuo T, Itabe H, Takano T, Tsukamoto Y, et al. Elevated Levels of Oxidized Low Density Lipoprotein Show a Positive Relationship With the Severity of Acute Coronary Syndromes. Circulation. 2001;103:1955–1960. doi: 10.1161/01.cir.103.15.1955. [DOI] [PubMed] [Google Scholar]
- 25.Chui H, Ramirez-Gomez L. Clinical and imaging features of mixed Alzheimer and vascular pathologies. Alzheimer's Research & Therapy. 2015;7:21. doi: 10.1186/s13195-015-0104-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pelegri C, Canudas AM, del Valle J, Casadesus G, Smith MA, Camins A, Pallas M, Vilaplana J. Increased permeability of blood-brain barrier on the hippocampus of a murine model of senescence. Mechanisms of ageing and development. 2007;128:522–8. doi: 10.1016/j.mad.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 27.Sureda FX, Gutierrez-Cuesta J, Romeu M, Mulero M, Canudas AM, Camins A, Mallol J, Pallas M. Changes in oxidative stress parameters and neurodegeneration markers in the brain of the senescence-accelerated mice SAMP-8. Experimental gerontology. 2006;41:360–7. doi: 10.1016/j.exger.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 28.Parathath SR, Parathath S, Tsirka SE. Nitric oxide mediates neurodegeneration and breakdown of the blood-brain barrier in tPA-dependent excitotoxic injury in mice. Journal of cell science. 2006;119:339–49. doi: 10.1242/jcs.02734. [DOI] [PubMed] [Google Scholar]
- 29.Vila A, Levchenko VV, Korytowski W, Girotti AW. Sterol carrier protein-2-facilitated intermembrane transfer of cholesterol- and phospholipid-derived hydroperoxides. Biochemistry. 2004;43:12592–605. doi: 10.1021/bi0491200. [DOI] [PubMed] [Google Scholar]
- 30.Saing L, Wei Y-C, Tseng C-J. Ergothioneine represses inflammation and dysfunction in human endothelial cells exposed to oxidized low-density lipoprotein. Clinical and Experimental Pharmacology and Physiology. 2015 doi: 10.1111/1440-1681.12374. n/a-n/a. [DOI] [PubMed] [Google Scholar]
- 31.Zeng Q, Song R, Ao L, Xu D, Venardos N, Fullerton DA, Meng X. Augmented Osteogenic Responses in Human Aortic Valve Cells Exposed to oxLDL and TLR4 Agonist: A Mechanistic Role of Notch1 and NF-κB Interaction. PLoS ONE. 2014;9:e95400. doi: 10.1371/journal.pone.0095400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang J, Sun L, Si YF, Li BM. Overexpression of actin-depolymerizing factor blocks oxidized low-density lipoprotein-induced mouse brain microvascular endothelial cell barrier dysfunction. Molecular and cellular biochemistry. 2012;371:1–8. doi: 10.1007/s11010-012-1415-7. [DOI] [PubMed] [Google Scholar]
- 33.Carbó C, Arderiu G, Escolar G, Fusté B, Cases A, Carrascal M, Abián J, Díaz-Ricart M. Differential Expression of Proteins From Cultured Endothelial Cells Exposed to Uremic Versus Normal Serum. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2008;51:603–612. doi: 10.1053/j.ajkd.2007.11.029. [DOI] [PubMed] [Google Scholar]
- 34.Yoshida H, Shoda T, Yanai H, Ikewaki K, Kurata H, Ito K, Furutani N, Tada N, Witztum JL, Tsimikas S. Effects of pitavastatin and atorvastatin on lipoprotein oxidation biomarkers in patients with dyslipidemia. Atherosclerosis. 2013;226:161–4. doi: 10.1016/j.atherosclerosis.2012.10.069. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.