Abstract
Background and Aims Angiosperms with simple vessel perforations have evolved many times independently of species having scalariform perforations, but detailed studies to understand why these transitions in wood evolution have happened are lacking. We focus on the striking difference in wood anatomy between two closely related genera of Adoxaceae, Viburnum and Sambucus, and link the anatomical divergence with climatic and physiological insights.
Methods After performing wood anatomical observations, we used a molecular phylogenetic framework to estimate divergence times for 127 Adoxaceae species. The conditions under which the genera diversified were estimated using ancestral area reconstruction and optimization of ancestral climates, and xylem-specific conductivity measurements were performed.
Key Results Viburnum, characterized by scalariform vessel perforations (ancestral), diversified earlier than Sambucus, having simple perforations (derived). Ancestral climate reconstruction analyses point to cold temperate preference for Viburnum and warm temperate for Sambucus. This is reflected in the xylem-specific conductivity rates of the co-occurring species investigated, showing that Viburnum lantana has rates much lower than Sambucus nigra.
Conclusions The lack of selective pressure for high conductive efficiency during early diversification of Viburnum and the potentially adaptive value of scalariform perforations in frost-prone cold temperate climates have led to retention of the ancestral vessel perforation type, while higher temperatures during early diversification of Sambucus have triggered the evolution of simple vessel perforations, allowing more efficient long-distance water transport.
Keywords: Adoxaceae, ancestral area and climate reconstruction, Baileyan wood trends, molecular dating, Sambucus, Viburnum, vessel perforation plate transition, wood anatomy
INTRODUCTION
Baileyan trends in wood anatomy are arguably one of the most common textbook examples of evolutionary patterns in plant anatomy. This assertion of a set of linear ancestral-to-derived transformation series in wood anatomical features found its origin in a broad comparison of the size of water-conductive cells in woody land plants (Bailey and Tupper, 1918). Later, Bailey’s student Frost (1930a, b) hypothesized that long and slender gymnosperm tracheids lost pit membranes in their scalariform end-wall pitting, and evolved into long, narrow angiosperm vessel elements with scalariform perforations including many bars (often >20). These vessel elements, considered ancestral within angiosperms due to their strong resemblance to tracheids of the gymnosperm outgroup, further developed into wider and thus hydraulically more efficient water conducting cells (Carlquist, 1975; Sperry et al., 2007). In addition, vessel elements also shortened and at the same time the number of bars in the perforation plates reduced to zero, leading to short vessel elements with simple perforations. Interestingly, scalariform perforations were much more abundant in the Cretaceous than in the Tertiary (Wheeler and Baas, 1991), but in-depth palaeobotanical evidence fully supporting the Baileyan trends remains weak due to the scarcity of early woody angiosperms in the fossil record. For instance, two of the earliest vessel-bearing wood types are found in taxa with scalariform perforations (Icacinoxylon) or simple perforations (Paraphyllantholoxylon) (Wheeler and Lehman, 2009; Falcon-Lang et al., 2012).
The scalariform-to-simple transition was established independently of any classification system, and was therefore hailed as a basis for identifying phylogenetic relationships among woody angiosperms (Bailey, 1957). However, Bailey and his students realized that vessel characters were prone to convergent evolution and that the use of homoplasious characters in the Tree of Life leads to erroneous conclusions (Bailey, 1944, 1957; confirmed in Baas and Wheeler, 1996). However, they never postulated a cause driving the transition, which is one of the major critiques of Bailey’s legacy (exhaustively discussed in Olson, 2012, 2014). It was only in the 1960s that Sherwin Carlquist placed the Baileyan trends in an adaptive framework. Carlquist pioneered the idea that evolution towards simple vessel perforations was driven by more hydraulic efficiency when plants moved from ever-wet or cold temperate habitats to (seasonally) dry habitats (Carlquist, 1966, 1975). Later studies confirmed Carlquist’s view, stating that scalariform perforations are retained in taxa that do not face selective pressure for high conductive efficiency, while a seasonal or permanent demand for great hydraulic efficiency in dry and/or warm areas has triggered species to evolve simple perforations, allowing more efficient long-distance water transport and thus more carbon fixation (e.g. Baas, 1976; Baas et al., 1983; Jansen et al., 2004). However, this scalariform-to-simple transition in vessel perforation plate morphology has been little tested in an evolutionary and ecophysiological framework (Sperry et al., 2007; Christman and Sperry, 2010).
We assessed the dynamics of these scalariform-to-simple transitions within the large asterid clade (angiosperms), and selected the Adoxaceae genera Viburnum (∼165 species) and Sambucus (∼28 species) – two closely related taxa with strikingly different wood anatomy (Metcalfe and Chalk, 1950; Schweingruber, 1990) – as a case study in asterids. We first evaluated the direction of perforation plate transition using phylogenetic estimates from existing sequence data for a set of carefully sampled taxa among the asterids. Then, we integrated original wood anatomical observations of Viburnum and Sambucus with an updated molecular phylogeny based on existing and original sequence data from five markers (Eriksson and Donoghue, 1997; Clement et al., 2014). A divergence time estimation analysis on both genera and analyses unravelling ancestral areas and ancestral climate preferences were carried out on this combined molecular dataset to find out when and where Viburnum and Sambucus diverged from each other. Furthermore, we assessed whether present-day precipitation and temperature BIOCLIM variables (Hijmans et al., 2005) can be linked to the wood anatomical variation observed, and we performed hydraulic conductivity measures in both genera to investigate our hypothesis that the scalariform-to-simple perforation plate shift is driven by peak conductive rates.
We set wished to test the following hypotheses: (1) simple-plated species have evolved many times independently within asterids from scalariform-plated relatives, and follow a unidirectional pattern; (2) Viburnum with scalariform perforations diversified first in habitats with low evaporative demands, while Sambucus, having simple vessel perforations, developed later, in hydraulically more demanding regions; (3) scalariform-to-simple perforation plate transitions are driven by selection acting on peak conductive rates; and (4) Viburnum and Sambucus differ dramatically in their wood anatomy, assuming a unique ecological niche for each of the genera based on present-day distribution patterns.
MATERIALS AND METHODS
Wood anatomy
Wood descriptions of Sambucus and Viburnum are scattered in the literature, and most wood anatomical studies include only a limited number of species from a restricted geographical area (e.g. Moll and Janssonius, 1920; Kanehira, 1921; Metcalfe and Chalk, 1950; Ogata, 1988; Schweingruber, 1990; Benkova and Schweingruber, 2004; InsideWood, 2004 onwards). To expand existing data and to achieve a more representative sampling, we performed original wood anatomical observations of both genera, covering the entire distribution range and all major subclades according to the latest molecular phylogenies (Eriksson and Donoghue, 1997; Clement et al., 2014). In total, 44 wood specimens belonging to 17 Sambuus species and 17 Viburnum species were investigated using light microscopy and scanning electron microscopy (Fig. 1, Table 1, Supplementary Data Notes S1 and S2). The methodology of wood sectioning and slide preparation is described in Lens et al. (2005, 2007). In short, wood sections 25 μm thick were made using a sledge microtome (Reichert, Germany). After sectioning, the tissues were bleached with sodium hypochlorite and stained with a mixture of safranin and alcian blue (35:65), dehydrated with 50–75–96 % ethanol and mounted in euparal. Slides were observed using a Leica DM2500 light microscope and photographed with a Leica DFC-425C digital camera (Leica Microscopes, Germany). Detailed wood anatomical descriptions for Viburnum and Sambucus are available in Supplementary Data Note S2 and Table S1, and follow the IAWA list of microscopic features for hardwood identification (IAWA Committee, 1989). For the terminology of the imperforate elements, we tend to agree with Carlquist (1984), who links the vessel distribution pattern with the presumed water-conducting capacity of the imperforate elements. Therefore, we prefer to name the imperforate elements in the ground tissue of Viburnum ‘tracheids’ rather than ‘fibres with distinctly bordered pits’, although more experimental studies in Viburnum should be carried out to support this statement.
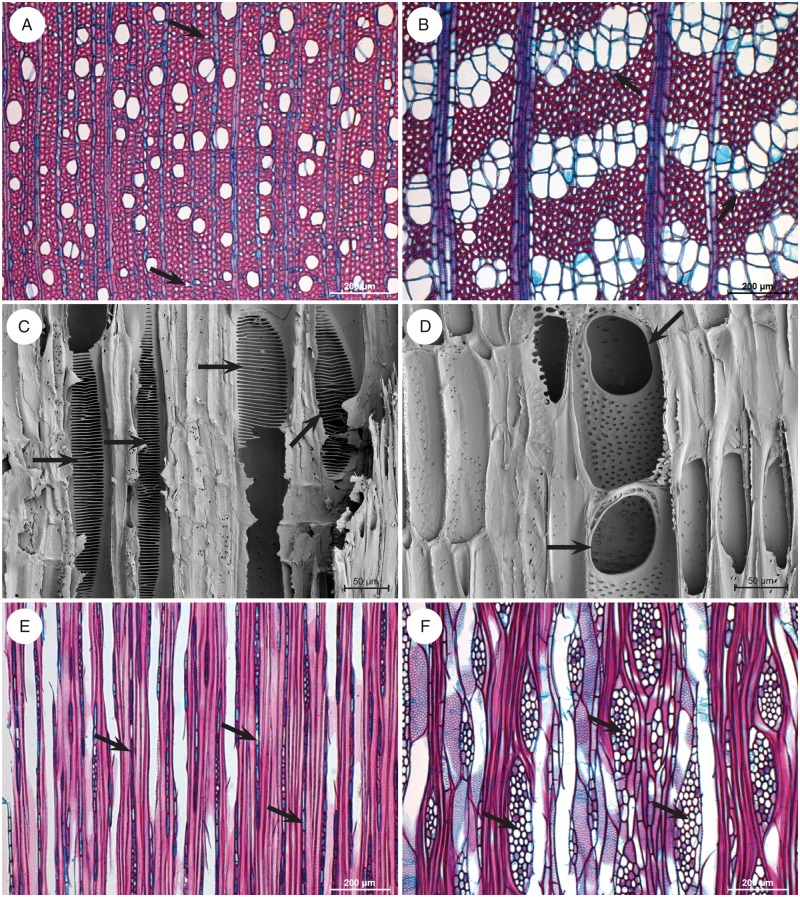
Fig. 1.
Illustrations of light microscope wood sections (A, B, E, F) and scanning electron microscope surfaces (C, D) showing the marked wood anatomical difference between Viburnum (A, C, E) and Sambucus (B, D, F). (A) V. prunifolium: transverse section, vessels mainly solitary, apotracheal axial parenchyma diffuse in aggregates (arrows). (B) S. glauca: transverse section, vessels in distinct clusters, axial parenchyma scanty paratracheal (arrows). (C) V. furcatum: radial section, scalariform vessel perforations with many bars (arrows). (D) S. javanica: radial section, simple vessel perforations (horizontal arrow). (E) V. opulus: tangential section, long multiseriate rays due to long uniseriate ends (arrows). (F) S. glauca: tangential section, multiseriate rays shorter and wider (arrows).
Table 1.
Overview of wood anatomical characters summarizing the most important differences between Sambucus and Viburnum
| Distinguishing wood features | Viburnum | Sambucus |
|---|---|---|
| Vessel perforation type | scalariform (often >20 bars) | Simple |
| Vessel element length (µm) | 700–1600 | 300–500 |
| Vessel distribution | Vessels solitary: VGI <1·5 | Vessels grouped: 2 < VGI < 5 |
| Intervessel pits | Opposite, scalariform or a mixture of both | Alternate |
| Vascular tracheids | – | + |
| Imperforate tracheary elements in the ground tissue | Elements with distinctly bordered pits | Elements with simple to minutely bordered pits |
| Axial parenchyma distribution | Diffuse apotracheal or diffuse in aggregates | Scanty paratracheal |
| Number of cells per axial parenchyma strand | Often 5–8 | Often 3 or 4 |
| Vessel-ray pitting | Distinct borders | Reduced borders |
| Number of rows of marginal ray cells per multiseriate ray | Variable: 1–15 | 1 or 2 |
| Multiseriate ray height (µm) | Often 600–2500 | 400–600 |
| Uniseriate ray density (mm−1) | Often 3–10 | 0–2 |
VGI, Vessel Grouping Index.
To assess the polarity of transitions between vessel perforation plates, we applied a Bayesian framework, using estimates of the phylogeny of 152 carefully selected asterid species, taking into account the variation in vessel perforation plate morphology across the asterids (Figs 2 and 3). We want to stress that we only intend to provide a realistic (but not final) estimation of the number of shifts in perforation plates in this huge clade, including about 100 000 (woody and herbaceous) species. Therefore, we used the SUPERSMART pipeline v.0.1.22 (Antonelli et al., 2014) to identify commonly sequenced markers and representative species with sufficient published sequence coverage (matK, n = 133; ndhF, n = 121; rbcL, n = 110 and rps16, n = 71; accession numbers are given in Supplementary Data Table S2). We aligned the markers using MAFFT v. 7.130b (Katoh et al., 2002) with default settings and autoselected alignment strategy, which chose FFT-NS-i for matK, ndhF and rbcL, and L-INS-i for rps16. We concatenated the alignments into a supermatrix and analysed this using ExaBayes v. 1.4.1 under default settings (Aberer et al., 2014) to construct a posterior sample of trees, which we rooted on Cornales.
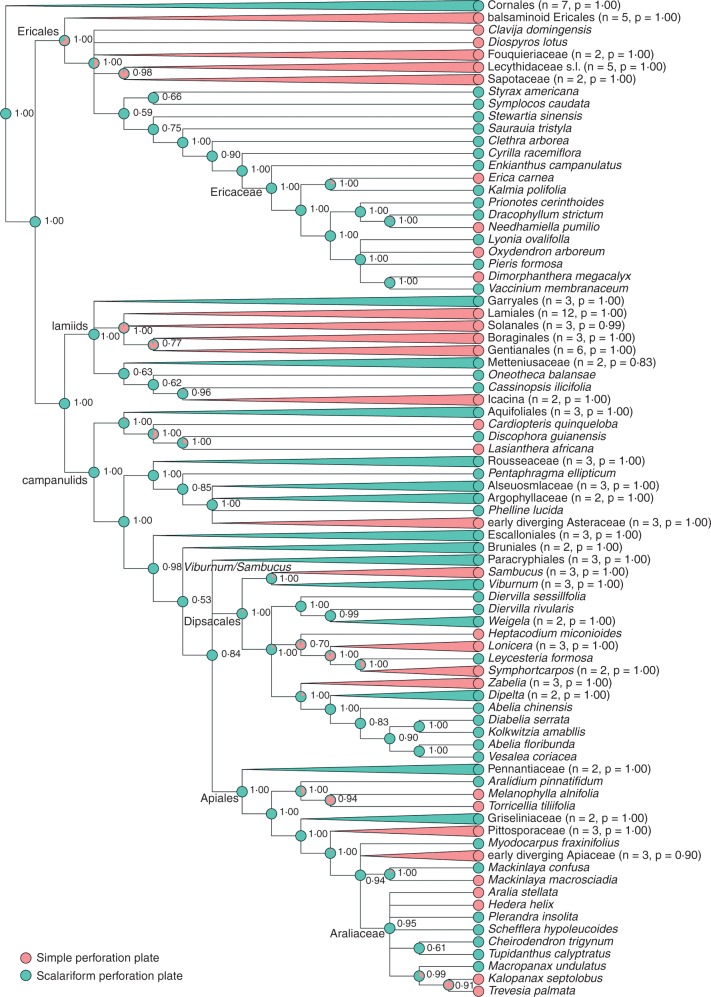
Fig. 2.
Majority-rule consensus tree topology summarizing the Bayesian comparative analysis of the evolutionary dynamics of transitions between scalariform (green) and simple (red) perforation plate types in asterids. Values on interior nodes are clade posterior probabilities. Pie charts on interior nodes are ancestral state reconstructions proportional to the probability of the respective reconstruction. Clades that are monophyletic as well as uniform in their character state are shown collapsed; the number of subtended species and the posterior probability of the root of the clade are given in parentheses. The scalariform plate perforation type is reconstructed as ancestral, with numerous independent transitions to the simple plate perforation type (at least 10), but note the apparent reversal for Leycesteria.
Fig. 3.
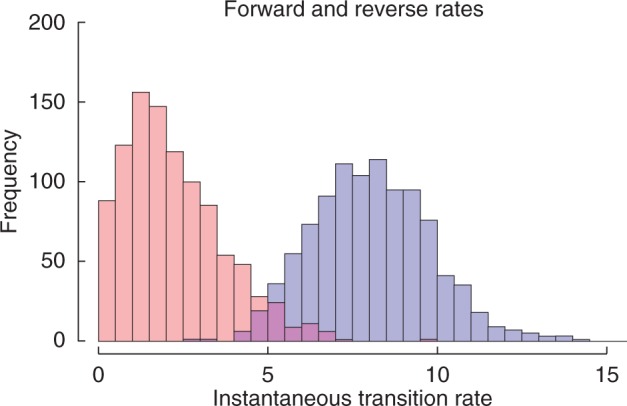
Distributions of instantaneous transition rates between scalariform and simple vessel perforation plates. Estimates of instantaneous transition rates from scalariform to simple (forward rates, violet) and simple to scalariform (reverse rates, salmon) across 106 iterations (less 10 k burn-in) of MCMC ancestral state reconstruction using BayesTraits on the posterior tree sample (n = 722) for the asterids. Transitions from scalariform to simple occur at significantly higher rates than reversals, and the latter are not significantly different from zero.
We used our posterior sample of trees to run comparative analyses of transitions between scalariform and simple using BayesTraits v. 2.0 (Meade and Pagel, 2014), which we ran as Markov chain Monte Carlo (MCMC) analyses using an exponential prior for the transition rates and using a stepping stone sampler (Xie et al., 2011) to obtain estimates of the marginal likelihoods. We did this for different instantaneous transition rate matrices: unconstrained; transition rate from simple to scalariform set to 0, i.e. no reversals; and forward and reverse rates set to equal. We also ran an MCMC analysis to reconstruct ancestral state distributions for all interior nodes
Taxon sampling phylogenetic analysis
We expanded existing phylogenetic studies for Sambucus based on original sequence data of five molecular markers that had already been used in several published Viburnum phylogenies (trnK, matK, trnS-G, atpB-rbcL, internal transcribed spacer (ITS). The newly generated Sambucus sequences were combined with the published sequences of Viburnum and the remaining genera of the Adoxaceae (Adoxa, Sinadoxa and Tetradoxa; Eriksson and Donoghue, 1997; Winkworth and Donoghue, 2005; Clement and Donoghue, 2011, 2012; Chatelet et al., 2013; Schmerler et al., 2012; Clement et al., 2014). In our sampling, Sambucus is represented by 27 species, Viburnum by 97 species and the small herbaceous genera Adoxa, Sinadoxa and Tetradoxa by one species each (see Note S1 for detailed species list). Members of the sister family Caprifoliaceae s.l., Weigela praecox and Diervilla sessilifolia, were selected as outgroup.
Species, subspecies and synonyms in Sambucus
The number of species in Sambucus is still under debate. During his revisions of the genus, von Schwerin (1909, 1920) reduced the number of Sambucus species from over 100 to 28. A more recent revision by Bolli (1994) further reduced the number of taxonomically valid species names to nine. However, Bolli’s morphological species concept remains ambiguous and needs to be adjusted, which was later confirmed by molecular phylogenetic studies (Eriksson and Donoghue, 1997; Clarke and Tobutt, 2006). An aim of the present study is to further contribute to clarifying species relationships within Sambucus, but fully resolving species boundaries is beyond the scope of this paper.
Molecular protocols and sequence analyses
DNA isolation followed the protocol of Janssens et al. (2006, 2009), whereas amplification of trnK, matK, trnS-G, atpB-rbcL and ITS was carried out following Young et al. (1999), Clement and Donoghue (2011), Manen et al. (1994) and White et al. (1990), respectively. Contiguous sequences were assembled using Geneious v. 7.0.6 (Biomatters, New Zealand). Automatic alignments were carried with MAFFT (Katoh et al., 2002) under an E-INS-i algorithm. Subsequent manual fine-tuning of the aligned dataset was done in Geneious v. 7.0.6. Gaps were treated as missing data, whereas potentially informative insertions and deletions were coded according to the ‘simple indel coding’ method of Simmons and Ochoterena (2000). Analyses performed without gap coding did not change the topology.
The best-fit nucleotide substitution model for each plastid and nuclear dataset was determined using jModelTest 2.1.4. (Posada, 2008) under the Akaike information criterion (AIC). The GTR + I + G model was found as best fit for trnS-G and trnK, whereas the GTR + G model was calculated as best substitution model for matK and ITS, and F81 + I as best substitution model for atpB-rbcL. A mixed-model approach was used in which the combined dataset was partitioned in order to apply a different model of evolution on each DNA region (Ronquist and Huelsenbeck, 2003). Bayesian inference analyses were conducted with MrBayes v. 3.1 (Huelsenbeck and Ronquist, 2001) on five individual data partitions and a combined data matrix. Each analysis was run twice for 10 million generations. Trees were sampled every 2500 generations. Inspection of chain convergence and effective sample size (ESS) parameters was done with TRACER v. 1.4 (Rambaut and Drummond, 2007). Only Bayesian posterior probabilities (BPPs) above 0·95 were taken into consideration (Suzuki et al., 2002).
Divergence time analysis
The node ages within the Adoxaceae were estimated based on a calibrated ultrametric phylogenetic tree (Fig. 4). The tree was calibrated using a combination of primary calibration points based on the ages of well-identified fossils available for Sambucus and Viburnum, and a secondary calibration point for the age of Adoxaceae from a previous dating analysis (Janssens et al., 2009). Three calibration points were used for age estimation: (1) minimum crown age constrained at 38 million years ago (Ma) for Sambucus (crown age) based on fossil endocarps from the late Eocene to Pliocene found in Europe (Reid and Chandler, 1926); (2) crown age of Viburnum constrained at a minimum age of 47·8 Ma, corresponding to the report of fossil leaves from the middle Eocene Jijuntun formation (Wang et al., 2010); and (3) Adoxaceae crown node set at 79·9 Ma, based on the large asterid analysis of Janssens et al. (2009), which matched the dating analysis of Bremer et al. (2004). The two fossil calibration points used in this study were modelled in BEAST v. 1.8.0 under a log-normal distribution (Drummond and Rambaut, 2007), with an offset that equals the age of the fossil calibration point, a mean of 1·0 and a standard deviation of 1·0. The third calibration point was given a normal distribution with a mean value and standard deviation of 5·0 (cf. Janssens et al., 2009).
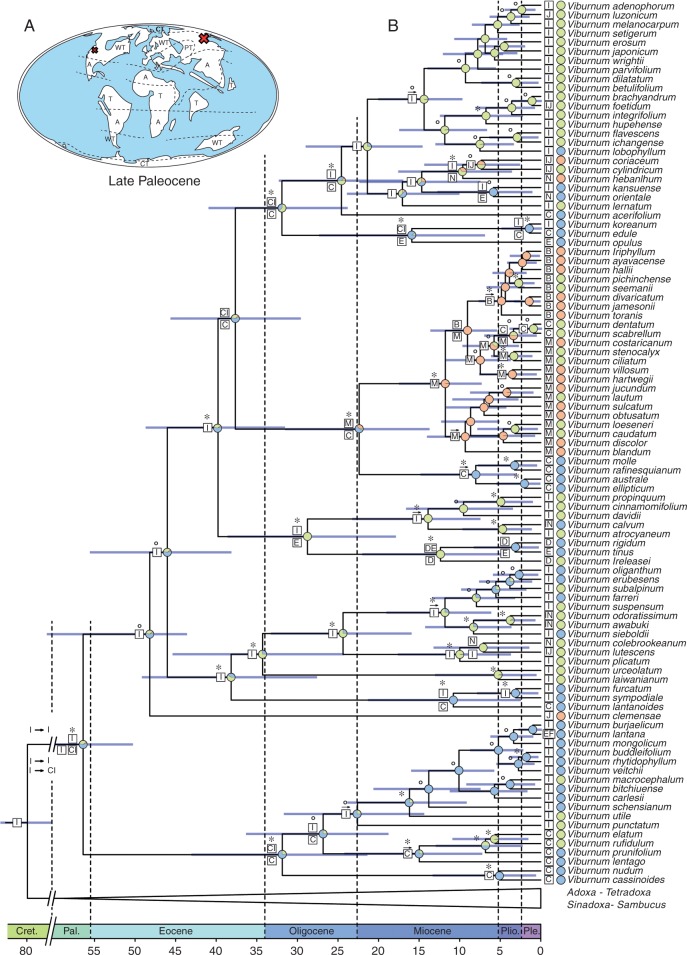
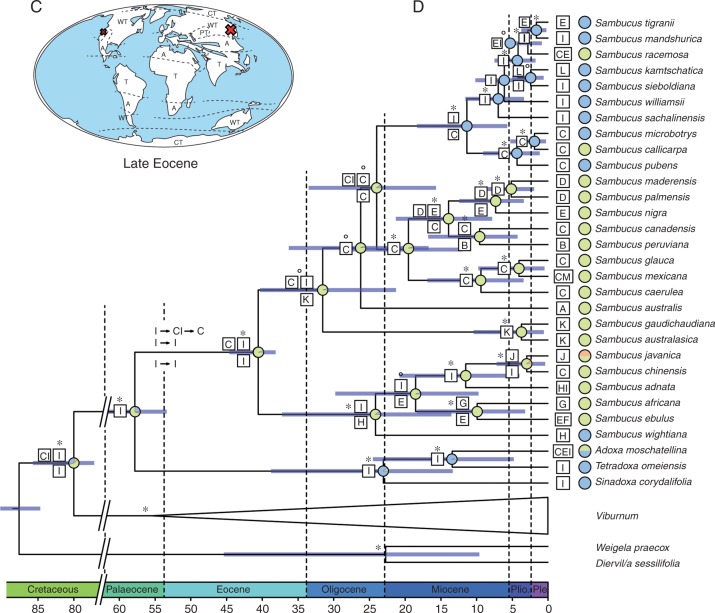
Fig. 4.
Palaeomaps and maximum clade credibility phylogeny for Viburnum (A, B) and Sambucus (C, D) inferred from combined ITS, trnK, matK, atpB-rbcL and trnS-G as obtained from BEAST. (A, C) Palaeomap of Viburnum (A) and Sambucus (C) during initial diversification of both genera. The red X marks the putative area(s) of initial diversification. A, Arid; WT, warm temperate; CT, cold temperate; T, tropical; PT, paratropical. (B, D) Maximum clade credibility phylogeny showing results from ancestral area reconstruction and ancestral climate reconstruction. Bayesian posterior probabilities (BPPs) are displayed above branches; asterisks indicate a BPP value ≥0·95, whereas BPP values between 0·5 and 0·95 are indicated by circles. Ancestral area reconstructions (AARs) with the highest likelihood value are shown as boxes at each node. A single box refers to a specific distribution range, whereas multiple boxes occurring either above or below indicate alternative AARs. The following abbreviations are used: A, South American Central Atlantic; B, South American Andes; C, North America; D, Macaronesia; E, Europe; F, North African Mediterranean and Atlas; G, Montane Eastern Africa; H, Western Himalaya; I, Subtropical-Temperate East Asia; J, Malesia and Indochina; K, Western Australia; L, Northeastern Siberia and Kamchatka; M, Caribbean; N, Western India and Sri Lanka. Pie charts at each node show the posterior probability of each possible climate type for the ancestral climate preference reconstruction (blue, cold temperate climate; green, warm temperate climate; orange, tropical climate).
A χ2 likelihood ratio test, used to assess rate heterogeneity among lineages (Felsenstein, 1988), indicated that the substitution rates in the combined dataset are not clock-like (P <0·001 for all markers). We therefore used a Bayesian approach as implemented in BEAST to calculate divergence times. To initiate the Bayesian dating analysis and to cope with the zero likelihood issue in BEAST, we used a starting tree that was obtained by carrying out a maximum likelihood analysis on the combined dataset in RAxML 7.2.8 (Stamatakis et al., 2008) under a GTR + G model with the rooted likelihood tree as input tree for a penalized likelihood analysis in r8s 1·70 (Sanderson, 2004), with all calibration points used as described above. Because of differences in substitution models among the individual chloroplast genes and ITS, we performed a partitioned Bayesian MCMC analysis under the assumption of the Yule speciation model and a relaxed log-normal clock. Partitions were unlinked for the model of DNA sequence evolution. All other priors were kept as defaults. Two runs of 20 million generations were performed, sampling every 2000 generations. Convergence of the chains (the ESS parameter exceeding 200) was carried out with TRACER v.1.6 (Rambaut and Drummond, 2007). The two runs were combined, discarding the initial 2 million generations as burn-in using Logcombiner v. 1.8.0, and a maximum clade credibility tree using a posterior probability limit of 0·5 was calculated using TreeAnnotator v.1.8.0 (Drummond and Rambaut, 2007).
Ancestral area reconstruction
Reconstruction of ancestral distribution ranges was carried out following the dispersal–extinction–cladogenesis model of Lagrange (Ree et al., 2005; Ree and Smith, 2008; Fig. 4). Lagrange scripts were generated using the online Lagrange configurator (www.reelab.net/lagrange/configurator/index). The maximum clade credibility tree obtained from the dating analysis with BEAST v. 1.8.0 was used as input tree. In total, 14 areas were defined based on known distribution ranges of species within the Adoxaceae and delimited using Takhtajan’s (1986) floristic regions of the world (Fig. 4). Maximum range size was defined at 2 (only three out of 141 taxa investigated in the ancestral area analysis occur in more than one distribution area, and were also set at 2). Dispersal effectiveness was set at ‘symmetric’, but configured between each distribution area. Whereas adjacent regions were set at 1·0, dispersal effectiveness was set at 0·7 between non-adjacent regions on the same continent and adjacent regions on different continents. Dispersal effectiveness was set at 0·2 between non-adjacent regions on different continents.
Due to the uncertain phylogenetic position of V. clemensae among the earlier-diverging lineages of Viburnum, we ran an alternative ancestral area reconstruction analysis in which V. clemensae was constrained as sister species to all other Viburnum species (cf. Moore and Donoghue, 2007; Schmerler et al., 2012). All parameters used were the same as in our original analysis.
Ancestral climate preference reconstruction
Bayesian stochastic character mapping (BSCM) as implemented in SIMMAP 1.5 was applied to reconstruct the ancestral preference in climate conditions in Adoxaceae (Bollback, 2006; Fig. 4). Climate preference was scored as follows: (1) tropical; (2) warm temperate; and (3) cold temperate. Distribution data from the Global Biodiversity Information Facility (GBIF) were used to assess climate types, after being screened for erroneous localities. The GBIF coordinates obtained from species investigated were plotted on the updated Köppen–Geiger climate classification (Kottek et al., 2006) using DIVA-GIS (Hijmans et al., 2011). Next, species were scored in one of the broad climate types according to the climate zone that corresponds to the plotted distribution range of each species analysed. In addition, elevation ranges were taken into consideration to assess the exact climate preference for each species. In order to guarantee a uniform method for evaluating the climate preference for all our Adoxaceae accessions, thereby avoiding putative bias that could affect the results of our overall climate preference analysis, we ignored the Schmerler et al. (2012) GBIF dataset for Viburnum and built our own.
Climate types are regarded as unordered with weight 1. Five thousand randomly sampled Bayesian topologies from the BEAST output file [generated by the ‘sub-sample tree’ function in BayesRate 1·5 (Silvestro et al., 2011)] were used as topology input. Hyperparameters defining the mean (E) and standard deviation accommodates the bias rate parameter I and the substitution rate parameter θ. A flat prior was used for the bias rate parameter I in all analyses. Due to the significant influence of E (θ) and standard deviation. (θ) on the overall outcome of each analysis, they were independently selected for each character using the ‘number of realizations sampled from priors’ function as implemented in SIMMAP (Couvreur et al., 2008; Janssens et al., 2012). Once the optimized hyperparameters are estimated, the impact of the standard deviation (θ) value becomes insignificant and the mean E (θ) value can be optimized for each character. As a result, the standard deviation (θ) is set at 2. We also carried out an alternative climate preference reconstruction in which V. clemensae was constrained as sister species to all remaining Viburnum species using the same parameters.
Present climate preference analysis
A phylogenetically broad selection of geographic locations obtained from the GBIF website for Viburnum and Sambucus species was analysed using principal components analysis (PCA). This PCA was used as a visualization tool to assess potential differences between the ecological niches occupied by the two genera based on present-day distribution data (using the 19 scaled/centred WorldClim bioclimatic variables; Hijmans et al., 2005). In total, 12 483 geographic locations from 30 Viburnum species were analysed. For Sambucus, 16 746 locations from 14 species were included in the PCA (see Supplementary Data Fig. S1 for species list). For Sambucus nigra, a random set of 1000 geographic locations from its full distribution was taken from the 206 000 that were available, preventing the niche of the genus from being represented by largely one species.
Xylem specific conductivity (Ks) measurements
Six equally sized (>1 m in length, 7–9 mm in diameter), leaf-bearing branches of Viburnum lantana and Sambucus nigra were sampled in the neighbourhood of Bordeaux in a public park in Gradignan (branches about 4–5 years old) and a more mesic forest in Madirac (branches about 2–3 years old; France), respectively, immediately put in a sealed plastic bag after removing the leaves, and stored in a refrigerator overnight. First, air was injected at one side of the branch at 2 bars to assess the maximum vessel length for both species. Then, we trimmed stem segments corresponding to ∼70 % open vessels under water (40 cm for V. lantana and 10 cm for S. nigra), and flushed the stems with 2 bars for 15–30 min using a solution of 10 mm KCl and 1 mm CaCl2 with the XYL’em apparatus (Bronkhorst, Montigny-les-Cormeilles, France). The maximum specific conductivity was determined based on the gravity method (Alder et al., 1997) using the software program graviflow v. 1.1 with four increasing pressure heads (1–3 kPa for each 10-cm branch, 3–5 kPa for each 40-cm branch). Conductance was calculated as the slope of the relation between flow rate and pressure gradient. After the measurements, cross-sections were made and photographed (according to the method described above in the section Wood anatomy) to calculate specific conductivity (Ks), by multiplying by sample length and dividing by xylem area. Feret diameter of each individual vessel and vessel density was measured by creating a binary image with an adjusted threshold and using the ‘analyze particles’ function of ImageJ software (http://rsb.info.nih.gov/ij) for about 3000 vessels per section (five sections per species).
RESULTS
Wood anatomy of Sambucus and Viburnum, and broad phylogenetic comparative analysis of vessel perforation plates in asterids
The wood anatomical descriptions of Sambucus and Viburnum are summarized in Notes S1 and S2 and Table 1, and illustrated in Fig. 1. Character coding of the perforation states for the broadly sampled asterids and the accession numbers of sequences used in their phylogenetic analysis are shown in Table S2.
The Bayesian phylogenetic supermatrix analysis for the broadly sampled asterids converged quickly and resulted in a posterior sample (n = 722, after discarding burn-in of 10 %) that implied well-supported clades [mean posterior probability (PP) on nodes under all-compatible consensus, i.e. on a fully resolved tree, 0·85 ± 0·26] that conform well to the common understanding of the systematics of this group. The ancestral state reconstructions suggest that the scalariform perforation type is ancestral for the asterids as a whole, as well as within the Paracryphiales–Dipsacales clade, and that there are three shifts in the latter towards simple perforations: one leading to Sambucus; one leading to Heptacodium and relatives (Caprifoliaceae) and one to Zabelia (Fig. 2).
Within Caprifoliaceae there is evidence for one reversal back to scalariform perforations in Leycesteria. However, constraining simple-to-scalariform reversals to a rate of 0 (i.e. no reversals, marginal lnL −76·079555) is overall statistically indistinguishable from the unconstrained model [marginal lnL −76·811068; in a Bayes factor (BF) analysis the test statistic is the absolute difference between these marginal likelihoods, which in this case does not exceed 1, i.e. not significant]. In contrast, scalariform-to-simple transitions far exceed reversals (Fig. 3), such that constraining forward and reverse transitions rates to equal yields a significantly poorer fit (marginal lnL -79·150205, BF=2·339137 compared with the unconstrained model).
Molecular phylogenetics of Adoxaceae
Since the phylogenetic relationships within Viburnum and Sambucus are not the focus of this paper, we list here the main results briefly, and comment in more detail about the relationships in Supplementary Data Note S3. Viburnum is sister to all remaining Adoxaceae genera and Sambucus is sister to the clade containing Adoxa, Sinadoxa and Tetradoxa (cf. previous papers listed in Note S3; Fig. 4). Our Viburnum topology supports previous work (cf. previous papers listed in Note S3), except for the position of Viburnum section Valvatotinus sensu Winkworth and Donoghue (2005), which comes out as the earliest-diverging clade in our study. Studies by Donoghue and collaborators fix V. clemensae as root prior, making this species sister to the remaining Viburnum species. In Sambucus, species of the section Ebulus sensu Hara (1983) are sister to the remainder of the genus (Fig. 4). The rest of the clade contains the monophyletic Sambucus section Botryosambucus and the paraphyletic section Sambucus sensu Bolli (1994).
Divergence time estimates
After 20 million generations, all parameters and age estimations had reached ESS values of >200. Adoxaceae were estimated to have a range in crown node age of 75·1–86·4 Ma [95 % highest posterior density (HPD), mean age 80.6 Ma, calibration point used 79·9 Ma]. This suggests an origin in the Late Cretaceous (Fig. 4). The range in crown node age for Viburnum was estimated to be 49·9 – 63·0 Ma (95 % HPD, mean age 56·1 Ma, calibration point used 47·8 Ma; Late Palaeocene), whereas its stem node age is similar to the crown node age of the Adoxaceae. The split of Sambucus and the lineage towards Adoxa, Tetradoxa and Sinadoxa occurred in the Palaeocene, with an estimated mean age of 58·4 Ma (95 % HPD 48·8 – 66·1 Ma). Diversification of Sambucus occurred in the Late Eocene at an estimated mean age of 40·9 Ma (95 % HPD 38·4 – 44·9 Ma). At the end of the Oligocene, Sinadoxa split off from the ancestor of Adoxa and Tetradoxa (95 % HPD 13·4–39·1 Ma, mean age estimate 23·2 Ma), whereas Adoxa diversified from Tetradoxa in the Miocene (95 % HPD 4·8–24·7 Ma, mean age estimate 13·5 Ma).
Within Sambucus, most of the diversification occurred between 20 and 4 Ma (Fig. 4). The initial diversification in the genus in the Late Eocene resulted in two major lineages (species of section Ebulus, and species of sections Sambucus and Botryosambucus, respectively), which in turn started to diversify during the Oligocene (Sambucus, 95 % HPD 13·6–37·5 Ma and mean age estimate 24·3 Ma; Botryosambucus, 95 % HPD 21·4 – 40·6 Ma and mean age estimate 31·7 Ma). The Sambucus clade that contains species from sections Sambucus and Botryosambucus is characterized by two small, early-diverging lineages successively branching off in the Oligocene, and the large split between section Botryosambucus and the remainder of species from section Sambucus occurred during the Late Oligocene (95 % HPD 15·8–33·8 Ma, mean age estimate 24·2 Ma). Divergence of both groups potentially occurred during the Miocene (Fig. 4).
For Viburnum, we observed an initial split during the Late Palaeocene, which resulted in two major lineages (a clade with species of section Valvatotinus and a clade with the remaining Viburnum species, respectively; Fig. 4). Whereas the clade containing species of V. section Valvotinus only started to diversify in the Oligocene (95 % HPD 21·6 – 43·5 Ma, mean age estimate 32·2 Ma), the latter clade had already diverged earlier during the Early to Middle Eocene (95 % HPD 41·2–58·6 Ma, mean age estimate 48·6 Ma).
Ancestral area reconstruction
Hardly any ambiguous ancestral area reconstruction was observed, with relative probability values for the nodes of interest nearly always at least 20 % higher than the next ancestral area alternative. From the 126 nodes analysed, 111 had a relative probability value for a certain ancestral area above 50 %, whereas 79 nodes had a relative probability value above 90 %. According to our analysis, Adoxaceae as a whole, as well as the ancestral lineage towards Sambucus, Adoxa, Sinadoxa and Tetradoxa, have an East Asian (I) origin (Fig. 4). Therefore, stem lineages of both Viburnum and Sambucus have an East Asian (I) origin as well. At the time of initial divergence of Sambucus, a split can be observed into an East Asian (I) and an East Asian (I) or North American (C) lineage. For Viburnum, the earliest split results in an East Asian (I) and an East Asian (I) or combined East Asian–North American (CI) lineage (Fig. 4). The alternative ancestral area reconstruction analysis in which V. clemensae was constrained as sister species to extant Viburnum species provided results similar to those of the original analysis, thereby demonstrating that the different position of V. clemensae did not have a significant impact on the overall ancestral area analysis of Viburnum and Sambucus.
Ancestral climate preference reconstruction
Plotting climate conditions onto the Adoxaceae phylogeny showed that the majority of lineages within Sambucus have a warm temperate preference, whereas the earliest diversification events within Viburnum show a cold temperate climate preference (Fig. 4). Nevertheless, several shifts from warm temperate to tropical or to cold temperate and vice versa have occurred within both genera. Our results reveal that the most recent common ancestor of Sambucus has a PP of 0·82 to be of warm temperate origin (0·13 cold temperate and 0·05 tropical origin). In contrast, the ancestor of Viburnum has a PP of 0·52 to be of cold temperate origin (0·36 warm temperate origin and 0·12 tropical origin). The alternative ancestral climate preference reconstruction analysis with V. clemensae constrained as sister to extant Viburnum species revealed similar but less pronounced results than in the original analysis: Sambucus has a PP of 0·80 to be of warm temperate origin (0·12 cold temperate and 0·08 tropical origin), whereas Viburnum has a PP of 0·45 to be of cold temperate origin (0·35 warm temperate and 0·21 tropical origin).
Present climate preference reconstruction
The climatic niche of Viburnum largely overlaps with that of Sambucus based on present-day distribution data. Viburnum has a broader climatic niche, while Sambucus is more confined to regions with smaller extremes in precipitation and temperature throughout the year (Fig. S1). More specifically, Sambucus species occur in regions with less extreme temperatures during the warmest period of the year, with less extreme precipitation differences throughout the year and lower mean temperatures.
Xylem specific conductivity (Ks) measures
Mean (± s.e.) Ks of S. nigra was three times higher than in V. lantana (5·86 ± 1·3 versus 1·92 ± 0·26 m2 s−1 MPa−1 10−4) for the stem segments representing ∼70 % open vessels. Standardizing sample length based on percentage of open vessels is important due to the shorter vessels in Sambucus compared with Viburnum (maximum vessel length 0·25 and 0·9 m, respectively). Mean (± s.e.) vessel diameter of both species was similar (25·86 ± 0·40 and 25·89 ± 0·27 μm, respectively), while the vessel density in S. nigra was significantly higher than in V. lantana (327 ± 2·2 and 215 ± 1·2 mm−2, respectively).
Discussion
Direction of the scalariform–simple perforation plate shifts within asterids is consistent with Baileyan trends, although reversals may occur
The wood anatomy of Viburnum perfectly fits with that of the Dipsacales outgroup Paracryphiales – characterized by an extremely large number of bars in scalariform perforation plates of up to 100 and more (Patel, 1973; Baas, 1975; Dickison and Baas, 1977) – and agrees with other early-diverging asterid lineages, thereby supporting its plesiomorphic nature (Fig. 2). Likewise, the evolution from scalariform towards simple perforation plates in Adoxaceae confirms the unidirectionality of the Baileyan trends, and represents one of numerous (at least ten) independent scalariform-to-simple transitions within asterids (Fig. 2; Baas and Wheeler, 1996; Lens et al., 2007, 2008). However, the unidirectionality may not always apply in asterids: Fig. 2 suggests one reversal from simple to scalariform vessel perforations in the caprifoloid Leycesteria, and additional reversals may occur in species having mixed simple and scalariform perforations that can occur even within the same vessel or vessel element, as in Araliaceae (Oskolski and Jansen, 2009) or Ericaceae (Lens et al., 2003, 2004b, c). Nevertheless, the preponderance of the evidence suggests that scalariform-to-simple transitions significantly exceed reversals, and that the paucity of reversals makes them statistically indistinguishable from their complete absence in our present analysis (Fig. 3).
The scalariform-to-simple perforation plate shift in Adoxaceae is driven by peak conductive rates
Our mean age estimates for the Adoxaceae are slightly older than those computed by Moore and Donoghue (2007) and Magallon et al. (2015), probably caused by differences in sampling, fossil calibration points and methods of analysis, but there is still an overlap between the 95 % confidence intervals of the two studies. We found that early diversification within Viburnum, showing scalariform vessel perforations with many (on average >20) bars, happened during the Late Palaeocene, which is about 15 million years earlier than the initial diversification of the simple-plated Sambucus clade during the Late Eocene (Fig. 4). Most likely, the difference in timing of early divergence between Viburnum and Sambucus allowed them to evolve in different niches, as supported by the ancestral climate preference reconstruction for Adoxaceae (Fig. 4): the earliest diversification events within Viburnum likely happened in cold temperate regions, while those of Sambucus occurred in warm temperate conditions. Thus, our analyses suggest that differences in temperature are associated with the scalariform-to-simple perforation plate transition in Adoxaceae.
The temperature scenario underlines our current ecological knowledge of wood anatomy, stating that species with scalariform perforations such as Viburnum typically occur in cool (and often mesic) regions that are characterized by low evaporative demands, while simple-plated species are often native to (seasonally) dry habitats (Carlquist, 1975; Baas et al., 1983; Jansen et al., 2004). This trade-off between type of vessel perforation and environment has been poorly investigated using xylem hydraulics. Based on the single-vessel technique, Christman and Sperry (2010) found experimental evidence that scalariform vessel perforations of diverse morphology double the lumen flow resistance, thereby impeding water flow much more than previously estimated based on models (e.g. Ellerby and Ennos, 1998; Schulte, 1999). Our hydraulic measures of V. lantana and S. nigra using standardized stem segments including ∼70 % open vessels and similar vessel diameters support this evidence. We found xylem-specific conductivity values three times higher in the simple-plated S. nigra compared with the scalariform-plated V. lantana, thereby confirming differences in Ks reported in the literature between Sambucus and Viburnum (25–33 and 13 m2 s−1 MPa−1 10−4 respectively; Sperry et al., 2005; Beikircher and Mayr, 2009). In other words, it is plausible to assume that selection for peak conductive rates has triggered scalariform-to-simple perforation plate transitions.
Although there is still controversy about the adaptive role of scalariform perforation plates, freezing temperatures may have been essential during the cold temperate climate in which the earliest diverging Viburnum species evolved (Fig. 4). Some authors claim that the closely spaced bars in the perforations can trap small freezing-induced air bubbles, thereby preventing detrimental levels of embolism when negative pressures in the transpiration stream increase (Zimmermann, 1983). However, other authors observed similar amounts of winter embolism in scalariform and simple-plated species of similar vessel size (Ewers, 1985; Davis et al., 1999) and many scalariform-plated species occur in tropical montane regions that are frost-free (Jansen et al., 2004).
Increasing vessel diameters may compensate for higher flow resistance in scalariform-plated species (cf. Viburnum, see below), but wider vessels make plants more vulnerable to freezing-induced embolism (Charrier et al., 2013, 2014). As mentioned by Davis et al. (1999) and Zanne et al. (2014), 44 µm is the mean hydraulic diameter above which freezing-induced embolisms are becoming frequent at mild negative pressures, agreeing with the mean diameters observed of the temperate viburnums experiencing frost (32 µm) compared with the tropical lowland species (60 µm). In addition to vessel perforation plate morphology and vessel diameter, woody angiosperms have evolved an array of solutions to improve their water flow efficiency. Apart from the increased vessel connectivity in Sambucus compared with Viburnum (cf. Loepfe et al., 2007; Sperry et al., 2007; Lens et al., 2011), other potential characters that could contribute to higher xylem-specific conductivity in Sambucus are thinner intervessel pit membranes (Choat et al., 2008; Lens et al., 2011, 2013) and higher ionic concentrations in xylem sap (Jansen et al., 2011).
Viburnum: out of the tropics?
The ancestral environment of Viburnum remains a matter of debate because of ambiguities in the relationships of the early diverging species. Based on our wider sampling within Adoxaceae, we found no evidence at all for a tropical ancestral habitat (PP 0·12) using our uniform methodology to code climate preferences for the species investigated, even in our analysis where we have constrained the tropical V. clemensae as root prior (PP 0·21). But in the latest Viburnum phylogeny with V. clemensae as root prior (Spriggs et al., 2015), tropical lowland forests were favoured as the ancestral state, although with poor support.
Out of ∼165 Viburnum species, only ∼22 truly lowland tropical species exist today that grow up to 10–20 m tall (Kern, 1951), with several of them positioned in deep branches within the tree, suggesting that Viburnum has been slowly dying off in its potentially ancestral habitat (dying embers hypothesis; Spriggs et al., 2015). According to this dying embers scenario, scalariform-plated species with decreased hydraulic efficiency growing in strongly competitive lowland forests would be outcompeted by more efficient, simple-plated eudicot lineages like Inga (Richardson et al., 2001) and mahoganies (Koenen et al., 2015) that have invaded these forests at a later stage (Sperry et al., 2007). This would probably also explain the worldwide decrease of species with exclusively scalariform perforations from the Cretaceous (70–50 %) towards the current situation (18 %) (Wheeler and Lehman, 2009). This declining trend is even more pronounced in the modern tropical lowlands, where only a minor fraction of the tree species (<10 %) has exclusively scalariform perforations (Baas, 1976; Jansen et al., 2004).
Wood anatomical variation between Viburnum and Sambucus is pronounced, but their ecological niches greatly overlap
Although Viburnum and Sambucus are undeniably close relatives, their wood anatomy can hardly be more different from each other with respect to the variation observed across angiosperms. The key difference lies in vessel characters, but a whole range of additional anatomical patterns co-evolved with the scalariform-to-simple transition in perforation plates (cf. Frost, 1931; Kribs, 1935, 1937; Metcalfe and Chalk, 1950; see Table 1 for a list of anatomical characters that co-evolved with perforation plate morphology). Surprisingly, despite their marked wood anatomical divergence, both genera largely overlap in their climatic niche based on present-day distribution data (Fig. S1). This means that the basic wood anatomical bauplan in Viburnum and Sambucus had already been established during initial diversification, after which no major evolutionary shifts in wood characters have taken place.
Within Viburnum, we did find fine-scale differences between temperate and tropical species. The mature wood samples of the tropical Viburnum species studied, which are scattered into several major subclades (Fig. 4; Spriggs et al., 2015), differ significantly at the 0·01 level in a number of quantitative features compared with the mature samples of the temperate relatives: the vessels are wider (on average 60 versus 32 µm) and less abundant (42 versus 110 mm−2), vessel elements and imperforate tracheary elements are longer (1310 versus 915 µm and 2120 versus 1340 µm, respectively) and rays are taller (1450 versus 670 µm; Table S1). This emphasizes that transitions to different climates within Viburnum have led to minor wood anatomical changes in specific cell types, as has been observed in other genera, such as Symplocos (van den Oever et al., 1981), Cornus (Noshiro and Baas, 2000) or Vaccinium (Lens et al., 2004a). Surprisingly, even the mean number of bars per scalariform perforation plate remains uniform based on our sampling (36 bars in tropical species versus 34 in temperate species). In other words, more efficient water transport in Viburnum seems to be caused by evolving wider vessels with more widely spaced bars in the perforation plates (following the Hagen–Poiseuille law) instead of developing fewer bars per plate. In addition to the more demanding hydraulic conditions in the tropics, the greater vessel diameters of tropical compared with temperate viburnums could also relate to the taller stature of the tropical species (Olson et al., 2014).
Concluding thoughts
In summary, asterids with simple vessel perforations have evolved many times independently from scalariform-plated relatives, although reversals may occasionally occur. Early diversification within Viburnum, characterized by ancestral scalariform perforation plates, occurred about 15 million years earlier than the initial diversification of Sambucus having simple perforations. The plesiomorphic perforation plates of Viburnum are in agreement with the frost-prone, cold temperate climates that were hypothesized in our ancestral climate preference reconstruction during the early diversification in the genus, while higher temperatures during early diversification of Sambucus may have triggered the evolution of simple vessel perforations, allowing improved long-distance water transport efficiency in the xylem. The much higher xylem-specific conductivity values of S. nigra compared with V. lantana confirm that the scalariform-to-simple perforation plate transitions are driven by selection acting on peak conductive rates. However, despite marked differences in wood anatomy and hydraulic conductivity between Viburnum and Sambucus, the ecological niches of the two genera based on present-day species distribution patterns largely overlap. Our study provides a first integrative approach to the underlying triggers behind the evolution of the scalariform-to-simple transition in perforation plates, but there is ample scope for further investigations of the convergent transitions within angiosperms. After Bailey’s pioneering work almost 100 years ago, we are starting to unravel the mechanism behind one of the most cited textbook examples in evolutionary plant anatomy.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of the following. Note S1: species list of wood and DNA samples studied. Note S2: wood description of Viburnum and Sambucus. Note S3: phylogenetic relationships within Viburnum and Sambucus. Table S1: overview of selected wood anatomical characters within Sambucus and Viburnum (Adoxaceae). Table S2: sequence accession numbers and vessel perforation types for asterids. Figure S1: climate niche of Sambucus and Viburnum.
ACKNOWLEDGEMENTS
We would like to thank the xylaria of Tervuren (Tw), Madison (MADw), Smithsonian USw, and Tsukuba (TWTw) for sending wood samples, and Bertie-Joan van Heuven for her valuable help with sectioning the wood samples. The Australian National Botanic Garden, Royal Botanic Garden Melbourne, Royal Botanic Garden Edinburgh, Botanic Garden University of Ghent, Arboretum Kalmthout (Belgium), Arboretum Hof ter Saksen (Belgium), and Arboretum Provincial Domain Bokrijk (Belgium) are acknowledged for sending leaf samples of Sambucus. F.L. received support from the Alberta Mennega Foundation. We are grateful to Andrew Meade for his help in interpreting BayesTraits results.
LITERATURE CITED
- Aberer AJ, Kobert K, Stamatakis A. 2014. ExaBayes: massively parallel Bayesian tree inference for the whole-genome era. Molecular Biology and Evolution 31: 2553–2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alder NN, Pockman WT, Sperry JS, Nuismer S. 1997. Use of centrifugal force in the study of xylem cavitation. Journal of Experimental Botany 48: 665–674. [Google Scholar]
- Antonelli A, Condamine FL, Hettling H. et al. 2014. SUPERSMART: ecology and evolution in the era of big data. PeerJ PrePrints 2: e501v1. [Google Scholar]
- Baas P. 1975. Vegetative anatomy and the affinities of Aquifoliaceae, Sphenostemon, Phelline, and Oncotheca. Blumea 22: 311–407. [Google Scholar]
- Baas P. 1976. Some functional and adaptive aspects of vessel member morphology In: Baas P, Bolton AJ, Catling DM, eds. Wood structure in biological and technological research. Leiden Botanical Series No. 3. The Hague, The Netherlands: Leiden University Press, 157–181. [Google Scholar]
- Baas P, Wheeler EA. 1996. Parallelism and reversibility in xylem evolution: a review. IAWA Journal 17: 351–364. [Google Scholar]
- Baas P, Werker E, Fahn A. 1983. Some ecological trends in vessel characters. IAWA Bulletin 4: 141–159. [Google Scholar]
- Bailey IW. 1944. The development of vessels in angiosperms and its significance in morphological research. American Journal of Botany 31: 421–428. [Google Scholar]
- Bailey IW. 1957. The potentialities and limitations of wood anatomy in the study of the phylogeny and classification of angiosperms. Journal of the Arnold Arboretum 38: 243–254. [Google Scholar]
- Bailey IW, Tupper WW. 1918. Size variation in tracheary cells: I. A comparison between the secondary xylems of vascular cryptogams, gymnosperms and angiosperms. Proceedings of the American Academy of Arts and Sciences USA 54: 147–204. [Google Scholar]
- Beikircher B, Mayr S. 2009. Intraspecific differences in drought tolerance and acclimation in hydraulics of Ligustrum vulgare and Viburnum lantana. Tree Physiology 29: 765–775. [DOI] [PubMed] [Google Scholar]
- Benkova VE, Schweingruber FH. 2004. Anatomy of Russian woods. Bern: Haupt. [Google Scholar]
- Bollback JP. 2006. SIMMAP version 1.0: a program for stochastic mapping of molecular and morphological characters. http://www.simmap.com/v1.0/simmap.html.
- Bolli R. 1994. Revision of the genus Sambucus. Dissertationes Botanicae. Vol. 223. [Google Scholar]
- Bremer K, Friis EM, Bremer B. 2004. Molecular phylogenetic dating of asterid flowering plants shows early Cretaceous diversification. Systematic Biology 53: 496–505. [DOI] [PubMed] [Google Scholar]
- Carlquist S. 1966. Wood anatomy of Compositae: a summary, with comments on factors controlling wood evolution. Aliso 6: 25–44. [Google Scholar]
- Carlquist S. 1975. Ecological strategies of xylem evolution. Berkeley, CA: University of California Press. [Google Scholar]
- Carlquist S. 1984. Vessel grouping in dicotyledon wood: significance and relationships to imperforate tracheary elements. Aliso 10: 505–525. [Google Scholar]
- Charrier G, Cochard H, Améglio T. 2013. Evaluation of the impact of frost resistances on potential altitudinal limit of trees. Tree Physiology 33: 891–902. [DOI] [PubMed] [Google Scholar]
- Charrier G, Charra-Vaskou K, Kasuga J, Cochard H, Mayr S, Améglio T. 2014. Freeze-thaw stress: effects of temperature on hydraulic conductivity and ultrasonic activity in ten woody angiosperms. Plant Physiology 164: 992–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatelet DS, Clement WL, Sack L, Donoghue MJ, Edwards EJ. 2013. The evolution of photosynthetic anatomy in Viburnum (Adoxaceae). International Journal of Plant Sciences 174: 1277–1291. [Google Scholar]
- Choat B, Cobb A, Jansen S. 2008. Structure and function of bordered pits: new discoveries and impacts on whole plant hydraulic function. New Phytologist 177: 608–626. [DOI] [PubMed] [Google Scholar]
- Christman MA, Sperry JS. 2010. Single-vessel flow measurements indicate scalariform perforation plates confer higher flow resistance than previously estimated. Plant, Cell & Environment 33: 431–443. [DOI] [PubMed] [Google Scholar]
- Clarke JB, Tobutt KR. 2006. Development of microsatellite primers and two multiplex polymerase chain reactions for the common elder (Sambucus nigra). Molecular Ecology Notes 6: 453–455. [Google Scholar]
- Clement WL, Arakaki M, Sweeney PW, Edwards EJ, Donoghue MJ. 2014. A chloroplast tree for Viburnum (Adoxaceae) and its implication for phylogenetic classification and character evolution. American Journal of Botany 101: 1029–1049. [DOI] [PubMed] [Google Scholar]
- Clement WL, Donoghue MJ. 2011. Dissolution of Viburnum section Megalotinus (Adoxaceae) of South East Asia and its implications for morphological evolution and biogeography. International Journal of Plant Sciences 172: 559–573. [Google Scholar]
- Clement WL, Donoghue MJ. 2012. Barcoding success as a function of phylogenetic relatedness in Viburnum, a clade of woody angiosperms. BMC Evolutionary Biology 12: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couvreur TLP, Richardson JE, Sosef MSM, Erkens RHJ, Chatrou LW. 2008. Evolution of syncarpy and other morphological characters in African Annonaceae: a posterior mapping approach. Molecular Phylogenetics and Evolution 47: 302–318. [DOI] [PubMed] [Google Scholar]
- Davis SD, Sperry JS, Hacke UG. 1999. The relationship between xylem conduit diameter and cavitation caused by freezing. American Journal of Botany 86: 1367–1372. [PubMed] [Google Scholar]
- Dickison WC, Baas P. 1977. The morphology and relationships of Paracryphia (Paracryphiaceae). Blumea 23: 417–438. [Google Scholar]
- Drummond AJ, Rambaut A. 2007. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Biology 7: 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellerby D, Ennos A. 1998. Resistances to fluid flow of model xylem vessels with simple and scalariform perforation plates. Journal of Experimental Botany 49: 979–985. [Google Scholar]
- Eriksson T, Donoghue MJ. 1997. Phylogenetic analyses of Sambucus and Adoxa (Adoxoideae, Adoxaceae) based on nuclear ribosomal ITS sequences and preliminary morphological data. Systematic Botany 22: 555–573. [Google Scholar]
- Ewers F. 1985. Xylem structure and water conduction in conifer trees, dicot trees, and lianas. IAWA Bulletin 6: 309–317. [Google Scholar]
- Falcon-Lang H, Wheeler EA, Baas P, Herendeen PS. 2012. A diverse charcoalified assemblage of Cretaceous (Santonian) angiosperm woods from Upatoi Creek, Georgia, USA. Part 1: wood types with scalariform perforation plates. Review of Palaeobotany and Palynology 184: 49–73. [Google Scholar]
- Felsenstein J. 1988. Phylogenies inferred from molecular sequences: inference and reliability. Annual Review of Genetics 22: 521–565. [DOI] [PubMed] [Google Scholar]
- Frost FH. 1930a. Specialization in secondary xylem of dicotyledons I. Origin of vessel. Botanical Gazette 89: 67–94. [Google Scholar]
- Frost FH. 1930b. Specialization in secondary xylem of dicotyledons II. Evolution of end wall of vessel segment. Botanical Gazette 90: 198–212. [Google Scholar]
- Frost FH. 1931. Specialization in secondary xylem of dicotyledons III. Specialization of lateral wall of vessel segment. Botanical Gazette 91: 88–96. [Google Scholar]
- Gleason SM, Westoby M, Jansen S. et al. 2016. Weak tradeoff between xylem safety and xylem-specific hydraulic efficiency across the world’s woody plant species. New Phytologist 209: 123–136. [DOI] [PubMed] [Google Scholar]
- Hara H. 1983. A revision of the Caprifoliaceae of Japan with reference to allied plants in other districts and the Adoxaceae. Tokyo: Academia Scientific Books. [Google Scholar]
- Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. 2005. Very high resolution interpolated climate surfaces for global land areas. International Journal of Climatology 25: 1965–1978. [Google Scholar]
- Hijmans RJ, Guarino L, Rojas E. 2011. DIVA-GIS, version 7.5. A geographical information system for the analysis of biodiversity data. http://www.diva-gis.org.
- Huelsenbeck JP, Ronquist F. 2001. MrBayes: Bayesian inference of phylogenetic trees. Bioinformatics 17: 754–755. [DOI] [PubMed] [Google Scholar]
- IAWA Committee. 1989. IAWA list of microscopic features for hardwood identification. IAWA Bulletin New Series 10: 219–332. [Google Scholar]
- InsideWood. 2004. onwards. Search the InsideWood database. http://insidewood.lib.ncsu.edu/search.
- Jansen S, Baas P, Gasson P, Lens F, Smets E. 2004. Variation in xylem structure from tropics to tundra: evidence from vestured pits. Proceedings of the National Academy of Sciences of the USA 101: 8833–8837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen S, Gortan E, Lens F. et al. 2011. Do quantitative vessel and pit characters account for ion-mediated changes in the hydraulic conductance of angiosperm xylem? New Phytologist 189: 218–228. [DOI] [PubMed] [Google Scholar]
- Janssens SB, Geuten K, Yuan Y-M, Song Y, Küpfer P, Smets E. 2006. Phylogenetics of Impatiens and Hydrocera (Balsaminaceae) using chloroplast atpB-rbcL spacer sequences. Systematic Botany 31: 171–180. [Google Scholar]
- Janssens SB, Knox EB, Huysmans S, Smets EF, Merckx VSFT. 2009. Rapid radiation of Impatiens (Balsaminaceae) during Pliocene and Pleistocene: result of a global climate change. Molecular Phylogenetics and Evolution 52: 806–824. [DOI] [PubMed] [Google Scholar]
- Janssens SB, Song Wilson Y, Yuan Y-M, Nagels A, Smets EF, Huysmans S. 2012. A total evidence approach using palynological characters to infer the complex evolutionary history of the Asian Impatiens (Balsaminaceae). Taxon 61: 392–404. [Google Scholar]
- Kanehira R. 1921. Anatomical characters and identification of Formosan woods with critical remarks from the climatic point of view. Taiwan: Government of Formosa. [Google Scholar]
- Katoh K, Misawa K, Kuma K, Miyata T. 2002. MAFFT: a novel method for rapid multiple sequence alignment based on Fourier transform. Nucleic Acids Research 30: 3059–3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern JH. 1951. The genus Viburnum (Caprifoliaceae) in Malaysia. Reinwardtia 1: 107–170. [Google Scholar]
- Koenen EJM, Clarkson JJ, Pennington TD, Chatrou LW. 2015. Recently evolved diversity and convergent radiations of rainforest mahoganies (Meliaceae) shed new light on the origins of rainforest hyperdiversity. New Phytologist 207: 327–339. [DOI] [PubMed] [Google Scholar]
- Kottek M, Grieser J, Beck C, Rudolf B, Rubel F. 2006. World Map of the Köppen-Geiger climate classification updated. Meteorologische Zeitschrift 15: 259–263. [Google Scholar]
- Kribs DA. 1935. Salient lines of structural specialization in the wood rays of dicotyledons. Botanical Gazette 96: 547–557. [Google Scholar]
- Kribs DA. 1937. Salient lines of structural specialization in the wood parenchyma of dicotyledons. Bulletin of the Torrey Botanical Club 64: 177–187. [Google Scholar]
- Lens F, Gasson P, Smets E, Jansen S. 2003. Comparative wood anatomy of epacrids (Styphelioideae, Ericaceae s.l.). Annals of Botany 91: 835–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lens F, Luteyn JL, Smets E, Jansen S. 2004a. Ecological wood anatomy of Vaccinioideae (Ericaceae s.l.). Flora 199: 309–319. [Google Scholar]
- Lens F, Smets E, Jansen S. 2004b. Comparative wood anatomy of Andromedeae s.s., Gaultherieae, Lyonieae and Oxydendreae (Vaccinioideae, Ericaceae s.l.). Botanical Journal of the Linnean Society 144: 161–179. [Google Scholar]
- Lens F, Kron KA, Luteyn JL, Smets E, Jansen S. 2004c. Comparative wood anatomy of the blueberry tribe (Vaccinieae, Ericaceae s.l.). Annals of the Missouri Botanical Garden 91: 566–592. [Google Scholar]
- Lens F, Dressler S, Jansen S, Van Evelghem L, Smets E. 2005. Relationships within balsaminoid Ericales: a wood anatomical approach. American Journal of Botany 92: 941–953. [DOI] [PubMed] [Google Scholar]
- Lens F, Schönenberger J, Baas P, Jansen S, Smets E. 2007. The role of wood anatomy in phylogeny reconstruction of Ericales. Cladistics 23: 229–254. [DOI] [PubMed] [Google Scholar]
- Lens F, Kårehed J, Baas P. et al. 2008. The wood anatomy of the polyphyletic Icacinaceae s.l., and their relationships within asterids. Taxon 57: 525–552. [Google Scholar]
- Lens F, Sperry JS, Christman MA, Choat B, Rabaey D, Jansen S. 2011. Testing hypotheses that link wood anatomy to cavitation resistance and hydraulic conductivity in the genus Acer. New Phytologist 190: 709–723. [DOI] [PubMed] [Google Scholar]
- Lens F, Tixier A, Cochard H, Sperry JS, Jansen S, Herbette S. 2013. Embolism resistance as a key mechanism to understand plant adaptive strategies. Current Opinion in Plant Biology 16: 287–292. [DOI] [PubMed] [Google Scholar]
- Loepfe L, Martinez-Vilalta J, Piñol J, Mencuccini M. 2007. The relevance of xylem network structure for plant hydraulic efficiency and safety. Journal of Theoretical Biology 247: 788–803. [DOI] [PubMed] [Google Scholar]
- Magallon S, Gomez-Acevedo S, Sanchez-Reyes LL, Hernandez-Hernandez T. 2015. A metacalibrated time-tree documents the early rise of flowering plant phylogenetic diversity. New Phytologist 207: 437–453. [DOI] [PubMed] [Google Scholar]
- Manen JF, Natali A, Ehrendorfer F. 1994. Phylogeny of Rubiaceae-Rubieae inferred from the sequence of a cpDNA intergene region. Plant Systematics and Evolution 190: 195–211. [Google Scholar]
- Meade A, Pagel M. 2014. BayesTraits V2.0. Reading, UK: Reading Evolutionary Biology Group; http://www.evolution.rdg.ac.uk/BayesTraits.html. [Google Scholar]
- Metcalfe CR, Chalk L. 1950. Anatomy of the dicotyledons, Vol. 2, 1st edn. Oxford: Clarendon Press. [Google Scholar]
- Moll JW, Janssonius HH. 1920. Mikrographie des Holzes der auf Java vorkommenden Baumarten, 4th edn. Gamopetalae. Leiden: Brill. [Google Scholar]
- Moore BR, Donoghue MJ. 2007. Correlates of diversification in the plant clade Dipsacales: geographic movement and evolutionary innovations. American Naturalist 170: S29–S55. [DOI] [PubMed] [Google Scholar]
- Noshiro S, Baas P. 2000. Latitudinal trends in wood anatomy within species and genera: case study in Cornus s.l. (Cornaceae). American Journal of Botany 87: 1495–1506. [PubMed] [Google Scholar]
- Ogata K. 1988. Wood anatomy of the Caprifoliaceae of Japan. IAWA Bulletin new series 9: 299–316. [Google Scholar]
- Olson ME. 2012. Linear trends in botanical systematics and the major trends of xylem evolution. Botanical Review 78: 154–183. [Google Scholar]
- Olson ME. 2014. Xylem hydraulic function, I.W. Bailey, and Nardini & Jansen: pattern and process. New Phytologist 203: 7–11. [DOI] [PubMed] [Google Scholar]
- Olson ME, Anfodillo T, Rosell JA. et al. 2014. Universal hydraulics of flowering plants: vessel diameter scales with stem length across angiosperm lineages, habits and climates. Ecology Letters 17: 988–997. [DOI] [PubMed] [Google Scholar]
- Oskolski AA, Jansen S. 2009. Distribution of scalariform and simple perforation plates within the vessel network in secondary xylem of Araliaceae and its implications for wood evolution. Plant Systematics and Evolution 278 41–51. [Google Scholar]
- Patel RN. 1973. Wood anatomy of the dicotyledons indigenous to New Zealand. 2. Escaloniaceae. New Zealand Journal of Botany 11: 421-434. [Google Scholar]
- Posada D. 2008. jModelTest: phylogenetic model averaging. Molecular Biology and Evolution 25: 1253–1256. [DOI] [PubMed] [Google Scholar]
- Rambaut A, Drummond AJ. 2007. Tracer v1.4. http:// beast.bio.ed.ac.uk/Tracer.
- Ree RH, Smith SA. 2008. Maximum likelihood inference of geographic range evolution by dispersal, local extinction, and cladogenesis. Systematic Biology 57: 4–14. [DOI] [PubMed] [Google Scholar]
- Ree RH, Moore BR, Webb CO, Donoghue MJ. 2005. A likelihood framework for inferring the evolution of geographic range on phylogenetic trees. Evolution 59: 2299–2311. [PubMed] [Google Scholar]
- Reid EM, Chandler MEJ. 1926. The Bembridge flora. Catalogue of Cainozoic plants in the Department of Geology, Vol. 1 London: British Museum (Natural History; ). [Google Scholar]
- Richardson JE, Pennington RT, Pennington TD, Hollingsworth PM. 2001. Rapid diversification of a species-rich genus of Neotropical rain forest trees. Science 293: 2242–2245. [DOI] [PubMed] [Google Scholar]
- Ronquist F, Huelsenbeck JP. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19: 1572–1574. [DOI] [PubMed] [Google Scholar]
- Sanderson MJ. 2004. R8s version 1.70 user’s manual. Davis, California.
- Schmerler SB, Clement WL, Beaulieu JM. et al. 2012. Evolution of leaf form correlates with tropical-temperate transitions in Viburnum (Adoxaceae). Proceedings of the Royal Society B 279: 3905–3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte PJ. 1999. Water flow though a 20-pore perforation plate in vessels of Liquidambar styraciflua. Journal of Experimental Botany 50: 1179-1187. [Google Scholar]
- Schweingruber FH. 1990. Anatomy of European woods. Bern: Haupt. [Google Scholar]
- Silvestro D, Schnitzler J, Zizka G. 2011. A Bayesian framework to estimate diversification rates and their variation through time and space. BMC Evolutionary Biology 11: 311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons MP, Ochoterena H. 2000. Gaps as characters in sequence-based phylogenetic analysis. Systematic Biology 49: 369–381. [PubMed] [Google Scholar]
- Sperry JS, Hacke UG, Wheeler JK. 2005. Comparative analysis of end wall resistivity in xylem conduits. Plant, Cell and Environment 28: 456–465. [Google Scholar]
- Sperry JS, Hacke UG, Feild TS, Sano Y, Sikkema EH. 2007. Hydraulic consequences of vessel evolution in angiosperms. International Journal of Plant Sciences 168 1127–1139. [Google Scholar]
- Spriggs EL, Clement WL, Sweeney PW, Madriñán S, Edwards EJ, Donoghue MJ. 2015. Temperate radiations and dying embers of a tropical past: the diversification of Viburnum. New Phytologist 207: 340–354. [DOI] [PubMed] [Google Scholar]
- Stamatakis A, Hoover P, Rougement J. 2008. A fast bootstrapping algorithm for the RAxML web servers. Systematic Biology 57: 758–771. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Glazko GV, Nei M. 2002. Overcredibility of molecular phylogenies obtained by Bayesian phylogenetics. Proceedings of the National Academy of Science of the USA 99: 16138–16143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taberlet P, Gielly L, Pautou G, Bouvet J. 1991. Universal primers for amplification of three non-coding regions of chloroplast DNA. Plant Molecular Biology 17: 1105–1109. [DOI] [PubMed] [Google Scholar]
- Takhtajan A. 1986. Floristic regions of the world. Berkeley, USA: University of California Press. [Google Scholar]
- Tel-Zur N, Abbo S, Myslabodski D, Mizrahi Y. 1999. Modified CTAB procedure for DNA isolation from epiphytic cacti of the genera Hylocereus and Selenicereus (Cactaceae). Plant Molecular Biology Reporter 17: 249–254. [Google Scholar]
- Van den Oever L, Baas P, Zandee M. 1981. Comparative wood anatomy of Symplocos and latitude and altitude of provenance. IAWA Bulletin New Series 2: 3-24. [Google Scholar]
- von Schwerin FG. 1909. Monographie der gattung Sambucus. Mitteilungen der Deutschen Dendrologischen Gesellschaft 18: 1–56. [Google Scholar]
- von Schwerin FG. 1920. Revisio generis Sambucus. Mitteilungen der Deutschen Dendrologischen Gesellschaft 29: 57–94. [Google Scholar]
- Wang Q, Ferguson DK, Feng G-P. et al. 2010. Climatic change during the Paleocene to Eocene based on fossil plants from Fushun, China. Palaeogeography, Palaeoclimatology, Palaeoecology 296: 323–331. [Google Scholar]
- Wheeler EA, Baas P. 1991. A survey of the fossil record for dicotyledonous wood and its significance for evolutionary and ecological wood anatomy. IAWA Journal 312: 275–318. [Google Scholar]
- Wheeler EA, Lehman TM. 2009. New late Cretaceous and Paleocene dicot woods of Big Bend National Park, Texas and a review of Cretaceous wood characteristics. IAWA Journal 30: 293–318. [Google Scholar]
- White TJ, Bruns T, Lee S, Taylor J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics In: Innis M, Gelfand D, Sninsky J, White TJ, eds. PCR protocols: a guide to methods and applications. San Diego, CA: Academic Press, 315–322. [Google Scholar]
- Winkworth RC, Donoghue MJ. 2005. Viburnum phylogeny based on combined molecular data: implications for taxonomy and biogeography. American Journal of Botany 92: 653–666. [DOI] [PubMed] [Google Scholar]
- Xie W, Lewis PO, Fan Y, Kuo L, Chen MH. 2011. Improving marginal likelihood estimation for Bayesian phylogenetic model selection. Systematic Biology 60: 150–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young ND, Steiner KE, de Pamphilis CW. 1999. The evolution of parasitism in Scrophulariaceae/Orobanchaceae: plastid gene sequences refute an evolutionary transition series. Annals of the Missouri Botanical Garden 86: 876–893. [Google Scholar]
- Zanne AE, Tank DC, Cornwell WK. et al. 2014. Three keys to the radiation of angiosperms into freezing environments. Nature 506: 89–92. [DOI] [PubMed] [Google Scholar]
- Zimmermann M. 1983. Xylem structure and the ascent of sap. Berlin: Springer. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.